Abstract
Background
This phase II CALGB trial evaluated the activity and safety of an extended induction schedule of galiximab (G) plus rituximab (R) in untreated follicular lymphoma (FL).
Patients and methods
Patients with previously untreated FL (grades 1, 2, 3a) received 4 weekly infusions of G + R, followed by an additional dose every 2 months four times. International Workshop Response Criteria were used to evaluate response.
Results
Sixty-one patients were treated and antibody infusions were well tolerated. The overall response rate (ORR) is 72.1% (95% confidence interval 59.2% to 82.9%): 47.6% complete response (CR)/unconfirmed complete response (CRu) and 24.6% partial response. At a median follow-up time of 4.3 years (range, 0.3–5.3 years) median progression-free survival (PFS) is 2.9 years. Notably, Follicular Lymphoma International Prognostic Index (FLIPI) correlated with ORR, CR rate, and PFS, and the low-risk FLIPI group (n = 12) achieved a 92% ORR, 75% CR/CRu rate, and 75% 3-year PFS.
Conclusions
An extended induction schedule of G + R in previously untreated FL is well tolerated and appears particularly efficacious in those patients with low-risk FLIPI scores. In addition, this trial served as the initial platform for additional CALGB ‘doublet’ combination regimes of rituximab plus other novel targeted agents.
Keywords: follicular lymphoma, Follicular Lymphoma International Prognostic Index (FLIPI), galiximab, immunotherapy, monoclonal antibodies, rituximab
introduction
Follicular lymphomas (FLs) are heterogeneous diseases with different histological grades (i.e. grades 1, 2, 3a, 3b), tumor- and patient-based prognostic indices [e.g. Follicular Lymphoma International Prognostic Index (FLIPI), tumor-associated macrophages, etc.] [1–3], and a variable therapeutic outcome. The majority of patients are initially treated with rituximab-based immunochemotherapy [4–10] with recent data suggesting benefit from rituximab maintenance (RM) therapy or a single infusion of Yttrium-90 ibritumumab tiuxetan [11]. However, based on the heterogeneity of FL, a single upfront treatment approach may not be appropriate for all FL patients. For example, a patient with non-bulky low-risk FLIPI disease may not require the same dose intensity as one with bulky symptomatic poor-risk disease. Rituximab monotherapy has been evaluated in the upfront treatment of FL and may be associated with prolonged progression-free survival (PFS) [12–15]. In fact, the randomized phase II SAKK 35/98 trial evaluated single-agent rituximab at two different schedules: standard therapy for four weekly doses, followed by randomization to observation or prolonged rituximab (i.e. 375 mg/m2 every 2 months for four times) in those patients responding or with stable disease at week 12 [16]. Median event-free survival in chemotherapy-naive patients was significantly improved in patients receiving prolonged treatment (n = 25) versus observation (n = 26): 36 months versus 19 months, respectively. In an unplanned long-term analysis of patients participating on this trial, 45% of previously untreated patients receiving prolonged rituximab were without events at 8 years [17]. Some limitations of this long-term analysis include the following: the evaluation was retrospective, computed tomography (CT) scan tumor evaluations were required for only 5 years, FLIPI scores were not prospectively collected and unavailable retrospectively, and a relatively small number of patients were included in this dataset. Antibody combination regimens are also under development in an attempt to improve treatment outcomes, avoid non-specific toxicities typically associated with chemotherapeutic agents, and theoretically decrease the risk of developing rituximab resistance by targeting more than a single antigen [18–20]. Galiximab, a primatized (i.e. human-macaque chimeric) anti-CD80 monoclonal antibody (mAb) has been evaluated in phase I and II studies of indolent and FL. CD80 (B7.1) is a transmembrane glycoprotein which is involved in regulating the balance between immune activation and suppression. Published data suggest that CD80 is involved in activation/regulation of T-cells [21], transiently expressed on activated B cells and dendritic cells [22], plays a role in regulation and activation of normal and malignant B cells [23], and is constitutively expressed on malignant B cells [24]. Cross-linking surface CD80 on lymphoma cells with anti-CD80 antibodies is associated with inhibition of cellular proliferation, induction of antibody-dependent cellular cytotoxicity (ADCC), and upregulation of proapoptotic molecules [23, 25, 26]. In addition, galiximab may inhibit tumor progression by binding to non-malignant, CD80-positive cells in the tumor microenvironment (e.g. macrophages, dendritic cells, myeloid-derived suppressor cells) that may alter the cytokine profile and/or cellular composition to favor tumor inhibition. An initial phase I/II dose-escalation trial of galiximab monotherapy (i.e. 4 weekly doses) was found to be well tolerated with modest antitumor activity in patients with relapsed FL [5]. A phase I/II trial of galiximab plus rituximab in a relapsed/refractory FL population demonstrated a 66% overall response rate (ORR) [33% complete response (CR)/ unconfirmed complete response (Cru); 33% partial response (PR)] with a median PFS of 12.1 months [27]. Based on these promising results, the CALGB decided to test this promising biological ‘doublet’ in previously untreated FL patients.
