Abstract
Advanced non-small cell lung cancer (NSCLC) prognosis is still poor and has recently been reformed by the development of immune checkpoint inhibitors and the approval of anti-PD-1 (programmed cell-death 1) treatments such as nivolumab and pembrolizumab in second line. More recently, atezolizumab (MDPL 3280A), a programmed cell-death-ligand 1 (PD-L1) inhibitor, was also studied in this setting. Here, we report a review of the literature assessing the efficacy, safety, and place of atezolizumab in the second-line treatment of advanced NSCLC. We performed a literature search of PubMed, American Society of Clinical Oncology, European Society of Medical Oncology and World Conference on Lung Cancer meetings. Atezolizumab showed a good tolerance profile and efficacy in comparison with docetaxel for second-line treatment of advanced NSCLC. Potential predictive biomarkers also have to be assessed.
Keywords: atezolizumab, immunotherapy, lung cancer, PD-L1, second-line
Introduction
Lung cancer is the leading cause of cancer death worldwide.1 Indeed, non-small cell lung Cancer (NSCLC) prognosis is poor with an overall survival rate at 5 years of 15% and until recently, treatment options in the second- or third-line setting for advanced diseases were limited.2,3 However, in the last few years, comprehension of cell biology has improved, and mechanisms allowing cancer cells to avoid immune destruction,4 relying both on innate and adaptive immunity, are better understood and addressed. Regarding adaptive immunity, there are two phases: during the first phase, dendritic cells interact with T cells which will then induce cancer cell destruction in the second phase. Inhibitory signals occur in both phases, involving membrane molecules of which the most important representative is the couple PD-1 (programmed cell-death 1) expressed by T cells with PD-L1 (programmed cell-death-ligand 1) on cancer cells. The last few years have seen the development and approval of anti-PD-1 therapies such as nivolumab in pretreated squamous5 and nonsquamous6 NSCLC, or pembrolizumab in previously treated, PD-L1-positive, advanced NSCLC.7 Therefore, recent guidelines established new recommendations including anti-PD-1 immunotherapy for second line treatment of NSCLC.8,9 However, PD-L1 blockade is distinct, since it allows PD-1 to bind its other ligand, PD-L2 which may be important in possibly preventing severe immune adverse effect events such as pneumonitis.10–12 Atezolizumab (MDPL 3280A) is a humanised engineered IgG1 monoclonal antibody targeting PD-L1 usually used with its specific companion immunohistochemistry (IHC) diagnostic assay, SP142 (Ventana Medical Systems, Inc. Arizona, USA), assessing PD-L1 expression both on tumour cells (TCs) and on tumour-infiltrating immune cells (ICs).
The percentage of PD-L1-positive cells is expressed by a score including PD-L1 expression levels on both TCs and ICs. On TCs, TC3, TC2, TC1 and TC0 are respectively correlated with PD-L1 expression levels of ⩾50%; ⩾5% and <50%; ⩾1% and <5%; and <1%. On tumour-infiltrating ICs IC3, IC2, IC1 and IC0 are respectively correlated with PD-L1 expression levels of ⩾10%; ⩾5% and <10%; ⩾1% and <5%; and <1%.13–16
In this article, we are proposing a review of the efficacy and safety of atezolizumab in locally advanced or metastatic NSCLC, focusing on its place as second-line treatment of NSCLC.
Methods
A literature search of PubMed, ASCO (American Society of Clinical Oncology), ESMO (European Society of Medical Oncology) and WCLC (World Conference on Lung Cancer) meeting abstracts, as well as a review of ClinicalTrials.gov was conducted using the following terms: ‘Atezolizumab’, ‘MDPL3280A’, ‘lung cancer’ or ‘Non-Small Cell Lung Cancer’. We chose to include clinical trials, meta-analyses and communications from international congresses between 2015 and 2017, since no clinical data were available on atezolizumab before 2013.
Results
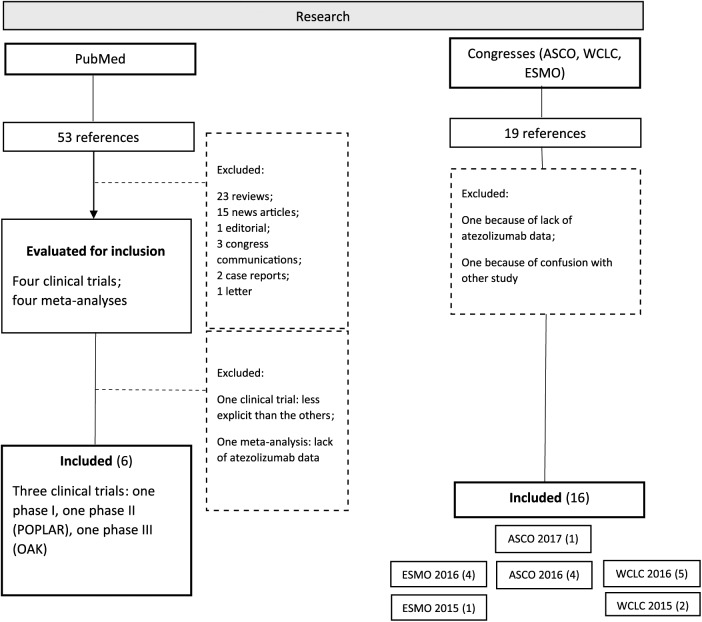
In PubMed, among 53 articles found, 4 were clinical trials. One phase I trial was excluded due to the lack of data compared with the others. Moreover, four articles were meta-analyses, including one with insufficient atezolizumab data, not reported here. Among congress reports, between 2015 and 2017, we found 19 abstracts. Two of them, being preliminary data further published in a larger trial or not reporting enough data about atezolizumab, were excluded. Figure 1 reports the flow chart of articles and reports included in this review.
