Abstract
Accumulated epidemiological, clinical and experimental evidence has indicated the beneficial health effects of the Mediterranean diet, which is typified by the consumption of virgin olive oil (VOO) as a main source of dietary fat. At the cellular level, compounds derived from various olive (Olea europaea), matrices, have demonstrated potent antioxidant and anti-inflammatory effects, which are thought to account, at least in part, for their biological effects. Research efforts are expanding into the characterization of compounds derived from Olea europaea, however, the considerable diversity and complexity of the vast array of chemical compounds have made their precise identification and quantification challenging. As such, only a relatively small subset of olive-derived compounds has been explored for their biological activity and potential health effects to date. Although there is adequate information describing the identification or isolation of olive-derived compounds, these are not easily searchable, especially when attempting to acquire chemical or biological properties. Therefore, we have created the OliveNet™ database containing a comprehensive catalogue of compounds identified from matrices of the olive, including the fruit, leaf and VOO, as well as in the wastewater and pomace accrued during oil production. From a total of 752 compounds, chemical analysis was sufficient for 676 individual compounds, which have been included in the database. The database is curated and comprehensively referenced containing information for the 676 compounds, which are divided into 13 main classes and 47 subclasses. Importantly, with respect to current research trends, the database includes 222 olive phenolics, which are divided into 13 subclasses. To our knowledge, OliveNet™ is currently the only curated open access database with a comprehensive collection of compounds associated with Olea europaea.
Database URL: https://www.mccordresearch.com.au
Introduction
The genus Olea (Oleaceae) contains approximately 40 taxa of evergreen shrubs and trees, found throughout southern Europe, Africa, Asia and Oceania (1). Native to the Mediterranean basin is Olea europaea is ubiquitously distributed throughout the region and is grown commercially for its fruit which contributes to the production of olive oil and table olives (2). Its hardiness and ubiquitous consumption have seen the cultivation of O. europaea endure from the Copper Age to the present day, where it is now one of the most valuable crops worldwide (3). Traditionally, the olive fruit and oil has been widely used in folk medicine, where it is used as a topical antiseptic and an analgesic for rheumatism and abdominal pain (4, 5). In more recent times, the beneficial health effects associated with the consumption of the extra-virgin olive has been highlighted by studies encompassing the Mediterranean diet, where olive oil is a primary source of dietary fat (6, 7).
Typified by the classical Seven Countries Study and more recently the Prevention with Mediterranean Diet study, the potential health benefits of the Mediterranean diet, associated with the consumption of extra-virgin olive oil (VOO), have emerged for a wide range of conditions including, cardiovascular disease, metabolic disease and diabetes, cancer prevention and neurodegeneration (8–14). In simple terms, extra-VOO is compromised of the major fatty acid fraction (98–99%), which comprised of predominantly oleic acid (55–83%) and linoleic acid (up to approximately 20%; Figure 1), and the minor constituents that incorporate the phenolic compounds (1–2%) (15).
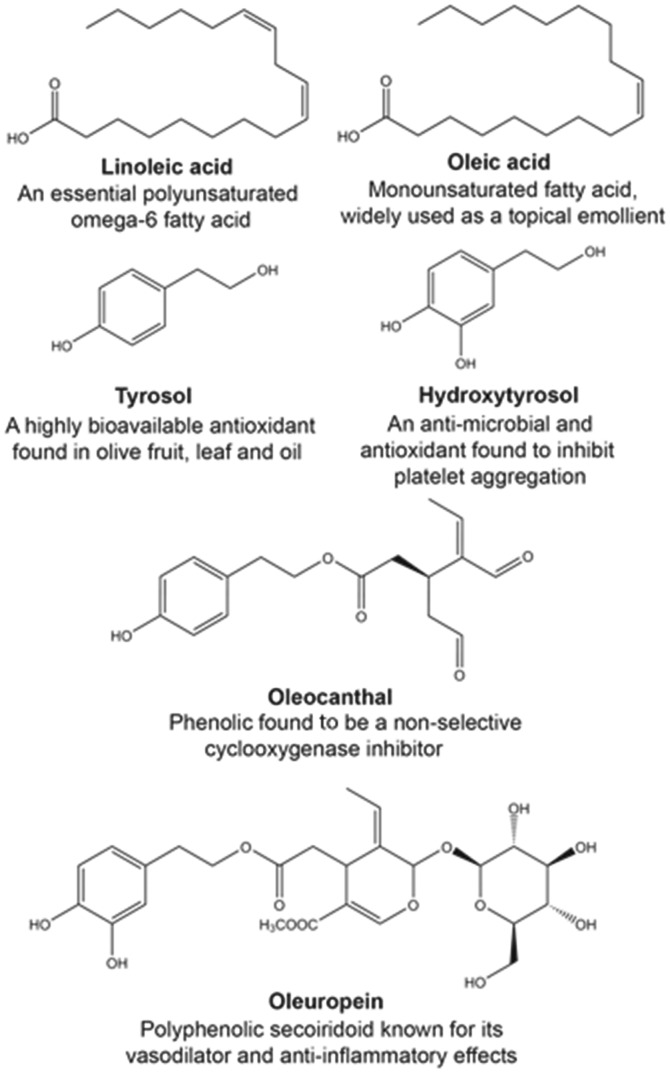
Figure 1.