patients and methods
study objectives
The primary objectives of the study were to determine the response rates (ORR and CR) and time to progression (TTP) of FL patients to G + R combination as initial therapy. Secondary objectives included: to evaluate the toxicity profile of this schedule, to determine the feasibility of this study design for future trials combining rituximab with other novel biologic agents, and to correlate Fc receptor polymorphism profile with treatment response.
eligibility criteria
Patients ≥18 years with previously untreated histologically confirmed FL, grades 1, 2, or 3a with stages III, IV, or bulky (i.e. single mass ≥7 cm in any unidimensional measurement) stage II disease were eligible for this study. Inclusion criteria also required: confirmation of CD20-positivity; Eastern Cooperative Oncology Group performance status of 0–2; measurable disease (e.g. tumor mass >1 cm), adequate hematological, renal, and hepatic function; no known central nervous system involvement, human immunodeficiency virus infection, or baseline human anti-chimeric antibody (HACA) positivity.
The study was conducted in accordance with the Declaration of Helsinki. Each participating clinical site obtained an Institutional Review Board-approved written informed consent from all study patients. As stated in the protocol, patients classified as poor-risk according to FLIPI were encouraged to be considered for participation on CALGB 50102/SWOG 50016 [a phase III trial of combination chemotherapy with cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) plus rituximab × 6 versus CHOP × 6, followed by Iodine-131-labeled anti-B1 (CD20) mAb for treatment of newly diagnosed FL]. The study was registered at www.clinicaltrials.gov as #: NCT00117975.
study treatment
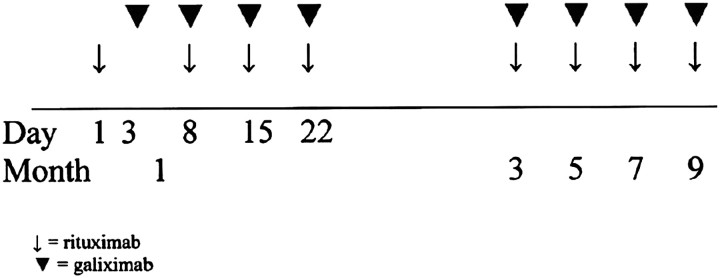
In this open-label multicenter, phase II study, patients received ‘induction’ rituximab (R) 375 mg/m2 by IV infusion on day 1, month 1; then galiximab (G) (500 mg/m2) over 60 min by IV infusion on day 3, month 1 because of expected longer infusion times typically associated with the first dose of rituximab. Both R + G (same doses) were given on days 8, 15, and 22 of month 1. ‘Extended induction’ therapy consisted of both R and G administered by IV infusion once in months 3, 5, 7, and 9. Overall, patients received a total of eight doses each of R and G over 9 months (see Figure 1).
Figure 1.
Treatment schema. Patients received a total of eight intravenous infusions of 375 mg/m2 of rituximab and eight infusions of 500 mg/m2 of galiximab over a period of 9 months.
study end points
efficacy
Disease assessments included CT scans or magnetic resonance imaging and physical examination at baseline, week 10, months 4, 6, 8, 10, and then every 4 months until disease progression or for a maximum of 10 years from study entry. Response to therapy was assessed using the IWG response criteria for non-Hodgkin's lymphoma (NHL) [28].
The primary efficacy end points were ORR and CR and PFS. ORR is defined as achievement of a CR or PR as the best observed response between trial entry and 12 months from enrollment on the trial. Secondary end points included correlation of Fc receptor polymorphism profile and FLIPI score with response to G + R therapy.
Follow-up evaluation included physical examinations, with vital sign measurements medical history/review of systems, adverse event (AE) documentation, complete blood counts, serum chemistry, serum immunoglobulin levels, HACA, and urinalysis.
statistical methods
The aim of this study was to evaluate the combination of galiximab plus rituximab in patients with previously untreated follicular NHL. If the data from this trial provided evidence of efficacy in this patient population, the treatment would be considered for further investigation. In evaluating response rate and PFS among FLIPI risk groups, the Cochran–Armitage test for trend and the log-rank test for differences in survival distributions were used, respectively.
The associations between each of ORR, CR, PFS, and FcR status (homozygous Val 158; homozygous Phe 158; heterozygous) were also evaluated in an exploratory fashion. In addition, FLIPI score versus ORR, CR rate, and PFS was also evaluated in an exploratory fashion.
A sample size of 51 patients was determined on the basis of the null hypothesis of an ORR of ≤0.65 tested against the alternative that the ORR is ≥0.80. An ORR of 0.80 would be considered worthy of further investigation. If ≥38 patients responded out of the total 51, then the study regimen would be considered for further investigation. The exact test had a one-sided α = 0.10 and 87% power.
