Abstract
Background and Aims
Regeneration dynamics in many arid zone grass species are regulated by innate seed dormancy mechanisms and environmental cues (temperature, moisture and fire) that result in infrequent germination following rainfall. This study investigated bet-hedging strategies associated with dormancy and germination in arid zone Triodia species from north-west Australia, by assessing (1) the effects of the mechanical restriction imposed by the indehiscent floral bracts (i.e. floret) covering the seed and (2) the impact of dormancy alleviation on florets and cleaned seeds (i.e. florets removed) when germinated under water stress.
Methods
The initial dormancy status and germination for six species were tested on intact florets and cleaned seeds, across temperatures (10–40 °C) with and without the fire-related stimulant karrikinolide (KAR1), and under alternating light or constant dark conditions. Physiological dormancy alleviation was assessed by wet/dry cycling florets over a period of 10 weeks, and germination was compared against untreated florets, and cleaned seeds across a water potential gradient between 0 and –1.5 MPa.
Key Results
Florets restricted germination (<45 %) at all temperatures and, despite partial alleviation of physiological dormancy (wet/dry cycling for 8 weeks), intact florets germinated only at high water potentials. Cleaned seeds showed the highest germination (40–90 %) across temperatures when treated with KAR1, and germinated at much lower water potentials (–0.4 and –0.9 MPa). Triodia pungens was the most responsive to KAR1, with both seeds and florets responding, while for the remaining five species, KAR1 had a positive effect for seeds only.
Conclusions
Only after seed dormancy was alleviated by removing florets and when KAR1 was applied did germination under water stress increase. This suggests that seeds of these Triodia species are cued to recruit following fire and during periods of high precipitation. Climate change, driven by large shifts in rainfall patterns, is likely to impact Triodia recruitment further in arid zone grasslands.
Keywords: Arid grass, floret, karrikinolide, KAR1, light, recruitment bottleneck, seed dormancy, temperature, spinifex, water potential, water stress, wet/dry cycling
INTRODUCTION
Germination in arid systems is a high-risk event, as sufficient rainfall to initiate germination may not necessarily be enough to support subsequent seedling establishment, plant growth and reproduction (Schwinning and Sala, 2004; Gremer et al., 2016; Liu et al., 2017). As a consequence, there is a tendency for arid zone species to spread this recruitment risk through ‘bet-hedging’, i.e. by dispersing a high and variable proportion of dormant seeds to avoid depletion in the soil seed bank (e.g. Gremer et al., 2016; Commander et al., 2017). Bet-hedging allows a seed cohort to forego a current recruitment opportunity for potentially enhanced establishment in a future one, thus accepting a short-term reduction in fitness for maximizing long-term fitness by ensuring a seed cohort as a whole is not lost to establishment failure in an unfavourable year (Clauss and Venable, 2000; Tielbörger et al., 2012). Through variably dormant seed cohorts, recruitment events therefore spread risk over several years, thus enabling survival in the soil seed bank.
Although risk-spreading via seed dormancy may provide a survival strategy in water-limited ecosystems, the regeneration of grasslands in arid ecosystems is often infrequent and slow, even under favourable rainfall conditions (Zimmermann et al., 2008; Lewandrowski et al., 2017). Furthermore, arid grasslands are particularly difficult to regenerate, because the recruitment of grass seedlings relies on the simultaneous alignment of several environmental factors (Muñoz-Rojas et al., 2016; Lewandrowski et al., 2017). For example, in fire-prone ecosystems, flushes of seedling recruitment are particularly evident after fire events following periods of high rainfall (Mott, 1978; Lewandrowski et al., 2017). Despite our knowledge of ecological recruitment patterns in natural landscapes, understanding of the physiological interactions driving recruitment processes in the context of bet-hedging for arid grass species is still limited.
Bet-hedging is influenced by the depth of dormancy in any given seed cohort. In the Poaceae, seed dormancy is regulated by two separate, but interacting mechanisms: (1) the ancillary bracts of the floret surrounding the seed, which are thought to limit oxygen and water uptake (Mott, 1974; Huarte et al., 2007) or modify sensitivity to light; and/or (2) the physiological restriction of embryo growth preventing germination (Gallart et al., 2008; Ma et al., 2008; Bewley et al., 2013). Consequently, seed germination from florets can be slow, resulting in delayed recruitment following favourable rainfall events or optimal moisture conditions. Many studies have shown that the removal of the covering floret structures from seeds promotes higher germination due to increased water imbibition and the reduction of mechanical pressure on embryo growth (Adkins et al., 2002; Bewley et al., 2013; Erickson et al., 2016).
Physiologically dormant grass seeds require suitable temperature and water availability for dormancy alleviation, and certain environmental cues such as smoke and light stimulation for germination to proceed (Baskin and Baskin, 2014). Particularly in fire-prone ecosystems, a considerable number of grass species have a positive germination response after exposure to karrikinolide (KAR1) (e.g. Long et al., 2011) – a smoke-derived chemical stimulant that is formed through the burning of plant material (Flematti et al., 2004). The overall sensitivity of seeds to KAR1 varies depending on dormancy state. For example, intact florets (seeds enclosed by the ancillary bracts) that receive a period of controlled after-ripening or burial in field conditions are often more responsive to KAR1 than freshly dispersed florets (Long et al., 2011; Erickson et al., 2016).
Wetting and drying events that mimic intermittent rainfall periods also influence seed dormancy in a range of arid zone species (Hoyle et al., 2008; Lewandrowski et al., 2017). For grasses specifically, four fortnight-long wetting and drying cycles increase germination performance of previously dormant material (Lewandrowski et al., 2017). From an ecological perspective, this may suggest that rainfall followed by drying periods can potentially alter bet-hedging of any given seed cohort by reducing dormancy under a range of environmental cues. If the floret influences seed germination events, understanding the interaction of seed dormancy and various environmental conditions, such as temperature, water availability and exposure to smoke-related cues, will aid our knowledge of the recruitment dynamics of grasses in natural systems.
