Abstract
Background and Aims
Wildfires are common in seasonally dry parts of the world with a Mediterranean climate. Prescribed burning is used to reduce fuel load and fire risk, but often without reliable information on its effects. This study investigated the effects of prescribed burns in different seasons on Pterostylis revoluta, an autumn-flowering Australian terrestrial orchid, and its orchid mycorrhizal fungi (OMFs) to find the least damaging season for a prescribed burn.
Methods
Burns were conducted mid-season in spring and summer 2011 and autumn and winter 2012. Orchids were enumerated and measured during their flowering season in autumn 2011–2014 and mycorrhizal fungi were isolated before and after the burns in autumn 2011, 2012 and 2014. Micro-organisms isolated were characterized. DNA was extracted from the OMFs, and the internal transcribed spacer region was amplified by PCR. Amplicons were clustered by restriction fragment length polymorphism (RFLP), and representative amplicons were sequenced. OMF were tested for sensitivity to smoke water.
Key Results
The number of plants increased up to 4-fold and 90 % of plants became vegetative during this study. Isolation of mycorrhizal fungi increased and isolation of bacteria decreased. Before the burns, the main OMF isolated was unexpectedly Tulasnella calospora (Boud.) Juel. By 2014, after the burns, the expected Ceratobasidium sp. D.P. Rogers was the only OMF isolated in most burnt quadrats, whereas T. calospora was confined to a minority of unburnt ‘control’ and the ‘spring burn’ quadrats, which were also the only ones with flowering plants.
Conclusions
The decline in rainfall during 2010–2012 probably caused the switch from mainly flowering to mainly vegetative plants and the change in OMFs. Burning in spring to summer was less damaging to this orchid than burning in autumn to winter, which should be noted by authorities in fire management plans for fire-prone areas in which this orchid occurs.
Keywords: Fire, burn, smoke, season, orchid, Pterostylis, fungi, Tulasnella, Ceratobasidium, mycorrhiza, phenology, conservation
INTRODUCTION
Orchids
The Orchidaceae is one of the most species-rich of all plant families and also contains a large proportion of threatened species (Backhouse and Jeanes, 1995). The State of Victoria (south-eastern Australia) contains 28 % of Australia’s terrestrial orchid species, 13 % of which are Nationally Threatened (Duncan, 2012). Most threatened orchids have populations of <100 plants, and their conservation is one of the main challenges faced by managers of public lands, e.g. Parks Victoria and the Department of Environment, Land, Water and Planning (DELWP) and its predecessors (Coates et al., 2006; Coates and Duncan, 2009).
Most terrestrial orchid species in temperate, seasonally hot, dry habitats such as Victoria are seasonally deciduous and dormant geophytes (Rasmussen, 1995). Soon after seed dispersal, the above-ground parts die back, leaving the dormant tubers 4–5 cm underground (Weston et al., 2005). On the return of suitable conditions, the dormant tuber produces new roots, leaves and flowers, and eventually a replacement tuber. In some species, the parent plant produces multiple tubers and each produces a new vegetative plant. Such clonally reproducing species tend to occur in large clusters with few flowering plants, e.g. mat-forming species of Pterostylis (Backhouse and Jeanes, 1995; Jones, 2006). In some of these species, flowering is anecdotally more common after late summer rains, e.g. P. coccina and P. revoluta. In other orchids worldwide, the frequency of flowering plants varies with rainfall received up to a year before the flowering season (Wells, 1981; Hutchings, 1987; Inghe and Tamm, 1988; Light and MacConnaill, 1991; Sieg and King, 1995; Kery and Gregg, 2004). Similarly, Morrison et al. (2015) found that patterns of precipitation explained 77 % of variation in the proportion of flowering plants in Platanthera praeclara.
Orchid seeds normally require an appropriate orchid mycorrhizal fungus (OMF) for germination and rely on one or more OMFs for nutrition throughout their lives (Bidartondo and Read 2008; Smith and Read, 2008). The roots or stem-collars are colonized by the OMFs, forming hyphal coils (pelotons) inside cortical cells (Ramsay et al., 1986). The OMFs are mostly basidiomycetes (mostly Rhizoctonia-like fungi in green orchids) although there are examples of orchids that form mycorrhiza with ascomycetes and some basidiomycetes that also form ectomycorrhiza with woody plants (Selosse et al., 2004; Dearnaley, 2007; Smith and Read, 2008; Gebauer et al., 2016). There is generally a specific relationship between the genera of Australian terrestrial orchids and their OMFs, e.g. Pterostylis with OMFs in Ceratobasidium (Warcup, 1973, 1981; Bougoure et al., 2005; Bonnardeaux et al., 2007; Dearnaley, 2007; Irwin et al., 2007). Conservation of terrestrial orchid species therefore also implies the need to conserve their OMFs (Rasmussen, 1995; Perkins et al., 1995).
Fire
In Australia, as well as in other countries with a Mediterranean climate, high intensity wildfires are common in seasonally dry areas, mainly in summer (Gill et al., 1981; Department of Environment, Land, Water and Planning, 2017b). Low-intensity prescribed (control/controlled/fuel reduction) burning at times of lesser fire risk is used to reduce fuel load and fire risk (Wade et al., 1989; Haines et al., 1998), and sometimes to protect biodiversity (including threatened orchids) (Kilgore and Curtis, 1987; Wade et al. 1989; Coates and Duncan, 2009). Autumn is particularly favoured for prescribed burns because of the ease of ignition and still conditions (Department of Environment, Land, Water and Planning, 2017b).
The effects of prescribed burns at times when they do not occur naturally may be very different from wildfire because of the differences in phenology of the plants and the intensity of the fire (Gill et al., 1981; Whelan, 1995). Inappropriate times of prescribed burning, such as during the active growth of seasonally dormant orchids, were predicted to be detrimental (Bond and van Wilgen, 1996; DeBano et al., 1998; Quarmby, 1999; Threatened Species Section, 2006). Burning during dormancy of the endangered orchid P. praeclara avoided fire-triggered changes in flowering but maintained the grassland that it needed to survive (Morrison et al., 2015). Prescribed burns may also affect OMFs. Prescribed burning when the soil was moist rather than dry resulted in greater micro-organism mortality in chaparral soil (Dunn et al., 1985) and so could affect the survival and activity of OMFs. It is therefore important to investigate the least damaging season for orchids and their OMFs in which to conduct prescribed burning.
Smoke from fires (and smoke water, i.e. water through which smoke has passed) may have significant effects on orchids and their OMFs. Smoke and smoke water contain butenolides derived from the burnt plant material (Van Staden et al., 2004) that stimulate seed germination in many species (Dixon and Barrett, 2003; Flematti et al., 2004; Jones, 2006). Smoke also has antifungal properties (Parmeter and Uhrenholdt, 1975; Zagory and Parmeter, 1984; Alam et al., 1999; Chumpookam et al., 2012; Lin et al., 2012) and so may reduce the activities of important micro-organisms, including OMFs, as soil micro-organism abundance is greatest in the topsoil (Neary et al., 1999). Bacteria are more resistant to heat than fungi, and so may be less affected by fire (Fuller et al., 1955; Wright and Tarrant, 1957; Dunn et al., 1985; Vázquez et al., 1993). Reports on the effects of fire on mycorrhizal fungi other than OMFs vary greatly, from reductions (Reddell and Malajczuk, 1984; Klopatek et al., 1988, 1994), to no changes (Visser, 1995; Baar et al., 1999) to increases (Korb et al., 2003). Smoke water might therefore be expected to increase seed germination in some plant species and decrease the abundance of some OMFs in soil in burnt areas.
Orchids and fire
Little information (mostly anecdotal) is available on the effects of wildfire on terrestrial Australian orchid species and their mycorrhizal fungi. Fire was stated to stimulate the growth of tubers from dormancy and may explain the appearance of large vegetative clusters of some Pterostylis species after fire (Backhouse and Jeanes, 1995). In contrast, wildfire was reported as having no effect (Quarmby 1999), reducing plant numbers (Backhouse and Jeanes, 1995; Quarmby, 1999) or inhibiting flowering in Pterostylis spp. (Jones et al., 1999; Jones and Clements, 2002; Duncan, 2012). Emergence and flowering were reduced by 66 % after wildfire in the Nationally Vulnerable P. chlorogramma (Duncan, 2012). Fire may damage shallow tubers (Backhouse and Jeanes, 1995; Quarmby, 1999), reduce plant size or reduce the rate of reproduction (Keith, 1996; Weston et al., 2005). In contrast, Morrison et al. (2015) concluded from a 20 year monitoring study that prescribed burning just before active growth of Platanthera praeclara in the USA had no effect on flowering once changes in rainfall were considered. Also, Ramsay et al. (1986) reported that fire increased OMF infection and the viability of OMFs isolated from pelotons in root-infected species in south-western Australia.
Only two Australian studies have compared orchid populations quantitatively before and after prescribed burns in autumn, and both were on spring-flowering Nationally Endangered species in Victoria. Increased flowering was observed in Prasophyllum correctum (expected to host Ceratobasidium spp. as the OMF) 2 years after prescribed burning (Coates et al., 2006) and in Caladenia orientalis (expected to host Sebacina spp. as the OMF) (Coates and Duncan, 2009). Because such threatened orchids frequently have very small populations, direct experimentation and statistical analysis are limited. Studying the effect of prescribed burning at different seasons on common (secure) orchids can provide valuable information to land managers, who must enact prescribed burns in fire-prone areas, and help them to manage populations of threatened orchids.
Aims
This study aimed to find the answers to three important questions about prescribed burning at different seasons on the common Victorian autumn-flowering orchid Pterostylis revoluta R.Br.: what are the effects on plant numbers and size, on colonization of the orchids by their OMF and on growth of the OMF?
MATERIALS AND METHODS
Site and orchid selection
The Greater Bendigo National Park site (E144°15.814′, S36°51.646′) was selected for the study because it had large clusters of orchids, the trees were sparsely spread 5–8 m away from one other for a uniform vegetation burn and the orchids studied were close to the park boundary, which facilitated access for fire services to the site. The vegetation was classified as Ecological Vegetation Class (EVC) 4.1 Box-Ironbark Forest (Department of Environment, Land, Water and Planning Victoria, 2017a).
