Abstract
Spider venom neurotoxins and cytolytic peptides are expressed as elongated precursor peptides, which are post-translationally processed by proteases to yield the active mature peptides. The recognition motifs for these processing proteases, first published more than 10 years ago, include the processing quadruplet motif (PQM) and the inverted processing quadruplet motif (iPQM). However, the identification of the relevant proteases was still pending. Here we describe the purification of a neurotoxin precursor processing protease from the venom of the spider Cupiennius salei. The chymotrypsin-like serine protease is a 28-kDa heterodimer with optimum activity at venom's pH of 6.0. We designed multiple synthetic peptides mimicking the predicted cleavage sites of neurotoxin precursors. Using these peptides as substrates, we confirm the biochemical activity of the protease in propeptide removal from neurotoxin precursors by cleavage C-terminal of the PQM. Furthermore, the PQM protease also cleaves the iPQM relevant for heterodimerization of a subgroup of neurotoxins. An involvement in the maturing of cytolytic peptides is very likely, due to high similarity of present protease recognition motifs. Finally, bioinformatics analysis, identifying sequences of homolog proteins from 18 spiders of 9 families, demonstrate the wide distribution and importance of the isolated enzyme for spiders. In summary, we establish the first example of a PQM protease, essential for maturing of spider venom neurotoxins. In the future, the here described protease may be established as a powerful tool for production strategies of recombinant toxic peptides, adapted to the maturing of spider venom toxins.
Keywords: antimicrobial peptide (AMP), enzyme purification, neurotoxin, protein processing, serine protease, processing quadruplet motif (PQM), propeptide
Introduction
The toxic action of spider venoms relies on a complex mixture of different components. The lead toxic components are mainly enzymes, as reported for Loxosceles (Sicariidae) (1), cytolytic peptides (e.g. in Lachesana tarabaevi (Zodariidae)) (2) or, in the majority of spider venoms, neurotoxins (3). The activity of neurotoxins can thereby be enhanced by cytolytic peptides, if co-occurring in the venom (4, 5). Cytolytic and neurotoxic peptides are expressed in the venom gland as precursors, which undergo proteolytic processing to yield the mature active peptide (6). Neurotoxic peptide precursors are reported to commonly comprise a signal peptide followed by an acidic propeptide and a mature peptide. The last four amino acid residues of the propeptide have been demonstrated to form a conserved motif featuring an Arg residue at position −1 and at least one Glu residue at positions −2, −3, and/or −4. This motif is named the processing quadruplet motif (PQM),2 and it is supposed to act as recognition site for a propeptide processing protease (6), henceforth referred to as PQM protease. In precursors of cytolytic peptides, an additional processing motif, featuring an Arg residue at position 1 and at least one Glu residue within the three following amino acids was identified and named inverted PQM (iPQM) (7, 8). Despite the description of the PQM and iPQM as potential protease recognition sites and the widespread existence of similar precursor structures of spider venom toxins, few is known about the proteases responsible for precursor maturation and there is still no experimental evidence for the involvement of a specific protease. Nevertheless, different venom proteases have been hypothesized to be involved in venom peptide maturation. Among them, a recently identified thiol protease from Lycosa singoriensis (Lycosidae) showing similarity to the cysteine protease cathepsin L (9) and a venom protein of Acanthoscurria geniculata (Theraphosidae) homologous to proprotein convertases (10). In addition, multiple metalloproteases and some serine proteases with unclear functions have been identified in spider venoms (3).
In conclusion, different proteins have been hypothesized to be involved in propeptide processing of spider venom peptides. Nevertheless, the involvement in the maturation process of venom neurotoxins has so far not been experimentally confirmed for any protease isolated from spider venom. Here, we report the isolation and characterization of a PQM processing protease from the venom of the Central American spider Cupiennius salei (Ctenidae). We demonstrate the specificity of the PQM protease for propeptide removal from spider venom neurotoxin precursors. Additionally, we provide evidence for the involvement of this protease in the generation of the two heterodimeric neurotoxins CsTx-13 (C. salei, P83919) (11) and ω-agatoxin-1A (Agelenopsis aperta, P15969) (12).
Results
Purification of the native protease
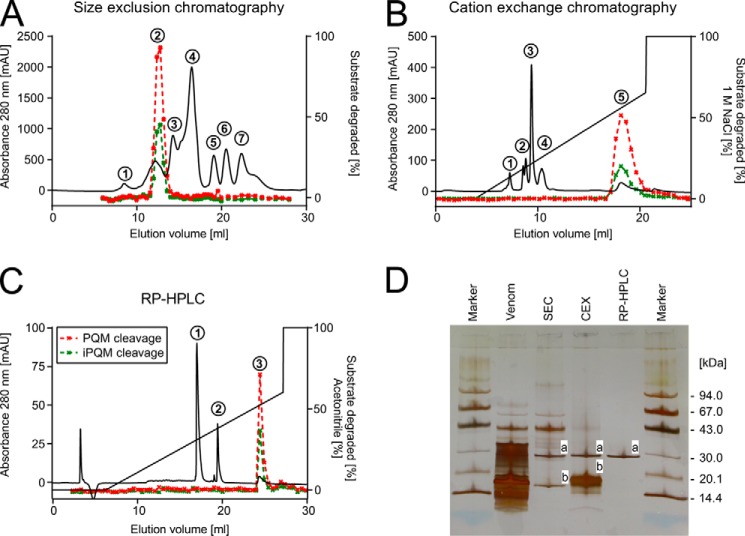
The results are based on processing a total of 11.5 ml of crude venom of C. salei, corresponding to the venom obtained from approximately 1150 spider milkings (13). We purified the protease by three successive chromatographic steps. All fractions of each chromatographic step were tested for proteolytic activity against two substrates mimicking a PQM and an iPQM, respectively (DABCYL-PFFENEQARKDDKN-Glu(EDANS)-NH2/DABCYL-FYPSQRSETD-Glu(EDANS)-NH2). Proteolytically active fractions were pooled, analyzed by SDS-PAGE, and subjected to the next chromatographic step. First, size exclusion chromatography (SEC) of venom on a Sephadex G-75 column allowed the separation of the active fraction (Fig. 1A, peak 2) from the majority of peptides and proteins appearing at masses smaller than ∼35 kDa in SDS-PAGE (Fig. 1D). Second, cation exchange chromatography (CEX) of size exclusion chromatography peak 2 on a MiniS PE 4.6/50 column led to the enrichment of the proteolytic activity in a broad peak (Fig. 1B, peak 5). We observed a loss in total proteolytic activity of this peak compared with SEC peak 2 (Table 1). This may be due to damnification of the protease leading to a reduced specific activity and/or due to the loss of protease material during purification. The assumed loss of protease is in accordance with our observations comparing SDS-PAGE lane SEC and lane CEX (Fig. 1D), where we noticed a reduction of the intensity of band a (protease) relative to the intensity of band b (contaminating peptides). Third, RP-HPLC on a butyl column enabled the final purification of the protease (Fig. 1C, peak 3). The contaminating peptides could be efficiently separated (Fig. 1C, peaks 1 and 2). Tandem mass spectrometry of chymotryptic fragments of the peptides in peaks 1 and 2 showed top hits for the neurotoxins CsTx-39, CsTx-243, CsTx-9, and CsTx-12 (Tables S1 and S2). The isolated enzyme appeared at a mass slightly below 30 kDa on SDS-PAGE (Fig. 1D, lane RP-HPLC), which is in good agreement with the measured mass of 28 kDa by MALDI-TOF-MS (Fig. 2A). Contamination of the purified enzyme could neither be detected by SDS-PAGE, nor by MALDI-TOF-MS with different matrices (Fig. 2A, Figs. S1 and S2). The specific activity of the pure enzyme was (6,364 ± 2,306) × 10−12 mol liter−1 s−1 μg−1. This is 1,097-fold higher than the specific activity of the venom (Table 1). The venom concentration of the isolated protease can be estimated to 0.78 ± 0.2 μm by quantification of the isolated enzyme (amino acid analysis). However, it is reasonable to assume that the venom concentration is underestimated due to loss of protease during the purification procedure.
Figure 1.
Purification of the native protease. A, size exclusion chromatography of 100 μl of C. salei venom in 100 mm NH4OAc, pH 5.0, on a Superdex 75 HR column. The proteolytic activity of collected fractions against the PQM substrate (red dashed line; DABCYL-PFFENEQARKDDKN-Glu(EDANS)-NH2) and the iPQM substrate (green dashed line; DABCYL-FYPSQRSETD-Glu(EDANS)-NH2) is given in percentage of degraded substrate. B, cation exchange chromatography of size exclusion chromatography peak 2 on a MiniS PE column equilibrated with 50 mm NH4OAc, pH 5.0. C, subsequent RP-HPLC separation of cation exchange chromatography peak 5 on a Nucleosil 300–5, C4 column equilibrated with 0.05% (v/v) TFA in water. D, SDS-PAGE (8–25%) of C. salei venom and pooled active fractions of each purification step. Bands referred to in the text are lettered.
