Abstract
Background and Aims
The rhizoids of leafy liverworts (Jungermanniales, Marchantiophyta) are commonly colonized by the ascomycete fungus Pezoloma ericae. These associations are hypothesized to be functionally analogous to the ericoid mycorrhizas (ErMs) formed by P. ericae with the roots of Ericaceae plants in terms of bi-directional phosphorus for carbon exchange; however, this remains unproven. Here, we test whether associations between the leafy liverwort Cephalozia bicuspidata and P. ericae are mutualistic.
Methods
We measured movement of phosphorus and carbon between C. bicuspidata and P. ericae using [33P]orthophosphate and 14CO2 isotope tracers in monoxenic cultures. We also measured leafy liverwort growth, with and without P. ericae.
Key Results
We present the first demonstration of nutritionally mutualistic symbiosis between a non-vascular plant and an ErM-forming fungus, showing transfer of fungal-acquired P to the liverwort and of liverwort-fixed C to the fungus alongside increased growth in fungus-colonized liverworts.
Conclusions
Thus, this ascomycete–liverwort symbiosis can now be described as mycorrhiza-like, providing further insights into ericoid mycorrhizal evolution and adding Ascomycota fungi to mycorrhizal fungal groups engaging in mutualisms with plants across the land plant phylogeny. As P. ericae also colonizes the rhizoids of Schistochilaceae liverworts, which originated in the Triassic and are sister to all other jungermannialean liverworts associated with fungi, our findings point toward an early origin of ascomycete–liverwort symbioses, possibly pre-dating their evolution in the Ericales by some 150 million years.
Keywords: Cephalozia bicuspidata, ericoid mycorrhizal fungi, liverwort, Pezoloma ericae, plant–fungus interactions, carbon-for-nutrient exchange, mycorrhizas, mutualism
INTRODUCTION
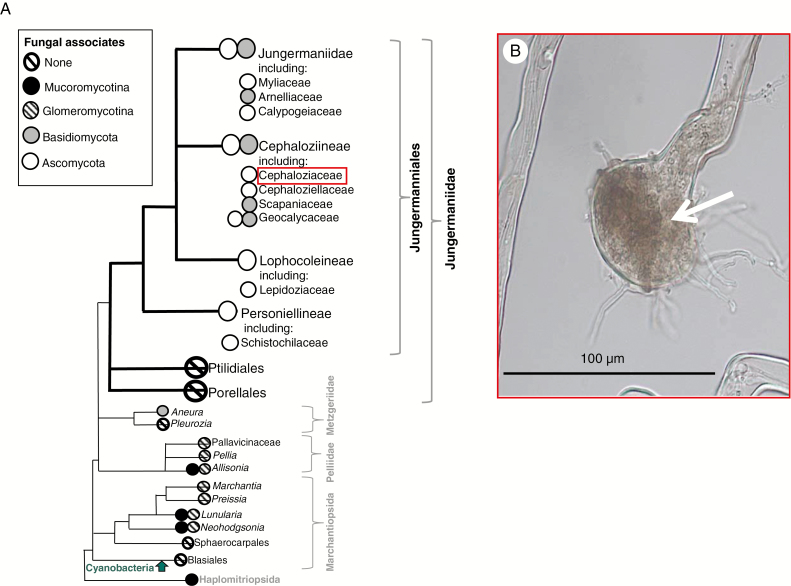
Mycorrhizas are intimate symbioses formed between plant roots and soil fungi that are prevalent across the globe in >80 % of extant land plants (Smith and Read, 2008). Through mycorrhizal associations, many plants engage in bi-directional exchange of photosynthesis-derived plant carbon (C) and fungal-acquired nutrients, scavenged from sources beyond the root depletion zone or from soil pores too small for roots to access (Smith and Read, 2008; Leake and Read, 2016). The role of mycorrhizas in supplying extant land plants with nutrients, together with evidence of mycorrhiza-like associations in Rhynie Chert plant fossils (Remy et al., 1994), has led to the hypothesis that mycorrhizal fungi probably facilitated the evolution of land plants >470 Mya (Pirozynski and Malloch, 1975; Bidartondo et al., 2011; Leake, 2015). Additionally, recent studies have shown that the genes required for mycorrhization are conserved across all land plant lineages (Wang et al., 2010), including the earliest diverging clade of liverworts – Haplomitriopsida (Fig. 1A, after Crandall-Stotler et al., 2009). The later-derived leafy liverworts (Jungermanniidae) have not been incorporated in such analyses, and a critical caveat of molecular studies is that the presence of genes does not necessarily imply functional significance. Indeed, for many groups of extant plants that form mycorrhizas and mycorrhiza-like associations where roots are absent, knowledge regarding the physiological function of the symbiosis has been severely limited (Read et al., 2000; Field et al., 2015a). To date, amongst fungus-associated early-branching land plants (i.e. liverworts, hornworts and lycophytes), nutritional mutualisms have only been demonstrated in a handful of early-diverging thalloid and Haplomitriopsida liverworts (Humphreys et al., 2010; Field et al., 2012, 2015a, 2016) (Fig. 1A), all of which form symbioses with Mucoromycota fungi (Glomeromycotina and/or Mucoromycotina) (Field et al., 2015b).
Fig. 1.
(A) Liverwort phylogram showing diversity in liverwort–fungal associations (after Crandall-Stotler et al., 2009) based on extensive sampling of nearly all the fungus-containing liverwort genera worldwide (Ligrone et al., 2007). (B) Cephalozia bicuspidata rhizoid resynthesized with Pezoloma ericae fungus (arrowed) in axenic culture.