The common orchid Pterostylis revoluta R.Br. (Large Autumn Greenhood) was chosen because it typically forms large dense colonies and because there were anecdotal reports of fire affecting flowering (Jones et al., 1999; Jones and Clements, 2002; Duncan, 2012) and plant numbers (Backhouse and Jeanes, 1995), but no quantitative survey before and after a fire. Pterostylis revoluta is dormant in spring to summer, flowers in autumn (April–May) soon after emergence and becomes dormant at the end of winter (Backhouse and Jeanes, 1995). Each tuber usually forms a single vegetative or flowering shoot. Vegetative plants have only a basal rosette of leaves and reproduce vegetatively by multiplication of daughter tubers, whereas flowering plants have only a few small cauline leaves and a single flower that forms seed when pollinated (Backhouse and Jeanes, 1995). The location of the OMFs is the stem-collar in all Pterostylis species examined (Ramsay et al., 1986; Irwin et al., 2007). Fungi in the genus Ceratobasidium D.P. Rogers [principally C. cornigerum (Bourdot) D.P. Rogers] were the only mycorrhizal fungi reported in studies of 16 of the then 55+ Pterostylis species (including P. revoluta) in south-eastern Australia (Warcup, 1981), although Warcup (1973) noted that he had ‘rarely’ found Tulasnella J. Schröt.
Quadrats for plant surveys
Fifteen quadrats, each 0.67 × 2 m, were systematically selected during the flowering seasons in autumn 2011 to contain the maximum number of plants within colonies. Three replicate quadrats were used as unburnt controls and the other quadrats for three replicates of each of four seasonal burns (spring, summer, autumn or winter). The location, number and size of quadrats were limited by the patchiness of orchid distributions (Batty et al., 2001; Bonnardeux et al., 2007) and the requirements of the managing authorities [Parks Victoria, the Country Fire Authority (CFA) and DELWP and its predecessors].
Prescribed burns
Burns were conducted in three quadrats in the middle of each season for spring 2011 and summer, autumn and winter 2012 based on suitable and safe conditions as advised by the managing authorities (Table 1). Three (‘control’) quadrats were unburnt. A distance of 1–2 m outside the quadrats was raked (to reduce fuel load) and watered before ignition. The ground fire was ignited from the perimeter as normal using a drip torch containing a mixture of petrol and diesel. A thermal imaging camera (Fluke P3, USA) measured the maximum temperature of the fire. Soil temperature was measured at 4 cm depth 5 min before and after each burn in each quadrat using a data logging system (TI-Nspire with easy temperature sensor, Texas Instruments).
Table 1.
Details of simulated prescribed burns in each season in in quadrats containing Pterostylis revoluta
| Season of burn | Date of burn | Ambient temperature (°C) | Maximum fire surface temperature (°C) | Soil temperature at 4 cm below ground 5 min before and after burns (°C) | |
|---|---|---|---|---|---|
| Pre-burn | Post-burn | ||||
| Spring | 18 October 2011 | 21.0 | * | 14.3 | 21.8 |
| Summer | 24 January 2012 | 28.1 | 387.7 | 28.6 | 47.3 |
| Autumn | 27 April 2012 | 22.0 | 189.6 | 19.0 | 23.0 |
| Winter | 24 July 2012 | 12.0 | 142.1 | 11.0 | 11.5 |
*Spring burn fire surface temperature was not measured.
Plant number and size
Plants were surveyed during their flowering seasons before the burns in 2011 and after the burns in 2012–2014. Measurements recorded were numbers of leafy rosettes (‘vegetative plants’) and of stems with single flowers (‘flowering plants’). In 2011–2012, the plant height, stem width, flower length and flower width (flowering plants only), and leaf length and leaf width (vegetative plants only) were also measured. Leaf length and width were not recorded for flowering plants because they had only small acute cauline leaves. Quantitative data were analysed using Minitab 16 or 18 statistical software (http://www.minitab.com). Significance was recorded at P ≤ 0.05. Where data were normally distributed or could be normalized by transformation, they were analysed by the parametric nested analysis of variance (ANOVA). The non-parametric Kruskal–Wallis and the Mood median tests were used as well or instead for non-normalized distributions. Plant numbers and proportions of flowering and vegetative plants were also analysed by the χ2 test.
Isolation of microbes from pelotons
In 2011, before the burns, the stem-collar was collected from one randomly selected flowering plant per quadrat for isolation of microbes from pelotons (within the limitations on replication imposed by the supervising authorities). This was repeated in 2012 and 2014 after the burns, except that both vegetative and flowering plant samples were collected where possible. In 2012, flowering plants were absent in ‘summer burn’, ‘autumn burn’ and ‘winter burn’ quadrats, and so only three samples from vegetative plants were collected, but three samples from both flowering and vegetative plants were collected from the ‘control’ and ‘spring burn’ quadrats. In 2014, two collars (one from flowering and one from vegetative plants) were collected from each of the ‘control’ quadrats and one from each of the burnt quadrats.
Pelotons were cultured from each collar for each treatment in 2011, 2012 and 2014 as in Huynh et al. (2009) in plates of fungal isolation medium (Clements et al., 1986) without streptomycin. Each plate contained 10 ± 3 pelotons. Plates were sealed with Parafilm™ and incubated at 25 °C for 3 weeks. Fungi were stained with lactophenol cotton blue and examined microscopically. Outcomes from pelotons were categorized as: (1) Rhizoctonia-like fungi (hyaline hyphae with chains of monilioid cells): (2) contaminant or other sporing or pigmented fungi; (3) bacteria; or (4) dead/no growth (Warcup, 1973; Huynh et al., 2009). Isolated fungi were labelled in the following order: name of the plant (p = P. revoluta), treatment (b = burnt, u = unburnt), fungal collection year (1 = 2011, 2 = 2012), quadrat ID (c = control, sp = spring, s = summer, a = autumn, w = winter), plant number (1–6), fungal isolate (a–z), e.g. pb2sp2c (P. revoluta, burnt, 2012, ‘spring burn’ quadrat, plant 2, isolate c). Data on proportions of categories 1–4 were analysed as for plants.
Molecular analysis of fungi
Two to three isolates were randomly selected from each plant, treatment and year. Agar blocks of 5 mm per side were sub-cultured into malt extract broth and grown on a slow shaker until large fluffy balls developed. Mycelia were harvested, drained and 100 mg ground to a fine powder using liquid nitrogen. DNA was extracted using a Favor Prep™ Plant Genomic DNA Extraction Mini Kit (Favorgen, Taiwan) according to the manufacturer’s instructions.
The ITS (internal transcribed spacer) region of each fungus was amplified using the universal primers ITS1 and ITS4 (White et al., 1990). Each 25 μL reaction contained: 12.5 μL of Promega GoTaq Green Master Mix, 8.5 μL of nuclease-free water, 1 μL of ITS1 (10 μm), 1 μL of ITS4 (10 μm) and 2 μL containing 2–5 ng of genomic DNA or sterile nuclease-free water. A G-Storm thermocycler was programmed for initial denaturation at 94 °C for 10 min; 35 cycles of 30 s denaturation at 94 °C, 1 min annealing at 51 °C and 1 min extension at 72 °C; and a final extension of 10 min at 72 °C. Aliquots of 5 μL of the PCR products were separated by electrophoresis on a 1.4% agarose gel in 1× TBE or 1× TAE buffer at 80–100 V alongside a GeneRuler™ 100 bp DNA Ladder Mix (Fermentas, Germany). Amplicons were stained with ethidium bromide and imaged using a Gel Doc™ XR+ System with Image Lab™Software (Bio-Rad, Australia) with Quantity One software.
The ITS products were digested for 24 h with each of two endonucleases (HhaI and TaqI) (Fermentas, Progen Australia) according to the manufacturer’s instructions. Each reaction contained 10 U of enzyme, 5 μL of ITS-PCR product, 1 μL of 10× buffer and 10 μL of sterile MilliQ® water. Restriction digests were electrophoresed and visualized as before. Each fragment was scored as a binary value (presence or absence) and results were pooled to generate a score plot showing 13 main clusters using principal components analysis in Minitab.
For 13 isolates representative of clusters, the remaining ITS-PCR product was purified of the other reaction ingredients using a Qiagen QIAquick PCR Purification Kit according to the manufacturer’s instructions. Purified products were sequenced using the BigDye Terminator v3.1 Cycle Sequencing Kit protocol (Applied Biosystems) according to the manufacturer’s instructions. DNA was precipitated using the Applied Biosystems Ethanol precipitation protocol 1 and products were electrophoresed at Micromon (Monash University) (www.micromon.com.au). Sequences were entered into MEGA7 (Kumar et al., 2016) (http://www.megasoftware.net/mega.php), searched for matches using Blast 2.2.29 (Morgulis et al., 2008) on the NCBI (National Center for Biotechnology Information) (http://www.ncbi.nlm.nih.gov/) and the closest matches for each were downloaded through MEGA7. Sequences and their closest matches were aligned using ClustalW (Thompson et al., 1994) and the ends trimmed to an even length. Phylogenetic trees were constructed from the ClustalW alignment using the maximum likelihood method based on the Tamura–Nei model (Tamura and Nei, 1993) with 500 bootstrap pseudoreplicates in MEGA7. The identity of isolates as either Tulasnella calospora or Ceratobasidium sp. was deduced from the size of the ITS amplicon (about 100 bp larger in Ceratobasidium sp. than in T. calospora), the patterns of restriction fragment length polymorphism (RFLP) clustering and ITS sequencing. Sequences were deposited in GenBank with accession numbers MF407553–MF407565.
Effect of burns and smoke water on fungal growth
Rhizoctonia-like cultures were cut into 5 mm side agar blocks, sub-cultured on malt extract agar (Oxoid) and incubated at 25 °C in darkness for 3 weeks. Fungi were then sub-cultured on malt extract agar prepared and autoclaved with one of six dilutions of smoke water (Regen-2000®) at 0, 10, 100, 1000, 5000 and 10 000 μL L–1 in 9 cm diameter plastic Petri dishes. There were three replicates for each isolate and each treatment. The diameter of each mycelial colony was measured at right angles at 6 d after sub-culturing. Data on diameter of fungal growth were analysed as for plants.
RESULTS
Prescribed burns and rainfall
All ‘burn’ quadrats burned satisfactorily. The maximum surface temperature recorded was 387.7 °C (summer burn) and the minimum was 142 °C (winter burn) (Table 1). The soil temperature ranged from 11–29 °C at 5 min pre-burn to 11–47 °C at 5 min post-burn. The rainfall in 2010, the year before the study began, was more than twice the average and then declined by 60 % in 2011 to average rainfall during 2012–2014 (Supplementary Data Fig. S1].
