Abstract
Background:
Myxofibrosarcoma (MFS), formerly considered as a myxoid variant of malignant fibrous histiocytoma, is the most common sarcoma of the extremities in adults and is characterized by a high frequency of local recurrence. The clinical behavior of MFS is unpredictable and the efficacy of chemotherapy is still not well documented. Furthermore, given the relatively recent recognition of MFS as a distinct pathologic entity its cellular and molecular biology has still not been extensively studied in patient-derived preclinical models. We examined the molecular biology and treatment outcomes of high-grade, patient-derived MFS primary cultures.
Methods:
A total of three patient-derived MFS primary cultures were analyzed. We evaluated the role of CD109 expression and also looked for a correlation between transforming growth factor-beta (TGF-β) expression and sensitivity of the primary cultures to different drugs.
Results:
CD109 was a promising marker for the identification of more aggressive high-grade MFS and a potential therapeutic target. The results also highlighted the potential role of TGF-β in chemoresistance. Pharmacological analysis confirmed the sensitivity of the cultures to chemotherapy. The most active treatments were epirubicin alone and epirubicin in combination with ifosfamide, the latter representing the current standard of care for soft tissue sarcomas (STSs), including MFS.
Conclusions:
Our results provide a starting point for further research aimed at improving the management of MFS patients undergoing chemotherapy.
Keywords: CD109, chemotherapy, high-grade myxofibrosarcoma, primary culture, TGF-β
Introduction
Myxofibrosarcoma (MFS) is one of the most common soft tissue sarcomas (STSs) usually affecting the extremities in adults.1 Currently, the World Health Organization describes MFS as a spectrum of malignant fibroblastic lesions with myxoid stroma, pleomorphism and curvilinear vessels.2,3 The majority of lesions occur on the lower limbs but have also been reported on the trunk and head/neck region. The retroperitoneum and abdominal cavity are very rare sites of occurrence. Clinically, low-grade lesions show a propensity for multiple local recurrence and are characterized by low metastatic capacity.4 Conversely, high-grade lesions, exhibit increasing rates of metastatic potential.1–6 Furthermore, 15–38% of local MFS recurrences evolve to higher histologic entities with increased metastatic potential.2 The prognosis for MFS is slightly better than that of other STSs, with an overall incidence of distant metastases ranging from 20% to 25%.7,8 At present there are no specific immunohistochemical markers for the standard differential diagnosis of MFS, which is based on the analysis of cytomorphologic findings comprising the presence of a myxoid background, nuclear atypia, pseudolipoblasts and curvilinear blood vessels. These histological characteristics also represent key features for distinguishing MFS from other sarcoma subtypes, for example, liposarcoma, in cases of dubious diagnosis.9,10
The current standard of care for localized disease is radical resection with clear surgical margins combined with neoadjuvant or adjuvant radiotherapy. The management of extremity MFS favors organ preservation, with amputation only considered in selected cases.11 However, despite treatments, local recurrence rates are between 50% and 60%.12–14 The use of chemotherapy in metastatic MFS, which represents the standard clinical care, is still limited and outcome remains very poor. As with other STS, no large clinical trials focusing on this tumor have been carried out to date, making it crucial to identify novel molecular markers that can be used for diagnostic purposes but also as new therapeutic targets. In this scenario, patient-derived specimens represent precious tools that have been widely used to focus on tumor pathophysiology and pharmacology.15–17 This is particularly evident in the case of rare tumors such as MFS where case series are inevitably small, limiting the number of surgical specimens. Furthermore, the number of commercially available immortalized cell lines is limited. Primary cultures thus represent a promising strategy for improving our understanding of the biology of these malignancies and for tailoring patient treatment.
The above consideration led us to analyze three patient-derived MFS primary cultures. Tumors from patients treated at our institute [Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST) IRCCS, Meldola, Italy] were harvested intraoperatively and MFS primary cultures were obtained. The cultures were characterized in terms of gene expression analysis. In particular, we evaluated the potential role of CD109, a transforming growth factor-beta (TGF-β) co-receptor first reported in this histotype by Emori and colleagues in 2015, as a novel marker and therapeutic target in MFS.18 The authors observed that CD109 was more frequently expressed in high-grade MFS than in low-grade disease. Although no statistically significant correlation was found between CD109 expression and tumor grade, an interesting trend was observed in this specific setting. The above results prompted us to explore the role of CD109 as an indicator of a more aggressive MFS histotype. We also investigated the sensitivity of our primary culture to different drugs currently used in clinical practice or under evaluation for the treatment of STS. Within this context, the expression of TGF-β was evaluated as a potential marker of chemoresistance.
To the best of our knowledge, this is the first study to examine the molecular biology and treatment outcomes of high-grade, patient-derived MFS primary cultures.
Materials and methods
Case series
The study involved three patients with high-grade MFS. All patients underwent surgical treatment performed by an experienced orthopedic surgeon at the Department of Orthopedics of Istituto Ortopedico Rizzoli, Bologna, Italy. Briefly, the tumor mass was surgically excised, as reported in the results section. The protocol was approved by the IRST-Area Vasta Romagna Ethics Committee, approval no. 4751/2015, and performed according to Good Clinical Practice standards and the Declaration of Helsinki. All patients gave written informed consent to take part in the study.
The surgical specimens were analyzed by a sarcoma pathologist (Pathology Unit, Morgagni-Pierantoni Hospital, Forlì, Italy) and processed within 3 h of surgical resection. Normal tissue characterized by adipose tissue, fascia, vessels and muscle tissue [Suppl. Figure 1(a) and (b)] obtained from one of the three patients was selected by a sarcoma pathologist and used as study control. In accordance with the protocol, all downstream preclinical analyses were performed at the Osteoncology and Rare Tumors Center [Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST) IRCCS].
Establishment of primary cell culture
Patient-derived MFS cell cultures were isolated from the surgical specimen. Tumor specimens were washed twice in sterile phosphate buffered saline (PBS) and shredded into 1–2 mm3 pieces with surgical scalpels. The obtained pieces were incubated with a PBS solution of 2 mg/ml collagenase type I (Millipore Corporation, Billerica, MA, USA) at 37°C in stirring conditions for 15 min and then stored overnight at room temperature. The next day the collagenase digestion was blocked by adding Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, 1% glutamine and 10% penicillin/streptomycin.
The cells were isolated from the aggregates using 100-µm sterile filters (CellTrics, Partec, Münster, Germany). Cells were counted and seeded in standard monolayer cultures at a density of 80,000 cells/cm2 and maintained in complete DMEM medium at 37°C in a 5% CO2 atmosphere. All the experiments were conducted using low-passage primary cultures and analyzed by an experienced sarcoma pathologist.