Table 1.
Summary of the purification process of the PQM protease
The data shown are from two independent purifications starting with 3 ml of crude venom each. The total activity was measured as degradation of the PQM substrate in triplicate for each purification. The data are given as mean ± S.D. The standard deviation of the specific activity was calculated according to the rules of error propagation.
| Step | Total activity | Protein content | Specific activity | Fold purification |
|---|---|---|---|---|
| mol l−1 s−1 (μl venom eq.)−1 | μg (μl venom eq.)−1 | mol l−1 s−1μg−1 | ||
| Venom | 5.93 ± 1.40 × 10−10 | 101.750 ± 6.124 | 5.8 ± 1.7 × 10−12 | 1 |
| SEC | 5.26 ± 0.53 × 10−10 | 3.581 ± 0.110 | 146.8 ± 19.2 × 10−12 | 25 |
| CEX | 0.85 ± 0.14 × 10−10 | 0.053 ± 0.009 | 1,601.7 ± 527.8 × 10−12 | 276 |
| HPLC | 1.40 ± 0.16 × 10−10 | 0.022 ± 0.006 | 6,364.3 ± 2,305.6 × 10−12 | 1,097 |
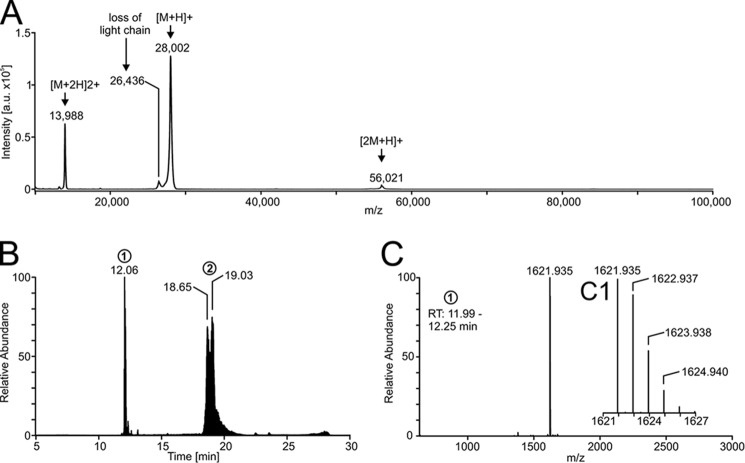
Figure 2.
Mass spectrometry of the PQM protease. A, mass spectrum of the intact protein acquired by MALDI-TOF-MS. The matrix used was α-cyano-4-hydroxycinnamic acid. B, chromatogram of the reduced and alkylated isolated protease given as total ion current. The heterodimerity of the protease is shown by separation of the light chain (peak 1) and the heavy chain (peak 2). C, mass spectrum of the light chain (retention time 11.99–12.25 min) with insertion (C1) showing the enlargement of the region at 1621–1627 m/z. The mass spectrum was deconvoluted to neutral monoisotopic masses with Xcalibur version 2.2 and its plug-in Xtract.
Structural characterization and cDNA sequence of the protease
The isolated protease was identified to be heterodimeric by liquid chromatography-mass spectrometry (LC-MS) of the reduced and alkylated protein (Fig. 2B). We sequenced the light chain (Fig. 2B, retention time 12.06 min) by tandem mass spectrometry (Fig. S3 and Table S3) and found it to be C-terminally amidated and connected to the heavy chain by one disulfide bridge. The monoisotopic mass of the uncharged light chain was determined to be 1,621.935 Da (Cys carboxyamidomethylated), which is in good agreement with its theoretical mass of 1,621.934 Da (Fig. 2C). The heavy chain eluted from the HPLC column in a broad peak (Fig. 2B, retention time 18.42–19.60 min). The mass spectra measured between these retention times provide evidence for the presence of multiple isoforms of the heavy chain (Fig. S4). Nevertheless, the number of isoforms and their accurate masses cannot be assessed due to unknown methionine oxidation state and possible adducts.
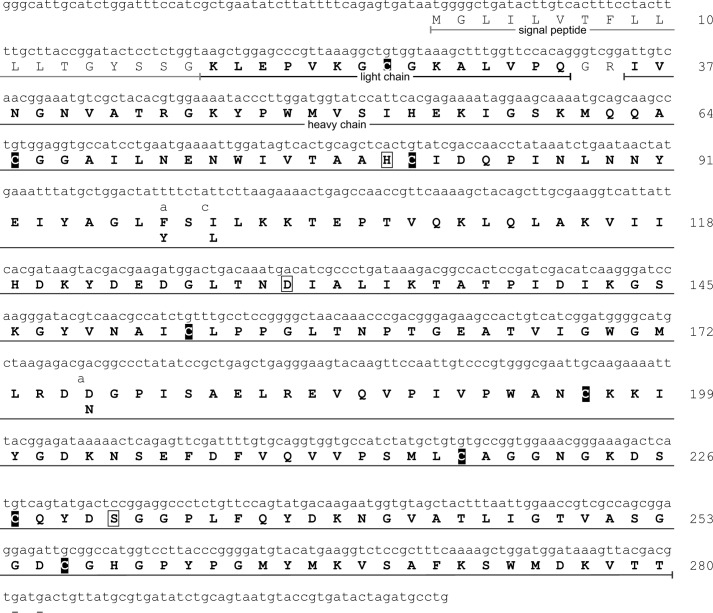
By analysis of the venom gland transcriptome, we identified two transcripts giving rise to two isoforms of a monomeric protease precursor, both differing in three amino acid residues of the later heavy chain (Fig. 3). The more abundant isoform (relative read count approximately 90%) was named venom serine protease 1a1 (VSP1_a1), the less abundant (relative read count approximately 10%) was named venom serine protease 1a2 (VSP1_a2). The amino acid sequences of the heavy chains were validated by tandem mass spectrometry of peptides resulting from trypsin, chymotrypsin, or proteinase K digests. The obtained data were searched against our in-house protein database venomProteome.fasta as described below, and the results were mapped against the cDNA-derived amino acid sequence of the two isoforms of the protease as identified at transcriptome level. The sequence coverage was 100% in the region 36Ile–Thr280 with a false discovery rate of ≤1% (Fig. 3). Alignment of the amino acid sequence to the cDNA sequence shows that the 840-bp open reading frame codes for a signal peptide (1Met–Gly18) followed by the light chain (19Lys–Gln33), a two-amino acid spacer (34Gly–Arg35), and the heavy chain (36Ile–Thr280). Functional analysis (14) of the amino acid sequence predicts the protein belongs to the serine peptidase S1A subfamily with active sites His80, Asp130, Ser231, and substrate-binding sites Asp225, Ala251, and Gly253. The amino acid sequence differences of the identified isoforms F98Y, and D176N were both validated on protein level. The isobaric amino acid change I100L cannot be detected by mass spectrometry.
Figure 3.
cDNA and amino acid sequence of the purified PQM protease. Shown are the cDNA and the amino acid sequence of the isolated protease main isoform. Non-silent mutations of the minor isoform are indicated below the main sequence. Amino acid residues in bold letters were validated on the protein level by either tandem mass spectrometry of the reduced and alkylated chain light chain, or tandem mass spectrometry of peptides generated by trypsin, chymotrypsin, or proteinase K digests of the heavy chain. Amino acids in boxes are part of the catalytic triad. Cysteines are black boxed.
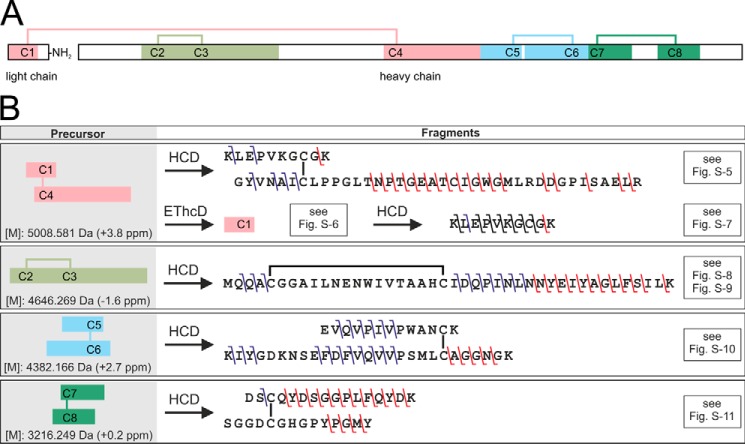
The disulfide bridge pattern of the PQM protease was elucidated by selective proteolysis followed by LC-MS/MS/MS analysis under nonreductive conditions (Fig. 4, A and B, Figs. S5–S11 and Tables S4–S8). The identified disulfide bridge pattern is identical to one of the peptide isomerase of A. aperta (15), the protein with the highest sequence identity to the PQM protease of all proteins with published disulfide bridge patterns.