Changes in orchid
In 2011, before the burns, orchid numbers per quadrat ranged from 17 to 35, and 36–74 % were flowering rather than vegetative (Fig. 1). By 2012, the numbers of vegetative plants had increased 4- to 6-fold in the unburnt ‘winter burn’ and burnt ‘spring burn’ quadrats but were unchanged in the others. Flowering plants had declined to only 0–15 % of the total and were absent from the unburnt ‘autumn burn’ and ‘winter burn’ quadrats (designated for burning but unburnt at that time) and burnt ‘summer burn’ quadrats. By 2013, after all quadrats had been burnt, compared with 2011 the numbers of total and vegetative plants had increased 4- to 6-fold in the ‘winter burn’ quadrats and more than doubled in the ‘spring burn’ quadrats, but were unchanged in the others; flowering plants comprised only 1–9 % of the total. By 2014, compared with 2011 numbers had increased 3- to 10-fold in total plants, 6- to 25-fold in vegetative plants and plants had become almost entirely vegetative (only 0–3 % flowering). Excavation in 2014 of the few plants allowed under the permit showed that some tubers outside the quadrats had abnormally produced two to three shoots (Supplementary Data Fig. S2), which looked like single overlapping plants above-ground; some tubers had produced both flowering and vegetative shoots.
Fig. 1.
Numbers of Pterostylis revoluta (mean ± s.e.) flowering and vegetative plants at 2011–2014 surveys. Note the different y-axis scales in figure parts. ‘Control’ quadrats had no burn in any year. *Quadrats burnt before the time of the survey. Bars on columns = 2 ×s.e. Means that do not share a letter are significantly different by Fisher’s test at P = 0.05. ANOVA: total plants = 9.62, P < 0.001; flowering plants = 34.38, P < 0.001; vegetative plants = 13.86, P < 0.001. LSD0.05: total plant number 84, flowering plant number 4.5, vegetative plant number 83. χ2 between years: total plant number = 489.34, P < 0.001, flowering plant number = 63.14, P < 0.001, vegetative plant number = 501.42, P < 0.001; for proportions of flowering vs. vegetative plants for: ‘control’ = 286.00, P < 0.001; ‘spring burn’ = 308.50, P < 0.001; ‘summer burn’ = 338.54; P < 0.001; ‘autumn burn’ = 140.61, P < 0.001; ‘winter burn’ = 768.02, P < 0.001.
Multiple regression analysis showed that the rainfall in the same or previous year affected the numbers of flowering and vegetative plants (P < 0.001–0.004). Burning by itself did not affect the numbers of total, flowering or vegetative plants (P = 0.359, 0.483 and 0.466, respectively). Season of burn and year affected all three parameters. Summer burns had no effect on any parameter (P = 0.949, 0.439 and 0.778, respectively). Burns in winter (P = 0.008 and 0.004) or spring (P = 0.013 and 0.006) significantly increased numbers of total and vegetative plants, respectively. Burns in autumn and winter significantly decreased flowering plant numbers (P = 0.001 and 0.020, respectively) whereas the spring burn significantly increased them (P = 0.003). The proportion of plants flowering decreased from 62 % to 1 % during 2011–2014 (χ2 for proportions among years = 57.872, P < 0.001). The optimum model from multiple regression explained 88 % of the variation in the data and showed that the proportion of flowering plants was significantly affected by rainfall in the same or previous year (P < 0.001) and by the autumn and winter burns (P < 0.001).
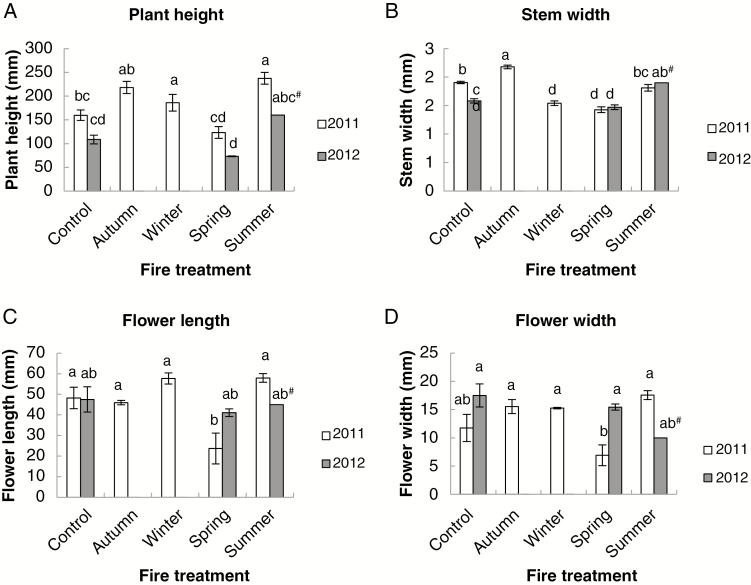
Only small changes occurred in the size of flowering plants during 2011–2012. Plant height did not change in any quadrat between 2011 and 2012 where flowering plants were present (Fig. 2). Stem width decreased in the ‘control’ quadrats in 2012, but was unchanged in others with flowering plants. Flower length did not change between years, but flower width increased in burnt ‘spring burn’ quadrats.
Fig. 2.
Plant data measurements for Pterostylis revoluta plants in 2011 pre-burn and 2012 post-burn quadrats (‘control’, ‘autumn burn’ and ‘winter burn’ quadrats had no burn by the flowering season in 2013). Data are the mean ± s.e. (A) Plant height (recorded as zero for vegetative plants), (B) stem width, (C) flower length, (D) flower width. Bars on columns = 2 × s.e. ANOVA: plant height F = 20.51, P < 0.001; stem width F = 38.07, P = 0.001; flower length F = 6.30, P = 0.002; flower width F = 5.91, P = 0.003. LSD0.05 from ANOVA: 43.357 (plant height), 0.149 (stem width), 15.456 (flower length), 5.257 (flower width). Means that do not share a letter are significantly different by Tukey’s family error test at P = 0.05. #The estimate was unreliable as there was only one flowering plant.
Changes in isolation of microbes from pelotons
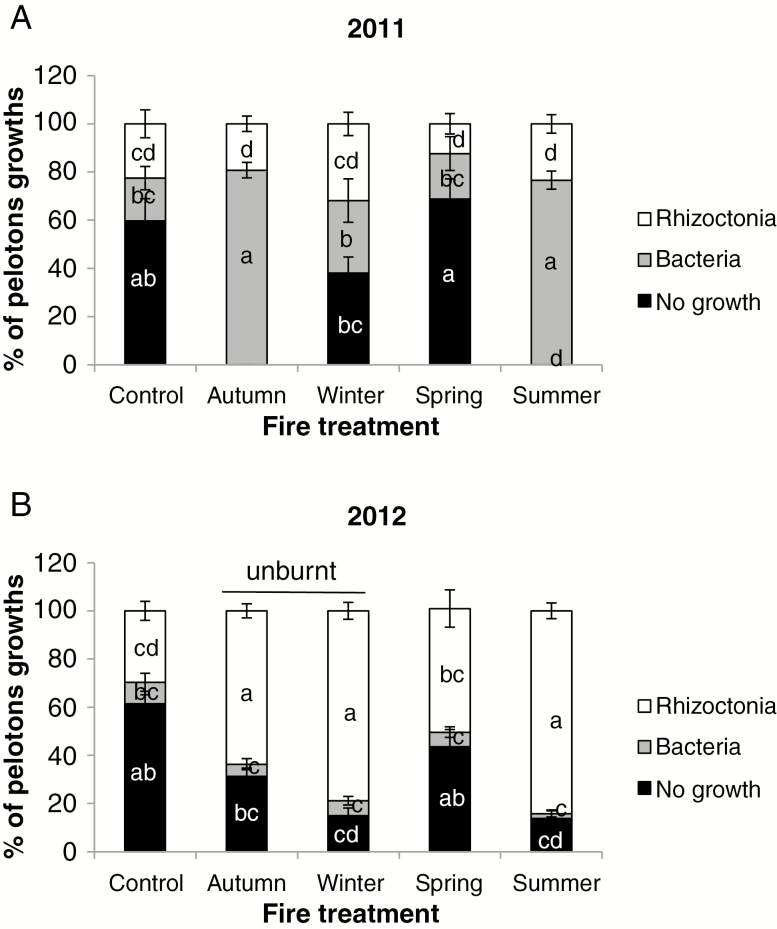
Pelotons were observed in the collars, and pelotons grew fungi and bacteria within 5 d (Fig. 3). In 2011, 10–30 % of pelotons from flowering plants (only) grew Rhizoctonia-like fungi and the remainder were dominated either by bacteria (20–80 %) or by no growth (0–70 %). In 2012, all treatments apart from the ‘control’ more than doubled the proportions of pelotons from which Rhizoctonia-like fungi were isolated (51–84 %) and more than quartered the proportions growing bacteria (2–6 %); however, this occurred in the unburnt ‘autumn burn’ and ‘winter burn’ treatments as well as in the burnt ‘spring burn’ and ‘summer burn’ treatments. In 2012, pelotons from vegetative plants grew mainly Rhizoctonia-like fungi (74 %) whereas those from flowering plants mainly had no growth (67 %) (Table 2). There was no difference between flowering and vegetative plants in the small proportion of pelotons that grew bacteria (5–7 %) and no ‘other fungi’ were isolated.
Fig. 3.
Isolation of micro-organisms in 2011 and 2012 from five treatments (‘control’, ‘spring burn’, ‘summer burn’, ‘autumn burn’ and ‘winter burn’) from Pterostylis revoluta plants. Data are the mean ± s.e. ‘Control’ treatments had no burn. Sampling in ‘autumn burn’ and ‘winter burn’ quadrats was before 2012 burns. Bars on columns = 2 × s.e. Means that do not share a letter are significantly different by Tukey’s family error test at P = 0.05. LSD0.05 from ANOVA in Rhizoctonia-like fungi = 17.01, other fungi = N/A (all zero), bacteria = 13.860, no growth = 18.598. χ2 among seasons: 2011 = 224.079, P < 0.001; 2012 = 100.429, P < 0.001; 2011 vs. 2012: ‘control’ = 0.549, P = 0.760; ‘spring burn’ = 36.430, P < 0.001; ‘summer burn’ = 119.978, P < 0.001; ‘autumn burn’ = 122.560, P < 0.001; ‘winter burn’ = 45.882, P < 0.001.