Chemical structures and key feature of widely investigated fatty acids (oleic and linoleic acids), and phenolic compounds (tyrosol, hydroxytyrosol, oleocanthal, and oleuropein) from Olea europaea.
Historically, the health benefits of extra-VOO was attributed to the high level of the mono- (oleic acid) and poly- (linoleic acid) unsaturated fatty acids, which, e.g. have been shown to have a wide range of beneficial cardiovascular effects (16–18). Further, it is now recognized that many of the beneficial effects of extra-VOO may be due to the unique minor compounds, including the flavonoids, lignans, secoiridoids and their hydrolysis products (19–21). These compounds have been shown to possess a broad range of bioactive properties, including antioxidant, anti-inflammatory and chemo-preventative activities, which have been elucidated over the past 20 years. The most widely investigated olive phenolic compounds include, oleuropein, hydroxytyrosol, tyrosol and oleocanthal (Figure 1). The potential beneficial health effects and cellular and molecular mechanisms of oleuropein, hydroxytyrosol and tyrosol have been reviewed extensively (22–29). Oleocanthal also represents an important phenolic compound in extra-VOO, which has been shown to possess potent antioxidant and anti-inflammatory effects, at least in part, through non-specific inhibition of cyclooxygenase-1 and -2 enzymes (30, 31).
Despite the promise of the Mediterranean diet with the consumption of extra-virgin olive and the encouraging results with specific fatty acids and phenolics, only a very small subset of compounds associated with O. europaea have been investigated to date. Importantly, many compounds that are structurally similar the well-known oleuropein, hydroxytyrosol, tyrosol and oleocanthal are either: (i) not commercially available, (ii) have not been synthesized in or isolated in quantities allowing meaningful experimentation and/or (iii) where available, have not been investigated for their biological effects.
OliveNet™: construction and content
The considerable diversity and complexity of compounds derived from O. europaea as well as the matrix (leaf, fruit, oil, pomace and wastewater), in which they are found, has posed challenges for characterization and isolation of many of these compound. However, there is adequate information available in the literature describing the identification and isolation of compounds from the olive. Whilst found exclusively in the literature, these compounds are not easily searchable, especially when attempting to acquire specific information or finding compounds with specific chemical or biological properties. Further, it is difficult to decipher which compounds from the literature have been included in existing small-molecule databases, such as the most comprehensive, PubChem (32). Therefore, we created the comprehensive OliveNet™ database incorporating the individual compounds derived from O. europaea. Curated and fully referenced, the OliveNet™ database serves as a valuable resource for those within various scientific disciplines interested in compounds found within the various matrices of the olive, the associated analytical techniques used in their identification and/or isolation and, where appropriate key information related to known biological activities.
Overall, OliveNet™ describes 676 compounds associated with O. europaea, including 222 phenolic compounds. Compounds were identified from a comprehensive review of 181 scientific publications, including journal articles and books, using relevant search terms including ‘olea europaea’, ‘olive’, ‘phenol’, ‘polyphenol’ in PubMed and Science Direct. Overall, the data entry goal was to source, extract and critically assess data from published reports concerned with the identification and biological effects of bioactive compounds in O. europaea. Original publications were selected based on the analytical methods used to identify and quantify the compounds present in the olive matrices. These involved a range of extraction processes, analytical separation and quantification techniques. Generally, high-performance liquid chromatography coupled to mass spectrometry/gas chromatography was employed to separate and then quantify the unsaponifiable compounds, including the phenolics (33–35). High resolution multinuclear (1 H, 13 C, 31 P) nuclear magnetic resonance spectroscopy was also used for elucidation of isolated compounds (36–39). These techniques represent a higher sensitivity compared to other spectrophotometric techniques, which have several limitations associated with their application (40). The importance of appropriate methodologies for isolation and characterization of bioactive phytonutrients such as phenolic compounds and flavonoids, which are included in our database, have been thoroughly reviewed, and research efforts for improving the quality of extracts are constantly evolving (41–45). For our OliveNet™ database, if the methodology was not sufficiently documented or considered inadequate, the compound was excluded, resulting in the reduction of the total 752 compounds initially identified to the final 676 individual compounds catalogued in the database.
Compound information regarding pharmacological activity was obtained from PubChem, Human Metabolome Database (HMDB) and Chemical Entities of Biological Interest (ChEBI), with links provided (46–48). Further, medical subject headings (MeSH) terms are provided where available. Similarly, concentrations of each compound in the various olive matrices (leaf, fruit, oil, pomace and wastewater) are documented where available. Known biological effects and pharmacological activity of the compounds are included, with references to biological efficacy determined by in vitro or in vivo studies as required. Important features of the OliveNet™ database are that it (i) easily adapted to include new compounds and (ii) encourages community input (Figure 2). Therefore, as further relevant research emerges, we can easily update compounds and biological activities.
Figure 2.
