Abstract
The identification of vulnerable atherosclerotic plaques in the coronary arteries is emerging as an important tool for guiding atherosclerosis diagnosis and interventions. Assessment of plaque vulnerability requires knowledge of both the structure and composition of the plaque. Intravascular photoacoustic (IVPA) imaging is able to show the morphology and composition of atherosclerotic plaque. With imminent improvements in IVPA imaging, it is becoming possible to assess human coronary artery disease in vivo. Although some challenges remain, IVPA imaging is on its way to being a powerful tool for visualising coronary atherosclerotic features that have been specifically associated with plaque vulnerability and clinical syndromes, and thus such imaging might become valuable for clinical risk assessment in the catheterisation laboratory.
Keywords: Vulnerable plaque, intravascular photoacoustic imaging, atherosclerosis, intravascular imaging, coronary artery disease
Vulnerable atherosclerotic plaque comprises a lipid-rich necrotic core, covered by a thin fibrous cap, that is weakened by macrophage infiltration.[1] Plaques that have this morphology and composition have a greater probability of being associated with acute coronary syndromes in clinical studies.[2,3] Coronary artery disease is most commonly triggered by the rupture of vulnerable plaque and thrombosis.[4] The identification of vulnerable plaque is emerging as an important element in coronary artery disease diagnosis and treatment.
In the past three decades, several imaging modalities have been employed to identify vulnerable plaque. Coronary angiography is the oldest of these modalities and can only visualise the artery lumen, not the artery wall and atherosclerotic lesions. Moreover, angiography creates images of a three-dimensional moving object that are presented in a two-dimensional plane. Intravascular, catheter-based imaging methods can be safely used in a routine catheterisation laboratory population[5] and offer direct visualisation of the artery wall. Intravascular ultrasound (IVUS) imaging has been used in routine clinical practice for more than 20 years. Due to the limited acoustic contrast between soft tissues, the conventional grayscale IVUS image is not sensitive enough to differentiate plaque composition, except for the presence of calcium.[6,7] While the application of an advanced analysis algorithm to IVUS images – virtual histology IVUS – has improved plaque composition characterisation,[8] no data yet support its accuracy in the detection of lipid-rich necrotic plaque core.[9–11] IVUS palpography is a technology used to assess local mechanical plaque properties based on tissue deformation caused by intraluminal pressure.[12] It can only sense the stiffness of the tissue, however, and cannot directly image the tissue, and it only assesses the first 450 μm of the arterial wall.[12] Light-based intravascular optical coherence tomography (IVOCT) creates high-resolution images (approximately 15 μm) with backscattered light from the tissue[13] and can image plaque components such as calcium, lipid and thrombus. IVOCT currently relies on qualitative image interpretation to distinguish these features.[14,15] The depth to which IVOCT penetrates is only 1–2 mm into the atherosclerotic tissue, which means it often cannot see the extent of the plaque up to the adventitia. Near-infrared spectroscopy (NIRS) is another light-based imaging modality for the detection of lipid-rich necrotic core in plaque.[16] It provides a probability for the presence of coronary lipid based on the spectral analysis of backscattered light;[17] however, NIRS cannot identify the amount and location of lipids, which may be important in risk assessment.[18]
Several other innovative optical modalities have been proposed recently[19–22] that are currently being evaluated in clinical trials. The ability of these technologies to provide reliable, spatially-precise, quantitative imaging of plaque lipid content is yet to be determined. In this paper, a newer modality is reviewed: intravascular photoacoustic (IVPA) imaging. This is an optical–acoustic hybrid imaging modality. IVPA is currently being developed to show both the morphology and chemical composition of the artery wall with a good imaging depth and resolution. This technology takes advantage of the unique optical absorption contrast between different tissues and reasonably large penetration depth owing to the low acoustic attenuation in soft tissues.[23,24] Published experimental data suggest it may be a strong contender for detailed plaque characterisation and the identification of plaque at increased risk of causing clinical sequelae.