As far as can be established from published surveys and incidental illustrations in floras (Pocock et al., 1984; Pocock and Duckett, 1985; Duckett et al., 1991; Ligrone et al., 2007), rhizoidal ascomycete associations occur in far more liverwort species than those containing basidiomycetes, glomeromycetes and mucoromycetes (Pressel et al., 2010). Following the accepted species names in The Plant List (2013), we estimate that up to 1000 late-diverging leafy liverworts are likely to have ascomycete fungal symbionts, i.e. around 20 % of liverwort species worldwide compared with <100 with basidiomycetes, glomeromycetes and mucoromycetes (see Supplemtnary Data Table S2 for a detailed breakdown). Reinforcing their symbiotic rather than opportunistic status, the ascomycetes (1) induce swelling, branching and septation of the rhizoids and (2) are ubiquitous rather than sporadic, in species where they occur. In addition, transmission electron micrographs show healthy hyphae in healthy host cells (Duckett et al., 1991; Pressel et al., 2010).
There are several families in the leafy liverworts (Jungermanniideae), including Schistochilaceae, Lepidoziaceae, Calypogeiaceae, Cephaloziaceae and Cephaloziellaceae, which consistently associate with Ascomycota fungi (Fig. 1A) (Pressel et al., 2010). These fungal symbionts include Pezoloma ericae (D.J. Read) Baral (syn. Rhizoscyphus ericae (D.J. Read) W.Y. Zhuang and Korf, 2004; Hymenoscyphus ericae (D.J. Read) Korf and Kernan, 1983; and Pezizella ericae (D.J. Read, 1974) (Duckett and Read, 1995; Read et al., 2000; Pressel et al., 2010). Notably, Pezoloma ericae is known to form ericoid mycorrhizas (ErMs) with the roots of Ericaceae plants and has previously been shown to provide nutrients to their vascular plant hosts in exchange for fixed C (Read et al., 2004; Smith and Read, 2008).
Ericaceous habitats are typically low in plant-available soil nutrients, including nitrogen (N) and/or phosphorus (P) (Stribley and Read, 1974; Mitchell and Read, 1981; Leake et al. 1990; Bolan, 1991; Myers and Leake, 1996). The vascular plants inhabiting these habitats, such as Calluna, Erica, Rhododendron and Vaccinium, grow together with non-vascular plants, including the widespread leafy liverworts in the Cephaloziaceae (Chambers et al., 1999; Upson et al., 2007) with which they ‘share’ fungal symbionts (Duckett and Read, 1995; Read et al., 2000). It was recently shown that that this shared mycobiont can bring benefits in terms of establishment and survival to ericaceous plants (Kowal et al., 2015), and that P. ericae-colonized liverworts may serve as a source of fungal inoculum for vascular plants. It is possible that this effect is driven by fungal-enhanced nutrition in liverworts and then in the vascular plant species sharing the fungal symbiont, analogous to the nutritional role of arbuscular mycorrhizal fungi (Glomeromycotina fungi) that associate with some thalloid liverworts (Field et al., 2012). It may be that P. ericae associates in leafy liverworts play a similar role to the Glomeromycotina or Mucoromycotina fungal partners of thalloid liverworts by supplementing plant P assimilation (Field et al., 2016).
Here, we aim to address the fundamental question of whether the ascomycete fungus P. ericae forms mycorrhiza-like associations with leafy liverworts equivalent to those formed by Glomeromycotina or Mucoromycotina fungi and thalloid liverworts. We traced the movement of P from P. ericae fungal hyphae to Cephalozia bicuspidata liverworts and the movement of C from liverworts to the fungi using isotope tracers. We determined liverwort growth responses to colonization by P. ericae fungi by measuring the size and mass of liverworts, grown both with and without P. ericae fungal symbionts.
MATERIALS AND METHODS
We collected Cephalozia bicuspidata from Thursley Common, Surrey, in autumn 2012 (OS grid reference SU900416). Mature sporophytes were harvested, surface-sterilized and spores were cultured axenically (Duckett and Read, 1995) on 1.5 % Phytagel™ (Sigma-Aldrich; ICP elemental analysis of Phytagel provided by Sigma-Aldrich: 0.85 % Ca, 0.35 % Mg, 1.70 % K, 0.15 % P and 0.45 % Na). No additional nutrients were added to the culture medium. Pezoloma ericae was isolated from the same liverwort collection and resynthesized with axenically-grown C. bicuspidata using published methods (Kowal et al., 2015), thus satisfying Koch’s postulates. Molecular identification of the fungal isolate as P. ericae was carried out previously (Kowal et al., 2015). Nomenclature for plants and fungi follows Hill et al. (2008) and www.speciesfungorum.org, respectively.