Synthesis of three-dimensional collagen scaffolds
The collagen scaffolds were synthesized in our laboratory as previously described, with slight modifications.19–22 A 1%-wt suspension of an insoluble type I microfibrillar collagen derived from bovine Achilles tendon (Sigma Aldrich, St. Louis, MO, USA) was prepared in 0.05 M of acetic acid solution (pH 3.2) and precipitated to pH 5.5 by adding 1 M of sodium hydroxide solution, resulting in a homogeneous suspension of fine collagen coprecipitates. The material was then chemically cross-linked with a 1 wt% 1,4-butanediol diglycidyl ether (BDDGE) for 24 h at 25°C to stabilize the collagen matrix and to control porosity and tortuosity. This suspension was blended at 20,000 rpm using an overhead homogenizer for 30 min at 4°C and, after mixing, was centrifuged at 2500 rpm for 5 min to remove air bubbles formed by blending. The mixed solution was frozen for 24 h and then freeze-dried for 24 h.
In order to consolidate the three-dimensional (3D) structure and to yield optimal levels of pore interconnectivity, the structure was freeze-dried with a controlled freezing and heating ramp from 25°C to −35°C and from −35°C to 25°C under vacuum conditions, p = 0.20 mbar. Finally, the scaffolds were sterilized by immersion in ethanol 70% for 1 h followed by three washes in PBS.
Immunohistochemical analysis
Hematoxylin and eosin (H&E) staining was performed to evaluate the morphological features and distribution of the cells. Briefly, tissue specimens were recovered, washed twice with PBS, and immediately paraffin-embedded in a cryomold (25 mm × 20 mm × 5 mm). Tissue blocks were cooled to −15°C and then sectioned into 5-µm-thick slices with a microtome. The slides were then stained using standard techniques and analyzed. MDM-2 gene amplification was performed to exclude the diagnosis of dedifferentiated liposarcoma. MDM-2 amplification was assessed by FISH analysis following the manufacturer’s instructions (Vysis MDM2/CEP12 dual color FISH probe kit). For primary cultures, 500,000 cells were cultured for 7 days in scaffolds which were paraffin-embedded in a cryomold (25 mm × 20 mm × 5 mm). The obtained slides were thawed, hydrated, stained with H&E and analyzed. Images were captured with an optical Zeiss Axioskop microscope equipped with a Polaroid camera. Immunohistochemistry analysis of CD109 expression in primary cultures was performed on 4 µm sections with the Ventana (Ventana Medical Systems, Tucson, Arizona, USA) according to the manufacturer’s instructions. The cultures were stained with mouse monoclonal antibody against CD109 (1:100, 496920 ThermoFisher Scientific, Waltham, Massachusetts, USA) and then with anti-mouse horseradish peroxidase (HRP)/diaminobenzidine (DAB). Counterstaining was performed with hematoxylin. Normal tissue was used as negative control. CD109 expression was determined as the percentage of immunopositive cells within the total analyzed area. Cells were considered positive in the presence of brown cytoplasmic staining.
Gene expression analysis
For gene expression analysis, tissue specimens were processed and the cells obtained were immediately stored with TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) without being cultured in vitro to avoid the risk of molecular changes. mRNA isolation was carried out according to the manufacturer’s instructions. A total of 150 ng of extracted RNA was reverse-transcribed using the iScript cDNA Synthesis Kit (BioRad, Hercules, CA, USA). Gene expression analysis was then carried out by 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) with the TaqMan gene expression assay mix (Applied Biosystems). For TGF-β analysis, amplification was performed in a total volume of 20 µl containing 2× TaqMan Universal PCR Master Mix (Applied Biosystems) and 2 µl of cDNA. ACTB and HPRT were used as housekeeping genes. The resulting amount the transcripts was normalized to the related housekeeping genes with the 2-ΔΔCT method. We used SYBR Select Master Mix (Applied Biosystems) with 2 µl of cDNA for CD109 analysis. B2 and HPRT were used as housekeeping genes. The primers for CD109 amplification were forward 5′-CCTGTGACCTTTGCAGTGATGT-3′ and reverse 5′-GAGTGATGATGGGAGCCTGAA-3′.23
Drug testing
10,000 cells/well were seeded in 96-well plates. Cells were allowed to recover for 3 days before treatment. The regimens were selected on the basis of the peak plasma concentration of each drug obtained from pharmacokinetic clinical data; ifosfamide (IFO)100 µm,24,25 epirubicin (EPI) 3.4 µm,26,27 EPI 3.4 µm plus IFO 100 µm, and trabectidin 2.2 × 10-5 µm.28 Survival percentages were assessed by the MMT assay (Sigma Aldrich) after 72 h of drug exposure according to the manufacturer’s instructions. The experiments were performed twice.
TUNEL assay
DNA fragmentation resulting from apoptotic signals was detected by the terminal deoxynucleotidyl transferase (TdT) nick end labeling (TUNEL) assay, as previously reported.29 After treatment, cells were washed twice with PBS, fixed in 1% formaldehyde on ice for 15 min and incubated in 70% ice cold ethanol for 1 h. Cells were then washed twice with PBS, permeabilized with 0.1% Triton-X100 for 5 min and incubated with TdT and FITC conjugated dUTP deoxynucleotides 1:1 solution (Roche Diagnostic GmbH, Mannheim, Germany) in a humidified atmosphere for 90 min at 37°C in the dark conditions. Images were captured with an inverted fluorescence microscope.
Statistical analysis
Three independent replicates were performed for each experiment. Data are presented as mean ± standard deviation (SD) or mean ± standard error, as stated, with n indicating the number of replicates. Differences between groups were assessed by a two-tailed Student’s t-test and accepted as significant at p < 0.05.
Results
Patient characteristics
MF1 history
Patient MF1 was a 66-year old female with a primary diagnosis of high-grade myxofibrosarcoma of the right thigh operated on July 2013 with negative surgical margins.
A chest computed tomography (CT) scan after surgery revealed the presence of bilateral nodules suggestive of lung metastases and subsequently confirmed by positron emission tomography (PET) CT scan with fludeoxyglucose F 18 (FDG). In September 2013, the patient underwent an atypical resection of the bilateral lung lesions, the histological report confirming the presence of adenocarcinoma of the lung, pT2a N0 LV0 G2 R0 staging with mutated K-ras and wild type EGFR. After surgery, there was no evidence of metastatic disease and she was treated with adjuvant chemotherapy for the lung cancer with carboplatin and gemcitabine for six cycles. She was monitored closely and was disease-free for about 9 months.
