Abstract
Purpose
The present study aimed to determine the safety and efficacy of a drug-coated balloon inflated within a thin-strut self-expanding bare-metal stent in patients with severe and complex femoropopliteal occlusive disease.
Methods
This prospective study used the Pulsar-self-expanding stent and Passeo-18 Lux drug-coated balloon in patients with severe and complex femoropopliteal occlusive disease. The primary endpoint was the 12-month primary patency, and the secondary endpoints included 24-month primary patency, assisted primary patency, secondary patency, and clinically associated target lesion revascularisation.
Results
The study included 44 patients (51 limbs). The mean age of the patients was 67.6 ± 10.2 years, with 73% men. Chronic limb severity was classified as Rutherford Category III in 41% of the patients, stage IV in 31%, and stage V in 27%. Lesions were predominantly Trans-Atlantic Inter-Society Consensus (TASC 2007) D (51%) and C (45%), with 32 (63%) chronic total occlusions. Procedural success was obtained in all cases. The mean lesion length was 200 ± 74.55 mm (95% CI = 167.09–208.01) with a mean number of stents per limb used of 1.57 ± 0.70 (95% CI = 1.37–1.76). Distal embolisation occurred in two patients. The primary patency rates at the 12- and 24-month follow-up were 94% (95% CI = 82.9–98.1) and 88% (95% CI = 75.7–94.5), respectively. The assisted primary was 94% (95% CI = 82.9–98.1) and secondary patency was 96% (95% CI = 85.2–99.0) at 24-month follow-up. The cumulative stent fracture rate at the 24-month follow-up was 10%. Freedom from clinically driven target lesion revascularisation was 94% (95% CI = 83–98%) at 12-month follow-up and 88% (95% CI = 76–94%) at 24-month follow-up, with two patients requiring a bypass graft.
Conclusion
Our novel approach involving the combination of a thin-strut bare-metal stent and a drug-coated balloon may be safe and effective, with sustainable and promising clinical outcomes up to 24 months after treatment.
Keywords: Superficial femoral artery, popliteal artery, stenting, angioplasty, paclitaxel
Introduction
There have been significant advancements in endovascular management options for atherosclerotic femoropopliteal disease over the last decade.1 Maintenance of luminal patency might be challenged by negative vascular remodelling resulting from neointimal hyperplasia.2 Percutaneous transluminal balloon angioplasty (PTA) alone has been shown to be associated with high restenosis rates.3 The early elastic recoil and late lumen loss (LLL) associated with PTA have been treated effectively with self-expanding nitinol bare-metal stents (BMSs).4
It is well described that PTA may damage the intima, particularly in complex Trans-Atlantic Inter-Society Consensus (TASC 2007) C and D lesions, often resulting in stent implantation.5 A well-documented issue of stenting is a high rate of restenosis, as it can induce a foreign body reaction, leading to neointimal proliferation and in-stent stenosis.6,7
However, successful use of drug-eluting technology in coronary intervention has led to the implementation of drug-eluting stents (DESs) and drug-coated balloons (DCBs) for infrainguinal vessels. DESs provide sustained release of an antiproliferative medication such as paclitaxel (most commonly used in peripheral stents) into the arterial wall.8–11 However, for use in longer femoropopliteal lesions (≥120 cm), it requires multiple overlapping stents for adequate covering of the disease segment, that may result in-stent restenosis and fracture/dislodgement due to the continuous dynamic stresses.12,13 Conversely, DCBs allow rapid, homogenous delivery of an anti-proliferative drug to the vessel wall without leaving prosthetic material in the vessel.14–17 The 12-month primary patency has been shown to be superior and freedom from target lesion revascularisation (TLR) has been shown to be better with DCBs than with PTA.18,19 Ideally, a DCB should have minimal drug loss during insertion, tracking and inflation. A limited number of DCB trials have investigated relatively short lesions, with an average stent length of 70 mm.14,15 As such, a DCB alone may be insufficient for complex, calcified lesions and offers no scaffolding or resistance to early elastic recoil and LLL due to negative remodelling. BMSs have been shown to be ideal for adjunctive use.20–26 Coronary studies have found that the use of a thin-strut device could significantly reduce the angiographic and clinical restenosis rates after stenting.27,28
The present study aimed to determine the safety and efficacy of a DCB inflated within a thin-strut self-expanding BMS in patients with severe and complex femoropopliteal occlusive disease.
Methodology
Study design
This Drug Eluting Balloon Angioplasty and Stenting (DEBAS) study was a prospective study and was performed at three hospitals. The Pulsar-18/35 self-expanding stent (Biotronik AG, Bulach, Switzerland) and Passeo-18 Lux DCB (Biotronik AG) were used to treat severe and complex femoropopliteal arterial occlusive disease. Pulsar-18 and Pulsar-35 contain exactly the same stents. The difference is in the delivery system (6F/0.035″ vs. 4F/0.018″, respectively). The Pulsar 35 stent has a tri-axial deployment over either a 0.035″ or 0.018″ wire. Ethics committee approval was obtained from all participating facilities prior to the start of this study. Patients with symptomatic femoropopliteal stenotic or occlusive lesions, who were candidates for angioplasty and stenting, were eligible for enrolment if they met the general and angioplasty-specific inclusion criteria and none of the exclusion criteria (Table 1). According to the protocol, eligible candidates had to have Rutherford Category II (moderate claudication) to stage V (ischaemic ulceration not exceeding ulcer of the digits of the foot) disease.