This study tested these concepts on seeds of six grass species in the widespread Australian genus Triodia (R.Br). Triodia grasslands represent one of the most widespread Australian habitats covering approximately one-third of the arid interior, and influence fire regimes that contribute to ecosystem functioning and composition over much of the continent (Nicholas et al., 2009; Nano and Clarke, 2010). Triodia recruitment has often been observed following fire events, with germination stimulated by smoke-related cues (Wright and Fensham, 2017). While a considerable body of knowledge exists on the recruitment of Triodia species, little is known about how the floret interacts with environmental variables such as temperature, moisture availability and smoke-related cues. Given their extensive distribution throughout the arid zone of Australia, it is believed that the resident soil seedbank of Triodia may provide a mechanism for bet-hedging recruitment events during unpredictable fire events and subsequent rainfall periods in the wet season (Wright and Fensham, 2017).
With the combination of floret- and seed-imposed physiological dormancy mechanisms believed to restrict germination in arid grass species to a limited environmental range, this study tested the hypotheses that florets: (1) would restrict germination regardless of the addition of a germination stimulant (KAR1) across a wide range of temperatures and available light; and (2) wet/dry cycling would improve germination in florets by alleviating physiological dormancy, but there would still be no advantage under water stress when compared with seeds with the floret removed (cleaned seeds), as the floret would restrict germination at decreasing water availability.
MATERIALS AND METHODS
Study region, study species, seed cleaning and quality control
Florets of six species [Triodia basedowii E. Pritz, T. epactia S.W.L. Jacobs, T. lanigera Domin, T. pungens R.Br., T. sp. Shovelanna Hill (S. van Leeuwen 3835) and T. wiseana C.A. Gardener] were commercially collected in March 2011 (Nindethana Seed Service and Native Bushland Seed Supply) from the Pilbara, a region characterized by cool (10–25 °C), dry winters and warm (25–45 °C) summers (BOM, 2016). Rainfall ranges between 300 and 350 mm per annum, and 60–70 % of this total average is received over summer (December–February). Rainfall events occur as small, infrequent and intermittent showers and thunderstorms, or as large events associated with tropical cyclones (Charles et al., 2013). After collection, seeds were maintained in an air-conditioned [approx. 25 °C/50 % relative humidity (RH)] room for 6 months. While after-ripening of florets may have occurred under these warmer and more humid storage conditions (Erickson and Merritt, 2016), germination from florets was still low, ranging between 0 and 40 % across the species tested. Batches of uncleaned florets were transferred to a cool (15 °C), dry (15 % RH) storage facility. The florets in the seed collections had low fill, where only 10–42 % of florets contained a seed (see Supplementary Data Appendix S1; Table S1), and were processed to remove unfilled florets for experimental purposes (sensuErickson and Merritt, 2016). Florets were often joined together as a spikelet, and were separated by gently rubbing through a 2 mm sieve using a rubber bung, followed by repeated passes through a vacuum aspirator (‘Zig Zag’ Selecta, Machinefabriek BV, Enkhuizen, The Netherlands), which separated light, unfilled floret fractions from heavy, filled floret fractions (i.e. florets that contained a seed). The final quality of the florets was assessed via X-ray analysis (Faxitron MX-20 x-ray cabinet, Tucson, AZ, USA), with most batches displaying 99 % fill (i.e. florets contained a healthy seed that possessed an intact and undamaged embryo and endosperm). In order to obtain pure seeds, florets were removed by gently rubbing them through a 1.4 mm sieve. Floret chaff and seeds were separated with the use of the vacuum aspirator. To remove any non-viable seeds damaged during the cleaning process, seeds were checked under a dissecting microscope and removed if any damage to the testa or embryo was visible. Once processed, batches of filled florets and cleaned seeds were stored at 15 °C and 15 % RH until experimental use.
Experiment 1: determining the effects of floret removal, temperature, light and karrikinolide on germination.
Intact florets and cleaned seeds were incubated at temperatures between 10 and 40 °C, under alternating light or constant dark, and in the presence or absence of the smoke-derived germination stimulant KAR1 synthesized following Flematti et al. (2004). Prior to germination testing, florets and cleaned seeds used in the experiment were surface-sterilized in a 2 % (w/v) calcium hypochlorite [Ca(OCl)2] solution for 30 min, under 10 min cycles of alternating vacuum (e.g. on/off/on at –70 kPa), and then rinsed in sterile deionized water. After sterilization, four replicates of 25 florets or cleaned seeds were plated on 90 mm Petri dishes containing 0.7 % (w/v) water–agar that was prepared with or without KAR1 at a 0.67 μm concentration (Long et al., 2011). Petri dishes were transferred into individual incubators set to alternating 12 h light/dark conditions and constant temperatures of either 10, 15, 20, 25, 30, 35 or 40 °C (Contherm Scientific Limited, Wellington, New Zealand). The arrangement of Petri dishes was randomized within treatments, with further daily randomization of dishes occurring within and between treatments within incubators. To exclude light in the constant dark treatments, Petri dishes were wrapped in aluminium foil until scoring at the end of the experiment. Light emitted in incubators was measured at 45 μmol m–2 s–1, from cool-white fluorescent lights. The germination test was conducted over 28 d to satisfy dormancy classification according to Baskin and Baskin (2014). Germination was defined as radicle emergence greater than one-third of the floret or seed coat length.
Experiment 2: improving germination in florets through wet/dry cycles
To improve germination of intact Triodia florets, a wet/dry cycling experiment was conducted following a modified method of the experiment described by Hoyle et al. (2008), under alternating light or constant dark conditions. Each 2-week cycle started with a 48 h wet exposure followed by a 12 d dry exposure at 50 % RH. Nylon mesh bags containing florets were placed onto 0.6 % water–agar during the wet exposure, to enable complete imbibition of the florets. After the conclusion of wet exposure, florets were dried and stored at 50 % RH maintained by a 370 g–1 L LiCl solution (Sigma-Aldrich Pty. Ltd, Sydney, NSW, Australia) in an airtight 270 × 270 × 130 mm polycarbonate electrical enclosure box (NHP Fibox, Australia) for 12 d. At the completion of each wet/dry cycle, four replicates of 25 florets were plated on to either water or KAR1–agar for 28 d at 30 °C (12 h alternating light/dark) and assessed for total germination percentage. The sterilization procedure prior to plating and Petri dish arrangement in the incubators followed the same protocol as described in Experiment 1.
