Abstract
Cholesterol content influences several important physiological functions due to its effect on membrane receptors. In this work, we tested the hypothesis that cellular cholesterol alters chemotactic response of monocytes to Monocyte Chemoattractant Protein -1 (MCP-1) due to their effect on the receptor, CCR2. We used Methyl-β-cyclodextrin (MβCD) to alter the baseline cholesterol in human monocytic cell line THP-1, and evaluated their chemotactic response to MCP-1. Compared to untreated cells, cholesterol enrichment increased the number of monocytes transmigrated in response to MCP-1 while depletion had opposite effect. Using imaging flow cytometry, we established that these differences were due to alterations in expression levels, but not the surface distribution, of CCR2.
Keywords: CCR2, MCP-1, transwell, AMNIS, THP-1, cholesterol, MβCD
Introduction
Chemotaxis is the directed movement of cells in response to chemical stimulus, and plays a vital role in inflammation (1). Monocyte chemotaxis is of particular importance in homeostatic inflammatory response and also in diseases such as atherosclerosis and cancer (2–4). Infection or injury trigger the production of pro-inflammatory signals and chemokines, which recruit circulating monocytes from flowing blood to the endothelial surface and transmigration into the extravascular space, respectively (5). The regulation of these phenomena become particularly crucial in the development and progression of pro-inflammatory diseases such as atherosclerosis.
We have recently shown that cellular cholesterol is an important regulator of monocyte recruitment to atherosclerotic foci (6). Cholesterol, which is an important risk factor of atherosclerosis alters the distribution of the endothelial adhesion receptor CD44, thus reshaping the dynamics of capture of monocytes from flowing blood (6). The majority of cellular cholesterol is present on the plasma membrane although it modulates the cell stiffness and hence overall cellular physiology (7). Despite the striking importance of cholesterol on monocyte function, its role on chemotaxis is not well understood. In this work, we tested the hypothesis that cholesterol content alters the chemotactic behavior of monocytes in response to MCP-1 by altering surface distribution/expression levels of the cognate receptor, CCR2. By depleting or enriching cholesterol levels using MβCD, we examined the response of monocytes to chemotactic stimulus, MCP-1, and changes in the MCP-1 levels and heterogeneity.
Materials and Methods
Cell Culture and cholesterol treatment
THP-1 (ATCC, Manassas, VA, USA), which is a human monocyte cell line, was cultured in RPMI 1640 (ATCC) supplemented with 10% heat inactivated FBS (Life technologies, Grand Island, NY, USA) and .05 mM mercaptoethanol (Sigma-Aldrich, St. Louis, MO, USA), at 37°C and 5% CO2. The cells were allowed to reach a density of 106/ml and were then passaged into fresh media. The cell viability was measured by Countess automated cell counter using the trypan blue exclusion assay (Life Technologies).
Cholesterol depletion and enrichment were performed using Methyl-β-cyclodextrin (MβCD; Sigma-Aldrich; stock solution of 200 mM) and MβCD-cholesterol complex (Sigma-Aldrich; stock solution of 25 mM), respectively. Cells were first washed with RPMI and centrifuged for 7 minutes at 130xG in room temperature (RT), and finally resuspended in RPMI at a concentration of 1×106/ml. Either 10 mM MβCD or 0.5 mM MβCD-cholesterol solution was mixed with the cell suspension, followed by incubation for 30 mins. at 37° C and 5% CO2. This was followed by estimation of cell viability using trypan blue exclusion assay. The cell suspension was centrifuged at 130xG for 7 minutes at RT, and then resuspended at a concentration of 1×106/ml in RPMI. This was repeated thrice, and cell viability was measured again. After the final centrifugation step, the supernatant was discarded and the cells were resuspended as required by the particular assay. At this point, the cell viability was assessed one final time.
Cholesterol content measurement
Cellular cholesterol content was estimated using Amplex Red Cholesterol Assay Kit (Life Technologies), as per established protocol (6). Approximately 0.75×105 cells were put in each well of a 96 well plate (Corning Inc., Corning, NY, USA). The Synergy2 plate reader (Biotek, Winooski, VT, USA) was used to measure fluorescence intensities at 540 nm(excitation)/600 nm(emission). The cholesterol contents were determined from a standard curve, using 1–20 μg/ml cholesterol standards.