Table 2.
Isolation (%) of micro-organisms from pelotons of flowering and vegetative plants of Pterostylis revoluta in ‘spring burn’ quadrats in 2012
| Isolates from pelotons | Plant reproductive status | |
|---|---|---|
| Flowering | Vegetative | |
| Rhizoctonia-like fungi | 28.2 ± 9.8b | 74.4 ± 8.7a |
| Other fungi | 0.0 | 0.0 |
| Bacteria | 4.6 ± 1.8a | 7.5 ± 4.1a |
| No growth | 68.1 ± 9.4a | 19.1 ± 6.0b |
Data are the mean ± s.e. Means within a row that do not share a letter are significantly different by Tukey’s family error test at P = 0.05.
LSD0.05 from ANOVA: Rhizoctonia-like fungi = 26.797, no growth = 23.306.
χ2 for proportions between flowering and vegetative plants = 49.035, P < 0.001.
Molecular analysis of fungi
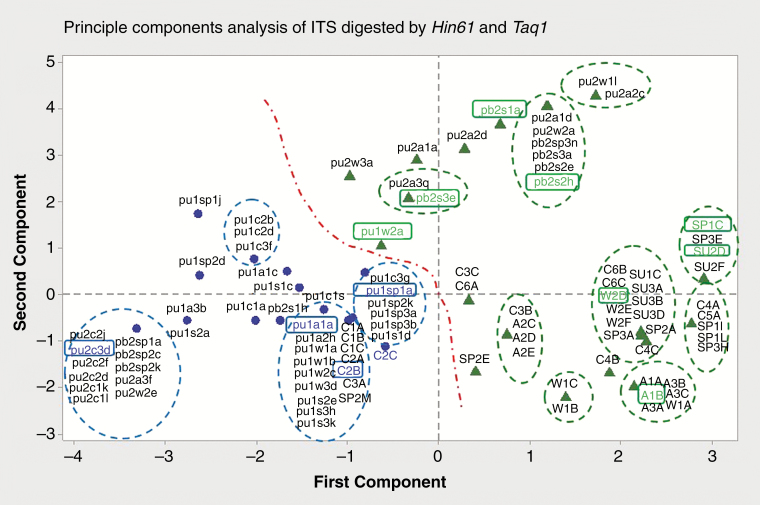
Internal transcribed spacer amplicons were produced from all fungi and RFLP fragments were produced with TaqI and Hin61 restriction enzymes (Supplementary Data Figs S3 and S4). Principal component analysis separated isolates into several main clusters (Fig. 4). Isolates from 2011 pre-burn sampling were clustered to the left. Isolates from 2012 were almost evenly divided between the left and right sides. All isolates from the 2014 ‘control’ quadrats clustered to the left while the remainder clustered to the right along with all isolates (except one) from burnt quadrats.
Fig. 4.
Principal components analysis of RFLP patterns of P. revoluta isolates from 2011–2014. Isolates from 2011–2012 were labelled as follows (lower case): (1) orchid (p = P. revoluta), (2) treatment (u = pre-burn, b = post-burn), (3) year of burn (1 = 2011, 2 = 2012), (4) season of burn (c = control, a = autumn, sp = spring, s = summer, w = winter), (5) plant replicate (1 = plant 1, 2 = plant 2, 3 = plant 3), (6) isolate identification (a–z). Isolates from 2014 were labelled as follows (upper case): (1) treatment (C = control, A = autumn, SP = spring, SU = summer, W = winter), (2) plant replicate (1 = plant 1, 2 = plant 2, 3 = plant 3, etc.), (3) isolate identification (A–Z). Coloured rectangles show sequenced isolates: blue = Tulasnella calospora, green = Ceratobasidium sp. Dotted ovals indicate boundaries of clusters. The central red dot-dash line indicates the boundary between isolates of different genera.
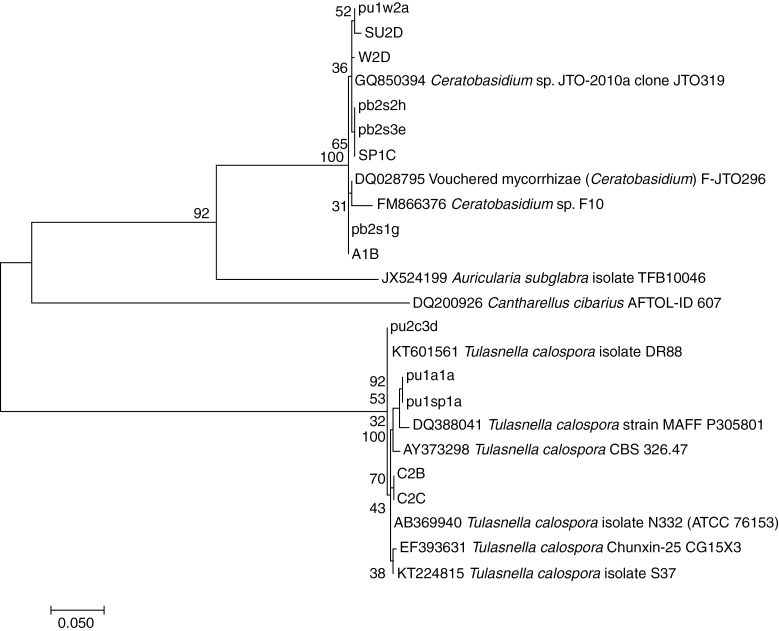
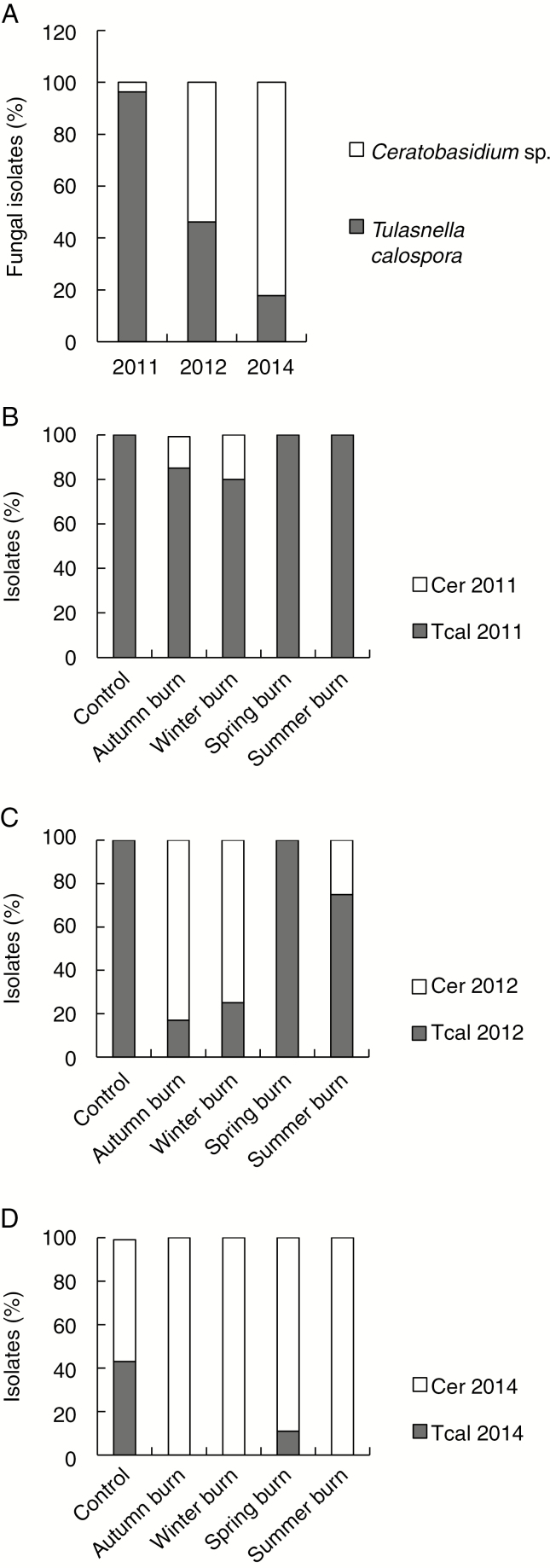
The ITS sequences of representative isolates from the clusters showed the closest matches (98–99 %) in GenBank to those of either T. calospora or Ceratobasidium sp. (Fig. 5). Most of the isolates from 2011 pre-burn sampling had closest matches to T. calospora; this declined progressively to 46 % in 2012 and only 18 % in post-burn sampling in 2014 (Fig. 6A). Ceratobasidium occupancy in individual treatments quickly progressed from a minority to a majority, and then the only isolates from quadrats burnt in summer, autumn and winter (Fig. 6B–D). By 2014, fungi with closest matches to T. calospora were isolated only in 11 % of ‘spring burn’ quadrats and 44 % of ‘control’ quadrats, which were the only quadrats with flowering plants. Multiple regression analysis showed that a majority of Ceratobasidium rather than T. calospora was associated with increased numbers of total and vegetative plants (P = 0.007 and P < 0.001, respectively) and decreased numbers of flowering plants (P < 0.001). However, flowering plants were not exclusively associated with T. calospora and vegetative plants with Ceratobasidium sp., e.g. plant 3 in the control treatment in 2014 was vegetative, but both T. calospora and Ceratobasidium sp. were isolated from pelotons.
Fig. 5.
Relationships of ITS sequences from 2011–2014 isolates from Pterostylis revoluta to those in GenBank. The evolutionary history was inferred by using the maximum likelihood method based on the Tamura–Nei model (Tamura and Nei, 1993). The tree with the highest log likelihood (–1665.26) is shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor–Joining and BioNJ algorithms to a matrix of pairwise distances estimated using the maximum composite likelihood (MCL) approach, and then selecting the topology with the superior log likelihood value. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. The analysis involved 24 nucleotide sequences. Codon positions included were first + second + third + non-coding. All positions containing gaps and missing data were eliminated. There were 344 positions in the final data set. Evolutionary analyses were conducted in MEGA7 (Kumar et al., 2016).
Fig. 6.
