Temsirolimus at up to 25 mg i.v. weekly was added to a backbone of rituximab and cladribine for first-line therapy of mantle cell lymphoma in an elderly cohort of patients with acceptable toxicity and promising activity .
Keywords: mantle cell lymphoma, temsirolimus, cladribine
Abstract
Background
We conducted this trial to determine the maximum tolerated dose (MTD) of temsirolimus added to an established regimen comprised of rituximab and cladribine for the initial treatment of mantle cell lymphoma.
Patients and methods
A standard phase I cohort of three study design was utilized. The fixed doses of rituximab and cladribine were 375 mg/m2 i.v. day 1 and 5 mg/m2/day i.v. days 1–5 of a 28-day cycle, respectively. There were five planned temsirolimus i.v. dose levels: 15 mg day 1; 25 mg day 1; 25 mg days 1 and 15; 25 mg days 1, 8 and 15; and 25 mg days 1, 8, 15, and 22.
Results
Seventeen patients were treated: three each at levels 1–4 and five at dose level 5. The median age was 75 years (52–86 years). Mantle Cell International Prognostic Index (MIPI) scores were low in 6% (1), intermediate in 59% (10), and high in 35% (6) of patients. Five patients were treated at level 5 without dose limiting toxicity. Hematologic toxicity was frequent: grade 3 anemia in 12%, grade 3 thrombocytopenia in 41%, grade 4 thrombocytopenia in 24%, grade 3 neutropenia in 6%, and grade 4 neutropenia in 18% of patients. The overall response rate (ORR) was 94% with 53% complete response and 41% partial response. The median progression-free survival was 18.7 months.
Conclusions
Temsirolimus 25 mg i.v. weekly may be safely added to rituximab and cladribine at 375 mg/m2 i.v. day 1 and 5 mg/m2/day i.v. days 1–5 of a 28-day cycle, respectively. This regimen had promising preliminary activity in an elderly cohort of patients with mantle cell lymphoma.
ClinicalTrials.gov Identifier
introduction
The treatment of mantle cell lymphoma (MCL) has diverged along two different pathways. In the first, dose intensity has been explored as a potential strategy to overcome resistance to chemotherapy. Regimens such as R-Hyper-CVAD (rituximab, cyclophosphamide, vincristine, doxorubicin, and dexamethasone) alternating with high-dose methotrexate and cytarabine have demonstrated high overall and complete response (CR) rates but are quite toxic, particularly in patients over 65 years of age [1, 2]. High-dose therapy followed by autologous stem-cell transplant in first remission is also commonly employed either after aggressive induction chemotherapy or after less intense therapy such as R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone), R-DHAP (rituximab, dexamethasone, cytarabine, and cisplatin) or RB (rituximab and bendamustine) [3–10]. A second less aggressive approach has typically been applied to older patients, patients with comorbidities, or those who desire a less aggressive approach. Options here have included R-CHOP [11], RB [12], and RC (rituximab and cladribine) [13, 14] often followed by rituximab maintenance [15]. Other agents shown to have significant activity in this disease at the point of relapse include ibrutinib [16], lenalidomide [17, 18], bortezomib [19, 20], and temsirolimus [21, 22]. Despite historical improvement in the median survival of patients with mantle cell lymphoma from ∼3–5 years, there is significant room for further development of effective combination therapies.
A series of trials in the North Central Cancer Treatment Group demonstrated that cladribine had a single-agent overall response rate (ORR) of 81% with 42% CR in newly diagnosed patients [13]. The addition of rituximab to the cladribine backbone increased the CR rate to 52% and appeared to improve the duration of response. Single-agent trials of the mammalian target of rapamycin (mTOR) inhibitor temsirolimus in patients with relapsed mantle cell lymphoma demonstrated an ORR of 38% at a dose of 250 mg i.v. weekly with a similar ORR of 41% at a 10-fold dose reduction to 25 mg weekly [21, 22]. Addition of rituximab to the low dose temsirolimus regimen resulted in a 59% ORR with 19% CR in relapsed mantle cell lymphoma [23]. Based on these promising results, we designed a phase I trial to combine rituximab, cladribine, and temsirolimus (RCT) for initial treatment of mantle cell lymphoma.
materials and methods
patient selection
Mantle cell lymphoma was confirmed by central pathology review. Patients were eligible if they had measurable disease as defined by at least one mass ≥2 cm, or based on splenomegaly extending at least 3 cm below the left costal margin. Patients were also eligible for study entry if they had assessable disease based on diffuse organ infiltration, though, in this case, response criteria did not include the possibility of partial response (PR). Additional eligibility criteria included: age ≥18 years; ECOG PS 0, 1, 2 or 3; platelets ≥100 000 × 109/l; neutrophils ≥1.500 × 109/l; and adequate renal and hepatic function. Exclusion criteria included: prior therapy for lymphoma; central nervous system involvement by lymphoma, HIV positive; prior mTOR inhibitor treatment; concurrent malignancy; or stem-cell transplant planned.
