Abstract
Background
Plant melatonin appears to be a multi-regulatory molecule, similar to those observed in animals, with many specific functions in plant physiology. In recent years, the number of studies on melatonin in plants has increased significantly. One of the most studied actions of melatonin in plants is its effect on biotic and abiotic stress, such as that produced by drought, extreme temperatures, salinity, chemical pollution and UV radiation, among others.
Scope
This review looks at studies in which some aspects of the relationship between melatonin and the plant hormones auxin, cytokinin, gibberellins, abscisic acid, ethylene, jasmonic acid and salicylic acid are presented. The effects that some melatonin treatments have on endogenous plant hormone levels, their related genes (biosynthesis, catabolism, receptors and transcription factors) and the physiological actions induced by melatonin, mainly in stress conditions, are discussed.
Conclusions
Melatonin is an important modulator of gene expression related to plant hormones, e.g. in auxin carrier proteins, as well as in metabolism of indole-3-acetic acid (IAA), gibberellins, cytokinins, abscisic acid and ethylene. Most of the studies performed have dealt with the auxin-like activity of melatonin which, in a similar way to IAA, is able to induce growth in shoots and roots and stimulate root generation, giving rise to new lateral and adventitious roots. Melatonin is also able to delay senescence, protecting photosynthetic systems and related sub-cellular structures and processes. Also, its role in fruit ripening and post-harvest processes as a gene regulator of ethylene-related factors is relevant. Another decisive aspect is its role in the pathogen–plant interaction. Melatonin appears to act as a key molecule in the plant immune response, together with other well-known molecules such as nitric oxide and hormones, such as jasmonic acid and salicylic acid. In this sense, the discovery of elevated levels of melatonin in endophytic organisms associated with plants has thrown light on a possible novel form of communication between beneficial endophytes and host plants via melatonin.
Keywords: ABA, auxin, cytokinin, ethylene, gibberellin, JA, melatonin, phytomelatonin, plant hormone, plant pathogen, plant stress, post-harvest, rhizogenesis, SA, senescence, tropism
INTRODUCTION
Melatonin (N-acetyl-5-methoxytryptamine) was discovered in 1958 in the bovine pineal gland (Lerner et al., 1958). Since then it has become one of the most studied biological molecules and its role has been much studied in mammals, birds, amphibians, reptiles and fish. Melatonin has many physiological roles in animals (Pandi-Perumal et al., 2008; Hardeland et al., 2012), such as influencing circadian rhythms, mood, sleep, body temperature, locomotor activity, food intake, retina physiology, sexual behaviour, seasonal reproduction and the immune system (Carrillo-Vico et al., 2013). Melatonin acts as a signal of darkness, providing information to the brain and peripheral organs and serving as an endogenous synchronizer for physiological rhythms (e.g. sleep–wake cycles, seasonal reproduction and endocrine release cycles).
Many advances in understanding the role of melatonin have been made since its discovery in plants in 1995 (Dubbels et al., 1995; Hattori et al., 1995). The very amplitude of its biological actions in plants has led it to being called a multi-regulatory molecule. Such actions include the ability to act as a plant biostimulator against stress, both biotic and abiotic; the ability to regulate plant growth; the ability to regulate processes of plant vegetative development, such as rooting, leaf senescence, photosynthetic efficiency and biomass yield; as well as a role as a potential regulator in the processes of flowering, and the formation and ripening of fruits and seeds (Arnao and Hernández-Ruiz, 2014b, 2015a, b; Hernández-Ruiz and Arnao, 2016; Nawaz et al., 2016).
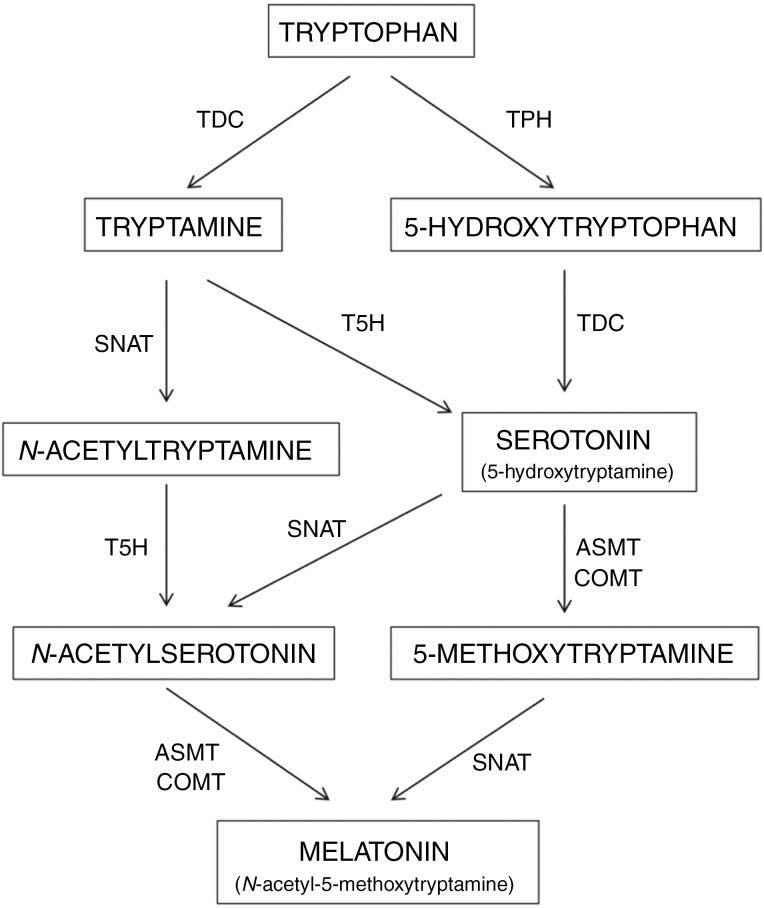
Melatonin is an indolic compound derived from serotonin (5-hydroxytryptamine). Both biogenic amines are synthesized from the amino acid tryptophan in a biosynthetic pathway that has been extensively studied in both animals and plants (Arnao and Hernández-Ruiz, 2006; Tan et al., 2015; Back et al., 2016). In plants, tryptophan is converted into tryptamine by tryptophan decarboxylase (TDC) (Fig. 1). Tryptamine is then converted into 5-hydroxytryptamine (commonly known as serotonin) by tryptamine 5-hydroxylase (T5H), an enzyme that has only been characterized in rice, and which possibly acts with a large number of substrates, although this has not been studied in depth. The N-acetylation of serotonin is catalysed by the enzyme serotonin N-acetyltransferase (SNAT). N-Acetylserotonin is then methylated by acetylserotonin methyl transferase (ASMT), a hydroxyindole-O-methyltransferase, which generates melatonin. In plants, the methylation of N-acetylserotonin can also be performed by a caffeic acid O-methyltransferase (COMT), a class of enzyme that can act on a diversity of substrates, including caffeic acid and quercetin (Byeon et al., 2014). Serotonin may also be transformed into 5-methoxytryptamine by ASMT (and by COMT) to generate melatonin through the action of SNAT. Also, melatonin can be generated through the formation of N-acetyltryptamine, which is converted into N-acetylserotonin. Finally, serotonin is also formed from 5-hydroxytryptophan after the action of tryptophan hydroxylase (TPH) and TDC, the latter step occurring mainly in animals but also, to a lesser extent, in plants. Moreover, melatonin can be generated through the formation of 5-methoxytryptamine, as proposed by several authors, suggesting that the melatonin biosynthesis pathway may follow various alternative routes compared with animal cells, with a greater capacity of adapt to metabolic changes in plants (Arnao and Hernández-Ruiz, 2014b; Tan et al., 2016). In short, melatonin in plants can be synthesized in many ways, the most relevant being the sequence: tryptophan → tryptamine → serotonin → N-acetylserotonin → melatonin (Fig. 1). All the named enzymes have been detected and characterized in rice and arabidopsis, except TPH, which is well known in animals but not in plants. Nevertheless, some authors have proposed that T5H can act as a hydroxylase with low substrate specificity and is capable of acting in all the hydroxylation steps described (Arnao and Hernández-Ruiz, 2014a, b). This same broad substrate specificity can also be attributed to SNAT, ASMT and COMT enzymes. Melatonin intermediates are produced in various subcellular compartments, such as the cytoplasm, endoplasmic reticulum and chloroplasts, which determine the subsequent enzymatic steps.
Fig. 1.
Biosynthetic pathway of melatonin in plants. The enzymes of the respective steps are: T5H, tryptophan 5-hydroxylase; TDC, tryptophan decarboxylase; SNAT, serotonin N-acetyltransferase; ASMT, acetylserotonin methyltransferase; and COMT, caffeic acid O-methyltransferase.
One of the most studied aspects related to melatonin has been its role as a protective agent against abiotic stress situations in plants. Melatonin acts as an effective free radical scavenger against hazardous reactive molecules, both reactive oxygen and reactive nitrogen species (ROS/RNS), among others. The excellent properties of melatonin as a natural antioxidant against ROS/RNS and the absence of pro-oxidant effects have been the subject of a great deal of research (Arnao and Hernández-Ruiz, 2015a; Reiter et al., 2014; Tan et al., 2000; Teixeira et al., 2003). In general, cold, heat, salinity, drought, UV radiation and chemical toxicity are countered or mitigated by the presence of melatonin. Higher plant survival rates, higher shoot and root growth, and photosynthetic efficiency, accompanied by improved chloroplast and stomatic morphologies, and high sucrose and proline levels have been observed in melatonin-treated plants, together with lower levels of ROS/RNS, lipid membrane peroxidation and cell damage (Arnao and Hernández-Ruiz, 2009a, 2013a, b, 2015b; Kolar and Machackova, 2005; Shi et al., 2016a). Taking into account the cross-relationship between stress-mediated signals and plant hormones, the involvement of melatonin in this area is very interesting, without addressing the role of melatonin in situations of abiotic stress. This last aspect can be consulted in several of the recent reviews on the subject (Arnao and Hernández-Ruiz, 2015b; Hernández-Ruiz and Arnao, 2016; Nawaz et al., 2016; Reiter et al., 2015; Tan et al., 2015).
In the present review, the relationships between melatonin and the plant hormones auxin, cytokinins (CKs), gibberellins (GA), abscisic acid (ABA), ethylene, jasmonic acid (JA) and salicylic acid (SA) are extensively discussed. The effects that melatonin treatments and the use of melatonin transgenic plants have on endogenous hormone levels, their related genes (biosynthesis, catabolism, receptors and transcription factors) and the physiological actions induced by exogenous or endogenous melatonin are presented.