Changes in relative isolation of Tulasnella calospora (Tcal) and Ceratobasidium sp. (Cer) during 2011–2014. (A) Changes in all burn seasons in 2011–2014, (B–D) changes among burn seasons in each year. Kruskal–Wallis for percentage of vegetative plants vs. fungus, H = 17.02, P < 0.001. χ2 for percentage of Ceratobasidium among years = 41.780, P < 0.001; for proportions among treatments = 161.469, P < 0.001. Pearson correlations: between percentage of Ceratobasidium and percentage flowering r = –0.677, P = 0.006; between percentage of Ceratobasidium and percentage vegetative r = 0.676, P = 0.006; between percentage flowering and percentage vegetative r = –0.591, PO = 0.020.
Effects of burns and smoke water on growth of Rhizoctonia-like fungi
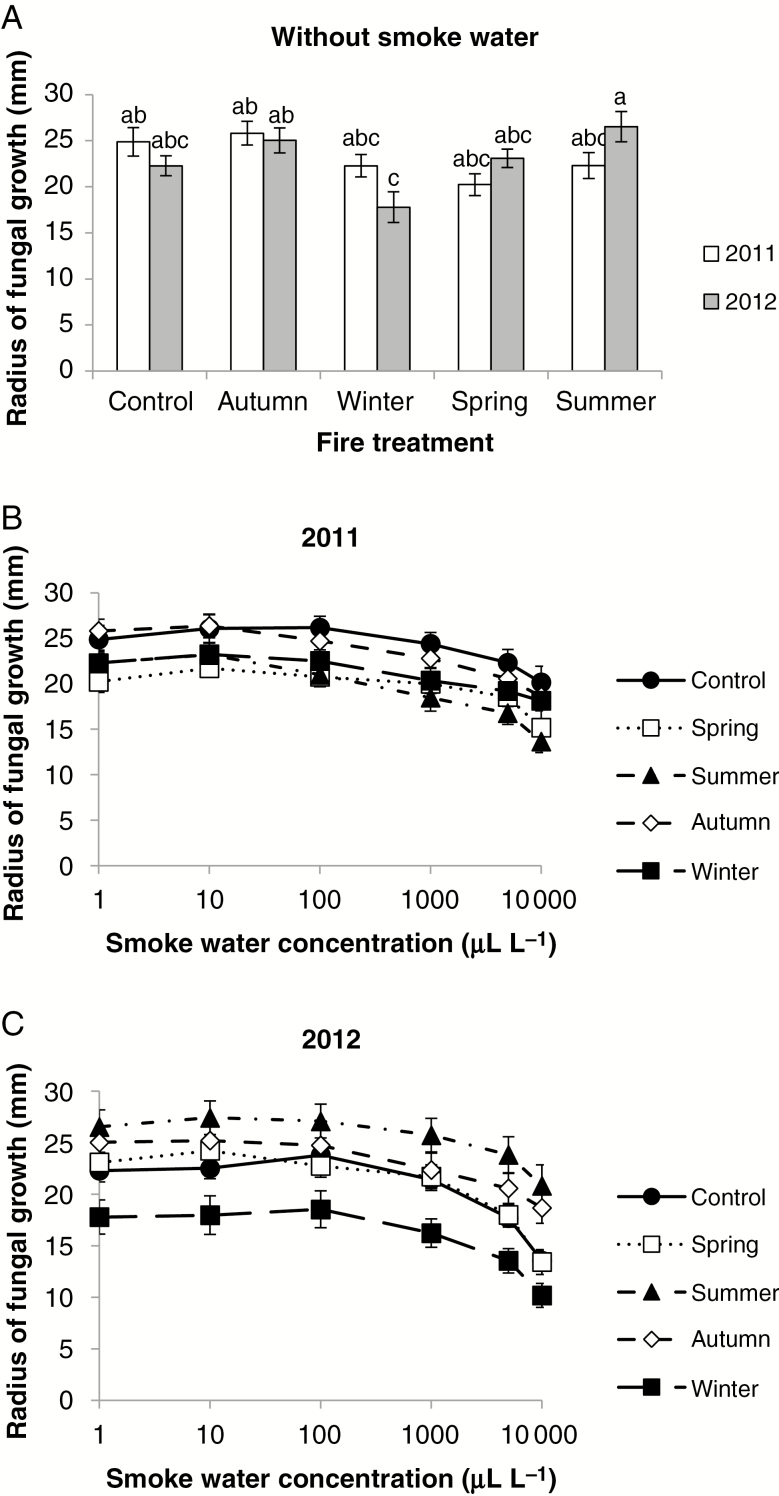
Without smoke water, the growth of fungi from all treatments was unchanged by any burn (Fig. 7A). The effect of smoke water was dose dependent, but fungal growth was reduced (by 19–43 %) only at 5000–10 000 µL L–1 (Fig. 7B, C). There was a significant interaction (ANOVA) between fire treatment and smoke water concentration. In 2011, fungal growth inhibition was greatest (39 %) in fungi from the ‘summer burn’ quadrats and least in fungi from the ‘control’ quadrats (19 %). In 2012, fungal growth inhibition was greatest (43 %) in fungi from the unburnt ‘winter burn’ quadrats and least (22 %) in fungi from the burnt ‘summer burn’ quadrats.
Fig. 7.
Effect of smoke water, burn and season of burn on growth of Rhizoctonia-like fungi isolated from Pterostylis revoluta on malt extract agar after 6 d growth. Data are the mean ± s.e. (A). 2011 vs. 2012 sampling, without smoke water, (B) 2011 sampling, (C) 2012 sampling. Samples from ‘spring burn’ and ‘summer burn’ quadrats in 2012 are post-burn; all others are pre-burn. Bars = 2 × s.e. Without smoke water, between-fire treatments ANOVA, F = 3.88, P < 0.001, LSD0.05 = 4.4; Mood median test, χ2 = 17.11, P = 0.047. Nested ANOVA: fire treatment, F = 4.903, P < 0.001; smoke water, F = 5.001, P < 0.001. LSD0.05 = 6.6. Two-way ANOVA: fire treatment, F = 24.52, P < 0.001; smoke water concentration, F = 46.11, P < 0.001; interaction F = 0.43, P = 1.000. LSD0.05 = 6.6.
DISCUSSION
This is the first long-term study to investigate the effect of prescribed burning at different seasons on autumn-flowering orchids using quantitative data before and after the burns. There were large changes in plant numbers, flowering and OMFs isolated from plants throughout the study. Were the large changes observed in the orchids the consequences of changes in rainfall, OMFs and season of burning, or were they merely coincidences?
Orchids
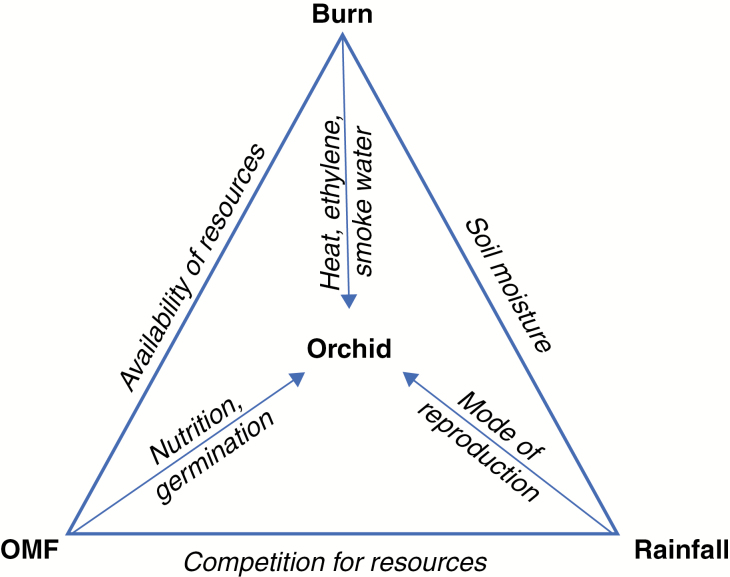
During 2011–2014, the number of P. revoluta plants increased up to 25-fold and the proportion of flowering orchids declined from 64 % in 2011 to ≤3 %. The increase in P. revoluta numbers after the prescribed burns is the opposite to the reported decline in plant numbers after wildfire in two species of Pterostylis in South Australia (Quarmby, 1999) and P. chlorogramma (Duncan, 2012), which was attributed to the shallowness of the tubers and their damage by fire (Quarmby, 1999). However, the subsequent increase in mainly vegetative plant numbers is like that noted by Quarmby (1999) and Backhouse and Jeanes (1995). The observed changes in P. revoluta phenology may have been caused by a combined effect of three factors with statistically significant effects: changes in rainfall (and consequently soil moisture), burning at different seasons and changes in OMFs, as summarized in Fig. 8.
Fig. 8.
Interactions of the orchid Pterostylis revoluta with possible factors in phenological changes during 2011–2014.
Rainfall
Changes in rainfall are a likely cause of the decrease in the numbers and proportion of flowering plants observed in this study. Increases in total and vegetative plants were also consistently associated with rainfall in the same or previous year. Late summer rain has previously been noted anecdotally to promote flowering in P. revoluta, with flowering rare during drought conditions (Backhouse and Jeanes, 1995). The rainfall in 2010, the year before the study began, was more than twice the average and then declined by 60 % in 2011 to average rainfall during 2012–2014. The large proportion of plants flowering in 2011 may have been unusual and related to the larger than normal rainfall in the previous year (2010). Flowering in some other orchid species is also sensitive to variation in rainfall; flowering was enhanced if greater rainfall was received before the flowering season and reduced if less rainfall was received in the same or previous year (Wells, 1981; Hutchings, 1987; Inghe and Tamm, 1988; Light and MacConnaill, 1991; Sieg and King, 1995; Kery and Gregg, 2004).
Seasonal burns
Burns would be expected to produce heat and ethylene and result in smoke water in the soil. Heat damage to underground parts of the plant by burning was unlikely during any season in the site studied here because the average soil temperature during burns, at the level of the stem-collars that the OMFs occupied, did not rise much above ambient, even in summer, as found by Morgan (1999). Therefore, ethylene or compounds in smoke water are more likely to have stimulated seed germination or growth from tubers, directly or indirectly through their effects on OMFs.