In February 2015, a new thigh lesion was surgically removed, the histology report indicating a relapse of high-grade myxofibrosarcoma; Ki67 70%; mitotic index 19 mitoses/10 high power fields (HPFs). Adjuvant local radiotherapy was administered.
In September 2015 evidence was found of inguinal lymph node involvement and the patient began chemotherapy with doxorubicin. After three cycles, she obtained a complete response of the lymph node disease and lung progression with the appearance of a new nodule of about 2 cm detected in the right pulmonary lobe. Chemotherapy was stopped and the patient underwent surgery to remove the lung nodule, confirmed as a relapse of the lung adenocarcinoma. The subsequent CT scan revealed progression in the lung, with the appearance of another nodule in the same lung. A biopsy revealed the presence of cells from high-grade myxofibrosarcoma. In October 2016, the patient began rechallenge with weekly anthracycline, initially obtaining SD but subsequently progression the lung. Her clinical conditions gradually deteriorated and she died in April 2017.
MF2 history
Patient MF2 was a 69-year-old male diagnosed with high-grade myxofibrosarcoma of the right knee in December 2013 and treated only with radical surgery. A recurrence occurred in the same knee in March 2014 and surgery was performed, with no evidence of residual microscopic tumor. After surgery, the patient was monitored closely and remained disease-free for 10 months. In June 2015, a magnetic resonance imaging (MRI) scan of the right knee revealed the presence of a new subcutaneous nodule near the previous surgical area. Surgery was performed, the histology confirming neoplastic cells from high-grade myxofibrosarcoma with myxoid areas, areas of necrosis and hemorrhage, and hypercellulated areas with marked pleomorphism. The tumor was completely excised. Our institute’s multidisciplinary team studied the case and scheduled radiotherapy of the right knee, which was concluded in September 2015. The patient continues to be disease-free.
MF3 history
Patient MF3 was a 64-year-old male diagnosed with high-grade myxofibrosarcoma of the left arm in March 2016. Surgery showed positive margins and so radicalization was performed, with complete excision of the tumor. Our institute’s multidisciplinary team recommended adjuvant radiotherapy, completed in November 2016. The patient is currently disease-free.
Patient characteristics are summarized in Table 1.
Table 1.
Patient characteristics.
| Patient | Sex | Age at surgery (years) | Site | Size (cm) | Histological subtype | IHC analysis |
Molecular cytogenetic analysis | Grade | Tumor | Radiotherapy post-surgery | Chemotherapy post-surgery | Follow up (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MF1 | Female | 66 | Thigh | 15 × 3 × 5.5 | Myxofibrosarcoma | SMA+ CD34- Myogenin + S100- |
MDM2/CEP12 = 1 | G3 | Local and distant recurrence | No | Yes | 22 |
| MF2 | Male | 69 | Knee | 6 × 4 × 3 | Myxofibrosarcoma with markedly pleomorphic areas | n/a | MDM2/CEP12 = 1 | G3 | Local recurrence | Yes | No | 14 |
| MF3 | Male | 64 | Lower arm | 14 × 7 × 2 | Myxofibrosarcoma | SMA+ CD34 + vimentin + cytokeratin - S100- |
MDM2/CEP12 = 1 | G3 | Primary tumor | Yes | No | 8 |
IHC, immunohistochemical; n/a, not applicable; SMA, smooth muscle actin.
Establishment of patient-derived myxofibrosarcoma cultures
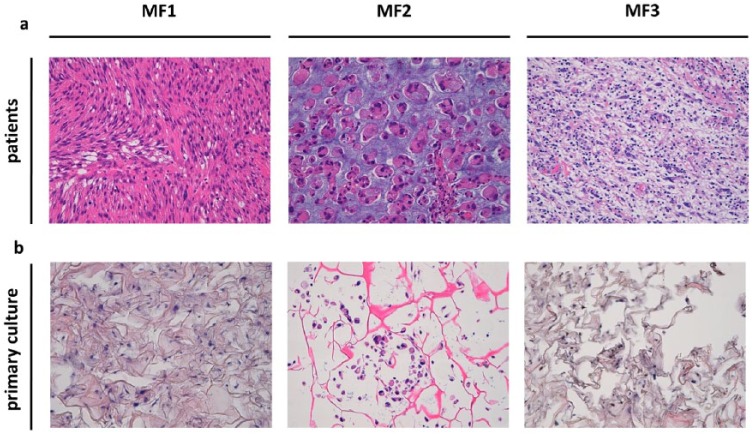
H&E-stained, surgically-resected tumor tissue was reviewed by an experienced sarcoma pathologist who confirmed the diagnosis of high-grade myxofibrosarcoma [Figure 1(a)]. MFS tissue specimen 1 (MF1) was a recurrence of hypodermic musculoskeletal myxofibrosarcoma [grade 3 Fédération Nationale des Centres de Lutte Contre le Cancer (FNCLCC)] with fibrotic scar tissue from the previous surgery. The dermis and the epidermis were undamaged. Tumor cells were positive for smooth muscle actin (SMA) and myogenin, and negative for CD34 and S100 protein. The proliferation index, estimated with Ki67 staining, was 70%. Moreover, areas of necrosis were detected and a mitotic index of 19 mitoses/10 HPF was calculated. MDM-2 gene amplification assessed by FISH was negative (MDM2/Cep12 ratio of 1.0) thus excluding a diagnosis of dedifferentiated liposarcoma.30,31 MF2 was characterized by fibroadipose tissue with an ‘apple-jelly’ nodule, mitotic index of 6 mitoses/10 HPF was determined. MDM-2 gene amplification assessed by FISH was negative. A diagnosis of recurrent high-grade myxofibrosarcoma with markedly pleomorphic areas was made. The lesion showed distinctive epithelioid features. The MF3 surgical material was a cutaneous lozenge with a whitish nodule. Tumor cells were positive for vimentin, focally positive for CD34 and SMA, and negative for cytokeratin and S100 protein. Ki67 was 15%. A mitotic index of 3 mitoses/10 HPF was determined. MDM2 gene amplification carried out to exclude a diagnosis of dedifferentiated liposarcoma, was negative (MDM2/Cep12 ratio of 1.0). The diagnosis was high-grade myxofibrosarcoma. A granulomatous reaction with giant cells and focal fibrosis of the previous surgery was observed in part of the surgical tissue. The primary cultures obtained from the surgical specimens continued to proliferate in monolayer cultures. Given that no specific immunohistochemical markers for the standard differential diagnosis of MFS are currently available, patient-derived cells were seeded in a tridimensional scaffold to investigate their cytomorphologic features [Figure 1(b)]. The evaluation of cytomorphologic features by an experienced sarcoma pathologist confirmed the establishment of MFS primary cultures. The proportion of MFS cells were MF1: 25%, MF2: 25% and MF3: 15%
Figure 1.