Figure 1.
Flow chart.
ESMO, European Society for Medical Oncology; ASCO, American Society of Clinical Oncology; WCLC, World Conference on Lung Cancer.
Efficacy
Phase I
Herbst and colleagues conducted a phase I study17 aiming to evaluate the single-agent safety and tolerability of MDPL3280A/atezolizumab administered by intravenous infusion every 3 weeks (q3w) to patients with locally advanced or metastatic solid tumours or haematological malignancies. A total of 277 patients were included, mostly suffering from NSCLC (31%), renal cell carcinoma (25%) and melanoma (16%). In the NSCLC subgroup, patients had a median age of 60 years and were mostly males (56%) performance status 0–1. A total of 76% had nonsquamous NSCLC, only 5% had central nervous system (CNS) metastasis, 80% had a tobacco history and 55% had received at least three prior systemic regimens.
In the dose-escalation phase of the study, patients received atezolizumab q3w from 0.01 mg/kg to 20 mg/kg and the expansion phase enrolled patients at 10 mg/kg, 15 mg/kg or 20 mg/kg q3w. Mean terminal serum half-life of 3 weeks was reached with a minimum dose of 1 mg/kg. A dose of 15 mg/kg q3w was sufficient to maintain target drug levels and the equivalent fixed dose of 1200 mg q3w was moved forward in clinical development of single-agent atezolizumab.
In this phase I study, 21% of patients with NSCLC showed a confirmed response according to Response Evaluation Criteria in Solid Tumours, version 1.1 (RECIST 1.1). The median progression-free survival (PFS) of all patients was 18 weeks. The association between response to atezolizumab and tumour-infiltrating ICs PD-L1 expression reached statistical significance in NSCLC (p = 0.015). These data were confirmed in a Japanese phase I study in 2016.14
Phase II – POPLAR trial
POPLAR15 was a phase II clinical trial testing atezolizumab 1200 mg q3w versus docetaxel 75 mg/m² q3w in the treatment of stage IIIb–IV NSCLC patients previously treated with platinum-based first-line chemotherapy. Overall survival (OS) favoured atezolizumab versus docetaxel with a hazard ratio (HR) of 0.73 [95% confidence interval (CI) 0.53–0.99], p = 0.040 in the intent-to-treat (ITT) population. Increasing improvement in OS was correlated with increased PD-L1 expression. However, PFS was not significantly improved in the atezolizumab arm: HR = 0.94 (95% CI 0.72–1.23), p = 0.645 (ITT population).
An objective response rate (ORR) of 38% was noticed in the TC3 or IC3 subgroup. Objective responses with atezolizumab were durable, with a median duration of 14.3 months (11.6–nonestimable) compared with 7.2 months (5.6–12.5 months) for docetaxel. This gap between atezolizumab and docetaxel was even wider in updated data presented at ASCO congress in 2016.18
An ongoing phase II trial, BIRCH, is currently conducted in first or more lines of treatment in preselected patients with IC2/3 or TC2/3 PD-L1 expression profile [ClinicalTrials.gov identifier: NCT02031458].19,20 In the first-line subgroup, ORR was 19%; 6-month PFS was 46%; 6-month OS was 82%; whereas in the second line subgroup, ORR was 17%; 6-month PFS was 29% and 6-month OS was 76%.19
Phase III – OAK trial
The following phase III trial, OAK,16,21 highlighted the efficacy of atezolizumab in second-line treatment of NSCLC, with a median OS of 13.8 months in the atezolizumab arm (95% CI 11.8–15.7) versus 9.6 months in the docetaxel arm [(8.6–11.2); HR 0.73 (95% CI 0.62–0.87), p = 0.0003]. PFS was similar between treatment groups in the ITT population [HR 0.95 (95% CI 0.82–1.10)]. There was no difference regarding objective response between the two groups with an ORR of 14% with atezolizumab and 13% with docetaxel in the ITT population.