Figure 4.
Disulfide bridge pattern of the PQM protease and overview on its elucidation. A, overview of the heterodimeric structure of the isolated protease. The light chain is C-terminally amidated. Disulfide bridges are shown as lines. Colored fragments represent cysteine-connected tryptic peptides detected by LC-MS of the digested, non-reduced PQM protease. B, overview of identified b- and y-fragment ions in the MS2 and MS3 spectra of the cysteine-connected tryptic peptides. The used fragmentation methods, higher-energy collisional dissociation (HCD), and electron transfer/higher-energy collision dissociation (EThcD) are abbreviated. Precursor masses are given as monoisotopic uncharged. The deviation of the experimental from the theoretic masses is given in brackets. Relevant mass spectra and tables of identified fragment ions are available in Fig. S5–S11 and Tables S4–S8.
Enzymatic activity characterization of the protease
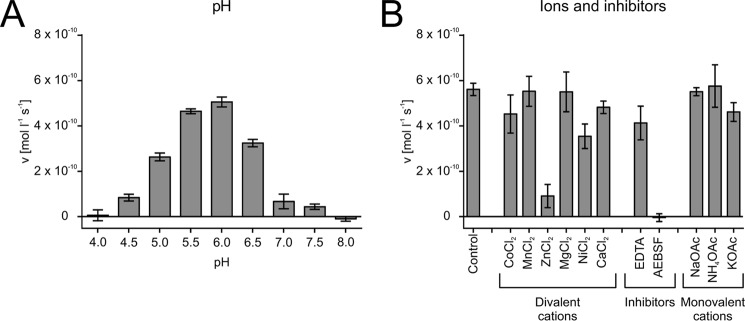
We measured the maximal activity of the isolated protease at pH 6. The enzyme was not active at buffer pH ≤ 4 or ≥ 8 (Fig. 5A). The divalent cations Co2+, Mn2+, Mg2+, Ni2+, and Ca2+ did not notably affect the activity of the isolated protease, whereas the presence of 5 mm Zn2+ in the assay buffer led to an activity decrease by a factor of 5 (Fig. 5B). A slight decrease in activity was observed in the presence of the chelating agent EDTA (10 mm), whereas the serine protease inhibitor AEBSF (4-(2-aminoethyl)benzenesulfonyl fluoride) (10 mm) caused a total loss of activity. The measured monovalent cations did not noteworthy affect the activity of the isolated protease (Fig. 5B).
Figure 5.
Enzymatic characterization of the purified PQM protease. A, pH dependence of the proteolytic activity. B, influence of cations and inhibitors on the proteolytic activity. The incubation buffers were 0.2 m NH4OAc, pH 6.0, supplemented with 5 mm of the indicated divalent cation or 10 mm protease inhibitor. Monovalent cations were tested at a concentration of 0.2 m of the indicated salt at pH 6.0. The data were measured in triplicate using the PQM substrate and are given as mean ± S.D.
Substrate specificity of the protease
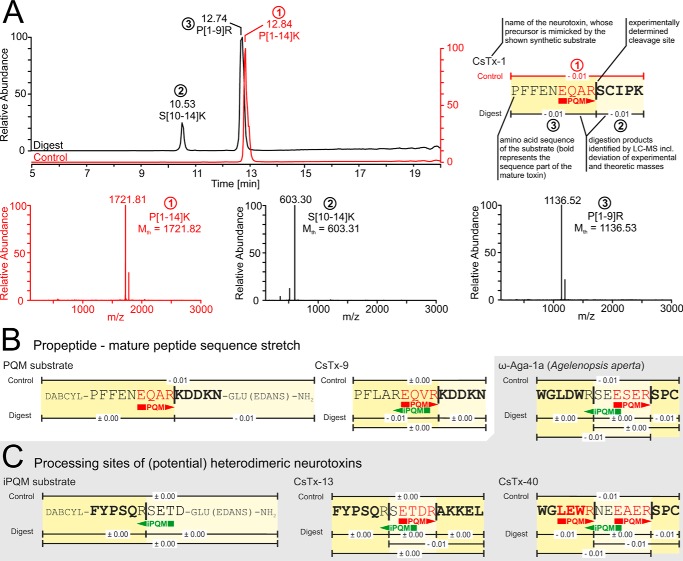
To assess the specificity of the isolated protease we: 1) analyzed known neurotoxic precursor sequences of C. salei for possible PQM protease processing sites, 2) synthesized peptides covering these sites, and 3) analyzed the digestion products of these peptides by LC-MS. The neurotoxins CsTx-1 and CsTx-9 exhibit the two most prominent PQMs in C. salei propeptides (EQAR and EQVR, respectively), present in ∼85% of all transcripts coding for neurotoxin precursors.3 We detected specific cleavage on the C-terminal side of these PQMs (Fig. 6, A and B). The involvement of the isolated protease in generating heterodimeric toxins, such as CsTx-13 (C. salei) or ω-agatoxin-1a (A. aperta), and the potential heterodimer CsTx-403 was investigated using appropriate substrates (Fig. 6C). The three substrates were all cut twice: 1) C-terminal of the PQM, and 2) C-terminal of Arg6. Arg6 is part of an iPQM in all three substrates and additionally part of a PQM in the substrate mimicking CsTx-40 (Fig. 6C). Cutting on the C-terminal side of the PQM of the heterodimeric precursors liberates the β-chain of the toxins. The C-terminal amino acid of the α-chains of the mature heterodimers corresponds to position 5 of the synthetic substrates. Nevertheless, cutting was observed C-terminal of Arg6 of the synthetic substrates. It is therefore tempting to assume that the C-terminal Arg from the liberated α-chain is removed by a carboxypeptidase during maturation of heterodimeric neurotoxins.
Figure 6.
Protease cleavage specificity on neurotoxin precursor mimicking substrates. A, LC-MS of the synthetic peptide PFFENEQARSCIPK after overnight incubation with the purified protease. This peptide mimics the propeptide-mature peptide sequence stretch of C. salei's main neurotoxin CsTx-1. The chromatogram is given as the total ion current and shows two peaks representing two fragments resulting from proteolytic digestion. The control (undigested peptide) is shown in red. The masses of the digestion fragments and the intact peptide were measured by online mass spectrometry and matched against the masses of all possible digestion fragments of the given synthetic substrate. Mass spectra are deconvoluted to neutral monoisotopic masses. Mth is the theoretical mass of a given peptide. An overview of identified digestion products is shown and explained in the top right corner. PQM and iPQM in the substrate sequence are indicated with red and green arrows. The Cys residues of all shown substrates are carboxamidomethyl-Cys. B, overview of the identified digestion products of the propeptide–mature peptide sequence stretch mimicking the C. salei neurotoxin CsTx-9 as well as the PQM substrate used for purification and characterization of the isolated protease. C, overview of identified digestion fragments of substrates mimicking processing sites of (potential) heterodimeric neurotoxins, and the iPQM substrate used for purification of the isolated protease. The substrates CsTx-13 and CsTx-40 refer to the respective neurotoxins of C. salei, ω-Aga-1a to the neurotoxin ω-Agatoxin-1a of the spider A. aperta. LC-MS data of digested substrates are available as Fig. S12–S18.
Discussion
Isolation and characterization of spider venom enzymes can be challenging due to: 1) their often up to 1000-fold lower venom concentration compared with the main toxins (e.g. for C. salei: venom hyaluronidase, 1.3–9.3 μm (16) versus main neurotoxin CsTx-1, 1.4–3.3 mm (13)), and 2) potential loss of activity during the purification. The latter requires optimization of the purification strategy, which is only possible if venom is accessible in high quantities.