All patients were to be followed until progression or a minimum of 10 years. TTP was measured from study entry until documented progression of disease or death due to NHL. All events were progressions or death due to NHL. Assuming a median TTP of 36 months and a period of 3 months to complete data entry, these analyses were scheduled to be analyzed at ∼40 and 55 months after study activation, respectively.
results
patient characteristics
Although 51 patients were originally planned to be enrolled on this trial, 61 assessable patients were actually enrolled between 3 August 2005 and 30 June 2006 at 21 CALGB institutions as the closure notice was sent on 15 June 2006 when accrual was at 52. In the last 2 weeks of accrual, 10 additional patients were registered, 8 within the last 2 days.
One patient never began treatment and was dropped from the analysis.
Baseline characteristics of all 61 treated patients are summarized in Table 1. Of note: 93% of patients had stage III/IV disease; 48% of patients were >60 years of age; 24% of patients had bulky (i.e. >7 cm) disease; and 37% of patients were classified as poor-risk by FLIPI stratification.
Table 1.
Summary of baseline patient characteristics (n = 61)
| Histology: FL, grades 1/2/3a | 44%/46%/10% |
| Male : female | 61% : 39% |
| Median age (years) | 57 (range: 22–85) |
| Age > 60 years | 48% |
| ECOG performance status 0/1/2 | 75%/22%/3% |
| Stage III/IV | 38%/55% |
| Bone marrow involvement | 46% |
| Elevated LDH | 23% |
| >4 nodal sites | 58% |
| Bulky disease (>7 cm) | 24% |
| FLIPI score | |
| 0–1 | 20.3% |
| 2 | 42.4% |
| 3–5 | 37.3% |
FL, follicular lymphoma; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; FLIPI, Follicular Lymphoma International Prognostic Index.
Eighty-two percent of patients completed all planned therapy. Reasons for not completing planned therapy are as follows: death (on day 5 due to NHL, n = 1); withdrawal from study (n = 2); non-protocol treatment given (n = 1); missed last treatment (n = 1); no response (n = 1); progression of disease (n = 5). Sixty-one patients were evaluable for safety and efficacy. Median follow-up is 4.3 years (range 0.3–5.3 years) as of 4 April 2011.
clinical AEs
Overall, G + R doublet biological therapy was well tolerated with only 13% of patients experiencing AEs. No patient experienced dose-limiting toxicity and none withdrew from the study due to toxicity. A total of 48% grade 2 and 13% grade 3 reversible AEs were reported. The following hematologic AEs were noted: 7% grade 2: leukopenia, n = 1; lymphopenia, n = 3; neutropenia, n = 1; and 2% grade 3: lymphopenia, n = 1. The following non-hematologic AEs were recorded: 44% grade 2 and 11% grade 3 and included fatigue, pain at tumor sites, cytokine release, allergy/rash, transient hypotension, and chills. Only three cases of grade 2 infections were reported.