Table 1.
DEBAS inclusion and exclusion criteria.
| Inclusion criteria |
| General |
| • De novo, restenotic or re-occluded lesion located in the femoropopliteal arteries suitable for endovascular treatment |
| • Patient presenting with a score from 2 to 5 according to the Rutherford classification |
| • Patient is willing to comply with specified follow-up evaluations at the predefined time intervals times |
| • Patient is >18 years old |
| • Patient understands the nature of the procedure and provides written informed consent, prior to enrollment in the study |
| • Prior to enrollment, the target lesion was crossed with standard guidewire manipulation |
| Angiographic |
| • The target lesions are located either within the native superficial femoral artery (SFA), the popliteal artery or the SFA and popliteal arteries |
| • The target lesion has angiographic evidence of stenosis or restenosis >50% or occlusion |
| • Target vessel diameter visually estimated is ≥4 mm and ≤6.5 m |
| • There is angiographic evidence of at least one tibial vessel runoff to the foot |
| Exclusion criteria |
| • Presence of another stent in the target vessel that was placed during a previous procedure |
| • Presence of an aortic thrombosis or significant common femoral ipsilateral stenosis |
| • Patients contraindicated for antiplatelet therapy, anticoagulants or thrombolytic |
| • Patients with known hypersensitivity to nickel-titanium or paclitaxel |
| • Patients with uncorrected bleeding disorders |
| • Female patient with child bearing potential not taking adequate contraceptives or currently breastfeeding |
| • Life expectancy of less than 12 months |
| • Ipsilateral iliac artery treatment before target lesion treatment with a residual stenosis >30% |
| • Use of thrombectomy, atherectomy or laser devices during procedure |
| • Any planned surgical intervention/procedure 30 days after the study procedure |
| • Any patient considered to be hemodynamically unstable at onset of procedure |
| • Patient is currently participating in another investigational drug or device study that has not reached the primary endpoint |
The treatment rationale was that in complex TASC C and D lesions, angioplasty alone would damage the intima, causing flow-limiting dissections often requiring stent implantation. Stent placement in long lesions has been associated with high rates of restenosis. However, inflating a DCB within the stent will ensure that the barotrauma is evenly spread across the stented length without substantially impeding drug transfer. The rationale for the use of thin-strut stents was that these stents decrease the distance between the DCB and the vessel wall owing to the low metal-to-artery ratio. This geometric principle of thin-struts reducing distance between drug coating and wall is independent of stent type or stent material. When the DCB is inflated within the stent, the scoring effect can cause plaque surface modification and may allow enhanced paclitaxel transfer, especially in calcified lesions.
Procedure
Vascular access was achieved using a 6F 45-cm Fortress sheath (Biotronik AG) for contralateral access and a 6F 11-cm Brite Tip® sheath (Cordis Corp., Miami, FL) for ipsilateral access. All inflow-limiting lesions were treated prior with either bare metal or covered stents.
It was mandatory for the guide-wire to cross the entire lesion before enrolment of the patient in the study. If several attempts at re-entry into the distal true lumen were unsuccessful using an antegrade approach, retrograde tibial (anterior tibial artery or posterior tibial artery) access was secured. This was achieved under direct ultrasound guidance using a micropuncture kit for placing a 4F sheath (Cook Medical, Bloomington, IN). According to the clinician’s discretion, the lesion could be treated with either direct primary stenting or predilation with a PTA balloon followed by BMS implantation. This was followed by the application of a DCB to the entire stented segment, extending 1 mm beyond the ends of the implanted stent, for a minimum of 90–120 s.
Systemic heparin was administered intravenously at 80–100 IU/kg prior to either PTA or stenting of the lesion. The study protocol mandated that patients subsequently commence clopidogrel (75 mg/day) for at least six months and lifelong aspirin (100–150 mg/day). Immediately after the procedure, a physical examination and ankle brachial index (ABI) assessment were performed. All complications and adverse events were recorded, along with the medications prescribed and the Rutherford Category at discharge.
Follow-up
Follow-up evaluations were mandated on day 1 and at 1, 6, 12, 18 and 24 months after the procedure. At each follow-up, a physical examination, ABI assessment, duplex ultrasound of the treated vessel and biplanar radiography were performed. For adequate interpretation of the biplanar radiographs, separate images were obtained for each stent in two planes, with maximal opacification and zoom. If there was evidence of restenosis, either computed tomography angiography or conventional angiography was performed to confirm the non-invasive findings.