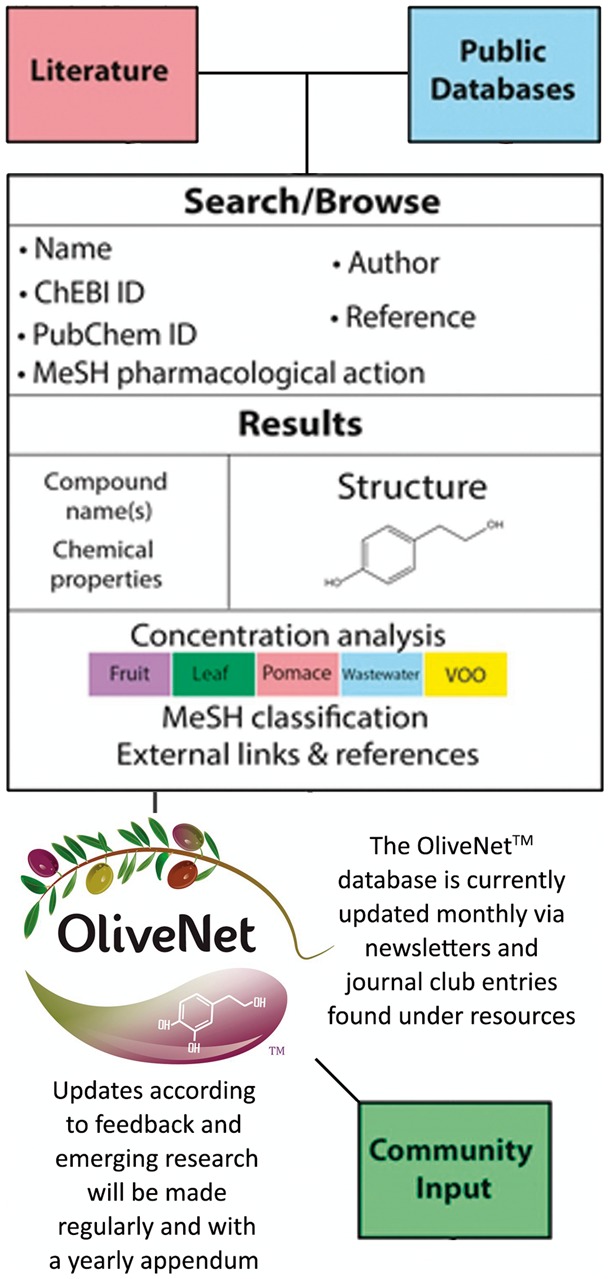
Integration of structure and composition of the OliveNet™ database. Compounds were identified from a comprehensive review of 181 scientific publications and compound information was obtained from public databases (PubChem, HMDB and ChEBI). Together with the chemical structure and basic chemical analyses, MeSH terms are provided where available. Concentrations of each compound in fruit, leaf, pomace, wastewater, VOO are indicated where available. Known biological effects and pharmacological activity of the compounds are included with references. As further relevant research emerges, we can easily update compounds and biological activities via community input.
Composition of OliveNet™
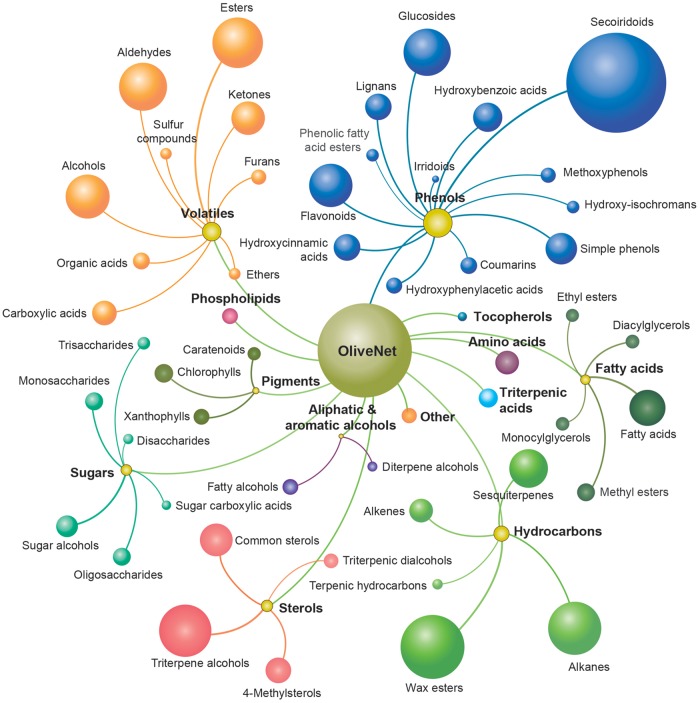
The 676 distinct chemical compounds derived from O. europaea in the OliveNet™ database are divided into 13 main classes, namely phenols, fatty acids, aliphatic and aromatic alcohols, sterols, phospholipids, triterpenic acids, volatiles, hydrocarbons, sugars, pigments, tocopherols, amino acids and a small number of other unclassified compounds (Figure 3). As described, 181 individual references were utilized to identify and classify the chemical compounds. The detailed classification process and the pertinent references for each compound have been documented in a table entitled ‘Original OliveNet™ Library: Quick Reference’, which can be easily be obtained from the web site. The table contains the complete list of compounds in their rightful overall class, an indication of the olive matrix in which they have been identified, and the key reference associated with the classification process.
Figure 3.
The OliveNet™ database consists of a total of 676 individual compounds categorized into 13 classes (bold and colour coordinated) and 47 subclasses (size of ball reflects the number of compounds in each subclass).
