Abstract
Purpose of review
CGI-58 is an important player in lipid metabolism. It acts as activator of triglyceride hydrolases and as acyl-CoA dependent lysophosphatidic acid acyl transferase. This review aims at establishing a structure-function relationship of this still rather enigmatic protein based on recent studies characterizing different functions of CGI-58.
Recent findings
Novel studies confirm the important regulatory role of CGI-58 as activator of the triglyceride hydrolase ATGL. New evidence, corroborated by the characterization of a CGI-58 knockout mouse model, also suggests the existence of yet unknown lipases that are activated by CGI-58. Additionally, CGI-58 was identified to exert acyl-CoA dependent lysophosphatidic acid acyl transferase activity, which implies possible roles in triglyceride/phospholipid synthesis or signaling processes. Unlike mammalian CGI-58 proteins, orthologs from plants and yeast additionally act as weak triglyceride and phospholipid hydrolases. A first 3D model was calculated and allows preliminary structural considerations for the functions of CGI-58.
Summary
Despite important progress concerning the different biochemical functions of CGI-58, the physiological importance of these activities requires better characterization. Furthermore, 3D structural data for CGI-58 are required to unveil the molecular mechanism of how CGI-58 acts as activator of lipases and exerts its enzymatic functions.
Keywords: lipid metabolism, ATGL activation, lysophosphatidic acid acyl transferase, αβ hydrolase
Introduction
During the last decade, the metabolism of neutral acylglycerols (triacylglycerol, TG; diacylglycerol, DG; and monoacylglycerol, MG) gained tremendous interest when it was realized that they represent more than just an inert compound for the storage of energy. It is now well established that in addition to their role as energy reserve, neutral lipids in cellular lipid droplets (LD) are an important reservoir for membrane phospholipids and a platform for the production of potent signaling lipids involved in energy homeostasis, cell cycle regulation, and inflammation. Consistent with this growing interest, new enzymes and regulatory proteins involved in the metabolism of acylglycerides were identified increasing the complexity of these pathways. Important recent discoveries disclosed numerous acyltransferases that are required for neutral lipid synthesis (1, 2) and identified lipins as essential phosphohydrolases in the formation of DG from phosphatidic acid (PA), which is an essential reaction for the subsequent formation of TG (3–5). Adipose triglyceride lipase (ATGL) was discovered to catalyze the initial step of lipolysis converting TG to DG (6). Complete hydrolysis of TG to fatty acids (FA) and glycerol requires two additional enzymes, hormone-sensitive lipase (HSL) and MG-lipase (MGL) (7–12). The activity of these enzymes is highly regulated by enzyme expression, post-translational enzyme modifications, and substrate access involving numerous hormones, (adipo)cytokines, and regulatory protein factors. Such protein factors include PAT proteins and members of the CideN family of proteins, which appear to control the access of lipolytic enzymes to TG contained within LD (13–18). Only two proteins are currently known to regulate lipolytic enzymes directly and independent of a cellular context: Comparative gene identification-58 (CGI-58, also annotated as αβ hydrolase domain containing 5, ABHD5) activates ATGL, whereas the protein product of G0/G1 switch gene 2 (G0S2) inhibits ATGL (19–21).
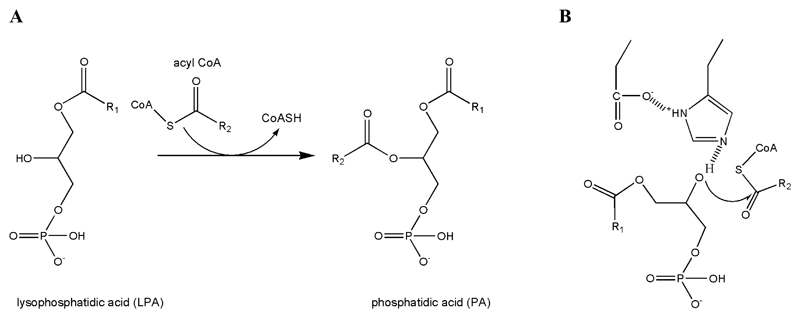
While the role of G0S2 in lipolysis and lipid metabolism still awaits in vivo confirmation, significant progress has been made in understanding the function of CGI-58 in various species. CGI-58 was first described in the year 2000 in an approach to identify proteins that are conserved between the human and Caenorhabditis elegans proteomes (22). In 2001, the link between CGI-58 and lipid metabolism became evident when Lefevre et al. reported that mutations in CGI-58 are causative for neutral lipid storage disease (NLSD) with ichthyosis (NLSD-I), also known as Chanarin Dorfman Syndrome (23). NLSD-I is characterized by the accumulation of neutral lipids in multiple tissues and ichthyosis. Depending on the mutation, variable phenotypes include liver steatosis, skeletal and cardiac myopathy, hearing loss, growth and mental retardation (reviewed in (24, 25) and (26–31)). In 2006, Lass et al. demonstrated that CGI-58 induces lipolysis by specifically activating ATGL and that NLSD-I variants of CGI-58 fail to stimulate ATGL (19). These results provided a plausible biochemical explanation for the systemic lipid accumulation in NLSD-I patients. They did not, however, explain the severe skin defect observed in CGI-58 deficient patients, because, as shown later, patients lacking ATGL suffer from NLSD but do not develop ichthyosis (32). This indicated that CGI-58 may have additional function(s) which are independent of ATGL. Consistent with this concept, CGI-58 was shown to exhibit enzymatic activity as acyl-CoA dependent lysophosphatidic acid acyltransferase (LPAAT) converting lysophosphatidic acid (LPA) to PA (33) (Figure 1A). Furthermore, non-mammalian CGI-58 (in plants and fungi) may act as TG hydrolase and phospholipase. These multiple functions of CGI-58 in lipid synthesis and lipolysis suggest a decisive role of the protein in lipid homeostasis connecting neutral lipid with phospholipid metabolism.
Figure 1.
A) CGI-58 exerts acyl-CoA dependent lysophosphatidic acid acyl transferase activity (LPAAT), where lysophosphatidic acid (LPA) and a Coenzyme A (CoA) activated acyl chain are esterified to form phosphatidic acid (PA). B) Catalytic His residues, which are properly positioned by an Asp and then activate the hydroxyl group for nucleophilic attack on acyl-CoA, are often found to be essential in the catalytic reaction of acyl- and acetyl-transferases.
In this review, we highlight recent findings concerning CGI-58 and its homologs i) as activator of ATGL and other TG hydrolases, ii) as TG hydrolase and phospholipase, and iii) as acyl-CoA dependent LPAAT. Moreover, we will relate these functions to the structure of CGI-58 on the basis of a homology model (34, 35).