efficacy
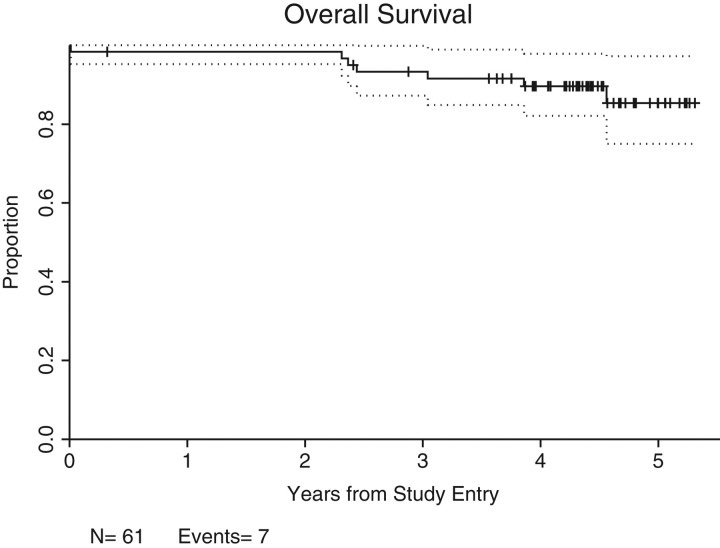
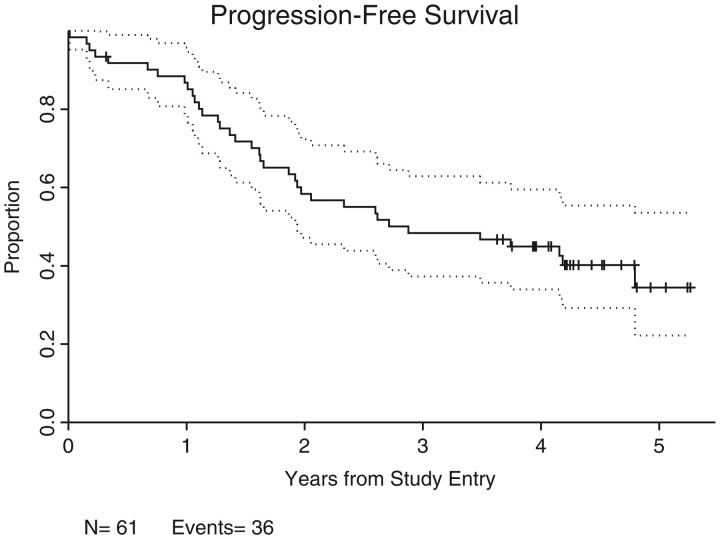
An ORR of 72.1% [44 of 61 patients; 95% confidence interval (CI): 59.2% to 82.9%; P = 0.1504] was achieved, with CR/CRu of 41%/6.6% (25/4 = 29 of 61 patients; 95% CI = 34.6% to 60.7%). Twenty-five percent of patients (n = 15) achieved PRs. The majority of the 44 responders achieved an initial ‘response’ at 2–3 months after initiating therapy; 7 patients had ‘delayed’ initial response ranging from 8 to 14 months after starting treatment; 12 patients converted from stable disease or PR to CR/CRu after ≥9 months on therapy. The median follow-up time is 4.3 years (0.3–5.3 years) in the 25 patients who have not progressed (Figure 2). Seven deaths secondary to progressive disease have occurred in this time period (no deaths due to other causes) and the overall median PFS is 2.9 years (Figure 3).
Figure 2.
Overall survival of 61 assessable patients over a median follow-up time of 4.3 years (7 patients died of post-progression).
Figure 3.
Overall progression-free survival curve. Kaplan–Meier analysis of overall progression-free survival, carried out in 61 assessable patients.
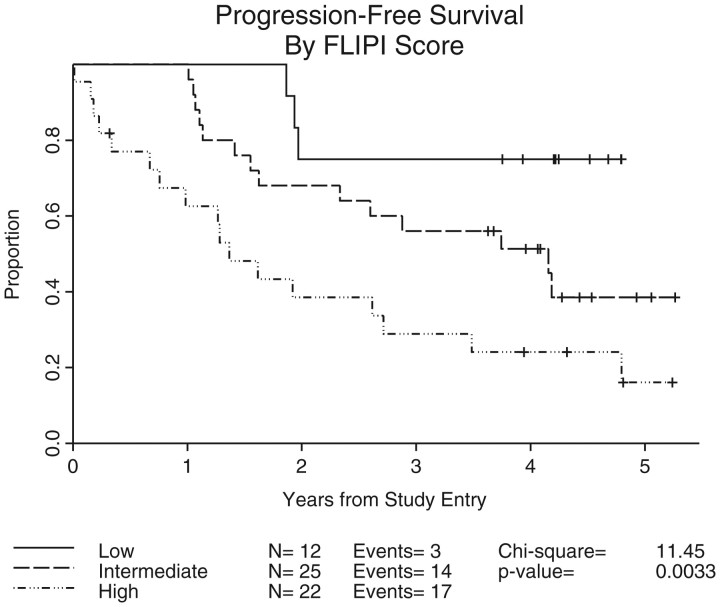
An analysis of outcome by FLIPI prognostic score was carried out and demonstrated a statistically significant association between FLIPI score and ORR (P = 0.004) and CR rate (P = 0.008), and PFS (P = 0.003); see Table 2 and Figure 4.
Table 2.
Association of FLIPI with ORR, CR rate, and DFS
| ORRa (P = 0.004)b | CR/CRu rate (P = 0.008)b | PFS (P = 0.007), 1 year | PFS (P = 0.007), 2 years | PFS (P = 0.007), 3 years | |
|---|---|---|---|---|---|
| Entire group | 72.1% | 47.5% | 0.87 | 0.58 | 0.48 |
| FLIPI 1 (good-risk) | 11/12 (92%) | 9/12 (75%) | 1.0 | 0.75 | 0.75 |
| FLIPI 2 (intermediate-risk) | 21/25 (84%) | 13/25 (52%) | 1.0 | 0.68 | 0.56 |
| FLIPI 3–5 (poor-risk) | 12/22 (55%) | 7/22 (32%) | 0.63 | 0.39 | 0.29 |
aORR was not associated with stage, gender, marrow involvement, age > 60 years, or Fc gamma receptor polymorphism status.
bOne-sided P-value for trend with respect to differences in FLIPI scores.
FLIPI, Follicular Lymphoma International Prognostic Index; ORR, overall response rate; CR, complete response; DFS, disease-free survival.
Figure 4.