The Principles of IVPA Imaging of Atherosclerotic Plaque
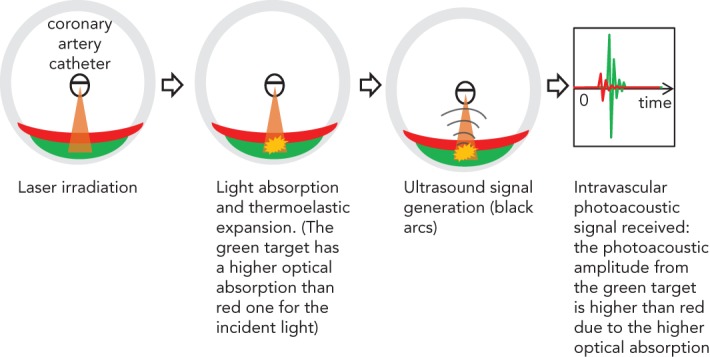
IVPA imaging requires short laser pulses, lasting a few nanoseconds, to be applied to irradiate the artery wall. Absorption of the light converts the optical energy into heat in the tissue and causes thermoelastic expansion, leading to a transient pressure rise (the laser pulse duration is shorter than both the stress relaxation and the thermal diffusion times). Figure 1 illustrates the principles involved in IVPA imaging.
Figure 1: Illustration of the Intravascular Photoacoustic Imaging Principle.
The specific optical absorption spectra of lipids and other pure substances can be related to the identification of plaque.[25] Various biomarkers present in plaques have been explored for their usefulness in IVPA imaging, with lipid being the most frequently used. IVPA imaging of plaque lipids can be performed at around 1.2 μm or 1.72 μm due to the high lipid-specific absorption contrast with relatively high resolution (~50 μm) and reasonable penetration depth.[26] At 1.2 μm the laser’s maximal permissible exposure limit is 20 mJ/cm2 and at 1.72 μm it is 1 J/cm2, according to the American National Standards Institute laser safety standard.[27,28] NIRS also makes use of lipid to identify potentially vulnerable plaque, but there is no depth resolution. The capability of IVPA to produce images of atherosclerotic plaque lipids has been demonstrated extensively with ex vivo tissues and in vivo animal models.[26,27,29–33]
Macrophages are an important cell type related to the progression of atherosclerosis[34] and the specific imaging of plaque macrophage content is a potentially interesting feature when identifying vulnerable plaque.[36] Macrophages do not exhibit a strong intrinsic contrast in the way lipids do, therefore exogenous imaging contrast agents with high optical absorption are applied in order to visualise them using IVPA imaging.[36,37] Furthermore, given that the activity of the enzyme matrix metalloproteinase (MMP) is an indicator of plaque instability, the localisation of MMP activity with an MMP-sensitive activatable probe in vulnerable plaques in human carotid specimens has been achieved using photoacoustic imaging.[38] More recently, an ApoE-/- mouse in vivo model injected with a photoacoustic ICG@PEG-Ag2S nanoprobe was successful in imaging plaque.[39]
Developing IVPA Imaging for Clinical Use
Encouraged by the capability of IVPA to create images of vulnerable atherosclerotic plaque, an intensive research effort is on-going to incorporate IVPA imaging into clinical applications.
Miniaturising the IVPA Catheter
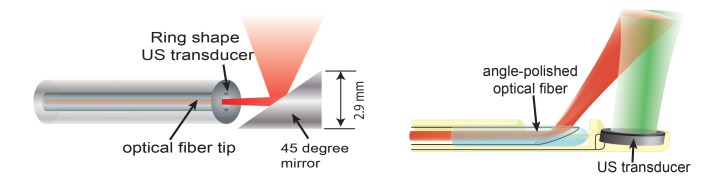
A miniature, and highly sensitive, catheter for IVPA imaging is an essential element if IVPA imaging technology is to enter common use. As IVPA is an intrinsically multimodal imaging technique, the catheter’s technical requirements are harder to meet than for single-modality probes like optical coherence tomography or IVUS catheters. A typical IVPA imaging catheter consists of an optical part for light delivery and a broadband ultrasound transducer. The optical part is usually a combination of an optical fibre and a mirror or a single-angle polished optical fibre.[40] Two IVPA catheter designs are shown in Figure 2. The unit shown in Figure 2A provides the greatest reported sensitivity. Miniaturisation is challenging, however, and this unit is currently too large for intravascular application, having a diameter of 2.9 mm.[27,28] Another design possibility – one that is favoured for miniaturisation – incorporates an offset between the optical fibre and ultrasound transducer, either longitudinally[41,42] (see Figure 2B) or laterally.[43] The smallest IVPA catheter reported to date that uses this design has an outer diameter of 0.9 mm, which is below the threshold of 1 mm desired for clinical translation into coronary arteries.[44]
Figure 2: Different Intravascular Photoacoustic (IVPA) Catheter Designs.