Expression and biochemical functions of CGI-58
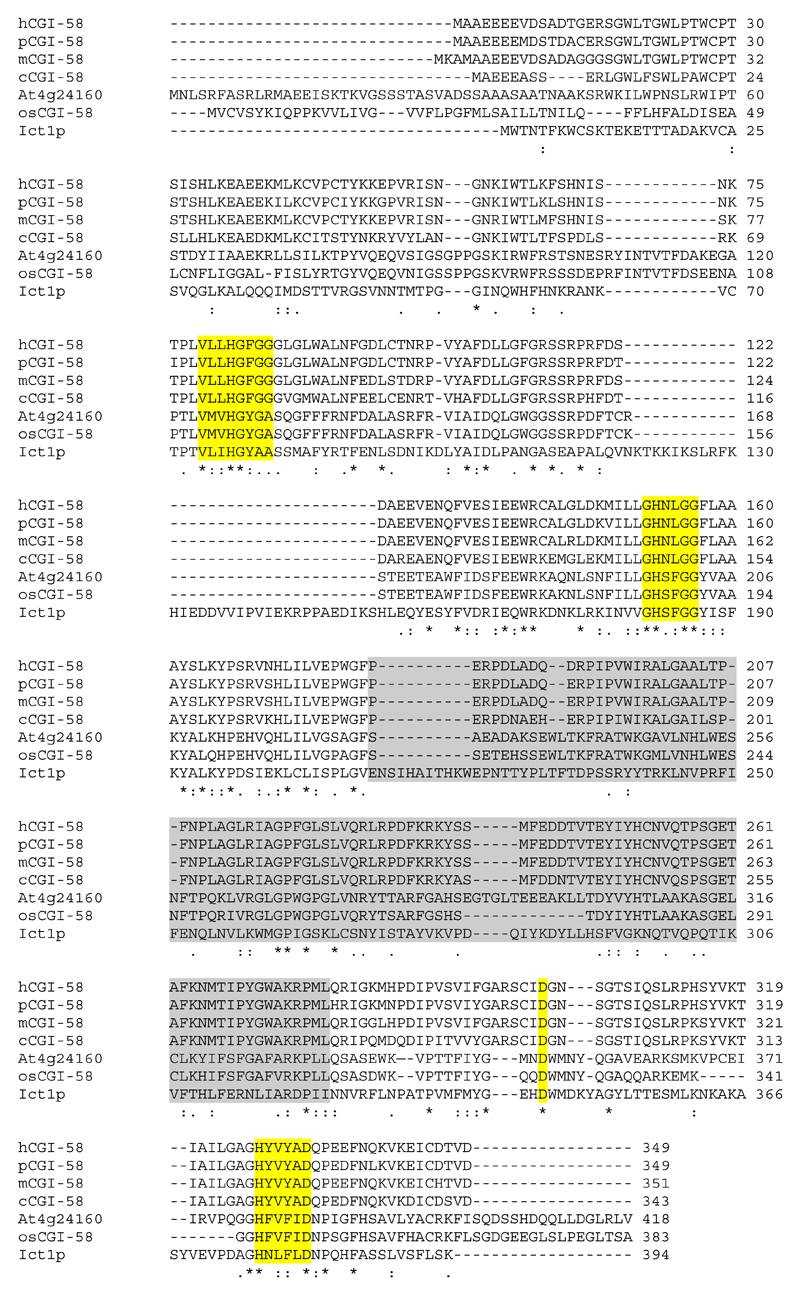
The human gene for CGI-58 is located on chromosome 3p21, comprises seven exons and encodes a 349 amino acid protein on a 5.4 kb mRNA. In mice, two mRNA species of approximately 1.4 and 3.2 kb are reported to code for the full length protein of 351 residues (19, 36). A splice variant of murine CGI-58 encodes a 202 residue isoform (37). CGI-58 mRNA is found in various tissues, including adipose tissue, testis, liver, neurons, muscle, and epidermis (19, 36, 38). Human and mouse CGI-58 (UniProt accession codes Q8WTS1 and Q9DBL9, respectively) share 94% sequence identity. Orthologs of CGI-58 were identified in different species including Arabidopsis thaliana (At4g24160) and Saccharomyces cerevisiae (Ict1p) indicating an evolutionary conserved role of CGI-58 in lipid metabolism (22, 36, 39–41).
Protein-protein interactions of CGI-58 regulate lipolysis at different levels
The mechanisms involved in the regulation of lipolysis require the interaction of CGI-58 with ATGL and several members of the perilipin family of LD-binding proteins (6–12, 17–19, 34, 42–46). Specifically, CGI-58 interacts with perilipin-1 (perilipin, PERI, PLIN), perilipin-2 (ADRP, ADFP, adipophilin), and perilipin-5 (PAT1, LSDP5, OXPAT, MLDP) (16, 36, 47–52).
CGI-58 as activator of ATGL
CGI-58 has been identified as activator of lipolysis by stimulating ATGL up to 20-fold (19). The CGI-58 mediated activation of ATGL has been shown in in vitro assays, in adipose tissue and also in non-adipose tissues.
In vitro lipolysis: Commonly used in vitro TG-hydrolase assays offer the TG substrate in an aqueous buffer system and use purified proteins or cellular lysates as supply for ATGL and CGI-58 (6, 19, 34, 53). In these assays, CGI-58 still stimulates ATGL, demonstrating that there is direct interaction between the two proteins and that CGI-58 does not require the presence of other LD-coating proteins to activate ATGL (6, 19, 34, 54–57). The molecular mechanism promoting ATGL activation by CGI-58 remains unknown.
Adipose tissue lipolysis: The involvement of CGI-58 in the regulation of the lipolytic process in adipocytes supports the so-called sequestration release model. In non-stimulated cells, CGI-58 preferentially interacts with unphosphorylated perilipin-1 at the surface of the LD. At the same time ATGL is located in the cytosol and on LD, whereas HSL is only cytosolic (46, 47). Upon β-adrenergic stimulation, PKA phosphorylates HSL and perilipin-1. This causes a translocation of HSL to the LD and the dissociation of CGI-58 from perilipin-1 (46, 58), which now promotes the interaction with its target lipase, ATGL. The interaction between ATGL and CGI-58 was shown to take place mainly with LD-associated ATGL and to a lesser degree in the cytoplasm (48).
Hormonal stimulation of lipolysis may also involve the translocation of cytosolic and ER-associated ATGL to the LD (20). The physiological role of these enzyme translocations in lipolysis and the involvement of CGI-58 in these processes remain to be determined.
Lipolysis in non-adipose cells: CGI-58 is also expressed in non-adipose tissues, where other members of the perilipin-family are considered to participate in the regulation of lipolysis (19, 36). These include the ubiquitously expressed perilipin-2 and perilipin-5, which is mostly found in oxidative tissue like muscle, heart, and liver. Recent findings demonstrate similar but not identical roles of perilipin-1 and perilipin-5 (47, 48, 51, 59). First detailed studies of cells expressing perilipin-5 showed co-localization of perilipin-5 with CGI-58 and ATGL (51, 59). Interaction of perilipin-5 and ATGL was demonstrated using FRET experiments (59). Perilipin-5 seems to bind either to CGI-58 or ATGL, but not to both proteins at the same time (59). It is suggested, that the interaction of perilipin-5 with ATGL and CGI-58 on the LD increases the local concentration of ATGL and CGI-58 on the LD and leads to functional interaction increasing lipolysis in the basal state (59). This is in contrast to cells expressing perilipin-1, where no co-localization of ATGL with perilipin-1 was observed (48, 59).
CGI-58 as activator of non-ATGL TG hydrolases
The inability of mutant forms of CGI-58 to activate ATGL provides a rational explanation for the excessive neutral lipid storage and abnormalities in phospholipid and TG metabolism in many tissues of patients with NLSD (60–62) (23, 32, 63–65). However, certain tissue-specific clinical features are not explained by defective ATGL activity. For example, patients and mice with CGI-58 deficiency (23, 63–65) suffer from severe ichthyosis, whereas this skin defect is not observed when ATGL is absent. This clearly suggests an important, ATGL-independent role of CGI-58 in the skin (32). An ATGL-independent function of CGI-58 is also suggested in the liver, however experimental data are still limited.
CGI-58 in the skin: Expression of CGI-58 in the skin was confirmed and was shown to be upregulated during skin development (38). Important insights into the epidermal function of CGI-58 came from the recent characterization of a CGI-58 knockout mouse model (65). These mice showed a severe skin permeability defect, systemic TG accumulation, severe hepatic steatosis, and died within 16 hours after birth. Importantly, the TG hydrolase activity in the epidermis of these animals was drastically reduced. In contrast, epidermal TG hydrolase activity was normal in ATGL knockout mice. Defective epidermal TG catabolism in CGI-58-deficient mice led to an impaired synthesis of acylceramides and defective formation of the corneocyte lipid envelop resulting in a dysfunctional permeability barrier of the skin (65). Increased TG accumulation, decreased acylceramide production, abnormal lamellular granule formation, and defective barrier function were also described in humans with CGI-58 deficiency (26, 38, 63, 64). To date, the epidermal TG hydrolase, which is speculated to be activated by CGI-58, has not been identified. Interestingly, mice that are unable to synthesize epidermal TG due to a lack in the rate-limiting TG synthetic enzyme diacylglycerol acyltransferase-2 develop a very similar barrier dysfunction as CGI-58 deficient mice (66). Apparently, functional TG synthesis and catabolism are essential for epidermal acylceramide synthesis and the formation of a functional epidermal permeation barrier.
CGI-58 in the liver: Patients and mice with mutations in ATGL and CGI-58 commonly develop hepatosteatosis, indicating prominent roles of CGI-58 and ATGL also in the TG homeostasis of the liver (63, 64, 67). A role of CGI-58 in lipoprotein secretion was indicated by different groups (68–70). However, it is still debated whether secreted TG result from exogenous FA or derive from stored TG. The levels of ATGL and HSL in the liver are relatively low and other lipases or TG degrading pathways (e.g. macrolipophagy) might play additional roles in hepatic TG turnover (67, 71–73).