Characteristics of TC3 or IC3 population were: median age of 64 years, mostly males (64.2%), White (77.4%), previous (65%) or current (19.7%) smokers, EGFR wild type (73.7%) and with nonsquamous NSCLC (70.1%).
Treatment beyond progression (TBP) is authorized if the investigator deemed the patient to be receiving clinical benefit and if patients consented to continuation. Clinical benefit is defined by an absence of unacceptable toxicity, a symptomatic deterioration attributed to disease progression after an integrated assessment of radiographic data, biopsy results (if available) and clinical status. New data from the OAK trial22 suggest that TBP with atezolizumab is efficient, as presented in ASCO 2017, where a pool of patients continue to receive the anti-PD-L1 agent after disease progression if a clinical benefit was still present. Among 332 patients with PD while treated by atezolizumab, 51% (168) continued anti-PD-L1 therapy. A total of 7% achieved subsequent response from new baseline (at PD), 49% had stable target lesions and median of OS (mOS) was 12.7 months (95% CI 9.3–14.9) while those who received other anticancer therapy (chemotherapy or new line of immunotherapy) had an mOS of 8.8 months (95% CI 6.0–12.1). Safety profile seemed to be tolerable. Consequently, there would be an interest of using atezolizumab in postprogression prolongation of survival.
Subgroup analyses
PD-L1 expression
In the POPLAR study,15 OS was correlated with PD-L1 expression level since OS in the TC1/2/3 or IC1/2/3 subgroups was higher in the atezolizumab [HR of 0.59 (95% CI 0.33–0.89), p = 0.014], whereas OS was not improved by atezolizumab in the TC0 and IC0 groups [HR 1.04 (0.62–1.75), p = 0.871].
Unlike in POPLAR, the OAK study16,21,23 showed a survival advantage for atezolizumab versus docetaxel even in the TC0 or IC0 subgroups (45% of the patients) with an HR of 0.75 (95% CI 0.59–0.96), p = 0.0215. It was consistent with the PD-L1 gene expression results: OS was improved by atezolizumab regardless of PD-L1 gene level expression. The difference in the two trials may be due to a statistically larger female population in the docetaxel group in POPLAR, overestimating the OS.
These data were consistent with a meta-analysis of three clinical trials with anti-PD-1 or PD-L1 antibodies such as nivolumab or atezolizumab24 and showing a significant improvement in OS, but not in PFS, except in the case of elevated levels of PD-L1 expression.
The main results of the OAK and POPLAR trials are showed in Table 2.
Table 2.
Efficacy data of POPLAR and OAK trials on atezolizumab in intention-to-treat and TC3 and IC3 populations.
| Phase | Population | Group | n | OS (months) | HR (95% CI ) | p | PFS (months) | HR (95% CI) | p | ORR (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fehrenbacher et al.15 (POPLAR) | II | ITT | Atezo | 144 | 12.6 | 0.73 (0.53–0.99) |
0.04 | 2.7 | 0.94 (0.72–1.23) |
0.645 | 14.6 |
| Docetaxel | 143 | 9.7 | 3 | 14.7 | |||||||
| TC3 or IC3 | Atezo | 24 | 15.5 | 0.49 (0.27–1.07) |
– | 7.8 | 0.60 (0.31–1.16) |
0.127 | 37.5 | ||
| Docetaxel | 23 | 11.1 | 3.9 | 13 | |||||||
| Rittmeyer et al.16 (OAK) | III | ITT | Atezo | 425 | 13.8 | 0.73 (0.62–0.87) |
0.0003 | 2.8 | 0.95 (0.82–1.10) |
0.49 | 14 |
| Docetaxel | 425 | 9.6 | 4.0 | 13 | |||||||
| TC3 or IC3 | Atezo | 72 | 20.5 | 0.41 (0.27–0.64) |
< 0.0001 | 4.2 | 0.63 (0.43–0.91) |
0.0123 | 30.6 | ||
| Docetaxel | 65 | 8.9 | 3.3 | 10.8 |
Atezo, atezolizumab; CI, confidence interval; HR, hazard ratio; IC3, immune cell 3; ITT, intention to treat; OS, overall survival; ORR, objective response rate; PFS, progression-free survival; TC3, tumour cell 3.
Clinical characteristics
The survival advantage of atezolizumab over docetaxel was statistically the same regardless of pathology subtype (squamous or nonsquamous),16,21 the presence of CNS metastases at baseline, or tobacco smoking history.16,21 However, a trend emerged, revealing that some subgroups benefited even more from atezolizumab than the global population, such as the elderly population (⩾65 years)16,21 [median OS was 14.1 months versus 9.2 months respectively, HR = 0.66 (95% CI 0.52–0.83) versus 0.80 (95% CI 0.64–1.00)] or the CNS metastases population19 [HR of 0.54 (95% CI 0.31–0.94) versus 0.75 (0.63–0.89)].