All study participants provided written informed consent. The protocol was approved by relevant Institutional Review Boards and carried out according to the guidelines of good clinical practice and with ethical standards for human experimentation.
trial design
phase I dosing regimen and escalation
This was an open-label cohort of three phase I dose escalation study designed to determine the maximum tolerated dose (MTD) of i.v. temsirolimus that could be added to a previously established combination of rituximab and cladribine at 375 mg/m2 i.v. day 1 and 5 mg/m2/day i.v. days 1–5 of a 28-day cycle, respectively. Either pegfilgrastim 6 mg day 6 or filgrastim 300–480 μm days 6–15 were routinely given. Prophylactic therapy for viral infections and/or pneumocystis was allowed but not required. The trial was conducted by the North Central Cancer Treatment Group. MTD is defined as the dose level below the lowest dose that induces dose-limiting toxicity (DLT) in at least one-third of patients (at least two of a maximum of six patients). The five dose levels were: 15 mg day 1; 25 mg day 1; 25 mg days 1, 15; 25 mg days 1, 8, 15; and 25 mg days 1, 8, 15, 22. Toxicity data were collected during all cycles of therapy and monitored.
DLTs were defined as any of the following occurring during the first cycle of therapy: grade 4 absolute neutrophil count (ANC) (<500/mm3) for ≥5 days; grade 4 ANC with fever >100.5 F or active infection; platelets <25 000/mm3; grade 4 infection; or ≥grade 3 nonhematologic toxicity at least possibly related to therapy as per NCI Common Terminology Criteria for Adverse Events v3.0 (CTCAE). Infusion reactions to rituximab were not considered DLTs.
patient evaluation
Baseline evaluation included medical history and examination; laboratory testing including hepatitis B and C serologies, chemistry group, complete blood count, and lipid panel; bone marrow biopsy; computed axial tomography (CT) scans of the chest, abdomen and pelvis; and central pathology review. Positron emission tomography (PET/CT) was recommended, but not required. Adverse event assessment was carried out at baseline and subsequently before each treatment cycle as well as at each monitoring visit as per NCIv3.0CTCAE. Patients were followed actively until either disease progression or 3 years following completion of therapy, whichever came first. CT or PET/CT was carried out every 3 months for the first 2 years and every 4 months for the third year of follow-up in responding patients. Event monitoring continued until 5 years from patient registration. Tumor response assessment was carried out after two cycles of therapy as per Cheson et al. [24]. Previously positive imaging studies were repeated. Patients achieving a CR received two more cycles of therapy and went to observation. A negative bone marrow was required for CR if previously positive. Patients with a PR or evidence of benefit but not meeting strict criteria for PR received two more cycles and were again restaged after the fourth cycle. If CR was achieved or there was evidence of further improvement, subjects then received two further cycles for a total of six cycles followed by end of therapy restaging. Patients with progressive disease at any time had protocol therapy discontinued. Patients with stable disease without benefit after two cycles or who had no improvement after the fourth cycle compared with after the second cycle after having a previous PR or stable disease with evidence of benefit went off treatment after cycle 4. Patients in CR at the end of therapy were not to receive maintenance therapy or undergo stem-cell transplant. Patients achieving less than a CR could proceed to other therapy at patient and physician discretion, and went to event monitoring at that point.
statistical methods
A standard phase I cohort of three study design was used for dose escalation. Sample size was determined by the dose escalation algorithm as described above and estimated to require 9–30 patients. Toxicity was assessed and attributed as per the NCI CTCAE v3.0, and DLT and MTD were defined as noted above. Hematologic toxicity was assessed as continuous variables as well as by standard toxicity grading. Nonhematologic toxicities were evaluated by ordinal CTCAE standard toxicity grading only. Overall toxicity incidences as well as toxicity profiles by dose level were analyzed by descriptive statistics. Overall and progression-free survival (PFS) curves were computed using the Kaplan–Meier method. Patients were censored for PFS if they were still alive and received subsequent treatment before progression occurred.
