Abstract
Mucopolysaccharidosis type I (MPS I) is a lysosomal disease resulting from deficiency in the α-L-iduronidase (IDUA) hydrolase and subsequent accumulation of glycosaminoglycan (GAG). Clinically, enzyme replacement therapy (ERT) with IDUA achieves negligible neurological benefits presumably due to blood-brain-barrier (BBB) limitations. To investigate the plant lectin ricin B chain (RTB) as a novel carrier for enzyme delivery to the brain, an IDUA:RTB fusion protein (IDUAL), produced in N. benthamiana leaves, was tested in a murine model of Hurler syndrome (MPS I). Affect mice (n=3 for each group) were intravenously injected with a single dose of IDUAL (0.58, 2 or 5.8 mg IDUA equivalents/kg) and analyzed after 24 hours. IDUA activities in liver, kidney and spleen increased significantly, and liver GAG levels were significantly reduced in all three groups. Plasma IDUA levels for all treated groups were high at 1 hour after injection and decreased by 95% at 4 hours, indicating efficient distribution into tissues. For long-term evaluations, IDUAL (0.58 or 2 mg/kg, 8 weekly injections) was intravenously injected into MPS I mice (n=12 for each group). Thirteen days after the 8th injection, significant IDUA activity was detected in the liver and spleen. GAG levels in tissues including the brain cortex and cerebellum were significantly reduced in treated animals. Treated MPS I mice also showed significant improvement in neurocognitive testing. ELISA results showed that while there was a significant antibody response against IDUAL and plant-derived IDUA, there was no significant antibody response to RTB. No major toxicity or adverse events were observed. Together, these results showed that infusion of IDUAL allowed for significant IDUA levels and GAG reduction in the brain and subsequent neurological benefits. This RTB-mediated delivery may have significant implications for therapeutic protein delivery impacting a broad spectrum of lysosomal, and potentially neurological diseases.
Keywords: lysosomal disease, RTB, enzyme replacement therapy, CNS protein delivery
1. INTRODUCTION
Lysosomal diseases represent a group of rare genetic disorders due to deficiencies of specific lysosomal enzymes and subsequent substrate accumulation. Enzyme replacement therapy (ERT) has provided systemic therapeutic benefits and improved quality of life for patients with lysosomal diseases including mucopolysaccharidosis type I (MPS I) [1]. However, ERT protocols achieve negligible neurological benefits due to the blood-brain-barrier (BBB). Therefore, there is keen interest in developing innovative enzyme delivery to treat neurological diseases.
Delivery of therapeutic proteins across the BBB remains a significant challenge for treating diseases with neurological involvement. Currently available ERT technology depends on interactions of the phosphorylated lysosomal enzyme and mannose 6-phosphate receptors (M6PR) or mannose receptors (MR) on target cells [2]. There have been studies using enzymes conjugated with antibodies [3,4] or peptides [5] that target receptors present on capillary endothelial cells. However, the limiting factors of these receptor-mediated approaches are uptake saturation and competition with native targets due to low abundance of receptors. The ribosome-inactivating toxin B subunit (RTB) lectin emerges as a novel protein carrier that utilizes adsorptive-mediated transcytosis, which may provide unique access to the central nervous system (CNS). RTB, as the nontoxic carbohydrate-binding B subunit of ricin from Ricinus communis, mediates uptake and trafficking of the ricin toxic A chain (RTA) in mammalian cells [6]. It has been reviewed that RTB exploits multiple endocytotic routes (e.g. clathrin-dependent, clathrin–independent and absorptive-mediated mechanisms) to enter cells [7]. The potential for RTB to facilitate delivery of therapeutic proteins to the brain is supported by several facts. Firstly, intravenous administration of ricin toxin led to multiple sites of focal hemorrhage in mice brains [8]. Secondly, the unique tropism of AAV9 into the CNS is mediated through galactose binding domain on the capsid [9]. Thirdly, it has been reported that wheat germ agglutinin, a lectin with strong affinity for N-acetyl galactosamin, could facilitate horseradish peroxidase across the BBB via transdocytosis [10]. Considering the fact that RTB is a galactose/N-acetyl galactosamine-specific lectin, it has the potential to facilitate conjugated proteins into the CNS through similar mechanisms used by agglutinin. Therefore, fusion of IDUA to RTB is expected to deliver the lysosomal enzyme to cells of the CNS that are not treated by current MPS I ERT. It has been shown that IDUA and RTB fusion protein retains both IDUA enzyme activity and lectin binding specificity. Further, the RTB and IDUA fusion proteins could efficiently enter MPS I patient fibroblasts through mechanisms independent of M6PR and MR, and more importantly, significantly reduced GAG storage in the lysosome [11].