MELATONIN AND AUXIN: ROLE IN GROWTH, RHIZOGENESIS AND TROPISM
The growth-promoting activity of melatonin is one of its more specific auxin-like roles. Table 1 shown studies in which exogenous melatonin has been seen to promote or inhibit growth. In many studies, melatonin has been seen to induce growth in the aerial parts and also in roots, e.g. Triticum, Hordeum, Avena, Oryza, Lupinus, arabidopsis, Brassica, Helianthus, Prunus, Cucumis and Punica, but also in tomato, soybean and maize plants (Arnao and Hernández-Ruiz, 2017a). Generally, growth inhibition only occurs in roots at high melatonin concentrations (>10 μm). The growth-promoting effect of melatonin is high when a stress condition affects plant development, as in the case of Helianthus (Mukherjee et al., 2014) and Zea mays (Kim et al., 2016) grown in saline conditions, in arabidopsis (Bajwa et al., 2014) and Cynodon (Shi et al., 2015b) exposed to cold stress, and in Malus grown in saline or mineral nutrient-deficient conditions (C. Li et al., 2016). Melatonin induced a 3- to 4-fold increase in growth compared with control plants in the aerial tissues of Lupinus, Phalaris, Triticum, Hordeum, arabidopsis and Cucumis, and a less pronounced increase in others (Arnao and Hernández-Ruiz, 2017a). More recently, the growth promotor activity of melatonin has been described in rice (Han et al., 2017), pepper (Korkmaz et al., 2017), perennial ryegrass (J. Zhang et al., 2017), cucumber (R. Zhang et al., 2017), lentil and bean (Aguilera et al., 2015).
Table 1.
Effects of melatonin on IAA
| Plant species | Melatonin treatment or overaccumulation | Effects observed | Reference |
|---|---|---|---|
| Around 20 species (monocot and dicot) | nm–μm | ↑ Aerial growth (especially in stress conditions) ↑↓ Root growth |
Arnao and Hernández-Ruiz (2017a) |
| Brassica juncea roots | 0.01–0.5 μm | ↑ IAA up to 2-fold ↑ Root growth |
Chen et al. (2009) |
| Tomato overexpressing sheep SNAT/ASMT* | ↑ Up to 6-fold | ↑ IAA up to 7-fold ↓ Apical dominance Branching phenotype ↑ Resistance to drought |
Wang et al. (2014) |
| Arabidopsis thaliana overexpressing apple ASMT | ↑ Up to 4-fold | ↓ IAA up to 1.4-fold ↑ Lateral roots, biomass ↑ Resistance to drought |
Zuo et al. (2014) |
| Arabidopsis seedlings | 1 mm | ↑ IAA-amino synthase genes | Weeda et al. (2014) |
| ↑ Auxin carrier proteins | |||
| Arabidopsis roots | 600 μm | ↓ IAA slightly ↓ Root meristem sizes |
Wang et al. (2016) |
| ↑↓ YUCCA genes ↓ Auxin-carrier proteins (PIN) |
|||
| Around eight species | 0.1–100 μm | ↑ New lateral roots ↑ Adventitious roots |
Arnao and Hernández-Ruiz (2007a, 2017a) |
| Tomato de-rooted plants | 12.5–100 μm | ↑ IAA up to 2-fold ↑ Auxin carrier proteins (PIN) ↑ Auxin signalling factors (IAA19, IAA24) ↑ Adventitious roots |
Wen et al. (2016) |
| Lupinus albus roots | 10–100 μm | ↑ Tropic response | Arnao and Hernández-Ruiz (2017a) |
*SNAT, serotonin N-acetyltransferase; ASMT, acetylserotonin methyltransferase.
↑ Increased content or increased action; ↓ decreased content or decreased acion.
In some cases, a relationship between melatonin treatment and endogenous indole-3-acetic acid (IAA) levels has been established. In general, melatonin treatment induces a slight (1.4- to 2.0-fold) increase in endogenous IAA compared with untreated plants, as has been observed in Brassica juncea (Chen et al., 2009) and tomato plants (Wen et al., 2016). However, in transgenic plants that overproduce melatonin, a substantial decrease in IAA levels has been reported. For example, tomato overexpressing sheep SNAT/HIOMT and arabidopsis overexpressing apple HIOMT accumulate endogenous melatonin, reaching levels up to six times that observed in wild-type plants, while the endogenous IAA content is decreased 7-fold in tomato and 1.4-fold in arabidopsis. The phenotypic effects in these transgenic plants clearly resemble auxin-like responses, promoting root growth and rhizogenesis, including reduced apical dominance, with a high degree of branching in the case of tomato overexpressing sheep SNAT/ASMT (Zuo et al., 2014) and a high number of lateral roots in the case of transgenic arabidopsis (Zuo et al., 2014). This suggests that melatonin does not replace IAA in the apical dominance function, but that both take part in the same physiological action. In a recent work, melatonin treatment was seen to reduce root meristem size in arabidopsis, accompanied by a decrease in the endogenous IAA content (Wang et al., 2016).
The rooting activity of melatonin has also been described. In a pioneering study by Arnao group’s (Arnao and Hernández-Ruiz, 2007a), where the strong induction of lupin roots by melatonin was compared with the effect of IAA; it was seen that melatonin induced the activity of root primordia from pericycle cells and clearly affected the appearance of both adventitious and lateral roots, modifying the pattern of distribution, the time course, the number and length of adventitious roots and the number of new lateral roots. Since this study, many other authors have confirmed that melatonin promotes the generation of lateral and adventitious roots in a variety of species. For example, in Arabidopsis thaliana, melatonin was seen to increase the appearance of adventitious roots 2-fold and the appearance of lateral roots by up to 3-fold, but to have no effect on root hair density (Pelagio-Flores et al., 2012; Koyama et al., 2013). Also, in three transgenic lines of A. thaliana which overproduce melatonin, a high number of lateral roots (compared with the wild type) was induced (Zuo et al., 2014). Similar rooting activity was obtained in sweet cherries (Sarropoulou et al., 2012a, b), cucumber (Zhang et al., 2013, 2014), pomegranate (Sarrou et al., 2014) and tomato plants (Wen et al., 2016).
Root primordial induction by melatonin is independent of the IAA signalling pathway. IAA, but not melatonin, is able to activate the auxin-inducible gene expression marker DR5:GUS in arabidopsis, (Pelagio-Flores et al., 2012; Koyama et al., 2013). These data suggest that melatonin can act in a parallel way to IAA, in both lateral and adventitious root induction (Arnao and Hernández-Ruiz, 2014b). Many genes related to rhizogenesis can be up- and downregulated by melatonin (by about 320 genes) in Cucumber sativus. Some members of these transcription factors and also some ethylene transcription factors have the ability to regulate root-related genes negatively and therefore suppress root formation. Thus, the downregulation of these genes by melatonin leads to a high number of lateral root primordia in cucumber (N. Zhang et al., 2014). A recent quantitative real-time PCR study in arabidopsis showed that some transcript levels of YUCCA (YUC) flavin monooxygenase-like proteins (YUC1, YUC2, YUC5 and YUC6) and TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS (TAA) family (TAA RELATED, TAR2) proteins, which play important roles in IAA biosynthesis, significantly decreased after treatment with 600 μm melatonin, while other transcripts (YUC3, YUC4, YUC7 and YUC8) increased. As a result, the endogenous IAA content in melatonin-treated roots was slightly lower than that of the control (Wang et al., 2016).
Another aspect recently proposed as being related to the auxin-like activity of melatonin is its possible role in the gravitropic response of roots. The only evidence concerning the possible participation of melatonin in tropic responses was that provided recently by Arnao and co-workers. This preliminary study described the effect that an exogenous imbalance of IAA or melatonin has on the tropic response of lupin roots. The authors suggested that the disruption (through IAA- or melatonin-enriched agar blocks) of the natural level of auxin (or melatonin) in lupin roots gives rise to an artificial internal gradient that provokes the high lateral growth of roots, with a consequent loss of verticality. Thus, melatonin induces negative tropism (roots grew away from the side to which it was applied). Once the auxin gradient was normalized on both sides of the root, vertical growth was re-established, guided by the force of gravity (Arnao and Hernández-Ruiz, 2017a).
In the first molecular studies carried out, the expression pattern of auxin metabolism-related genes exhibited minimal changes in melatonin-treated arabidopsis plants with respect to untreated plants. Thus, only one IAA-amino synthase was upregulated, with no change in the expression of auxin biosynthesis genes (Weeda et al., 2014). More significant was the downregulation of several auxin-influx carrier proteins (AUX1/LAX) in response to melatonin. AUX1 regulates lateral root development, root gravitropism, root hairs and leaf phyllotaxy (Swarup and Péret, 2012), suggesting that melatonin can interfere in the action of auxin through changes in auxin carriers that modify local IAA gradients (Weeda et al., 2014; Arnao and Hernández-Ruiz, 2015b; Wang et al., 2016). Curiously, in lupin roots and some monocot roots, a melatonin gradient similar to the IAA gradient has been described, suggesting that both gradients co-participate in plant growth (Hernández-Ruiz et al., 2004; Hernández-Ruiz and Arnao, 2008). Recently, Wang and co-workers demonstrated that melatonin negatively regulated auxin biosynthesis and the auxin response in arabidopsis . Triiodobenzoic acid (TIBA; an auxin polar transporter inhibitor) did not enhance the melatonin-mediated reduction of root meristem size, indicating that polar auxin transport may be necessary for the regulation of root meristem size by melatonin treatment (Wang et al., 2016). More recently in rice, substantial changes in the expression of many auxin-related transcription factors, including WRKY, NAC, MYB, bHLH, HD-ZIP and ERF (ethylene-related factor), exhibited up- and downregulated expression profiles following melatonin treatment. The authors confirmed that melatonin is an important mediator for shaping root architecture (inhibiting embryonic root growth and promoting lateral root formation and development) via modulation of the auxin response in rice (Liang et al., 2017).
A model to explain some of the actions of melatonin in its role an as auxin-like regulator has recently been proposed (Arnao and Hernández-Ruiz, 2017a). The signalling molecule nitric oxide (NO), which is involved in a variety of physiological processes during plant growth and development and is an important modulator of stress responses and pathophysiological processes, seems to be a key player in this respect (Di et al., 2015; Hussain et al., 2016). NO has been extensively reported to regulate auxin responses in roots, adventitious roots, lateral roots, root hair formation and root gravitropism (Pagnussat et al., 2002; Correa-Aragunde et al., 2004; Hu et al., 2005; Lombardo et al., 2006). Interestingly, the application of NO donors can mimic the effect of auxin, which suggests an important role for NO in auxin-induced processes (Chen and Kao, 2012). Also, NO mediates the auxin response leading to adventitious root formation (Pagnussat et al., 2004; Correa-Aragunde et al., 2015). Many of these effects can be attributed to the action of NO as a signalling molecule.
In the case of melatonin, adventitious root formation induced by melatonin in tomato seedlings was mediated by NO acting as a downstream signal. Melatonin increased the endogenous NO level, upregulating nitrate reductase (which reduces nitrite to NO using NADPH). Curiously, in tomato roots NO increased the level of melatonin, indicating a possible NO feedback mechanism. From this, it is difficult to elucidate whether melatonin is upstream or downstream of NO. So, it is also interesting that melatonin can act as an NO scavenger (Wen et al., 2016). Also, melatonin treatment induced changes in acropetal auxin transport through the upregulation of several auxin efflux genes (PIN1, PIN3 and PIN7) and auxin signalling transduction genes (IAA19 and IAA24). In contrast, these same auxin PIN proteins (PIN1/3/7) were downregulated in arabidopsis, influencing auxin transport (Wang et al., 2016).