Progression-free survival by FLIPI score. Log-rank test analysis of progression-free survival versus FLIPI subgroup, carried out in 61 assessable patients. FLIPI, Follicular Lymphoma International Prognostic Index.
Fc gamma receptor IIIA-VV [29] and receptor IIA-HH [30] genotypes have been associated with an improved response rate in FL patients treated with rituximab monotherapy. In the present study, no correlation was identified between Fc gamma RIIIA or RIIA polymorphisms with ORR, CR rate, or PFS.
discussion
Over the past decade, the initial therapy of FL has been evolving. Although many novel agents and treatment approaches have been tested, no single regimen or approach has been shown to be optimal for all patients [31–37]. Single-agent rituximab or rituximab-containing immunochemotherapy (e.g. R-CVP, R-CHOP) have been the most common induction therapy for FL patients in the United States in the past decade [33]. Two recent studies already appear to be changing the induction treatment paradigm for FL. Bendamustine plus rituximab has been reported to be superior to R-CHOP as the initial treatment of patients with symptomatic grades 1 and 2, and FL [10]. Second, the Primary Rituximab Maintenance (PRIMA) study demonstrated that use of q 2 month RM therapy following induction R-chemotherapy (R-CHOP, R-CVP, or R-FCM) demonstrated a 14% improvement in 2-year PFS compared with those patients in an observation arm [38]. Nevertheless, these treatment approaches would be considered excessive for most patients with low-risk FL. Galiximab, a primatized anti-CD80 mAb, is a costimulatory molecule that is constitutively expressed on neoplastic B cells, is capable of mediating ADCC against NHL cells following its binding to surface CD80, and is also found on non-malignant CD80-expressing immunoeffector cells present in the tumor microenvironment (which may contribute to its antitumor activity). Galiximab is also well tolerated as a 1-h infusion in prior clinical trials [5, 21] and has an excellent toxicity profile.
The current CALGB trial evaluated the efficacy and safety of G + R in a unique schedule: G + R for four consecutive weeks, followed by extended dosing with G + R (every 2 months for four times) in previously untreated FL, grades 1, 2, and 3a. The stratification of various FL-risk groups in current clinical trials will ultimately help guide the choice of therapy for individual patients and avoid under-treating poorer risk patients, but at the same time avoiding over-treating lower risk patients. Furthermore, trials that utilize mAbs having different mechanisms-of-action that target unique CD20 epitopes or other unique surface antigens or that limit the total amount of rituximab exposure (e.g. unnecessary use of prolonged maintenance rituximab schedules) to minimize the development of rituximab resistance are needed in order to continue to advance the field of lymphoma therapeutics and to optimize upfront and subsequent therapy in different risk-stratified subgroups.
Of interest in this study is the correlation between FLIPI and ORR, CR rate, and PFS with this biological doublet. FLIPI has been shown to be predictive in patients treated with immunochemotherapy [39], predicts transformation [40], and can be applied in first relapse [41]. In our database, we have demonstrated the validity of the prognostic significance of the FLIPI when combination immunotherapy (i.e. G + R) is utilized in the upfront setting.
Early phase I/II trials of four weekly infusions of galiximab alone [5] or in combination with rituximab [27] were evaluated in patients with relapsed or refractory FL. G monotherapy demonstrated modest (i.e. 11% ORR) clinical activity; however, G + R produced a 66% ORR (33% CR/CRu) with a median PFS of 12.1 months. Results with an eight infusion rituximab monotherapy dosing schedule (i.e. patients received 375 mg/m2 weekly four times and if responding or with stable disease were randomized to receive an additional 375 mg/m2 every 2 months for an additional four times or observation) published by Ghielmini et al. [16] demonstrated prolonged PFS rates in the chemotherapy-naive subset of patients and served as the basis for the choice of schedule for our G + R trial. Unfortunately, a formal comparison between the potential benefit of adding an additional biological (i.e. galiximab) to rituximab in this extended induction schedule is not carried out since specific patient characteristics (i.e. FLIPI) of the 25 previously untreated patients receiving ‘prolonged’ rituximab in the Ghielmini et al. [16] trial were not prospectively collected and not currently obtainable [17]. In addition, significant differences in the required monitoring of study patients on these two studies exist (e.g. the G + R study required a more extensive and frequent evaluation of disease assessment). In the present study, no correlation between Fc gamma RIIIA or RIIA polymorphisms and ORR, CR rate, TTP, or PFS was demonstrated in patients treated with galiximab plus rituximab. This finding suggests that the mechanism(s) of antitumor activity seen with G + R differs and may be unique from that associated with rituximab monotherapy alone.