On the contrary, there was a disadvantage of atezolizumab versus docetaxel in the EGFR (epidermal growth factor receptor) mutant population [OS HR 1.24 (95% CI 0.71–2.18)],21 as previously reported with other immune checkpoint inhibitors such as nivolumab or pembrolizumab [OS HR = 1.05 (95% CI 0.70–1.55), p < 0.81] in a meta-analysis recently published.25
Other biomarkers
In the phase II trial, POPLAR, OS in the atezolizumab population seemed to be correlated to higher gene level expression of PD-L1, PD-1, PD-L2, B7.1 and T-effector interferon gamma,15 but since it was not confirmed for PD-L1 in OAK, further analyses should be performed based on tumour mutation burden researches as shown in Rizvi and colleagues’ study: high nonsynonymous mutation burden was associated with clinical benefit of pembrolizumab.26
Safety
In the phase I study by Herbst and colleagues,17 the maximum tolerated dose was not reached and no dose-limiting toxicities were observed.
The rates of adverse effect events in clinical trials of atezolizumab are reported in Table 1.
Table 1.
Adverse event rates in the POPLAR and OAK trials in intention-to-treat populations.
| Trial name | Arms | Any grade adverse events (%) | Grade 3–4 adverse events (%) | Adverse events leading to dose modification, delay or interruption (%) | Immune-related adverse events (%) |
|---|---|---|---|---|---|
| Hervst et al.13 | Atezo | 94.9 | 39 | – | – |
| POPLAR | Atezo | 96 | 40 | 24 | 3.2* |
| Doc | 96 | 53 | 33 | – | |
| OAK | Atezo | 94 | 37 | 25 | 10.5** |
| Doc | 96 | 54 | 36 | – |
Pneumonitis: 6; colitis: 2; hepatitis: 2.
Pneumonitis: 4; increased aspartate aminotransferase: 6; increased alanine aminotransferase: 6; colitis: 4; hepatitis: 1.
Atezo, atezolizumab; Doc, docetaxel.
The most common side effects of any grade were fatigue (26.8%), decreased appetite (23.5%), cough (23.2%), asthenia (19%), dyspnoea (19%), nausea (17.7%), pyrexia (17.7%), constipation (17.6%), diarrhoea (15.4%) and arthralgia (12%).16
Pooled safety analyses were conducted on patients suffering from asymptomatic untreated CNS metastases or stable previously treated brain metastases at baseline27 in several studies as POPLAR. A total of 27 patients were analysed: 44% had any neurological adverse event (AE) versus 28% in the population with no CNS metastasis, but there were no more AEs of any type and no discontinued treatment due to AE in the subgroup of patients with CNS metastases.
PD-1/PD-L1 AEs compared with docetaxel in a meta-analysis had an odds ratio (OR) of 0.36 (95% CI 0.28–0.46); p < 0.001, all grades included and of 0.18 (95% CI 0.14–0.22), p < 0.001 for grade 3–5 AEs, showing a better tolerability profile of immune checkpoint inhibitors in comparison with chemotherapy by docetaxel.28
Another meta-analysis compared anti-PD-1 and anti-PD-L1 tolerance profile and did not show any difference on AE rates (including immune AE and pneumonitis) on a population of more than 4400 patients.29
In conclusion, phase I–III trials showed a good tolerance profile and efficacy of atezolizumab for the treatment of pretreated NSCLC patients, especially in comparison with standard chemotherapy with docetaxel.
Discussion
Based on clinical trials and meta-analyses, we report that atezolizumab is efficient and safe for second-line treatment of advanced NSCLC, irrespective of PD-L1 expression. But its place compared with other immune checkpoint inhibitors, including anti-PD-1 treatments already approved in this setting is still to be proven. Otherwise, relevance of PD-L1 expression according to SP142 IHC should be reassessed, since atezolizumab is effective in any PD-L1 expression group, and other predictive biomarkers should be studied.
Furthermore, there are remaining questions regarding potential combination of atezolizumab with other therapeutics, such as systemic treatment (chemotherapy, other immunotherapy, and targeted therapy), radiotherapy or surgery; the existence of a targeted population of interest and biomarkers to guide atezolizumab use; the place of atezolizumab in the treatment strategy of advanced NSCLC; the potential extension of atezolizumab use to other histology like small cell lung cancer (SCLC) or in other settings such as adjuvant or neoadjuvant treatment of early-stage NSCLC.
All upcoming trials referring to atezolizumab are presented in Table 3. Atezolizumab is being tested alone in the neoadjuvant or adjuvant setting, in first-line treatment of NSCLC, in small SCLC or in association with other therapies.
Table 3.