Fungus to plant phosphorus transfer
We grew C. bicuspidata and P. ericae together in one compartment (‘liverwort and fungus’) of 9 cm split-plate microcosms (Supplementary Data Fig. S1) filled on both sides of the divide (‘B’ in Fig. S1a) with 1.5 % sterile Phytagel. Fragments of P. ericae isolate (approx. 7 mm2) were inserted beneath the surface of the Phytagel, and an axenically grown leafy liverwort stem (two per microcosm, approx. 2 cm apart) was gently pressed onto the surface, directly above the fungus fragment. Following establishment of liverwort–fungal symbiosis, which is confined to the rhizoids (Fig. 1B), and growth of extraradical fungal hyphae (11 weeks after planting), we introduced 0.1 MBq H333PO4 (i.e. 0.03 µg of 33P, specific activity 111 GBq mmol–1; Hartmann Analytics, Braunschweig, Germany) into a well within the medium in the contiguous compartment of the plate. Each 33P-labelled well was then filled with 1.5 % sterile Phytagel. The barrier dividing the microcosm prevented the liverwort from encroaching into the compartment containing 33P while fungal hyphae were able to grow over the barrier and colonize the medium in both compartments (see Supplemtnary Data Fig. S1). We prepared a total of 16 microcosms with an additional fungus-free (control) microcosm to measure non-fungal-mediated diffusion of 33P into liverwort tissue. We also tested the effectiveness of the barriers in eight undivided microcosms containing C. bicuspidata without fungus, with 33P-labelled wells placed at the same distance from plants as in divided microcosms. All microcosms were sealed with Parafilm ‘M’ (Sigma), and placed in a controlled environment chamber (BDR16, Conviron, Winnipeg, MB, Canada). The temperature regime was typical of late-spring/summer for south-eastern England with light intensity reflecting that at ground level beneath canopy vegetation, similar to that experienced by the liverworts in their natural environment (irradiance of 50 μmol m–2 s–1, 12 h:12 h, light:dark, 16 °C:14 °C day:night, 80 % relative humidity and 440 ppm [CO2]). After 8 weeks, we removed the liverworts from the microcosms, and freeze-dried all plant tissues and growth medium containing fungal hyphae.
Between 10 and 30 mg of plant tissue and growth medium for each microcosm were digested in 1 mL of concentrated H2SO4 for 2 h and then heated to 365 °C for 15 min. After cooling, 100 μL of hydrogen peroxide was added to each sample before reheating to 365 °C for 2 min, resulting in a clear solution. Samples were diluted up to 10 mL with distilled water before 2 mL of the diluted digest solution was mixed with 10 mL of the liquid scintillant Emulsify Safe (Perkin Elmer). The activity of the samples was determined using liquid scintillation counting (Packard TriCarb 3100, Isotech, Chesterfield, UK). The amount of 33P transferred to the plants by the fungus in each microcosm was calculated using equations from Cameron et al. (2007). This figure was then adjusted for passive movement of 33P from the substrate via diffusion by subtracting the mean amount of 33P measured in liverworts harvested from fungus-free control microcosms.
Plant to fungus carbon transfer
We filled nine cube-shaped vessels (Sigma Magenta GA-7-3, 77 × 77 × 97 mm) with Phytagel (1.5 %) to a depth of 30 mm to prepare three replicate microcosms with fungus only and six ‘complete’ microcosms with liverwort and fungus. A fragment of P. ericae isolate (approx. 7 mm2) was inserted beneath the surface of the Phytagel for the fungus-only and complete microcosms. For the complete microcosms, an axenically grown leafy liverwort stem was gently pressed onto the surface of the medium, directly above the fungus fragment. We maintained the plants in the same controlled environment chamber and conditions as above. After 6 months of growth, we labelled the microcosms with 14CO2, separating the growth medium from the chamber headspace with polythene (pre-cut with 25 mm diameter holes for the liverworts) in order to minimize direct diffusion of 14CO2 into the substrate. The polythene was sealed with anhydrous lanolin where it met the substrate and along the edges of the Magenta vessels (Supplemetnary Data Fig. S2b). We generated 0.5 MBq of 14CO2 gas by adding 6.8 μL of [14C]sodium bicarbonate to tubes in each microcosm before introducing 500 μL of 25 % lactic acid. We allowed plants to fix 14CO2 for 5 h in the middle of the day before introducing two Eppendorf tubes containing 1 mL of 2 m KOH into each microcosm for 30 min to trap any remaining 14CO2 from the headspace of each microcosm. We then harvested, separated and freeze-dried the liverworts and Phytagel-containing fungal hyphae (see Supplementary Data Methods S1) before weighing, homogenizing and determining 14C content by sample oxidation (Packard Sample Oxidiser) and liquid scintillation counting (Packard Tri-Carb 3100).
We calculated total C (12C plus 14C) fixed by the plant and transferred to the fungus as a function of the total volume and CO2 content of the vessel headspace and the proportion of supplied 14CO2 label fixed by the plants, using equations from Cameron et al. (2008). The specific activity of the source was 2.04 TBq mol–1.
To account for C movement through passive diffusion, and to assess the effectiveness of the polythene–lanolin barrier, total plant-fixed fungal C in the complete microcosms was determined by subtracting the mean C measured in the fungus from the fungus-only control microcosms from each of the complete microcosms.
Liverwort growth
We measured C. bicuspidata growth both with and without fungal inoculation, looking first at changes in leafy liverwort surface area and secondly at liverwort biomass. In the first experiment, we introduced small fragments (approx. 7 mm2) of P. ericae to 9 cm plates containing 1.5 % Phytagel with axenically grown leafy liverworts (approx. 1 year old). After fungal colonization of the liverwort rhizoids was confirmed microscopically (Fig. 1B), we re-plated colonized liverworts and fungus-free control liverworts individually using the same medium. Surface area measurements of each liverwort stem (including leaves) were made before and after 6 weeks using images taken with a Nikon Coolpix S10 digital camera and analysed using ImageJ software (Rasband, 1997–2012) (Supplementary Data Fig. S3). For the second measurement of the effect of P. ericae on C. bicuspidata growth, we freeze-dried and weighed the liverworts from the complete microcosms used for the 14C transfer experiment (above) after they were harvested at 6 months and compared the mean with that of plants from liverwort-only microcosms (n = 6). This allowed us to test for differences in mean plant biomass in liverwort growth with or without fungus over a longer time period than the surface area growth experiment described above. Liverwort stems were weighed before initial planting to ensure similarity in mass between sample groups.