In this study, recombinant proteins that fuse the human IDUA enzyme with RTB (termed IDUAL for IDUA-lectin) were produced using a proprietary plant-based bioproduction platform (BioStrategies LC, State University, AR). It has been shown that plant-based bioproduction provides advantages in reduced manufacturing time and cost [12]. Further, the safety and efficacy of IDUAL-mediated ERT in treating MPS I diseases, including neuropathology, were evaluated in an IDUA-deficient mouse model. The specific study design is summarized in Figure 1. In the short-term (24 hours) study, a single infusion of IDUAL into MPS I mice led to detectable IDUA activities in the CNS. The therapeutic potential of IDUAL concerning the CNS was substantiated by GAG reduction in the brain and improvements in neurocognitive testing after 8 weekly infusions.
Figure 1. Schematic study design.
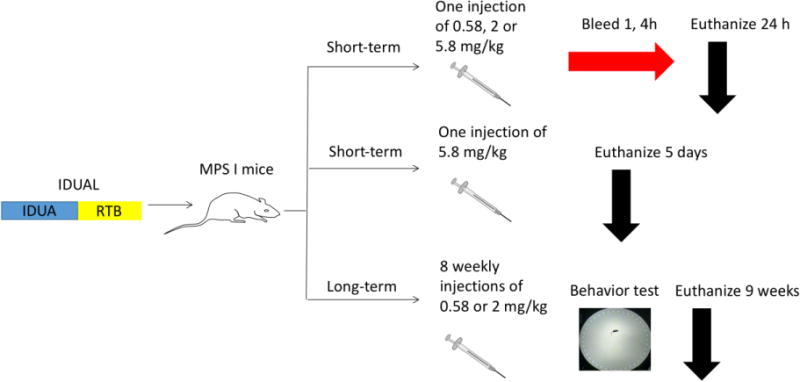
In the short-term study, IDUAL was injected into MPS I mice (n=3 for each group). All treated groups were sacrificed and tissues were harvested. In the long-term study, MPS I mice (n=12 for each group), after 8 weekly injections, all mice underwent Barnes maze test and were sacrificed.
2. MATERIALS AND METHODS
2.1 MPS I mice
MPS I knockout mice (Idua−/−), a kind gift from Dr. Elizabeth Neufeld, UCLA, were generated by insertion of neomycin resistance gene into exon 6 of the 14-exon IDUA gene on the C57BL/6 background [13]. MPS I mice (Idua−/−) were randomized based on gender, weight and age, age-matched heterozygous mice (Idua−/+) were included as controls. All animals were observed daily for signs of morbidity and mortality from Day 1 to necropsy. All mouse care and handling procedures were in compliance with the rules of the Institutional Animal Care and Use Committee (IACUC) of the University of Minnesota.
2.2 IDUA enzyme assay
IDUA enzyme assay was conducted as previously described [14]. IDUA activity was determined by a fluorometric assay using 4-methylumbelliferyl α-L-iduronide (Glycosynth, Cheshire, UK) as the substrate. 4-MU iduronide was diluted with sodium formate buffer (0.4 M, pH 3.5). Then, 25 μL aliquots of substrate (360 μM) were mixed with 25 μL aliquots of tissue homogenates. The mixture was incubated at 37°C for 30 min, and 200 μL glycine carbonate buffer (pH 10.4) was added to quench the reaction. IDUA catalyzed the cleavage of the non-fluorescent substrate (4-MU iduronide) into a fluorescent product (4-MU). 4-methylumbelliferone (Sigma-Aldrich, St. Louis, MO) was used to make the standard curve. The resulting fluorescence was measured using a microplate reader (BioTek, Winooski, VT) with excitation at 355 nm and emission at 460 nm. IDUA enzyme activity was expressed in units (nmol converted to product per hour) per mg protein as determined with a Pierce protein assay kit (Thermo Fisher Scientific, Waltham, MA). All reactions were run in triplicate.