Expected atezolizumab (MDPL3280A) trials.
| Histology | Stage | Histology subgroup | Therapeutic place | Phase | Experimental arm | Control arm | ClinicalTrials.gov identifier |
|---|---|---|---|---|---|---|---|
| NSCLC | Stage I | I | MDPL3280A + stereotaxic radiotherapy | NCT02599454 | |||
| Stage Ib-IIIa | After adjuvant cisplatin-based chemotherapy | III | MDPL3280A | Best supportive care | NCT02486718 | ||
| Neoadjuvant +/– adjuvant | II | MDPL3280A | NCT02927301 | ||||
| Neoadjuvant | II | MDPL3280A + carboplatin + nab-paclitaxel | NCT02716038 | ||||
| Stage IIIb–IV | Nonsquamous | First line | III | MDPL3280A + carboplatin + nab-paclitaxel | Carboplatin + nab-paclitaxel | NCT02367781 | |
| III | MDPL3280A + carboplatin/cisplatin + pemetrexed | Carboplatin/cisplatin + pemetrexed | NCT02657434 | ||||
| III | MDPL3280A + carboplatin + paclitaxel +/– bevacizumab | Carboplatin + paclitaxel + bevacizumab | NCT02366143 | ||||
| First line or higher in EGFR mutant | Ib–II | MDPL3280A + rociletinib | NCT02630186 | ||||
| Squamous | First line | III | MDPL3280A + carboplatin + paclitaxel or nab-paclitaxel | Carboplatin + nab-paclitaxel | NCT02367794 | ||
| III | MDPL3280A + carboplatin/cisplatin + gemcitabine | Carboplatin/cisplatin + gemcitabine | NCT02409355 | ||||
| Both | After previous treatment with PD-1-directed therapy | II | MDPL3280A | NCT03014648 | |||
| First line or higher | Ib | MDPL3280A + alectinib or erlotinib | NCT02013219 | ||||
| First line or higher in preselected PD-L1 (+) | II | MDPL3280A |
NCT02031458 or NCT01846416 |
||||
| First line or higher in NY-ESO-1 (+) | II | CDX-1401 + MDPL3280A | NCT02495636 | ||||
| Second line or higher | IB-II | MDPL3280A + daratumumab (anti-CD38) | MDPL3280A | NCT03023423 | |||
| SCLC | Stage IIIb–IV | First line | I-II | MDPL3280A + carboplatin + etoposide | Carboplatin–etoposide | NCT02748889 | |
| II | MDPL3280A + carboplatin + etoposide + trilaciclib | MDPL3280A + carboplatin + etoposide | NCT03041311 | ||||
| I–III | MDPL3280A + carboplatin + etoposide | Carboplatin + etoposide | NCT027633579 | ||||
| Second line | II | MDPL3280A | Carboplatin + etoposide or topotecan | NCT03059667 | |||
EGFR, epidermal growth factor receptor; NSCLC, non-small cell lung cancer; NY-ESO-1, cancer-testis antigen; PD-1, programmed cell-death 1; PD-L1, programmed cell-death-ligand 1; SCLC, small cell lung cancer.
Atezolizumab combined with other treatments
Atezolizumab is currently tested in association with chemotherapy, anti-VEGF, EGFR tyrosine kinase inhibitor, vaccine therapy, anti-CD38, anti-Cyclin Dependent Kinase (CDK)4/6 and radiation therapy (Table 2).
Combinations of atezolizumab with chemotherapy shouldn’t convey more toxicities than chemotherapy alone as shown as in a phase Ib study examining safety of the anti-PD-L1 associated with either paclitaxel, pemetrexed or weekly nab-paclitaxel.30
Other trials should provide more information about the efficacy and safety of the combination with chemotherapy.31,32
Targeted population and potential biomarkers
Updated data from the BIRCH trial were presented at WCLC 2016,20 where the ORR in the investigated group was 32% for TC3 or IC3 and 24% for TC2/3 or IC2/3. In this last subgroup, median duration of response was 13.1 months, median OS was 20.1 months. ORR was surprisingly better in the EGFR mutant subgroup (31%) than in the EGFR wild-type subgroup (20%) and ORR was also better in the Kirsten rat sarcoma virus oncogene (KRAS) mutant subgroup (27% versus 21% for KRAS wild-type NSCLC).
The FIR [ClinicalTrials.gov identifier: NCT01846416] study is currently assessing the efficacy of atezolizumab on preselected patients with CNS metastasis at baseline.