The phenolic class of compounds is of particular importance with respect to human nutrition. Compounds in this class contribute to the stability and organoleptic properties of the oil and possess a range of biological activities, which are of particular importance. Indeed, the European Food Safety Authority has approved claims stating that the dietary intake of extra-VOO phenolic compounds prevent oxidation of low-density lipoprotein, display anti-inflammatory properties and contribute to body defences against external agents (49). As described earlier, research has focussed on a few phenolic compounds such as hydroxytyrosol and oleuropein, which have been shown to prevent oxidative stress and selectively induce cell-cycle arrest and induce apoptosis in cancer cells (50–54). It is generally recognized that their orthodiphenolic structure confers strong antioxidant activity, while their lipophilicity profiles provide insight into their anti-inflammatory function (51, 55).
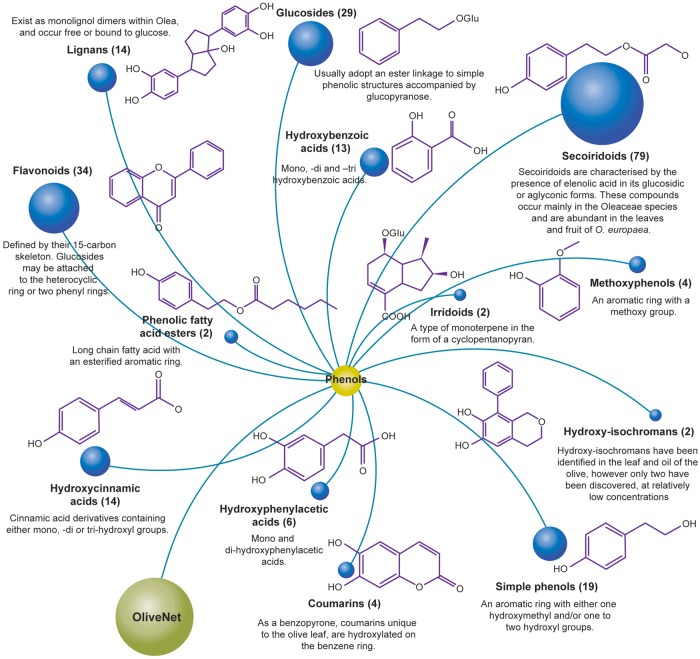
Phenolic compounds encompass a diverse subset of chemical structures found within the leaf, fruit, oil, pomace and wastewater from processing. Structurally, they are characterized by an aromatic ring with one or more hydroxyl groups. In the OliveNet™ database, we describe a total of 222 phenolic compounds derived from O. europaea, which are further divided into 13 subclasses comprised of the: simple phenols, methoxyphenols, hydroxybenzoic acids, hydroxyphenylacetic acids, hydroxycinnamic acids, secoiridoids, glucosides, flavonoids, hydroxyisochromans, coumarins, irridoids, lignans and phenolic fatty acid esters (Figure 4). Among the phenolic compounds presented in OliveNet™, approximately 45% are not currently commercially available and only a small subset has been investigated in biological experiments. For example, one of the most widely investigated olive phenolics is hydroxytyrosol and as can identified in the ‘Original OliveNet™ Library: Quick Reference’ table on the website, hydroxytyrosol, is a simple phenolic compound found in various concentrations in the olive fruit, leaf, pomace, and in extra-VOO (56–59). In contrast, much less is known about other more obscure olive-derived phenolics, such as dihydro-p-coumaric acid (phloretic acid), certain hydroxyphenylacetic acids (e.g. 4-hydroxy-3-methoxyphenylacetic acid, and caffeoylglucose, to name a few examples (60–62). In this context, one of the major aims of producing the OliveNet™ database is to identify and classify compounds to inspire potential synthesis of non-commercially available compounds and to encourage further exploration of the less well-studied compounds.
Figure 4.
The OliveNet™ database consists of 222 individual phenolic compounds divided into 13 subclasses. The number of individual compounds in each subclass, representative chemical structures, and relevant key information are shown.
Website content and utility
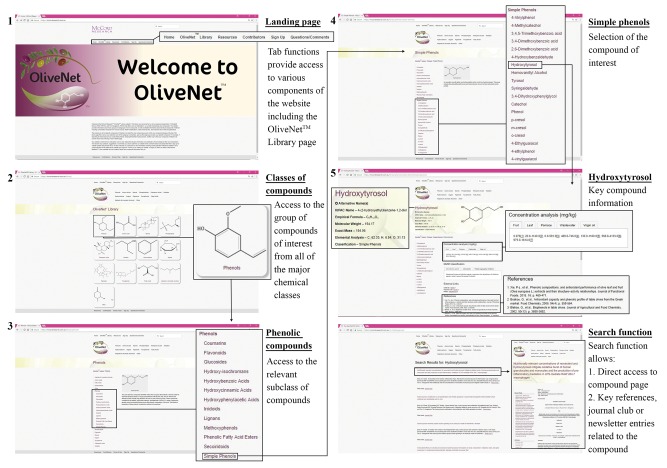
OliveNet™ employs a web interface, which is simple and easy to use and allows for compounds to be searched by a number of different criteria. As the step-by-step workflow indicates users may browse for compounds of interest via the OliveNet™ Library homepage (Figure 5). Alternatively, direct website search engine is provided on each page, where compound names or keywords can be entered directly (Figure 5). Users are initially directed to the site homepage, where user-friendly links for searching and browsing compounds are provided. On each compound information page, the chemical structure is displayed and all of the chemical names, including the IUPAC name, common name and any synonyms for that particular compound are listed. The compound properties, including the molecular weight, exact mass and elemental analysis is also provided. Where available, the concentration (mg/kg) quantified in the leaf, fruit, oil and in the oil processing products (pomace and wastewater), is provided with relevant references. Similarly, where relevant, references are provided in relation to the classification and bioactivity of individual compounds.