2.3 Tissue and urine GAG assay
GAG assays were conducted as described previously [15,16]. Creatinine levels in urine were assessed with the creatinine assay kit (Sigma-Aldrich, St. Louis, MO). Urine GAG levels were expressed as μg GAG/mg creatinine.
2.4 Barnes maze test
Barnes maze test was conducted as described previously [17]. Data (including escape latency, distance moved, and velocity) were collected and analyzed using the EthoVision program (Noldus, Wageningen, Netherlands).
2.5 Enzyme-linked immunosorbent assay (ELISA)
Indirect ELISA was performed as previously described [15]. IDUA (R&D Systems, Minneapolis, MN), RTB (Vector Laboratories, Burlingame, CA), planted derived IDUA or IDUAL (BioStrategies LC, State University, AR) was diluted with Tris-buffered saline (pH=7.4) to a final concentration of 4 μg/mL. In a 96-well microplate, 50 μL of each diluted proteins were incubated at 4°C overnight. After washing and blocking with Tris-buffered saline containing 1% BSA at room temperature for 2 hours, 100 μL serum (1:50 dilutions) was incubated at room temperature for 1 hour. After washing, the wells were incubated with 100 μL anti-mouse IgG alkaline phosphatase conjugate (Abcam, Cambridge, MA) at room temperature for 1 hour. After washing, the wells were incubated with 200 μL of 1 mg/mL p-nitrophenyl phosphate chromogenic substrate (Sigma-Aldrich, St. Louis, MO) at room temperature for 1h. Then, 50 μL EDTA (0.1 M, pH 8.0) was added to stop the reaction. Absorbance at 405 nm was quantified by using a microplate reader (BioTek, Winooski, VT).
2.6 Statistical analysis
For evaluation of differences between samples, one-way or two-way analysis of variance (ANOVA) and Dunnett’s multiple comparisons test were applied. Statistical significant level was set at p<0.05. Data analysis was conducted with Prism 7 (Graphpad, La Jolla, CA).
3. RESULTS
3.1 Establishing an effective dose and monitoring biodistribution after a single infusion of IDUAL in MPS I mice
The previously reported IDUA and RTB fusion construct [11] was optimized by incorporating a plant signal peptide, tobacco codon-optimized IDUA, a truncated RTB (removal of 6 amino acids), and placing RTB at the C-terminus of IDUA. Then, the resulting proteins (further referenced as IDUAL) were produced using a tobacco-based expression system and characterized as previously described [11]. A single injection of IDUAL (at 3 different doses) was administered via tail vein into adult MPS I mice (n=3 for each dose, 6-10 weeks old). The 3 doses were selected to flank the FDA-approved human equivalent dose for laronidase (0.58 mg/kg body weight): e.g., IDUAL equivalents at 0.58 mg/kg (low dose), 2 mg/kg (middle dose), and 5.8 mg/kg (high dose). Mice were phlebotomized at 1 hour (n=3) or 4 hours (n=3) post injection, and euthanized at 24 hours immediately after perfusion with saline. Serum clearance was analyzed in these mice at 1, 4 and 24 hours after injection. Serum levels were dose-dependent with the majority mobilized into tissues by 4 hours and serum clearance by 24 hours (Figure 2A).
Figure 2. Enzyme activity and GAG levels in MPS I mice after a single infusion of IDUAL.