In the proposed model, a double control of NO levels by IAA and melatonin probably permits the fine-tuning of responses through auxin carrier proteins in growth, rooting and tropic processes (Arnao and Hernández-Ruiz, 2017a), without forgetting that NO is also involved in the signalling cascades of other plant hormones. Also NO takes part in innate immune pathogenic responses (see below).
MELATONIN AND CYTOKININS: SENESCENCE RETARDANT, ROLE IN PHOTOSYNTHESIS
Melatonin also retards the induced-senescence process, and it has been reported that barley leaves treated with melatonin delay dark-induced senescence in a concentration-dependent manner, slowing down chlorophyll loss in detached leaves (Arnao and Hernández-Ruiz, 2009b). The incubation of leaf sections with different concentrations of kinetin (a synthetic cytokinin) also retarded the senescence process, inducing lower chlorophyll loss in the treated leaves (high levels of total chlorophyll retained) than in control leaves. Chlorophyll retention in leaves was proportional to the concentration of kinetin (1, 10 and 100 μm), although retention was slightly less than in leaves treated with 1 mm melatonin. The assayed combinations of kinetin and melatonin prevented chlorophyll losses in all cases where the concentration of melatonin was 0.1 and 1 mm (Table 2). CKs (also kinetin) do not prevent senescence completely but delay it considerably by inducing nutrient mobilization. Nevertheless, the possible synergistic effect of kinetin and melatonin has not been studied.
Table 2.
Effects of melatonin on giberrellins, abscisic acid and citokinins
| Plant hormone | Plant species | Melatonin treatment | Effects observed | Reference |
|---|---|---|---|---|
| Gibberellins (GAs) | Cucumber seedlings (in saline conditions) | 1 μm | ↑ GAs ↑ GA biosynthesis genes ↑ Germination rate |
Zhang et al. (2014a, b) |
| ABA and CKs | Barley leaves (in dark-induced senescence) | 0.1–1 mM | ↑ Chlorophyll retention ↓ Senescence ↓ ABA action ↑ Kinetin action |
Arnao and Hernández-Ruiz (2009b) |
| Cucumber seedlings (in saline conditions) | 1 μm | ↓ ABA ↓ ABA biosynthesis genes ↑ ABA catabolism genes |
Zhang et al. (2014a) | |
| Apple leaves (in drought) | 10–500 μm | ↓ ABA ↓ ABA biosynthesis genes ↑ ABA catabolism genes |
Li et al. (2015) | |
| Barley leaves (in drought/cold) | 1 mm | ↑ ABA ↑ Photosynthesis efficiency ↑ Resistance to drought, cold |
(X. Li et al., 2016) | |
| Wild ryegrass leaves (Elymus nutans) (in cold) | 1–300 μm | ↑ ABA ↑ Cold-responsive genes ↑ Resistance to cold ↓ Cell membrane damage ↑ ROS level ↑ Antioxidative enzymes; ABA-dependent and ABA-independent pathways |
Fu et al. (2017) | |
| Watermelon plants (in cold) | 1.5–150 μm | ↑ Cold responsive genes ↑ Resistance to cold ↑ IAA, GAs, ethylene, JA related-genes ↓ ABA receptor PYL8 gene |
Li et al. (2017) | |
| Perennial ryegrass leaves (Lolium perenne) (in heat-induced senescence) | 20 μm | ↑ Growth, tiller, turf quality ↑ Chlorophyll retention ↑ Photosynthesis efficiency ↑ Cell membrane stability ↓ Senescence ↓ ABA ↓ ABA biosynthesis genes ↓ ABA signalling transcription factors ↓ Senescence-associated genes ↑ CKs ↑ CK biosynthesis genes ↑↓ CKs signalling transcription factors |
Zhang et al. (2017a) |
↑ Increased content or increased action; ↓ decreased content or decreased acion.
This anti-senescence effect of melatonin was confirmed in other species such as apple (Wang et al., 2012; Wang et al., 2013), cucumber (Zhang et al., 2013), rice (Liang et al., 2015), peach (Gao et al., 2016), ryegrass (J. Zhang et al., 2016), cassava tuber (Hu et al., 2016) and arabidopsis (Shi et al., 2015d). In arabidopsis, chlorophyllase and pheophorbide a oxygenase (PaO), two chlorophyll-degrading enzymes, were both downregulated by melatonin (Weeda et al., 2014), and in Malus hupehensis suppression of the upregulation of some senescence markers and PaO by melatonin clearly indicated its role as a regulating factor in induced senescence (Wang et al., 2013). Melatonin has also been related to a protective effect on photosynthetic pigments (chlorophylls and carotenoids), enhancing photosynthetic efficiency in photosystems and alleviating the photoinhibition caused by abiotic stressors, preserving chloroplasts, increasing the capacity for CO2 assimilation and stomatal conductance; melatonin also promotes high osmolyte levels, inducing proline biosynthesis and optimizing cell turgor in drought conditions (Arnao and Hernández-Ruiz, 2014b, 2015b). In a recent paper, melatonin-treated tomato seedlings showed an increase in the effective quantum yield of photosystem II (PSII), the photochemical quenching coefficient and the proportion of ‘open’ PSII centres that control plants under saline conditions. In this way, damage to the photosynthetic electron transport chain in PSII was mitigated. In addition, melatonin pre-treatment facilitated the repair of PSII by maintaining the availability of D1 protein, which was otherwise reduced by salinity (X. Zhou et al., 2016). Transcriptome analysis revealed that melatonin upregulated the expression of genes related to photosynthesis, carbohydrate and fatty acid metabolism, and also ascorbate biosynthesis (Arnao and Hernández-Ruiz, 2015b).
More recently, the protective role of melatonin in heat-induced senescence of the perennial ryegrass (Lolium perenne L.) has been demonstrated (J. Zhang et al., 2017). Melatonin increases heat-induced growth inhibition, delays leaf senescence and downregulates senescence-associated genes (LpSAG12 and Lph36). It also increases tiller numbers, cell membrane stability, chlorophyll retention and photosynthesis rate (Table 2). Melatonin-treated plants increase CK levels during heat stress. Thus, isopentenyladenine and trans-zeatin riboside levels, which decrease in heat stress, recovered in melatonin-treated plants, showing a positive correlation between endogenous levels of melatonin and CKs. In contrast, under non-stress conditions, the application of melatonin did not alter CKs levels. As a novelty, the authors demonstrated that two CK biosynthesis genes (LpIPT2 and LpOG1) were upregulated by melatonin in stress conditions. Also, two types of transcription factor (A-ARRs and B-ARRs) involved in the CK signalling pathways were down- and upregulated, respectively. Taken together, the above demonstrates that the alleviation of heat stress by melatonin is the result of an interesting cross-talk between melatonin and CKs (J. Zhang et al., 2017).
MELATONIN, GIBBERELLINS AND ABSCISIC ACID
The levels of both GAs and ABA were altered by melatonin treatment (Table 2). Thus, melatonin upregulated GA biosynthesis genes such as GA20ox and GA3ox in cucumber seedlings in saline conditions, contributing to a high level of active GAs such as GA3 and GA4, promoting the salt-inhibited germination process (H. J. Zhang et al., 2014; N. Zhang et al., 2014). Also, melatonin treatment induced the upregulation of ABA catabolism genes (two CYP707 monooxygenases) and the downregulation of 9-cis-epoxycarotenoid dioxygenase (NCED), a key enzyme in ABA biosynthesis, which resulted in a rapid decrease in ABA levels during seed germination under salt stress (H. J. Zhang et al., 2014). Similar data were obtained in apple leaves in drought conditions, where melatonin pre-treatment halved the ABA content through regulation of the s0ame ABA biosynthesis and catabolism enzymes as mentioned above (Li et al., 2015).
Using the wild type and an ABA-deficient mutant of barley plants, the effect of melatonin treatment (applied to leaves or roots) on drought priming-induced cold tolerance was investigated. All stressing treatments significantly increased the levels of endogenous ABA and melatonin in wild-type barley leaves (ABA did not increase in the mutant), suggesting that melatonin increased drought priming-induced ABA accumulation under cold stress. Thus, the application of melatonin resulted in a higher ABA concentration in the drought-primed plants when exposed to cold stress, which might help to maintain a better plant water status, modulating sub-cellular antioxidant systems and ABA levels in barley. In addition, both drought priming and exogenous melatonin application increased the endogenous melatonin concentration in the barley mutant, indicating that the response was ABA independent. Also, exogenous application of melatonin significantly enhanced the chlorophyll content of the primed plants under cold stress. Together with the positive effects of melatonin on photosynthetic electron transport and Rubisco activity, both drought priming and the exogenous application of melatonin could alleviate cold stress-induced photosynthetic limitations in barley (X. Li et al., 2016).
In heat-induced senescence in perennial ryegrass leaves, exogenous melatonin decreased the ABA content, delaying the senescence process (J. Zhang et al., 2017). The decrease in ABA was correlated with the downregulation of two ABA biosynthesis genes (LpZEP and LpNCED1), which were upregulated by heat stress, although melatonin suppressed this effect. Melatonin also suppressed the heat-induced upregulation of two ABA signalling transcription factor genes (LpABI3 and LpABI5) and of two senescence-associated genes (Table 2). Thus, the response of ABA in heat-induced senescence was delayed by melatonin through the decrease of ABA biosynthesis and the downregulation of signalling pathway factors, while inducing the biosynthesis of CKs that act as senescence retardant agents (J. Zhang et al., 2017).
Recently, in a cold tolerance study of two genotypes of Chinese wild ryegrass (Elymus nutans Griseb.), the cold-tolerant Damxung and the cold-sensitive Gannan, the role of melatonin in two ABA-dependent and ABA-independent pathways was studied (Fu et al., 2017). Melatonin-treated plants increased ABA production, and both ABA and melatonin alleviated cold-induced oxidative damage, decreasing reactive oxygen species (ROS) and malondialdehyde (MDA) levels and enhancing antioxidant metabolites [ascorbate (AsA) and glutathione (GSH)] and related-enzymes [superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX) and glutathione reductase (GR)] (Table 2). Also, melatonin upregulates the expression of important cold-responsive genes such as CBF and COR genes. Neither ABA nor fluridone (an ABA biosynthesis inhibitor) changed endogenous melatonin levels, suggesting that ABA might act as a downstream signal of melatonin in the stress response. Thus, cold-induced melatonin increased ABA levels through an ABA-dependent pathway. Also the upregulation of cold-responsive genes by melatonin, in an ABA-independent form, agrees with the pathway proposed in the ABA-treated barley in cold stress (Skinner et al., 2005).
Lastly, in an interesting work with watermelon plants, local melatonin application to the roots was able to induce cold tolerance-responsive genes in other non-treated organs such as leaves, where it induced a systemic response (Table 2). The long-distance transport of melatonin through the xylem, together with other plant hormones, has been measured and quantified. Furthermore, melatonin induces changes in the expression of most genes in plant hormones such as IAA, CKs, GAs, ethylene and jasmonic acid (JA), generally upregulating the expression of biosynthesis enzymes, receptors and elements of the hormone signalling pathways, but only in cold-induced stress. In the case of the ABA receptor PYL8, its gene was downregulated by melatonin treatment during cold stress in watermelon plants (Li et al., 2017).