Experiment 3: determining germination responses of treated florets and cleaned seeds under water stress
To determine if the floret limits germination under water stress, a germination test was conducted on different dormancy states (untreated florets, wet/dry cycled florets and cleaned seeds) across osmotic potentials imposed by polyethylene glycol solutions (PEG-8000, Sigma-Aldrich Pty. Ltda). Osmotic potentials are well suited to simulate water stress in soil (Michel, 1983). The various PEG solutions were prepared as described by Michel (1983) for 30 °C incubator conditions, and validated using a dewpoint psychrometer (WP4C Dew Point PotentiaMeter, Decagon Devices, Inc., Pullman, WA, USA). For this experiment, an 8-week wet/dry cycling (four 2-week cycles) treatment was used for all species, and was conducted as described in Experiment 2. Intact florets and cleaned seeds were plated on 90 mm Petri dishes containing two filter papers that were hydrated with 20 mL of PEG solutions for –0.01, –0.1, –0.2, –0.3, –0.4, –0.6, –0.8, –1 and –1.5 MPa, or a control that was hydrated with water only. All plates were incubated at 30 °C (12 h alternating light/dark). Filter papers maintained moisture for the total duration of the trial and did not require re-wetting. PEG concentrations were re-tested with the dewpoint psychrometer from plates at the conclusion of the experiment to ensure solutions were maintained at the target concentrations. The sterilization procedure prior to plating and Petri dish arrangement in the incubators followed the same protocol as described in Experiment 1. Germination was scored daily over 28 d.
Statistical analysis
Factor analysis.
Germination data were analysed using binomial logistic regressions [general linearised models (GLMs)] in the statistical environment R (R Core Team, 2014); data were fitted with a logit-link function followed by a likelihood test. For each experiment, a full model including all main factor terms and their two-way interactions was undertaken for each species separately. In Experiment 1, temperature was analysed as a continuous factor, while dormancy state (intact florets and cleaned seeds), stimulant (water and KAR1) and light (constant dark and alternating light/dark) were categorical factors. In Experiment 2, the number of wet/dry cycles was analysed as a continuous factor, while stimulant (water and KAR1) was a categorical factor. Finally, in Experiment 3, water stress was continuous, while the dormancy state (untreated floret, wet/dry cycled floret and cleaned seed) was analysed as a categorical factor.
Parameterization and comparison of environmental thresholds for water stress.
In order to determine differences in germination following dormancy alleviation treatment across water stress, non-linear functions were fitted to the germination response for water stress. The thresholds were calculated by fitting a three-parameter Weibull function (Weibull, 1951) available from the ‘drc’ package in R (Ritz and Streibig, 2005),
where d is the parameter for the upper limit or maximum germination on a germination curve, c the lower limit equal to 0, b proportional to the slope of the curve of F and water stress x, and the parameter e equal at the median response or curve inception point (Ψb50). Estimated water stress limiting germination at fractions at 95 % response (i.e. the initial reduction in germination by 5 % from the maximum germination response, Ψb95); median 50 % (50 % reduction in germination from the maximum germination response Ψb50); and the minimum response (i.e. the water potential reducing germination to a 5 % response close to the base, Ψb5) were calculated from the fitted curve. These parameters were chosen to represent different germination fractions of intact florets and seeds that are limited by increasing water stress, assuming that the upper limit for germination represents the maximum response possible in the germination treatment. The three-parameter Weibull functions were most suitable for determining the responses over other non-linear functions, with the curve selection determined as described in Ritz and Streibig (2005). The best-fitting curve was selected based on the log-likelihood, Aikike information criterion (AIC) and lack-of-fit test. Comparison of calculated parameters was performed through pairwise comparisons of means. All analyses were conducted in R (R Core Team, 2014).
RESULTS
Experiment 1: determining the effects of floret removal, temperature, light and karrikinolide on germination
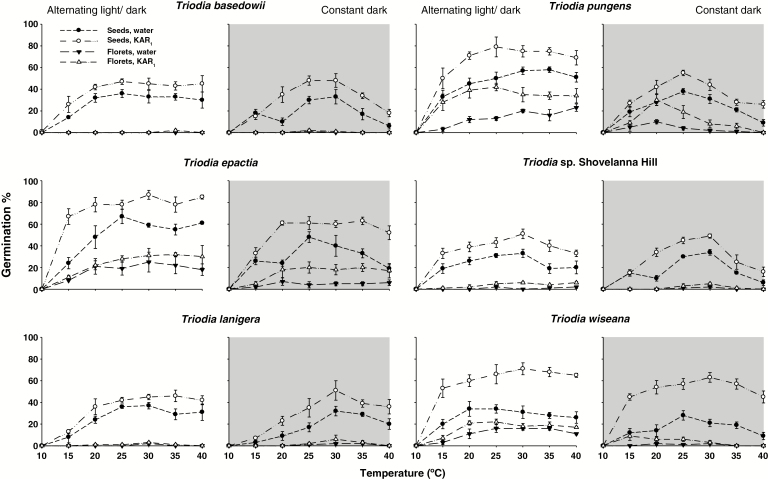
Overall germination responses were between 0 and 85 % and varied significantly for species, dormancy state (intact florets and cleaned seeds), temperature, light and KAR1 (Table 1). For all species, germination after seed cleaning outperformed germination from florets (all P < 0.001), with KAR1 further stimulating germination on average by 15 % in cleaned seeds (P < 0.001). While cleaned seeds germinated between 30 and 85 % on KAR1, germination from florets even after exposure to KAR1 was somewhat limited (0–45 %). Additionally, germination was only significantly improved in florets in T. pungens on KAR1 across temperatures between 20 and 40 °C, while only seeds were affected from most temperatures in the other species (Fig. 1; Table 1). From both intact florets and cleaned seeds, germination proceeded across a wide temperature range under alternating light conditions (P < 0.001), while showing a slightly reduced response under constant dark conditions (Fig. 1). Under alternating light, maximum germination responses were between 20 and 40 °C, while germination from constant dark conditions decreased at temperatures >30 °C (Fig. 1).
Table 1.