Cell chemotaxis
Monocyte chemotaxis was performed using 5μm transwell inserts (Corning) in 24 well plates (Corning). 30 ng/ml of MCP-1 (R&D Systems, Minneapolis, MN, USA) was used as chemoattractant, and 600 μL of complete media with or without chemoattractant was put in each well. The cells were treated with 1μM Calcein-AM (Life Technologies), immediately before seeding in the transwell inserts. The transwell inserts were initially primed using complete media without MCP-1 for 30 minutes at 37°C. Following the priming, 100000 cells/insert were seeded in a final volume of 100 μL. At least 6 inserts were used per condition. The cells were allowed to pass through the insert pores for 1.5, 3 and 6 hours. This was followed by measuring the fluorescence intensity using a plate reader (BioTek). Untreated cells were used as control. Cells from all condition in the absence of MCP-1 was used as negative control. The cell number was estimated from a standard curve for Calcein-AM. After the time points were reached, the inserts were imaged under the microscope at 20X and cell count estimates were obtained to account for the number of cells stuck to the transwell membrane during chemotaxis. At least three inserts were imaged per condition for every experiment.
Monocyte chemotaxis receptor
Changes in CCR2 distribution and expression due to changes in cholesterol were assessed using multispectral imaging flow cytometry (ImagestreamX Mark II; Amnis corp., Seattle, WA, USA). Fluorescently tagged anti-CCR2 (Miltenyi Biotec, Auburn, CA, USA) was used for visualization and quantification of CCR2. THP-1 cells were initially centrifuged at 130xG for 7 minutes at room temperature, and then resuspended in 100 μL PBS (cell concentration of 107/ml.) in 1.5 ml. tubes (Eppendorf). 10 μL the antibody reagent was added to the cells and they were incubated for 10 minutes at 4°C. The cell suspension was then centrifuged at 130xG for 7 minutes at 4°C. Following this, the cells were fixed using 4% formaldehyde solution in PBS, maintaining a final concentration of 107 cells/ml. The intensity and distribution of CCR2 on the plasma membrane were estimated using mean fluorescence intensity and H Variance SD, respectively. A higher mean fluorescence intensity indicates higher expression of CCR2, and vice versa. A lower H variance SD value implies that CCR2 is more uniformly distributed, and vice versa.
Statistics
All the experiments were performed in triplicates, and each experiment was repeated at least thrice under independent conditions, on different days, unless otherwise mentioned. The results are represented as mean ± SEM from one representative experiment. Statistical differences were evaluated using one-way ANOVA with Tukey’s post hoc test, and significance was reported at α=0.05.
Results and Discussion
We have previously established the importance of cellular cholesterol on the tethering and adhesion of monocytes to endothelial-mimetic surfaces, which is the first critical step in its inflammatory response (7). In this work, we have explored the effects of cellular cholesterol content on monocyte chemotaxis, which remains ill-explored, despite its significant importance in inflammation.
Firstly, we altered the baseline cholesterol levels in human monocytic cell line THP-1 cells and estimated cholesterol levels using cholesterol oxidase based assay (Amplex red cholesterol assay). Depletion resulted in ~50% decrease and enrichment caused ~170% increase in cholesterol levels as compared to the untreated cells (6). We have shown that the MβCD treatment results in comparable changes in cholesterol levels between THP-1 and freshly isolated human peripheral blood monocytes (6,7). Having successfully altered the cholesterol levels, we investigated the chemotactic response of THP-1 cells to Monocyte Chemoattractant Protein – 1 (MCP-1), which is a physiologically relevant chemoattractant for monocytes with established importance in inflammatory response (8,9).