Companion diagnostic assays for anti-PD-1/PD-L1 therapies are discussed because of many differences and heterogeneity of measures and practical use. For instance, the nivolumab companion assay is Dako 28-8, targeting membrane tumour cells, with a threshold of positivity ⩾5%, pembrolizumab uses Dako 22C3 (Agilent, CA, United States), staining PD-L1 present on membrane tumour cells with a distinction for ‘weak’ expression (1–49%) and strong (⩾50%), SP142 attendant atezolizumab detect PD-L1 on tumour and tumour-infiltrating immune cells with a the positivity score TC1/2/3 or IC1/2/3.33
However, SP142, used with atezolizumab, is distinguishing itself due to its ability to detect PD-L1 expression on two different types of cells: TCs and tumour-infiltrating ICs. Furthermore, there is a strong correlation between PD-L1 IHC status and PD-L1 messenger RNA (mRNA) expression also with T-effector mRNA expression.34
One interesting fact in PD-L1 expression is that its intrapatient heterogeneity is low in metachronous tissues, indicating distinct types of tumour samples, including fresh or archival, can be reliably used to assess PD-L1 expression.35
The phase I study of atezolizumab17 for the treatment of NSCLC, melanoma, renal cell carcinoma, other solid tumours and haematological malignancies showed that atezolizumab was more effective in patients with pre-existing immunity suppressed by PD-L1, thus immunotherapy helps to reinvigorate immune cells. Therefore, high levels of PD-L1 and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) expression at baseline, such as low levels of CX3CL-1 (fractalin) expression were correlated with a positive response, in all tumour types. In addition, in melanoma, great expression of interferon gamma and indoleamine 2,3-dioxygénase (IDO-1) or Chemokine (C-X-C motif) ligand 9 (CXCL-9) was correlated with a good response to anti-PD-L1. The authors of this phase I study proposed three models of nonresponders: (1) ‘immunological ignorance’ (little or no tumour-infiltrating immune cell infiltration); (2) ‘nonfunctional immune response’ (presence of an immune infiltrate with minimal to no expression of PD-L1); and (3) ‘excluded infiltrate’ (presence of an immune infiltrate that resided solely around the outer edge of the tumour cell mass).
However, atezolizumab has shown efficacy in phase II and III studies regardless of SP142 PD-L1 expression results, and other biomarkers should be investigated further. For example, tumour mutation load, which was quantified by Kowanetz and colleagues using the Fundation Medicine 1 (FM1) panel of 315 cancer-related genes on tumour specimens of patients from the POPLAR, BIRCH and FIR studies. OS, PFS and ORR were improved in patients with increased mutation load treated with atezolizumab in both unselected and PD-L1-positive patients.36
PD-L2 expression also seems to be correlated with atezolizumab efficacy in NSCLC, melanoma, renal cell carcinoma and urothelial carcinoma but further evidence is required.37
Moreover, PD-L1 staining is usually performed on tumour samples and sometimes leads to rebiopsy, but PD-1/PD-L1/PD-L2 expression on peripheral blood cells could be an easiest way to characterize PD-L1 expression and predict response to atezolizumab.38
The place of atezolizumab in therapy and potential extension of use
So far, atezolizumab was studied for second-line treatment of advanced NSCLC, such as previously approved other immune checkpoint inhibitors.
A phase II study of atezolizumab as neoadjuvant and adjuvant therapy in patients with resectable NSCLC is currently ongoing39 aiming to enrol 180 patients with stage I, II and IIIa NSCLC prior to curative intent, who will receive two injections of atezolizumab 1200 mg before surgery. A second phase of this study will assess atezolizumab as an adjuvant therapy for patients who benefited from the neoadjuvant phase.
A multicentre phase III double-blinded, placebo-controlled study, IMpower133 [ClinicalTrials.gov identifier: NCT02763579],40 will try to extend the indication of atezolizumab to SCLC treatment in first line and in association with standard chemotherapy: carboplatin–etoposide.
Conclusions
Atezolizumab (MDPL3280A) clearly is an added value in the treatment of advanced stage pretreated NSCLC. Its interest in contrast with other immune checkpoint inhibitors relies on its efficacy, even in low or no PD-L1 expression subgroups. Considering the efficacy of anti-PD-1 such as pembrolizumab or nivolumab is overall higher in PD-L1-positive patients, atezolizumab might be preferable in PD-L1-negative patients. It will be necessary to consider other variant methods of PD-L1 testing used for each therapy to further explore this hypothesis.
Nonetheless, toxicity profile does not seem to differ between anti-PD-1 and anti-PD-L1 treatments and, in order, there is no argument for choosing atezolizumab over pembrolizumab or nivolumab, based on toxicity profile.
We are still far from fully understanding cancer immunity and the mechanisms leading to the success or not of immunotherapy agents. For this reason, we should investigate to a greater level the factors of failure, such as EGFR-mutant tumours.
Supplementary Material
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: Consultations for Astra-Zeneca, Bristol-Myers Squibb, Boehringer–Ingelheim, Clovis Oncology, Eli Lilly Oncology, F. Hoffmann–La Roche Ltd., Novartis, Merck, MSD, Pierre Fabre and Pfizer.
Contributor Information
Fanny Jean, Multidisciplinary Oncology and Therapeutic Innovations Department, Aix Marseille University, Marseille, France.
Pascale Tomasini, Multidisciplinary Oncology and Therapeutic Innovations Department, Aix Marseille University, Marseille, France Aix Marseille University, Inserm U911 CRO2, Marseille, France.
Fabrice Barlesi, Service d’Oncologie Multidisciplinaire & Innovations Thérapeutiques, Pôle Cardio-Vasculaire et Thoracique, Aix Marseille University, Assistance Publique – Hôpitaux de Marseille Hôpital Nord – Chemin des Bourrely, 13915 Marseille Cedex 20, France.