Structural characterization
We identified the isolated PQM protease to be a heterodimer. The heterodimeric structure is likely to be a result of proteolytic cleavage of a monomeric precursor C-terminal of Arg35. Nevertheless, the C-terminal amino acid of the light chain was identified to be Gln33. We propose that this is a result of exoproteolytical removal of Arg35 followed by peptidylglycine α-amidating monooxygenase (PAM)-mediated C-terminal amidation of Gln33, using Gly34 as amide donor (7). A PAM has been identified in our C. salei venom gland transcriptome.3
The biological function of the heterodimeric structure of the PQM protease is unclear. Some proteases are expressed as zymogens that are activated by limited proteolysis. Trypsin, for example, emerges from its precursor trypsinogen by activation through proteolytic removal of a six-amino acid propeptide (17). In analogy, cleavage of the monomeric precursor of the PQM protease into a light and a heavy chain may be required for the activation of the protease. Thus, the light chain might be comparable with the propeptide of trypsin, but stays connected to the activated protease due to the presence of an interchain disulfide bridge. Nevertheless, the structural advantage of heterodimerity of the PQM protease needs further investigation requiring an active protease obtained by recombinant expression.
Isoforms
We detected isoforms of the PQM protease heavy chain by transcriptome analysis and validated the respective amino acid sequences by bottom-up proteomics. Isoforms of venom proteins have also been described for other enzymes, such as phospholipases D from spiders of the genus Loxosceles (18). In addition, isoforms are reported for many venom neurotoxins and are thought to be a result of gene duplication (19).
Homologous proteins
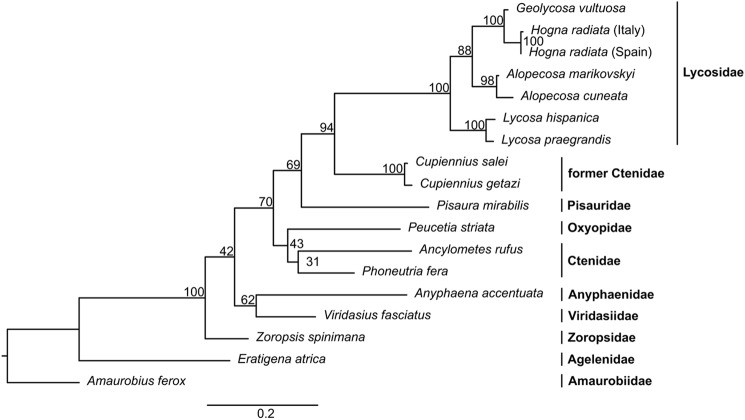
The isolated protease shows high amino acid sequence identity with two proteins isolated from spider venom: U21-ctenitoxin-Pn1a (76%) (P84033) a monomeric venom protease from Phoneutria nigriventer (20), and a heterodimeric venom peptide isomerase (56%) (Q9TWH3/Q9TXD8) from A. aperta (15). Comparable identities (59–98%) were calculated for putative protease homologs from 18 spiders of nine families (if we regard Cupiennius as non-ctenidae, see below) identified in our in-house spider venom gland cDNA libraries of RTA clade spiders (Fig. S19). The high amino acid sequence conservation and the presence of the enzyme in the venom glands of all investigated spider families demonstrate the high biological relevance of the protease for spiders. Phylogenetic analysis (Fig. 7) shows a topology in good agreement with a recently published phylogeny (21, 22). Interestingly, the sequences of the two Cupiennius species cluster distantly from their currently assigned family Ctenidae and group close to Lycosidae and Pisauridae. Corresponding observations concerning ambiguous family affiliation of Cupiennius have recently been discussed in taxonomic studies (22, 23), indicating that the structure of venom components can provide relevant phylogenetic arguments.
Figure 7.
Evolution and distribution of the PQM protease. Maximum likelihood phylogenetic tree calculated from an amino acid alignment of the C. salei PQM protease and its homologs identified in venom gland cDNA libraries from other spiders (alignment available as Fig. S19). The node support is given as bootstrap proportions obtained from 1000 rapid bootstrapping replicates. Spider families are indicated in bold.
Multiple sequence alignment of the homolog amino acid sequences shows conserved catalytic residues, substrate-binding sites, and high overall identities (Fig. S19). All shown sequences feature the same signal peptidase cleavage site (Gly18, prediction using SignalP 4.1 (24)), a conserved cysteine pattern, and conserved amino acid residues (35Arg–Val37) at the predicted proteolytic cleavage site for heterodimerization of the monomeric precursor. It is therefore reasonable to propose, that the homologs undergo all the same maturation mechanism resulting in a heterodimeric structure. Nevertheless, this is not in agreement with the description of U21-ctenitoxin-Pn1a of Phoneutria nigriventer (P84033) as a monomeric enzyme (20). However, the cDNA-derived amino acid sequence of another closely related species in the same genus, Phoneutria fera, exhibits the structural features as prerequisite for heterodimerization and a high sequence identity of 95% with U21-ctenitoxin-Pn1a (Fig. S19).
The light chain of the protease from C. salei is C-terminally amidated. This amidation seems not to be conserved, as some sequences lack Gly34, the supposed amide-donor for the amidation by a PAM. Interestingly, the peptide isomerase from A. aperta (Q9TWH3/Q9TXXD8) exhibits the same amino acid residues in the predicted active center and substrate-binding sites, as well as the same disulfide bridge pattern as the PQM protease of C. salei. Functional analysis of the amino acid sequence predicts the enzyme to belong to the peptidase S1A subfamily. Therefore, it seems conceivable that the one-step purification procedure of the peptide isomerase from the venom of the funnel web spider (15) was not appropriate to purify the enzyme to homogeneity, resulting in a misinterpretation of the enzymatic function due to contamination of the purified protein by an isomerase. A comparable case has been reported for Tex31, a cysteine-rich venom protein belonging to the CAP superfamily (IPR014044 and IPR001283), with homologs in spider venom (10). Tex31, isolated from the venom of the cone snail Conus textile, was initially thought to proteolytically process conotoxin precursors (25). However, Qian et al. (26) could demonstrate that the reported proteolytic activity of Tex31 is rather caused by a contamination of Tex31 with an unknown protease than by the protein itself.
Specificity of the PQM protease
Spider venom protoxin-processing sites have already been well investigated at the transcriptome level, resulting in the description of the protease recognition motifs PQM and iPQM (6, 7). In our study, we investigated the cutting specificity of the isolated protease on different synthetic substrates. We identified a specific cutting C-terminal of all Arg residues of PQMs. Furthermore, cutting on the C-terminal side of Arg residues of all iPQMs and PQMs was observed for synthetic substrates mimicking the processing sites of the heterodimeric neurotoxin precursors of CsTx-13, CsTx-40,3 and ω-Aga-1a. In contrast, we could not observe processing of the iPQM present in close proximity to the PQM in the substrate mimicking the CsTx-9 propeptide–mature peptide sequence stretch. It seems conceivable that the PQM protease lacks the specificity for this iPQM, and/or that the short distance between the Arg residues of the PQM and the nearby iPQM may impede correct substrate recognition (three amino acids in the case of the CsTx-9 precursor and four to five amino acids in the case of the precursors of heterodimeric neurotoxins).
Not all predicted cutting sites were cleaved with the same efficiency. This is followed from the observed HPLC peak height ratios between digestion fragments and undigested substrates. It is well known that the turnover number of a protease is highly dependent on the substrate (27), which may explain the observed differences in cleavage efficiency.
The here described cleavage specificity of the isolated protease is congruent with the predicted specificity of a propeptide peptidase (7) and points toward an involvement of the PQM protease in generation of heterodimeric toxins from their monomeric precursors. Our data indicates that heterodimeric neurotoxins are generated by: 1) removal of the inter-chain spacer of the monomeric precursors by the PQM protease, followed by 2) removal of the C-terminal Arg of the α-chain of heterodimeric neurotoxins by a carboxypeptidase. This is in accordance with the supposed maturation process of toxin precursors based on transcriptomic studies (7). The number of reported heterodimeric neurotoxins is rather low in proportion to all described spider venom neurotoxins (28). This raises the question if heterodimerization of monomeric neurotoxins is of biological benefit. In C. salei venom, a family of heterodimeric neurotoxins (CsTx-8, -12, and -13) are present in millimolar concentrations (29). The LD50 values of these heterodimeric neurotoxins (CsTx-13: 16.3 pmol/mg of fly; CsTx-8: 6.3 pmol/mg of fly) are significantly higher than the ones of the main monomeric neurotoxins (CsTx-1: 0.35 pmol/mg of fly; CsTx-9: 3.12 pmol/mg of fly) (11, 29). Surprisingly, CsTx-13 enhances synergistically the paralytic activity of CsTx-1 and CsTx-9 toward Drosophila flies even in nontoxic concentrations (11).