MELATONIN AND ETHYLENE
As regards the effect of exogenous melatonin on ethylene metabolism, post-harvest ripening and the quality of tomato fruit, Table 3 shows that treating tomatoes with 50 μm melatonin for 2 h led to substantial changes in their fruit ripening parameters, such as lycopene levels, fruit softening, flavour, and ethylene signalling and biosynthesis enzymes with respect to untreated tomatoes (Sun et al., 2015). Exogenous melatonin slightly increased ethylene generation and the subsequent timing of the climacteric peak through the up-regulation of 1-aminocyclopropane-1-carboxylic acid (ACC) synthase expression. Also, the ethylene receptor genes, NR and ETR4, and the transducing elements, EIL1, EIL3 and ERF2, were upregulated by melatonin.
Table 3.
Effects of melatonin on ethylene
| Plant species | Melatonin treatment | Effects observed | Reference |
|---|---|---|---|
| Tomato fruits | 50 μm | ↑ Ethylene, climacteric peak Changes in flavours, lycopene, softening ↑ ACC synthase genes, anthocyanins ↑ Ethylene receptor genes |
Sun et al. (2015, 2016) |
| Tomato plants | 0.1 mm | ↑ Ascorbic acid, lycopene, Ca, P ↑ Quality and yield of tomato fruits |
Liu et al. (2016a) |
| Lupin seedlings | 0.01–1 mm | ↓ Ethylene rate | Arnao and Hernández-Ruiz (2007b) |
| Arabidopsis seedlings | 1 mm | ↑ ACC synthase genes | Weeda et al. (2014) |
| Cassava roots | 100 μm | ↓ Post-harvest deterioration ↑ Shelf-life of tuberous |
Hu et al. (2016) |
| Peach fruits | 0.1 mm | ↓ Post-harvest senescence ↑ Weight, firmness, ascorbic acid |
Gao et al. (2016) |
| Strawberry fruits | 100 μm | ↓ Post-harvest senescence ↑ ATP, antioxidants, shelf-life |
Aghdam and Fard (2017) |
↑ Increased content or increased action; ↓ decreased content or decreased acion.
In contrast, melatonin strongly inhibited the rate of ethylene production in etiolated lupin seedlings (by up to 65 % in roots, compared with a control) (Arnao and Hernández-Ruiz, 2007b). This inhibitory effect on ethylene production is similar to that shown by auxin, which was able to induce ACC synthase expression, while blocking the induction of ACC oxidase expression by ethylene, in agreement with the model proposed by Kang’s group in mungbean hypocotyls (Kim et al., 2001). Also, in melatonin-treated arabidopsis, two ACC synthases were up-regulated, one auxin inducible according to the above model (Weeda et al., 2014). This opposite effect may have been due to differences in the auxin-mediated response between vegetative and reproductive tissues (Pattison and Catalá, 2012). Exogenous melatonin treatment induced the upregulation of genes related to lycopene biosynthesis, aroma/flavour, cell wall structure and aquaporins in tomatoes, leading to the conclusion that melatonin promotes post-harvest tomato fruit ripening through increased ethylene production and signalling (Sun et al., 2015).
In a recent differential proteomic analysis of tomato fruits, 241 proteins involved in several ripening-related pathways, including cell wall metabolism, oxidative phosphorylation, carbohydrate, flavonoid biosynthesis and fatty acid metabolism, were significantly influenced by melatonin. Also, the levels of eight proteins related to anthocyanin accumulation during fruit ripening increased, as previously observed in Brassica (N. Zhang et al., 2016). Although the processes of ripening and senescence in fruits are intrinsically linked, the exogenous application of melatonin positively regulates some parameters linked to fruit ripening (ethylene generation, cell wall changes, increases in pigments and aroma/flavour), while others linked to fruit senescence (senescence-related proteins and antioxidant enzyme proteins) are delayed (Sun et al., 2016). Also in tomato, melatonin-treated seeds produced plants with much higher yields as well as higher ascorbic acid, lycopene and Ca levels, while the content of N, Mg, Cu, Zn, Fe and Mn decreased. In contrast, plants irrigated weekly with melatonin-supplemented nutrient solutions showed significant improvements in their contents of soluble solids, ascorbic acid, lycopene, citric acid and phosphorus compared with control plants that received only a standard solution. The higher sucrose and glucose contents observed indicated that melatonin increased the fruit sugar content. As tomato fruits develop over time, the levels of soluble galactose increase, thereby stimulating ethylene production and, subsequently, promoting ripening. Also, melatonin-treated plants have significantly higher levels of citric acid. The flavour of fruits is optimal when there are high concentrations of both sugars and organic acids. In general, melatonin increased both fruit yield (up to 13%) and quality in tomato fruits (J. Liu et al., 2016).
In peach fruits, melatonin treatment effectively retarded the senescence in the two peach cultivars assayed, as indicated by reduced weight loss, decay incidence and respiration rate, while the firmness, total soluble solids and ascorbic acid contents were maintained (Table 3). Similarly, the post-harvest application of melatonin delayed senescence and maintained the quality of peach fruit, which may be attributed to its capacity to mediate antioxidative actions, suggesting that melatonin treatment may be a promising method for delaying senescence and maintaining fruit quality of post-harvest peach fruit (Gao et al., 2016). Also in melatonin-treated strawberry, improvements in post-harvest decay indicators have been described, along with high ATP contents, antioxidant activity and polyphenol levels (Aghdam and Fard, 2017).
MELATONIN, JASMONIC ACID AND SALICYLIC ACID
In animals, melatonin has important immunomodulatory, antioxidant, anti-inflammatory and neuroprotective effects, suggesting that this methoxyindole may be a good therapeutic alternative for fighting bacterial, viral and parasitic infections (Bonilla et al., 2004; Wu et al., 2011; Carrillo-Vico et al., 2013; Vielma et al., 2014). Also, its use as an in vivo antibiotic has been suggested, for example in human newborns suffering from septicaemia (Gitto et al., 2001).
In agriculture, pathogenic diseases are responsible for major production and economic losses in crops, and particular attention has been paid recently to the action(s) of melatonin in plant biotic stress. In the first study related to plant pathogen infection, treating trees through their roots by irrigation with melatonin at different concentrations improved resistance of Malus prunifolia against the fungus Diplocarpon mali (Marssonina apple blotch). At 20 d, melatonin-treated apple trees showed a lower number of damaged leaves, a higher chlorophyll content, more efficient PSII and less defoliation than infected untreated trees. Melatonin contributed to greater resistance to fungal infection, reduced lesions, inhibited pathogen expansion and generally alleviated disease damage (Yin et al., 2013). On the other hand, different concentrations of melatonin showed growth inhibition activity against several plant fungal pathogens such as Alternaria spp., Botrytis spp. and Fusarium spp. growing in standard media. Melatonin decreased the rate of infection in plant pathogen attacks by Penicillium spp. in non-sterilized Lupinus albus seeds (Arnao and Hernández-Ruiz, 2015b).
The most widely used model in plant–bacterial pathogen interaction studies is arabidopsis/Pseudomonas syringae (avirulent-Rpt2). In general, the application of melatonin to arabidopsis (also tobacco) induces pathogenesis-related genes, which further supports the idea that melatonin may be a defence signalling molecule in plants against pathogens. Melatonin upregulates pathogen-related, SA- and ethylene-dependent genes, an effect that was suppressed in mutants defective in SA and ethylene signalling. Also, melatonin increased NO- and SA-related genes, accompanied by a reduced susceptibility to the pathogen, leading to an increase in both melatonin and NO. SNAT knockout mutants not only exhibited reduced levels of melatonin, but also lower levels of SA, along with greater susceptibility to the pathogen (Lee et al., 2014, 2015). Recently, it has been found that mitogen-activated protein kinase (MAPK) signalling through diverse MAPKKK kinase cascades is also required for melatonin-mediated innate immunity in plants (Lee and Back, 2016, 2017). The data obtained to date suggest that melatonin should be incorporated in the exhaustively described JA models (Wasternack and Hause, 2013; Shyu and Brutnell, 2015). Melatonin acts upstream of the defence gene signalling pathway, inducing the biosynthesis of SA, JA, NO and ethylene, which, acting together, elicit disease resistance in a well known co-action (Fig. 2) (Zhu and Lee, 2015). The emergence of an oxidative burst during the early stages of the plant–pathogen interaction seems to increase endogenous melatonin levels (Arnao and Hernández-Ruiz, 2015b). In conclusion, melatonin seems to be involved in innate plant immunity against fungal and bacterial pathogens via an SA/JA/ethylene- and NO-dependent pathway (Lee et al., 2014; Qian et al., 2015; Shi et al., 2015a, c, 2016b; Lee and Back, 2017).
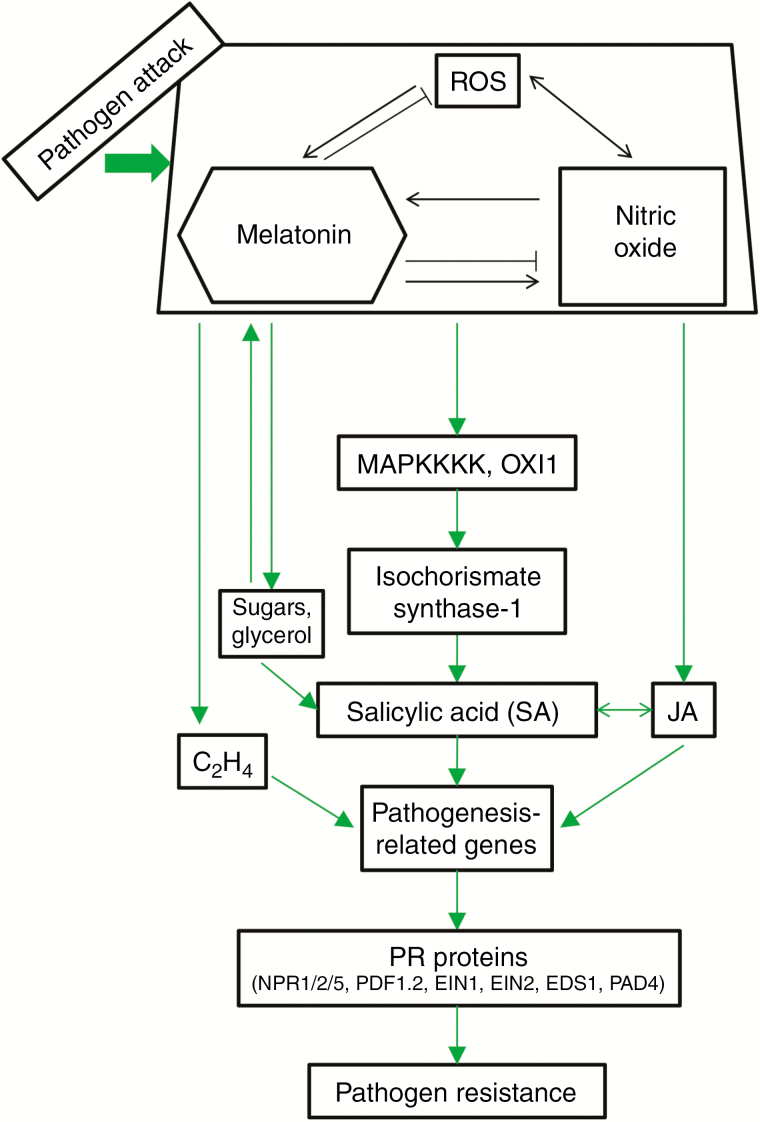
Fig. 2.