G + R achieved an ORR of 72.1% (95% CI 59.2% to 82.9%) in the entire group and did not achieve our pretreatment estimation that an ORR ≥ 80% would be considered worthy of further investigation. However, clinical characteristics/prognostic factors (e.g. FLIPI) strongly influenced the therapeutic response. For example, although they were not formally excluded, eligibility criteria clearly stated that patients classified as poor-risk FLIPI should be considered for participation in CALGB 50102/SWOG 50016. Nonetheless, 37.3% of patients participating on our trial had poor-risk FLIPI which ultimately influenced the therapeutic outcomes. Indeed, if the 22 high-risk FLIPI patients were excluded from our database, the resultant ORR would be 86.5% and the CR rate 60%. The excellent results achieved in low-risk FLIPI patients treated with G + R suggests that this combination is highly effective with an excellent toxicity profile and has the advantage of delaying and perhaps avoiding myelotoxic chemotherapy and limiting the amount of rituximab exposure associated with prolonged RM schedules which adds expense, potential risk for the development of rituximab resistance, and does not appear to add benefit to good-risk patient populations. Of interest are data presented by Ardeshna et al. [42] at the 2010 annual ASH meeting from a UK intergroup randomized trial comparing rituximab with watchful waiting in asymptomatic non-bulky FL (grades 1, 2, and 3a) patients. Arm C (rituximab weekly four times, then q 2 months for 12 times) resulted in a 49% CR/CRu rate and the median time to initiation of new therapy (i.e. chemotherapy or radiotherapy) not reached at 4 years. Our CR/CRu rate of 48% and overall 3-year PFS of 48% in the entire group (but 75% in the good-risk FLIPI subgroup) are promising and achieved with only using a total of 8 rituximab infusions (in combination with galiximab) compared with a total of 16 rituximab infusions in the UK intergroup study. G + R is the first of several rituximab-based doublets which are being evaluated sequentially by the CALGB in an attempt to determine an optimal upfront non-chemotherapy-based therapeutic approach for the treatment of FL and will serve as the benchmark for future comparisons.
In conclusion, G + R combination immunotherapy is a novel, active, and well-tolerated upfront treatment strategy for FL patients, especially those with ‘low-risk’ FLIPI and warrants comparison with other front-line regimens in this population.
funding
Cancer and Leukemia Group B (CALGB); Biogen Idec to MSC.
disclosure
Consultant/advisory role: MSC (Biogen Idec and Genentech). All remaining authors have declared no conflicts of interest.
acknowledgements
Presented in part at the 10th International Conference on Malignant Lymphoma (ICML), Lugano, Switzerland, 4–7 June 2008 and at the 50th Annual Meeting of the American Society of Hematology, San Francisco, CA, USA, 6–9 December 2008.
references
- 1.Martin AR, Weisenburger DD, Chan WC, et al. Prognostic value of cellular proliferation and histologic grade in follicular lymphoma. Blood. 1995;85:3671–3678. [PubMed] [Google Scholar]
- 2.Relander T, Johnson NA, Farinha P, et al. Prognostic factors in follicular lymphoma. J Clin Oncol. 2010;28:2902–2913. doi: 10.1200/JCO.2009.26.1693. [DOI] [PubMed] [Google Scholar]
- 3.Solal-Celigny P, Roy P, Colombat P, et al. Follicular lymphoma international prognostic index. Blood. 2004;104:1258–1265. doi: 10.1182/blood-2003-12-4434. [DOI] [PubMed] [Google Scholar]
- 4.Czuczman MS, Grillo-Lopez AJ, White CA, et al. Treatment of patients with low-grade B-cell lymphoma with the combination of chimeric anti-CD20 monoclonal antibody and CHOP chemotherapy. J Clin Oncol. 1999;17:268–276. doi: 10.1200/JCO.1999.17.1.268. [DOI] [PubMed] [Google Scholar]
- 5.Czuczman MS, Thall A, Witzig TE, et al. Phase I/II study of galiximab, an anti-CD80 antibody, for relapsed or refractory follicular lymphoma. J Clin Oncol. 2005;23:4390–4398. doi: 10.1200/JCO.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 6.Czuczman MS, Weaver R, Alkuzweny B, et al. Prolonged clinical and molecular remission in patients with low-grade or follicular non-Hodgkin's lymphoma treated with rituximab plus CHOP chemotherapy: 9-year follow-up. J Clin Oncol. 2004;22:4711–4716. doi: 10.1200/JCO.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 7.Hiddemann W, Kneba M, Dreyling M, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106:3725–3732. doi: 10.1182/blood-2005-01-0016. [DOI] [PubMed] [Google Scholar]