(A) A collinear IVPA catheter. (B) An IVPA catheter with a longitudinal offset between optical and acoustic beams, where red is the optical beam and green the ultrasound beam [adapted from Wu et al., 2010[41]].
Efficient and Specific Plaque Identification
Lipids are found in the plaque inside the artery wall, which is rich in cholesterol and cholesteryl esters,[45,46] as well as in peri-adventitial fat tissue around the artery, which contains a mixture of fatty acids.[47] The spectral contrast between plaque lipids and peri-adventitial fat makes it possible to differentiate between them using spectroscopic IVPA imaging (sIVPA).[26] As a consequence of this, the presence and location of lipids within a plaque can be reliably and rapidly displayed during catheter pullback, using both cross-sectional and longitudinal data display. Performing multiple wavelength sIVPA imaging, however, has an impact on the complexity, cost andspeed of the imaging system. Minimisation of the number of wavelengths for the differentiation of different lipids or improving the efficiency for specific lipid differentiation is necessary for practical application. The possibility of specific plaque lipid imaging using sIVPA with limited number of wavelengths has been investigated (see Figure 3).[26,48] It is possible to achieve about 70 % true positive and 15 % false positive pixel identification of plaque lipids based on the relative difference between amplitudes of only two wavelengths (see Figure 4).[49]
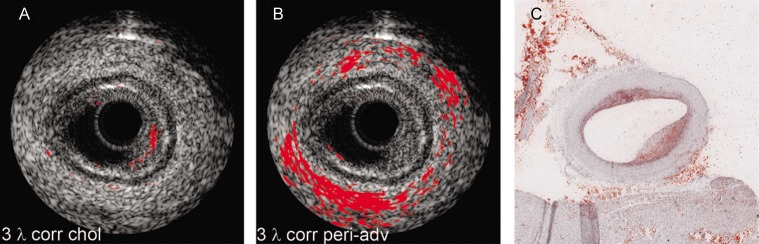
Figure 3: Lipid Identification in an Atherosclerotic Human Coronary Artery.
(A) Plaque lipid identification based on a three-wavelength correlation with the reference spectra of cholesterol in the 1.2 mm spectral range. (B) Peri-adventitial fat identification based on three-wavelength correlation with the reference spectra of human peri-adventitial fat tissue. (C) Histology result following Oil Red O staining. All lipid identifications have been laid over registered intravascular ultrasound images. Adapted from Jansen et al., 2013.[48]
Figure 4: Ex Vivo Lipid Differentiation Result of the Atherosclerotic Left Anterior Ascending Coronary Artery of an 80-year-old Man.