(a) H&E staining of the surgical specimen showing high-grade myxofibrosarcoma (light blue stroma) infiltrating fibrotic tissue. 20× magnification. (b) H&E staining of MFS primary culture (light blue spots) seeded into tridimensional scaffold. 20× magnification.
H&E, hematoxylin and eosin; MFS, myxofibrosarcoma.
Gene expression analysis of patient-derived myxofibrosarcoma cultures
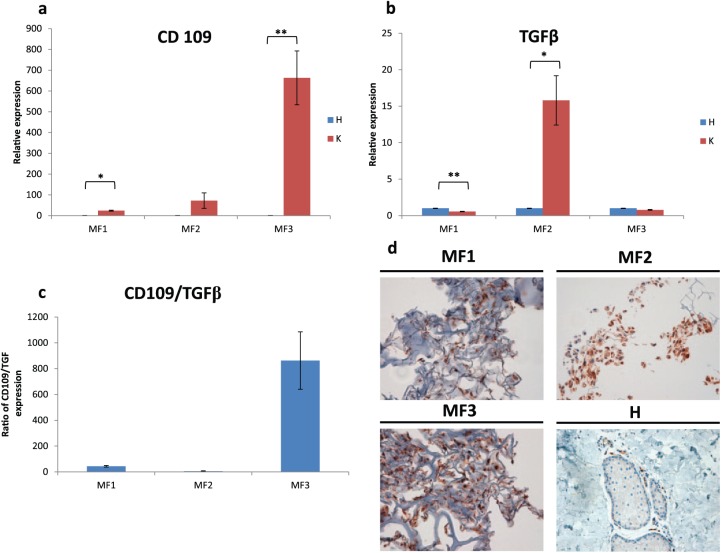
Gene expression analysis was performed to investigate whether CD109 represents a useful marker of diagnosis and a promising therapeutic target for MFS. Higher levels of CD109 were observed in all primary MFS cultures (K) with respect to matched normal tissue (H) [Figure 2(a)]. CD109 expression in MF1 was almost 24-fold higher than that of control tissue (p = 0.008), whereas in MF2 and MF3 it was 72-fold (p = 0.196) and 663-fold (p = 0.036) higher than that of the control, respectively. Since CD109 is a negative regulator of the TGF-β signaling pathway, we also carried out TGF-β gene expression profiling [Figure 2(b)], observing that TGF-β expression was 0.5-fold (p = 0.008) and 0.7-fold (p = 0.064) lower than that of control in MF1 and MF3 respectively, but 15-fold (p =0.048) higher than control in MF2. The CD109/TGF-β expression ratio was 43 in MF1, 5 in MF2 and 862 in MF3 [Figure 2(c)].
Figure 2.
(a) CD109 relative expression analysis in MFS primary cultures. (b) TGF-β relative expression analysis in MFS primary cultures. (c) CD109/TGF-β relative expression ratio in MFS primary cultures. (d) CD109 (brown stain) IHC analysis of MFS primary cultures and normal tissue (H). 20× magnification.
IHC, immunohistochemical; MFS, myxofibrosarcoma; TGF-β, transforming growth factor-beta.
Immunohistochemical analysis of CD109
Immunohistochemical analysis of the paraffin-embedded primary cultures was performed to define the role of CD109 in high-grade myxofibrosarcoma. Results showed that tumor cells, when present, were strongly positive for CD109 [Figure 2(d)]. Conversely, CD109 was weakly expressed in normal tissue (H). The slides were reviewed by an experienced sarcoma pathologist who determined the percentage of CD109 immunopositive cells of 80% in the primary cultures.
Chemotherapy in high-grade primary myxofibrosarcoma cultures
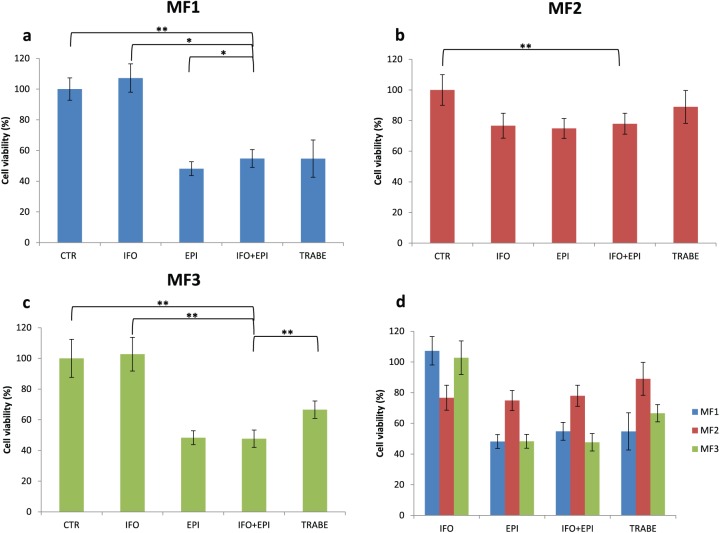
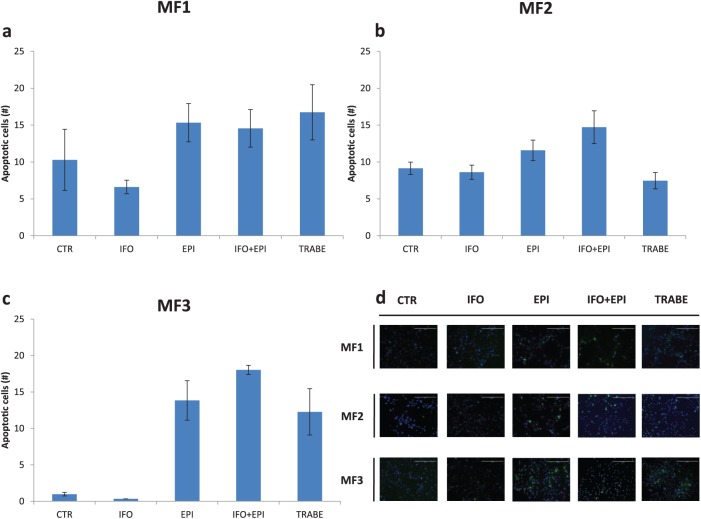
We assessed the sensitivity of our primary cultures to IFO, EPI and a combination of IFO and EPI, which represents the standard chemotherapy for MFS. We also evaluated the efficacy of trabectedin, a marine-derived, DNA-binding compound, approved for the treatment of patients with advanced STS after failure of anthracyclines, or for those who are not candidates for these agents.32 In MF1, IFO did not affect survival [Figure 3(a)]. MF1 treated with EPI showed a 48% survival compared with control, IFO plus EPI 54% (IFO + EPI versus IFO, p = 0.0001; IFO + EPI versus EPI, p = 0.03, IFO + EPI versus trabectedin, p = 0.49) and trabectedin 54%. In MF2 [Figure 3(b)], cell viability with IFO was 76%, 74% with EPI, 77% with IFO + EPI (IFO + EPI versus IFO, p = 0.06, IFO + EPI versus EPI, p = 0.40, IFO + EPI versus trabectedin, p = 0.37) and 88% with trabectedin. IFO did not affect survival in MF3 [Figure 3(c)]. MF3 treated with EPI showed a 48% survival, IFO plus EPI 47% (IFO + EPI versus IFO, p = 0.00002, IFO + EPI versus EPI, p = 0.41, IFO + EPI versus TRABE, p = 0.00036) and trabectedin 66%. Drug activity was also analyzed by TUNEL assay and the number of apoptotic cells corroborated previous data obtained by 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) assay (Figure 4).