Statistics
After analysing the data for normal distribution and homogeneity of variance, we applied the appropriate statistical tests, i.e. parametric or non-parametric, using GraphPad Prism (version 6.0h). The Mann–Whitney U-test was used both for the dry mass experiment and for testing the barrier effect in the 33P experiment, owing to the low number of data points; Student’s t-test was used for other data sets.
RESULTS AND DISCUSSION
The leafy liverwort–Pezoloma ericae symbiosis is mutualistic and mycorrhiza-like
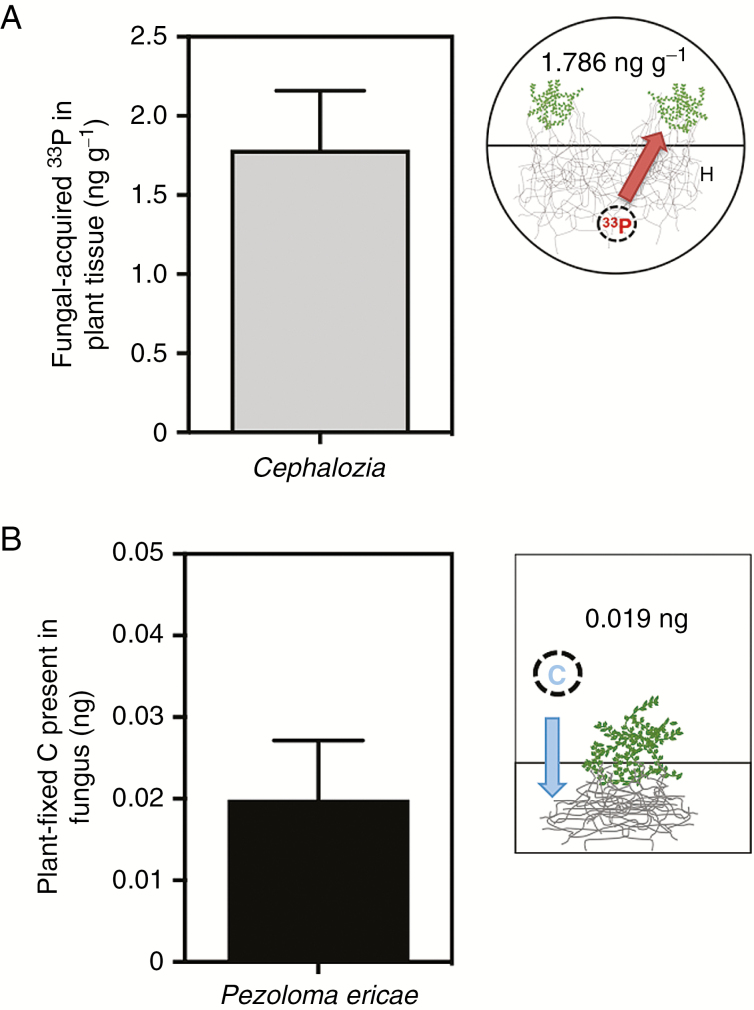
In our radiolabelled liverwort microcosms, 1.786 ng g –1 of 33P was assimilated from P. ericae into C. bicuspidata tissues (Fig. 2A; Supplementry Data Table S1). There was a significant difference in the liverwort 33P content and concentration between plant-only microcosms with barriers and plant-only microcosms without barriers [Mann–Whitney U = 0; n1 = 2, n2 = 13; P = 0.019 two-tailed (data not shown)], further demonstrating the efficacy of the barriers in our microcosms for preventing direct plant access to 33P-labelled wells. While we cannot exclude the possibility that the P content of the Phytagel substrate used in our microcosms may have been directly assimilated by the liverworts within the systems and therefore reduced plant demand for fungal-acquired 33P, our data unequivocally show movement of 33P from the fungus to the leafy liverwort.
Fig. 2.
(A) Concentration of fungal-acquired 33P in Cephalozia bicuspidata tissue (ng g–1). (B) Total plant-derived carbon present in extraradical fungal mycelium after a 5 h labelling period (ng). Error bars denote 1 s.e. in both panels.