Enzymatic function of CGI-58
In addition to its regulatory role in the lipolytic process, CGI-58 has also been shown to exhibit enzymatic functions. An acyl-CoA dependent LPAAT activity was observed for each biochemically characterized CGI-58 ortholog. In contrast, the activity as TG hydrolase and glycerophospholipase was only observed for CGI-58 from Saccharomyces cerevisiae (Ict1p) and Arabidopsis thaliana (At4g24160) but not for mammalian orthologs (19, 39, 50).
CGI-58 as LPAAT
Acyl-CoA dependent LPAAT activity of CGI-58 was reported in 2008 and subsequently analyzed in more detail (33, 34, 37, 74) (Figure 1). The specific activity of CGI-58 in vitro is relatively low and was determined to be in the range of 10 nmol PA/minute/mg purified protein (33, 34, 74). Detergents or divalent cations are reported to inhibit CGI-58’s LPAAT activity (74). The best acyl donors were arachidonoyl-CoA and oleoyl-CoA for mouse CGI-58, and oleoyl-CoA for human CGI-58 (33, 74). Ict1p and At4g24160 are reported to exhibit very similar substrate specificities and activities (39, 40). Most other known LPAATs are membrane proteins and only a few recombinant proteins were characterized (33, 34, 39, 40, 74–79).
Whether the LPAAT activity of CGI-58 plays a role in vivo is currently unknown. However, it is tempting to speculate that this activity participates in the local (possibly LD-associated) biosynthesis of PA controlling PA-dependent signaling processes. Other interesting hypotheses for CGI-58’s LPAAT activity suggest i) that it could be involved in reducing end-product inhibition when FA are formed during lipolysis or ii) that CGI-58 plays a role in the production of eicosanoid signaling lipids due to its selectivity for arachidonoyl-CoA (74). An important role of the LPAAT activity of CGI-58 in neutral lipid synthesis is unlikely considering that CGI-58 deficiency leads to increased cellular TG levels. Overexpression of CGI-58 in S. cerevisiae or fibroblasts from NLSD-I patients, as well as knock-down of CGI-58 in mice resulted in altered levels of total and/or specific PL (33, 70, 74). Earlier studies also reported defects in the catabolism of long-chain TG and in PL remodeling (60, 61, 80). Disruption of At4g24160 in Arabidopsis thaliana was reported to result in increased TG and glycolipid content, and the accumulation of LD in mature leaves (81). Steryl esters were reduced, whereas the levels of PL were not significantly changed (81).
CGI-58 as TG and PL hydrolase
In contrast to human and murine CGI-58 (19, 50), the orthologous proteins in S. cerevisia (Ict1p) and A. thaliana (At4g24160) harbor a catalytic serine in their nucleophilic elbow (Figure 2) and exhibit hydrolytic activities towards TG and PL in the range of 5-25 pmol FA/hour/mg purified protein (39, 40). These levels are extremely low and it is currently not established, if the lipolytic activities of Ict1p and At4g24160 have any physiological function.
Figure 2.
Sequence alignment of CGI-58 homologs from human (hCGI-58), pig (pCGI-58), mouse (mCGI-58), chicken (cCGI-58), Arabidopsis thaliana (At4g24160), rice (osCGI-58), and Saccharomyces cerevisiae (Ict1p). Conserved regions harboring the G-X-S/N-X-G motif, HX4D motif, and the proposed catalytic Asp in a long loop region are highlighted in yellow. The cap region is highlighted in gray. The sequence alignment was obtained with ClustalW and manual finishing.
Structure-function relationship of CGI-58
CGI-58 displays protein sequence similarities to epoxide hydrolases, iminopeptidases, and dehalogenases harboring αβ hydrolase folds. This fold is also observed in different hydrolytic enzymes including esterases, lipases, transacetylases, and thioesterase acyl transferases (82). Typical αβ hydrolases share low overall sequence identity and harbor a central, mostly parallel β-sheet flanked by α-helices (82–86). It should also be noted, that some αβ hydrolases do not have any known enzymatic activity or do not exert their enzymatic functions via the classical catalytic triad mechanisms (82, 84, 87).
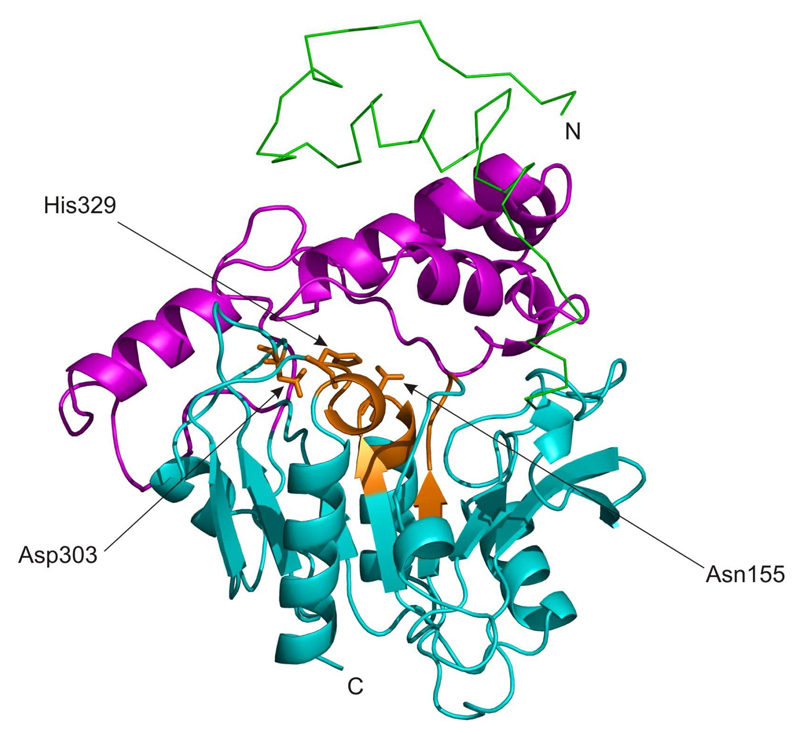
Due to the lack of experimental 3D information, homology models of CGI-58 comprising an αβ hydrolase core were calculated and provide interesting information on gross features of CGI-58 (34, 88, 89) (Figure 3).
Figure 3.
3D model of murine CGI-58. The αβ hydrolase core structure and the mostly helical cap are shown as cartoon colored in cyan and magenta, respectively. Conserved regions harboring the G-X-S/N-X-G motif, HX4D motif, and the proposed catalytic Asp303 are highlighted in orange. A conserved region around amino acid 86, which is predicted to participate in forming the oxyanion-hole is also colored in orange. The N-terminal extension is displayed as a green line.
The 3D homology model of CGI-58
One 3D model of mouse CGI-58 (mCGI-58) was deduced from the structure of a soluble epoxide hydrolase (AnEH, 17 % identity, 36 % similiarity) harboring an αβ hydrolase fold as template (34, 90). The homology model ranges from Ala5 to Val350 and includes an N-terminal region (Ala5 to Val48) consisting of α helices and loop regions, and an αβ-hydrolase core domain (Val48 to Val350). A cap (Pro180 to Leu280), which is inserted after β-strand 6 of the core domain, covers the proposed catalytic site (34). Other typical features of αβ hydrolases include the oxyanion hole and residues forming the catalytic triad. Generally, the triad consists of a nucleophile (Ser/Asp), a histidine, and an Asp or Glu, which positions the catalytic histidine. The nucleophile is located in the catalytic elbow forming a G-X-S/D-X-G consensus sequence. In the 3D model of mCGI-58, Phe86 is considered to participate in the oxyanion hole. The predicted catalytic triad residues Asn155, His329, and Asp303 are at equivalent positions to the AnEH template (Asp192, His374, and Asp348). Interestingly, in CGI-58 an asparagine residue (Asn155) is found within the consensus sequence of the catalytic elbow. Due to the chemical nature of asparagine, it cannot exert an equivalent contribution to the catalytic reaction mechanism as the corresponding aspartic acid of the template AnEH. Conservation of the third triad residue (Asp or Glu) is not so obvious and can only be detected upon manual intervention of alignment programs. According to the 3D model, this catalytic Asp is located in a loop region and therefore could allow substantial sequence variation (Figures 2 and 3). In the 3D model of CGI-58, a potential substrate binding pocket is also located between the cap and the αβ hydrolase core domain (Figure 3). In analogy to other αβ hydrolases, the cap might play an important role in determining the shape and chemical properties of the substrate binding pocket. Parts of the cap and the core (Leu177, Glu179, Trp181, Thr269, and Ile270) line the entrance to the potential substrate binding pocket. Mutations of corresponding residues in soluble epoxide hydrolase showed an important role of these residues in substrate specificity and positioning (91).