Model of melatonin/NO/ROS action on biotic stress responses (pathogen resistance). Pathogen attack increases NO and melatonin levels through ROS. In the model of arabidopsis/Pseudomonas syringae DC3000 (avrRpt2), the plant–pathogen interaction revealed that the MAPKKK3 and OXI1 (oxidative signal-inducible1) kinases are responsible for triggering melatonin-induced defence signalling pathways. Isochorismate synthase-1, a key enzyme in salicylic acid synthesis, was upregulated, increasing SA levels and triggering a pathogen-induced response. Also, melatonin and NO were able to induce jasmonic acid (JA) biosynthesis and increase fructose, glucose, sucrose and glycerol levels, all of which activate pathogenesis-related gene expression. Also the melatonin induction of ethylene biosynthesis, through ACC synthase (ACS6) collaborates in the induction of pathogen resistance (PR) proteins such as NPR1, plant defensin (PDF), ethylene insensitive (EIN), enhanced disease susceptibility 1 (EDS1) and phytoalexin deficient 4 (PAD4) factors, all key signalling components in the plant SA- and ethylene-mediated defence responses.
Interestingly, endophytes (i.e. soil microbes) form symbiotic relationships with plants. These symbiotic organisms are important in defending their hosts against pathogens and may also promote the growth of their host plants via nitrogen fixation, phosphorus solubilization and the enhancement of plant hormone levels. The occurrence of melatonin biosynthesis in endophytic bacteria in Vitis sp. provides evidence for a novel form of communication between beneficial endophytes and host plants via melatonin. Thus, symbiotic interaction induces high melatonin level in roots, more lateral roots of greater length and also larger plants with higher chlorophyll levels. So, a lower ROS burst was measured (Jiao et al., 2016; Ma et al., 2017). In this sense, high endogenous melatonin levels have recently been detected in the soil fungus Trichoderma spp., which may be related to the proposed function of these fungi as root/stem growth promoters, plant pathogen resistance inducers and as alleviating bioagents in plants against various environmental stressors such as heavy metals, radiation, acid and alkaline soils and extreme temperatures, among others (T. Liu et al., 2016).
CONCLUSIONS AND FUTURE CHALLENGES
The observed field of action of melatonin in plants has grown as research has progressed. The first application of plant melatonin as a nutraceutical (Reiter et al., 2007; Arnao and Hernández-Ruiz, 2015c, 2017b) has also expanded, giving rise to specific roles in the physiology of plants. Environmental factors induce high endogenous melatonin levels, which can change the expression of many genes, regulation factors and stress-responsive genes that attenuate or reverse the negative effects of biotic/abiotic stressors on physiological processes, acting in this way as a positive effector against stress. Clearly, the action of melatonin becomes more evident and concise in challenging physiological situations, when plants are subjected to stressful environments or aggressive conditions such as severe abiotic stress and/or pathogen infections. Undoubtedly, plant hormones play a key role in these situations. One or more modes of interaction with melatonin have been described for plant hormones. As has been seen throughout this review, melatonin regulates genes related to plant hormones, ranging from those involved in the biosynthesis or catabolism of IAA, CKs, GAs, ABA, JA and ethylene to genes encoding auxin carriers such as PINs, ethylene-related proteins (e.g. NR and ETR4), ABA receptor (PYL8) and signalling/transducing elements, among others. A mediating role for NO in the melatonin response has been proposed in some cases, mainly in its auxin-like and plant immune responses.
Nitric oxids and ROS are key signals that appear in stress conditions and have been extensively studied in recent years (Astier et al., 2016). Generally, these signals tend to act together, maintaining their respective networks (RNS and ROS) in a co-ordinated way. NO levels are self-regulated and also regulate the ROS network through NO-dependent post-translational modifications (Romero-Puertas and Sandalio, 2016). NO modulates several functions via protein modifications, including the nitration, S-nitrosylation and ligation of NO to transition metals, but also through modification in lipids (nitro-fatty acids) and DNA (8-nitroguanine) (Damiani et al., 2016; del Rio and López-Huertas, 2016; Molassiotis et al., 2016; Saxena et al., 2016; Pucciariello and Perata 2017). Moreover, NO triggers a set of responses to alleviate stress and cellular damage that includes transient metabolic reprogramming involving both primary and secondary metabolism (León et al., 2016).
The NO and ROS signals interplay with plant hormones under biotic and abiotic stress situations. Excellent reviews on this subject can be found in Asgher et al. (2017) and Freschi (2013). Three ways in which NO can modify hormonal action have been described: (1) by chemical modifications of transcription factors and other regulatory proteins, thereby influencing transcription of genes; (2) by chemical modifications of proteins and enzymes involved in plant hormone metabolism, transport and signalling; and (iii) by direct chemical reaction between NO (or derivatives) and plant hormones. All this can lead to changes in plant hormone levels, distribution and signalling.
One or more examples of the action of NO on plant hormones can be found. Synergistic effects of auxin and NO have been observed in rhizogenesis, gravitropic and root nodulation processes, among others. Also, NO might enhance IAA levels in roots under stress conditions, inhibit acropetal auxin transport, reducing some PIN proteins, and promote auxin-dependent genes through the S-nitrosylation of the auxin receptor protein (TIR1). With respect to GAs, antagonistic effects with NO have been described in many of the physiological processes involving both these signalling molecules, such as seed germination, de-etiolation transition processes and root growth, mainly in diverse abiotic stresses. Thus, a mutual antagonism controls endogenous levels of NO and GAs. NO inhibits GA signalling by promoting DELLA protein accumulation, whose presence represses GA-regulated genes, and also the inhibition of the E3 ubiquitin ligase SLY1. Also with CKs, a complex antagonistic interaction with NO has been described. Zeatin can react with peroxynitrite (ONOO–), generating biologically inactive CK derivates. NO may react with the signalling element AHP1, making it inactive, and inhibiting the phosphorelay mechanism that activates CK regulators (ARRs). NO acts as a downstream element in the ABA signalling pathway, activating ABA-mediated responses together with H2O2. NO induces changes in cGMP, cytosolic calcium and protein kinases/phosphatases, key downstream elements in NO/ABA signalling. NO can also negatively affect ethylene biosynthesis. Thus, up to five Yang cycle enzymes might be S-nitrosylated, inactivating them. Also, NO can inhibit the transcript levels of ACC synthase and ACC oxidase, decreasing ethylene levels. Also, NO promotes the formation of conjugated forms of ACC. In the plant pathogen responses, NO stimulates SA biosynthesis, and in its interaction with the SA receptor promotes the denitrosylation of NPR1, a key nuclear factor in SA response genes. NO also activates JA biosynthesis, whose JA-response is repressed by cytosolic S-nitrosylated oligomeric NPR1. In all cases, NO was identified as acting downstream of plant hormones.
Figures 2 and 3 compile much of the information known so far on the melatonin–NO binomial. The models outline the data concerning NO mediation in pathogen resistance involving melatonin, NO, JA, SA and ethylene (Fig. 2), and in the action of plant hormones in abiotic stress conditions (Fig. 3). The so-called auxin-like activity of melatonin (through the modulation of auxin carriers such as PIN1/3/7) is depicted in Fig. 3 as a particular case. According to present data, melatonin seems to be mediated by NO, and possibly vice versa. Melatonin increased endogenous NO levels and, curiously, NO also increased the level of melatonin, indicating a possible NO feedback mechanism. It is also interesting that melatonin can act as a scavenger of NO (Wen et al., 2016). Although some data indicate that melatonin might act as an upstream signal of plant hormones in stress responses, the increases/decreases of melatonin and NO levels through the action of some plant hormones complicate interpretation of the model. Also, because of the complexity of the melatonin–NO interaction, it is difficult to elucidate whether melatonin is upstream or downstream of NO. H2O2 (an important ROS) mainly acts as a signal molecule in stress situations and, curiously, seems to be of relevance in the upregulation of several melatonin biosynthesis enzymes, resulting in an increase in melatonin levels in stressed plants (Arnao and Hernández-Ruiz, 2015b; Nawaz et al., 2016). Thus, melatonin interacts with H2O2 and NO, representing post-translational modifications and hormone gene expression changes which determine a global hormone-mediated response to alleviate stress effects. It seems that melatonin and IAA co-participate in some of the classic auxin responses such as cell growth and root formation (Woodward and Bartel, 2005; Slovak et al., 2016). Modulation of the expression of different auxin carriers (PINs) by melatonin and IAA appears to be at the centre of the physiologically mediated response (Fig. 3).
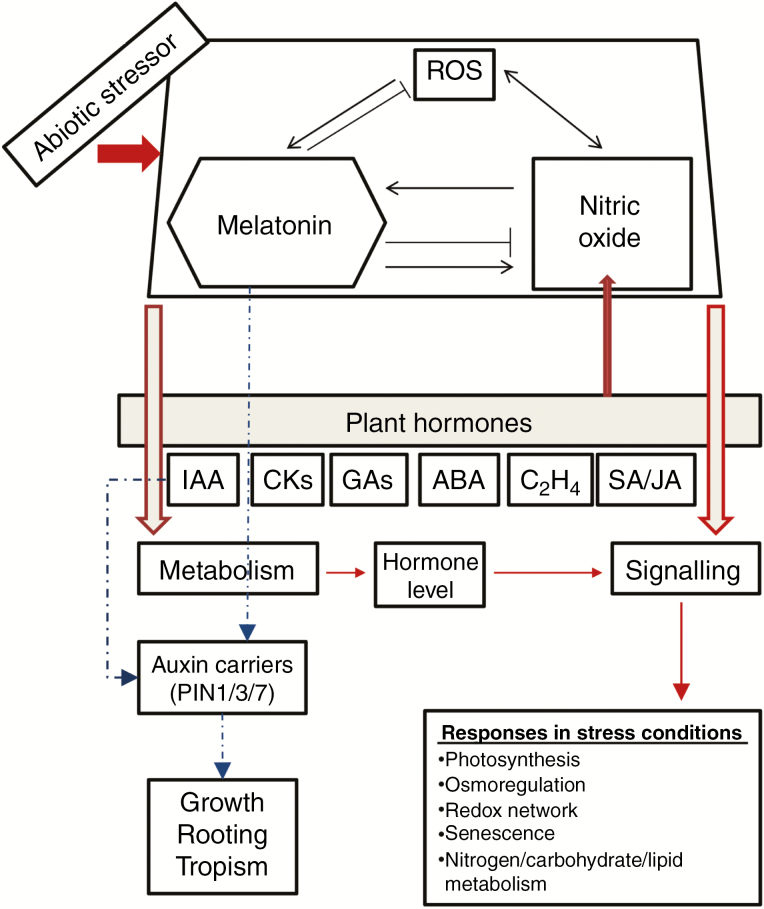
Fig. 3.