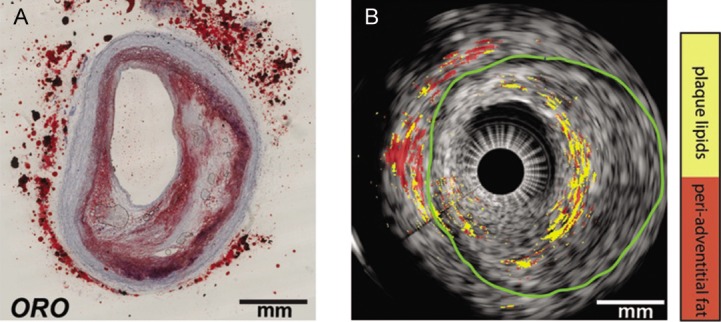
(A) Histology: Oil Red O (ORO) staining of the intravascular photoacoustic/intravascular ultrasound image in cross-section. The lipids are shown in red. (B) Lipid differentiation map overlaid on the co-registered intravascular ultrasound image of the coronary artery. The lipids in the plaques are yellow and lipids in the peri-adventitial tissue are red. The green contour indicates the external elastic lamina. Adapted from Wu et al., 2015.[49]
High-speed IVPA Imaging of Atherosclerotic Plaque
Despite successful plaque identification by IVPA imaging, the low imaging speed limits its application in practice. Lipid specificity remains limited by the single-wavelength operation and the average optical power remains quite high (up to 1 W), which raises concerns about thermal damage to the patient’s artery and the potential hazard to the operator resulting from skin and eye exposure. With the introduction of higher pulse-rate laser systems (2 kHz), IVPA imaging speed has been increased from about 0.3 to 1 frame per second.[27,28,50] This represents a significant step forward, but is still not practical. More and faster IVPA imaging systems are, however, on the horizon. A fast multiple-wavelength sIVPA imaging system with potentially ~5 frames per second has been developed that is capable of imaging at a low-energy pulse.[44]
Discussion
IVPA imaging shows great promise in detecting the morphology and composition (e.g. lipid deposition) of arterial lipid plaque burden. Owing to the extensive research on IVPA imaging, several important milestones have been passed, with the development of a miniature flexible catheter (<1 mm in diameter), high-efficiency specific imaging of vulnerable plaque composition and the development of a high-speed IVPA imaging system (~ 5 frames per second). Some challenges remain, however, in the further development of IVPA imaging.
Most current IVPA imaging systems require the artery to be cleared of blood by flushing for a better photoacoustic signal-to-noise ratio. It is in principle possible to perform IVPA imaging at 1.72 μm without flushing, due to the similarity in the optical absorption property of blood and water-based tissues. Wang et al. performed the first in vivo IVPA imaging (at 1,720 nm) in a hypercholesterolemic rabbit model through blood.[29] Further IVPA imaging through blood – and in and ex vivo human tests – must await the resolution of other clinical implementation issues described in this article. Although the imaging speed has been significantly improved,[51–53] it is still not fast enough for clinical use. Recently, it was found that the sensitivity of IVPA catheters can be increased by an order of magnitude by matching the frequency response of the receiving transducer to the low-frequency range.[54,55] This enables imaging with lower pulse energy, allowing for fast laser sources with moderate output power. Other challenges in adapting IVPA imaging to the clinical setting include the cost and robustness of the laser system and the choice of catheter sheath material, which needs to be transparent for ultrasound and infrared light. These topics being investigated in on-going engineering research.
Conclusion
IVPA imaging is unique in its biochemical specificity and can offer the clinician direct, validated visualisation of coronary plaque lipid. To many clinicians, the ideal tool for guiding atherosclerotic plaque diagnosis and interventions requires high resolution to characterise the thin cap, high sensitivity to detect lipids for plaque visualisation in the artery, and a reasonable depth of penetration for plaque burden assessment. To achieve this, the combination of IVOCT and IVPA appears to be a promising choice, but at the cost of integrating an extra optical coherence tomography laser system. However, the natural progression of plaque with vulnerable characteristics is complex and the development and application of clinical tools for its assessment is, likewise, challenging. To date, IVPA imaging remains a research tool; future advances in technology will determine to what degree it will be of use in clinical practice.