Interaction of CGI-58 with LD and proteins
Growing evidence suggests that the different activities of CGI-58 (ATGL activation, interaction with ATGL, interaction with LD, LPAAT) are not directly linked to each other. In table 1, selected biochemically characterized variants of CGI-58 and the corresponding changes in protein function are listed. These data show that interaction of CGI-58 with ATGL is a requirement, but not sufficient for ATGL activation (34). LPAAT activity seems to be very robust and was shown for point mutants hQ130P and hE260K, N-terminal truncations, and the short splice variant of CGI-58 (33, 34, 37). Interestingly, these mutants were unable to activate ATGL and unable to localize to the LD as wild-type CGI-58 (34, 37, 48, 49). No mutations have been identified, which i) abolish TG-hydrolase or PL-hydrolase activity in the CGI-58 orthologs Ict1p and At4g24160 and ii) abolish LPAAT activity while retaining other known functions of CGI-58.
Table 1.
Biochemical properties of CGI-58 mutants and the short isoform CGI-58S. Experimentally determined activities and interactions are marked as "+" or "-" depending on whether they were observed or not, respectively. If no experimental data are available the boxes were left empty.
| Isoform/Mutant ($) | ATGL-activation | ATGL-interaction | LPAAT activity | LD-association | Perilipin-1 interaction | Perilipin-2 interaction | Perilipin-5 interaction | Ref |
|---|---|---|---|---|---|---|---|---|
| mCGI-58S | - | + | - | - | (37) | |||
| mE9K | + | + | + | (49) | ||||
| hQ130P, mQ132P | - | + | - | - | - | (19, 33, 49) | ||
| h190ter | - | (19) | ||||||
| hE260K, mE262K | -(*) | + | - | - | - | - | (19, 33, 48, 49, 51) | |
| m10-351 | + | + | + | (34) | ||||
| mW21A | + | + | + | (34) | ||||
| mW21A/W25A/W29A | - | + | + | - | (34) | |||
| m32-351 | - | (+) | + | - | (34) | |||
| m19-351 | + | (+) | +/-, + | +/- | +/- | (34, 49) | ||
| m100ter-m317ter | - | - | - | (49) | ||||
| m325ter, m329ter, m335ter | - | (34) |
Mutations described in human or mouse CGI-58 are indicated with h or m, respectively.
CGI-58 E262K was reported to stimulate ATGL in the absence of perilipin-5 (51).
The N-terminal Trp-rich region was shown to be involved in ATGL stimulation and localization of CGI-58 to LD (34). Deletion of this N-terminal region abolished LD binding of CGI-58 but did not affect its interaction with ATGL or the LPAAT activity (34). Experiments with N-terminal peptides and LD-mimicking micelles suggested that CGI-58 might directly interact with the LD thereby enabling access of ATGL to its TG substrate, or promoting interaction of CGI-58 with ATGL at the LD (34).
CGI-58 harboring the point mutation E262K is unable to interact with perilipin-1 and perilipin-2 (49, 51). Consequently, it might be speculated, that the cap harbors an important protein-protein surface of CGI-58. It is unknown, whether the cap or the N-terminal extension undergo structural rearrangements upon binding of CGI-58 with interaction partners, which could play a role in accessibility of the predicted substrate binding pocket or change the surface properties of CGI-58. Based on analogy to other αβ hydrolases, the cap could also be involved in governing the substrate specificity of CGI-58. The cap and the N-terminal region of CGI-58 from animals (human, mouse, chicken), plants (rice, A. thaliana), and S. cerevisia show significant differences at the sequence level: The overall identity of these regions is low (ca. 15%), whereas the corresponding sequences within vertebrates and within plants show 95% and 85% sequence identity, respectively. This indicates different biological functions for CGI-58 in mammalian and non-mammalian species.
Structural elements of CGI-58 related to enzymatic function
In analogy to other αβ hydrolases, the reported hydrolytic activities of the non-mammalian orthologs could be exerted by the predicted triad residues of the αβ-hydrolase core. Structure-function considerations concerning the LPAAT activity of CGI-58 are speculative because residues involved in the LPAAT activity are unknown. Data bank searches reveal protein sequences for more than 500 lysophosphatidic acid acyl transferases, however no 3D structural entry is available to date (92, 93).
TG and PL hydrolase activity: CGI-58 orthologs from S. cerevisia, A. thaliana, and rice have a serine in the catalytic elbow, which could act as nucleophile in the hydrolysis of TG and PL. The presence of an asparagine in the catalytic elbow of human and mouse CGI-58 explains the lack of hydrolytic activity (Figure 2) (19, 50). Substitution of Asn155 with Ser in mouse CGI-58 did not result in lipolytic activity (42). This could stem from slight rearrangement of the catalytic site, extremely low levels of catalytic activity, or non-exhaustive testing of suitable substrates.
LPAAT activity: 3D structures of other O-acyl- and O-acetyl-transferases reveal different folds (including αβ hydrolases) and catalytic mechanisms, but generally attribute a central and predominant role to a catalytic histidine. The reaction mechanism is reminiscent to those seen in catalytic triads of αβ hydrolases (94) (Figure 1B). A conserved HX4D motif is thought to form an ion pair and thus facilitate the His-mediated activation of the hydroxyl group at the C1 or C2 positions of the glycerol-3-phosphate in glycerol-3-phosphate-1-acyltransferases (GPAT) and 1-acylglycerol-3-phosphate-O-acyltransferases (AGPAT), respectively (94–97). The hydroxyl group can then act as a nucleophile and attack the acetyl- or acyl donor. A conserved HX4D motif is found in essentially all orthologs of CGI-58, however, experiments testing its contribution to LPAAT activity have not been reported. Interestingly, the conserved His-329 within the HX4D motif is identical with the catalytic residue predicted for the classical catalytic triad. It should also be noted that many epoxide-hydrolases (e.g. AnEH, human epoxide hydrolase 2) or epoxide-hydrolase like haloalkane dehalogenases harbor an HX4E or HX4D motif. To our knowledge, no acyltransferase activity was reported for these enzymes.
AGPATs and GPATs from bacteria, plants, and mammals also have conserved amino acid sequences important for binding of the glycerol-phosphate (96, 97). A reminiscent cluster of positive charges is also present in mCGI-58 ranging from Arg229 to Lys237, which might adopt a corresponding function.
Conclusion
A variety of biochemical functions of CGI-58 and its orthologs emphasize the importance of the protein in lipid metabolism. CGI-58 is now established to act as regulator of lipolysis in multiple tissues affecting both TG and PL metabolism. The function of CGI-58 as co-activator of ATGL provides an excellent rationale for some (e.g. systemic lipid accumulation), but not all (e.g. skin defect, mental retardation) phenotypic manifestations in NLSD-I patients. Based on the acyl-CoA dependent LPAAT activity, CGI-58 may play an important role in the biogenesis of lipid mediators in signaling processes.
Despite the recent progress, numerous aspects of CGI-58 need to be addressed i) to understand the activation of ATGL at molecular detail, ii) to identify non-ATGL target lipases of CGI-58, iii) to pinpoint the physiological relevance of the LPAAT function, and v) to understand the different functions of CGI-58 at a structural level. Finally, it will be interesting to see how the various activities of CGI-58 are integrated to maintain functional glycerolipid homeostasis in essentially all eukaryotes.
Key points.
-
-
CGI-58 is a key regulatory protein in lipolysis and activates ATGL and other TG hydrolases.
-
-
The TG hydrolase activating function of CGI-58 is influenced by protein-protein interaction with members of the perilipin family.
-
-
The enzymatic function of CGI-58 as LPAAT might play a role in signaling processes.
-
-
CGI-58 belongs to the αβ hydrolase fold family.
-
-
In contrast to mammalian CGI-58, which does not act as a TG hydrolase, non-mammalian orthologs of CGI-58 are reported to have TG and PL hydrolyzing activity.