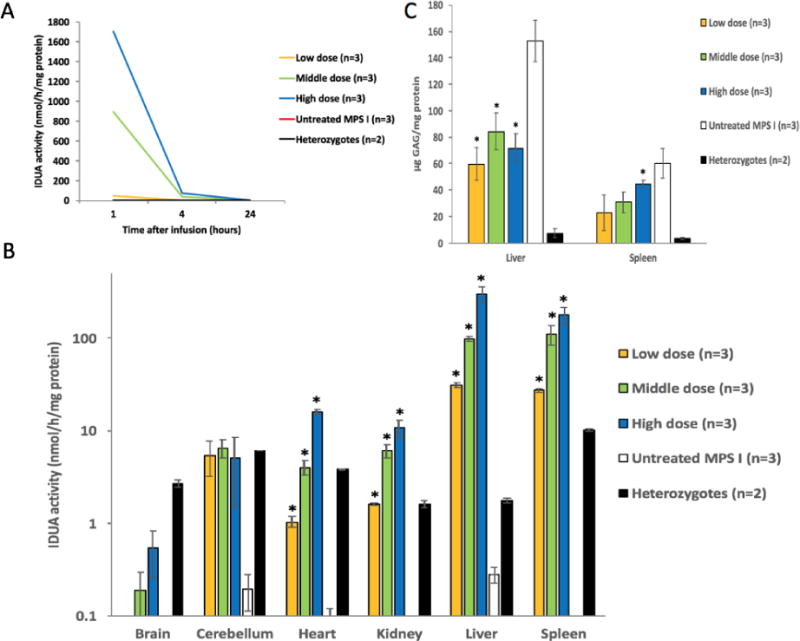
(A) Plasma IDUA activity at 1, 4 and 24 hours post injection; (B) IDUA activities in different tissues; (C) GAG levels in liver and spleen. Low dose group, 0.58 mg/kg; Middle dose group, 2 mg/kg. Data are mean ± SEM. * indicates p<0.05 when comparing with untreated MPS I group.
Based on tissue IDUA enzyme activity, IDUAL was well distributed into peripheral organs (Figure 2B). Administration with low dose (0.58 mg/kg, equivalent to current human dose) were as high as, or exceeded, enzyme activity levels of normal mice (unaffected heterozygous mice) in spleen, liver and kidney. With high dose administration, enzyme activity levels reached 4, 6, 12 and 15 fold higher levels in heart, kidney, liver and spleen, respectively (compared with normal mice). Interestingly, there is a dose-dependent trend towards increasing enzyme activity in the brain and cerebellum of treated mice from all dose groups. Additionally, significant GAG reduction was observed in liver (all three treated groups) and spleen (high-dose) 24 hours after injection, indicating the rapid clearance of GAG storage (Figure 2C).
Three additional mice were treated with the high dose and monitored for 5 days. No evidence of general toxicity (morbidity and mortality) was observed. IDUA levels in peripheral organs (heart, kidney, liver and spleen) were equivalent or greater than that in normal mice, indicating the stability of IDUAL in tissues after 5 days. More importantly, IDUA levels in cerebellum significantly increased to 25% of normal levels (p<0.05, Figure S1A), indicating that a small amount of enzyme may have entered the CNS. GAG levels in liver and spleen were reduced by 80% and 90% compared to untreated MPS I mice, respectively (p<0.05, Figure S1B).
3.2 IDUA enzyme activity increase in tissues of treated MPS I mice after 8 weekly infusions of IDUAL
Based on results of the dose-finding study, 2 doses (low dose and middle dose) of IDUAL were selected for repeated administrations to MPS I mice. MPS I mice (5–9 weeks old, n=12, 6 for each gender) were treated with 8 weekly injections. Normal heterozygous mice and untreated MPS I mice were analyzed in parallel with treated mice. Blood was collected 1 day before the 1st, 3rd, 6th injection and immediately before euthanasia. No plasma IDUA levels were detected, confirming the rapid plasma clearance of IDUAL. All mice were euthanized 13 days after the 8th injection, and tissues were weighed and processed for further assessment. The relative weights of spleens from the treated groups were significantly lower than untreated animals, but comparable to healthy heterozygous animals. This indicates that treatment with IDUAL led to resolution of splenomegaly in the murine model, which is a common phenotypic abnormality of MPS I patients (p<0.05, Figure 3A). Notably, there are up to 42% of normal IDUA levels in liver from the middle dose group 13 days after the 8th injection (Figure 3B), suggesting a long tissue half-life of IDUAL.