- 8.Marcus R, Imrie K, Belch A, et al. CVP chemotherapy plus rituximab compared with CVP as first-line treatment for advanced follicular lymphoma. Blood. 2005;105:1417–1423. doi: 10.1182/blood-2004-08-3175. [DOI] [PubMed] [Google Scholar]
- 9.Montoto S, Moreno C, Domingo-Domenech E, et al. High clinical and molecular response rates with fludarabine, cyclophosphamide and mitoxantrone in previously untreated patients with advanced stage follicular lymphoma. Haematologica. 2008;93:207–214. doi: 10.3324/haematol.11671. [DOI] [PubMed] [Google Scholar]
- 10.Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab is superior in respect of progression free survival and CR rate when compared to CHOP plus rituximab as first-line treatment of patients with advanced follicular, indolent, and mantle cell lymphomas: Final results of a randomized Phase III study of the StiL (Study Group Indolent Lymphomas, Germany) Blood. 2009;114:168–169. [Google Scholar]
- 11.Morschhauser F, Radford J, Van Hoof A, et al. Phase III trial of consolidation therapy with yttrium-90-ibritumomab tiuxetan compared with no additional therapy after first remission in advanced follicular lymphoma. J Clin Oncol. 2008;26:5156–5164. doi: 10.1200/JCO.2008.17.2015. [DOI] [PubMed] [Google Scholar]
- 12.Colombat P, Salles G, Brousse N, et al. Rituximab (anti-CD20 monoclonal antibody) as single first-line therapy for patients with follicular lymphoma with a low tumor burden: clinical and molecular evaluation. Blood. 2001;97:101–106. doi: 10.1182/blood.v97.1.101. [DOI] [PubMed] [Google Scholar]
- 13.Hainsworth JD. First-line and maintenance treatment with rituximab for patients with indolent non-Hodgkin's lymphoma. Semin Oncol. 2003;30:9–15. doi: 10.1053/sonc.2003.50023. [DOI] [PubMed] [Google Scholar]
- 14.Hainsworth JD, Burris HA, III, Morrissey LH, et al. Rituximab monoclonal antibody as initial systemic therapy for patients with low-grade non-Hodgkin lymphoma. Blood. 2000;95:3052–3056. [PubMed] [Google Scholar]
- 15.Witzig TE, Vukov AM, Habermann TM, et al. Rituximab therapy for patients with newly diagnosed, advanced-stage, follicular grade I non-Hodgkin's lymphoma: a phase II trial in the North Central Cancer Treatment Group. J Clin Oncol. 2005;23:1103–1108. doi: 10.1200/JCO.2005.12.052. [DOI] [PubMed] [Google Scholar]
- 16.Ghielmini M, Schmitz SF, Cogliatti SB, et al. Prolonged treatment with rituximab in patients with follicular lymphoma significantly increases event-free survival and response duration compared with the standard weekly x 4 schedule. Blood. 2004;103:4416–4423. doi: 10.1182/blood-2003-10-3411. [DOI] [PubMed] [Google Scholar]
- 17.Martinelli G, Schmitz SF, Utiger U, et al. Long-term follow-up of patients with follicular lymphoma receiving single-agent rituximab at two different schedules in trial SAKK 35/98. J Clin Oncol. 2010;28:4480–4484. doi: 10.1200/JCO.2010.28.4786. [DOI] [PubMed] [Google Scholar]
- 18.Faderl S, Ferrajoli A, Wierda W, et al. Alemtuzumab by continuous intravenous infusion followed by subcutaneous injection plus rituximab in the treatment of patients with chronic lymphocytic leukemia recurrence. Cancer. 2010;116:2360–2365. doi: 10.1002/cncr.24958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leonard JP, Schuster SJ, Emmanouilides C, et al. Durable complete responses from therapy with combined epratuzumab and rituximab: final results from an international multicenter, phase 2 study in recurrent, indolent, non-Hodgkin lymphoma. Cancer. 2008;113:2714–2723. doi: 10.1002/cncr.23890. [DOI] [PubMed] [Google Scholar]
- 20.Maddipatla S, Hernandez-Ilizaliturri FJ, Knight J, Czuczman MS. Augmented antitumor activity against B-cell lymphoma by a combination of monoclonal antibodies targeting TRAIL-R1 and CD20. Clin Cancer Res. 2007;13:4556–4564. doi: 10.1158/1078-0432.CCR-07-0680. [DOI] [PubMed] [Google Scholar]
- 21.Coyle AJ, Gutierrez-Ramos JC. The expanding B7 superfamily: increasing complexity in costimulatory signals regulating T cell function. Nat Immunol. 2001;2:203–209. doi: 10.1038/85251. [DOI] [PubMed] [Google Scholar]
- 22.Chambers CA, Allison JP. Costimulatory regulation of T cell function. Curr Opin Cell Biol. 1999;11:203–210. doi: 10.1016/s0955-0674(99)80027-1. [DOI] [PubMed] [Google Scholar]
- 23.Suvas S, Singh V, Sahdev S, Vohra H, et al. Distinct role of CD80 and CD86 in the regulation of the activation of B cell and B cell lymphoma. J Biol Chem. 2002;277:7766–7775. doi: 10.1074/jbc.M105902200. [DOI] [PubMed] [Google Scholar]