Acknowledgements
This work was supported by grants from “GOLD III” which is part of the Austrian Genome Project “GEN-AU Genome Research in Austria” and is funded by the Austrian Federal Ministry of Science and Research and FFG. Support is also provided by project P22170, the SFB LIPOTOX (F30), and the doctoral school “DK Molecular Enzymology” (W901-B05), which are funded by the Austrian Science Fund (FWF).
Footnotes
The authors declare no conflict of interest.
References
- 1.Turkish AR, Sturley SL. The genetics of neutral lipid biosynthesis: An evolutionary perspective. Am J Physiol Endocrinol Metab. 2009;297:E19–27. doi: 10.1152/ajpendo.90898.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turkish A, Sturley SL. Regulation of triglyceride metabolism. I. Eukaryotic neutral lipid synthesis: "Many ways to skin acat or a dgat". Am J Physiol Gastrointest Liver Physiol. 2007;292:G953–957. doi: 10.1152/ajpgi.00509.2006. [DOI] [PubMed] [Google Scholar]
- 3.Siniossoglou S. Lipins, lipids and nuclear envelope structure. Traffic. 2009;10:1181–1187. doi: 10.1111/j.1600-0854.2009.00923.x. [DOI] [PubMed] [Google Scholar]
- 4.Reue K. The lipin family: Mutations and metabolism. Curr Opin Lipidol. 2009;20:165–170. doi: 10.1097/MOL.0b013e32832adee5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reue K, Dwyer JR. Lipin proteins and metabolic homeostasis. J Lipid Res. 2009;50(Suppl):S109–114. doi: 10.1194/jlr.R800052-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger G, Eisenhaber F, Hermetter A, Zechner R. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383–1386. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]
- 7.Ahmadian M, Wang Y, Sul HS. Lipolysis in adipocytes. Int J Biochem Cell Biol. 2010;42:555–559. doi: 10.1016/j.biocel.2009.12.009. [*Review on adipocyte lipolysis including a discussion of therapeutic approaches to regulate lipolysis.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bezaire V, Langin D. Regulation of adipose tissue lipolysis revisited. Proc Nutr Soc. 2009;68:350–360. doi: 10.1017/S0029665109990279. [*Proceedings of the Nutrition Society reviewing adipocyte lipolysis including a special discussion of lipolysis and adipose tissue inflammation.] [DOI] [PubMed] [Google Scholar]
- 9.Watt MJ, Spriet LL. Triacylglycerol lipases and metabolic control: Implications for health and disease. Am J Physiol Endocrinol Metab. 2010;299:E162–168. doi: 10.1152/ajpendo.00698.2009. [*Review on lipolysis also discussing the role of released fatty acids in PPAR signaling.] [DOI] [PubMed] [Google Scholar]
- 10.Zechner R, Kienesberger PC, Haemmerle G, Zimmermann R, Lass A. Adipose triglyceride lipase and the lipolytic catabolism of cellular fat stores. J Lipid Res. 2009;50:3–21. doi: 10.1194/jlr.R800031-JLR200. [* This review summarizes lipolysis also discussing tissue specificity and regulation.] [DOI] [PubMed] [Google Scholar]
- 11.Zimmermann R, Lass A, Haemmerle G, Zechner R. Fate of fat: The role of adipose triglyceride lipase in lipolysis. Biochim Biophys Acta. 2009;1791:494–500. doi: 10.1016/j.bbalip.2008.10.005. [* This paper reviews the current knowledge of lipid homeostasis.] [DOI] [PubMed] [Google Scholar]
- 12.Lass A, Zimmermann R, Oberer M, Zechner R. Lipolysis - a highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog Lipid Res. 2011;50:14–27. doi: 10.1016/j.plipres.2010.10.004. [*Comprehensive review on lipolysis in adiopose and non-adipose tissues including discussion on physiological function.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gong J, Sun Z, Li P. Cide proteins and metabolic disorders. Curr Opin Lipidol. 2009;20:121–126. doi: 10.1097/MOL.0b013e328328d0bb. [DOI] [PubMed] [Google Scholar]
- 14.Li F, Gu Y, Dong W, Li H, Zhang L, Li N, Li W, Song Y, Jiang L, Ye J, Li Q. Cell death-inducing dff45-like effector, a lipid droplet-associated protein, might be involved in the differentiation of human adipocytes. FEBS J. 2010;277:4173–4183. doi: 10.1111/j.1742-4658.2010.07806.x. [DOI] [PubMed] [Google Scholar]
- 15.Christianson JL, Boutet E, Puri V, Chawla A, Czech MP. Identification of the lipid droplet targeting domain of the cidea protein. J Lipid Res. 2010;51:3455–3462. doi: 10.1194/jlr.M009498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bickel PE, Tansey JT, Welte MA. Pat proteins, an ancient family of lipid droplet proteins that regulate cellular lipid stores. Biochim Biophys Acta. 2009;1791:419–440. doi: 10.1016/j.bbalip.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brasaemle DL. Thematic review series: Adipocyte biology. The perilipin family of structural lipid droplet proteins: Stabilization of lipid droplets and control of lipolysis. J Lipid Res. 2007;48:2547–2559. doi: 10.1194/jlr.R700014-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Ducharme NA, Bickel PE. Lipid droplets in lipogenesis and lipolysis. Endocrinology. 2008;149:942–949. doi: 10.1210/en.2007-1713. [DOI] [PubMed] [Google Scholar]
- 19.Lass A, Zimmermann R, Haemmerle G, Riederer M, Schoiswohl G, Schweiger M, Kienesberger P, Strauss JG, Gorkiewicz G, Zechner R. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by cgi-58 and defective in chanarin-dorfman syndrome. Cell Metab. 2006;3:309–319. doi: 10.1016/j.cmet.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Yang X, Lu X, Lombes M, Rha GB, Chi YI, Guerin TM, Smart EJ, Liu J. The g(0)/g(1) switch gene 2 regulates adipose lipolysis through association with adipose triglyceride lipase. Cell Metab. 2010;11:194–205. doi: 10.1016/j.cmet.2010.02.003. [**This paper is the first report describing the protein G0S2 as inhibitor of ATGL.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu X, Yang X, Liu J. Differential control of atgl-mediated lipid droplet degradation by cgi-58 and g0s2. Cell Cycle. 2010;9:2719–2725. doi: 10.4161/cc.9.14.12181. [* This paper gives new hints concerning ATGL mediated TG breakdown and G0S2 mediated inhibition of ATGL activity.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai CH, Chou CY, Ch'ang LY, Liu CS, Lin W. Identification of novel human genes evolutionarily conserved in caenorhabditis elegans by comparative proteomics. Genome Res. 2000;10:703–713. doi: 10.1101/gr.10.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lefevre C, Jobard F, Caux F, Bouadjar B, Karaduman A, Heilig R, Lakhdar H, Wollenberg A, Verret JL, Weissenbach J, Ozguc M, et al. Mutations in cgi-58, the gene encoding a new protein of the esterase/lipase/thioesterase subfamily, in chanarin-dorfman syndrome. Am J Hum Genet. 2001;69:1002–1012. doi: 10.1086/324121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamaguchi T, Osumi T. Chanarin-dorfman syndrome: Deficiency in cgi-58, a lipid droplet-bound coactivator of lipase. Biochim Biophys Acta. 2008 doi: 10.1016/j.bbalip.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 25.Schweiger M, Lass A, Zimmermann R, Eichmann TO, Zechner R. Neutral lipid storage disease: Genetic disorders caused by mutations in adipose triglyceride lipase/pnpla2 or cgi-58/abhd5. Am J Physiol Endocrinol Metab. 2009;297:E289–296. doi: 10.1152/ajpendo.00099.2009. [* This review gives an overview on mutations in CGI-58 and ATGL causing NLSD.] [DOI] [PubMed] [Google Scholar]