Figure 5.
Screenshots from the OliveNet™ database. An example of navigation from the OliveNet™ Library homepage, to the phenolic compound landing page, and ultimately to an individual phenolic compound page (in this example, hydroxytyrosol), is shown. The direct search function is also highlighted
Overall, the OliveNet™ database provides a comprehensive record of compounds associated with O. europaea in one easily searchable database. We intend OliveNet™ to be a valuable resource for those conducting chemical, biochemical or biological research related to O. europaea. It is anticipated that primary users will be from the food industry where structural analyses, synthesis of novel compounds and chemical characterization, may ultimately lead to additional standards for assessment of the quality of extra-VOOs. More broadly in the biological field, OliveNet™ is intended to serve as a platform for initial structure-activity relationship analyses, in silico modeling and characterization to preferred biological pathway targets and potentially identification of lead compounds for isolation or synthesis. Ultimately, novel compounds may emerge for various diseases and these potentially suitable candidates may be isolated or synthesized in experimentally meaningful purities and concentrations, for further in vitro and in vivo exploration and validation.
With respect to community input and evolution of the website, with further research and understanding, additional compounds, or even classes of compounds will incorporated into the database. For example, we are currently scoping the seleno-amino acids and phytoprostanes, which, represent classes of important bioactive compounds (63–65). The antioxidant and anti-inflammatory properties of seleno-amino acids (such as selenocysteine) have been widely studied and their presence in extra-VOO is emerging (66, 67). Similarly, phytoprostanes are emerging as important bioactive compounds found in extra-VOO and these also targets for inclusion in the database (68–70).
Conclusions and future enhancements
In summary, OliveNet™ is a freely available database containing compounds identified from O. europaea. As an online interface, OliveNet™ allows olive researchers and industry users to easily find information about 676 common and mostly obscure compounds associated with O. europaea, as well as to examine their chemical structure for structure-activity relationship analyses and for potential use in virtual screening. OliveNet™ represents a comprehensively referenced database linked to peer-reviewed literature, which will be updated as required. Further, modifications and additions to reflect new discoveries from the research community are encouraged. It is anticipated that as analytical techniques advance, the integration of past and future work by various disciplines will drive forward the validation of possible bioactives. Since there are still many unknown compounds present in the polar fraction of olive oil, it remains important to carry out fundamental chemical analyses of the various O. europaea matrices. New findings can be easily incorporated into the OliveNet™ database.
Acknowledgements
The authors are grateful to Ms Kariel Post of McCord Research (Iowa, USA) for designing and creating the OliveNet™ website.
Funding
T.C.K. was supported by an Australian Research Council Future Fellowship, and the Epigenomic Medicine Laboratory was supported by McCord Research (Iowa, USA). This study was supported in part by the Victorian Government's Operational Infrastructure Support Program.
Conflict of interest
There is no conflict of interest with respect to the OliveNet™ database. However, it should be known that Dr Elizabeth D McCord is the owner and CEO of McCord Research, Iowa USA and inventor of the olive-based formulation Olivamine®.
References
- 1. Guillaume B., Rafael Rubio de C., Pascal-Antoine C.. et al. (2009) Phylogenetics of Olea (Oleaceae) based on plastid and nuclear ribosomal DNA sequences: tertiary climatic shifts and lineage differentiation times. Ann. Botany, 104, 143.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Green P.S. (2002) A Revision of Olea L. (Oleaceae). Kew Bull., 57, 91–140. [Google Scholar]
- 3. Galili E., Weinstein-Evron M., Zohary D. (1989) Appearance of olives in submerged neolithic sites along the carmel coast. J. Israel Perhistoric Soc., 22, 95–97. [Google Scholar]
- 4. Aikaterini P.-K. (2006) The culture of the olive tree (Mediterranean World) In: Boskou D. (ed). Olive oil: Chemistry and Technology. AOCS press, Ill, USA, pp. 1–2. [Google Scholar]
- 5. Ray N.B., Lam N.T., Luc R.. et al. (2015) Cellular and molecular effects of bioactive phenolic compounds in olives and olive oil In: Boskou D. (ed.). Olive and Olive Oil Bioactive Constituents. AOCS Press, Ill, USA, pp. 53–91. [Google Scholar]
- 6. la Lastra C., Barranco M., Motilva V.. et al. (2001) Mediterranean diet and health: biological importance of olive oil. Curr. Pharm. Des., 7, 933–950. [DOI] [PubMed] [Google Scholar]