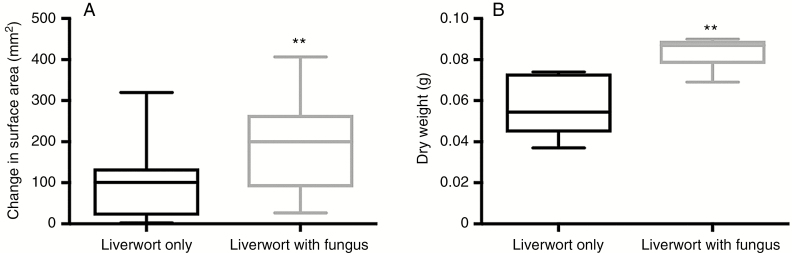
The leafy liverworts transferred 0.019 ng (0.22 ng g–1) of plant-fixed C to their fungal partners (Fig. 2B), equivalent to 0.27 % of the total amount of C fixed during the labelling period (Supplementary Data Table S1). By demonstrating unequivocal exchange of fungal-acquired P and plant-fixed C between symbionts, our results provide the first experimental evidence that associations between non-vascular plants and ascomycete fungal symbionts are nutritionally mutualistic. This confirms previous hypotheses based on cytological evidence (van der Heijden et al., 2015) and culturing experiments (Duckett and Read, 1991; Upson et al., 2007; Kowal et al., 2015). Additionally, liverworts resynthesized with P. ericae grew significantly larger than liverworts grown under identical conditions without the fungus for both growth experiments (Fig. 3A, B). Surface area after 6 weeks almost doubled in the P. ericae-treated microcosms compared with the liverwort-only microcosms (t-test; n1 = 22, n2 = 16; P < 0.01), and total biomass of the liverworts (dry weight; g) after 6 months of growth was significantly greater with P. ericae (Mann–Whitney U = 2; P ≤ 0.01; n1,2 = 6).
Fig. 3.
(A) Change in surface area of leafy liverworts after 6 weeks of growth with and without Pezoloma ericae fungal symbiont (n = 16, 22), **P < 0.01 (using t-test). (B) Total biomass of liverworts (dry weight; g) after 6 months of growth with and without P. ericae (Mann–Whitney U = 2; **P = 0.0087; n1,2 = 6 two-tailed). Error bars denote 1 s.e. in both panels.
Together with our evidence of C for nutrient exchange between symbionts, it is clear that the symbiosis between non-vascular leafy liverworts and their fungal partners is mutualistic and mycorrhiza-like. This lays the foundation to investigate further functional differences between liverwort–ascomycete partnerships and previously documented exchanges between P. ericae and vascular plants (Pearson and Read, 1973; Read and Stribley, 1973; Upson et al., 2008, Kowal et al., 2015).
Our experiments aimed to uncover whether there is any movement of plant-fixed C and fungal-acquired P between C. bicuspidata liverworts and P. ericae. The evidence of 33 P transfer from fungus to plant in our experimental microcosms (Fig. 2A) strongly supports a nutritional role for P. ericae that is particularly significant given that P can be one of the most limiting nutrients in the ericaceous habitats where C. bicuspidata grows. It is possible that the enhanced growth we observed in liverworts with fungal symbionts compared with those without (Fig. 3) was a result of fungal remineralization of organic compounds leached from the liverwort, rather than a direct result of increased uptake of fungal-acquired P. However, the provision of 33P by the fungus, as shown here, supports the hypothesis that the fungus plays a role in leafy liverwort P nutrition in natural environments.
Evolutionary context
Our demonstration that the association between a leafy liverwort and the ericoid mycorrhizal fungus P. ericae can be mutually beneficial provides additional clues about the evolution of ErMs, and lends further weight to the idea that mutualisms between leafy liverworts and ascomycete fungi pre-dated their formation in the Ericales, estimated at <100 Mya (Brundrett, 2004). Following the discovery that ascomycetes belonging to the P. ericae group colonize the rhizoids of Schistochilaceae liverworts, thought to have originated in the Triassic (Heinrichs et al., 2007) as the sister group to all other fungus-containing lineages in the Jungermanniales, Pressel et al. (2008) suggested that this association arose >250 Mya. Nonetheless, divergence time estimates for ascomycete lineages remain a matter of debate, particularly as the presumed recent origin of Leotiomycetes, the clade containing P. ericae, is in the Cretaceous (James et al., 2006; Prieto and Wedin, 2013). Until ever-improving phylogenetic methods integrating fossil evidence (Beimforde et al., 2014) provide more accurate date estimates, recent origins for the association with multiple instances of host shifting between leafy liverworts and Ericaceae plants remain a plausible alternative scenario (Selosse, 2005; Pressel et al., 2008).
Observations on previously reported liverwort–fungal symbioses
Although not directly comparable, given considerable differences in the physiology of the liverwort hosts and in experimental design (i.e. axenic and edaphic conditions), our finding of mycorrhiza-like associations between ascomycete fungi and leafy liverworts now invites further comparisons with more ancient lineages of liverworts, i.e. Haplomitriopsida (Treubia and Haplomitrium) and complex thalloid liverworts (Neohodgsonia, Allisonia and Marchantia) and fungi, i.e. Mucoromycotina and/or Glomeromycotina (Field et al., 2015a, 2016) (see Fig. 1A; Supplementary Data Table S1). When fungal-acquired 33P uptake is normalized to biomass (Fig. 2A; Table S1), it appears that C. bicuspidata gains significantly less 33P from its fungal partner than Haplomitrium, Treubia (with Mucoromycotina only), Allisonia and Neohodgsonia (with Mucoromycotina and Glomeromycotina), but a roughly similar amount to Marchantia (with Glomeromycotina only). Cephalozia bicuspidata C allocation (in terms of absolute amount, Fig. 2B) to its symbiotic fungus also points to a relatively low C demand by P. ericae on its liverwort host, especially when compared with other liverwort lineages (0.27 % of plant-fixed C transferred to the symbiotic fungus vs. 2.2–14.2 %, see Table S1). This may be partially influenced by the facultative biotrophic nature of P. ericae allowing it to gain at least some organic C from dead organic matter, and the extent of colonization (i.e. restricted to rhizoids vs. extensive through the thallus). It is possible that the fungus in our experiments was able to derive some C directly from the Phytagel substrate (which contains glucose and trace levels of nutrients; see the Materials and Methods). Regardless of the P for C measurements presented in Table S1, it is evident that the relative ‘cost’ of maintaining mycorrhiza-like symbioses varies between liverwort–fungal symbioses, with ErM-like associations potentially requiring less liverwort investment in terms of photosynthate allocation, and the liverwort–Mucoromycotina partnership requiring the most (Field et al., 2016).