Cytolytic venom peptide precursors have been reported to feature a comparable organization to neurotoxic precursors, but in some cases to give rise to more than one mature peptide. Multiple mature peptides on one precursor are separated by short spacers flanked by iPQMs and PQMs. These spacers are proteolytically removed during the maturation process and are comparable with the above-mentioned inter-chain spacers of heterodimeric neurotoxin precursors. In analogy to our findings for heterodimeric neurotoxins, maturation of some cytolytic precursors is thought to involve a carboxypeptidase (7). We assume that maturation of cytolytic precursors is comparable with the maturation of heterodimeric neurotoxins and involves the same proteases. Furthermore, we expect the PQM protease to occur in all spiders producing neurotoxic and/or cytolytic peptide precursors.
To the best of our knowledge, this is the first report about the isolation and characterization of the protease responsible for the removal of propeptides from spider venom neurotoxin precursors. In summary, our data confirm the proposed maturation mechanism of monomeric neurotoxins by proteolytic cleavage C-terminal of the PQM (6). Moreover, specificity studies imply analogous maturation mechanisms of heterodimeric neurotoxins and complex cytolytic precursors. We provide experimental evidence to support the described maturation mechanism of complex precursors by PQM and iPQM cleavage followed by exoproteolytical removal of the residual arginine of the iPQM (7). As consequence, the PQM protease may enable efficient production strategies for recombinant peptides and proteins, adapted to the maturing of complex cytolytic peptides as supposed already by Vassilevski et al. (30).
Experimental procedures
Spider maintenance and venom collection
C. salei (Ctenidae) were laboratory bred and milked as previously reported (13). For the purification and characterization of the PQM protease, venom of adult male and female spiders was used. For transcriptomic studies, Ancylometes rufus, P. fera, Cupiennius getazi, Viridasius fasciatus, Zoropsis spinimana, Hogna radiata (Italy), and H. radiata (Spain) were laboratory bred. Eratigena atrica (formerly Tegenaria atrica), Pisaura mirabilis, Anyphaena accentuata, Amaurobius ferox, and Alopecosa cuneata were collected in Switzerland, Lycosa hispanica in Spain, Geolycosa vultuosa in Hungary, Peucetia striata in Tansania, Lycosa praegrandis in Greece, and Alopecosa marikovskyi in Kasachstan. To reduce gene pool variation, spiders of the same species were collected from one population. Collection of spiders did not require specific permissions because we collected on public and/or private land of the authors without any protection status or because spiders were laboratory bred. None of the above-mentioned spiders belongs to a protected or endangered species.
cDNA libraries of venom glands
The cDNA venom gland library of C. salei is based on 454 sequencing and has been previously described (31). The libraries of other spiders were built using the following procedure: venom glands of adult spiders (see on top) were dissected in different time intervals after milking and stored in RNAlater (Qiagen). Total RNA was extracted using an in-house protocol combining phenol/chloroform extraction with the RNeasy mini kit (Qiagen). cDNA libraries were prepared using Illumina's TruSeq-stranded mRNA prep kit. We used double barcoding and selected fragments with lengths between 300 and 600 bp for further sequencing (Pippin HT system, Sage Science). Each spider venom gland cDNA library was multiplexed (25% per lane) with other libraries (mostly genome) from non-arthropods and sequenced by the next generation sequencing platform of the University of Bern on an Illumina HiSeq3000 platform using 2 × 150 bp paired-end sequencing cycles. The resulting reads have been assembled using Trinity version 2.1.1 (32).
PQM protease cDNA sequences
The sequences of the ORFs encoding the two isoforms of the C. salei PQM protease (VSP1_a1 and VSP1_a2) were established from 189 contigs comprising 1960 reads. Sequences of homolog proteins from other spiders were obtained by a venom gland cDNA library search using a combination of Blast and Exonerate 2.2.0 (33) with the sequence of the C. salei PQM protease as template. Sequences were manually reviewed at read level.
Substrate design and enzymatic assays
The proteolytic activity was measured in a fluorescence resonance energy transfer (FRET)-based assay using two peptide-substrates carrying a PQM and an iPQM, respectively. The PQM substrate (DABCYL-PFFENEQARKDDKN-Glu (EDANS)-NH2, PQM underlined) was adapted to the propeptide of CsTx-1 (P81694) and to the mature peptide of CsTx-9 (P58604). CsTx-1, the most abundant neurotoxin in the venom of C. salei, comprises a cysteine at position 2 (13). To prevent any possible effect of a free cysteine residue on proteolytic processing of the synthetic substrate or on the activity test, we adapted the mature peptide mimicking region of the PQM substrate to CsTx-9 (the second most abundant neurotoxin in C. salei venom), whereas retaining the PQM of the main neurotoxin CsTx-1. The iPQM substrate (DABCYL-FYPSQRSETD-Glu(EDANS)-NH2, iPQM underlined) was adapted to C. salei's main heterodimeric neurotoxin CsTx-13 (P83919). For the activity test, 1.2 μg of the substrates, (supplied by GeneCust, Luxembourg) were resolved in 130 μl of activity test buffer (200 mm NH4OAc, pH 6.0, 100 mm KCl, 1 mm CaCl2) and incubated for 30–120 min with 20 μl of each fraction of a purification step. Incubation was performed at room temperature in assay plates (96-well, flat bottom, non-binding surface, black polystyrene; Corning). End point fluorescence measurements were performed at 25 °C using a Synergy HT Microplate reader (BioTek, Winooski, VT) equipped with the following filters: 340/30 and 485/20 nm. The activity of a given fraction was calculated as percentage of substrate digested after incubation relative to the negative control (buffer only) and the positive control (1 μg of trypsin).
Isolation and purification
We purified the native protease by applying three successive steps of chromatographic separation, starting with a total of 2–3 ml of venom per batch. First, size exclusion chromatography of 100 μl of venom per separation diluted with 300 μl of running buffer (100 mm NH4OAc, pH 5.0) was performed on a Superdex 75 HR (10 × 300 mm, GE Healthcare Life Sciences) column at a flow rate of 0.8 ml/min. Second, fractions exhibiting proteolytic activity were combined, diluted 1:1 (v/v) by addition of H2O, and reseparated on a MiniS PE (4.6 × 50 mm, GE Healthcare Life Sciences) column equilibrated with 50 mm NH4OAc, pH 5.0. Analytes were eluted by applying a gradient of 0–0.65 m NaCl in 21 min at a flow rate of 0.8 ml/min. Third, the eluted proteolytic active fraction was further separated by RP-HPLC on a Nucleosil 300–5, C4 (5 μm, 4.6 × 250 mm, Macherey-Nagel, Düren, Germany) column equilibrated with 0.05% (v/v) TFA in water. Proteins were eluted with 0.05% (v/v) TFA in acetonitrile applying a gradient of 0–60% acetonitrile in 42 min at a flow rate of 0.5 ml/min.
Polyacrylamide gel electrophoresis
SDS-PAGE and silver staining was performed using a PhastGel System and PhastGel Gradient 8–25 (all GE Healthcare).
Amino acid analysis
Amino acid analysis was performed as previously reported (34).
Enzymatic characterization
We determined the effect of different cations, protease inhibitors, and pH on the activity of the isolated protease. First, 55 ng of the isolated protease was preincubated in the respective buffer in assay plates at 37 °C for 15 min. Second, the substrate (DABCYL-PFFENEQARKDDKN-Glu(EDANS)-NH2) was added to a final concentration of 5 μm and the increase in fluorescence was immediately measured in intervals of 1 min during 15 min at 37 °C using a Synergy HT Microplate reader (BioTek) as described above. The following assay buffers were used: 0.2 m NH4OAc, 0.2 m NaOAc, or 0.2 m KOAc, all pH 6.0, for monovalent cations: 0.2 m NH4OAc, pH 6.0, and 5 mm of one of the following additives: CoCl2, MnCl2, ZnCl2, NiCl2, or CaCl2 for divalent cations, 10 mm EDTA or AEBSF for inhibitors, or 0.2 m NH4OAc, pH 3.5 to 8.5, by steps of 0.5 for pH. All assay buffers contained additionally 7.5% (v/v) acetonitrile. For each condition, a negative control (no protease) and a positive control (10 μg trypsin) were included. Trypsin was preincubated with peptide prior to the addition of the respective buffer to ensure full digestion of the substrate at t = 0. The proteolytic activity was calculated as an increase in fluorescence per time relative to the respective negative and positive controls. All measurements were done in triplicate.