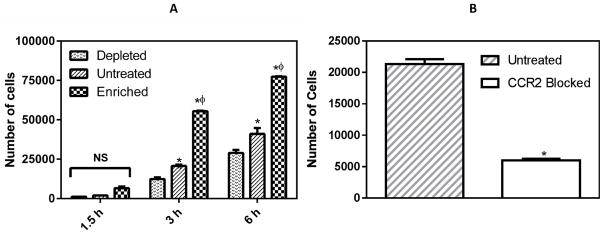
A transwell assay was used to assess the chemotactic response of THP-1 cells to the chemoattractant MCP-1. After 1.5 hours, only a few cells passed through the wells towards to the chemoattractant for all the conditions, albeit following a similar trend as longer durations. After 3 hours, there was a 40% decrease in the number of cholesterol depleted cells that transmigrated compared to untreated control. In contrast, cholesterol enrichment resulted in a 170% increase in the number of transmigrated cells as compared to untreated control. After 6 h, depletion resulted in 30% fewer cells and enrichment resulted in 90% more cells transmigrated across the membrane in comparison to the untreated control (Figure 1A). We observed that only a small fraction of the cells were stuck in the transwell membrane during the experiment: 5±1.5% of untreated, 4±0.75 % of depleted, and 6±1% of enriched cells, after 3 hours of transmigration. This observation indicates that the data obtained is indeed reflective of the true chemotactic behavior of the cells, rather than gravity-driven, non-specific movement.
Figure 1.
Monocyte chemotaxis in response to MCP-1 gradient. (A) Number of cells transmigrated to the bottom well after 1.5h, 3h and 6h. (B) Number of cells transmigrated at 3 h in the presence of anti-CCR2 antibody (25 μg/ml). * and φ represent statistically significant difference (p< 0.05, n = 3) in comparison to depleted and untreated cells, respectively.
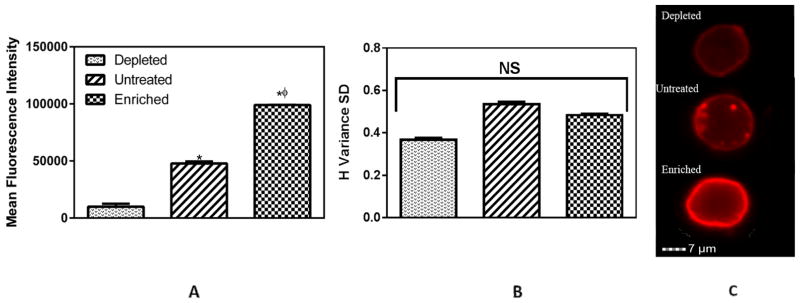
Having established that cholesterol changes the chemotactic response of monocytes, we investigated changes in specific receptor to MCP-1 on THP-1 cells, to understand the underlying molecular mechanism. CCR2 is the most well-known receptor towards MCP-1 (9). To confirm the role of MCP-1/CCR2 in our system, we blocked the CCR2 receptors on the THP1 cells using an anti-CCR2 antibody. After 3 hours, we observed a substantial decrease (~80%) in chemotaxis when the THP-1 cells were blocked with saturating concentration (25μg/ml) of anti-CCR2 antibody (Figure 1B). We used imaging flow cytometry to investigate the expression and distribution of CCR2 receptor in control and cholesterol-treated cells. We observed significant changes in the CCR2 expression levels due to cholesterol content (Figure 2A). Untreated THP-1 cells had a 3 fold expression level of CCR2 as compared to cholesterol depleted cells. Cholesterol enrichment demonstrated an increase of 1.2 fold as compared to untreated cells. Cholesterol depletion has been previously reported to inhibit cell chemotaxis (10). THP-1 cells exhibited a similar behavior and cholesterol depleted cells demonstrated significantly less chemotactic response as compared to untreated and cholesterol enriched cells. We can attribute this to the reduction in expression of CCR2. A plausible reason for this decline in CCR2 levels might be receptor internalization due to cholesterol depletion (11,12). Expectedly, cholesterol enriched cells exhibited opposite effects. In case of hypercholesterolemia, it has been shown that as a consequence of metabolic and inflammatory signaling, monocytes upregulate atherogenic chemoreceptors (13,14). Consistent with these findings, we observed a significant increase in the expression levels of CCR2 in case of cholesterol enrichment.