Having established that there is a mycorrhizal-like nutritional exchange between the leafy liverwort C. bicuspidata and its fungal symbiont P. ericae, investigations are now needed which include fungus to plant N transfer and more natural experimental conditions to provide a robust platform to investigate functional variation in plant–fungal symbioses across evolutionary lineages and ecological gradients.
Future directions
The diversity of fungal symbionts across the land plant phylogeny is becoming increasingly apparent; however, there is still a relative dearth of information regarding the function of plant–fungal symbioses in many clades, particularly the bryophytes. The largest remaining functional knowledge gaps now are the basidiomycete symbioses in thalloid Aneuraceae and leafy Scapaniaceae and Arnelliaceae liverworts (Bidartondo and Duckett, 2010). In pteridophytes, only two pioneering studies to date have demonstrated the bi-directional exchange of plant-fixed C for fungal-acquired N and P between green sporophytes of Osmunda regalis and Ophioglossum vulgatum and their Glomeromycotina fungal symbionts (Field et al., 2012; 2015c; Pressel et al., 2016).
Although we show transfer of C from C. bicuspidata to fungi in vitro, in habitats where C. bicuspidata grows, often in deep shade under a canopy of ericaceous plants, the fungal symbiont of C. bicuspidata may be getting a large proportion of its organic C from surrounding vascular plants via a shared fungal hyphal network, as demonstrated previously between Betula and the mycoheterotrophic liverwort Cryptothallus (Aneura) mirabilis via a shared basidiomycete fungus (Read et al., 2000; Bidartondo et al., 2003). How this affects ecosystem C and nutrient budgets in terms of storage and cycling remains to be uncovered.
In conclusion, our findings provide a novel and important example of mycorrhiza-like functioning in an additional fungal lineage with non-vascular plants. Thus, our demonstration of symbiotic ErM-like functioning of P. ericae with a leafy liverwort now adds the Ascomycota to the list of fungal groups engaging in mutualistic C for nutrient exchange across the land plant phylogeny. It has been proposed that C. bicuspidata liverworts with ErM fungal symbionts can be used to restore threatened heathlands by promoting establishment of native ericaceous plants (Kowal et al., 2015) that are limited by ErM inoculum availability (Diaz et al., 2006). Our results suggest that at least one of the mechanisms underpinning the potential role of C. bicuspidata in habitat restoration strategies is likely to be nutritional. The potential ecological applications of pioneer non-vascular plants as mycorrhizal reservoirs and vectors, particularly in habitat restoration, are only just becoming apparent. Evidence of a mutualism between a widespread non-vascular plant and ascomycete fungi that form mycorrhizas with dominant vascular plants should now encourage further ecological and physiological experiments.
SUPPLEMENTARY DATA
Supplementary data are available online a https://academic.oup.com/aob and consist of the following. Figure S1: diagrams and photographs of the 33P transfer experiment showing the split plate experimental design set up before and after the fungus crossed the barrier. Figure S2: diagram showing microcosm design for carbon transfer experiments. Figure S3: example of digitized pictures used to measure perimeter area of liverworts. Table S1: summary of carbon for nutrient exchange between Cephalozia bicuspidata and Pezoloma ericae, alongside data from previous studies of early-diverging liverwort lineages and their fungal partners. Table S2: estimates of likely species numbers of liverworts worldwide with fungal symbionts. Methods S1: methods for harvesting plants and fungus for 33P acid digestion, liquid scintillation and nutrient budgeting.
ACKNOWLEDGEMENTS
J.K. thanks the BSBI for funding for laboratory work and the University of Sheffield for use of growth and analytical facilities. K.F. is supported by a BBSRC Translational Fellowship (BB/M026825/1). All authors are supported by NERC (NE/N009665/1). We thank the editor and reviewers for their constructive comments on an earlier version of this manuscript.