results
general
This study opened in April 2009 and closed to accrual February 2012 with a total of 17 patients treated. The original plan was to transition to a phase II trial at the MTD; however, support for the phase II portion of the trial was withdrawn after the original sponsor (Wyeth Pharmaceuticals, Inc.) was acquired by Pfizer, Inc. Pretreatment characteristics are as reported in Table 1. The median age was 75 and 94% had intermediate or high-risk disease per the Mantle Cell International Prognostic Index (MIPI), reflecting selection of patients who were poor candidates for dose intense therapy. There were three patients treated on each of the first four dose levels, and five patients were treated on the highest planned dose level at temsirolimus 25 mg days 1, 8, 15, and 22. Dose-reduction tables were specified for both cladribine and temsirolimus at the start of each cycle and dose-reduction tables for day 8, 15, and 22 temsirolimus were predetermined. For dose levels 1 and 2, there were no dose reductions nor omitted doses and all but two patients (both dose level 2 and off for adverse events) completed treatment per protocol. For dose level 3, two patients had no dose reductions nor omitted doses and went off treatment of physician discretion and completed per protocol, respectively. The other dose level 3 patient had cladribine reduction, a missed dose of temsirolimus, and went off treatment of adverse events after three cycles. All three patients at dose level 4 had dose reductions of cladribine, at least one omitted dose of temsirolimus, and went off treatment of adverse events before receiving six cycles. For dose level 5, two patients completed treatment per protocol (one had two omitted temsirolimus doses while the other did not and there were no dose reductions), two patients had omitted temsirolimus doses and cladribine reductions (discontinued for alternative therapy and adverse event, respectively), and one patient discontinued due to progression after two cycles with no reductions nor omitted doses. In all, seven patients completed therapy per protocol, seven discontinued therapy for adverse events after three to five cycles (three in CR; four in PR), one progressed on therapy, one went to alternative therapy in PR after four cycles, and one chose to go off therapy in PR after four cycles.
Table 1.
Patient characteristics
| Total (n = 17) | |
|---|---|
| Age (years) | |
| Median | 75.0 (52.0–86.0) |
| Range | |
| Sex | |
| Female | 6 (35.3%) |
| Male | 11 (64.7%) |
| Extranodal site involvement | |
| Yes | 16 (94.1%) |
| No | 1 (5.9%) |
| Clinical stage | |
| II | 1 (5.9%) |
| III | 1 (5.9%) |
| IV | 15 (88.2%) |
| International Prognostic Index | |
| Low | 2 (11.8%) |
| Low-intermediate | 2 (11.8%) |
| High-intermediate | 10 (58.8%) |
| High | 3 (17.6%) |
| Mantle Cell International Prognostic Index | |
| Low | 1 (5.9%) |
| Intermediate | 10 (58.8%) |
| High | 6 (35.3%) |
dose-limiting toxicity and maximum tolerated dose
The protocol as originally written resulted in a grade 3 rituximab infusion-related adverse event being categorized as a DLT. There were no other DLTs, and the protocol was amended to exclude rituximab infusion-related adverse events as DLT to prevent premature closure unrelated to the addition of temsirolimus. The protocol was closed to accrual after five patients had completed cycle 1 at the maximum planned dose without DLT, as the statistical design would determine the MTD at that level regardless of the outcome of a sixth patient.
safety profile
All patients were assessable for adverse events. Table 2 reports grade ≥3 adverse events at least possibly related to treatment. Adverse events regardless of attribution occurring during therapy are reported in supplementary Table S1, available at Annals of Oncology online. Data reported covers all cycles delivered. Hematologic toxicity was frequent. Three patients experienced thrombotic episodes, two of which were attributed to therapy. There were three episodes of pneumonitis. One case was documented as pneumocystis by polymerase chain reaction. This patient went off protocol and was not rechallenged with chemotherapy. The second was in the setting of blood-culture-positive sepsis during the last cycle of therapy in complete remission. The third case had no organism identified, was treated with empiric antibiotics only, and did not recur with subsequent chemotherapy, making this unlikely to have been pulmonary toxicity from temsirolimus.
Table 2.