- 24.Munro JM, Freedman AS, Aster JC, et al. In vivo expression of the B7 costimulatory molecule by subsets of antigen-presenting cells and the malignant cells of Hodgkin's disease. Blood. 1994;83:793–798. [PubMed] [Google Scholar]
- 25.Bhat S, Czuczman MS. Galiximab: a review. Expert Opin Biol Ther. 2010;10:451–458. doi: 10.1517/14712591003596318. [DOI] [PubMed] [Google Scholar]
- 26.Vinjamaram S, Czuczman MS, Hernandez-Ilizaliturri FJ. The use of galiximab in non-Hodgkin lymphoma. Clin Lymphoma Myeloma. 2008;8:277–282. doi: 10.3816/CLM.2008.n.038. [DOI] [PubMed] [Google Scholar]
- 27.Leonard JP, Friedberg JW, Younes A, et al. A phase I/II study of galiximab (an anti-CD80 monoclonal antibody) in combination with rituximab for relapsed or refractory, follicular lymphoma. Ann Oncol. 2007;18:1216–1223. doi: 10.1093/annonc/mdm114. [DOI] [PubMed] [Google Scholar]
- 28.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 29.Cartron G, Dacheux L, Salles G, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–758. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- 30.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 2003;21:3940–3947. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 31.Dreyling M. Newly diagnosed and relapsed follicular lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v181–v183. doi: 10.1093/annonc/mdq184. [DOI] [PubMed] [Google Scholar]
- 32.Feuerlein K, Zucca E, Ghielmini M. First-line treatment of follicular lymphoma: a patient-oriented algorithm. Leuk Lymphoma. 2009;50:325–334. doi: 10.1080/10428190802713513. [DOI] [PubMed] [Google Scholar]
- 33.Friedberg JW, Taylor MD, Cerhan JR, et al. Follicular lymphoma in the United States: first report of the national LymphoCare study. J Clin Oncol. 2009;27:1202–1208. doi: 10.1200/JCO.2008.18.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kahl BS, Bartlett NL, Leonard JP, et al. Bendamustine is effective therapy in patients with rituximab-refractory, indolent B-cell non-Hodgkin lymphoma: results from a Multicenter Study. Cancer. 2010;116:106–114. doi: 10.1002/cncr.24714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seymour JF. Hematology: Follicular lymphoma: maintenance therapy is (often) indicated. Nat Rev Clin Oncol. 2009;6:624–626. doi: 10.1038/nrclinonc.2009.159. [DOI] [PubMed] [Google Scholar]
- 36.Sharkey RM, Press OW, Goldenberg DM. A re-examination of radioimmunotherapy in the treatment of non-Hodgkin lymphoma: prospects for dual-targeted antibody/radioantibody therapy. Blood. 2009;113:3891–3895. doi: 10.1182/blood-2008-11-188896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Witzig TE, Wiernik PH, Moore T, et al. Lenalidomide oral monotherapy produces durable responses in relapsed or refractory indolent non-Hodgkin's Lymphoma. J Clin Oncol. 2009;27:5404–5409. doi: 10.1200/JCO.2008.21.1169. [DOI] [PubMed] [Google Scholar]
- 38.Salles G, Seymour JF, Offner F, et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet. 2011;377:42–51. doi: 10.1016/S0140-6736(10)62175-7. [DOI] [PubMed] [Google Scholar]
- 39.Buske C, Hoster E, Dreyling M, et al. The Follicular Lymphoma International Prognostic Index (FLIPI) separates high-risk from intermediate- or low-risk patients with advanced-stage follicular lymphoma treated front-line with rituximab and the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) with respect to treatment outcome. Blood. 2006;108:1504–1508. doi: 10.1182/blood-2006-01-013367. [DOI] [PubMed] [Google Scholar]
- 40.Gine E, Montoto S, Bosch F, et al. The Follicular Lymphoma International Prognostic Index (FLIPI) and the histological subtype are the most important factors to predict histological transformation in follicular lymphoma. Ann Oncol. 2006;17:1539–1545. doi: 10.1093/annonc/mdl162. [DOI] [PubMed] [Google Scholar]
- 41.Montoto S, Lopez-Guillermo A, Altes A, et al. Predictive value of Follicular Lymphoma International Prognostic Index (FLIPI) in patients with follicular lymphoma at first progression. Ann Oncol. 2004;15:1484–1489. doi: 10.1093/annonc/mdh406. [DOI] [PubMed] [Google Scholar]
- 42.Ardeshna KM, Smith P, Qian W, et al. An intergroup randomized trial of rituximab versus a watch and wait strategy in patients with stage II, III, IV, asymptomatic, non-bulky follicular lymphoma (Grades 1, 2, and 3a). A preliminary analysis. Blood. 2010;116:6. [Google Scholar]