CASE
An 83-year-old female with history of cerebral meningioma, multiple spinal hemangiomas with postresection lower extremity weakness, urinary incontinence, and 25-year history of recurrent urinary tract infections (UTIs) presented to our infectious disease clinic in December 2010 with 5 UTIs over 4 months. Symptoms abated for only a week between antibiotic courses. Allergies to ciprofloxacin, nitrofurantoin, and sulfa drugs limited treatment options. Prophylactic cephalexin gave temporary relief only. From October 2013 to February 2014, she was treated with 5 antibiotic courses for UTIs. Two urine cultures grew extended-spectrum β-lactamase (ESBL)-producing multidrug-resistant (MDR) Escherichia coli. Another culture in September grew a different MDR E. coli.
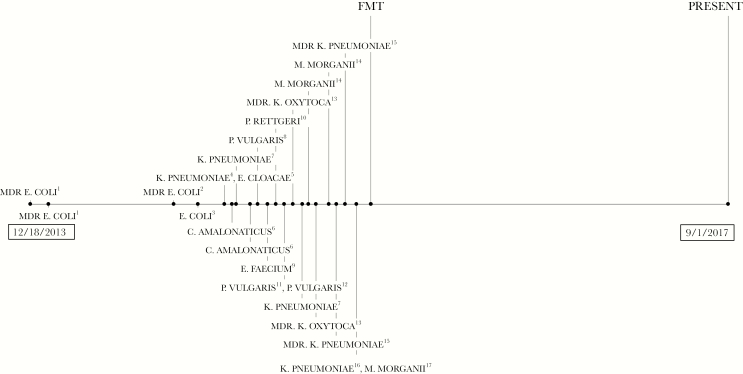
In October and November 2014, she had 3 ED presentations with MDR E. coli UTIs, with signs of sepsis on her third presentation. Cystoscopy with pyelograms revealed no fistula or nidus of infection. Subsequently, multiple symptomatic episodes of cystitis yielded 16 positive urine cultures demonstrating increasing antimicrobial resistance (Figure 1, Table 1). Cultures grew E. coli, Enterobacter cloacae, Citrobacter amalonaticus, Proteus vulgaris, Enterococcus faecium, Providencia rettgeri, Morganella morganii, ESBL K. pneumoniae, and ESBL K. oxytoca. Antibiotics used to treat these isolates included nitrofurantoin, cephalexin, cefuroxime, ceftriaxone, levofloxacin, doxycycline, trimethoprim-sulfamethoxazole, vancomycin, fosfomycin, piperacillin-tazobactam, meropenem, and ertapenem.
Figure 1.
(1) Extended-spectrum β-lactamase (ESBL) Escherichia coli, resistant to ampicillin, ampicillin/sulbactam, aztreonam, cefazolin, cefepime, ceftazidime, ceftriaxone, cefuroxime, gentamicin, levofloxacin, piperacillin/tazobactam, tobramycin, and trimethoprim/sulfa. (2) ESBL Escherichia coli, resistant to ampicillin, ampicillin/sulbactam, aztreonam, cefazolin, cefepime, ceftazidime, ceftriaxone, cefuroxime, levofloxacin, piperacillin/tazobactam, tetracycline, and trimethoprim/sulfa. (3) ESBL Escherichia coli, resistant to ampicillin. (4) Klebsiella pneumoniae, resistant to ampicillin; intermediate resistance to cefoxitin. (5) Enterobacter cloacae, resistant to ampicillin, ampicillin/sulbactam, cefazolin, cefoxitin, and nitrofurantoin; intermediate resistance to cefuroxime. (6) Citrobacter amalonaticus, resistant to ampicillin, ampicillin/sulbactam, cefazolin, ceftriaxone, cefuroxime, levofloxacin, tetracycline, and trimethoprim/sulfa; intermediate resistance to aztreonam. (7) Klebsiella pneumoniae, resistant to ampicillin. (8) Proteus vulgaris, resistant to ampicillin, ampicillin/sulbactam, cefazolin, ceftriaxone, cefuroxime, nitrofurantoin, and tetracycline. (9) Enterococcus faecium, resistant to ampicillin and penicillin. (10) Providencia rettgeri, resistant to ampicillin, ampicillin/sulbactam, cefazolin, and nitrofurantoin; intermediate resistance to tetracycline. (11) Proteus vulgaris, resistant to ampicillin, ampicillin/sulbactam, cefazolin, cefuroxime, nitrofurantoin, tetracycline; intermediate resistance to aztreonam and ceftriaxone. (12) Proteus vulgaris, resistant to ampicillin, ampicillin/sulbactam, cefazolin, cefuroxime, nitrofurantoin, and tetracycline. (13) ESBL Klebsiella oxytoca, resistant to ampicillin, ampicillin/sulbactam, aztreonam, cefazolin, cefepime, ceftazidime, ceftriaxone, cefuroxime, gentamicin, piperacillin/tazobactam, tetracycline, tobramycin, and trimethoprim/sulfa; intermediate resistance to levofloxacin. (14) Morganella morganii, resistant to ampicillin, ampicillin/sulbactam, cefazolin, cefuroxime, nitrofurantoin, and tetracycline; intermediate resistance to cefoxitin. (15) ESBL Klebsiella pneumoniae, resistant to ampicillin, ampicillin/sulbactam, aztreonam, cefazolin, cefepime, ceftazidime, ceftriaxone, cefuroxime, nitrofurantoin, piperacillin/tazobactam, tetracycline, tobramycin, and trimethoprim/sulfa. (16) Klebsiella pneumoniae, resistant to ampicillin, ampicillin/sulbactam, nitrofurantoin, tetracycline, tobramycin, and trimethoprim/sulfa. (17) Morganella morganii, resistant to ampicillin, ampicillin/sulbactam, cefazolin, ceftazidime, cefuroxime, and tetracycline; intermediate resistance to cefoxitin and nitrofurantoin. Abbreviations: FMT, fecal microbiota transplantation; MDR, multidrug-resistant.