Summary statistics (z-statistics and P-values) from binomial logistic regressions to determine the effects of dormancy state (untreated florets; seeds), temperature (10–40 °C), light (constant dark; alternating light/ dark) and germination stimulant (water; KAR1) in Experiment 1; wet/dry cycles (0–10 weeks), germination stimulant (water; KAR1) in Experiment 2; and, water stress (0 to –1.5 MPa) and dormancy state (untreated florets; wet/dry-cycled florets; cleaned seeds) in Experiment 3
| Factor terms | d.f. | Triodia basedowii | Triodia epactia | Triodia lanigera | Triodia pungens | Triodia sp. Shovelanna Hill | Triodia wiseana |
|---|---|---|---|---|---|---|---|
| Experiment 1 | |||||||
| Dormancy state | 1 | 3.41*** | 7.26*** | 3.70*** | 7.01*** | 6.78*** | 6.87*** |
| Temperature | 1 | 2.80** | 4.90*** | 2.58** | 5.12*** | 2.63** | 3.52*** |
| Light | 1 | –2.01* | 3.38*** | –0.16 | 3.48*** | 1.60 | 5.54*** |
| Stimulant | 1 | 2.87*** | 3.33*** | 2.03* | 4.12*** | 2.64*** | 3.10*** |
| Dormancy state × temperature | 1 | –0.01 | –1.22 | 0.25 | –1.15 | –1.76 | 0.65 |
| Dormancy state × light | 1 | –1.01 | –2.58** | 2.08* | 2.03* | –1.63 | –6.89*** |
| Dormancy state × stimulant | 1 | –1.02 | –1.05 | –0.90 | 3.12*** | –2.22* | 1.72 |
| Temperature × light | 1 | 1.55 | 0.95 | –1.57 | 0.85 | 0.58 | 1.83 |
| Temperature × stimulant | 1 | 0.43 | 0.12 | –0.44 | 0.34 | 0.42 | –0.06 |
| Light × stimulant | 1 | –1.18 | –2.54* | –1.39 | 2.32* | –0.06 | –2.79** |
| Experiment 2 | |||||||
| Wet/dry cycling | 1 | –0.13 | 4.52*** | –1.36 | 4.12*** | 0.16 | 5.63*** |
| Stimulant | 1 | –0.36 | 2.15 | 0.17 | 3.10*** | 1.56 | 2.08* |
| Wet/dry cycling × stimulant | 2 | –0.05 | –0.81 | –0.26 | –1.49 | –0.59 | 0.18 |
| Experiment 3 | |||||||
| Water stress | 1 | 4.64*** | 14.10*** | 9.06*** | 4.62 | 4.84*** | 12.00*** |
| Dormancy state (seeds) | 2 | 7.37*** | 5.45*** | 4.92*** | 4.62*** | 4.10*** | 4.41*** |
| Dormancy state (wet/dry-cycled florets) | 2 | 0.01 | 2.28*** | –0.01 | 1.89 | –0.02 | 1.84 |
| Water stress × dormancy state (seeds) | 2 | –3.73*** | –5.80*** | –3.13** | –3.48*** | –3.74*** | –4.89*** |
| Water stress × dormancy state (wet/dry-cycled florets) | 2 | –0.004 | –0.97 | –0.004 | –0.21 | –0.01 | –0.66 |
Main factor effects and their two-way interactions are shown for each species separately.
Asterisks show the significance level at *P < 0.05, **P < 0.01, or ***P < 0.001.
Fig. 1.
Effects of temperature, germination stimulant and alternating light/dark or constant dark treatments on mean germination (± s.e., n = 4) of intact florets and cleaned seeds of six Australian Triodia species. Intact florets or cleaned seeds were incubated at temperatures between 10 and 40 °C on either water or KAR1 (0.67 μm) agar plates and exposed to 12 h alternating light/dark conditions, or constant dark by wrapping plates in aluminium foil for 28 d.
Overall, the lowest germination was observed in florets of T. basedowii, T. lanigera and T. sp. Shovelanna Hill, which never exceeded 2.5 %, while germination of cleaned seeds ranged between 35 and 45 % (P ≤ 0.05) on KAR1. In contrast, KAR1 more strongly affected germination in T. epactia, T. pungens and T. wiseana, with maximum germination under optimal temperature between 80 and 85 % for cleaned seeds (Fig. 1).
Experiment 2: improving germination in florets through wet/dry cycles
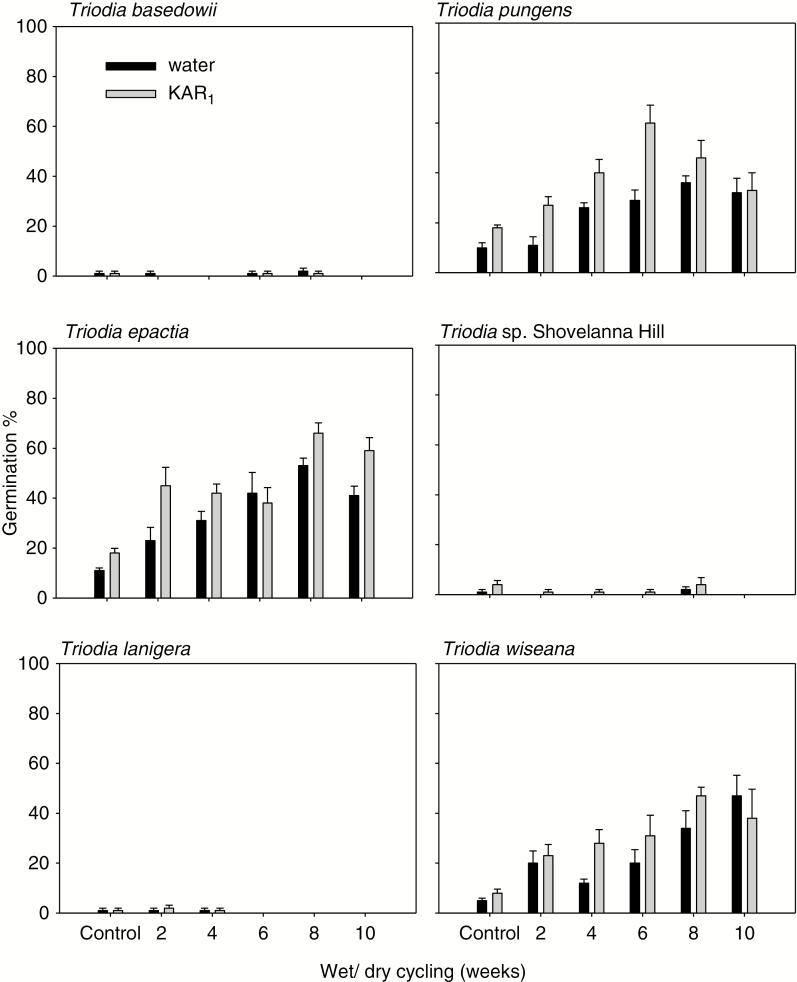
Out of the six Triodia species tested, only three responded significantly to wet/dry cycling (Table 1). In seeds of T. epactia, T. pungens and T. wiseana, germination improved up to 4-fold under optimal treatment of eight weeks of wet/dry cycling (P < 0.01), when compared with germination from control treatments (Fig. 2). Following eight weeks of wet/dry cycling, germination was 45 % in T. epactia, 40 % in T. pungens and 38 % in T. wiseana. Plating onto KAR1 further enhanced germination, with maximum germination of 65 % in T. epactia, 60 % in T. pungens and 47 % in T. wiseana (all P < 0.001; Fig. 2).