Study devices
The Pulsar stent mounted on the Pulsar-18 or Pulsar-35 system is a self-expanding nitinol stent deployed using either a 4F (Pulsar-18) or 6F (Pulsar-35) introducer sheath. The Pulsar stent has a thin-strut design with high multi-axial flexibility that provides an optimised chronic outward force. This stent is available in different lengths (20, 40, 60, 80, 100, 120, 150, 170 and 200 mm) and diameters (4, 5, 6 and 7 mm). Standard PTA techniques were followed for balloon dilation before and after stent deployment.
The Passeo-18 Lux paclitaxel-coated balloon is available in diameters of 2–7 mm and lengths of 40–120 mm. Its design is based on that of the Passeo-18 uncoated balloon catheter, and it has a balloon coating containing a matrix of anti-proliferative paclitaxel and butyryl-trihexyl citrate, which is a biocompatible excipient for enabling optimal drug transfer to the target lesion tissue. The Passeo-18 Lux balloon also has a unique safeguard, which improves ease of handling and protects the user and balloon coating from contact and damage. After insertion into the introducer sheath, the safeguard can simply be peeled away.
Definitions and outcomes
The primary endpoint was 12-month primary patency defined as absence of >50% restenosis with an increase in the peak systolic velocity ratio (PSVR) ≥2.5 and no clinically associated re-intervention at the stented segment, within 5 mm of each side of the stented area.
The secondary endpoints were (1) technical success defined as the ability to cross and stent the lesion, and achieve angiographic residual stenosis <30% and residual stenosis <50% on duplex imaging; (2) sustained clinical success in follow-up defined as an improvement in the Rutherford Category by ≥1 level compared to the stage before the procedure, at the 6-, 12- and 24-month follow-up; (3) number and type of stent fractures at the 12- and 24-month follow-ups; (4) assisted primary patency and secondary patency at 12- and 24-month follow-up; (5) freedom from major adverse events at the 12- and 24-month follow-up; (6) freedom from TLR and target vessel revascularisation at 12- and 24-month follow-up; (7) freedom from major target limb amputation and death at the 12- and 24-month follow-up; and (8) secondary patency at 12- and 24-month follow-up.
Statistical analysis
Primary analysis of all baseline characteristics and study outcomes was based on the available data from all enrolled participants, unless otherwise indicated. Continuous data are presented as mean ± standard deviation (95% confidence interval (CI)) or median with interquartile range (IQR). Categorical data are presented as the number of patients and percentage. TASC (2007) II classification was used in this study. A patient with more than one event was counted only once towards the event rate based on the total number of participants with adverse events. Sub-group differences in rates of patency at 24 months were investigated using logistic regression. Kaplan–Meier analysis was used to evaluate time-to-event data, including the primary effectiveness endpoint. Further analysis was performed according to lesion length (lesion length <120 mm vs. lesion length ≥120 mm) and severity of calcification (mild or moderate vs. severe vessel calcification). When two legs from the same patient were enrolled in the study, it was necessary to recognise that the vessels were genetically identical and therefore the outcomes could be correlated. All analyses were therefore adjusted for potential intra-cluster correlation, using robust sandwich variance estimates, originally developed by Huber and White and later extended by Lin and Wei29 to produce the adjusted log-rank P-value and perform the survival analyses.
All hypotheses tests were performed using two-sided tests, and the critical value for statistical significance was set at a value of p < 0.05. All analyses were performed using PASW 22 (SPSS, Chicago, IL), SAS 9.4 (SAS Institute, Inc., Cary, NC) and Stata 14 (StataCorp LP, College Station, TX) statistical software.
Results
Patient characteristics
The study included 51 limbs from 44 patients between October 2007 and April 2010. The patient characteristics are summarised in Table 2. The mean age of the patients was 67.6 years, and 73 were men. Chronic limb severity was classified as Rutherford Category III in 41%, stage 4 in 31% and stage 5 in 27% of limbs. The most common pre-existing risk factors were hypertension (70%), hyperlipidaemia (52%), diabetes mellitus (55%) and smoking (39%). Of particular note, 16% of the treated lesions were in popliteal arteries, and the lesions were predominantly TASC D (51%) and C (45%), with 32 (63%) chronic total occlusions (Table 3).
Table 2.
Demographic, comorbidities and clinical characteristics of 44 patients at Baseline.
| Variable | n (%) |
|---|---|
| Age (years) | 67.6 ± 10.2 |
| Men | 32 (72.7%) |
| Race | |
| White | 43 (97.7%) |
| Asian | 1 (2.3%) |
| Smoking | 17 (38.6%) |
| Diabetes | 24 (54.6%) |
| CAD | 16 (36.4%) |
| Hypertension | 31 (70.4%) |
| Hyperlipidemia | 23 (52.3%) |
| CVA | 2 (4.5%) |
| CRF | 3 (6.8%) |
| Indication for treatment – n (%) | |
| Rest pain | 18 (35.3%) |
| Acute ischemia | 3 (5.9%) |
| Claudication | 27 (52.9%) |
| Ulcer/gangrene | 14 (27.4) |
| ASA classification | |
| ASA 2 | 20 (45.5%) |
| ASA 3 | 18 (40.9%) |
| ASA 4 | 6 (14.6%) |
CAD: coronary artery disease; CVA: cerebral vascular disease; CRF: chronic renal failure; ASA: American Society of Anesthesiologists.