LITERATURE CITED
- Beimforde C, Feldberg K, Nylinder S et al. . 2014. Estimating the Phanerozoic history of the Ascomycota lineages: combining fossil and molecular data. Molecular Phylogenetics and Evolution 78: 386–398. [DOI] [PubMed] [Google Scholar]
- Bidartondo MI, Duckett JG. 2010. Conservative ecological and evolutionary patterns in liverwort–fungal symbioses. Proceedings of the Royal Society B: Biological Sciences 277: 485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidartondo MI, Bruns TD, Weiss M, Sergio S, Read DJ. 2003. Specialized cheating of the ectomycorrhizal symbiosis by an epiparasitic liverwort. Proceedings of the Royal Society B: Biological Sciences 270: 835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidartondo MI, Read DJ, Trappe JM, Merckx V, Ligrone R, Duckett JG. 2011. The dawn of symbiosis between plants and fungi. Biology Letters 7: 574–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolan NS. 1991. A critical review on the role of mycorrhizal fungi in the uptake of phosphorus by plants. Plant and Soil 134: 189–207. [Google Scholar]
- Brundrett MC. 2002. Coevolution of roots and mycorrhizas of land plants. New Phytologist 154: 275–304. [DOI] [PubMed] [Google Scholar]
- Brundrett MC. 2004. Diversity and classification of mycorrhizal associations. Biological Reviews 79: 473–495. [DOI] [PubMed] [Google Scholar]
- Cameron DD, Johnson I, Leake JR, Read DJ. 2007. Mycorrhizal acquisition of inorganic phosphorus by the green-leaved terrestrial orchid Goodyera repens. Annals of Botany 99: 831–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron DD, Johnson I, Read DJ, Leake JR. 2008. Giving and receiving: measuring the carbon cost of mycorrhizas in the green orchid, Goodyera repens. New Phytologist 180: 176–184. [DOI] [PubMed] [Google Scholar]
- Chambers SM, Williams PG, Seppelt RD, Cairney JWG. 1999. Molecular identification of Hymenoscyphus sp. from rhizoids of the leafy liverwort Cephaloziella exiliflora in Australia and Antarctica. Mycological Research 103: 286–288. [Google Scholar]
- Crandall-Stotler B, Stotler RE, Long DG. 2009. Phylogeny and classification of the Marchantiophyta. Edinburgh Journal of Botany 66: 155–198. [Google Scholar]
- Diaz A, Green I, Benvenuto M, Tibbett M. 2006. Are ericoid mycorrhizas a factor in the success of Calluna vulgaris heathland restoration?Restoration Ecology 14: 187–195. [Google Scholar]
- Duckett JG, Read DJ. 1991. The use of the fluorescent dye, 3,3’-dihexyloxacarbocyanine iodide, for selective staining of ascomycete fungi associated with liverwort rhizoids and ericoid mycorrhizal roots. New Phytologist 118: 259–272. [DOI] [PubMed] [Google Scholar]
- Duckett JG, Read DJ. 1995. Ericoid mycorrhizas and rhizoid–ascomycete associations in liverworts share the same mycobiont – isolation of the partners and resynthesis of the associations in-vitro. New Phytologist 129: 439–477. [Google Scholar]
- Duckett JG, Renzaglia KS, Pell K. 1991. A light and electron microscope study of rhizoid–ascomycete associations and flagelliform axes in British hepatics: with observations on the effects of the fungus on host morphology. New Phytologist 118: 233–257. [DOI] [PubMed] [Google Scholar]
- Field KJ, Cameron DD, Leake JR, Tille S, Bidartondo MI, Beerling DJ. 2012. Contrasting arbuscular mycorrhizal responses of vascular and non-vascular plants to a simulated Palaeozoic CO2 decline. Nature Communications 3: 1–8. [DOI] [PubMed] [Google Scholar]
- Field KJ, Pressel S, Rimington WR, Duckett JG, Bidartondo MI. 2015a. Symbiotic options for the conquest of land. Trends in Ecology and Evolution 30: 477–486. [DOI] [PubMed] [Google Scholar]
- Field KJ, Rimington WR, Bidartondo MI et al. . 2015b. First evidence of mutualism between ancient plant lineages (Haplomitriopsida liverworts) and Mucoromycotina fungi and its response to simulated Palaeozoic changes in atmospheric CO2. New Phytologist 205: 743–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field KJ, Leake JR, Tille S et al. . 2015c. From mycoheterotrophy to mutualism: mycorrhizal specificity and functioning in Ophioglossum vulgatum sporophytes. New Phytologist 205: 1492–1502. [DOI] [PubMed] [Google Scholar]
- Field KJ, Rimington WR, Bidartondo MI et al. . 2016. Functional analysis of liverworts in dual symbiosis with Glomeromycota and Mucoromycotina fungi under a simulated Palaeozoic CO2 decline. ISME Journal 10: 1514–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heijden MGA, Martin FM, Selosse M-A, Sanders IR. 2015. Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytologist 205: 1406–1423. [DOI] [PubMed] [Google Scholar]
- Heinrichs J, Hentschel J, Wilson R, Feldberg K, Schneider H. 2007. Evolution of leafy liverworts (Jungermanniidae, Marchantiophyta): estimating divergence times from chloroplast DNA sequences using penalized likelihood with integrated fossil evidence. Taxon 56: 31–44. [Google Scholar]
- Hill MO, Blackstock TH, Long DG, Rothero GP. 2008. A checklist and census catalogue of British and Irish bryophytes. Cheshire: British Bryological Society. [Google Scholar]
- Humphreys CP, Franks PJ, Rees M, Bidartondo MI, Leake JR, Beerling DJ. 2010. Mutualistic mycorrhiza-like symbiosis in the most ancient group of land plants. Nature Communications 1: 1–7. [DOI] [PubMed] [Google Scholar]