References
- 1.Schaar JA, Muller JE, Falk E et al. Terminology for high- risk and vulnerable coronary artery plaques. Eur Heart J. 2004;25:1077–82. doi: 10.1016/j.ehj.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Kini AS, Motoyama S, Vengrenyuk Y et al. Multimodality intravascular imaging to predict periprocedural myocardial infarction during percutaneous coronary intervention. JACC Cardiovasc Interv. 2015;8:937–45. doi: 10.1016/j.jcin.2015.03.016. 10.1016/j.jcin.2015.03.016 [DOI] [PubMed] [Google Scholar]
- 3.Goldstein JA, Maini B, Dixon SR et al. Detection of lipid- core plaques by intracoronary near-infrared spectroscopy identifies high risk of periprocedural myocardial infarction. Circ Cardiovasc Interv. 2011;4:429–37. doi: 10.1161/CIRCINTERVENTIONS.111.963264. 10.1161/CIRCINTERVENTIONS.111.963264 [DOI] [PubMed] [Google Scholar]
- 4.http://www.who.int/topics/cardiovascular_diseases/en/ http://www.who.int/topics/cardiovascular_diseases/en/ World Health Organization. Cardiovascular diseases (CVDs). January 2015. Available at: (accessed 9 September 2016)
- 5.de van der Sijde JN, Karanasos A, van Ditzhuijzen NS Safety of optical coherence tomography in daily practice: a comparison with intravascular ultrasound. Eur Heart J Cardiovasc Imag. 2016. 10.1093/ehjci/jew037 jew037. [DOI] [PubMed]
- 6.Allen TJ, Hall A, Dhillon AP et al. Spectroscopic photoacoustic imaging of lipid-rich plaques in the human aorta in the 740 to 1400 nm wavelength range. J Biomed Opt. 2012;17:061209–10. doi: 10.1117/1.JBO.17.6.061209. 10.1117/1.JBO.17.6.061209 [DOI] [PubMed] [Google Scholar]
- 7.Choudhury RP, Fuster V, Fayad ZA. Molecular, cellular and functional imaging of atherothrombosis. Nat Rev Drug Discov. 2004;3:913–25. doi: 10.1038/nrd1548. [DOI] [PubMed] [Google Scholar]
- 8.Nair A, Kuban BD, Tuzcu EM et al. Coronary plaque classification with intravascular ultrasound radiofrequency data analysis. Circulation. 2002;106:2200–6. doi: 10.1161/01.cir.0000035654.18341.5e. [DOI] [PubMed] [Google Scholar]
- 9.Thim T, Hagensen MK, Wallace-Bradley D et al. Unreliable assessment of necrotic core by virtual histology intravascular ultrasound in porcine coronary artery disease. Circ Cardiovasc Imaging. 2010;3:384–91. doi: 10.1161/CIRCIMAGING.109.919357. 10.1161/CIRCIMAGING.109.919357 [DOI] [PubMed] [Google Scholar]
- 10.Shin ES, Garcia-Garcia HM, Ligthart JM et al. In vivo findings of tissue characteristics using iMap IVUS and virtual histology IVUS. EuroIntervention. 2011;6:1017–9. doi: 10.4244/EIJV6I8A175. 10.4244/EIJV6I8A175 [DOI] [PubMed] [Google Scholar]
- 11.Puri R, Worthley MI, Nicholls SJ. Intravascular imaging of vulnerable coronary plaque: current and future concepts. Nat Rev Cardiol. 2011;8:131–9. doi: 10.1038/nrcardio.2010.210. 10.1038/nrcardio.2010.210 [DOI] [PubMed] [Google Scholar]
- 12.Schaar JA, van der Steen AF, Mastik F et al. Intravascular palpography for vulnerable plaque assessment. J Am Coll Cardiol. 2006;47:C86–91. doi: 10.1016/j.jacc.2006.01.035. [DOI] [PubMed] [Google Scholar]
- 13.Tearney GJ, Regar E, Akasaka T et al. International Working Group for Intravascular Optical Coherence Tomography (IWG-IVOCT). Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol. 2012;59:1058–72. doi: 10.1016/j.jacc.2011.09.079. 10.1016/j.jacc.2011.09.079 [DOI] [PubMed] [Google Scholar]
- 14.Jansen K, van Soest G, van der Steen AFW. Intravascular photoacoustic imaging: A new tool for vulnerable plaque identification. Ultrasound Med Biol. 2014;40:1037–48. doi: 10.1016/j.ultrasmedbio.2014.01.008. 10.1016/j.ultrasmedbio.2014.01.008 [DOI] [PubMed] [Google Scholar]
- 15.van Soest G, Goderie T, Regar E et al. Atherosclerotic tissue characterization in vivo by optical coherence tomography attenuation imaging. J Biomed Opt. 2010;15:011105. doi: 10.1117/1.3280271. 10.1117/1.3280271 [DOI] [PubMed] [Google Scholar]