Grade 3+ adverse events at least possibly related to treatment (maximum grade per patient during treatment cycles)
| Toxicity | Grade |
|||
|---|---|---|---|---|
| 3 |
4 |
|||
| N | % | N | % | |
| Platelet count decreased | 7 | 41.2 | 4 | 23.5 |
| Lymphocyte count decreased | 1 | 5.9 | 8 | 47.1 |
| Leukocyte count decreased | 3 | 17.6 | 3 | 17.6 |
| Neutrophil count decreased | 1 | 5.9 | 3 | 17.6 |
| Fatigue | 3 | 17.6 | ||
| Cytokine release syndrome | 2 | 11.8 | ||
| Hemoglobin decreased | 2 | 11.8 | ||
| Thrombosis | 2 | 11.8 | ||
| Blood glucose increased | 1 | 5.9 | ||
| Febrile neutropenia | 1 | 5.9 | ||
| Hemorrhage nasal | 1 | 5.9 | ||
| Nausea | 1 | 5.9 | ||
| Pneumonia (grade 0/1/2 ANC) | 1 | 5.9 | ||
| Pneumonia (grade 3/4 ANC) | 1 | 5.9 | ||
| Pneumonitis | 1 | 5.9 | ||
| Vomiting | 1 | 5.9 | ||
Cholesterol and triglycerides were assessed before therapy and monitored during therapy because of the known association of mTOR inhibitors and hyperlipidemia. Grade 1 increased cholesterol occurred in 41% and grade 1 or 2 elevated triglycerides in 47% of patients, respectively. There were treatment delays on two dose level 2 patients, three dose level 4 patients, and two dose level 5 patients. The median (range) number of cycles received was 5 (2–6) with 17 patients completing at least two cycles, 13 patients completing at least four cycles, and 6 patients completing all six cycles. Three patients had a new primary malignancy (basal cell carcinoma of the left lower eyelid, prostate adenocarcinoma, and myelodysplasia).
tumor response
The ORR was 94% with 53% CR and 41% PR in this elderly patient cohort. The distribution of responses across dose levels is shown in Table 3. One patient had stable disease after two cycles of therapy and subsequently achieved a CR after six cycles of therapy. One patient progressed after cycle 2. All other responders had at least a PR after two cycles of therapy. Figure 1A and B shows the PFS and OS for all patients with a median follow-up of 30.7 months in 11 alive patients.
Table 3.
Best response by dose level
| Dose level | Number of patients | Best response |
||
|---|---|---|---|---|
| CR | PR | PD | ||
| 1 | 3 | 2 | 1 | |
| 2 | 3 | 3 | ||
| 3 | 3 | 1 | 2 | |
| 4 | 3 | 1 | 2 | |
| 5 | 5 | 2 | 2 | 1 |
Figure 1.
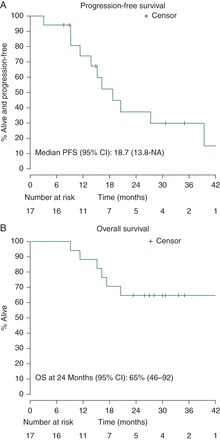
(A) Progression-free survival following RCT therapy (median 18.7 months). Five patients were censored for transitioning to other therapy before progression (one radiation, three other chemotherapy, one stem-cell transplant). (B) Overall survival following RCT therapy (median not reached).
discussion
This investigator-initiated study demonstrated that temsirolimus can be safely added to rituximab and cladribine at a dose of 25 mg i.v. weekly without any reduction in the dose of cladribine. This dose was chosen as the maximum tested dose and schedule based on previous studies in relapsed mantle cell lymphoma which demonstrated no suggestion of increased activity of this single agent at 250 mg i.v. weekly (ORR 38%) [21] versus 25 mg i.v. weekly (ORR 41%) [22]. It is quite likely that the true MTD may be significantly higher, as there were no DLTs at this level. Other investigators are studying the combination of temsirolimus with other agents, including rituximab and bendamustine at higher doses of temsirolimus.
This combination was well tolerated despite the advanced age of the patients (median 75 years). While the study is small and phase I by design, limiting the predictive value for response, the ORR of 94% with 53% CR is encouraging given the advanced age of the cohort. Historical comparisons include an ORR of 94% with 34% CR in a group of median age 61.5 treated with R-CHOP [25] and a recent publication suggesting superior PFS for RB compared with R-CHOP in a subset analysis of MCL patients of median age 70, though specific response rates and MIPI scores were not reported [26]. A more intense regimen combined cytarabine with a reduced bendamustine dose RB in 40 patients with MCL with median age 70 years. Subset analysis of 20 patients with previously untreated disease revealed an ORR of 100% with a 95% CR reported [27].