Fig. 2.
Effects of wet/dry cycling on germination of intact florets in six Australian Triodia species. Florets were untreated (Control) or wet/dry cycled for 2–10 weeks. Following wet/dry cycling treatment, intact florets were incubated in Petri dishes containing either water or KAR1 (0.67 μm) agar at constant 30 °C under 12 h alternating light/dark for 28 d.
Experiment 3: determining germination responses of treated florets and cleaned seeds under water stress
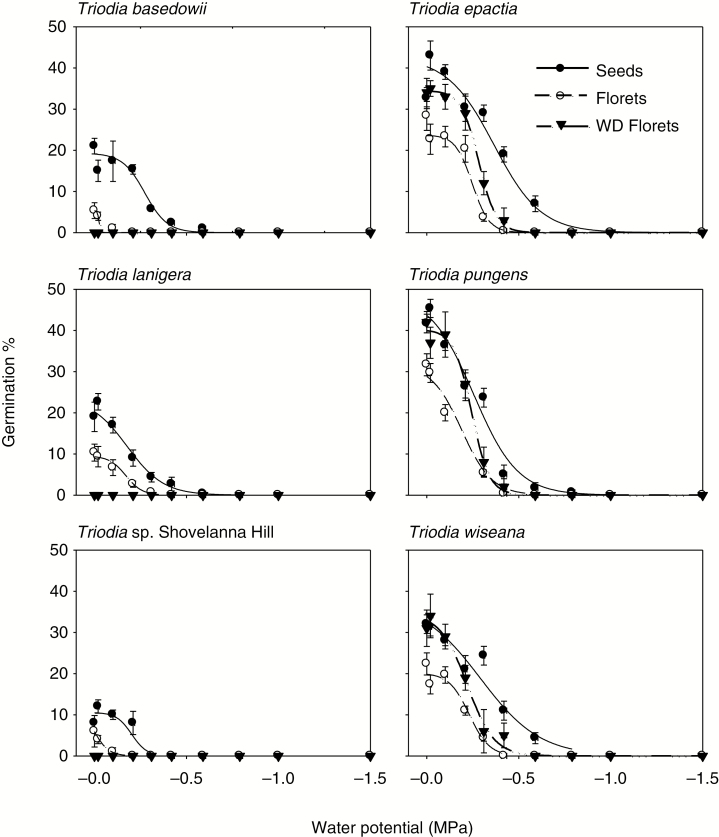
Under well-watered conditions, germination for all species was highest in cleaned seeds, although 8-week wet/dry-cycled florets demonstrated similar responses at 0 MPa in T. epactia, T. pungens and T. wiseana (P ≥ 0.131; Fig. 3). The highest germination in cleaned seeds was ≥40% in T. epactia and T. pungens, 31 % in T. wiseana, 21% in T. basedowii and T. lanigera, and 10 % in T. sp. Shovelanna Hill when compared to the two floret treatments (P < 0.001).
Fig. 3.
Effects of water potential on mean germination (± s.e., n = 4) of untreated florets, wet/dry-cycled (WD) florets and cleaned seeds in six Triodia species. Untreated florets, WD florets and cleaned seeds were plated into Petri dishes containing temperature-corrected PEG solutions at 0, –0.01, –0.1, –0.2, –0.3, –0.4, –0.6, –0.8, –1.0, and –1.5 MPa concentrations, and incubated at constant 30 °C under alternating light/dark conditions for 28 d. Water potential thresholds limiting germination at the maximum (Ψb95), median (Ψb50) and minimum (Ψb5) are presented in Table 2.
Overall, the reduction in germination due to water stress was always significantly greater (all P < 0.001) for intact florets, when compared with cleaned seeds in all species (Fig. 3; Table 2). The overall range for germination under water stress was also consistently greater for cleaned seeds when compared with intact florets in all species, as shown by lower water potential thresholds (Table 1; all P < 0.001). Regardless of wet/dry cycling, intact florets germinated between –0.05 and –0.49 MPa, while cleaned seeds ceased to germinate between –0.4 and –0.9 MPa (Fig. 3; Table 2).
Table 2.
Water potential thresholds describing the maximum (Ψb95), median (Ψb50) and minimum (Ψb5) (mean ± s.e., n = 4) responses of untreated florets, wet/dry-cycled (WD) florets and cleaned seeds in Triodia
| Triodia species | Dormancy state | Ψ b95 [MPa] | Ψ b50 [MPa] | Ψ b5 [MPa] |
|---|---|---|---|---|
| Triodia basedowii | Florets | 0.0 ± 0.0 | −0.01 ± 0.01 | −0.05 ± 0.01 |
| Seeds | −0.1 ± 0.01 t= 6.31*** | −0.24 ± 0.01 t= 6.31*** | −0.49 ± 0.05 t= 6.31*** | |
| Triodia epactia | Florets | −0.14 ± 0.03 | −0.27 ± 0.01 | −0.36 ± 0.04 |
| WD Florets | −0.12 ± 0.03t=−0.21 | −0.30 ± 0.02t=−1.06*** | −0.39 ± 0.07t=0.65 | |
| Seeds | −0.11 ± 0.01t=0.66 | −0.44 ± 0.01t=−3.04*** | −0.88 ± 0.1t=-7.14*** | |
| Triodia lanigera | Florets | −0.04 ± 0.03 | −0.18 ± 0.03 | −0.32 ± 0.07 |
| Seeds | −0.03 ± 0.01t=0.19 | −0.24 ± 0.02t=−1.21 | −0.49 ± 0.06t=2.20*** | |
| Triodia pungens | Florets | −0.12 ± 0.02 | −0.24 ± 0.01 | −0.36 ± 0.04 |
| WD Florets | −0.13 ± 0.02t=−0.21 | −0.27 ± 0.01t=−1.06*** | −0.40 ± 0.04t=-0.65 | |
| Seeds | −0.11 ± 0.01t=0.34 | −0.37 ± 0.01t=−6.78*** | −0.73 ± 0.06t=-8.01*** | |
| Triodia sp. Shovelanna Hill | Florets | 0.0 ± 0.0 | −0.01 ± 0.01 | −0.1 ± 0.1 |
| Seeds | 0.0 ± 0.0t=0.03 | −0.18 ± 0.02t=−5.81*** | −0.4 ± 0.05t=-6.91*** | |
| Triodia wiseana | Florets | −0.09 ± 0.02 | −0.23 ± 0.01 | −0.38 ± 0.03 |
| WD Florets | −0.07 ± 0.03t=0.5 | −0.26 ± 0.03t=2.59 | −0.41 ± 0.03t=−0.46 | |
| Seeds | −0.07 ± 0.01t=0.73 | −0.41 ± 0.01t=−5.06*** | −0.78 ± 0.07t=−8.32*** |
Untreated, WD florets and cleaned seeds were plated onto Petri dishes containing different PEG solutions simulating moisture stress. Experimental responses in T. basedowii, T. lanigera and T. sp Shovelanna Hill were omitted due to lack of germination following wet/dry cycling.