- 16.Gardner CM, Tan H, Hull EL et al. Detection of lipid core coronary plaques in autopsy specimens with a novel catheter-based near-infrared spectroscopy system. JACC Cardiovasc Imaging. 2008;1:638–48. doi: 10.1016/j.jcmg.2008.06.001. 10.1016/j.jcmg.2008.06.001 [DOI] [PubMed] [Google Scholar]
- 17.Waxman S, Dixon SR, L’Allier P et al. In vivo validation of a catheter-based near-infrared spectroscopy system for detection of lipid core coronary plaques: initial results of the SPECTACL study. J Am Coll Cardiol Img. 2009;2:858–68. doi: 10.1016/j.jcmg.2009.05.001. 10.1016/j.jcmg.2009.05.001 [DOI] [PubMed] [Google Scholar]
- 18.Niccoli G, Liuzzo G, Montone RA et al. Advances in mechanisms, imaging and management of the unstable plaque. Atherosclerosis. 2014;233:467–77. doi: 10.1016/j.atherosclerosis.2014.01.036. 10.1016/j.atherosclerosis.2014.01.036 [DOI] [PubMed] [Google Scholar]
- 19.Vinegoni C, Botnaru I, Aikawa E et al. Indocyanine green enables near-infrared fluorescence imaging of lipid-rich, inflamed atherosclerotic plaques. Sci Transl Med. 2011;3:84ra–45. doi: 10.1126/scitranslmed.3001577. 10.1126/scitranslmed.3001577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ughi GJ, Wang H, Gerbaud E Clinical characterization of coronary atherosclerosis with dual-modality OCT and near- infrared autofluorescence imaging. JACC Cardiovasc Imaging. 2016. 10.1016/j.jcmg.2015.11.020 epub ahead of press. [DOI] [PMC free article] [PubMed]
- 21.Nadkarni SK, Bouma BE, Helg T et al. Characterization of atherosclerotic plaques by laser speckle imaging. Circulation. 2005;112:885–92. doi: 10.1161/CIRCULATIONAHA.104.520098. 10.1161/CIRCULATIONAHA.104.520098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Soest G, van der Steen AFW, Regar E. Autofluorescence: a new NIR on the block. JACC Cardiovasc Imaging. 2016. 10.1016/j.jcmg.2015.12.011 epub ahead of press. [DOI] [PubMed]
- 23.Jansen K, van Soest G, van der Steen T. Coronary Atherosclerosis: Current Management and Treatment. 2012. pp. 166–74. Photoacoustic imaging of coronary arteries: current status and potential clinical application. In: Arampatzis C, McFadden EP, Michalis LK, Virmani R, Serruys PW (eds) First edn. London: Informa Healthcare,
- 24.Schoenhagen P, Vince DG. Intravascular photoacoustic tomography of coronary atherosclerosis riding the waves of light and sound. J Am Coll Cardiol. 2014;64:391–3. doi: 10.1016/j.jacc.2014.05.018. 10.1016/j.jacc.2014.05.018 [DOI] [PubMed] [Google Scholar]
- 25.Fleming CP, Eckert J, Halpern EF et al. Depth resolved detection of lipid using spectroscopic optical coherence tomography. Biomed Opt Express. 2013;4:1269–84. doi: 10.1364/BOE.4.001269. 10.1364/BOE.4.001269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jansen K, Wu M, van der Steen AF et al. Photoacoustic imaging of human coronary atherosclerosis in two spectral bands. Photoacoustics. 2014;2:12–20. doi: 10.1016/j.pacs.2013.11.003. 10.1016/j.pacs.2013.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang P, Ma T, Slipchenko MN et al. High-speed Intravascular photoacoustic imaging of lipid-laden atherosclerotic plaque enabled by a 2-kHz barium nitrite raman laser. Sci Rep. 2014;4:6889. doi: 10.1038/srep06889. 10.1038/srep06889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hui J, Yu Q, Ma T et al. High-speed intravascular photoacoustic imaging at 1.7 μm with a KTP-based OPO. Biomed Opt Express. 2015;6:4557–66. doi: 10.1364/BOE.6.004557. 10.1364/BOE.6.004557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang B, Karpiouk A, Yeager D et al. Intravascular photoacoustic imaging of lipid in atherosclerotic plaques in the presence of luminal blood. Opt Lett. 2012;37:1244–6. doi: 10.1364/OL.37.001244. 10.1364/OL.37.001244 [DOI] [PubMed] [Google Scholar]
- 30.Allen TJ, Hall A, Dhillon A 2010. 10.1117/12.842205 (eds). Photoacoustic imaging of lipid rich plaques in human aorta. In: Oraevsky AA, Wang LV (eds) Photons Plus Ultrasound: Imaging and Sensing 2010 San Francisco, California: SPIE.