Substrate specificity
To investigate the function of the isolated protease in the maturation process of venom neurotoxins, we analyzed the digestion products of different substrates (all supplied by GeneCust, Luxembourg) after incubation with the isolated enzyme. Two substrates have been designed to mimic the propeptide–mature peptide cutting site of C. salei neurotoxins: PFFENEQARSCIPK (CsTx-1) and PFLAREQVRKDDKN (CsTx-9). Additionally, two substrates mimic potential processing sites within the mature chain region of C. salei venom neurotoxin precursors: WGLEWRNEEAERSPC (CsTx-40) and FYPSQRSETDRAKKEL (CsTx-13). One substrate (WGLDWRSEESERSPC) mimics the cutting site within ω-agatoxin-1A (P15969) isolated from the venom of the spider A. aperta (12). Substrates containing Cys residues were reduced and alkylated to prevent polymer formation. Reduced and alkylated substrates (20 μm final) were mixed with 75 ng of isolated protease in 200 mm NH4OAc, pH 6.0, in assay plates (96-well, flat bottom, non-binding surface, black polystyrene; Corning). After overnight incubation at 37 °C and acidification of the sample by addition of formic acid to 1% (v/v), samples were stored at −80 °C until analysis of the digestion products by LC-MS. Liquid chromatography was performed using a Reprosil-Pur 200 C18-aq column (5 μm, 2 × 100 mm, Dr. Maisch, Ammerbuch-Entringen, Germany). After 5 min of isocratic wash with 2% acetonitrile, 0.5% (v/v) formic acid in water, the analytes were eluted by applying a gradient of 2–80% acetonitrile, 0.5% (v/v) formic acid. The gradient length was 20 min, the flow rate during load and isocratic wash was 0.15 ml/min, and during the gradient was 0.3 ml/min. The eluted sample compounds were identified by online mass spectrometry in full scan positive ion mode on a LTQ Orbitrap Velos mass spectrometer (ThermoFisher Scientific). The scan range was 110–2000 m/z, and the resolution was set to 100,000. Data were processed using Excalibur version 2.2. LC-MS data are available as Fig. S12–S18.
MALDI-TOF-MS
MALDI-TOF-MS was performed on an Autoflex III Smartbeam mass spectrometer (Bruker Daltonics). 1 μl of acidified sample was directly mixed with 1 μl of matrix solution (α-cyano-4-hydroxycinnamic acid, sinapic acid, or 2′,6′-dihydroxyacetophenone) on a ground steel target plate and air dried. The spectra were acquired in linear positive mode between 10 and 100 kDa and analyzed using the Flex analysis software (Bruker Daltonics). Spectra are shown baseline subtracted.
Tandem mass spectrometry
For protein identification, dried protein fractions were reconstituted in Laemmli buffer containing 20 mm DTT and separated on a 12.5% SDS-PAGE. Protein bands were excised and prepared for mass spectrometry as described elsewhere (35). For enzymatic digests with proteases other than trypsin, the procedure was slightly modified. For chymotrypsin digestion, incubations were done at room temperature for 6 h. For proteinase-K digestion, no ProteaseMax (Promega) was used and the digestion was carried out at 37 °C for 30 min. Peptide extracts were analyzed by nano-LC-MS/MS as described elsewhere (36).
For the mass spectrometric characterization of the protease light–heavy chain dimer, 3 μg of HPLC purified and dried protein was reconstituted in 10 μl of 8 m urea, 50 mm Tris-HCl, pH 8.0, reduced with 10 mm DTT at 37 °C for 30 min, followed by alkylation with 50 mm iodoacetamide at 37 °C for 30 min in the dark. Excess iodoacetamide was quenched with DTT. The protein was diluted to a concentration of 0.05 μg/μl with 0.1% TFA and 2 μl of this solution were analyzed by nano-LC-MS/MS on an Ultimate3000 chromatograph connected to an Orbitrap Fusion Lumos mass spectrometer (ThermoFisher Scientific). Analytes were trapped on a Pepmap 100 Trap C18 column (300 μm × 5 mm, ThermoFisher Scientific) and separated by backflush over a C18 column (3 μm, 100 Å, 75 μm × 150 mm, Nikkyo Technos Co. Ltd., Japan) applying a gradient of 0 to 38% acetonitrile, 0.1% formic acid in 10 min at a flow rate of 300 nl min−1. MS scans were recorded in data-dependent acquisition mode acquiring MS1 and MS2 spectra in the Orbitrap detector with a cycle time of 3 s and the following settings: scan range 400–2000 m/z; MS1 and MS2 resolution at 120,000 and 30,000; AGC of 400,000 and 50,000; and a maximum injection time of 50 ms for both, respectively; including 2+ and 3+ ions only without exclusion time and an isolation width of 1.6 m/z; HCD fragmentation with a relative collision energy of 30%. All fragment spectra peak list files in Mascot generic file format were created with ProteomeDiscoverer version 2.1 (ThermoFisher Scientific) keeping the six most intense fragment ions in a sliding window of 20 m/z.
Tandem mass spectrometry data analysis
LC-MS data were analyzed with Excalibur (ThermoFisher Scientific, version 2.2), fragment spectra were interpreted with SearchGUI (version 3.2.14) (37) and Peptide Shaker (version 1.16.5) (38). We used an in-house database (venomProteome.fasta) containing: 1) all Araneae sequences in Swiss-Prot (as of 04/03/2017, n = 1490), 2) additionally identified peptides and proteins from our C. salei venom gland transcriptome,3 including the cDNA-derived amino acid sequences of the two isoforms of the PQM protease (n = 85), and 3) known contaminants (n = 218). The search engine was X! TANDEM (version 2015.12.15.2) and the search parameters: maximum missed cleavages, 3 (for trypsin and chymotrypsin) and option “unspecific” (for proteinase K); precursor ions tolerance, 10 ppm; fragment ion tolerance, 0.01 Da; fixed modifications, carbamidomethylation of C; variable modifications, oxidation of M. The results were filtered by applying a false discovery rate of ≤1%. Data are available via ProteomeXchange with identifier PXD007126. Details of identified peptides and proteins are given in Tables S1, S2, and S9.
Elucidation of the disulfide bridge pattern
2.5 μg of the dried purified enzyme were reconstituted in 20 μl of 8 m urea, 50 mm Tris-HCl, pH 8.0, by strong vortexing for 30 s and sonication for 1 min in a water bath. The protein was digested by incubation for 6 h at 37 °C with 100 ng of trypsin after dilution of urea to 1.6 m with 20 mm Tris-HCl, 2 mm CaCl2, pH 8.0. For the digestion with agarose-bound trypsin (Sigma), the enzyme was reconstituted in 20 μl of 50 mm Tris-HCl, pH 8.0, and incubated with immobilized enzyme for 22 h at 37 °C. After digestion, half of the samples were reduced by addition of DTT to a final concentration of 10 mm and incubation for 30 min at 50 °C. Reduced and native samples were acidified with TFA to a final concentration of 1% and 100 ng of digested enzyme were analyzed as described under “Tandem mass spectrometry” with a few changes. Only peptide ions with a charge state between 3 and 8 were subjected to HCD fragmentation and the three most intense fragments were subjected to another HCD fragmentation (MS3) using a normalized fragmentation energy of 35%. MS2 spectra were recorded in the orbitrap with resolution 15,000 and AGC of 50,000, whereas MS3 spectra were recorded in the ion trap with AGC of 10,000 and maximum injection times of 35 ms for both detectors, respectively. Additionally, the MS3 acquisition mode was repeated with a mixed MS2 fragmentation mode of ETD and HCD (EThcD) (25%) for peptide ions of charge 4–8, whereas peptides with charge 2–3 were fragmented with HCD as above. Relevant spectra were annotated manually and are available as Fig. S5–S11.
Phylogenetic analysis
The amino acid sequence alignment was generated using Clustal Omega (39) and was manually reviewed (alignment available in Fig. S19). The best fitting substitution model was identified to be WAG+G+I by using MEGA 7.0.26 (40). The maximum likelihood analysis was conducted in RAxML 8.2.9 (41) using default parameters and 1000 random starting trees under the mentioned model. The clade support was obtained by rapid bootstrapping (42) executing 1000 rapid bootstrap inferences. The bootstrap support was drawn on the best-scoring tree. Calculations were performed on UBELIX, the HPC cluster at the University of Bern.