Note: Continuous data are presented as the means ± standard deviation; categorical data are given as the counts (percentage).
Table 3.
Lesion and clinical characteristics at baseline.
| Characteristic | n = 51 | % (95% CI) |
|---|---|---|
| Study leg | ||
| Left limb | 27 | 52.9 (39–66) |
| Right limb | 24 | 47.1 (34–60) |
| Lesion location | ||
| Mid SFA | 4 | 8 (3–20) |
| Distal SFA | 4 | 8 (3–20) |
| SFA full length | 21 | 42 (27–58) |
| Popliteal artery | 8 | 16 (7–31) |
| SFA + popliteal artery | 13 | 26 (14–43) |
| Lesion characteristics | ||
| Pre procedure RVD [mm] | 6.02 ± 0.33 | (5.93 – 6.11) |
| Lesion length [mm] | 200 (IQR: 140–250)* | n/a |
| Total occlusions | 32 | 62.7 (46–77) |
| Calcification, n (%) | ||
| None or mild | 17 | 33.3 (19–46) |
| Moderate | 22 | 43.2 (29–58) |
| Severe | 12 | 23.5 (13–38) |
| TASC classification | ||
| TASC B | 2 | 3.9 (1–15) |
| TASC C | 23 | 45.1 (30–61) |
| TASC D | 26 | 51 (36–66) |
| Rutherford Becker (RB) category | ||
| RB3 | 21 | 41.2 (27–57) |
| RB4 | 16 | 31.4 (19–48) |
| RB5 | 14 | 27.4 (16–43) |
| Pre-operative ABI | 0.39 (IQR: 0.3–0.42) | |
n: number of limbs; IQR: interquartile range; SFA: superficial femoral artery; RVD: reference vessel diameter; TASC: trans-Atlantic inter-society consensus; ABI: ankle brachial index; n/a: non-applicable.
Note: Continuous data are presented as the means ± standard deviation or median (interquartile range); categorical data are given as the counts (percentage).
Procedural results
Technical success was achieved in all 51 (100%) treated limbs. Contralateral femoral access was used in the majority of cases (80%). A total of 80 stents were implanted in the 51 limbs, and the mean number of stents per limb was 1.57 ± 0.70 (95% CI = 1.37–1.76). A single stent was used in 55% of the patients, and 98% of the implanted stents had a diameter ≥6 mm. A total of 125 DCBs were used in the 51 limbs, and the mean number of balloons per limb was 2.45 ± 1.08 (95% CI = 2.13–2.78). Only DCBs with diameters of 6 and 7 mm were used. The mean inflation time was 1.80 ± 0.27 min (95% CI = 1.72–1.89). The mean lesion length was 200 ± 74.55 mm (95% CI = 167.09–208.01) with the normal-to-normal site method (Tables 3 and 4).
Table 4.
Baseline angiographic and interventional data.
| Variable | n = 51 | % (95% CI) |
|---|---|---|
| No. of crural runoff vessels | ||
| One vessel | 4 (7.8%) | 7.8 (2–24) |
| Two vessels | 18 (35.3%) | 35.3 (0.22–0.51) |
| Three vessels | 29 (56.9%) | 56.9 (0.41–0.71) |
| Vascular access | ||
| Femoral | 41 (80.4%) | 80.4 (0.66–0.89) |
| Retrograde tibial | 10 (19.6%) | 19.6 (0.11–0.33) |
| Mean lesion length | 200 ± 74.55 | (167.09–208.01) |
| No. of stents implanted | 1.57 ± 0.70 | (1.37–1.76) |
| Diameter of stents implanted | 6.21 ± 0.41 | (6.10–6.33) |
| Length of stents implanted | 200 (IQR: 120–300) | n/a |
| No. of DCB used/patient | 2.45 ± 1.08 | (2.13–2.78) |
| Diameter of DCB used/patient | 6.22 ± 0.42 | (6.10–6.33) |
| Balloon inflation time (min) | 1.80 ± 0.27 | (1.72–1.89) |
DCB: drug coated balloon; n: number of limbs; IQR: interquartile range.
Note: Continuous data are presented as the means ± standard deviation or median (interquartile range); categorical data are given as the counts (percentage).