Water potentials were calculated from a three-parameter Weibull function for germination data across water potentials.
Summary statistics (t-statistics and P-values) are shown for WD florets and cleaned seeds for comparison with untreated florets only. Asterisks show the significance level at P < 0.001 (***).
DISCUSSION
The floret restricts Triodia germination to a narrow water stress envelope
This study examined bet-hedging strategies for seed germination in Triodia species by testing how the floret impacted seed germination across temperatures and under water stress. While other studies demonstrate that the removal of the restrictive floret improves seed germination under optimal temperature and water availability (Adkins et al., 2002; Ma et al., 2008; Erickson et al., 2016), we show that the floret influences seed germination performance under water-limiting conditions in arid zone grass species. Seeds contained within the florets of all six Triodia species showed limited germination under relatively low water stress conditions (i.e. high water potentials; see Fig. 3 and Table 1), suggesting that their seeds were overall highly sensitive to water stress during germination. For all species tested, the median and base water potential thresholds were consistently higher in florets when compared with cleaned seeds, further demonstrating how the floret restricts germination to a narrow range, under water-limited conditions. Such restrictions have significant implications for Triodia recruitment in arid zone grasslands (Lewandrowski et al., 2017).
Given that the seeds tested in this study were relatively small (approx. 0.7–1.6 mg; see Supplementary Data Appendix S1) and exhibited improved germination responses under alternating light and dark conditions compared with constant dark conditions, the germination niche appears to be close to the soil surface. This is due to smaller seeds lacking the penetrative strength to emerge from depth and they therefore are more frequently found in the upper soil layers (Fenner and Thompson, 2005). Additionally, the wide temperature range (20–40 °C) over which Triodia germination occurred is a common trait in many arid zone grass species (Mott, 1978; Jurado and Westoby, 1992; Erickson et al., 2016), and one that may allow seeds to take advantage of prolonged rainfall events across seasons. While rainfall in arid zone Triodia grasslands occurs predominantly during summer, high evaporative water stress during summer may disadvantage establishing seedlings. A wide germination envelope for temperature may provide a window of opportunity for germination to occur during cooler low evaporation periods, i.e. early/ late summer periods, although this remains to be tested.
The processes of spreading germination over time (i.e. as controlled by physiological dormancy) and restricting seedling emergence to periods of greatest water availability (i.e. high water potentials) probably represent a recruitment strategy to reduce seedling mortality in environments where rainfall is sporadic and unpredictable. Because of the different germination envelopes between intact florets and cleaned seeds, we suggest that the floret acts to limit Triodia germination when rainfall is low or sporadic. Furthermore, if the floret imposes a mechanical restriction on the seed under water stress, any compromise to floret structure by abrasion or scarification would influence moisture sensitivity, i.e. low water potentials (e.g. Ma et al., 2008; Stevens et al., 2015; Guzzomi et al., 2016).
Implications for regeneration of Triodia grasslands in the arid zone
Our results offer insight into why Triodia germination is often so unpredictable in degraded and/or arid landscapes (Bateman et al., 2016; Sluiter et al., 2016; Shackelford et al., 2017). While positive responses to KAR1 confirm the presence of a smoke-derived cue in Triodia seeds (Erickson et al., 2016), we demonstrate that intact florets hamper germination when exposed to KAR1, and that sensitivity to KAR1 increases with dormancy loss through wet/dry cycling. Intact florets still seem to hamper seed germination (0–45 %) when exposed to KAR1 in most Triodia species, and maximum germination rates were only achieved when florets were cleaned from seeds (germination 40–90 %). Out of all six species examined in this study, T. pungens florets were the most responsive to KAR1, corroborating the high regeneration potential this species shows following exposure to smoke (Gamage et al., 2014). With intact florets showing a reduced sensitivity to KAR1 in comparison with cleaned seeds, our findings suggest that the floret has a significant impact on seedling recruitment in fire-prone ecosystems. Although cued to germinate following fire (Wright and Fensham, 2017), slower germination speed (t50 rates >3 d) observed in intact florets (see Supplementary Data Appendix S2, Table S2) indicates that post-fire germination delay is contingent on a requirement for extended water availability after rainfall. Water retention in semi-arid ecosystems is typically short lived during summer periods, while high water stress through surface evaporation would also limit water availability to support seed germination. Triodia florets enable further bet-hedging, by preventing immediate post-fire depletion of the soil seed bank and facilitating the potential for some seeds to germinate only after later, extended rainfall events.
Although a number of studies have reported increased germination pulses following soil wetting and drying (Mott, 1978; Battaglia et al., 2000; Fay and Schultz, 2009), our results indicate that natural short-term wetting and drying cycles may be a significant ecological mechanism influencing recruitment bet-hedging in T. epactia, T. pungens and T. wiseana. As wetting and drying events occur through intermittent rainfall in the arid zone, an increasing number of florets are likely to respond following increasing rainfall in the season. Specifically, for the responsive species, optimal treatment consisted of four cycles of wetting and drying (treatment duration of eight weeks), with responses to KAR1 improving significantly with an increasing number of cycles. For the species that did not respond to wet/dry cycles, other environmental conditions mimicking natural seed bank dynamics may be required to improve germination (Erickson et al., 2016). Taken together, varying responsiveness following wet/dry cycling and exposure to KAR1 indicates that the degree to which bet-hedging occurs varies between species. While this study only investigated six Triodia species, further research is required in order to understand whether species responses within the genus are environmentally and/or genetically driven (Anderson et al., 2017; Dayrell et al., 2017).