Database deposits
The nucleotide sequences of the two PQM protease isoforms of C. salei have been deposited in the GenBankTM database under accession numbers MF537412 and MF537413. Nucleotide sequences of homologous transcripts form other spiders are accessible under the following GenBankTM accession numbers: L. hispanica accession number MF537414, G. vultuosa accession number MF537415, A. rufus accession number MF537416, P. fera accession number MF537417, C. getazi accession number MF537418, P. striata accession number MF537419, V. fasciatus accession number MF537420, P. mirabilis accession number MF537421, E. atrica accession number MF537422, A. accentuata accession number MF537423, Z. spinimana accession number MF537424, H. radiata (Italy) accession number MF537425, L. praegrandis accession number MF537426, A. ferox accession number MF537427, A. marikovskyi accession number MF537428, H. radiata (Spain) accession number MF537429, and A. cuneata accession number MF537430. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE (43) partner repository with the dataset identifier PXD007126.
Author contributions
N. L. conceived the study, designed and conducted most experiments, analyzed the results and transcriptomic data, and wrote the paper. L. K. N. conceived the study, analyzed the results and transcriptomic data, wrote the paper, bred and milked the spiders, and dissected the venom glands. D. K. contributed to transcriptomic data analysis. S. S. provided technical assistance for mass spectrometry, and contributed to data analysis. M. H. provided technical assistance for nano-LC-MS/MS, contributed to data analysis, and wrote the methods part for tandem mass spectrometry. W. N. conceived transcriptomic studies, and collected and identified spider species. All authors reviewed the results, corrected the manuscript, and approved its final version.
Supplementary Material
Acknowledgments
We are grateful to Benjamin Eggs, Christian Kropf, Miguel Richard, and André Leetz for help in spider sampling, Seraina Klopfstein for help with phylogenetic analyses, and Benoît Zuber for providing access to his fluorimeter.
This work was supported by Swiss National Science Foundation Grant 31003A_162564 (to W. N.). The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Tables S1–S9 and Fig. S1–S19.
The nucleotide sequence(s) reported in this paper has been submitted to the GenBankTM/EBI Data Bank with accession number(s) MF537412, MF537413, MF537414, MF537415, MF537416, MF537417, MF537418, MF537419, MF537420, MF537421, MF537422, MF537423, MF537424, MF537425, MF537426, MF537427, MF537428, MF537429, and MF537430.
The mass spectrometry proteomics data are available from ProteomeXchange with the accession number PXD007126.
L. Kuhn-Nentwig, personal communication.
- PQM
- processing quadruplet motif
- DABCYL
- 4-((4-(dimethylamino)phenyl)azo)benzoic acid
- EDANS
- 5–((2-aminoethyl)amino)naphthalene-1-sulfonic acid
- SEC
- size exclusion chromatography
- CEX
- cation exchange chromatography
- RP-HPLC
- reversed-phase HPLC
- AEBSF
- 4-(2-aminoethyl)benzenesulfonyl fluoride
- iPQM
- inverted processing quadruplet motif
- PRIDE
- proteomics identifications
- HCD
- higher-energy collisional dissociation
- EThcD
- electron transfer/higher-energy collision dissociation
- PAM
- peptidylglycine α-amidating monooxygenase
- AGC
- automatic gain control.
References
- 1. Planas E., Zobel-Thropp P. A., Ribera C., and Binford G. (2015) Not as docile as it looks?: loxosceles venom variation and loxoscelism in the Mediterranean basin and the Canary Islands. Toxicon 93, 11–19 10.1016/j.toxicon.2014.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kozlov S. A., Vassilevski A. A., Feofanov A. V., Surovoy A. Y., Karpunin D. V., and Grishin E. V. (2006) Latarcins, antimicrobial and cytolytic peptides from the venom of the spider Lachesana tarabaevi (Zodariidae) that exemplify biomolecular diversity. J. Biol. Chem. 281, 20983–20992 10.1074/jbc.M602168200 [DOI] [PubMed] [Google Scholar]
- 3. Kuhn-Nentwig L., Stöcklin R., and Nentwig W. (2011) Venom composition and strategies in spiders: is everything possible? Adv. In Insect Phys. 40, 1–86 10.1016/B978-0-12-387668-3.00001-5 [DOI] [Google Scholar]
- 4. Corzo G., Villegas E., Gómez-Lagunas F., Possani L. D., Belokoneva O. S., and Nakajima T. (2002) Oxyopinins, large amphipathic peptides isolated from the venom of the wolf spider Oxyopes kitabensis with cytolytic properties and positive insecticidal cooperativity with spider neurotoxins. J. Biol. Chem. 277, 23627–23637 10.1074/jbc.M200511200 [DOI] [PubMed] [Google Scholar]
- 5. Kuhn-Nentwig L., Schaller J., and Nentwig W. (2004) Biochemistry, toxicology and ecology of the venom of the spider Cupiennius salei (Ctenidae). Toxicon 43, 543–553 10.1016/j.toxicon.2004.02.009 [DOI] [PubMed] [Google Scholar]
- 6. Kozlov S., Malyavka A., McCutchen B., Lu A., Schepers E., Herrmann R., and Grishin E. (2005) A novel strategy for the identification of toxinlike structures in spider venom. Proteins 59, 131–140 10.1002/prot.20390 [DOI] [PubMed] [Google Scholar]
- 7. Kozlov S. A., and Grishin E. V. (2007) The universal algorithm of maturation for secretory and excretory protein precursors. Toxicon 49, 721–726 10.1016/j.toxicon.2006.11.007 [DOI] [PubMed] [Google Scholar]
- 8. Dubovskii P. V., Vassilevski A. A., Kozlov S. A., Feofanov A. V., Grishin E. V., and Efremov R. G. (2015) Latarcins: versatile spider venom peptides. Cell. Mol. Life Sci. 72, 4501–4522 10.1007/s00018-015-2016-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang Y., Chen J., Tang X., Wang F., Jiang L., Xiong X., Wang M., Rong M., Liu Z., and Liang S. (2010) Transcriptome analysis of the venom glands of the Chinese wolf spider Lycosa singoriensis. Zoology 113, 10–18 10.1016/j.zool.2009.04.001 [DOI] [PubMed] [Google Scholar]
- 10. Sanggaard K. W., Bechsgaard J. S., Fang X., Duan J., Dyrlund T. F., Gupta V., Jiang X., Cheng L., Fan D., Feng Y., Han L., Huang Z., Wu Z., Liao L., Settepani V., et al. (2014) Spider genomes provide insight into composition and evolution of venom and silk. Nat. Commun. 5, 3765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wullschleger B., Kuhn-Nentwig L., Tromp J., Kämpfer U., Schaller J., Schürch S., and Nentwig W. (2004) CSTX-13, a highly synergistically acting two-chain neurotoxic enhancer in the venom of the spider Cupiennius salei (Ctenidae). Proc. Natl. Acad. Sci. U.S.A. 101, 11251–11256 10.1073/pnas.0402226101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Santos A. D., Imperial J. S., Chaudhary T., Beavis R. C., Chait B. T., Hunsperger J. P., Olivera B. M., Adams M. E., and Hillyard D. R. (1992) Heterodimeric structure of the spider toxin omega-agatoxin IA revealed by precursor analysis and mass spectrometry. J. Biol. Chem. 267, 20701–20705 [PubMed] [Google Scholar]
- 13. Kuhn-Nentwig L., Schaller J., and Nentwig W. (1994) Purification of toxic peptides and the amino acid sequence of CSTX-1 from the multicomponent venom of Cupiennius salei (Araneae:Ctenidae). Toxicon 32, 287–302 10.1016/0041-0101(94)90082-5 [DOI] [PubMed] [Google Scholar]
- 14. Finn R. D., Attwood T. K., Babbitt P. C., Bateman A., Bork P., Bridge A. J., Chang H. Y., Dosztanyi Z., El-Gebali S., Fraser M., Gough J., Haft D., Holliday G. L., Huang H., Huang X., et al. (2017) InterPro in 2017-beyond protein family and domain annotations. Nucleic Acids Res. 45, D190–D199 10.1093/nar/gkw1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shikata Y., Watanabe T., Teramoto T., Inoue A., Kawakami Y., Nishizawa Y., Katayama K., and Kuwada M. (1995) Isolation and characterization of a peptide isomerase from funnel web spider venom. J. Biol. Chem. 270, 16719–16723 10.1074/jbc.270.28.16719 [DOI] [PubMed] [Google Scholar]