- 31.Jansen K, van der Steen AFW, van Beusekom HMM et al. Intravascular photoacoustic imaging of human coronary atherosclerosis. Opt Lett. 2011;36:597–9. doi: 10.1364/OL.36.000597. 10.1364/OL.36.000597 [DOI] [PubMed] [Google Scholar]
- 32.Wang B, Su JL, Amirian J et al. Detection of lipid in atherosclerotic vessels using ultrasound-guided spectroscopic intravascular photoacoustic imaging. Opt Express. 2010;18:4889–97. doi: 10.1364/OE.18.004889. 10.1364/OE.18.004889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang P, Wang HW, Sturek M et al. Bond-selective imaging of deep tissue through the optical window between 1600 and 1850 nm. J Biophotonics. 2012;5:25–32. doi: 10.1002/jbio.201100102. 10.1002/jbio.201100102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 35.Tearney GJ, Yabushita H, Houser SL et al. Quantification of macrophage content in atherosclerotic plaques by optical coherence tomography. Circulation. 2003;107:113–9. doi: 10.1161/01.cir.0000044384.41037.43. [DOI] [PubMed] [Google Scholar]
- 36.Wang B, Joshi P, Sapozhnikova V (eds). Intravascular photoacoustic imaging of macrophages using molecularly targeted gold nanoparticles. Proc. SPIE 7564, Photons Plus Ultrasound: Imaging and Sensing 2010. 10.1117/12.841070 2010:75640A.
- 37.Wang B, Yantsen E, Larson T et al. Plasmonic intravascular photoacoustic imaging for detection of macrophages in atherosclerotic plaques. Nano Lett. 2009;9:2212–7. doi: 10.1021/nl801852e. 10.1021/nl801852e [DOI] [PubMed] [Google Scholar]
- 38.Razansky D, Harlaar NJ, Hillebrands JL et al. Multispectral optoacoustic tomography of matrix metalloproteinase activity in vulnerable human carotid plaques. Mol Imaging Biol. 2012;14:277–85. doi: 10.1007/s11307-011-0502-6. 10.1007/s11307-011-0502-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu C, Zhang Y, Li Z et al. A novel photoacoustic nanoprobe of ICG@ PEG-Ag 2 S for atherosclerosis targeting and imaging in vivo. Nanoscale. 2016;8:12531–9. doi: 10.1039/c6nr00060f. 10.1039/c6nr00060f [DOI] [PubMed] [Google Scholar]
- 40.Karpiouk AB, Wang B, Emelianov SY. Development of a catheter for combined intravascular ultrasound and photoacoustic imaging. Rev Sci Instrum. 2010;81:014901. doi: 10.1063/1.3274197. 10.1063/1.3274197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu M, Jansen K, Springeling G et al. Impact of device geometry on the imaging characteristics of an intravascular photoacoustic catheter. Appl Opt. 2014;53:8131–9. doi: 10.1364/AO.53.008131. 10.1364/AO.53.008131 [DOI] [PubMed] [Google Scholar]
- 42.Jansen K, Springeling G, Lancee C An intravascular photoacoustic imaging catheter. Presented at: IEEE International Ultrasonics Symposium, San Diego, 11–14 October 2010.