Model of melatonin/NO/ROS action on plant hormone-mediated abiotic stress responses. Abiotic stressors induce an increase in endogenous melatonin through the upregulation of melatonin biosynthetic genes. Melatonin increases the nitric oxide (NO) level through (at least) the upregulation of nitrate reductase (which usually reduces nitrate to nitrite but can also reduce nitrite to NO using NADPH as a cofactor). NO induces melatonin biosynthesis, while melatonin can also act as an NO scavenger. Melatonin and NO induce changes in hormone levels through the up-/downregulation of hormone biosynthesis/catabolism enzymes. Also, melatonin and NO change the expression of several transcription factors and hormone signalling elements, which determines the global antistress response. Some plant hormones such as IAA, CKs and ABA can stimulate NO production. As a particular case, melatonin and NO can generate changes in the auxin carriers of cells (PINs 1/3/7), and in auxin signalling transduction genes (IAA19 and IAA24, in the case of melatonin), determining auxin-like responses such as growth, rooting and tropism (localized gradients).
However, it must be taken into account that only molecular studies between melatonin and plant hormones have been carried out since 2014 (Weeda et al., 2014). Many more experiments are necessary, for example using plant hormone mutants. Unfortunately, only one ABA-deficient mutant in barley has been used in treatments with melatonin (X. Li et al., 2106). Regarding melatonin transgenic lines, there are many transgenic melatonin-deficient or melatonin-overaccumulating lines (Arnao and Hernández-Ruiz, 2015b). Unfortunately, there are no studies with them and plant hormones yet. One of the most promising fields of study related to melatonin in plants is that of endophytic relationships. The direct relationship between melatonin levels and the decrease in ROS due to activation of the redox defences (enzymes and metabolites) in Vitis sp. suggests that high levels of endogenous melatonin induced by endophytic microbes, such as Bacillus amyloliquefaciens, B. thuringiensis, B. cereus, Agrobacterium tumefaciens and Pseudomonas fluorescens, among others (or their own levels of melatonin), work in unison to cope with oxidative stress, acting as an antistress agent, and also as a root-promoting agent. The easy incorporation of melatonin through the roots, and its subsequent distribution throughout the rest of the plant, raises the possibility that the melatonin synthesized by soil microbes can be an intercommunicating agent between host plants and microbes (pathogens or not). Other plant regulator substances such as brassinosteroids and strigolactones have not been dealt with in this review because no data are available concerning their joint action with melatonin. There are only three papers in the case of polyamines: in one of them, the protective role of melatonin against cold-induced apoptosis was related to polyamine levels in carrot suspension cells (Lei et al., 2004), while a more recent study in arabidopsis dealt with the tolerance to iron deficiencies induced by melatonin and the possible intermediate role of polyamines (C. Zhou et al., 2016). The levels of putrescine, spermidine and spermine, together with sucrose and proline, increased in tomato plants under cold and melatonin treatments, suggesting a relationship between melatonin and polyamine-mediated stress responses (Ding et al., 2017). The enhanced production of NO by some polyamines under chilling stress in tomato seedlings suggested cross-talk between polyamines and ABA, via an NO/ROS-dependent mechanism involved in the chilling response (Diao et al., 2017).
Identification of a possible melatonin receptor is a priority task to clarify the mechanisms that activate the responses in plants. Thus, although its double action (as an antioxidant agent and as a modulator of gene expression) seems evident, it still poses questions concerning its transport between cells (free or protein assisted, taking into account that it is an amphipatic molecule) and between tissues and organs (through the xylem and phloem), its possible conjugation with carbohydrates or amino acids, the signal transduction elements involved and the possible sharing with plant hormone mechanisms, among others. Although in this work there are many indications of connections between plant hormones and melatonin, there is still a long way to go before any firm conclusions can be reached. However, future studies on melatonin and individual plant hormones will undoubtedly throw light on the degree of interaction that exists at cellular and tissular level, and possible synergies or antagonisms in the mediated responses.
LITERATURE CITED
- Aghdam MS, Fard JR. 2017. Melatonin treatment attenuates postharvest decay and maintains nutritional quality of strawberry fruits (Fragaria×anannasa cv. Selva) by enhancing GABA shunt activity. Food Chemistry 221: 1650–1657. [DOI] [PubMed] [Google Scholar]
- Aguilera Y, Herrera T, Liébana R et al. . 2015. Impact of melatonin enrichment during germination of legumes on bioactive compounds and antioxidant activity. Journal of Agricultural and Food Chemistry 63: 7967–7974. [DOI] [PubMed] [Google Scholar]
- Arnao MB, Hernández-Ruiz J. 2006. The physiological function of melatonin in plants. Plant Signaling and Behavior 1: 89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnao MB, Hernández-Ruiz J. 2007a. Melatonin promotes adventitious- and lateral root regeneration in etiolated hypocotyls of Lupinus albus L. Journal of Pineal Research 42: 147–152. [DOI] [PubMed] [Google Scholar]
- Arnao MB, Hernández-Ruiz J. 2007b. Inhibition of ACC oxidase activity by melatonin and IAA in etiolated lupin hypocotyls. In: Ramina A, Chang C, Giovannoni J, Klee H, Perata P, Woltering E, eds. Advances in plant ethylene research. Dordrecht: Springer, 101–103. [Google Scholar]
- Arnao MB, Hernández-Ruiz J. 2009a. Chemical stress by different agents affects the melatonin content of barley roots. Journal of Pineal Research 46: 295–299. [DOI] [PubMed] [Google Scholar]
- Arnao MB, Hernández-Ruiz J. 2009b. Protective effect of melatonin against chlorophyll degradation during the senescence of barley leaves. Journal of Pineal Research 46: 58–63. [DOI] [PubMed] [Google Scholar]
- Arnao MB, Hernández-Ruiz J. 2013a. Growth conditions determine different melatonin levels in Lupinus albus L. Journal of Pineal Research 55: 149–155. [DOI] [PubMed] [Google Scholar]
- Arnao MB, Hernández-Ruiz J. 2013b. Growth conditions influence the melatonin content of tomato plants. Food Chemistry 138: 1212–1214. [DOI] [PubMed] [Google Scholar]
- Arnao MB, Hernández-Ruiz J. 2014a. Melatonin: possible role as light-protector in plants. In: Radosevich JA, ed. UV radiation: properties, effects, and applications. New York: Nova Science Publishers, 79–92. [Google Scholar]
- Arnao MB, Hernández-Ruiz J. 2014b. Melatonin: plant growth regulator and/or biostimulator during stress?Trends in Plant Science 19: 789–797. [DOI] [PubMed] [Google Scholar]
- Arnao MB, Hernández-Ruiz J. 2015a. Melatonin: synthesis from tryptophan and its role in higher plants. In: D’ Mello JPF, ed. Amino acids in higher plants. Boston: CAB International, 390–435. [Google Scholar]
- Arnao MB, Hernández-Ruiz J. 2015b. Functions of melatonin in plants: a review. Journal of Pineal Research 59: 133–150. [DOI] [PubMed] [Google Scholar]
- Arnao MB, Hernández-Ruiz J. 2015c. Phytomelatonin: searching for plants with high levels as a natural source of nutraceuticals. In: Atta-ur-Rahman FRS, ed. Studies in Natural products chemistry (Bioactive natural products), Vol. 46 Amsterdam, The Netherlands: Elsevier Science Publishers, 519–545. [Google Scholar]
- Arnao MB, Hernández-Ruiz J. 2017a. Growth activity, rooting capacity, and tropism: three auxinic precepts fulfilled by melatonin. Acta Physiologiae Plantarum 39: 127. [Google Scholar]
- Arnao MB, Hernández-Ruiz J. 2017b. Phyto-melatonin: a natural substance from plants with interesting nutraceutical properties. In: Motohashi N, ed. Nutraceuticals: prospects, sources and role in health and disease. New York: NOVA Science Publishers, 123–157. [Google Scholar]
- Asgher M, Per TS, Masood A et al. . 2017. Nitric oxide signaling and its crosstalk with other plant growth regulators in plant responses to abiotic stress. Environmental Science and Pollution Research 24: 2273–2285. [DOI] [PubMed] [Google Scholar]
- Astier J, Loake G, Velikova V, Gaupels F. 2016. Editorial: interplay between NO signaling, ROS, and the antioxidant system in plants. Frontiers in Plant Science 7: 1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back K, Tan DX, Reiter RJ. 2016. Melatonin biosynthesis in plants: multiple pathways catalyze tryptophan to melatonin in the cytoplasm or chloroplasts. Journal of Pineal Research 61: 426–437. [DOI] [PubMed] [Google Scholar]
- Bajwa VS, Shukla MR, Sherif SM, Murch SJ, Saxena PK. 2014. Role of melatonin in alleviating cold stress in Arabidopsis thaliana. Journal of Pineal Research 56: 238–245. [DOI] [PubMed] [Google Scholar]
- Bonilla E, Valero N, Chacín-Bonilla L, Medina-Leendertz S. 2004. Melatonin and viral infections. Journal of Pineal Research 36: 73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byeon Y, Lee HY, Lee K, Back K. 2014. Caffeic acid O-methyltransferase is involved in the synthesis of melatonin by methylating N-acetylserotonin in Arabidopsis. Journal of Pineal Research 57: 219–227. [DOI] [PubMed] [Google Scholar]
- Carrillo-Vico A, Lardone PJ, Alvarez-Sanchez N, Rodríguez-Rodriguez A, Guerrero JM. 2013. Melatonin: buffering the immune system. International Journal of Molecular Sciences 14: 8638–8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Qi WB, Reiter RJ, Wei W, Wang BM. 2009. Exogenously applied melatonin stimulates root growth and raises endogenous IAA in roots of etiolated seedling of Brassica juncea. Journal of Plant Physiology 166: 324–328. [DOI] [PubMed] [Google Scholar]
- Chen Y, Kao C. 2012. Calcium is involved in nitric oxide- and auxin-induced lateral root formation in rice. Protoplasma 249: 187–195. [DOI] [PubMed] [Google Scholar]
- Correa-Aragunde N, Graziano M, Lamattina L. 2004. Nitric oxide plays a central role in determining lateral root development in tomato. Planta 218: 900–905. [DOI] [PubMed] [Google Scholar]
- Correa-Aragunde N, Cejudo F, Lamattina L. 2015. Nitric oxide is required for the auxin-induced activation of NADPH-dependent thioredoxin reductase and protein denitrosylation during root growth responses in arabidopsis. Annals of Botany 116: 695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiani I, Pauly N, Puppo A, Brouquisse R, Boscari A. 2016. Reactive oxygen species and nitric oxide control early steps of the legume–Rhizobium symbiotic interaction. Frontiers in Plant Science 7: 454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di D, Zhang C, Guo G. 2015. Involvement of secondary messengers and small organic molecules in auxin perception and signaling. Plant Cell Reports 34: 895–904. [DOI] [PubMed] [Google Scholar]