- 26.Uchida Y, Cho Y, Moradian S, Kim J, Nakajima K, Crumrine D, Park K, Ujihara M, Akiyama M, Shimizu H, Holleran WM, et al. Neutral lipid storage leads to acylceramide deficiency, likely contributing to the pathogenesis of dorfman-chanarin syndrome. J Invest Dermatol. 2010;130:2497–2499. doi: 10.1038/jid.2010.145. [*Letter to the editor, describing a role of CGI-58 in acylceramide production and corneocyte-bound lipid envelope formation, which are both required to build a functional skin permeability barrier.] [DOI] [PubMed] [Google Scholar]
- 27.Ujihara M, Nakajima K, Yamamoto M, Teraishi M, Uchida Y, Akiyama M, Shimizu H, Sano S. Epidermal triglyceride levels are correlated with severity of ichthyosis in dorfman-chanarin syndrome. J Dermatol Sci. 2010;57:102–107. doi: 10.1016/j.jdermsci.2009.10.016. [* This study reveals a novel mutation in the cgi-58 gene leading to NLSD-I and shows a positive correlation of TG accumulation in the skin with the severity of ichthyosis.] [DOI] [PubMed] [Google Scholar]
- 28.Cakir M, Bruno C, Cansu A, Cobanoglu U, Erduran E. Liver cirrhosis in an infant with chanarin-dorfman syndrome caused by a novel splice-site mutation in abhd5. Acta Paediatr. 2010;99:1592–1594. doi: 10.1111/j.1651-2227.2010.01869.x. [* In this paper, a new mutation in the CGI-58 gene causing NLSD-I is described.] [DOI] [PubMed] [Google Scholar]
- 29.Redaelli C, Coleman RA, Moro L, Dacou-Voutetakis C, Elsayed SM, Prati D, Colli A, Mela D, Colombo R, Tavian D. Clinical and genetic characterization of chanarin-dorfman syndrome patients: First report of large deletions in the abhd5 gene. Orphanet J Rare Dis. 2010;5:33. doi: 10.1186/1750-1172-5-33. [* In this study new NLSD-I causing mutations in CGI-58 are described; interestingly, one patient with NLSD-I did not have a mutation within CGI-58.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruno C, Bertini E, Di Rocco M, Cassandrini D, Ruffa G, De Toni T, Seri M, Spada M, Li Volti G, D'Amico A, Trucco F, et al. Clinical and genetic characterization of chanarin-dorfman syndrome. Biochem Biophys Res Commun. 2008;369:1125–1128. doi: 10.1016/j.bbrc.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 31.Emre S, Unver N, Evans SE, Yuzbasioglu A, Gurakan F, Gumruk F, Karaduman A. Molecular analysis of chanarin-dorfman syndrome (cds) patients: Identification of novel mutations in the abhd5 gene. Eur J Med Genet. 2010;53:141–144. doi: 10.1016/j.ejmg.2010.03.002. [* New mutations in the CGI-58 gene causing NLSD-I are described.] [DOI] [PubMed] [Google Scholar]
- 32.Fischer J, Lefevre C, Morava E, Mussini JM, Laforet P, Negre-Salvayre A, Lathrop M, Salvayre R. The gene encoding adipose triglyceride lipase (pnpla2) is mutated in neutral lipid storage disease with myopathy. Nat Genet. 2007;39:28–30. doi: 10.1038/ng1951. [DOI] [PubMed] [Google Scholar]
- 33.Ghosh AK, Ramakrishnan G, Chandramohan C, Rajasekharan R. Cgi-58, the causative gene for chanarin-dorfman syndrome, mediates acylation of lysophosphatidic acid. J Biol Chem. 2008;283:24525–24533. doi: 10.1074/jbc.M801783200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gruber A, Cornaciu I, Lass A, Schweiger M, Poeschl M, Eder C, Kumari M, Schoiswohl G, Wolinski H, Kohlwein SD, Zechner R, et al. The n-terminal region of comparative gene identification-58 (cgi-58) is important for lipid droplet binding and activation of adipose triglyceride lipase. J Biol Chem. 2010;285:12289–12298. doi: 10.1074/jbc.M109.064469. [** This study points out that the N-terminal region of CGI-58 is important for LD localization and ATGL activation but not for LPAAT activity and gives a first 3D model of the protein.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamaguchi T. Crucial role of cgi-58/alpha/beta hydrolase domain-containing protein 5 in lipid metabolism. Biol Pharm Bull. 2010;33:342–345. doi: 10.1248/bpb.33.342. [*Short review on CGI-58 and its function in adipose and non-adipse tissues.] [DOI] [PubMed] [Google Scholar]
- 36.Subramanian V, Rothenberg A, Gomez C, Cohen AW, Garcia A, Bhattacharyya S, Shapiro L, Dolios G, Wang R, Lisanti MP, Brasaemle DL. Perilipin a mediates the reversible binding of cgi-58 to lipid droplets in 3t3-l1 adipocytes. J Biol Chem. 2004;279:42062–42071. doi: 10.1074/jbc.M407462200. [DOI] [PubMed] [Google Scholar]
- 37.Yang X, Lu X, Liu J. Identification of a novel splicing isoform of murine cgi-58. FEBS Lett. 2010;584:903–910. doi: 10.1016/j.febslet.2009.12.058. [* This paper describes a splicing form of CGI-58 in mice which is incapable of LD localization and activation of ATGL but exhibits LPAAT activity.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akiyama M, Sakai K, Takayama C, Yanagi T, Yamanaka Y, McMillan JR, Shimizu H. Cgi-58 is an alpha/beta-hydrolase within lipid transporting lamellar granules of differentiated keratinocytes. Am J Pathol. 2008;173:1349–1360. doi: 10.2353/ajpath.2008.080005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghosh AK, Chauhan N, Rajakumari S, Daum G, Rajasekharan R. At4g24160, a soluble acyl-coenzyme a-dependent lysophosphatidic acid acyltransferase. Plant Physiol. 2009;151:869–881. doi: 10.1104/pp.109.144261. [** In this study, a CGI-58 homolog in Arabidopsis thaliana is identified and the recombinant protein was demonstrated to exhibit LPAAT, TG hydrolase and PL hydrolase activities.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghosh AK, Ramakrishnan G, Rajasekharan R. Ylr099c (ict1) encodes a soluble acyl-coa-dependent lysophosphatidic acid acyltransferase responsible for enhanced phospholipid synthesis on organic solvent stress in saccharomyces cerevisiae. J Biol Chem. 2008;283:9768–9775. doi: 10.1074/jbc.M708418200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saarela J, Jung G, Hermann M, Nimpf J, Schneider WJ. The patatin-like lipase family in gallus gallus. BMC Genomics. 2008;9:281. doi: 10.1186/1471-2164-9-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lass A, Zimmermann R, Oberer M, Zechner R. Lipolysis - a highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog Lipid Res. 2010 doi: 10.1016/j.plipres.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, Heldmaier G, Maier R, Theussl C, Eder S, Kratky D, et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–737. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- 44.Yamaguchi T, Omatsu N, Omukae A, Osumi T. Analysis of interaction partners for perilipin and adrp on lipid droplets. Mol Cell Biochem. 2006;284:167–173. doi: 10.1007/s11010-005-9045-y. [DOI] [PubMed] [Google Scholar]
- 45.Di Marzo V, Bisogno T, De Petrocellis L, Melck D, Orlando P, Wagner JA, Kunos G. Biosynthesis and inactivation of the endocannabinoid 2-arachidonoylglycerol in circulating and tumoral macrophages. Eur J Biochem. 1999;264:258–267. doi: 10.1046/j.1432-1327.1999.00631.x. [DOI] [PubMed] [Google Scholar]
- 46.Granneman JG, Moore HP. Location, location: Protein trafficking and lipolysis in adipocytes. Trends Endocrinol Metab. 2008;19:3–9. doi: 10.1016/j.tem.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 47.Granneman JG, Moore HP, Granneman RL, Greenberg AS, Obin MS, Zhu Z. Analysis of lipolytic protein trafficking and interactions in adipocytes. J Biol Chem. 2007;282:5726–5735. doi: 10.1074/jbc.M610580200. [DOI] [PubMed] [Google Scholar]