Figure 3.
Cytotoxicity analysis of (a) MF1 primary culture, (b) MF2 primary culture and (c) MF3 primary culture treated with IFO, EPI, IFO + EPI, and trabectedin. Differences between groups were assessed by a two-tailed Student’s t-test and accepted as significant (*) at p < 0.05. (d) Cumulative cytotoxicity analysis data.
EPI, epirubicin; IFO, ifosfamide; TRABE, trabectedin.
Figure 4.
Number of TUNEL-positive MFS cells in (a) MF1 primary culture, (b) MF2 primary culture and (c) MF3 primary culture. (d) TUNEL staining of MFS primary cultures (10× magnification) showing apoptotic cells (green spots) and cell nuclei (blue spots). Scale bar 400 µm.
MFS, myxofibrosarcoma; TUNEL, terminal deoxynucleotidyl transferase nick end labeling.
Discussion
MFS is one of the most common STSs in adults, occurring mainly on the extremities but also on the trunk and head and neck region. Despite having a better prognosis than other subtypes, the clinical behavior of MFS is characterized by a propensity for multiple local recurrences and a lower incidence of distant metastases.4
The diagnosis of MFS is based on the presence of specific morphological features including prominent curvilinear blood vessels, myxoid stroma, spindle and pleomorphic cells. There are still no specific diagnostic markers available. The cornerstone of therapy for localized disease is surgery, with radiotherapy administered pre- or post-surgery in selected cases. However, local recurrences are frequently reported.33 The standard of care for metastatic MFS is chemotherapy, but outcomes are still very limited.
A better understanding of the molecular biology and treatment outcomes of high-grade MFS is needed to improve patient management. In the field of translational research, primary cultures provide a valuable material for studying tumor pathophysiology and pharmacology. This is especially true for rare tumors such as STS in which clinical outcome is very poor and large clinical trials are limited. In the present work, near-patient cells derived from high-grade MFS were harvested immediately after surgery. The cells were seeded in 3D scaffolds to confirm the establishment of MFS patient-derived cultures. The assessment of the morphological features in H&E-stained 3D primary cultures was reviewed by an experienced sarcoma pathologist who confirmed the diagnosis of high-grade MFS.
As previously mentioned, MFS was recently acknowledged as a separate pathologic entity and as such there is still little documentation of its clinical behavior and patient outcome. It is therefore crucial to identify a marker that can act as a tool for diagnosis but as a therapeutic target.
TGF-β is a multifunctional cytokine belonging to the transforming growth factor superfamily which is involved in several physiological and tumor-associated pathways. It also appears to have a role in chemotherapy resistance, as reported in several tumors including breast cancer and squamous cell carcinoma.34,35 CD109, a TGF-β co-receptor, is a cell surface glycoprotein expressed in endothelial cells, hematopoietic cells, and several tumors. Its activation is mediated by T-lymphocytes, a subpopulation of bone marrow CD34+ cell and platelets. CD109 promotes TGF-β receptor degradation and inhibits TGF-β/Smad signaling.36–39 The prognostic significance of CD109 overexpression has been reported in several tumors including breast cancer,40,41 squamous cell/adenosquamous carcinoma of the lung, esophagus, uterus and gallbladder,42–45 and also STS. Recently, the prognostic impact of CD109 expression was also reported in MFS.18 Emori and colleagues observed 5-year overall survival rates of 77% and 0% for CD109-negative and CD109-positive patients, respectively. Although they did not find a statistically significant correlation between CD109 expression and tumor grade (p = 0.2877) an interesting trend was seen for high-grade MFS which showed higher positivity for CD109 than grade 1 and 2.
Furthermore, although CD109 mRNA overexpression is observed in various sarcoma cell lines, it is weakly expressed in normal adult tissues.46
On the basis of these results, we conducted an analysis of CD109 and TGF-β gene expression profiling. CD109 was overexpressed in all high-grade MFS primary cultures with respect to matched patient-derived normal tissue. Interestingly, CD109 overexpression was markedly higher in the primary tumor MF3 (663-fold higher compared with the normal tissue) than in local recurrences MF1 and MF2 (24- and 72-fold higher than that of normal tissue) [Figure 2(a) and (c)]. These data are consistent with findings from other studies in which CD109, albeit preferentially expressed during the early stages of tumorigenesis in tumors such as oral and urothelial carcinomas, was also associated with advanced stage in STS.18 We hypothesized that the lower CD109 expression observed in local recurrences MF1 and MF2 could be correlated to disease relapse, suggesting a similar behavior of the advanced stage. Further research is needed to confirm this. Although MF1 and MF2 were both local recurrences, differences in CD109 gene expression were detected which could be attributable to the variability of the specimens and the clinical characteristics of patients.
The potential prognostic role of CD109 was not investigated in this study due to the small sample size and the limited patient follow up. Of note, patient MF1, who had the longest follow up, died in 2016 after developing MFS-derived lung metastases. This would seem to correlate with the results presented by Emori and colleagues, who found that CD109 overexpression was significantly associated with distant metastases (p = 0.01), otherwise further analysis are needed to confirm this. Furthermore, immunohistochemical analysis of CD109 in the primary cultures [Figure 2(d)] confirmed its higher expression than in normal tissue (H), suggesting its potential role as an aid to support the identification of high-grade MFS.