- Diao Q, Song Y, Shi D, Qi H. 2017. Interaction of polyamines, abscisic acid, nitric oxide, and hydrogen peroxide under chilling stress in tomato (Lycopersicon esculentum Mill.) seedlings. Frontiers in Plant Science 8: 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding F, Liu B, Zhang S. 2017. Exogenous melatonin ameliorates cold-induced damage in tomato plants. Scientia Horticulturae 219: 264–271. [Google Scholar]
- Dubbels R, Reiter RJ, Klenke E et al. . 1995. Melatonin in edible plants identified by radioimmunoassay and by HPLC-MS. Journal of Pineal Research 18: 28–31. [DOI] [PubMed] [Google Scholar]
- Freschi L. 2013. Nitric oxide and phytohormone interactions: current status and perspectives. Frontiers in Plant Science 4: 398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Wu Y, Miao Y et al. . 2017. Improved cold tolerance in Elymus nutans by exogenous application of melatonin may involve ABA-dependent and ABA-independent pathways. Scientific Reports 7: 39865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Zhang ZK, Chai HK et al. . 2016. Melatonin treatment delays postharvest senescence and regulates reactive oxygen species metabolism in peach fruit. Postharvest Biology and Technology 118: 103–110. [Google Scholar]
- Gitto E, Karbownik M, Reiter RJ. 2001. Effects of melatonin treatment in septic newborns. Pediatric Research 50: 756–760. [DOI] [PubMed] [Google Scholar]
- Han QH, Huang B, Ding CB et al. . 2017. Effects of melatonin on anti-oxidative systems and Photosystem II in cold-stressed rice seedlings. Frontiers in Plant Science 8: 785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardeland R, Madrid JA, Tan DX, Reiter RJ. 2012. Melatonin, the circadian multioscillator system and health: the need for detailed analysis of peripheral melatonin signal. Journal of Pineal Research 52: 139–166. [DOI] [PubMed] [Google Scholar]
- Hattori A, Migitaka H, Iigo M et al. . 1995. Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochemistry and Molecular Biology International 35: 627–634. [PubMed] [Google Scholar]
- Hernández-Ruiz J, Arnao MB. 2008. Distribution of melatonin in different zones of lupin and barley plants at different ages in the presence and absence of light. Journal of Agricultural and Food Chemistry 56: 10567–10573. [DOI] [PubMed] [Google Scholar]
- Hernández-Ruiz J, Arnao MB. 2016. Phytomelatonin, an interesting tool for agricultural crops. Focus on Science 2: 1–7. [Google Scholar]
- Hernández-Ruiz J, Cano A, Arnao MB. 2004. Melatonin: growth-stimulating compound present in lupin tissues. Planta 220: 140–144. [DOI] [PubMed] [Google Scholar]
- Hu W, Kong H, Guo Y et al. . 2016. Comparative physiological and transcriptomic analyses reveal the actions of melatonin in the delay of postharvest physiological deterioration of cassava. Frontiers in Plant Science 7: 736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Neill S, Tang Z, Cai W. 2005. Nitric oxide mediates gravitropic bending in soybean roots. Plant Physiology 137: 663–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain A, Mun BG, Imran QM et al. . 2016. Nitric oxide mediated transcriptome profiling reveals activation of multiple regulatory pathways in Arabidopsis thaliana. Frontiers in Plant Science 7: 975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao J, Ma Y, Chen S et al. . 2016. Melatonin-producing endophytic bacteria from grapevine roots promote the abiotic stress-induced production of endogenous melatonin in their hosts. Frontiers in Plant Science 7: 1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JE, Kim WT, Kang BG. 2001. IAA and N6-benzyladenine inhibit ethylene-regulated expression of ACC oxidase and ACC synthase genes in mungbean hypocotyls. Plant and Cell Physiology 42: 1056–1061. [DOI] [PubMed] [Google Scholar]
- Kim M, Seo H, Park C, Park WJ. 2016. Examination of the auxin hypothesis of phytomelatonin action in classical auxin assay systems in maize. Journal of Plant Physiology 190: 67–71. [DOI] [PubMed] [Google Scholar]
- Kolar J, Machackova I. 2005. Melatonin in higher plants: occurrence and possible functions. Journal of Pineal Research 39: 333–341. [DOI] [PubMed] [Google Scholar]
- Korkmaz A, Karakas A, Kocacinar F, Cuci Y. 2017. The effects of seed treatment with melatonin on germination and emergence performance of pepper seeds under chilling stress. Journal of Agricultural Sciences 23: 167–176. [Google Scholar]
- Koyama FC, Carvalho TLG, Alves E et al. . 2013. The structurally related auxin and melatonin tryptophan-derivatives and their roles in Arabidopsis thaliana and in the human malaria parasite Plasmodium falciparum. Journal of Eukaryotic Microbiology 60: 646–651. [DOI] [PubMed] [Google Scholar]
- Lee HY, Back K. 2016. Mitogen-activated protein kinase pathways are required for melatonin-mediated defense responses in plants. Journal of Pineal Research 60: 327–335. [DOI] [PubMed] [Google Scholar]
- Lee HY, Back K. 2017. Melatonin is required for H2O2- and NO-mediated defense signaling through MAPKKK3 and OXI1 in Arabidopsis thaliana. Journal of Pineal Research 62: e12379. [DOI] [PubMed] [Google Scholar]
- Lee HY, Byeon Y, Back K. 2014. Melatonin as a signal molecule triggering defense responses against pathogen attack in Arabidopsis and tobacco. Journal of Pineal Research 57: 262–268. [DOI] [PubMed] [Google Scholar]
- Lee HY, Byeon Y, Tan DX, Reiter RJ, Back K. 2015. Arabidopsis serotonin N-acetyltransferase knockout mutant plants exhibit decreased melatonin and salicylic acid levels resulting in susceptibility to an avirulent pathogen. Journal of Pineal Research 58: 291–299. [DOI] [PubMed] [Google Scholar]
- Lei XY, Zhu RY, Zhang GY, Dai YR. 2004. Attenuation of cold-induced apoptosis by exogenous melatonin in carrot suspension cells: the possible involvement of polyamines. Journal of Pineal Research 36: 126–131. [DOI] [PubMed] [Google Scholar]
- León J, Costa A, Castillo MC. 2016. Nitric oxide triggers a transient metabolic reprogramming in Arabidopsis. Scientific Reports 6: 37945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner AB, Case JD, Takahashi Y, Lee TH, Mori W. 1958. Isolation of melatonin, a pineal factor that lightens melanocytes. Journal of American Chemical Society 80: 2587. [Google Scholar]
- Li C, Liang D, Chang C, Jia D, Ma F. 2015. Melatonin mediates the regulation of ABA metabolism, free-radical scavenging, and stomatal behavior in two Malus species under drought stress. Journal of Experimental Botany 66: 669–680. [DOI] [PubMed] [Google Scholar]
- Li C, Liang B, Chang C, Wei Z, Zhou S, Ma F. 2016. Exogenous melatonin improved potassium content in Malus under different stress conditions. Journal of Pineal Research 61: 218–229. [DOI] [PubMed] [Google Scholar]
- Li H, Chang J, Zheng J et al. . 2017. Local melatonin application induces cold tolerance in distant organs of Citrullus lanatus L. via long distance transport. Scientific Reports 7: 40858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Tan DX, Jiang D, Liu F. 2016. Melatonin enhances cold tolerance in drought-primed wild-type and abscisic acid-deficient mutant barley. Journal of Pineal Research 61: 328–339. [DOI] [PubMed] [Google Scholar]
- Liang C, Zheng G, Li W et al. . 2015. Melatonin delays leaf senescence and enhances salt stress tolerance in rice. Journal of Pineal Research 59: 91–101. [DOI] [PubMed] [Google Scholar]
- Liang C, Li A, Yu H et al. . 2017. Melatonin regulates root architecture by modulating auxin response in rice. Frontiers in Plant Science 8: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhang R, Sun Y, Liu Z, Jin W, Sun Y. 2016. The beneficial effects of exogenous melatonin on tomato fruit properties. Scientia Horticulturae 207: 14–20. [Google Scholar]
- Liu T, Zhao F, Liu Z, Zuo Y, Hou J, Wang Y. 2016. Identification of melatonin in Trichoderma spp. and detection of melatonin content under controlled-stress growth conditions from T. asperellum. Journal of Basic Microbiology 56: 843. [DOI] [PubMed] [Google Scholar]
- Lombardo M, Graziano M, Polacco J, Lamattina L. 2006. Nitric oxide functions as a positive regulator of root hair development. Plant Signaling and Behavior 1: 28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Jiao J, Fan X et al. . 2017. Endophytic bacterium Pseudomonas fluorescens RG11 may transform tryptophan to melatonin and promote endogenous melatonin levels in the roots of four grape cultivars. Frontiers in Plant Science 7: 2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molassiotis A, Job D, Ziogas V, Tanou G. 2016. Citrus plants: a model system for unlocking the secrets of NO and ROS-inspired priming against salinity and drought. Frontiers in Plant Science 7: 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, David A, Yadav S, Baluska F, Bhatla SC. 2014. Salt stress-induced seedling growth inhibition coincides with differential distribution of serotonin and melatonin in sunflower seedling roots and cotyledons. Physiologia Plantarum 152: 714–728. [DOI] [PubMed] [Google Scholar]
- Nawaz MA, Huang Y, Bie Z et al. . 2016. Melatonin: current status and future perspectives in plant science. Frontiers in Plant Science 6: 1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagnussat G, Lanteri M, Lombardo M, Lamattina L. 2004. Nitric oxide mediates the indole acetic acid induction activation of a mitogen-activated protein kinase cascade involved in adventitious root development. Plant Physiology 135: 179–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagnussat G, Simontacchi M, Puntarulo S, Lamattina L. 2002. Nitric oxide is required for root organogenesis. Plant Physiology 129: 954–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandi-Perumal SR, Trakht I, Srinivasan V et al. . 2008. Physiological effects of melatonin: role of melatonin receptors and signal transduction pathways. Progress in Neurobiology 85: 335–353. [DOI] [PubMed] [Google Scholar]
- Pattison RJ, Catalá C. 2012. Evaluating auxin distribution in tomato (Solanum lycopersicum) through an analysis of the PIN and AUX/LAX gene families. The Plant Journal 70: 585–598. [DOI] [PubMed] [Google Scholar]
- Pelagio-Flores R, Muñoz-Parra E, Ortiz-Castro R, Lopez-Bucio J. 2012. Melatonin regulates Arabidopsis root system architecture likely acting independently of auxin signaling. Journal of Pineal Research 53: 279–288. [DOI] [PubMed] [Google Scholar]
- Pucciariello C, Perata P. 2017. New insights into reactive oxygen species and nitric oxide signalling under low oxygen in plants. Plant, Cell and Environment 40: 473–482. [DOI] [PubMed] [Google Scholar]