Implications for regeneration from the restoration perspective
Arid zone restoration success is significantly impaired by low and erratic rainfall, and causes slow and infrequent regeneration (Merino-Martín et al., 2017; Shackelford et al., 2017). With seeds being the primary means to restore vegetation, removing the floret and treating seeds with KAR1 in conjunction with other seed enhancement technologies (e.g. Erickson et al., 2017) may improve establishment success in smoke-responsive grass species; however, this remains untested at large scales. Additionally, we show that successful germination is limited to windows of high water availability. As such, targeted irrigation that mimics large rainfall events to raise soil water potentials for extended periods could enhance recruitment in restoration sites, as long as water potentials are held above critical thresholds for germination.
While active restoration through seed enhancements or irrigation of sites may promote plant regeneration in a restoration context, recruitment events in natural systems may become altered, if not compromised, under predicted climate change scenarios (Parmesan and Hanley, 2015). Climate change is anticipated to negatively influence the timing, duration and intensity of rainfall in many ecosystems worldwide (Millennium Ecosystem Assessment, 2005; IPCC, 2013), including the Australian arid zone (Charles et al., 2013). As a consequence, if arid zone species such as Triodia demonstrate such tightly regulated recruitment patterns, shifts in rainfall patterns caused by climate change will significantly alter arid zone plant regeneration worldwide. Only a few studies highlight seedbank resilience to climate change (Parmesan and Hanley, 2015), and further research is required to understand the influence of climate change on seed dormancy, and subsequent plant recruitment in general.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Appendix S1: seed quality of commercial seed collections used for experimentation. Appendix S2: methodology and results for temperature and water stress effects on germination rate of treated Triodia seeds.
ACKNOWLEDGEMENTS
The authors acknowledge financial support from BHP Billiton Iron Ore, under the Pilbara Seed Atlas project (2008–2013) and the Restoration Seedbank Initiative (2013–present; Community Development Project Contract no. 8600048550), a partnership between BHP Billiton Iron Ore, The University of Western Australia and the Botanic Gardens and Parks Authority (BGPA). The authors thank staff and students at BGPA, particularly David Symons for providing logistical support and assistance during the study. The authors also thank Dr Mick Hanley and two anonymous reviewers for valuable comments that improved a previous version of this manuscript.
LITERATURE CITED
- Adkins SW, Bellairs SM, Koch DS. 2002. Seed dormancy mechanisms in warm season grass species. Euphytica 126: 13–20. [Google Scholar]
- Anderson BM, Thiele KR, Krauss SL, Barrett MD. 2017. Genotyping-by-sequencing in a species complex of Australian hummock grasses (Triodia): methodological insights and phylogenetic resolution. PLoS One 12: e0171053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin CC, Baskin JM. 2014. Seeds: ecology, biogeography, and evolution of dormancy and germination. Elsevier. [Google Scholar]
- Bateman A, Lewandrowski W, Stevens JC, Muñoz-Rojas M. 2016. Ecophysiological indicators to assess drought responses of arid zone native seedlings in reconstructed soils. Land Degradation and Development doi: 10.1002/ldr.2660. [Google Scholar]
- Battaglia LL, Fore SA, Sharitz RR. 2000. Seedling emergence, survival and size in relation to light and water availability in two bottomland hardwood species. Journal of Ecology 88: 1041–1050. [Google Scholar]
- Bewley JD, Bradford KJ, Hilhorst HWM, Nonogaki H. 2013. Seeds: physiology of development, germination and dormancy. Berlin: Springer. [Google Scholar]
- BOM 2016. Bureau of Meterology, Australia. [Google Scholar]
- Charles SP, Fu G, Silberstein RP et al. . 2013. Interim report on the hydroclimate of the Pilbara past, present and future. A report to the Western Australian Government and industry partners from the CSIRO Pilbara Water Resource Assessment, CSIRO Water for a Healthy Country, Australia. [Google Scholar]
- Clauss M, Venable D. 2000. Seed germination in desert annuals: an empirical test of adaptive bet hedging. American Naturalist, 155: 168–186. [DOI] [PubMed] [Google Scholar]
- Commander LE, Golos PJ, Miller BP, Merritt DJ. 2017. Seed germination traits of desert perennials. Plant Ecology 218: 1077–1091. [Google Scholar]
- Dayrell RL, Garcia QS, Negreiros D, Baskin CC, Baskin JM, Silveira FA. 2017. Phylogeny strongly drives seed dormancy and quality in a climatically buffered hotspot for plant endemism. Annals of Botany 119: 267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson TE, Merritt DJ. 2016. Seed collection, cleaning, and storage procedures. In: Erickson TE, Barrett RL, Merritt DJ, Dixon KW, eds. Pilbara seed atlas and field guide: plant restoration in Australia’s arid northwest. Dickson, Australian Capital Territory: CSIRO Publishing: 7–16. [Google Scholar]
- Erickson TE, Shackelford N, Dixon KW, Turner SR, Merritt DJ. 2016. Overcoming physiological dormancy in seeds of Triodia (Poaceae) to improve restoration in the arid zone. Restoration Ecology 24: S64–S76. [Google Scholar]
- Erickson TE, Muñoz-Rojas M, Kildisheva OA. et al. . 2017. Benefits of adopting seed-based technologies for rehabilitation in the mining sector: a Pilbara perspective. Australian Journal of Botany: in press. doi: 10.1071/BT17154. [Google Scholar]
- Fay PA, Schultz MJ. 2009. Germination, survival, and growth of grass and forb seedlings: effects of soil moisture variability. Acta Oecologica 35: 679–684. [Google Scholar]
- Fenner M, Thompson K. 2005. The ecology of seeds. Cambridge: Cambridge University Press. [Google Scholar]
- Flematti GR, Ghisalberti EL, Dixon KW, Trengove RD. 2004. A compound from smoke that promotes seed germination. Science 305: 977–977. [DOI] [PubMed] [Google Scholar]
- Gallart M, Verdú AMC, Mas MT. 2008. Dormancy breaking in Digitaria sanguinalis seeds: the role of the caryopsis covering structures. Seed Science and Technology 36: 259–270. [Google Scholar]