Figure 2.
Monocyte receptor CCR2 for MCP-1. (A) Changes in expression levels of CCR2. (B) Distribution levels of CCR2 on plasma membrane. (C) Fluorescence visualization of CCR2. * and φ represent statistically significant difference (p< 0.05, n = 3) in comparison to depleted and untreated cells, respectively. CCR2 is shown in red.
However, cholesterol treatment did not significantly alter CCR2 distribution on the plasma membrane (Figure 2B). Representative images of the different conditions corroborate these findings (Figure 2C). These results suggest that the expression levels of CCR2 dictate the chemotactic response of THP-1 cells. This observation is in contrast to our recent finding that cholesterol alters the rolling of monocytes primarily through the redistribution of the receptor CD44 rather than through its expression levels (7,15). However, it must be kept in consideration that the distribution levels were considered at basal levels, without MCP-1 stimulus. In the presence of chemokine stimulus, the corresponding receptors (16), including CCR2 (17), get polarized in the direction of the signal (leading edge). An increased availability of CCR2 (as seen in cholesterol enriched cells) on the plasma membrane thus enables more receptors to respond to the chemotactic signal, and this strong response can possibly lead to an enhanced transmigration of monocytes through the endothelium (18), further exacerbating the inflammation (4,19).
In conclusion, we have demonstrated that cholesterol plays an important role in the chemotactic response of THP-1 cells, with higher cholesterol content leading to enhanced chemotaxis by altering CCR2 expression levels. Our results show that with increase in cholesterol content, it is easier for the cells to transmigrate in response to a chemokine signal, which may have important consequences in atherosclerosis, a pro-inflammatory disease with cholesterol being a risk factor. Although we have focused on the MCP-1/CCR-2 receptor-ligand pair, the analyses described in this work may be further expanded to investigate the cholesterol modulation of chemotaxis involving other receptor ligand pairs, such as IL-8/CXCR1 and MCP3/CC CKR2B.
Highlights.
Monocyte chemotaxis is dependent on cellular cholesterol content.
Higher cholesterol content corresponds to stronger chemotactic response.
CCR2 expression levels dictate the changes in chemotaxis.
Acknowledgments
This work was supported by a grant from the NIH (HL112629). The authors would like to acknowledge the assistance of the Immune Defense Core Facility at The University of Texas at San Antonio, supported by a grant (G12MD007591) from the National Institute on Minority Health and Health Disparities from the National Institutes of Health (NIH), and faculty start-up funds from the San José State University. Author contributions: A.K.S and A.K.R designed the study, analyzed the data and wrote the manuscript. A.K.S performed the experiments. M.M assisted in CCR2 blocking experiments. S.F.D and S.J.E participated in experimental design. Competing financial interest statement: The authors declare no conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wong CHY, Heit B, Kubes P. Molecular regulators of leucocyte chemotaxis during inflammation. Cardiovasc Res. 2010;86:183–191. doi: 10.1093/cvr/cvq040. [DOI] [PubMed] [Google Scholar]
- 2.Gerszten RE, Garcia-Zepeda EA, Lim YC, Yoshida M, Ding HA, Gimbrone MA, Jr, Luster AD, Luscinskas FW, Rosenzweig A. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999;398:718–723. doi: 10.1038/19546. [DOI] [PubMed] [Google Scholar]