In summary, we were able to combine temsirolimus 25 mg i.v. weekly with full-dose rituximab and cladribine for up to six cycles in newly diagnosed MCL patients of median age 75 years without undue toxicity. Preliminary data suggests significant antitumor activity. These results suggest that further trials of combination therapy including temsirolimus and other newer agents are warranted, particularly in patients who are older or ineligible for more dose intensive strategies such as hematopoietic stem-cell transplantation.
funding
This trial was an investigator-initiated and written trial conducted through the North Central Cancer Treatment Group, which is now part of Alliance. Wyeth Pharmaceuticals provided temsirolimus as well as clinical trial support funding (Grant 3066K1–1163). Wyeth was subsequently purchased by Pfizer Pharmaceuticals. The manuscript was investigator written.
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
references
- 1.Romaguera JE, Fayad L, Rodriguez MA, et al. High rate of durable remissions after treatment of newly diagnosed aggressive mantle-cell lymphoma with rituximab plus hyper-CVAD alternating with rituximab plus high-dose methotrexate and cytarabine. J Clin Oncol. 2005;23:7013–7023. doi: 10.1200/JCO.2005.01.1825. [DOI] [PubMed] [Google Scholar]
- 2.Romaguera JE, Fayad LE, Feng L, et al. Ten-year follow-up after intense chemoimmunotherapy with Rituximab-HyperCVAD alternating with Rituximab-high dose methotrexate/cytarabine (R-MA) and without stem cell transplantation in patients with untreated aggressive mantle cell lymphoma. Br J Haematol. 2010;150:200–208. doi: 10.1111/j.1365-2141.2010.08228.x. [DOI] [PubMed] [Google Scholar]
- 3.Dreyling M, Lenz G, Hoster E, et al. Early consolidation by myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission significantly prolongs progression-free survival in mantle-cell lymphoma: results of a prospective randomized trial of the European MCL Network. Blood. 2005;105:2677–2684. doi: 10.1182/blood-2004-10-3883. [DOI] [PubMed] [Google Scholar]
- 4.Siddiqui M, Johnston P, Inwards D, et al. Autologous stem cell transplantation (ASCT) in first remission for patients with mantle cell lymphoma is associated with a prolonged survival. Ann Oncol. 2005;16:199. [Google Scholar]
- 5.Murali S, Winton E, Waller EK, et al. Long-term progression-free survival after early autologous transplantation for mantle-cell lymphoma. Bone Marrow Transplant. 2008;42:529–534. doi: 10.1038/bmt.2008.201. [DOI] [PubMed] [Google Scholar]
- 6.Van't Veer MB, Notenboom A, McKenzie M, et al. First report of the HOVON 45: a phase II study with rituximab, high dose Ara-C and autologous stem cell transplantation in the primary treatment of mantle cell lymphoma. Blood. 2006;108:2734. [Google Scholar]
- 7.Geisler CH, Kolstad A, Laurell A, et al. Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo-purged stem cell rescue: a nonrandomized phase 2 multicenter study by the Nordic Lymphoma Group. Blood. 2008;112:2687–2693. doi: 10.1182/blood-2008-03-147025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Till BG, Gooley TA, Crawford N, et al. Effect of remission status and induction chemotherapy regimen on outcome of autologous stem cell transplantation for mantle cell lymphoma. Leuk Lymphoma. 2008;49:1062–1073. doi: 10.1080/10428190801923725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Damon LE, Johnson JL, Niedzwiecki D, et al. Immunochemotherapy and autologous stem-cell transplantation for untreated patients with mantle-cell lymphoma: CALGB 59909. J Clin Oncol. 2009;27:6101–6108. doi: 10.1200/JCO.2009.22.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delarue R, Haioun C, Ribrag V, et al. CHOP and DHAP plus rituximab followed by autologous stem cell transplantation in mantle cell lymphoma: a phase 2 study from the Groupe d'Etude des Lymphomes de l'Adulte. Blood. 2013;121:48–53. doi: 10.1182/blood-2011-09-370320. [DOI] [PubMed] [Google Scholar]
- 11.Howard OM, Gribben JG, Neuberg DS, et al. Rituximab and CHOP induction therapy for newly diagnosed mantle-cell lymphoma: molecular complete responses are not predictive of progression-free survival. J Clin Oncol. 2002;20:1288–1294. doi: 10.1200/JCO.2002.20.5.1288. [DOI] [PubMed] [Google Scholar]