- 16. Biner O., Trachsel C., Moser A., Kopp L., Langenegger N., Kämpfer U., von Ballmoos C., Nentwig W., Schürch S., Schaller J., and Kuhn-Nentwig L. (2015) Isolation, N-glycosylations and function of a hyaluronidase-like enzyme from the venom of the spider Cupiennius salei. PLoS ONE 10, e0143963 10.1371/journal.pone.0143963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hanquier J., Sorlet Y., Desplancq D., Baroche L., Ebtinger M., Lefevre J. F., Pattus F., Hershberger C. L., and Vertes A. A. (2003) A single mutation in the activation site of bovine trypsinogen enhances its accumulation in the fermentation broth of the yeast Pichia pastoris. Appl. Environ. Microbiol. 69, 1108–1113 10.1128/AEM.69.2.1108-1113.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Catalan A., Cortes W., Sagua H., González J., and Araya J. E. (2011) Two new phospholipase D isoforms of Loxosceles laeta: cloning, heterologous expression, functional characterization, and potential biotechnological application. J. Biochem. Mol. Toxicol. 25, 393–403 10.1002/jbt.20399 [DOI] [PubMed] [Google Scholar]
- 19. Haney R. A., Clarke T. H., Gadgil R., Fitzpatrick R., Hayashi C. Y., Ayoub N. A., and Garb J. E. (2016) Effects of gene duplication, positive selection, and shifts in gene expression on the evolution of the venom gland transcriptome in widow spiders. Genome Biol. Evol. 8, 228–242 10.1093/gbe/evv253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Richardson M., Pimenta A. M., Bemquerer M. P., Santoro M. M., Beirao P. S., Lima M. E., Figueiredo S. G., Bloch C. Jr, Vasconcelos E. A., Campos F. A., Gomes P. C., and Cordeiro M. N. (2006) Comparison of the partial proteomes of the venoms of Brazilian spiders of the genus Phoneutria. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 142, 173–187 10.1016/j.cbpc.2005.09.010 [DOI] [PubMed] [Google Scholar]
- 21. Nentwig W. (2013) Spider Ecophysiology. in Spider Ecophysiology (Nentwig W., ed) 1 Ed., pp. 505–508, Springer-Verlag Berlin, Heidelberg [Google Scholar]
- 22. Wheeler W. C., Coddington J. A., Crowley L. M., Dimitrov D., Goloboff P. A., Griswold C. E., Hormiga G., Prendini L., Ramírez M. J., Sierwald P., Almeida-Silva L., Alvarez-Padilla F., Arnedo M. A., Benavides Silva L. R., et al. (2016) The spider tree of life: phylogeny of Araneae based on target-gene analyses from an extensive taxon sampling. Cladistics 33, 574–616 [DOI] [PubMed] [Google Scholar]
- 23. Polotow D., Carmichael A., and Griswold C. E. (2015) Total evidence analysis of the phylogenetic relationships of Lycosoidea spiders (Araneae, Entelegynae). Invertebr. Syst. 29, 124–163 10.1071/IS14041 [DOI] [Google Scholar]
- 24. Petersen T. N., Brunak S., von Heijne G., and Nielsen H. (2011) SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat. Methods 8, 785–786 10.1038/nmeth.1701 [DOI] [PubMed] [Google Scholar]
- 25. Milne T. J., Abbenante G., Tyndall J. D., Halliday J., and Lewis R. J. (2003) Isolation and characterization of a cone snail protease with homology to CRISP proteins of the pathogenesis-related protein superfamily. J. Biol. Chem. 278, 31105–31110 10.1074/jbc.M304843200 [DOI] [PubMed] [Google Scholar]
- 26. Qian J., Guo Z. Y., and Chi C. W. (2008) Cloning and isolation of a Conus cysteine-rich protein homologous to Tex31 but without proteolytic activity. Acta Biochim. Biophys. Sin. (Shanghai) 40, 174–181 [DOI] [PubMed] [Google Scholar]
- 27. Oliva M. L., Andrade S. A., Juliano M. A., Sallai R. C., Torquato R. J., Sampaio M. U., Pott V. J., and Sampaio C. A. (2003) Kinetic characterization of factor Xa binding using a quenched fluorescent substrate based on the reactive site of factor Xa inhibitor from Bauhinia ungulata seeds. Curr. Med. Chem. 10, 1085–1093 10.2174/0929867033457548 [DOI] [PubMed] [Google Scholar]
- 28. Herzig V., Wood D. L., Newell F., Chaumeil P. A., Kaas Q., Binford G. J., Nicholson G. M., Gorse D., and King G. F. (2011) ArachnoServer 2.0, an updated online resource for spider toxin sequences and structures. Nucleic Acids Res. 39, D653–657 10.1093/nar/gkq1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kuhn-Nentwig L., Schaller J., Schürch S., and Nentwig W. (2016) Venom of Cupiennius salei (Ctenidae). in Spider Venoms (Gopalakrishnakone P., Corzo G. A., de Lima M. E., and Diego-García E., eds) pp. 47–70, Springer Netherlands, Dordrecht [Google Scholar]
- 30. Vassilevski A. A., Kozlov S. A., and Grishin E. V. (2008) Antimicrobial peptide precursor structures suggest effective production strategies. Recent Pat. Inflamm. Allergy Drug Discov. 2, 58–63 10.2174/187221308783399261 [DOI] [PubMed] [Google Scholar]
- 31. Kuhn-Nentwig L., Fedorova I. M., Lüscher B. P., Kopp L. S., Trachsel C., Schaller J., Vu X. L., Seebeck T., Streitberger K., Nentwig W., Sigel E., and Magazanik L. G. (2012) A venom-derived neurotoxin, CsTx-1, from the spider Cupiennius salei exhibits cytolytic activities. J. Biol. Chem. 287, 25640–25649 10.1074/jbc.M112.339051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grabherr M. G., Haas B. J., Yassour M., Levin J. Z., Thompson D. A., Amit I., Adiconis X., Fan L., Raychowdhury R., Zeng Q., Chen Z., Mauceli E., Hacohen N., Gnirke A., Rhind N., di Palma F., Birren B. W., Nusbaum C., Lindblad-Toh K., Friedman N., and Regev A. (2011) Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 29, 644–652 10.1038/nbt.1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Slater G. S., and Birney E. (2005) Automated generation of heuristics for biological sequence comparison. BMC Bioinformatics 6, 31 10.1186/1471-2105-6-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Trachsel C., Siegemund D., Kämpfer U., Kopp L. S., Bühr C., Grossmann J., Lüthi C., Cunningham M., Nentwig W., Kuhn-Nentwig L., Schürch S., and Schaller J. (2012) Multicomponent venom of the spider Cupiennius salei: a bioanalytical investigation applying different strategies. FEBS J. 279, 2683–2694 10.1111/j.1742-4658.2012.08650.x [DOI] [PubMed] [Google Scholar]
- 35. Gunasekera K., Wüthrich D., Braga-Lagache S., Heller M., and Ochsenreiter T. (2012) Proteome remodelling during development from blood to insect-form Trypanosoma brucei quantified by SILAC and mass spectrometry. BMC Genomics 13, 556 10.1186/1471-2164-13-556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brügger V., Duman M., Bochud M., Munger E., Heller M., Ruff S., and Jacob C. (2017) Delaying histone deacetylase response to injury accelerates conversion into repair Schwann cells and nerve regeneration. Nat. Commun. 8, 14272 10.1038/ncomms14272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vaudel M., Barsnes H., Berven F. S., Sickmann A., and Martens L. (2011) SearchGUI: An open-source graphical user interface for simultaneous OMSSA and X!Tandem searches. Proteomics 11, 996–999 10.1002/pmic.201000595 [DOI] [PubMed] [Google Scholar]
- 38. Vaudel M., Burkhart J. M., Zahedi R. P., Oveland E., Berven F. S., Sickmann A., Martens L., and Barsnes H. (2015) PeptideShaker enables reanalysis of MS-derived proteomics data sets. Nat. Biotechnol. 33, 22–24 10.1038/nbt.3109 [DOI] [PubMed] [Google Scholar]
- 39. Sievers F., Wilm A., Dineen D., Gibson T. J., Karplus K., Li W., Lopez R., McWilliam H., Remmert M., Söding J., Thompson J. D., and Higgins D. G. (2011) Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7, 539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kumar S., Stecher G., and Tamura K. (2016) MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stamatakis A. (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22, 2688–2690 10.1093/bioinformatics/btl446 [DOI] [PubMed] [Google Scholar]
- 42. Stamatakis A., Hoover P., and Rougemont J. (2008) A rapid bootstrap algorithm for the RAxML Web servers. Syst. Biol. 57, 758–771 10.1080/10635150802429642 [DOI] [PubMed] [Google Scholar]
- 43. Vizcaíno J. A., Csordas A., del-Toro N., Dianes J. A., Griss J., Lavidas I., Mayer G., Perez-Riverol Y., Reisinger F., Ternent T., Xu Q. W., Wang R., and Hermjakob H. (2016) 2016 update of the PRIDE database and its related tools. Nucleic Acids Res. 44, D447–D456 10.1093/nar/gkv1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.