References
- 1. Stewart BW, Wild CP. (eds). World cancer report 2014. Lyon, France: International agency for research on cancer. [PubMed] [Google Scholar]
- 2. Masters GA, Temin S, Azzoli CG, et al. Systemic therapy for stage IV non-small-cell lung cancer: American society of clinical oncology clinical practice guideline update. J Clin Oncol 2015; 33: 3488–3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Besse B, Adjei A, Meldgaard P, et al. 2nd ESMO consensus conference on lung cancer: non-small-cell lung cancer first line/second and further lines of treatment in advanced disease. Ann Oncol 2014; 25: 1475–1484. [DOI] [PubMed] [Google Scholar]
- 4. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674. [DOI] [PubMed] [Google Scholar]
- 5. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab vs docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015; 373: 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab vs docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015; 373: 1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016; 387: 1540–1550. [DOI] [PubMed] [Google Scholar]
- 8. Novello S, Barlesi F, Califano R, et al. Metastatic non-small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016; 27(Suppl. 5): V1–V27. [DOI] [PubMed] [Google Scholar]
- 9. National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology (NCCN guidelines). Non-small cell lung cancer v6.2017, shttps://www.nccn.org/professionals/physician_gls/PDF/nscl.pdf (2017, accessed 12 May 2017). [DOI] [PubMed]
- 10. Xia B, Herbst RS. Immune checkpoint therapy for non-small-cell cancer: an update. Immunotherapy 2016; 8: 265–277. [DOI] [PubMed] [Google Scholar]
- 11. Matsumoto K, Fukuyama S, Eguchi-Tsuda M, et al. B7-DC induced by IL-13 works as a feedback regulator in the effector phase of allergic asthma. Biochem Biophys Res Commun 2008; 365: 170–175. [DOI] [PubMed] [Google Scholar]
- 12. Akbari O, Stock P, Singh AK, et al. PD-L1 and PD-L2 modulate airway inflammation and iNKT-cell-dependent airway hyperreactivity in opposing directions. Mucosal Immunol 2010; 3: 81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gaule P, Smithy JW, Toki M, et al. A quantitative comparison of antibodies to programmed cell death 1 ligand 1. JAMA Oncol 2016; 3: 256–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mizugaki H, Yamamoto N, Murakami H, et al. Phase I-dose finding study of monotherapy with atezolizumab, an engineered immunoglobulin monoclonal antibody targeting PD-L1, in Japanese patients with advanced solid tumours. Invest New Drug 2016; 34: 596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016; 387: 1837–1846. [DOI] [PubMed] [Google Scholar]
- 16. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017; 389: 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MDPL3280A in cancer patients. Nature 2014; 515: 563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smith DA, Vansteenkiste JF, Fehrenbacher L, et al. Updated survival and biomarker analyses of a randomized phase II study of atezolizumab vs docetaxel in 2L/3L NSCLC (POPLAR). Paper presented at 2016. American Society of Clinical Oncology, June 2016; Chicago, USA. Abstract 9028. [Google Scholar]
- 19. Besse B, Johnson M, Janne PA, et al. Phase II, single-arm trial (BIRCH) of atezolizumab as first-line or subsequent therapy for locally advanced or metastatic PD-L1 selected non-small cell lung cancer (NSCLC). Paper presented at 2015. European Society of Medical Oncology, September 2015; Vienna, Austria. Abstract 16LBA. [Google Scholar]
- 20. Garassino M, Rizvi N, Besse B, et al. , et al. Atezolizumab as 1L therapy for advanced NSCLC in PD-L1-selected patents: updated ORR, PFS and OS data from BIRCH study. Paper presented at 2016. World Conference on Lung Cancer, December 2016; Vienna, Austria. S251–S252.