Primary endpoint
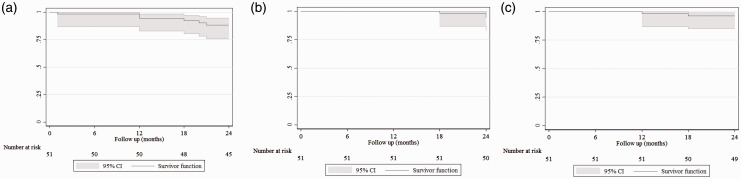
The primary patency rates at the 12 and 24-month follow-up were 94% (95% CI = 82.9–98.1) and 88% (95% CI = 75.7–94.5), respectively (Figure 1(a)). Logistic regression analyses according to lesion length demonstrate that there was no statistical difference between the two groups of patients (OR = 2.5; 95% CI = 0.29–21.24; p = 0.40).
Figure 1.
Kaplan–Meir curves representing primary patency (a), assisted primary patency (b) and secondary patency (c). Curve shows patency up to 24 months after combined stenting and drug-coated balloon angioplasty. The grey shadow indicates 95% confidence interval. Standard error did not exceed 10% at any time of follow-up. Figure 1(a) and (b) are courtesy of BIOTRONIK AG.
Secondary endpoints
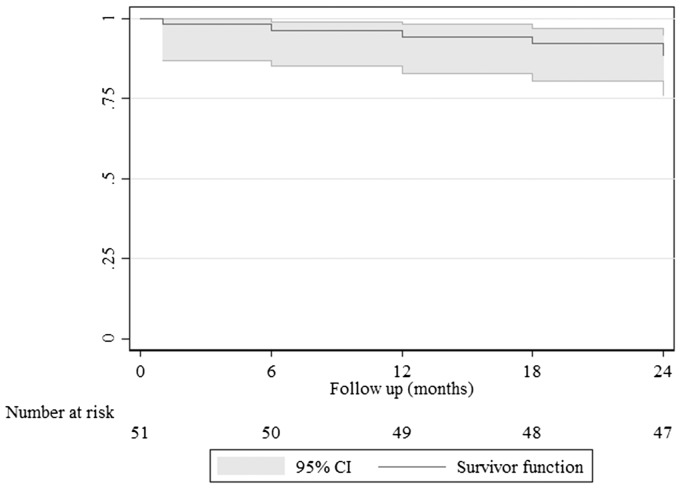
Freedom from clinically driven TLR (Figure 2) was 94% (95% CI = 83–98%) at 12-month follow-up and 88% (95% CI = 76–94%) at 24-month follow-up, with two patients requiring a bypass graft, and the rate of freedom from cumulative major amputation was 98% (Table 5). Analysis of freedom from TLR according to lesion length was 94% (95% CI = 63–99%) for lesions less than 120 mm length versus 86% (95% CI = 69–94%) for lesions ≥120 mm length at 24-month follow-up and this was not statistically significant (P = 0.44). The stent fracture rate at the 12-month follow-up was only 2%, and the cumulative stent fracture rate at the 24-month follow-up was 10% (Table 5). Type I stent fracture occurred in one patient at the 12-month follow-up, type II stent fracture occurred in three patients at the 18-month follow-up and type III stent fracture occurred in one patient at the 24-month follow-up. Only the type III stent fracture was associated with restenosis that required re-intervention.
Figure 2.
Kaplan–Meier curve representing freedom from revascularisation (TLR. Curve shows freedom from TLR up to 24 months follow-up. The grey shadow indicates 95% confidence interval. Standard error did not exceed 10% at any time of follow-up.
Table 5.
Two-years clinical and outcome safety data.
| Variables | 1 month (%) | 6 month (%) | 12 month (%) | 18 month (%) | 24 month (%) |
|---|---|---|---|---|---|
| Freedom from major amputation | 100 | 100 | 100 | 100 | 98 |
| Freedom from minor amputation | 98 | 96.1 | 96.1 | 94.1 | 96.1 |
| Freedom from stent fracturea | 100 | 100 | 98b | 94.1c | 98d |
CD-TLR: clinically driven target lesion revascularisation.
Stent fracture was confirmed on plain X-ray screening of the implanted stents.
Type I stent fracture.
Type II stent fracture.
Type III stent fracture.
The assisted primary patency rates (Figure 1(b)), at the 12- and 24-month follow-up were 98% (95% CI = 86.9–99.7) and 94% (95% CI = 82.9–98.1), respectively, and the secondary patency rates at the 12- and 24-month follow-up were 100% (95% CI = 86.9–100) and 96% (95% CI = 85.2–99.0), respectively (Figure 1(c)). The mean ABI improved immediately after treatment (with mean ABI at baseline was 0.39 ± 0.02, 95% CI = 0.36–0.42) and improvement was sustained up to 24-month follow up (mean ABI was 1.06 ± 0.02, 95% CI = 1.02–1.09). There was a satisfactory improvement among the patients, with 76% of the patients at the 12-month follow-up and 86% of the patients at the 24-month follow-up showing clinical improvement to Rutherford Category 0/I.
Procedure-related complications were identified in five patients (10%). Of the five patients, two had distal embolisation in the peroneal arteries that was managed conservatively, one had retroperitoneal bleeding requiring surgery, one had a false aneurysm requiring thrombin injection, and one had a haematoma that did not require any treatment or blood product transfusion. The overall median length of hospital stay was 2 days (IQR: 2–3).