- 43.Li X, Wei W, Zhou Q et al. Intravascular photoacoustic imaging at 35 and 80 MHz. J Biomed Opt. 2012;17:106005–1. doi: 10.1117/1.JBO.17.10.106005. 10.1117/1.JBO.17.10.106005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Y, Gong X, Liu C et al. High-speed intravascular spectroscopic photoacoustic imaging at 1000 A-lines per second with a 0.9-mm diameter catheter. J Biomed Opt. 2015;20:065006. doi: 10.1117/1.JBO.20.6.065006. 10.1117/1.JBO.20.6.065006 [DOI] [PubMed] [Google Scholar]
- 45.Lundberg B. Chemical composition and physical state of lipid deposits in atherosclerosis. Atherosclerosis. 1985;56:93–110. doi: 10.1016/0021-9150(85)90087-5. [DOI] [PubMed] [Google Scholar]
- 46.Stegemann C, Drozdov I, Shalhoub J et al. Comparative lipidomics profiling of human atherosclerotic plaques. Circ Cardiovasc Genet. 2011;4:232–42. doi: 10.1161/CIRCGENETICS.110.959098. 10.1161/CIRCGENETICS.110.959098 [DOI] [PubMed] [Google Scholar]
- 47.Tsai CL, Chen JC, Wang WJ. Near-infrared absorption property of biological soft tissue constituents. J Med Biol Eng. 2001;21:7–14. [Google Scholar]
- 48.Jansen K, Wu M, van der Steen AF et al. Lipid detection in atherosclerotic human coronaries by spectroscopic intravascular photoacoustic imaging. Opt Express. 2013;21:21472–84. doi: 10.1364/OE.21.021472. 10.1364/OE.21.021472 [DOI] [PubMed] [Google Scholar]
- 49.Wu M, Jansen K, van der Steen AF et al. Specific imaging of atherosclerotic plaque lipids with two-wavelength intravascular photoacoustics. Biomed Opt Express. 2015;6:3276–86. doi: 10.1364/BOE.6.003276. 10.1364/BOE.6.003276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Piao Z, Ma T, Li J et al. High speed intravascular photoacoustic imaging with fast optical parametric oscillator laser at 1.7 μm. Appl Phys Lett. 2015;107:083701. doi: 10.1063/1.4929584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sethuraman S, Aglyamov SR, Amirian JH et al. Intravascular photoacoustic imaging using an IVUS imaging catheter. IEEE Trans Ultrason Ferroelectr Freq Control. 2007;54(5):978–978. doi: 10.1109/tuffc.2007.343. [DOI] [PubMed] [Google Scholar]
- 52.Sethuraman S, Amirian JH, Litovsky SH et al. Ex vivo characterization of atherosclerosis using intravascular photoacoustic imaging. Opt Express. 2007;15:16657–66. doi: 10.1364/oe.15.016657. [DOI] [PubMed] [Google Scholar]
- 53.Sethuraman S, Amirian JH, Litovsky SH et al. Spectroscopic intravascular photoacoustic imaging to differentiate atherosclerotic plaques. Opt Express. 2008;16:3362–7. doi: 10.1364/oe.16.003362. [DOI] [PubMed] [Google Scholar]
- 54.Daeichin V, Wu M, de Jong N et al. Frequency analysis of the photoacoustic signal generated by coronary atherosclerotic plaque. Ultrasound Med Biol. 2016;42:2017–25. doi: 10.1016/j.ultrasmedbio.2016.03.015. 10.1016/j.ultrasmedbio.2016.03.015 [DOI] [PubMed] [Google Scholar]
- 55.Daeichin V, Chen C, Ding Q et al. A broadband polyvinylidene difluoride-based hydrophone with integrated readout circuit for intravascular photoacoustic imaging. Ultrasound Med Biol. 2016;42:1239–43. doi: 10.1016/j.ultrasmedbio.2015.12.016. 10.1016/j.ultrasmedbio.2015.12.016 [DOI] [PubMed] [Google Scholar]