- Qian Y, Tan DX, Reiter RJ, Shi H. 2015. Comparative metabolomic analysis highlights the involvement of sugars and glycerol in melatonin-mediated innate immunity against bacterial pathogen in Arabidopsis. Scientific Reports 5: 15815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter RJ, Tan DX, Manchester LC et al. . 2007. Melatonin in edible plants (phytomelatonin): identification, concentrations, bioavailability and proposed functions. World Review of Nutrition & Dietetics 97: 211–230. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Tan DX, Galano A. 2014. Melatonin: exceeding expectations. Physiology (Bethesda) 56: 371–381. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Tan DX, Zhou Z, Coelho-Cruz MH, Fuentes-Broto L, Galano A. 2015. Phytomelatonin: assisting plants to survive and thrive. Molecules 20: 7396–7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rio LA, López-Huertas E. 2016. ROS generation in peroxisomes and its role in cell signaling. Plant, Cell and Environment 57: 1364–1376. [DOI] [PubMed] [Google Scholar]
- Romero-Puertas MC, Sandalio LM. 2016. Nitric oxide level is self-regulating and also regulates its ROS partners. Frontiers in Plant Science 7: 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarropoulou VN, Therios IN, Dimassi-Theriou KN. 2012a. Melatonin promotes adventitious root regeneration in in vitro shoot tip explants of the commercial sweet cherry rootstocks CAB-6P (Prunus cerasus L.), Gisela 6 (P. cerasus×P. canescens), and MxM 60 (P. avium×P.mahaleb). Journal of Pineal Research 52: 38–46. [DOI] [PubMed] [Google Scholar]
- Sarropoulou VN, Dimassi-Theriou KN, Therios IN, Koukourikou-Petridou M. 2012b. Melatonin enhances root regeneration, photosynthetic pigments, biomass, total carbohydrates and proline content in the cherry rootstock PHL-C (Prunus avium×Prunus cerasus). Plant Physiology and Biochemistry 61: 162–168. [DOI] [PubMed] [Google Scholar]
- Sarrou E, Therios IN, Dimassi-Theriou KN. 2014. Melatonin and other factors that promote rooting and sprouting of shoot cuttings in Punica granatum cv. Wonderful. Turkist Journal of Botany 38: 293–301. [Google Scholar]
- Saxena I, Srikanth S, Chen Z. 2016. Cross talk between H2O2 and interacting signal molecules under plant stress response. Frontiers in Plant Science 7: 570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Chen Y, Tan DX, Reiter RJ, Chan Z, He C. 2015a. Melatonin induces nitric oxide and the potential mechanisms relate to innate immunity against bacterial pathogen infection in Arabidopsis. Journal of Pineal Research 59: 102–108. [DOI] [PubMed] [Google Scholar]
- Shi H, Jiang C, Ye T et al. . 2015b. Comparative physiological, metabolomic, and transcriptomic analyses reveal mechanisms of improved abiotic stress resistance in bermudagrass [Cynodon dactylon (L). Pers.] by exogenous melatonin. Journal of Experimental Botany 66: 681–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Qian Y, Tan DX, Reiter RJ, He C. 2015c. Melatonin induces the transcripts of CBF/DREB1s and their involvement in both abiotic and biotic stresses in Arabidopsis. Journal of Pineal Research 59: 334–342. [DOI] [PubMed] [Google Scholar]
- Shi H, Reiter RJ, Tan DX, Chan Z. 2015d. INDOLE-3-ACETIC ACID INDUCIBLE 17 positively modulates natural leaf senescence through melatonin-mediated pathway in Arabidopsis. Journal of Pineal Research 58: 26–33. [DOI] [PubMed] [Google Scholar]
- Shi H, Chen K, Wei Y, He C. 2016a. Fundamental issues of melatonin-mediated stress signaling in plants. Frontiers in Plant Science 7: 1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Wei Y, He C. 2016b. Melatonin-induced CBF/DREB1s are essential for diurnal change of disease resistance and CCA1 expression in Arabidopsis. Plant Physiology and Biochemistry 100: 150–155. [DOI] [PubMed] [Google Scholar]
- Shyu C, Brutnell TP. 2015. Growth–defence balance in grass biomass production: the role of jasmonates. Journal of Experimental Botany 66: 4165–4176. [DOI] [PubMed] [Google Scholar]
- Skinner JS, von Zitzewitz J, Szucs P et al. . 2005. Structural, functional, and phylogenetic characterization of a large CBF gene family in barley. Plant Molecular Biology 59: 533–551. [DOI] [PubMed] [Google Scholar]
- Slovak R, Ogura T, Satbhai SB, Ristova D, Busch W. 2016. Genetic control of root growth: from genes to networks. Annals of Botany 117: 9–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun QQ, Zhang N, Wang J et al. . 2015. Melatonin promotes ripening and improves quality of tomato fruit during postharvest life. Journal of Experimental Botany 66: 657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Zhang N, Wang J et al. . 2016. A label-free differential proteomics analysis reveals the effect of melatonin on promoting fruit ripening and anthocyanin accumulation upon postharvest in tomato. Journal of Pineal Research 61: 138–153. [DOI] [PubMed] [Google Scholar]
- Swarup R, Péret B. 2012. AUX/LAX family of auxin influx carriers – an overview. Frontiers in Plant Science 3: 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan DX, Manchester LC, Reiter RJ, Qi WB, Karbownik M, Calvo JR. 2000. Significance of melatonin in antioxidative defense system: reactions and products. Biological Signals and Receptors 9: 137–159. [DOI] [PubMed] [Google Scholar]
- Tan DX, Manchester CL, Esteban-Zubero E, Zhou Z, Reiter JR. 2015. Melatonin as a potent and inducible endogenous antioxidant: synthesis and metabolism. Molecules 20: 18886–18906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan DX, Hardeland R, Back K, Manchester LC, Aatorre-Jimenez MA, Reiter RJ. 2016. On the significance of an alternate pathway of melatonin synthesis via 5-methoxytryptamine: comparisons across species. Journal of Pineal Research 61: 27–40. [DOI] [PubMed] [Google Scholar]
- Teixeira A, Morfim MP, de Cordova CAS, Charao CCT, Rodrigues de Lima V, Creczynski-Pasa TB. 2003. Melatonin protects against pro-oxidant enzymes and reduces lipid peroxidation in distinct membranes induced by the hydroxyl and ascorbyl radicals and by peroxynitrite. Journal of Pineal Research 35: 262–268. [DOI] [PubMed] [Google Scholar]
- Vielma JR, Bonilla E, Chacín-Bonilla L, Mora M, Medina-Leendertz S, Bravo Y. 2014. Effects of melatonin on oxidative stress, and resistance to bacterial, parasitic, and viral infections: a review. Acta Tropica 137: 31–38. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhao Y, Reiter RJ et al. . 2014. Changes in melatonin levels in transgenic Micro-Tom tomato overexpressing ovine AANAT and ovine HIOMT genes. Journal of Pineal Research 56: 134–142. [DOI] [PubMed] [Google Scholar]
- Wang P, Sun X, Li C, Wei Z, Liang D, Ma F. 2013. Long-term exogenous application of melatonin delays drought-induced leaf senescence in apple. Journal of Pineal Research 54: 292–302. [DOI] [PubMed] [Google Scholar]
- Wang P, Yin L, Liang D, Li C, Ma F, Yue Z. 2012. Delayed senescence of apple leaves by exogenous melatonin treatment: toward regulating the ascorbate–glutathione cycle. Journal of Pineal Research 53: 11–20. [DOI] [PubMed] [Google Scholar]
- Wang Q, An B, Wei Y et al. . 2016. Melatonin regulates root meristem by repressing auxin synthesis and polar auxin transport in Arabidopsis. Frontiers in Plant Science 7: 1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasternack C, Hause B. 2013. Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Annals of Botany 111: 1021–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeda S, Zhang N, Zhao X et al. . 2014. Arabidopsis transcriptome analysis reveals key roles of melatonin in plant defense systems. PLoS One 9: e93462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen D, Gong B, Sun S et al. . 2016. Promoting roles of melatonin in adventitious root development of Solanum lycopersicum L. by regulating auxin and nitric oxide signaling. Frontiers in Plant Science 7: 718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward AW, Bartel BON. 2005. Auxin: regulation, action, and interaction. Annals of Botany 95: 707–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu UI, Mai FD, Sheu JN et al. . 2011. Melatonin inhibits microglial activation, reduces pro-inflammatory cytokine levels, and rescues hippocampal neurons of adult rats with acute Klebsiella pneumoniae meningitis. Journal of Pineal Research 50: 159–170. [DOI] [PubMed] [Google Scholar]
- Yin L, Wang P, Li M et al. . 2013. Exogenous melatonin improves Malus resistance to Marssonina apple blotch. Journal of Pineal Research 54: 426–434. [DOI] [PubMed] [Google Scholar]
- Zhang HJ, Zhang N, Yang RC et al. . 2014. Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA4 interaction in cucumber (Cucumis sativus L.). Journal of Pineal Research 57: 269–279. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li H, Xu B, Li J, Huang B. 2016. Exogenous melatonin suppresses dark-induced leaf senescence by activating the superoxide dismutase–catalase antioxidant pathway and down-regulating chlorophyll degradation in excised leaves of perennial ryegrass (Lolium perenne L.). Frontiers in Plant Science 7: 1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Shi Y, Zhang X, Du H, Xu B, Huang B. 2017. Melatonin suppression of heat-induced leaf senescence involves changes in abscisic acid and cytokinin biosynthesis and signaling pathways in perennial ryegrass (Lolium perenne L.). Environmental and Experimental Botany 138: 36–45. [Google Scholar]
- Zhang N, Zhao B, Zhang HJ et al. . 2013. Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.). Journal of Pineal Research 54: 15–23. [DOI] [PubMed] [Google Scholar]
- Zhang N, Zhang HJ, Zhao B et al. . 2014. The RNA-seq approach to discriminate gene expression profiles in response to melatonin on cucumber lateral root formation. Journal of Pineal Research 56: 39–50. [DOI] [PubMed] [Google Scholar]
- Zhang N, Sun Q, Li H et al. . 2016. Melatonin improved anthocyanin accumulation by regulating gene expressions and resulted in high reactive oxygen species scavenging capacity in cabbage. Frontiers in Plant Science 7: 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Sun Y, Liu Z, Jin W, Sun Y. 2017. Effects of melatonin on seedling growth, mineral nutrition, and nitrogen metabolism in cucumber under nitrate stress. Journal of Pineal Research 62: e12403. [DOI] [PubMed] [Google Scholar]
- Zhou C, Liu Z, Zhu L, Ma Z, Wang J, Zhu J. 2016. Exogenous melatonin improves plant iron deficiency tolerance via increased accumulation of polyamine-mediated nitric oxide. International Journal of Molecular Sciences 17: 1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Zhao H, Cao K, Hu L, Du T, Baluska F, Zou Z. 2016. Beneficial roles of melatonin on redox regulation of photosynthetic electron transport and synthesis of D1 protein in tomato seedlings under salt stress. Frontiers in Plant Science 7: 1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Lee B. 2015. Friends or foes: new insights in jasmonate and ethylene co-actions. Plant and Cell Physiology 56: 414–420. [DOI] [PubMed] [Google Scholar]
- Zuo B, Zheng X, He P et al. . 2014. Overexpression of MzASMT improves melatonin production and enhances drought tolerance in transgenic Arabidopsis thaliana plants. Journal of Pineal Research 57: 408–417. [DOI] [PubMed] [Google Scholar]