Discussion
Our results suggest that the hybrid approach of DCB inflation following BMS implantation is safe, with good mid-term clinical results at 24 months after the procedure. Complex TASC C and D lesions were found to have a good primary patency at 24 months after the procedure. The improved mid-term luminal patency translated into low rates of TLR and amputations. The health care costs with this hybrid approach might be lower than the costs with the BMS only approach reported previously owing to the lower TLR rates with the hybrid approach than with the BMS only approach.
Scheinert et al.30 performed an international multicentre randomised control trial (BIOLUX P-1) to investigate the safety and efficacy of the Passeo-18 Lux DCB, which was used in our series. The authors found that the 6-month rates of TLR and binary restenosis were lower with this approach than with PTA, and these findings are comparable to the findings in our study. However, at the 12-month follow-up, the TLR rate was 16% in the as-treated population in this previous study, a rate four times higher than that observed in the present study. Furthermore, 57% of the cases developed post-procedure vessel dissections in the BIOLUX P-I study. It is unclear whether these outcomes were clinically significant; however, a BMS scaffold might help avoid such negative outcomes.
Multiple studies have compared DCB with PTA (LEVANT-1, THUNDER Pacifier, FemPac and BIOLUX P-116,30–32); however, most of these studies appear to be limited by the relatively short lesion length (57–81 mm). The authors of the previous studies might have been reluctant to randomise patients with longer lesions owing to the perceived inadequacy of dilation alone. The platform provided by the BMS in our series allowed for uniform application of paclitaxel along a reasonably long lesion.
In the Drug-Eluting Balloon in Peripheral Intervention for the Superficial Femoral Artery (DEBATE-SFA) trial, Liistro et al.17 randomised 104 patients presenting with critical limb ischaemia or claudication to either a drug-eluting balloon (DEB) and BMS group or a PTA and BMS group.17 All lesions underwent predilation with an uncoated balloon, and thereafter, the DEB and BMS group underwent further dilation with a paclitaxel-coated balloon (In.Pact Admiral, Invatec/Medtronic, Santa Rosa, CA) and subsequently received a self-expanding nitinol stent (Maris, Invatec/Medtronic). At the 12-month follow-up, the rate of freedom from TLR was 83.0%, which is lower than the rate noted in the present study. DCB inflation without stent presence may cause irregular distribution of paclitaxel. We used a thin-strut Pulsar-18 stent; however, Liistro et al. used the Maris stent (Invatec/Medtronic), which has slightly thicker struts, and this might have inhibited drug delivery to the arterial wall owing to the small paclitaxel footprint at the lesion and vessel wall.
Previous coronary studies found that the use of a thin-strut device was responsible for a significant reduction in angiographic and clinical restenosis rates after stenting.27,28 This concept has generated interest in combining DCB with thin-strut stents in order to reduce the distance between the drug coating and the vessel wall, with a well-established reduction of barotrauma on the vessel wall.
We did not find any difference in the primary patency rates between patients with lesions ≥120 mm in length and those with lesions <120 mm in length that is comparable to the findings of the VIPER study.33 The VIPER study was a single-arm study of 119 patients with long SFA lesions (mean length = 19 cm), and 56% of the lesions had chronic total occlusions and 61.0% had moderate-to-severe calcification. In this previous study, the GORE® Viabahn® endoprosthesis (WL Gore & Associates, Inc., Flagstaff, AZ) with a heparin bioactive surface exhibited 73% primary patency in long SFA lesions. Additionally, the patency rate was independent of lesion length, with equivalent results for long lesions (>20 cm) and medium lesions (5–20 cm) at 1 year. The VIASTAR study compared the GORE® Viabahn® endoprosthesis to a BMS for the treatment of complex, long lesions and noted similar results.34
Similar to our study, the BIOLUX 4EVER trial, currently at early phase, is evaluating the combination of a Pulsar-18 stent and Passeo-18 Lux Balloon in a multicentre, international study. However, in contrast to our practice of inflating the DCB within the BMS, the authors of this trial are attempting to initially treat the lesion with a DCB, followed by BMS implantation. It will be interesting to note the differences in the results between these two treatment strategies.
Limitations
This study included a very small study sample. Therefore, selection bias may exist and may have affected the conclusion and restricted the achievement of robust results. However, this is the first time this combination of therapeutic modalities has been used in complex femoropopliteal lesions (long lesions with or without major calcification). Type II error may occur due to the small sample size. The outcome of this study would have been boosted if it were compared with DCB angioplasty and bail out spot stenting; however, this constitutes complicatedly a different study that our institution will pursue in the near future.
Conclusion
Our novel approach involving the combination of a thin-strut BMS and a DCB may be safe and effective, with sustainable and promising clinical outcomes up to 24 months after treatment. This approach may be considered as a part of routine practice mainly in patients with complex and long occlusive lesions.