- 48.Granneman JG, Moore HP, Krishnamoorthy R, Rathod M. Perilipin controls lipolysis by regulating the interactions of ab-hydrolase containing 5 (abhd5) and adipose triglyceride lipase (atgl) J Biol Chem. 2009;284:34538–34544. doi: 10.1074/jbc.M109.068478. [** This study provides new insights into the interactions of perilipin-1, CGI-58, and ATGL.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamaguchi T, Omatsu N, Matsushita S, Osumi T. Cgi-58 interacts with perilipin and is localized to lipid droplets. Possible involvement of cgi-58 mislocalization in chanarin-dorfman syndrome. J Biol Chem. 2004;279:30490–30497. doi: 10.1074/jbc.M403920200. [DOI] [PubMed] [Google Scholar]
- 50.Yamaguchi T, Omatsu N, Morimoto E, Nakashima H, Ueno K, Tanaka T, Satouchi K, Hirose F, Osumi T. Cgi-58 facilitates lipolysis on lipid droplets but is not involved in the vesiculation of lipid droplets caused by hormonal stimulation. J Lipid Res. 2007;48:1078–1089. doi: 10.1194/jlr.M600493-JLR200. [DOI] [PubMed] [Google Scholar]
- 51.Granneman JG, Moore HP, Mottillo EP, Zhu Z. Functional interactions between mldp (lsdp5) and abhd5 in the control of intracellular lipid accumulation. J Biol Chem. 2009;284:3049–3057. doi: 10.1074/jbc.M808251200. [** This is the first paper characterizing the functional interaction of CGI-58 and perilipin 5 (mldp).] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kimmel AR, Brasaemle DL, McAndrews-Hill M, Sztalryd C, Londos C. Adoption of perilipin as a unifying nomenclature for the mammalian pat-family of intracellular lipid storage droplet proteins. J Lipid Res. 2010;51:468–471. doi: 10.1194/jlr.R000034. [*Proposal of a uniform nomenclature of proteins from the perilipin family.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holm C, Osterlund T. Hormone-sensitive lipase and neutral cholesteryl ester lipase. Methods Mol Biol. 1999;109:109–121. doi: 10.1385/1-59259-581-2:109. [DOI] [PubMed] [Google Scholar]
- 54.Schweiger M, Schoiswohl G, Lass A, Radner FP, Haemmerle G, Malli R, Graier W, Cornaciu I, Oberer M, Salvayre R, Fischer J, et al. The c-terminal region of human adipose triglyceride lipase affects enzyme activity and lipid droplet binding. J Biol Chem. 2008;283:17211–17220. doi: 10.1074/jbc.M710566200. [DOI] [PubMed] [Google Scholar]
- 55.Bezaire V, Mairal A, Ribet C, Lefort C, Girousse A, Jocken J, Laurencikiene J, Anesia R, Rodriguez AM, Ryden M, Stenson BM, et al. Contribution of adipose triglyceride lipase and hormone-sensitive lipase to lipolysis in hmads adipocytes. J Biol Chem. 2009;284:18282–18291. doi: 10.1074/jbc.M109.008631. [** In this paper a current model of lipolysis is approved in a human cell culture system especially pointing out the independent function of ATGL/CGI-58 and HSL.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bezaire V, Mairal A, Anesia R, Lefort C, Langin D. Chronic tnfalpha and camp pre-treatment of human adipocytes alter hsl, atgl and perilipin to regulate basal and stimulated lipolysis. FEBS Lett. 2009;583:3045–3049. doi: 10.1016/j.febslet.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 57.Alsted TJ, Nybo L, Schweiger M, Fledelius C, Jacobsen P, Zimmermann R, Zechner R, Kiens B. Adipose triglyceride lipase in human skeletal muscle is upregulated by exercise training. Am J Physiol Endocrinol Metab. 2009;296:E445–453. doi: 10.1152/ajpendo.90912.2008. [DOI] [PubMed] [Google Scholar]
- 58.Brasaemle DL, Subramanian V, Garcia A, Marcinkiewicz A, Rothenberg A. Perilipin a and the control of triacylglycerol metabolism. Mol Cell Biochem. 2009;326:15–21. doi: 10.1007/s11010-008-9998-8. [DOI] [PubMed] [Google Scholar]
- 59.Granneman JG, Moore HP, Mottillo EP, Zhu Z, Zhou L. Interactions of perilipin-5 (plin5) with adipose trigylceride lipase (atgl) J Biol Chem. 2010 doi: 10.1074/jbc.M110.180711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Igal RA, Coleman RA. Acylglycerol recycling from triacylglycerol to phospholipid, not lipase activity, is defective in neutral lipid storage disease fibroblasts. J Biol Chem. 1996;271:16644–16651. doi: 10.1074/jbc.271.28.16644. [DOI] [PubMed] [Google Scholar]
- 61.Igal RA, Coleman RA. Neutral lipid storage disease: A genetic disorder with abnormalities in the regulation of phospholipid metabolism. J Lipid Res. 1998;39:31–43. [PubMed] [Google Scholar]
- 62.Williams ML, Coleman RA, Placezk D, Grunfeld C. Neutral lipid storage disease: A possible functional defect in phospholipid- linked triacylglycerol metabolism. Biochim Biophys Acta. 1991;1096:162–169. doi: 10.1016/0925-4439(91)90055-e. [DOI] [PubMed] [Google Scholar]
- 63.Schweiger M, Lass A, Zimmermann R, Eichmann TO, Zechner R. Neutral lipid storage disease: Genetic disorders caused by mutations in adipose triglyceride lipase/pnpla2 or cgi-58/abhd5. Am J Physiol Endocrinol Metab. 2009 doi: 10.1152/ajpendo.00099.2009. [DOI] [PubMed] [Google Scholar]
- 64.Yamaguchi T, Osumi T. Chanarin-dorfman syndrome: Deficiency in cgi-58, a lipid droplet-bound coactivator of lipase. Biochim Biophys Acta. 2009;1791:519–523. doi: 10.1016/j.bbalip.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 65.Radner FP, Streith IE, Schoiswohl G, Schweiger M, Kumari M, Eichmann TO, Rechberger G, Koefeler HC, Eder S, Schauer S, Theussl HC, et al. Growth retardation, impaired triacylglycerol catabolism, hepatic steatosis, and lethal skin barrier defect in mice lacking comparative gene identification-58 (cgi-58) J Biol Chem. 2010;285:7300–7311. doi: 10.1074/jbc.M109.081877. [** This study characterizes the CGI-58 knock out mouse with impaired acylceramide synthesis and describes an ATGL-independent function of CGI-58 in the skin.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stone SJ, Myers HM, Watkins SM, Brown BE, Feingold KR, Elias PM, Farese RV., Jr Lipopenia and skin barrier abnormalities in dgat2-deficient mice. J Biol Chem. 2004;279:11767–11776. doi: 10.1074/jbc.M311000200. [DOI] [PubMed] [Google Scholar]
- 67.Ong KT, Mashek MT, Bu SY, Greenberg AS, Mashek DG. Adipose triglyceride lipase is a major hepatic lipase that regulates triacylglycerol turnover and fatty acid signaling and partitioning. Hepatology. 2011;53:116–126. doi: 10.1002/hep.24006. [*This study investigates the role of ATGL in the liver and shows that ATGL is a major hepatic triacylglycerol lipase involved in hepatic fatty acid partitioning and signaling.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brown JM, Chung S, Das A, Shelness GS, Rudel LL, Yu L. Cgi-58 facilitates the mobilization of cytoplasmic triglyceride for lipoprotein secretion in hepatoma cells. J Lipid Res. 2007;48:2295–2305. doi: 10.1194/jlr.M700279-JLR200. [DOI] [PubMed] [Google Scholar]
- 69.Caviglia JM, Sparks JD, Toraskar N, Brinker AM, Yin TC, Dixon JL, Brasaemle DL. Abhd5/cgi-58 facilitates the assembly and secretion of apolipoprotein b lipoproteins by mca rh7777 rat hepatoma cells. Biochim Biophys Acta. 2009;1791:198–205. doi: 10.1016/j.bbalip.2008.12.018. [** This study reveals that CGI-58 plays a crucial role in the process of lipoprotein assembly in the liver.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brown JM, Betters JL, Lord C, Ma Y, Han X, Yang K, Alger HM, Melchior J, Sawyer J, Shah R, Wilson MD, et al. Cgi-58 knockdown in mice causes hepatic steatosis but prevents diet-induced obesity and glucose intolerance. J Lipid Res. 2010;51:3306–3315. doi: 10.1194/jlr.M010256. [** This paper describes the triacylglycerol, diacylglycerol and ceramide accumulation in mice on a high fed diet caused by CGI-58 knock down which does not cause obesity.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Okazaki H, Igarashi M, Nishi M, Tajima M, Sekiya M, Okazaki S, Yahagi N, Ohashi K, Tsukamoto K, Amemiya-Kudo M, Matsuzaka T, et al. Identification of a novel member of the carboxylesterase family that hydrolyzes triacylglycerol: A potential role in adipocyte lipolysis. Diabetes. 2006;55:2091–2097. doi: 10.2337/db05-0585. [DOI] [PubMed] [Google Scholar]