The early increases in numbers of total and vegetative plants after winter and spring burns may be due more to the effects of smoke or smoke water rather than to the temperatures reached. Smoke water has many stimulant effects, including flowering and seed germination, in many plant species (Dixon et al., 1995; Flematti et al., 2004; Morrison et al., 2015). Smoke water may have stimulated dormant tubers to emerge, seeds to germinate or protocorms to develop into leafy plants (Jones, 2006; Chumpookam et al., 2012). The abnormal production of multiple shoots per tuber (as noted in 2014) would have accelerated the increase in apparent plant number and may also have been caused by ethylene produced during the fire (Jeanes and Backhouse, 1995; Brown et al., 2008). Further research into the effect of smoke water on the development of P. revoluta may clarify this.
The greatest changes from burning would be expected in P. revoluta by burning in autumn to winter (when the plants are active above-ground) and the least changes by burning in spring to summer (when the plants are dormant underground). Morrison et al. (2015) observed that burning avoided fire-triggered changes in flowering when the endangered orchid P. praeclara was dormant. However, the numbers and proportions of flowering plants of the P. revoluta decreased and the number and proportion of vegetative plants increased in all treatments, including the control, suggesting that burning had less effect than changes in rainfall and OMFs. Also, the differences between burn treatments had disappeared by 2014 (2–3 years after the burns), suggesting that changes were relatively short lived and induced less by burns and seasons than by changes in rainfall and OMFs.
OMFs
Increases in the numbers of vegetative and total plants were associated with the replacement of T. calospora by Ceratobasidium sp. This may have led to the germination of dormant orchid seed, as C. cornigerum germinated seeds of Pterostylis spp. whereas Tulasnella spp. (including T. calospora) did not (Warcup, 1973; Bonnardeaux et al., 2007). The emergence of vegetative seedlings could explain the rapid increase in vegetative plant number, starting in 2012 and continuing through to 2014. Alternatively, stimulation of vegetative reproduction (including multiple plants from single tubers) through Ceratobasidium sp. (the most common symbiont in Pterostylis spp.) may have increased the proportion of tubers producing emergent growth through greater nutrition. The change in OMF could also have contributed to the change from flowering to vegetative growth, as flowering occurred only in the quadrats with plants occupied by T. calospora (alone or with Ceratobasidium). Rhizosphere micro-organisms (including Rhizoctonia) produce auxins, which can affect many aspects of plant growth and reproduction (Tsavkelova et al., 2006). Several previous studies have shown differences in symbiotic effectiveness and orchid development and phenology even among strains of the same species of OMF (e.g. Rasmussen, 1995; Huynh et al., 2009).
Changes in OMFs
The possible causes of the replacement of T. calospora by Ceratobasidium sp. are the decreases in rainfall, decreases in available nutrients as litter was consumed by fire and competition for the available resources, including the orchids (Fig. 8).
Rainfall may also be the main driving force behind the change in the OMF isolated from the orchids. Changes in rainfall were also associated with the replacement of one ISSR type of Tulasnella by another in the orchid Goodyera pubescens during drought (McCormick et al., 2006). Similarly, Kartzinel et al. (2013) found that a decrease in seasonal precipitation correlated with a decrease in diversity of the Tulasnellaceae in a tropical epiphytic orchid. Ceratobasidium sp. may have been outcompeted by T. calospora during the abnormally large rainfall in 2010–2011, and the re-occupancy of the orchids by Ceratobasidium sp. during more ‘normal’ rainfall may represent the restoration of the ‘normal’ OMF of Pterostylis spp. (Warcup, 1981; Bougoure et al., 2005; Dearnaley 2007; Bonnardeux et al., 2007).
During the transition in OMF from T. calospora to Ceratobasidium, both T. calospora and Ceratobasidium sp. were isolated from pelotons in some individual plants. This is unlike previous reports that only one OMF occupied an orchid at any one time (McCormick and Jacquemyn, 2004; Shefferson et al., 2005; McCormick et al., 2006; Roche et al., 2010). However, the common Australian orchid Microtis intermedia formed mycorrhiza with fungi of both the Sebacinales and the Ceratobasidiaceae (Bonnardeaux et al., 2007), and Cypripedium californicum contained fungi in the Tulasnellaceae, Sebacinaceae and Ceratobasidiaceae (Shefferson et al., 2005).
The presence of both T. calospora and Ceratobasidium sp. in individual plants suggests that adult plants of P. revoluta may be non-specific at the study site. The ability to form symbioses with the OMFs available in changing conditions would be an ecological advantage in the variable climate in south-eastern Australia (Swarts and Dixon, 2009). Jacquemyn et al. (2010) argued that stressful sites with shortages of water and nutrients should result in a prevalence of generalist orchids that symbiose with whatever OMFs are available at the site. Although seed germination experiments (Warcup, 1973; Bonnardeaux et al., 2007) showed specificity of Pterostylis spp. to Ceratobasidium spp., common widespread adult orchids may have broader symbiotic capabilities and be able to use the OMFs available as conditions change. Extraction of DNA from the litter and soil around the orchid, as in Voyron et al. (2017), may solve the question of whether only one or both are present and (if the latter) if one is preferred depending on environmental conditions. However, T. calospora was found in pelotons inside 40 % of plants of Anacamptis morio but not in the associated soil and litter, although other tulasnelloid and ceratobasidioid fungi were detected (Voyron et al., 2017), and so its apparent absence from soil and litter would not be conclusive.
The discovery of fungi in the T. calospora complex as the dominant (96 %) OMF in 2011 was unexpected, as Warcup (1973) noted that he had only ‘rarely’ found Tulasnella. in Pterostylis species. Ceratobasidium (principally C. cornigerum) was almost the only mycorrhizal genus named in studies of 16 of the then 55+ Pterostylis species (including P. revoluta) in south-eastern Australia (Warcup, 1981). Since then, members of the Ceratobasidiaceae have been the only OMF reported from species of Pterostylis (Bougoure et al., 2005; Bonnardeux et al., 2007; Dearnaley, 2007; Irwin et al. 2007). Tulasnella calospora has previously been isolated and identified as the main OMF in Australia from species of 11 genera other than Pterostylis, principally Acianthus, Diuris, Thelymitra and Dendrobium (Warcup, 1971, 1973, 1981; Bougoure et al., 2005; Smith et al., 2010) (summarized in Linde et al., 2017). Worldwide, T. calospora has also been noted from regions with a wide range of rainfall as the dominant OMF from a wide variety of orchids, both epiphytic (Suárez et al., 2006; Yuan et al., 2010; Nogeira et al., 2014; Yu et al., 2015) and terrestrial (Hadley, 1970; Masuhara and Katsuya, 1994; McCormick et al., 2004; Ding et al., 2014; Voyron et al., 2017). Hadley (1970) considered T. calospora to be a universal orchid symbiont, and this may explain its occupancy of P. revoluta under wetter than average conditions, though Kottke et al. (2013) showed that orchids shared OMFs regardless of environmental conditions. Further research is needed on the OMFs occupying Pterostylis species to clarify the role of soil moisture on occupancy.
Changes in microbial isolation from orchids
Between 2011 and 2012, the proportion of OMF isolates increased while the proportion of bacterial isolates decreased in all but the ‘control’ quadrats. This was the opposite of the expected increase after burns, as bacterial populations were expected to decrease less than fungal populations in fires (Fuller et al., 1955; Wright and Tarrant, 1957; Dunn et al., 1985; Vázquez et al., 1993). However, bacterial populations may have been unusually large in 2011 because a wet or moist soil encourages bacterial growth (Wilkinson et al., 1989) and isolations of bacteria decreased as rainfall decreased to the average in 2012. The proportion of OMF isolates was greater from vegetative than flowering plants and so the greater proportion of vegetative plants in 2012 may have led to a greater proportion of OMF isolates, as in Caladenia formosa (Huynh et al., 2004, 2009).
Burning affected the replacement of T. calospora by Ceratobasidium, as the proportion of Ceratobasidium infection progressed more rapidly in ‘burnt’ than ‘control’ quadrats. Fire generally reduced soil fungal populations (Wright and Tarrant, 1957; Renbuss et al., 1972; Vázquez et al., 1993) irrespective of season and moisture levels (Saravanan et al., 2013). However, both OMFs isolated from P. revoluta were equally and relatively insensitive to smoke water, as growth declined by only 19–43 % at 5000–10 000 µL L–1. In comparison, only 3 % (3000 µL L–1) smoke water caused structural damage in Pythium species (Lin et al., 2012). The relative insensitivity to smoke water of both OMFs isolated from P. revoluta may have led to them outcompeting more inhibited fungi for available nutrients and so may be a cause of the increased numbers of P. revoluta after fire.
Conservation implications of this study
The timing of a burn is critical for the re-emergence of a plant (Jones and Laude, 1960). While for the autumn-flowering P. revoluta the least damaging time for a prescribed burn was spring to summer, it grows along with several spring-flowering orchids in Greater Bendigo National Park that are active in spring and for which fire would be very damaging. Burning during the common dormant season for all orchids in late spring to summer is preferable to burning at other times for conservation of the orchids on this site. Following this research, Parks Victoria now conducts burns in late spring whenever possible. Further similar studies on other species and over a longer time are needed to base management practices on scientific study of their effects on the flora that such reserves are intended to conserve.
Conclusions
Changes in rainfall may have caused rapid increases in plant numbers, reductions in the proportion of flowering plants and changes in the dominant OMF in P. revoluta from T. calospora to Ceratobasidium sp. in 2011–2014. Burning during the season of active emergent growth (autumn) led to greater proportions of OMF isolation, but this may be because of greater proportions of vegetative plants. Both OMFs (T. calospora and Ceratobasidium sp.) were relatively insensitive to burning and smoke water, but Ceratobasidium occupancy proceeded faster in burnt quadrats. Prescribed burning late in spring is therefore preferable to burning in autumn or winter.
SUPPLEMENTARY DATA
Supplementary data are available at https://academic.oup.com/aob and consist of the following. Figure S1: annual rainfall and mean maximum and minimum temperatures in 2008–2014 at Bendigo Airport (Australian Government Bureau of Meteorology, 2017). Figure S2: abnormal growth of vegetative and flowering stems from single tubers of Pterostylis revoluta. Figure S3: patterns of Pterostylis revoluta isolates from 2011-2012 from pre-burn (A) and post-burn (B) plots cleaved with TaqI and or Hin61. Figure S4: patterns of Pterostylis revoluta isolates from 2014 cleaved with Hin61 and TaqI.