It has also been reported that CD109 negatively regulates TGF-β pathway through TGF-β receptor degradation in numerous diseases, including fibrosis and cancer.47 Given that TGF-β regulates its own expression by a positive feedback mechanism,48 we decided to analyze it in our specimens, observing that it was markedly overexpressed in MF2 with respect to MF1 and MF3. Furthermore, TGF-β expression was downregulated when CD109 was overexpressed, suggesting that CD109 exerts a negative effect on TGF-β expression and its signaling pathway [Figure 2(b)].
Further analyses are needed to elucidate the molecular mechanisms of both CD109 and TGF-β expression in MFS. In this regard, it would also be useful to compare CD109 expression with that of primary cultures of other STS subtypes to assess the potential role of CD109 in the differential diagnosis of MFS. Furthermore, TGF-β expression in STS histotypes could be studied to clarify whether it plays a part in resistance to chemotherapy.
In our study, patient-derived cultures were treated with some of the drugs currently used for STS to investigate the impact of chemotherapy on MFS. The chemosensitivity assay revealed that MF1 and MF3, albeit clinically different, responded in a similar way to the drugs. Conversely, MF2 generally exhibited a lower response to the treatments [Figure 3(d)]. Notably, the MF2 specimen was characterized by the presence of markedly pleomorphic areas (Figure 1) and a higher expression of TGF- β than the other two specimens [Figure 2(b)], which could explain its lower sensitivity to chemotherapy. Further analyses are needed to confirm these observations.
The most active regimens in our analysis were EPI alone or in combination with IFO, both of which proved significant in all of the cultures. Anthracycline-based chemotherapy represents the current standard first-line treatment of STS, including MFS. Clinically, the combination of anthracyclines and IFO is widely used to increase the clinical benefit of patients. In other settings, anthracyclines alone are preferred, especially for patients undergoing palliative care.49,50
Interestingly, the antiproliferative activity of IFO was more effective in MF2 than in the other cultures, suggesting a higher sensitivity of this drug in high-grade MFS with markedly pleomorphic areas.51,52
Trabectedin showed a higher cytotoxic activity in MF1 and MF3 than in MF2 cultures. The efficacy of this drug in STS, in particular the myxoid liposarcoma histotype, has been demonstrated in numerous clinical trials, but there are also reports of its activity in other sarcoma subtypes.53–55 Although, there is limited evidence of the impact of trabectedin on MFS,56 our results are suggestive of some activity in this STS subtype. Moreover, its antitumor activity appears to be related to its direct effect on both cancer cells and the tumor microenvironment. Immunomodulatory and anti-angiogenetic effects have also been reported.57,58 Given the pleiotropic mechanism of action of trabectedin, we can thus hypothesize that its full spectrum of activity was underestimated in our in vitro models. The TUNEL assay was performed to analyze the number of apoptotic cells after treatment with each of the tested drugs. Results were consistent with those obtained from the cytotoxicity assay, confirming the sensitivity of the MFS patient-derived cultures to most of the drugs (Figure 4).
In conclusion, the results from the present study highlight the importance of using near-patient models in STS research to obtain a better understanding of the biology of the disease. Moreover, our findings increase MFS cell characterization which, given the few MFS cell lines commercially available,59 could represent a starting point for further studies on this poorly explored cancer. Gene and protein expression analysis indicated that CD109 could represent an aid to the diagnosis of high-grade MFS and a potential therapeutic target for this histotype. Our findings also suggest that TGF-β expression might be a useful marker for resistance to chemotherapy. However, further analyses are needed to elucidate the role of these genes in the management of MFS patients. However, the study is not without limitations, that is, small sample size, cellular heterogeneity in primary cultures and decreasing reliability of the preclinical model over time to recapitulate the tumor microenvironment.
Finally, our results provide a starting point for further investigations aimed at improving the current management of MFS patients undergoing chemotherapy.
Acknowledgments
ADV and TI conceived the idea for the study. ADV, LM, GM, CL, CS and FP performed the experiments. RC performed the surgery and provided the surgical specimens. FR, LM, AB, NR, DA and TI were responsible for data interpretation. ADV drafted the paper. All authors read and approved the final version of the manuscript for submission.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Alessandro De Vita, Osteoncology and Rare Tumors Center, Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST) IRCCS, Meldola, Italy.
Federica Recine, Osteoncology and Rare Tumors Center, Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST) IRCCS, Meldola, Italy.
Laura Mercatali, Osteoncology and Rare Tumors Center, Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST) IRCCS, Via Piero Maroncelli 40, 47014 Meldola (FC), Italy.
Giacomo Miserocchi, Osteoncology and Rare Tumors Center, Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST) IRCCS, Meldola, Italy.
Chiara Liverani, Osteoncology and Rare Tumors Center, Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST) IRCCS, Meldola, Italy.
Chiara Spadazzi, Osteoncology and Rare Tumors Center, Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST) IRCCS, Meldola, Italy.
Roberto Casadei, Department of Orthopedics, Istituto Ortopedico Rizzoli, University of Bologna, Bologna, Italy.
Alberto Bongiovanni, Osteoncology and Rare Tumors Center, Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST) IRCCS, Meldola, Italy.
Federica Pieri, Pathology Unit, Morgagni-Pierantoni Hospital, Forlì, Italy.
Nada Riva, Osteoncology and Rare Tumors Center, Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST) IRCCS, Meldola, Italy.
Dino Amadori, Osteoncology and Rare Tumors Center, Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST) IRCCS, Meldola, Italy.
Toni Ibrahim, Osteoncology and Rare Tumors Center, Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST) IRCCS, Meldola, Italy.