- 7. Hertog M.G., Kromhout D., Aravanis C.. et al. (1995) Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Archiv. Int. Med., 155, 381–386. [PubMed] [Google Scholar]
- 8. Filik L., Ozyilkan O. (2003) Olive-oil consumption and cancer risk. Eur. J. Clin. Nutr., 57, 191.. [DOI] [PubMed] [Google Scholar]
- 9. Hu F.B. (2003) The Mediterranean diet and mortality–olive oil and beyond. N. Eng. J. Med., 348, 2595–2596. [DOI] [PubMed] [Google Scholar]
- 10. Visioli F., Galli C. (1995) Natural antioxidants and prevention of coronary heart disease: the role of olive oil and its minor constituents. Nutr. Metabol. Cardiovasc. Diseases, 5, 306–306. [Google Scholar]
- 11. Willett W.C., Sacks F., Trichopoulou A.. et al. (1995) Mediterranean diet pyramid: a cultural model for healthy eating. Am. J. Clin. Nutr., 61, 1402S–1406S. [DOI] [PubMed] [Google Scholar]
- 12. Estruch R., Ros E., Salas-Salvado J.. et al. (2013) Primary prevention of cardiovascular disease with a Mediterranean diet. N. Eng. J. Med., 368, 1279–1290. [DOI] [PubMed] [Google Scholar]
- 13. Aboul-Enein B.H., Puddy W.C., Bernstein J. (2017) Ancel Benjamin Keys (1904-2004): his early works and the legacy of the modern Mediterranean diet. J. Med. Biograph., doi: 10.1177/0967772017727696. [DOI] [PubMed] [Google Scholar]
- 14. Keys A., Menotti A., Karvonen M.J.. et al. (1986) The diet and 15-year death rate in the seven countries study. Am. J. Epidemiol., 124, 903–915. [DOI] [PubMed] [Google Scholar]
- 15. Viola P., Viola M. (2009) Virgin olive oil as a fundamental nutritional component and skin protector. Clin. Dermatol., 27, 159–165. [DOI] [PubMed] [Google Scholar]
- 16. Singer P., Jaeger W., Berger I.. et al. (1990) Effects of dietary oleic, linoleic and alpha-linolenic acids on blood pressure, serum lipids, lipoproteins and the formation of eicosanoid precursors in patients with mild essential hypertension. J. Human Hypertension, 4, 227–233. [PubMed] [Google Scholar]
- 17. Seppanen-Laakso T., Vanhanen H., Laakso I.. et al. (1993) Replacement of margarine on bread by rapeseed and olive oils: effects on plasma fatty acid composition and serum cholesterol. Ann. Nutr. Metabol., 37, 161–174. [DOI] [PubMed] [Google Scholar]
- 18. Aviram M., Eias K. (1993) Dietary olive oil reduces low-density lipoprotein uptake by macrophages and decreases the susceptibility of the lipoprotein to undergo lipid peroxidation. Ann. Nutr. Metabol., 37, 75–84. [DOI] [PubMed] [Google Scholar]
- 19. Servili M., Montedoro G. (2002) Contribution of phenolic compounds to virgin olive oil quality. Eur. J. Lipid Sci. Technol., 104, 602–613. [Google Scholar]
- 20. Frankel E.N. (2011) Nutritional and biological properties of extra virgin olive oil. J. Agric. Food Chem., 59, 785–792. [DOI] [PubMed] [Google Scholar]
- 21. Vissers M.N., Zock P.L., Katan M.B. (2004) Bioavailability and antioxidant effects of olive oil phenols in humans: a review. Eur. J Clin. Nutr., 58, 955–965. [DOI] [PubMed] [Google Scholar]
- 22. Shamshoum H., Vlavcheski F., Tsiani E. (2017) Anticancer effects of oleuropein. BioFactors, 43, 517–528. [DOI] [PubMed] [Google Scholar]
- 23. Fayyaz S., Aydin T., Cakir A.. et al. (2016) Oleuropein mediated targeting of signaling network in cancer. Curr. Top. Med. Chem., 16, 2477–2483. [DOI] [PubMed] [Google Scholar]
- 24. Peyrol J., Riva C., Amiot M.J. (2017) Hydroxytyrosol in the prevention of the metabolic syndrome and related disorders. Nutrients, 9, 306.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fabiani R. (2016) Anti-cancer properties of olive oil secoiridoid phenols: a systematic review of in vivo studies. Food Function, 7, 4145–4159. [DOI] [PubMed] [Google Scholar]
- 26. Rigacci S., Stefani M. (2016) Nutraceutical properties of olive oil polyphenols. An itinerary from cultured cells through animal models to humans. Int. J. Mol. Sci., 17, 843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stefani M., Rigacci S. (2014) Beneficial properties of natural phenols: highlight on protection against pathological conditions associated with amyloid aggregation. BioFactors, 40, 482–493. [DOI] [PubMed] [Google Scholar]
- 28. Carito V., Ceccanti M., Tarani L.. et al. (2016) Neurotrophins' modulation by olive polyphenols. Curr. Med. Chem., 23, 3189–3197. [DOI] [PubMed] [Google Scholar]
- 29. De Nicolo S., Tarani L., Ceccanti M.. et al. (2013) Effects of olive polyphenols administration on nerve growth factor and brain-derived neurotrophic factor in the mouse brain. Nutrition, 29, 681–687. [DOI] [PubMed] [Google Scholar]
- 30. Beauchamp G.K., Keast R.S., Morel D.. et al. (2005) Phytochemistry: ibuprofen-like activity in extra-virgin olive oil. Nature, 437, 45–46. [DOI] [PubMed] [Google Scholar]