- 12.Rummel MJ, Niederle N, Maschmeyer G. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial (vol 381, pg 1203, 2013) Lancet. 2013;381:1184. doi: 10.1016/S0140-6736(12)61763-2. [DOI] [PubMed] [Google Scholar]
- 13.Inwards DJ, Fishkin PA, Hillman DW, et al. Long-term results of the treatment of patients with mantle cell lymphoma with cladribine (2-CDA) alone (95–80–53) or 2-CDA and rituximab (N0189) in the North Central Cancer Treatment Group. Cancer. 2008;113:108–116. doi: 10.1002/cncr.23537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spurgeon SE, Pindyck T, Okada C, et al. Cladribine plus rituximab is an effective therapy for newly diagnosed mantle cell lymphoma. Leuk Lymphoma. 2011;52:1488–1494. doi: 10.3109/10428194.2011.575489. [DOI] [PubMed] [Google Scholar]
- 15.Kahl BS, Longo WL, Eickhoff JC, et al. Maintenance rituximab following induction chemoimmunotherapy may prolong progression-free survival in mantle cell lymphoma: a pilot study from the Wisconsin Oncology Network. Ann Oncol. 2006;17:1418–1423. doi: 10.1093/annonc/mdl127. [DOI] [PubMed] [Google Scholar]
- 16.Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369:507–516. doi: 10.1056/NEJMoa1306220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Habermann TM, Lossos IS, Justice G, et al. Lenalidomide oral monotherapy produces a high response rate in patients with relapsed or refractory mantle cell lymphoma. Br J Haematol. 2009;145:344–349. doi: 10.1111/j.1365-2141.2009.07626.x. [DOI] [PubMed] [Google Scholar]
- 18.Witzig TE, Vose J, Zinzani PL, et al. An international phase II trial of single-agent lenalidomide for relapsed or refractory aggressive B-cell non-Hodgkin's lymphoma. Ann Oncol. 2011;22:1622–1627. doi: 10.1093/annonc/mdq626. [DOI] [PubMed] [Google Scholar]
- 19.Fisher RI, Bernstein SH, Kahl BS, et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2006;24:4867–4874. doi: 10.1200/JCO.2006.07.9665. [DOI] [PubMed] [Google Scholar]
- 20.Belch A, Kouroukis CT, Crump M, et al. A phase II study of bortezomib in mantle cell lymphoma: the National Cancer Institute of Canada Clinical Trials Group trial IND.150. Ann Oncol. 2007;18:116–121. doi: 10.1093/annonc/mdl316. [DOI] [PubMed] [Google Scholar]
- 21.Witzig TE, Geyer SM, Ghobrial I, et al. Phase II trial of single-agent temsirolimus (CCI-779) for relapsed mantle cell lymphoma. J Clin Oncol. 2005;23:5347–5356. doi: 10.1200/JCO.2005.13.466. [DOI] [PubMed] [Google Scholar]
- 22.Ansell SM, Inwards DJ, Rowland KM, Jr, et al. Low-dose, single-agent temsirolimus for relapsed mantle cell lymphoma: a phase 2 trial in the North Central Cancer Treatment Group. Cancer. 2008;113:508–514. doi: 10.1002/cncr.23580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ansell SM, Tang H, Kurtin PJ, et al. Temsirolimus and rituximab in patients with relapsed or refractory mantle cell lymphoma: a phase 2 study. Lancet Oncol. 2011;12:361–368. doi: 10.1016/S1470-2045(11)70062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheson BD. The International Harmonization Project for Response Criteria in Lymphoma Clinical Trials. Hematol Oncol Clin North Am. 2007;21:841–854. doi: 10.1016/j.hoc.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 25.Lenz G, Dreyling M, Hoster E, et al. Immunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improves response and time to treatment failure, but not long-term outcome in patients with previously untreated mantle cell lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG) J Clin Oncol. 2005;23:1984–1992. doi: 10.1200/JCO.2005.08.133. [DOI] [PubMed] [Google Scholar]
- 26.Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381:1203–1210. doi: 10.1016/S0140-6736(12)61763-2. [Erratum appears in Lancet: 1184]. [DOI] [PubMed] [Google Scholar]
- 27.Visco C, Finotto S, Zambello R, et al. Combination of rituximab, bendamustine, and cytarabine for patients with mantle-cell non-Hodgkin lymphoma ineligible for intensive regimens or autologous transplantation. J Clin Oncol. 2013;31:1442–1449. doi: 10.1200/JCO.2012.45.9842. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