- Gamage HK, Memmott P, Firn J, Schmidt S. 2014. Harvesting as an alternative to burning for managing spinifex grasslands in Australia. Advances in Ecology 2014: 1–11. [Google Scholar]
- Gremer JR, Kimball S, Venable DL. 2016. Within- and among-year germination in Sonoran Desert winter annuals: bet hedging and predictive germination in a variable environment. Ecology Letters 19: 1209–1218. [DOI] [PubMed] [Google Scholar]
- Guzzomi AL, Erickson TE, Ling KY, Dixon KW, Merritt DJ. 2016. Flash flaming effectively removes appendages and improves the seed coating potential of grass florets. Restoration Ecology 24: S98–S105. [Google Scholar]
- Hoyle GL, Daws MI, Steadman KJ, Adkins SW. 2008. Mimicking a semi-arid tropical environment achieves dormancy alleviation for seeds of Australian native Goodeniaceae and Asteraceae. Annals of Botany 101: 701–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huarte R, Staltari S, Chorzempa S, Garćia MD. 2007. Tripsacum dactyloides (L) L. caryopses water uptake dynamics and germination responses to gibberellic acid, fluctuating temperature and pericarp scarification. Seed Science and Technology 35: 255–265. [Google Scholar]
- IPCC 2013. Climate change 2013: the physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change.Cambridge: Cambridge University Press. [Google Scholar]
- Jurado E, Westoby M. 1992. Germination biology of selected central Australian plants. Australian Journal of Ecology 17: 341–348. [Google Scholar]
- Lewandrowski W, Erickson TE, Dixon KW, Stevens JC. 2017. Increasing the germination envelope under water stress improves seedling emergence in two dominant grass species across different pulse rainfall events. Journal of Applied Ecology 54: 997–1007. [Google Scholar]
- Liu Y, Barot S, El-Kassaby YA, Loeuille N. 2017. Impact of temperature shifts on the joint evolution of seed dormancy and size. Ecology and Evolution 7: 26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long RL, Stevens JC, Griffiths EM, Adamek M, Powles SB, Merritt DJ. 2011. Detecting karrikinolide responses in seeds of the Poaceae. Australian Journal of Botany 59: 610. [Google Scholar]
- Ma HY, Liang ZW, Wang ZC, Chen Y, Huang LH, Yang F. 2008. Lemmas and endosperms significantly inhibited germination of Leymus chinensis (Trin.) Tzvel. (Poaceae). Journal of Arid Environments 72: 573–578. [Google Scholar]
- Merino-Martín L, Courauld C, Commander LE, Turner SR, Lewandrowski W, Stevens JC. 2017. Interactions between seed functional traits and burial depth regulate germination and seedling emergence under water stress in species from semi-arid environments. Journal of Arid Environments 147: 25–33. [Google Scholar]
- Michel BE. 1983. Evaluation of the water potentials of solutions of polyethylene glycol 8000 both in the absence and presence of other solutes. Plant Physiology 72: 66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millennium Ecosystem Assessment 2005. Ecosystems and human well-being: desertification synthesis. Washington, DC: World Resources Institute. [Google Scholar]
- Mott JJ. 1974. Mechanisms controlling dormancy in the arid zone grass Aristida contorta. I. Physiology and mechanisms of dormancy. Australian Journal of Botany 22: 635–645. [Google Scholar]
- Mott JJ. 1978. Dormancy and germination in five native grass species from savannah woodland communities of the Northern Territory. Australian Journal of Botany 26: 621–631. [Google Scholar]
- Muñoz-Rojas M, Erickson TE, Martini DC, Dixon KW, Merritt DJ. 2016. Climate and soil factors influencing seedling recruitment of plant species used for dryland restoration. Soil 2: 287–298. [Google Scholar]
- Nano CEM, Clarke PJ. 2010. Woody–grass ratios in a grassy arid system are limited by multi-causal interactions of abiotic constraint, competition and fire. Oecologia 162: 719–732. [DOI] [PubMed] [Google Scholar]
- Nicholas AM, Franklin DC, Bowman DM. 2009. Coexistence of shrubs and grass in a semi-arid landscape: a case study of mulga (Acacia aneura, Mimosaceae) shrublands embedded in fire-prone spinifex (Triodia pungens, Poaceae) hummock grasslands. Australian Journal of Botany 57: 396–405. [Google Scholar]
- Parmesan C, Hanley ME. 2015. Plants and climate change: complexities and surprises. Annals of Botany 116: 849–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, http://www.r-project.org/. [Google Scholar]
- Ritz C, Streibig JC. 2005. Bioassay analysis using R. Journal of Statistical Sofware 12: 1–21. [Google Scholar]
- Schwinning S, Sala OE. 2004. Hierarchy of responses to resource pulses in arid and semi-arid ecosystems. Oecologia 141: 211–220. [DOI] [PubMed] [Google Scholar]
- Shackelford N, Miller BP, Erickson TE. 2017. Restoration of open-cut mining in semi-arid systems: a synthesis of long-term monitoring data and implications for management. Land Degradation and Development doi: 10.1002/ldr.2746. [Google Scholar]
- Sluiter IR, Schweitzer A, Mac Nally R. 2016. Spinifex–mallee revegetation: implications for restoration after mineral-sands mining in the Murray–Darling Basin. Australian Journal of Botany 64: 547–554. [Google Scholar]
- Stevens J, Chivers I, Symons D, Dixon K. 2015. Acid-digestion improves native grass seed handling and germination. Seed Science and Technology 43: 313–317. [Google Scholar]
- Tielbörger K, Petruů M, Lampei C. 2012. Bet‐hedging germination in annual plants: a sound empirical test of the theoretical foundations. Oikos 121: 1860–1868. [Google Scholar]
- Weibull W. 1951. A statistical distribution function of wide applicability. Journal of Applied Mechanics 103: 293–297. [Google Scholar]
- Wright BR, Fensham RJ. 2017. Relationships between fire severity and recruitment in arid grassland dominated by the obligate-seeding soft spinifex (Triodia pungens). International Journal of Wildland Fire 25: 1264–1272. [Google Scholar]
- Zimmermann J, Higgins SI, Grimm V, Hoffmann J, Münkemüller T, Linstädter A. 2008. Recruitment filters in a perennial grassland: the interactive roles of fire, competitors, moisture and seed availability. Journal of Ecology 96: 1033–1044. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.