Table 1.
Antimicrobial Susceptibility Patterns of Urine Isolates
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antibiotic | ESBL Escherichia coli |
ESBL Escherichia coli |
Escherichia coli | Klebsiella pneumoniae | Enterobacter cloacae | Citrobacter amalonaticus | Klebsiella pneumoniae | Proteus vulgaris | Enterococcus faecium | Providencia rettgeri | Proteus vulgaris | Proteus vulgaris | MDR Klebsiella oxytoca | Morganella morganii | MDR Klebsiella pneumoniae | Klebsiella pneumoniae | Morganella morganii |
| Amikacin | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | |
| Ampicillin | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R |
| Amp/Sul | R | R | S | S | R | R | S | R | R | R | R | R | R | R | R | R | |
| Aztreonam | R | R | S | S | S | I | S | S | S | I | S | R | S | R | S | S | |
| Cefazolin | R | R | S | S | R | R | S | R | R | R | R | R | R | R | S | R | |
| Cefepime | R | R | S | S | S | S | S | S | S | S | S | R | S | R | S | S | |
| Cefoxitin | S | I | R | S | S | S | S | S | S | I | R | S | I | ||||
| Ceftazidime | R | R | S | S | S | S | S | S | S | S | S | R | S | R | S | R | |
| Ceftriaxone | R | R | S | S | S | R | S | R | S | I | S | R | S | R | S | S | |
| Cefuroxime | R | R | S | S | I | R | S | R | S | R | R | R | R | R | S | R | |
| Colistin | b | ||||||||||||||||
| Fosfomycin | S | a | a | ||||||||||||||
| Gentamicin | R | S | S | S | S | S | S | S | S | S | S | R | S | S | S | S | |
| Levofloxacin | R | R | S | S | S | R | S | S | S | S | S | I | S | S | S | S | |
| Meropenem | S | S | S | S | S | S | S | S | S | S | S | ||||||
| Nitrofurantoin | S | S | S | S | R | S | S | R | S | R | R | R | S | R | R | R | I |
| Penicillin | R | ||||||||||||||||
| Pip/Tazo | R | R | S | S | S | S | S | S | S | S | S | R | S | R | S | S | |
| Tetracycline | S | R | S | S | S | R | S | R | S | I | R | R | R | R | R | R | R |
| Tigecycline | S | ||||||||||||||||
| Tobramycin | R | S | S | S | S | S | S | S | S | S | S | R | S | R | R | S | |
| Trim/Sulfa | R | R | S | S | S | R | S | S | S | S | S | R | S | R | R | S | |
| Vancomycin | S |
Abbreviations: Amp/Sul, ampicillin/sulbactam; Pip/Tazo, piperacillin/tazobactam; Trim/Sulfa, trimethoprim/sulfamethoxazole.
aMinimum inhibitory concentration (MIC) >1024 ug/mL.
bMIC 0.125 ug/mL.
Across 20 presentations from November 2013 to October 2015, symptoms included urinary urgency and worsened incontinence 11 times, generalized fatigue (without urgency or incontinence) 5 times, both sets of symptoms 3 times, and dysuria with suprapubic tenderness once. Though her symptoms were somewhat atypical of cystitis, she was treated for urinary infection given significant pyuria on urinalyses, neurologic changes, and limited ability to review systems with her comorbidities. Symptoms prompted each urinalysis and urine culture, and all antibiotics were prescribed according to susceptibility results. No antibiotics were prescribed without symptoms and a positive urine culture. Except for 2 doses of fosfomycin, which she could not obtain, and 1 course of levofloxacin interrupted for rash, all antibiotic courses were completed. Extensive nonantibiotic UTI prevention was attempted from 2010 to 2014, including solifenacin succinate, vaginal estrogen, increased fluid intake, scheduled voiding, stool softeners, methenamine hippurate, and vitamin C.
In March 2015, she developed diarrhea, tested positive for C. difficile, and completed 16 days of metronidazole. The following 6 months, her symptomatic UTIs (with bacteriuria and pyuria) continued but with decreased symptom-free intervals between infections from weeks to days. After episodes with MDR K. oxytoca and K. pneumoniae were treated with intravenous ertapenem, a surgical port was placed in anticipation of further parenteral therapy.