References
- 1. Sanfilippo R, Miceli R, Grosso F, et al. Myxofibrosarcoma: prognostic factors and survival in a series of patients treated at a single institution. Ann Surg Oncol 2011; 18: 720–725. [DOI] [PubMed] [Google Scholar]
- 2. Fletcher CDM, Bridge JA, Hogendoorn P, et al. WHO classification of tumours of soft tissue and bone. 4th ed. Lyon, France: IARC Press, 2013. [Google Scholar]
- 3. Merck C, Angervall L, Kindblom LG, et al. A malignant soft tissue tumor of fibroblastic-histiocytic origin. A clinicopathologic and prognostic study of 110 cases using a multivariate analysis. Acta Pathol Microbiol Immuno Scand Suppl 1983; 282: 1–40. [PubMed] [Google Scholar]
- 4. Haglund KE, Raut CP, Nascimento AF, et al. Recurrence patterns and survival for patients with intermediate- and high-grade myxofibrosarcoma. Int J Radiat Oncol Biol Physics 2012; 82: 361–367. [DOI] [PubMed] [Google Scholar]
- 5. Weiss SW, Goldblum JR. Malignant fibrohistiocytic tumors. In: Enzinger FM, Weiss SW. (eds) Soft tissue tumors. 4th ed. St. Louis: Mosby-Year Book, 2001, pp.535–569. [Google Scholar]
- 6. Mentzel T, Calonje E, Wadden C, et al. Clinicopathologic analysis of 75 cases with emphasis on the low-grade variant. Am J Surg Path 1996; 20: 391–405. [DOI] [PubMed] [Google Scholar]
- 7. Gronchi A, Lo Vullo S, Colombo C, et al. Extremity soft tissue sarcoma in a series of patients treated at a single institution: local control directly impacts survival. Ann Surg 2010; 251: 506–511. [DOI] [PubMed] [Google Scholar]
- 8. Lin CN, Chou SC, Li CF. Prognostic factors of myxofibro- sarcomas: implications of margin status, tumor necrosis, and mitotic rate on survival. J Surg Oncol 2006; 93; 294–303. [DOI] [PubMed] [Google Scholar]
- 9. Huang HY, Brennan MF, Singer S, et al. Distant metastasis in retroperitoneal dedifferentiated liposarcoma is rare and rapidly fatal: a clinicopathological study with emphasis on the low-grade myxofibrosarcoma-like pattern as an early sign of dedifferentiation. Mod Pathol 2005; 18: 976–984. [DOI] [PubMed] [Google Scholar]
- 10. Hisaoka M, Morimitsu Y, Hashimoto H, et al. Retroperitoneal liposarcoma with combined well-differentiated and myxoid malignant fibrous histiocytoma-likemyxoid areas. Am J Surg Pathol 1999; 23:1480–1492. [DOI] [PubMed] [Google Scholar]
- 11. Rosenberg SA, Tepper J, Glatstein E, et al. The treatment of soft-tissue sarcomas of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg 1982; 196: 305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Manoso MW, Pratt J, Healey JH, et al. Infiltrative MRI pattern and incomplete initial surgery compromise local control of myxofibrosarcoma. Clin Orthop Relat Res 2006; 450: 89–94. [DOI] [PubMed] [Google Scholar]
- 13. Mentzel T, Hogendoorn PCW, Huang HY. Myxofibrosarcoma. In: Fletcher CDM, Bridge JA, Hogendoorn PCW, et al. (eds) WHO classification of tumours of soft tissue and bone. Lyon: IARC Press, 2013, pp.93–94. [Google Scholar]
- 14. Sambri A, Bianchi G, Righi A, et al. Surgical margins do not affect prognosis in high grade myxofibrosarcoma. Eur J Surg Oncol 2016; 42: 1042–1048. [DOI] [PubMed] [Google Scholar]
- 15. Raouf A, Sun YJ. In vitro methods to culture primary human breast epithelial cells. Methods Mol Biol 2013; 946: 363–381. [DOI] [PubMed] [Google Scholar]
- 16. Nishikata T, Ishikawa M, Matsuyama T, et al. Primary culture of breast cancer: a model system for epithelial-mesenchymal transition and cancer stem cells. Anti Cancer Res 2013; 33: 2867–2873. [PubMed] [Google Scholar]
- 17. Steinstraesser L, Jacobsen F, Schubert C, et al. Establishment of pimary human sarcoma model in athymic nude mice. Hum Cell 2010; 23: 50–57. [DOI] [PubMed] [Google Scholar]
- 18. Emori M, Tsukahara T, Murata K, et al. Prognostic impact of CD109 expression in myxofibrosarcoma. J Surg Oncol 2015; 111: 975–979. [DOI] [PubMed] [Google Scholar]
- 19. Minardi S, Sandri M, Martinez JO, et al. Multiscale patterning of a biomimetic scaffold integrated with composite microspheres. Small 2014, 10; 3943–3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Calabrese G, Giuffrida R, Fabbi C, et al. Hydroxyapatite scaffolds induce human adipose derived stem cells osteogenic differentiation in vitro. PLoS One 2016; 11: e0151181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cunniffe GM, Dickson GR, Partap S, et al. Development and characterization of a collagen nano-hydroxyapatitem composite scaffold for bone tissue engineering. J Mater Sci Mater Med 2010; 21: 2293–2298. [DOI] [PubMed] [Google Scholar]
- 22. Liverani C, La Manna F, Groenewoud A, et al. Innovative approaches to establish and characterize primary cultures: an ex vivo 3D system and the zebrafish model. Biol Open 2017; 6: 133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ye BG, Sun HC, Zhu XD, et al. Reduced expression of CD109 in tumor-associated endothelial cells promotes tumor progression by paracrine interleukin-8 in hepatocellular carcinoma. Oncotarget 2016; 7: 29333–29345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Highley MS, Momerency G, Sawyers D, et al. The neurotoxicity and pharmacokinetics of oral ifosfamide. J Anal Oncol 2015; 15: 13–23. [Google Scholar]
- 25. Cerny T, Leyvraz S, von Briel T, et al. Saturable metabolism of continuous high-dose ifosfamide with mesna and GM-CSF: a pharmacokinetic study in advanced sarcoma patients. Swiss Group for Clinical Cancer Research (SAKK) Ann Oncol 1999; 10:1087–1094. [DOI] [PubMed] [Google Scholar]
- 26. Fogli S, Danesi R, Gennari A, et al. Gemcitabine, epirubicin And paclitaxel: pharmacokinetic and pharmacodynamic interactions in advancedbreast cancer. Ann Oncol 2002; 13: 919–927. [DOI] [PubMed] [Google Scholar]
- 27. Danesi R, Innocenti F, Fogli S, et al. Pharmacokinetics and pharmacodynamics of combination chemotherapy with paclitaxel and epirubicin breast cancer patients. Br J Clin Pharmacol 2002; 53: 508–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van Kesteren CH, de Vooght MM, López-Lázaro L, et al. Yondelis (trabectedin, ET-743): the development of an anticancer agent of marine origin. Anticancer Drugs 2003; 14: 487–502. [DOI] [PubMed] [Google Scholar]