- 3.Jaipersad AS, Lip GY, Silverman S, Shantsila E. The role of monocytes in angiogenesis and atherosclerosis. J Am Coll Cardiol. 2014;63:1–11. doi: 10.1016/j.jacc.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 4.Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, Rollins BJ. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell. 1998;2:275–281. doi: 10.1016/s1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- 5.Feige E, Yacov N, Salem Y, Levi I, Mendel I, Propheta-Meiran O, Shoham A, Hait- Darshan R, Polonsky O, George J, Harats D, Breitbart E. Inhibition of monocyte chemotaxis by VB-201, a small molecule lecinoxoid, hinders atherosclerosis development in ApoE(−)/(−) mice. Atherosclerosis. 2013;229:430–439. doi: 10.1016/j.atherosclerosis.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Saha AK, Osmulski P, Dallo SF, Gaczynska M, Huang TH, Ramasubramanian AK. Cholesterol Regulates Monocyte Rolling through CD44 Distribution. Biophys J. 2017;112:1481–1488. doi: 10.1016/j.bpj.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saha AK, Dallo SF, Detmar AL, Osmulski P, Gaczynska M, Huang TH, Ramasubramanian AK. Cellular cholesterol regulates monocyte deformation. J Biomech. 2017;52:83–88. doi: 10.1016/j.jbiomech.2016.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takahashi M, Galligan C, Tessarollo L, Yoshimura T. Monocyte chemoattractant protein-1 (MCP-1), not MCP-3, is the primary chemokine required for monocyte recruitment in mouse peritonitis induced with thioglycollate or zymosan A. J Immunol. 2009;183:3463–3471. doi: 10.4049/jimmunol.0802812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte Chemoattractant Protein-1 (MCP-1): An Overview. J Interf Cytok Res. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pierini LM, Eddy RJ, Fuortes M, Seveau S, Casulo C, Maxfield FR. Membrane lipid organization is critical for human neutrophil polarization. J Biol Chem. 2003;278:10831–10841. doi: 10.1074/jbc.M212386200. [DOI] [PubMed] [Google Scholar]
- 11.Borroni V, Baier CJ, Lang T, Bonini I, White MM, Garbus I, Barrantes FJ. Cholesterol depletion activates rapid internalization of submicron-sized acetylcholine receptor domains at the cell membrane. Mol Membr Biol. 2007;24:1–15. doi: 10.1080/09687860600903387. [DOI] [PubMed] [Google Scholar]
- 12.Brejchova J, Vosahlikova M, Roubalova L, Parenti M, Mauri M, Chernyavskiy O, Svoboda P. Plasma membrane cholesterol level and agonist-induced internalization of delta-opioid receptors; colocalization study with intracellular membrane markers of Rab family. J Bioenerg Biomembr. 2016;48:375–396. doi: 10.1007/s10863-016-9667-7. [DOI] [PubMed] [Google Scholar]
- 13.Foster GA, Xu L, Chidambaram AA, Soderberg SR, Armstrong EJ, Wu H, Simon SI. CD11c/CD18 Signals Very Late Antigen-4 Activation To Initiate Foamy Monocyte Recruitment during the Onset of Hypercholesterolemia. J Immunol. 2015;195:5380–5392. doi: 10.4049/jimmunol.1501077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han KH, Tangirala RK, Green SR, Quehenberger O. Chemokine receptor CCR2 expression and monocyte chemoattractant protein-1-mediated chemotaxis in human monocytes. A regulatory role for plasma LDL. Arterioscler Thromb Vasc Biol. 1998;18:1983–1991. doi: 10.1161/01.atv.18.12.1983. [DOI] [PubMed] [Google Scholar]
- 15.Evani SJ, Ramasubramanian AK. Biophysical regulation of Chlamydia pneumoniae-infected monocyte recruitment to atherosclerotic foci. Sci Rep. 2016;6:19058. doi: 10.1038/srep19058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao Z, Zhang N, Murphy DB, Devreotes PN. Dynamic distribution of chemoattractant receptors in living cells during chemotaxis and persistent stimulation. J Cell Biol. 1997;139:365–374. doi: 10.1083/jcb.139.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nieto M, Frade JM, Sancho D, Mellado M, Martinez AC, Sanchez-Madrid F. Polarization of chemokine receptors to the leading edge during lymphocyte chemotaxis. J Exp Med. 1997;186:153–158. doi: 10.1084/jem.186.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langer HF, Chavakis T. Leukocyte-endothelial interactions in inflammation. J Cell Mol Med. 2009;13:1211–1220. doi: 10.1111/j.1582-4934.2009.00811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyle JJ. Macrophage activation in atherosclerosis: pathogenesis and pharmacology of plaque rupture. Curr Vasc Pharmacol. 2005;3:63–68. doi: 10.2174/1570161052773861. [DOI] [PubMed] [Google Scholar]