In May 2015, a C. difficile relapse was treated with oral vancomycin with prolonged taper. After another C. difficile relapse in October, fecal microbiota transplantation (FMT) was planned. Antibiotics were discontinued 48 hours prior. Stool from an unrelated donor who underwent routine serological and stool testing was delivered via colonoscopy without complications. Nine days post-FMT, she had complete resolution of all UTI and Clostridium difficile infection (CDI) symptoms. At 25 months post-FMT, there have been no recurrences. Of 2 urinalyses obtained since FMT for symptoms of increased fatigue, neither has indicated bacteriuria or pyuria, and she has not been prescribed antibiotics.
DISCUSSION
FMT is safe and effective for recurrent CDI [1]. It has also emerged as potential therapy for decolonization of MDR organisms (MDROs), offering a safe and possibly cost-effective strategy for tackling antibiotic resistance beyond current efforts to improve antibiotic stewardship and prevent infection [2].
Intestinal MDRO decolonization after FMT has been described in case reports, retrospective studies, and a prospective single-center study [3–5]. However, MDRO eradication from nongastrointestinal body sites is not as well described [6]. A recent review found that FMT for recurrent Clostridium difficile infection (RCDI) was associated with decreased UTI frequency and improved antibiotic susceptibility profiles [7]. Case reports have also described interruption of recurrent UTIs with a Verona integron-encoded metallo-β-lactamase-positive MDR P. aeruginosa, recurrent renal allograft pyelonephritis with ESBL E. coli, and carbapenemase-producing K. pneumoniae osteomyelitis and sepsis [8–10].
Here, we describe resolution of recurrent symptomatic UTI after FMT for RCDI in a woman who had been treated with nearly continuous antibiotics in the preceding 2 years. As common sources of morbidity, UTIs make significant contributions toward antibiotic resistance and antibiotic-associated infections. Though FMT may have changed provider behavior, post-FMT urine cultures were not indicated in the absence of further symptoms. Pre- or post-FMT stool cultures were not obtained for microbiota analysis. However, our case adds evidence for FMT as a safe and potentially efficacious intervention for the prevention and treatment of infections outside of C. difficile, suggesting a possible role for FMT in combatting antimicrobial resistance.
Acknowledgements
Potential conflicts of interest. The authors have no conflicts of interest.
References
- 1. Halpin AL, McDonald LC. Editorial commentary: the dawning of microbiome remediation for addressing antibiotic resistance. Clin Infect Dis 2016; 62:1487–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kelly CR, Khoruts A, Staley C et al. . Effect of fecal microbiota transplantation on recurrence in multiply recurrent Clostridium difficile infection: a randomized trial. Ann Intern Med 2016; 165:609–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. García-Fernández S, Morosini MI, Cobo M et al. . Gut eradication of VIM-1 producing ST9 Klebsiella oxytoca after fecal microbiota transplantation for diarrhea caused by a Clostridium difficile hypervirulent R027 strain. Diagn Microbiol Infect Dis 2016; 86:470–1. [DOI] [PubMed] [Google Scholar]
- 4. Bilinski J, Grzesiowski P, Sorensen N et al. . Fecal microbiota transplantation in patients with blood disorders inhibits gut colonization with antibiotic-resistant bacteria: results of a prospective, single-center study. Clin Inect Dis 2017; 65:364–70. [DOI] [PubMed] [Google Scholar]
- 5. Stripling J, Kumar R, Baddley JW et al. . Loss of vancomycin-resistant enterococcus fecal dominance in an organ transplant patient with Clostridium difficile colitis after fecal microbiota transplant. Open Forum Infect Dis 2015; 2:ofv078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Crum-Cianflone NF, Sullivan E, Ballon-Landa G. Fecal microbiota transplantation and successful resolution of multidrug-resistant-organism colonization. J Clin Microbiol 2015; 53:1986–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tariq R, Pardi DS, Tosh PK et al. . Fecal microbiota transplantation for recurrent Clostridium difficile infection reduces recurrent urinary tract infection frequency. Clin Infect Dis 2017; 65:1745–7. [DOI] [PubMed] [Google Scholar]
- 8. Stalenhoef JE, Terveer EM, Knetsch CW et al. . Fecal microbiota transfer for multidrug-resistant gram-negatives: a clinical success combined with microbiological failure. Open Forum Infect Dis 2017; 4:ofx047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Singh R, van Nood E, Nieuwdorp M et al. . Donor feces infusion for eradication of extended spectrum beta-lactamase producing Escherichia coli in a patient with end stage renal disease. Clin Microbiol Infect 2014; 20:O977–8. [DOI] [PubMed] [Google Scholar]
- 10. Freedman A, Eppes S. Use of stool transplant to clear fecal colonization with carbapenem-resistant enterobacteriaceae (CRE): proof of concept. Open Forum Infect Dis 2017; 1:ofu051. [Google Scholar]