ACKNOWLEDGEMENTS
T.H. designed the study; all authors contributed to field and laboratory work, analysed the data and wrote the paper. N.J. thanks RMIT University for a scholarship. This work was supported by the Australian Orchid Foundation and the Helen Macpherson Smith Trust. All authors thank DELWP Bendigo (especially Julie Whitfield and Russell Brown) for invaluable field support; the CFA Bendigo for effective and safe conduct of the seasonal burns; and undergraduate student volunteers for assistance with field and laboratory work in 2014. This research was conducted under DELWP Permit No. 10004525.
LITERATURE CITED
- Alam S, Alam MS, Mahal F. 1999. Growth inhibition (in vitro) of chilli fruit rot pathogen Alternaria tenuis. Journal of the Asiatic Society for Bangladeshi Science 25: 211–216. [Google Scholar]
- Australian Government Bureau of Meteorology 2015. Climate data online. Station 081123 (Bendigo Airport). http://www.bom.gov.au/climate/data (4 September 2017). [Google Scholar]
- Baar J, Horton TR, Kretzer A, Bruns TD. 1999. Mycorrhizal colonization of Pinus muricata from resistant propagules after a stand‐replacing wildfire. New Phytologist 143: 409–418. [Google Scholar]
- Backhouse GN, Jeanes J. 1995. Orchids of Victoria.Carlton: The Miegunyah Press at Melbourne University Press. [Google Scholar]
- Batty AL, Dixon KW, Brundrett M, Sivasithamparam K. 2001. Constraints to symbiotic germination of terrestrial orchid seed in a Mediterranean bushland. New Phytologist 152: 511–520. [DOI] [PubMed] [Google Scholar]
- Bidartondo MI, Read DJ. 2008. Fungal specificity bottlenecks during orchid germination and development. Molecular Ecology 17: 3707–3716. [DOI] [PubMed] [Google Scholar]
- Bond WJ, van Wilgen BW. 1996. Fire and plants. Vol. 14 in Population and Community Biology Series. London: Chapman and Hall. [Google Scholar]
- Bonnardeaux Y, Brundrett M, Batty A, Dixon K, Koch J, Sivasithamparam K. 2007. Diversity of mycorrhizal fungi in terrestrial orchids: compatibility webs, brief encounters, lasting relationships and alien invasions. Mycological Research 111: 51–61. [DOI] [PubMed] [Google Scholar]
- Bougoure JJ, Bougoure DS, Cairney JWG, Dearnaley JDW. 2005. ITS-RFLP and sequence analysis of endophytes from Acianthus, Caladenia and Pterostylis (Orchidaceae) in southeastern Queensland. Mycological Research 109: 452–460 [DOI] [PubMed] [Google Scholar]
- Brown AP, Dundas P, Dixon KW, Hopper SD. 2008. Orchids of Western Australia. Nedlands: University of Western Australia Press. [Google Scholar]
- Chumpookam J, Lin HL, Shiesh CC, Ku KL. 2012. Effect of smoke water on seed germination and resistance to Rhizoctonia solani inciting papaya damping-off. Horticulture NCHU 37: 13–29. [Google Scholar]
- Clements MA, Muir H, Cribb PJ. 1986. A preliminary report on the symbiotic germination of European terrestrial orchids. Kew Bulletin 41: 437–445. [Google Scholar]
- Coates F, Lunt ID, Tremblay RL. 2006. Effects of disturbance on population dynamics of the threatened orchid Prasophyllum correctum DL Jones and implications for grassland management in south-eastern Australia. Biological Conservation 129: 59–69. [Google Scholar]
- Coates F, Duncan M. 2009. Demographic variation between populations of Caladenia orientalis – a fire-managed threatened orchid. Australian Journal of Botany 57: 326–339. [Google Scholar]
- Dearnaley JDW. 2007. Further advances in orchid mycorrhizal research. Mycorrhiza 17: 475–486. [DOI] [PubMed] [Google Scholar]
- DeBano LF, Neary DG, Ffolliott PF. 1998. Fire’s effects on ecosystems.New Jersey, USA: John Wiley and Sons. [Google Scholar]
- Department of Environment, Land, Water and Planning Victoria 2017a. NatureKit (biodiversity interactive map). http://maps.biodiversity.vic.gov.au/viewer/?viewer=NatureKit (10 August 2017). [Google Scholar]
- Department of Environment, Land, Water and Planning Victoria 2017b. Forest fire management Victoria: past bushfires 1851–2013. https://www.ffm.vic.gov.au/history-and-incidents/past-bushfires (10 August 2017). [Google Scholar]
- Ding R, Chen X-H, Zhang L-J et al. 2014. Identity and specificity of Rhizoctonia-like fungi from different populations of Liparis japonica (Orchidaceae) in Northeast China. PLoS One 9: e105573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon K, Barrett R. 2003. Defining the role of fire in south-west Western Australian plants. In: Abbott I, Burrows N, eds. Fire in ecosystems of south-west Western Australia: impacts and management.Leiden: Backhuys Publishers, 205–223. [Google Scholar]
- Dixon KW, Roche S, Pate JS. 1995. The promotive effect of smoke derived from burnt native vegetation on seed germination of Western Australian plants. Oecologia 101: 185–192. [DOI] [PubMed] [Google Scholar]
- Duncan M. 2012. Response of orchids to bushfire. Black Saturday Victoria 2009 – natural values fire recovery program.Heidelberg, Victoria, Australia: Department of Sustainability and Environment. [Google Scholar]
- Dunn PH, Barro SC, Poth M. 1985. Soil moisture affects survival of microorganisms in heated chaparral soil. Soil Biology and Biochemistry 17: 143–148. [Google Scholar]
- Flematti GR, Ghisalberti EL, Dixon KW, Trengove RD. 2004. A compound from smoke that promotes seed germination. Science 305: 977–977. [DOI] [PubMed] [Google Scholar]
- Fuller WH, Shannon S, Burgess PS. 1955. Effect of burning on certain forest soils of northern Arizona. Forest Science 1: 44–50. [Google Scholar]
- Gebauer G, Preiss K, Gebauer AC. 2016. Partial mycoheterotrophy is more widespread among orchids than previously assumed. New Phytologist 211: 11–15. [DOI] [PubMed] [Google Scholar]
- Gill MA, Groves RH, Noble IR, eds 1981. Fire and the Australian biota. Canberra: Australian Academy of Science. [Google Scholar]
- Hadley G. 1970. Non-specificity of symbiotic infection in orchid mycorrhiza. New Phytologist 69: 1015–1023. [Google Scholar]
- Haines TK, Martinez J, Cleaves DA. 1998. Influences on prescribed burning activity in the United States national forest system. International Forest Fire News 19: 43–46. [Google Scholar]
- Hutchings MJ. 1987. The population biology of the early spider orchid, Ophrys sphegodes Mill. I: a demographic study from 1975 to 1984. Journal of Ecology 75: 711–727. [Google Scholar]
- Huynh TT, McLean CB, Coates F, Lawrie AC. 2004. Effect of developmental stage and peloton morphology on success in isolation of mycorrhizal fungi in Caladenia formosa (Orchidaceae). Australian Journal of Botany 52: 231–241. [Google Scholar]
- Huynh TT, Thomson R, McLean CB, Lawrie AC. 2009. Functional and genetic diversity of mycorrhizal fungi from single plants of Caladenia formosa (Orchidaceae). Annals of Botany 104: 757–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inghe O, Tamm CO. 1988. Survival and flowering of perennial herbs. 5. Patterns of flowering. Oikos 51: 203–219. [Google Scholar]
- Irwin MJ, Bougoure JJ, Dearnaley JDW. 2007. Pterostylis nutans (Orchidaceae) has a specific association with two Ceratobasidium root-associated fungi across its range in eastern Australia. Mycoscience 48: 231–239. [Google Scholar]
- Jacquemyn H, Honnay O, Cammue BPA, Brys R, Lievens B. 2010. Low specificity and nested subset structure characterize mycorrhizal associations in five closely-related species of the genus Orchis. Molecular Ecology 19: 4086–4095. [DOI] [PubMed] [Google Scholar]
- Jones DL. 2006. A complete guide to native orchids of Australia, including the island territories.Sydney: Reed New Holland. [Google Scholar]
- Jones DL, Clements MA. 2002. Australian orchid research Volume 4: a review of pterostylis. Melbourne: Australian Orchid Foundation. [Google Scholar]
- Jones D, Wapstra H, Tonelli P, Harris S. 1999. The orchids of Tasmania. Melbourne: Melbourne University Press. [Google Scholar]
- Jones MB, Laude HM. 1960. Relationships between sprouting in chamise and the physiological condition of the plant. Journal of Range Management 13: 210–214. [Google Scholar]
- Kartzinel TR, Trapnell DW, Shefferson RP. 2013. Highly diverse and spatially heterogeneous mycorrhizal symbiosis in a rare epiphyte is unrelated to broad biogeographic or environmental features. Molecular Ecology 22: 5949–5961. [DOI] [PubMed] [Google Scholar]
- Keith D. 1996. Fire-driven extinction of plant populations: a synthesis of theory and review of evidence from Australian vegetation. Proceedings of the Linnean Society of New South Wales 116: 37–78. [Google Scholar]
- Kery M, Gregg KB. 2004. Demographic analysis of dormancy and survival in the terrestrial orchid Cypripedium reginae. Journal of Ecology 92: 686–695. [Google Scholar]
- Kilgore BM, Curtis GA. 1987. Guide to understory burning in ponderosa pine–larch–fir forests in the Intermountain West. United States Department of Agriculture, Forest Service Intermountain Research Station: General Technical Report INT-233. [Google Scholar]
- Klopatek CC, DeBano LF, Klopatek JM. 1988. Effects of simulated fire on vesicular-arbuscular mycorrhizae in pinyon-juniper woodland soil. Plant and Soil 109: 245–249. [Google Scholar]
- Klopatek CC, Freise CF, Allen MF, Klopatek JM. 1994. Comparisons of laboratory and field burning experiments on mycorrhizae distribution, density and diversity. Journal of Society of American Foresters 94: 762–776. [Google Scholar]
- Korb JE, Johnson NC, Covington WW. 2003. Arbuscular mycorrhizal propagule densities respond rapidly to ponderosa pine restoration treatments. Journal of Applied Ecology 40: 101–110. [Google Scholar]