- James TY, Kauff F, Schoch CL et al. . 2006. Reconstructuing the early evolution of fungi using a six-gene phylogeny. Nature 443: 818–822. [DOI] [PubMed] [Google Scholar]
- Kowal J, Pressel S, Duckett JG, Bidartondo MI. 2015. Liverworts to the rescue: an investigation of their efficacy as mycorrhizal inoculum for vascular plants. Functional Ecology 30: 1014–1023. [Google Scholar]
- Leake JR. 2015. Biogeochemical effects of coevolution of plants and fungi. In: Bronstein JL, ed. Mutualism. Oxford: Oxford University Press, 226. [Google Scholar]
- Leake JR, Read DJ. 2016. Mycorrhizal symbiosis and pedogenesis throughout Earth’s history. In: Johnson NC, Gehring C, Jansa J, eds. Mycorrhizal mediation of soil: fertility, structure and carbon storage Amsterdam: Elsevier, 9–33. [Google Scholar]
- Leake JR, Shaw G, Read DJ. 1990. The role of ericoid mycorrhizas in the ecology of ericaceous plants. Agriculture Ecosystems and Environment 29: 237–250. [Google Scholar]
- Ligrone R, Carafa A, Lumini E, Bianciotto V, Bonfante P, Duckett JG. 2007. Glomeromycotean associations in liverworts: a molecular cellular and taxonomic analysis. American Journal of Botany 94: 1756–1777. [DOI] [PubMed] [Google Scholar]
- Mitchell DT, Read DJ. 1981. Utilization of organic and inorganic phosphates by the mycorrhizal endophytes of Vaccinium macrocarpon and Rhododendron ponticum. Transactions of the British Mycological Society 76: 255–260. [Google Scholar]
- Myers MD, Leake JR. 1996. Phosphodiesters as mycorrhizal P sources II. Ericoid mycorrhiza and the utihzation of nuclei as a phosphorus and nitrogen source by Vaccinium macrocarpon. New Phytologist 132: 445–451. [DOI] [PubMed] [Google Scholar]
- Pearson V, Read DJ. 1973. The biology of mycorrhiza in the Ericaceae. New Phytologist 72: 1325–1331. [Google Scholar]
- Pirozynski KA, Malloch DW. 1973. The origin of land plants: a matter of mycotropism. Biosystems 6: 153–164. [DOI] [PubMed] [Google Scholar]
- The Plant List 2013. Version 1.1. Published on the Internet; http://www.theplantlist.org/ (accessed 1 January 2017).
- Pocock K, Duckett JG. 1985. On their occurrence of branched and swollen rhizoids in British hepatics: their relationships with the substratum and associations with fungi. New Phytologist 99: 281–304. [Google Scholar]
- Pocock K, Duckett JG, Grolle R, Mohamed MAH, Pang WC. 1984. Branched and swollen rhizoids in hepatics from montane rain forest in Peninsular Malaya. Journal of Bryology 13: 241–246. [Google Scholar]
- Pressel S, Ligrone R, Duckett JD, Davis EC. 2008. A novel ascomycetous endophytic association in the rhizoids of the leafy liverwort family, Schistochilaceae (Jungermanniidae, Hepaticopsida). Americal Journal of Botany 95: 531–541. [DOI] [PubMed] [Google Scholar]
- Pressel S, Bidartondo MI, Ligrone R, Duckett JG. 2010. Fungal symbioses in bryophytes: new insights in the twenty first century. Phytotaxa 9: 238–253. [Google Scholar]
- Pressel S, Bidartondo MI, Field KJ, Rimington WR, Duckett JG. 2016. Pteridophyte fungal associations: current knowledge and future perspectives. Journal of Systematics and Evolution 54: 666–678. [Google Scholar]
- Prieto M, Wedin M. 2013. Dating the diversification of the major lineages of Ascomycota (Fungi). PLoS One 8: e65576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband WS. 1997–2012. ImageJ. Maryland, USA: US National Institutes of Health. [Google Scholar]
- Read DJ. 1993. Mycorrhiza in plant communities. Advances in Plant Pathology 9: 1–31. [Google Scholar]
- Read DJ, Stribley DP. 1973. Effect of mycorrhizal infection on nitrogen and phosphorus nutrition of ericaceous plants. Nature New Biology 244: 81–82. [DOI] [PubMed] [Google Scholar]
- Read DJ, Duckett JG, Francis R, Ligrone R, Russell AJ. 2000. Symbiotic fungal associations in ‘lower’ land plants. Philosophical Transactions of the Royal Society B: Biological Sciences 355: 815–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read DJ, Leake JR, Perez-Moreno J. 2004. Mycorrhizal fungi as drivers of ecosystem processes in heathland and boreal forest biomes. Canadian Journal of Botany 82: 1243–1263. [Google Scholar]
- Remy W, Taylor TN, Hass H, Kerp H. 1994. Four hundred million year old vesicular arbuscular mycorrhizae. Proceedings of the National Academy of Sciences, USA 91: 11841–11843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selosse M-A. 2005. Are liverworts initiating mycorrhizas?New Phytologist 165: 345–349. [DOI] [PubMed] [Google Scholar]
- Smith SE, Read DJ. 2008. Mycorrhizal symbiosis. Cambridge: Academic Press. [Google Scholar]
- Stribley DP, Read DJ. 1974. Biology of mycorrhiza in Ericaceae IV. Effect of mycorrhizal infection on uptake of N-15 from labelled soil by Vaccinium macrocarpon Ait. New Phytologist 73: 1149–1155. [Google Scholar]
- Upson R, Read DJ, Newsham KK. 2007. Widespread association between the ericoid mycorrhizal fungus Rhizoscyphus ericae and a leafy liverwort in the maritime and sub-Antarctic. New Phytologist 176: 460–471. [DOI] [PubMed] [Google Scholar]
- Wang B, Yeun LH, Xue JY, Liu Y, Ane JM, Qiu YL. 2010. Presence of three mycorrhizal genes in the common ancestor of land plants suggests a key role of mycorrhizas in the colonization of land by plants. New Phytologist 186: 514–525. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.