- 21. Barlesi F, Park K, Ciardiello F, et al. Primary analysis from OAK, a randomized phase III study comparing atezolizumab with docetaxel in 2L/3L NSCLC. Paper presented at 2016. European Society of Medical Oncology, October 2016; Copenhagen, Denmark. Abstract LBA44_PR. [Google Scholar]
- 22. Gandara DR, Pawel JV, Sullivan R, et al. Impact of atezolizumab treatment beyond disease progression (TBP) in advanced NSCLC: results from the randomized Ph III OAK Study. Paper presented at 2017 American Society of Clinical Oncology, June 2017; Chicago, USA. Abstract 9001. [Google Scholar]
- 23. Gadgeel SM, Ciardiello F, Rittmeyer A, et al. OAK, a randomized phase III study of atezolizumab vs docetaxel in patients with advanced NSCLC: results from subgroup analyses. Paper presented at 2016 World Conference on Lung Cancer, December 2016; Vienna, Austria Plenary session 430. [Google Scholar]
- 24. Zhou GW, Xiong Y, Chen S, et al. Anti-PD-1/PD-L1 antibody therapy for pre-treated advanced non-small-cell lung cancer: a meta-analysis of randomized clinical trials. Medicine 2016; 95: e4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee CK, Man J, Lord S, et al. Checkpoint inhibitors in metastatic EGFR-mutated NSCLC – A meta-analysis. J Thorac Oncol 2017; 12: 403–407. [DOI] [PubMed] [Google Scholar]
- 26. Rizvi NA, Hellmann MD, Snyder A, et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015; 348: 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lukas R, Gandhi M, O’Hear C, et al. Atezolizumab in advanced NSCLC patients with baseline brain metastases: a pooled cohort safety analysis. Paper presented at 2016 World Conference on Lung Cancer, December 2016; Vienna, Austria. Abstract P2.03b-014, Poster 5214. [Google Scholar]
- 28. Wang C, Yu X, Wang W. A meta-analysis of efficacy and safety of antibodies targeting PD-1/PD-L1 in treatment of advanced NSCLC. Medicine 2016; 95: e5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pillai RN, Behera M, Owonikoko TK, et al. Evaluation of toxicity profile of PD-1 versus PD-L1 inhibitors in NSCLC. Paper presented at 2016 American Society of Clinical Oncology, June 2016; Chicago, USA. Abstract 9035. [Google Scholar]
- 30. Camidge R, Liu SV, Powderly J, et al. Atezolizumab (MDPL3280A) combined with platinum-based chemotherapy in non-small cell lung cancer (NSCLC): a phase IB safety and efficacy update. Paper presented at 2015 World Conference on Lung Cancer, September 2015; Denver, CO, USA Oral session, S173–S260. [Google Scholar]
- 31. Reck M, Papadimitrakopoulou VA, Capuzzo F, et al. Phase III clinical trials in chemotherapy-naive patients with advanced NSCLC assessing the combination of atezolizumab with chemotherapy. Paper presented at 2016 European Society of Medical Oncology, October 2016; Copenhagen, Denmark. vi416–vi454. [Google Scholar]
- 32. Papadimitrakopoulou VA, Cappuzzo F, Jotte RM, et al. Phase III clinical trials of atezolizumab combined with chemotherapy in chemotherapy-naive patients with advanced NSCLC. Paper presented at 2016 American Society of Clinical Oncology, June 2016; Chicago, IL, USA. Abstract TPS9103. [Google Scholar]
- 33. Texido C, Karachaliou N, Gonzalez-Cao M, et al. Assays for predicting and monitoring responses to lung cancer immunotherapy. Cancer Biol Med 2015; 12: 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Williams J, Kowanetz M, Koeppen H, et al. The SP142 PD-L1 IHC assay for atezolizumab reflects pre-existing immune status in NSCLC and correlates with PD-L1 mRNA. Paper presented at 2016 European Society of Medical Oncology, October 2016; Copenhagen, Denmark. Abstract 1171P. [Google Scholar]
- 35. Chaft JE, Chao B, Akerley WL, et al. Evaluation of PD-L1 expression in metachronous tumour samples and FDG-PET as a predictive biomarker in Ph2 study (FIR) of atezolizumab (MDPL3280A). Paper presented at 2015 World Conference on Lung Cancer, September 2015; Denver, CO, USA 1556–0864. [Google Scholar]
- 36. Kowanetz M, Zou W, Shames DS, et al. Tumour mutation load assessed by Foundation One (FM1) is associated with improved efficacy of atezolizumab in patients with advanced NSCLC. Paper presented at 2016 European Society of Medical Oncology, October 2016; Copenhagen, Denmark. Abstract 77P. [Google Scholar]
- 37. Schmid P, Hegde PS, Zou W, et al. Association of PD-L2 expression in human tumours with atezolizumab activity. Paper presented at 2016 American Society of Clinical Oncology, June 2016; Chicago, USA. Abstract 11506. [Google Scholar]
- 38. Arrieta O, Montes-Servin E. Evaluation of PD1/PDL1 expression on peripheral blood cells subpopulations in patients with NSCLC. Paper presented at 2016 World Conference on Lung Cancer, December 2016; Vienna, Austria. Abstract MA 14.02. [Google Scholar]
- 39. Owen D, Bunn PJ, Johnson B, et al. A phase II study of atezolizumab as neoadjuvant and adjuvant therapy in patients with resectable non-small-cell lung cancer (NSCLC). Paper presented at 2016 World Conference on Lung Cancer, December 2016; Vienna, Austria. S1082. [Google Scholar]
- 40. Horn L, Reck M, Mok TSK, et al. A phase III study of atezolizumab with carboplatin plus etoposide in patients with extensive-stage small-cell lung cancer (IMpower 133). Paper presented at 2016 European Society of Medical Oncology, October 2016; Copenhagen, Denmark vi493–vi496. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.