Acknowledgements
Figure 1(a) and 1(b) are courtesy of BIOTRONIK AG.
Patient consent
Patient understands the nature of the procedure and provides written informed consent, prior to enrollment in the study.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Mwipatayi has received research support from Biotronik AG.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Bates MC, Aburahma AF. An update on endovascular therapy of the lower extremities. J Endovasc Ther 2004; 11(Suppl 2): 107–127. [DOI] [PubMed] [Google Scholar]
- 2.Nikol S, Huehns TY, Höfling B. Molecular biology and post-angioplasty restenosis. Atherosclerosis 1996; 123: 17–31. [DOI] [PubMed] [Google Scholar]
- 3.Rocha-Singh KJ, Jaff MR, Crabtree TR, et al. Performance goals and endpoint assessments for clinical trials of femoropopliteal bare nitinol stents in patients with symptomatic peripheral arterial disease. Catheter Cardiovasc Interv 2007; 69: 910–919. [DOI] [PubMed] [Google Scholar]
- 4.Gray WA, Granada JF. Drug-coated balloons for the prevention of vascular restenosis. Circulation 2010; 121: 2672–2680. [DOI] [PubMed] [Google Scholar]
- 5.Ihnat DM, Duong ST, Taylor ZC, et al. Contemporary outcomes after superficial femoral artery angioplasty and stenting: the influence of TASC classification and runoff score. J Vasc Surg 2008; 47: 967–974. [DOI] [PubMed] [Google Scholar]
- 6.Hoffmann R, Mintz GS, Dussaillant GR, et al. Patterns and mechanisms of in-stent restenosis. A serial intravascular ultrasound study. Circulation 1996; 94: 1247–1254. [DOI] [PubMed] [Google Scholar]
- 7.Gray BH, Sullivan TM, Childs MB, et al. High incidence of restenosis/reocclusion of stents in the percutaneous treatment of long-segment superficial femoral artery disease after suboptimal angioplasty. J Vasc Surg 1997; 25: 74–83. [PubMed] [Google Scholar]
- 8.Gershlick A, De Scheerder I, Chevalier B, et al. Inhibition of restenosis with a paclitaxel-eluting, polymer-free coronary stent: the European evaluation of paclitaxel Eluting Stent (ELUTES) trial. Circulation 2004; 109: 487–493. [DOI] [PubMed] [Google Scholar]
- 9.Moses JW, Mehran R, Nikolsky E, et al. Outcomes with the paclitaxel-eluting stent in patients with acute coronary syndromes: analysis from the TAXUS-IV trial. J Am Coll Cardiol 2005; 45: 1165–1171. [DOI] [PubMed] [Google Scholar]
- 10.Creel CJ, Lovich MA, Edelman ER. Arterial paclitaxel distribution and deposition. Circ Res 2000; 86: 879–884. [DOI] [PubMed] [Google Scholar]
- 11.Axel DI, Kunert W, Göggelmann C, et al. Paclitaxel inhibits arterial smooth muscle cell proliferation and migration in vitro and in vivo using local drug delivery. Circulation 1997; 96: 636–645. [DOI] [PubMed] [Google Scholar]
- 12.Klein AJ, Chen SJ, Messenger JC, et al. Quantitative assessment of the conformational change in the femoropopliteal artery with leg movement. Catheter Cardiovasc Interv 2009; 74: 787–798. [DOI] [PubMed] [Google Scholar]
- 13.Scheinert D, Scheinert S, Sax J, et al. Prevalence and clinical impact of stent fractures after femoropopliteal stenting. J Am Coll Cardiol 2005; 45: 312–315. [DOI] [PubMed] [Google Scholar]
- 14.Tepe G, Zeller T, Albrecht T, et al. Local delivery of paclitaxel to inhibit restenosis during angioplasty of the leg. N Engl J Med 2008; 358: 689–699. [DOI] [PubMed] [Google Scholar]
- 15.Werk M, Albrecht T, Meyer DR, et al. Paclitaxel-coated balloons reduce restenosis after femoro-popliteal angioplasty: evidence from the randomized PACIFIER trial. Circ Cardiovasc Interv 2012; 5: 831–840. [DOI] [PubMed] [Google Scholar]
- 16.Werk M, Langner S, Reinkensmeier B, et al. Inhibition of restenosis in femoropopliteal arteries: paclitaxel-coated versus uncoated balloon: femoral paclitaxel randomized pilot trial. Circulation 2008; 118: 1358–1365. [DOI] [PubMed] [Google Scholar]
- 17.Liistro F, Grotti S, Porto I, et al. Drug-eluting balloon in peripheral intervention for the superficial femoral artery: the DEBATE-SFA randomized trial (drug eluting balloon in peripheral intervention for the superficial femoral artery). JACC Cardiovasc Interv 2013; 6: 1295–1302. [DOI] [PubMed] [Google Scholar]