- 29. De Vita A, Miserocchi G, Recine F, et al. Activity of eribulin in a primary culture of well-differentiated/dedifferentiated adipocytic sarcoma. Molecules 2016; 21: pii: E1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weaver J, Downs-Kelly E, Goldblum JR, et al. Fluorescence in situ hybridization for MDM2 gene amplification as a diagnostic tool in lipomatous. Mod Pathol 2008; 21: 943–949. [DOI] [PubMed] [Google Scholar]
- 31. De Vita A, Mercatali L, Recine F, et al. Current classification, treatment options, and new perspectives in the management of adipocytic sarcomas. Onco Targets Ther 2016; 9: 6233–6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Demetri GD, von Mehren M, Jones RL, et al. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: results of a phase III randomized multicenter clinical trial. J Clin Oncol 2016; 34: 786–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Look Hong NJ, Hornicek FJ, Raskin KA, et al. Prognostic factors and outcomes of patients with myxofibrosarcoma. Ann Surg Oncol 2013; 20: 80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bhola NE, Balko JM, Dugger TC, et al. TGF-β inhibition enhances chemotherapy action against triple-negativebreast cancer. J Clin Invest 2014; 123: 1348–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Oshimori N, Oristian D, Fuchs E. TGF-β promotes heterogeneity and drug resistance in squamous cellcarcinoma. Cell 2015; 160: 963–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hashimoto M, Ichihara M, Watanabe T, et al. Expression of CD109 in human cancer. Oncogene 2004, 23: 3716–3720. [DOI] [PubMed] [Google Scholar]
- 37. Murray LJ, Bruno E, Uchida N, et al. CD109 is expressed on a subpopulation of CD34þ cells enriched in hematopoietic stem and progenitor cells. Exp Hematol 1999; 27, 1282–1294. [DOI] [PubMed] [Google Scholar]
- 38. Bizet AA, Liu K, Tran-Khanh N, et al. The TGF-beta co-receptor, CD109, promotes internalization and degradation of TGF-beta receptors. Biochim Biophys Acta 2011; 1813: 742–753. [DOI] [PubMed] [Google Scholar]
- 39. Hagiwara S, Murakumo Y, Mii S, et al. Processing of CD109 by furin and its role in the regulation of TGF-beta signaling. Oncogene 2010; 29: 2181–2191. [DOI] [PubMed] [Google Scholar]
- 40. Tao J, Li H, Li Q, et al. CD109 is a potential target for triple-negative breast cancer. Tumour Biol 2014; 35: 12083–12090. [DOI] [PubMed] [Google Scholar]
- 41. Hasegawa M, Moritani S, Murakumo Y, et al. CD109 expression in basal-like breast carcinoma. Pathol Int 2008; 58: 288–294. [DOI] [PubMed] [Google Scholar]
- 42. Sato T, Murakumo Y, Hagiwara S, et al. High-level expression of CD109 is frequently detected in lung squamous cell carcinomas. Pathol Int 2007; 57: 719–724. [DOI] [PubMed] [Google Scholar]
- 43. Hagiwara S, Murakumo Y, Sato T, et al. Up-regulation of CD109 expression is associated with carcinogenesis of the squamous epithelium of the oral cavity. Cancer Sci 2008; 99: 1916–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang JM, Hashimoto M, Kawai K, et al. CD109 expression in squamous cell carcinoma of the uterine cervix. Pathol Int 2005; 55: 165–169. [DOI] [PubMed] [Google Scholar]
- 45. Dong F, Lu C, Chen X, et al. CD109 is a novel marker for squamous cell/adenosquamous carcinomas of the gallbladder. Diagn Pathol 2015; 7: 130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Emori M, Tsukahara T, Murase M, et al. High expression of CD109 antigen regulates the phenotype of cancer stem-like cells/cancer-initiating cells in the novel epithelioid sarcoma cell line ESX and is related to poor prognosis of soft tissue sarcoma. PLoS One 2013; 8: e84187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li C, Hancock MA, Sehgal P, et al. Soluble CD109 binds TGF-β and antagonizes TGF-β signalling and responses. Biochem J 2016; 473: 537–547. [DOI] [PubMed] [Google Scholar]
- 48. Miyazono K. Positive and negative regulation of TGF-beta signaling. J Cell Sci 2000; 113: 1101–1109. [DOI] [PubMed] [Google Scholar]
- 49. Judson I, Verweij J, Gelderblom H. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft tissue sarcoma: a randomized controlled phase 3 trial. Lancet Oncol 2014; 15: 415–423. [DOI] [PubMed] [Google Scholar]
- 50. Gronchi A, Stacchiotti S, Verderio P, et al. Short, full-dose adjuvant chemotherapy (CT) in high-risk adult soft tissue sarcomas (STS): long-term follow up of a randomized clinical trial from the Italian Sarcoma Group and the Spanish Sarcoma Group. Ann Oncol 2016; 27: 2283–2288. [DOI] [PubMed] [Google Scholar]
- 51. Le Cesne A, Antoine E, Spielmann M, et al. High-dose ifosfamide: circumvention of resistance to standard-dose ifosfamide in advanced soft tissue sarcomas. J Clin Oncol 1995; 13: 1600–1608. [DOI] [PubMed] [Google Scholar]
- 52. Verma S, Younus J, Stys-Norman D. Meta-analysis of ifosfamide-based combination chemotherapy in advanced soft tissue sarcoma. Cancer Treat Rev 2008; 34: 116–123. [DOI] [PubMed] [Google Scholar]
- 53. De Sanctis R, Marrari A, Marchetti S, et al. Efficacy of trabectedin in advanced soft tissue sarcoma: beyond lipo- and leiomyosarcoma. Drug Des Devel Ther 2015; 9: 5785–57891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Recine F, Bongiovanni A, Riva N, et al. Update on the role of trabectedin in the treatment of intractable soft tissue sarcomas. Onco Targets Ther 2017; 10: 1155–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Demetri GD, Chawla SP, von Mehren M. Efficacy and safety of trabectedin in patients with advanced or metastatic liposarcoma or leiomyosarcoma after failure of prior anthracyclines and ifosfamide: results of a randomized Phase II study of two different schedules. J Clin Oncol 2009; 27: 4188–4196. [DOI] [PubMed] [Google Scholar]
- 56. Garcia-Carbonero R, Supko JG, Manola V, et al. Phase II and pharmacokinetic study of ecteinascidin 743 in patients with progressive sarcomas of soft tissues refractory to chemotherapy. J Clin Oncol 2004; 22:1480–1490. [DOI] [PubMed] [Google Scholar]
- 57. Germano G, Frapolli R, Belgiovine C, et al. Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell 2013; 23: 249–262. [DOI] [PubMed] [Google Scholar]
- 58. D’Incalci M, Galmarini CM. A review of trabectedin (ET-743): a unique mechanism of action. Mol Canc Ther 2010; 9: 2157–2163. [DOI] [PubMed] [Google Scholar]
- 59. Kawashima H, Ogose A, Gu W, et al. Establishment and characterization of a novel myxofibrosarcoma cell line. Cancer Genet Cytogenet 2005; 161: 28–35. [DOI] [PubMed] [Google Scholar]