- Kottke I, Setaro SD, Haug I et al. 2013. Mycorrhiza networks promote biodiversity and stabilize the tropical mountain rain forest ecosystem: perspectives for understanding complex communities. In: Bendix J, Beck E, Bräuning A. et al. , eds. Ecosystem services, biodiversity and environmental change in a tropical mountain ecosystem of South Ecuador. Berlin: Springer, 187–203. [Google Scholar]
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33: 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light MH, MacConnaill M. 1991. Patterns of appearance in Epipactis helleborine (L.) Crantz. In: Wells TCE, Willems JH, eds. Population ecology of terrestrial orchids. The Hague: SPB Academic Publishing, 77–87. [Google Scholar]
- Lin H-L, Chumpookam J, Shiesh C-C, Chung W-H. 2012. Smoke-water controls Pythium damping-off in papaya seedling. HortScience 47: 1453–1456. [Google Scholar]
- Linde CC, May TW, Phillips RD, Ruibal M, Smith LM, Peakall R. 2017. New species of Tulasnella associated with terrestrial orchids in Australia. IMA Fungus 8: 27–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuhara G, Katsuya K. 1994. In situ and in vitro specificity between Rhizoctonia spp. and Spiranthes sinensis (Persoon) Ames. var. amoena (M. Bieberstein) Hara (Orchidaceae). New Phytologist 127: 711–718. [DOI] [PubMed] [Google Scholar]
- McCormick MK, Jacquemyn H. 2014. What constrains the distribution of orchid populations?New Phytologist 202: 392–400. [Google Scholar]
- McCormick MK, Whigham DF, O’Neill J. 2004. Mycorrhizal diversity in photosynthetic terrestrial orchids. New Phytologist 163: 425–438. [DOI] [PubMed] [Google Scholar]
- McCormick MK, Whigham DF, Sloan D, O’Malley K, Hodkinson B. 2006. Orchid–fungus fidelity: a marriage meant to last?Ecology 87: 903–911. [DOI] [PubMed] [Google Scholar]
- Morgan JW. 1999. Defining grassland fire events and the response of perennial plants to annual fire in temperate grasslands of south-eastern Australia. Plant Ecology 144: 127–144. [Google Scholar]
- Morgulis A, Coulouris G, Raytselis Y, Madden T, Agarwala R, Schäffer A. 2008. Database indexing for production MegaBLAST searches. Bioinformatics 24: 1757–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison LW, Jaack-Gaynor JL, Young CC, DeBacker MD. 2015. A 20-year record of the Western Prairie Fringed Orchid (Platanthera praeclara): population dynamics and modelling of precipitation effects. Natural Areas Journal 35: 246–255. [Google Scholar]
- Neary DG, Klopatek CC, DeBano LF, Ffolliott PF. 1999. Fire effects on belowground sustainability: a review and synthesis. Forest Ecology and Management 122: 51–71. [Google Scholar]
- Nogeira RE, van den Berg C, Pereira OL, Kasuya MCM. 2014. Isolation and characterisation of Rhizoctonia-like fungi associated with orchid roots in the Quadrilátero Ferrifero and Zona da Mata regions of the state of Minas Gerais, Brazil. Acta Botanica Brasilica 28: 298–300. [Google Scholar]
- Parmeter J, Uhrenholdt B. 1975. Some effects of pine-needle or grass smoke on fungi. Phytopathology 65: 28–31. [Google Scholar]
- Perkins AJ, Masuhara G, McGee PA. 1995. Specificity of the associations between Microtis parviflora (Orchidaceae) and its mycorrhizal fungi. Australian Journal of Botany 43: 85–91. [Google Scholar]
- Quarmby JP. 1999. Recovery plan for twelve threatened orchids in the Lofty Block region of South Australia 2010.South Australia: Department of Environment and Natural Resources. [Google Scholar]
- Ramsay RR, Dixon KW, Sivasithamparam K. 1986. Patterns of infection and endophytes associated with Western Australian orchids. Lindleyana 1: 203–214. [Google Scholar]
- Rasmussen HN. 1995. Terrestrial orchids: from seed to mycotrophic plant. Cambridge: Cambridge University Press. [Google Scholar]
- Reddell P, Malajczuk N. 1984. Formation of mycorrhizae by jarrah (Eucalyptus marginata Donn ex Smith) in litter and soil. Australian Journal of Botany 32: 511–520. [Google Scholar]
- Renbuss MA, Chilvers GA, Pryor LD. 1972. Microbiology of an ashbed. Proceedings of the Linnean Society of New South Wales 97: 302–310. [Google Scholar]
- Roche SA, Carter RJ, Peakall R, Smith LM, Whitehead MR, Linde CC. 2010. A narrow group of monophyletic Tulasnella (Tulasnellaceae) symbiont lineages are associated with multiple species of Chiloglottis (Orchidaceae): implications for orchid diversity. American Journal of Botany 97: 1313–1327. [DOI] [PubMed] [Google Scholar]
- Saravanan V, Santhi R, Kumar P, Kalaiselvi T, Vennila S. 2013. Effect of forest fire on microbial diversity of the degraded shola forest ecosystem of Nilgiris Eastern Slope Range. Research Journal of Agriculture and Forestry Sciences 1: 5–8. [Google Scholar]
- Selosse M-A, Faccio A, Scappaticci G, Bonfante P. 2004. Chlorophyllous and achlorophyllous specimens of Epipactis microphylla (Neottieae, Orchidaceae) are associated with ectomycorrhizal septomycetes, including truffles. Microbial Ecology 47: 416–426. [DOI] [PubMed] [Google Scholar]
- Shefferson RP, Weiss M, Kull T, Taylor D. 2005. High specificity generally characterizes mycorrhizal association in rare lady’s slipper orchids, genus Cypripedium. Molecular Ecology 14: 613–626. [DOI] [PubMed] [Google Scholar]
- Sieg CH, King RM. 1995. Influence of environmental factors and preliminary demographic analyses of a threatened orchid, Platanthera praeclara. American Midland Naturalist 134: 307–323. [Google Scholar]
- Smith SE, Read DJ. 2008. Mycorrhizal symbiosis. San Diego: Academic Press. [Google Scholar]
- Smith ZF, James EA, McLean CB. 2010. Mycorrhizal specificity of Diuris fragrantissima (Orchidaceae) and persistence in a reintroduced population. Australian Journal of Botany 58: 97–106. [Google Scholar]
- Suárez JP, Weiss M, Abele A, Garnica S, Oberwinkler F, Kottke I. 2006. Diverse tulasnelloid fungi form mycorrhizas with epiphytic orchids in an Andean cloud forest. Mycological Research 110: 1257–1270. [DOI] [PubMed] [Google Scholar]
- Swarts ND, Dixon KW. 2009. Terrestrial orchid conservation in the age of extinction. Annals of Botany 104: 543–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Nei M. 1993. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Molecular Biology and Evolution 10: 512–526. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. 1994. Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research 122: 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threatened Species Section 2006. Flora recovery plan: Tasmanian threatened orchids 2006–2010.Hobart: Department of Primary Industries, Water and Environment. [Google Scholar]
- Tsavkelova EA, Klimova SY, Cherdyntseva TA, Netrosov AI. 2006. Microbial producers of plant growth stimulators and their practical use: a review. Applied Biochemistry and Microbiology 42: 117–126. [PubMed] [Google Scholar]
- Van Staden J, Jager AK, Light ME, Burger BV. 2004. Isolation of the major germination cue from plant-derived smoke. South African Journal of Botany 70: 654–659. [Google Scholar]
- Vázquez FJ, Acea MJ, Carballas T. 1993. Soil microbial populations after wildfire. FEMS Microbiology Ecology 13: 93–103. [Google Scholar]
- Visser S. 1995. Ectomycorrhizal fungal succession in jack pine stands following wildfire. New Phytologist 129: 389–401. [Google Scholar]
- Voyron S, Ercole E, Ghignone S, Perotto S, Girlanda M. 2017. Fine-scale spatial distribution of orchid mycorrhizal fungi in the soil of host-rich grasslands. New Phytologist 213: 1428–1439. [DOI] [PubMed] [Google Scholar]
- Wade DD, Lunsford JD, Dixon MJ, Mobley HE. 1989. A guide for prescribed fire in southern forests. United States Department of Agriculture: Forest Service Southern Region Technical Publication R8-TP 11. [Google Scholar]
- Warcup JH. 1971. Specificity of mycorrhizal association in some Australian terrestrial orchids. New Phytologist 70: 41–46. [Google Scholar]
- Warcup JH. 1973. Symbiotic germination of some Australian terrestrial orchids. New Phytologist 72: 387–392. [Google Scholar]
- Warcup JH. 1981. The mycorrhizal relationships of Australian orchids. New Phytologist 87: 371–381. [Google Scholar]
- Wells TCE, Rothery P, Cox R, Bamford S. 1981. Flowering dynamics of Orchis morio L. and Herminium monorchis (L.) R.Br. at two sites in eastern England. Botanical Journal of the Linnean Society 126: 39–48. [Google Scholar]
- Weston PH, Perkins AJ, Entwisle TJ. 2005. More than symbioses: orchid ecology, with examples from the Sydney region. Cunninghamia 9: 1–15. [Google Scholar]
- Whelan RJ. 1995. The ecology of fire.Cambridge: Cambridge University Press. [Google Scholar]
- White JJ, Bruns TD, Lee S, Taylor JJ. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Gelfand MA, Sninsky JJ, White J, eds. PCR protocols – a guide to methods and applications. New York: Academic Press, 315–322. [Google Scholar]
- Wilkinson KG, Dixon KW, Sivasithamparam K. 1989. Interaction of soil bacteria, mycorrhizal fungi and orchid seed in relation to germination of Australian orchids. New Phytologist 112: 429–435. [Google Scholar]
- Wright E, Tarrant RF. 1957. Microbiological soil properties after logging and slash burning. United States Department of Agriculture Forest Service: Pacific Northwest Forest and Range Experiment Station Research Note No. 157. [Google Scholar]
- Yu Y, Cui Y-H, Hsiang T, Zeng ZQ, Yu Z-H. 2015. Isolation and identification of endophytes from roots of Cymbidium goeringii and Cymbidium faberi (Orchidaceae). Nova Hedwigia 101: 57–64. [Google Scholar]
- Yuan L, Yang ZL, Li S-Y, Hu H, Huang J-L. 2010. Mycorrhizal specificity, preference, and plasticity of six slipper orchids from South Western China. Mycorrhiza 20: 559–568. [DOI] [PubMed] [Google Scholar]
- Zagory D, Parmeter JR. 1984. Fungitoxicity of smoke. Phytopathology 74: 1027–1031. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.