- 31. Parkinson L., Keast R. (2014) Oleocanthal, a phenolic derived from virgin olive oil: a review of the beneficial effects on inflammatory disease. Int. J. Mol. Sci., 15, 12323–12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bolton E.E., Wang Y., Thiessen P.A.. et al. (2008) PubChem: integrated platform of small molecules and biological activities. Ann. Rep. Comput. Chem., 4, 217–241. [Google Scholar]
- 33. de la Torre-Carbot K., Jauregui O., Gimeno E.. et al. (2005) Characterization and quantification of phenolic compounds in olive oils by solid-phase extraction, HPLC-DAD, and HPLC-MS/MS. J. Agric. Food Chem., 53, 4331–4340. [DOI] [PubMed] [Google Scholar]
- 34. Boskou G., Salta F.N., Chrysostomou S.. et al. (2006) Antioxidant capacity and phenolic profile of table olives from the Greek market. Food Chem., 94, 558–564. [Google Scholar]
- 35. Quirantes‐Piné R., Lozano‐Sánchez J., Herrero M.. et al. (2013) HPLC–ESI–QTOF–MS as a powerful analytical tool for characterising phenolic compounds in olive‐leaf extracts. Phytochem. Anal., 24, 213–223. [DOI] [PubMed] [Google Scholar]
- 36. Christophoridou S., Dais P., Tseng L.H.. et al. (2005) Separation and identification of phenolic compounds in olive oil by coupling high-performance liquid chromatography with postcolumn solid-phase extraction to nuclear magnetic resonance spectroscopy (LC-SPE-NMR). J. Agric. Food Chem., 53, 4667–4679. [DOI] [PubMed] [Google Scholar]
- 37. Hatzakis E., Koidis A., Boskou D.. et al. (2008) Determination of phospholipids in olive oil by 31P NMR spectroscopy. J. Agric. Food Chem., 56, 6232–6240. [DOI] [PubMed] [Google Scholar]
- 38. Karkoula E., Skantzari A., Melliou E.. et al. (2012) Direct measurement of oleocanthal and oleacein levels in olive oil by quantitative (1)H NMR. Establishment of a new index for the characterization of extra virgin olive oils. J. Agric. Food Chem., 60, 11696–11703. [DOI] [PubMed] [Google Scholar]
- 39. Scano P., Casu M., Lai A.. et al. (1999) Recognition and quantitation of cis-vaccenic and eicosenoic fatty acids in olive oils by 13C nuclear magnetic resonance spectroscopy. Lipids, 34, 757–759. [DOI] [PubMed] [Google Scholar]
- 40. Gómez-Caravaca A.M., Lozano-Sánchez J., Gámez MdMC.. et al. (2015) Bioactive phenolic compounds from Olea europaea: a challenge for analytical chemistry In: Boskou D. (ed). Olive and Olive Oil Bioactive Constituents. AOCS Press, Ill, USA, pp. 261–298. [Google Scholar]
- 41. La Barbera G., Capriotti A.L., Cavaliere C.. et al. (2017) Liquid chromatography-high resolution mass spectrometry for the analysis of phytochemicals in vegetal-derived food and beverages. Food Res. Int., 100, 28–52. [DOI] [PubMed] [Google Scholar]
- 42. Zenezini Chiozzi R., Capriotti A.L., Cavaliere C.. et al. (2017) Evaluation of column length and particle size effect on the untargeted profiling of a phytochemical mixture by using UHPLC coupled to high-resolution mass spectrometry. J. Sep. Sci., 40, 2541–2557. [DOI] [PubMed] [Google Scholar]
- 43. Gallina-Toschi T., Cerretani L., Bendini A.. et al. (2005) Oxidative stability and phenolic content of virgin olive oil: an analytical approach by traditional and high resolution techniques. J. Sep. Sci., 28, 859–870. [DOI] [PubMed] [Google Scholar]
- 44. Goldsmith C.D., Vuong Q.V., Stathopoulos C.E.. et al. (2014) Optimization of the aqueous extraction of phenolic compounds from olive leaves. Antioxidants, 3, 700–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stamatopoulos K., Chatzilazarou A., Katsoyannos E. (2013) Optimization of multistage extraction of olive leaves for recovery of phenolic compounds at moderated temperatures and short extraction times. Foods, 3, 66–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bolton E.E., Wang Y., Thiessen P.A.. et al. (2008) Chapter 12—PubChem: integrated platform of small molecules and biological activities In: Ralph A.W., David C.S. (eds). Annual Reports in Computational Chemistry. Elsevier, Amsterdam, Netherlands, Vol. 4, pp. 217–241. [Google Scholar]
- 47. Wishart D.S., Tzur D., Knox C.. et al. (2007) HMDB: the Human Metabolome Database. Nucleic Acids Res., 35, D521–D526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Degtyarenko K., de Matos P., Ennis M.. et al. (2008) ChEBI: a database and ontology for chemical entities of biological interest. Nucleic Acids Res., 36, D344–D350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. EFSA Panel on Dietetic Products, N., Allergies (2011) Scientific Opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage (ID 1333, 1638, 1639, 1696, 2865), maintenance of normal blood HDL cholesterol concentrations (ID 1639), maintenance of normal blood pressure (ID 3781), “anti-inflammatory properties” (ID 1882), “contributes to the upper respiratory tract health” (ID 3468), “can help to maintain a normal function of gastrointestinal tract” (3779), and “contributes to body defences against external agents” (ID 3467) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J., 9, 2033. [Google Scholar]