- 18.Tepe G, Laird J, Schneider P, et al. Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12-month results from the IN.PACT SFA randomized trial. Circulation 2015; 131: 495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Micari A, Cioppa A, Vadalà G, et al. 2-Year results of paclitaxel-eluting balloons for femoropopliteal artery disease: evidence from a multicentre registry. JACC Cardiovasc Interv 2013; 6: 282–289. [DOI] [PubMed] [Google Scholar]
- 20.Steiner S, Schmidt A, Bausback Y, et al. Midterm patency after femoropopliteal interventions: a comparison of standard and interwoven nitinol stents and drug-coated balloons in a single-center, propensity score-matched analysis. J Endovasc Ther 2016; 23: 347–355. [DOI] [PubMed] [Google Scholar]
- 21.Schillinger M, Sabeti S, Loewe C, et al. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N Engl J Med 2006; 354: 1879–1888. [DOI] [PubMed] [Google Scholar]
- 22.Krankenberg H, Schlüter M, Steinkamp HJ, et al. Nitinol stent implantation versus percutaneous transluminal angioplasty in superficial femoral artery lesions up to 10 cm in length: the femoral artery stenting trial (FAST). Circulation 2007; 116: 285–292. [DOI] [PubMed] [Google Scholar]
- 23.Mwipatayi BP, Hockings A, Hofmann M, et al. Balloon angioplasty compared with stenting for treatment of femoropopliteal occlusive disease: a meta-analysis. J Vasc Surg 2008; 47: 461–469. [DOI] [PubMed] [Google Scholar]
- 24.Acin F, de Haro J, Bleda S, et al. Primary nitinol stenting in femoropopliteal occlusive disease: a meta-analysis of randomized controlled trials. J Endovasc Ther 2012; 19: 585–595. [DOI] [PubMed] [Google Scholar]
- 25.Bosiers M, Deloose K, Callaert J, et al. 4-French-compatible endovascular material is safe and effective in the treatment of femoropopliteal occlusive disease: results of the 4-EVER trial. J Endovasc Ther 2013; 20: 746–756. [DOI] [PubMed] [Google Scholar]
- 26.Matsumura JS, Yamanouchi D, Goldstein JA, et al. The United States stuDy for evalUating endovasculaR treAtments of lesions in the superficial femoral artery and proximal popliteal by usIng the protege everfLex nitinol stent system II (DURABILITY II). J Vasc Surg 2013; 58: 73.e1–83.e1. [DOI] [PubMed] [Google Scholar]
- 27.Kastrati A, Mehilli J, Dirschinger J, et al. Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO) trial. Circulation 2001; 103: 2816–2821. [DOI] [PubMed] [Google Scholar]
- 28.Pache J, Kastrati A, Mehilli J, et al. Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO-2) trial. J Am Coll Cardiol 2003; 41: 1283–1288. [DOI] [PubMed] [Google Scholar]
- 29.Lin DY, Wei LJ. The robust inference for the cox proportional hazards model. J Am Stat Assoc 1989; 84: 1074–1078. [Google Scholar]
- 30.Scheinert D, Schulte KL, Zeller T, et al. Paclitaxel-releasing balloon in femoropopliteal lesions using a BTHC excipient: twelve-month results from the BIOLUX P-I randomized trial. J Endovasc Ther 2015; 22: 14–21. [DOI] [PubMed] [Google Scholar]
- 31.Scheinert D, Duda S, Zeller T, et al. The LEVANT I (Lutonix paclitaxel-coated balloon for the prevention of femoropopliteal restenosis) trial for femoropopliteal revascularization: first in human randomized trial of low-dose drug-coated balloon versus uncoated balloon angioplasty. JACC Cardiovasc Interv 2014; 7: 10–19. [DOI] [PubMed] [Google Scholar]
- 32.Tepe G, Schnorr B, Albrecht T, et al. Angioplasty of femoral-popliteal arteries with drug-coated balloons: 5-year follow-up of the THUNDER trial. JACC Cardiovasc Interv 2015; 8: 102–108. [DOI] [PubMed] [Google Scholar]
- 33.Saxon RR, Chervu A, Jones PA, et al. Heparin-bonded, expanded polytetrafluoroethylene-lined stent graft in the treatment of femoropopliteal artery disease: 1-year results of the VIPER (Viabahn Endoprosthesis with Heparin Bioactive Surface in the Treatment of Superficial Femoral Artery Obstructive Disease) trial. J Vasc Interv Radiol 2013; 24: 165–173; quiz 174. [DOI] [PubMed] [Google Scholar]
- 34.Lammer J, Zeller T, Hausegger KA, et al. Heparin-bonded covered stents versus bare-metal stents for complex femoropopliteal artery lesions: the randomized VIASTAR trial (Viabahn endoprosthesis with PROPATEN bioactive surface [VIA] versus bare nitinol stent in the treatment of long lesions in superficial femoral artery occlusive disease). J Am Coll Cardiol 2013; 62: 1320–1327. [DOI] [PubMed] [Google Scholar]