- 73.Reid BN, Ables GP, Otlivanchik OA, Schoiswohl G, Zechner R, Blaner WS, Goldberg IJ, Schwabe RF, Chua SC, Jr, Huang LS. Hepatic overexpression of hormone-sensitive lipase and adipose triglyceride lipase promotes fatty acid oxidation, stimulates direct release of free fatty acids, and ameliorates steatosis. J Biol Chem. 2008;283:13087–13099. doi: 10.1074/jbc.M800533200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Montero-Moran G, Caviglia JM, McMahon D, Rothenberg A, Subramanian V, Xu Z, Lara-Gonzalez S, Storch J, Carman GM, Brasaemle DL. Cgi-58/abhd5 is a coenzyme a-dependent lysophosphatidic acid acyltransferase. J Lipid Res. 2010;51:709–719. doi: 10.1194/jlr.M001917. [** This study provides kinetic characterization of the LPAAT activity of mouse CGI-58 and describes the effect of CGI-58 overexpression in human fibroblasts from NLSD-I patients.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hollenback D, Bonham L, Law L, Rossnagle E, Romero L, Carew H, Tompkins CK, Leung DW, Singer JW, White T. Substrate specificity of lysophosphatidic acid acyltransferase beta -- evidence from membrane and whole cell assays. J Lipid Res. 2006;47:593–604. doi: 10.1194/jlr.M500435-JLR200. [DOI] [PubMed] [Google Scholar]
- 76.Sukumaran S, Barnes RI, Garg A, Agarwal AK. Functional characterization of the human 1-acylglycerol-3-phosphate-o-acyltransferase isoform 10/glycerol-3-phosphate acyltransferase isoform 3. J Mol Endocrinol. 2009;42:469–478. doi: 10.1677/JME-09-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim HU, Huang AH. Plastid lysophosphatidyl acyltransferase is essential for embryo development in arabidopsis. Plant Physiol. 2004;134:1206–1216. doi: 10.1104/pp.103.035832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maisonneuve S, Bessoule JJ, Lessire R, Delseny M, Roscoe TJ. Expression of rapeseed microsomal lysophosphatidic acid acyltransferase isozymes enhances seed oil content in arabidopsis. Plant Physiol. 2010;152:670–684. doi: 10.1104/pp.109.148247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yamashita A, Nakanishi H, Suzuki H, Kamata R, Tanaka K, Waku K, Sugiura T. Topology of acyltransferase motifs and substrate specificity and accessibility in 1-acyl-sn-glycero-3-phosphate acyltransferase 1. Biochim Biophys Acta. 2007;1771:1202–1215. doi: 10.1016/j.bbalip.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 80.Hilaire N, Salvayre R, Thiers JC, Bonnafe MJ, Negre-Salvayre A. The turnover of cytoplasmic triacylglycerols in human fibroblasts involves two separate acyl chain length-dependent degradation pathways. J Biol Chem. 1995;270:27027–27034. doi: 10.1074/jbc.270.45.27027. [DOI] [PubMed] [Google Scholar]
- 81.James CN, Horn PJ, Case CR, Gidda SK, Zhang D, Mullen RT, Dyer JM, Anderson RG, Chapman KD. Disruption of the arabidopsis cgi-58 homologue produces chanarin-dorfman-like lipid droplet accumulation in plants. Proc Natl Acad Sci U S A. 2010;107:17833–17838. doi: 10.1073/pnas.0911359107. [** This paper describes an analogy of CGI-58 disruption in plants and animals producing a CDS like phenotype.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Carr PD, Ollis DL. Alpha/beta hydrolase fold: An update. Protein Pept Lett. 2009;16:1137–1148. doi: 10.2174/092986609789071298. [DOI] [PubMed] [Google Scholar]
- 83.Heikinheimo P, Goldman A, Jeffries C, Ollis DL. Of barn owls and bankers: A lush variety of alpha/beta hydrolases. Structure. 1999;7:R141–146. doi: 10.1016/s0969-2126(99)80079-3. [DOI] [PubMed] [Google Scholar]
- 84.Holmquist M. Alpha/beta-hydrolase fold enzymes: Structures, functions and mechanisms. Curr Protein Pept Sci. 2000;1:209–235. doi: 10.2174/1389203003381405. [DOI] [PubMed] [Google Scholar]
- 85.Nardini M, Dijkstra BW. Alpha/beta hydrolase fold enzymes: The family keeps growing. Curr Opin Struct Biol. 1999;9:732–737. doi: 10.1016/s0959-440x(99)00037-8. [DOI] [PubMed] [Google Scholar]
- 86.Ollis DL, Cheah E, Cygler M, Dijkstra B, Frolow F, Franken SM, Harel M, Remington SJ, Silman I, Schrag J, et al. The alpha/beta hydrolase fold. Protein Eng. 1992;5:197–211. doi: 10.1093/protein/5.3.197. [DOI] [PubMed] [Google Scholar]
- 87.Bugg TD. Diverse catalytic activities in the alphabeta-hydrolase family of enzymes: Activation of h2o, hcn, h2o2, and o2. Bioorg Chem. 2004;32:367–375. doi: 10.1016/j.bioorg.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 88.Arnold K, Kiefer F, Kopp J, Battey JN, Podvinec M, Westbrook JD, Berman HM, Bordoli L, Schwede T. The protein model portal. J Struct Funct Genomics. 2009;10:1–8. doi: 10.1007/s10969-008-9048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kiefer F, Arnold K, Kunzli M, Bordoli L, Schwede T. The swiss-model repository and associated resources. Nucleic Acids Res. 2009;37:D387–392. doi: 10.1093/nar/gkn750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zou J, Hallberg BM, Bergfors T, Oesch F, Arand M, Mowbray SL, Jones TA. Structure of aspergillus niger epoxide hydrolase at 1.8 a resolution: Implications for the structure and function of the mammalian microsomal class of epoxide hydrolases. Structure. 2000;8:111–122. doi: 10.1016/s0969-2126(00)00087-3. [DOI] [PubMed] [Google Scholar]
- 91.Reetz MT, Bocola M, Wang LW, Sanchis J, Cronin A, Arand M, Zou J, Archelas A, Bottalla AL, Naworyta A, Mowbray SL. Directed evolution of an enantioselective epoxide hydrolase: Uncovering the source of enantioselectivity at each evolutionary stage. J Am Chem Soc. 2009;131:7334–7343. doi: 10.1021/ja809673d. [DOI] [PubMed] [Google Scholar]
- 92.Chang A, Scheer M, Grote A, Schomburg I, Schomburg D. Brenda, amenda and frenda the enzyme information system: New content and tools in 2009. Nucleic Acids Res. 2009;37:D588–592. doi: 10.1093/nar/gkn820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Geer LY, Marchler-Bauer A, Geer RC, Han L, He J, He S, Liu C, Shi W, Bryant SH. The ncbi biosystems database. Nucleic Acids Res. 2010;38:D492–496. doi: 10.1093/nar/gkp858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Turnbull AP, Rafferty JB, Sedelnikova SE, Slabas AR, Schierer TP, Kroon JT, Simon JW, Fawcett T, Nishida I, Murata N, Rice DW. Analysis of the structure, substrate specificity, and mechanism of squash glycerol-3-phosphate (1)-acyltransferase. Structure. 2001;9:347–353. doi: 10.1016/s0969-2126(01)00595-0. [DOI] [PubMed] [Google Scholar]
- 95.Coleman RA, Lee DP. Enzymes of triacylglycerol synthesis and their regulation. Prog Lipid Res. 2004;43:134–176. doi: 10.1016/s0163-7827(03)00051-1. [DOI] [PubMed] [Google Scholar]
- 96.Takeuchi K, Reue K. Biochemistry, physiology, and genetics of gpat, agpat, and lipin enzymes in triglyceride synthesis. Am J Physiol Endocrinol Metab. 2009;296:E1195–1209. doi: 10.1152/ajpendo.90958.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang YM, Rock CO. Thematic review series: Glycerolipids. Acyltransferases in bacterial glycerophospholipid synthesis. J Lipid Res. 2008;49:1867–1874. doi: 10.1194/jlr.R800005-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]