- 50. Rafehi H., Smith A.J., Balcerczyk A.. et al. (2012) Investigation into the biological properties of the olive polyphenol, hydroxytyrosol: mechanistic insights by genome-wide mRNA-Seq analysis. Genes Nutr., 7, 343–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Saija A., Trombetta D., Tomaino A.. et al. (1998) In vitro'evaluation of the antioxidant activity and biomembrane interaction of the plant phenols oleuropein and hydroxytyrosol. Int. J. Pharm., 166, 123–133. [Google Scholar]
- 52. Benavente-García O., Castillo J., Lorente J.. et al. (2000) Antioxidant activity of phenolics extracted from Olea europaea L. leaves. Food Chem., 68, 457–462. [Google Scholar]
- 53. Visioli F., Bellomo G., Galli C. (1998) Free radical-scavenging properties of olive oil polyphenols. Biochem. Biophys. Res. Commun., 247, 60–64. [DOI] [PubMed] [Google Scholar]
- 54. Carluccio M.A., Siculella L., Ancora M.A.. et al. (2003) Olive oil and red wine antioxidant polyphenols inhibit endothelial activation antiatherogenic properties of mediterranean diet phytochemicals. Arterioscler. Thromb. Vasc. Biol., 23, 622–629. [DOI] [PubMed] [Google Scholar]
- 55. Galli C., Visioli F. (1999) Antioxidant and other activities of phenolics in olives/olive oil, typical components of the Mediterranean diet. Lipids, 34, S23–S26. [DOI] [PubMed] [Google Scholar]
- 56. Lozano-Sanchez J., Giambanelli E., Quirantes-Pine R.. et al. (2011) Wastes generated during the storage of extra virgin olive oil as a natural source of phenolic compounds. J. Agric. Food Chem., 59, 11491–11500. [DOI] [PubMed] [Google Scholar]
- 57. Dierkes G., Krieger S., Duck R.. et al. (2012) High-performance liquid chromatography-mass spectrometry profiling of phenolic compounds for evaluation of olive oil bitterness and pungency. J. Agric. Food Chem., 60, 7597–7606. [DOI] [PubMed] [Google Scholar]
- 58. Maiuri M.C., De Stefano D., Di Meglio P.. et al. (2005) Hydroxytyrosol, a phenolic compound from virgin olive oil, prevents macrophage activation. Naunyn Schmiedeberg's Arch. Pharmacol., 371, 457–465. [DOI] [PubMed] [Google Scholar]
- 59. Lozano-Sanchez J., Castro-Puyana M., Mendiola J.A.. et al. (2014) Recovering bioactive compounds from olive oil filter cake by advanced extraction techniques. Int. J. Mol. Sci., 15, 16270–16283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Owen R.W., Haubner R., Mier W.. et al. (2003) Isolation, structure elucidation and antioxidant potential of the major phenolic and flavonoid compounds in brined olive drupes. Food Chem. Toxicol., 41, 703–717. [DOI] [PubMed] [Google Scholar]
- 61. Bendini A., Cerretani L., Carrasco-Pancorbo A.. et al. (2007) Phenolic molecules in virgin olive oils: a survey of their sensory properties, health effects, antioxidant activity and analytical methods. An overview of the last decade. Molecules, 12, 1679–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jaiswal R., Matei M.F., Glembockyte V.. et al. (2014) Hierarchical key for the LC-MSn identification of all ten regio- and stereoisomers of caffeoylglucose. J. Agric. Food Chem., 62, 9252–9265. [DOI] [PubMed] [Google Scholar]
- 63. Karg K., Dirsch V.M., Vollmar A.M.. et al. (2007) Biologically active oxidized lipids (phytoprostanes) in the plant diet and parenteral lipid nutrition. Free Radical Res., 41, 25–37. [DOI] [PubMed] [Google Scholar]
- 64. Rahmanto A.S., Davies M.J. (2012) Selenium-containing amino acids as direct and indirect antioxidants. IUBMB Life, 64, 863–871. [DOI] [PubMed] [Google Scholar]
- 65. Skaff O., Pattison D.I., Morgan P.E.. et al. (2012) Selenium-containing amino acids are targets for myeloperoxidase-derived hypothiocyanous acid: determination of absolute rate constants and implications for biological damage. Biochem. J., 441, 305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Torres S., Cerutti S., Raba J.. et al. (2014) Preconcentration of seleno-amino acids on a XAD resin and determination in regional olive oils by SPE UPLC-ESI-MS/MS. Food Chem., 159, 407–413. [DOI] [PubMed] [Google Scholar]
- 67. Torres S., Gil R., Silva M.F.. et al. (2016) Determination of seleno-amino acids bound to proteins in extra virgin olive oils. Food Chem., 197, 400–405. [DOI] [PubMed] [Google Scholar]
- 68. Collado-Gonzalez J., Medina S., Durand T.. et al. (2015) New UHPLC-QqQ-MS/MS method for quantitative and qualitative determination of free phytoprostanes in foodstuffs of commercial olive and sunflower oils. Food Chem., 178, 212–220. [DOI] [PubMed] [Google Scholar]
- 69. Collado-Gonzalez J., Perez-Lopez D., Memmi H.. et al. (2015) Water deficit during pit hardening enhances phytoprostanes content, a plant biomarker of oxidative stress, in extra virgin olive oil. J. Agric. Food Chem., 63, 3784–3792. [DOI] [PubMed] [Google Scholar]
- 70. Collado-Gonzalez J., Grosso C., Valentao P.. et al. (2017) Inhibition of alpha-glucosidase and alpha-amylase by Spanish extra virgin olive oils: the involvement of bioactive compounds other than oleuropein and hydroxytyrosol. Food Chem., 235, 298–307. [DOI] [PubMed] [Google Scholar]