Figure 3. Enzyme activity and GAG levels in MPS I mice after 8 weekly infusions of IDUAL.
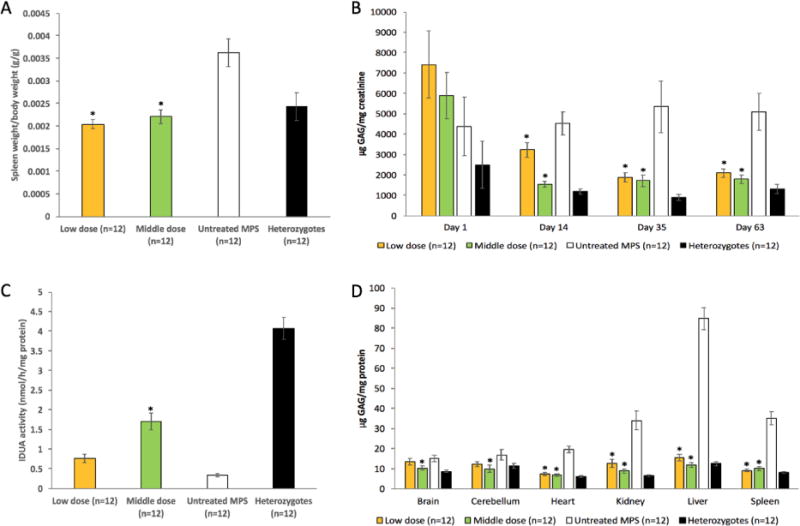
(A) Relative spleen weights; (B) urine GAG levels on Day 1, 14, 35 and 63; (C) IDUA activities in liver; (D) GAG levels in different tissues. Low dose group, 0.58 mg/kg; Middle dose group, 2 mg/kg. Data are mean ± SEM. * indicates p<0.05 when comparing with untreated MPS I group.
3.3 GAG storage reduction in urine and tissues of treated MPS I mice after 8 weekly infusions of IDUAL
Urine samples were collected from all mice on Day 1, 14, 35 and 63. Urine GAG levels were significantly reduced in both low and middle dose groups starting from Day 14 (p<0.05) (Figure 3C). On Day 14, mice from the low dose group had higher urine GAG levels than the middle dose group (p<0.05). However, there was no such difference on Day 35 and 63, indicating that the middle dose treatment reduces GAG storage more rapidly. Tissue GAG levels were significantly reduced in liver (by up to 86%), spleen (74%), heart (65%), kidney (74%) and brain (31%) (p<0.05) (Figure 3D). There was no difference in GAG levels between mice treated with different doses. These results showed that both low and middle doses of IDUAL achieved significant GAG reduction.
3.4 Neurological response in treated MPS I mice after 8 weekly infusions of IDUAL
All mice underwent Barnes maze test from Day 54 to 60 post-first infusion. The results showed that normal mice improved over time, as indicated by decrease in latency to find the target escape hole over the 6 days of the test. In contrast, the escape latencies in untreated MPS I mice were not significantly different from baseline after 6 days of learning (Figure 4). Interestingly, starting from Day 2 to 6 of learning, both low and middle dose groups showed an improvement in maze performance over time as compared to the untreated MPS I group (p<0.05), and performed equally well as the wild type animals. By Day 6 of learning, mice from the low and middle dose groups showed a statistically significant difference in learning behavior with an average of 46 ± 12 (mean ± SEM) and 39 ± 12, respectively. However, untreated MPS I mice took 100 ± 15 seconds to find the target. Normal control mice reached the target in 49 ± 10 seconds on Day 6 of the test. These results showed that mice receiving both low and middle doses of enzyme were able to achieve significant neurological benefits.
Figure 4. Barnes maze: escape latencies in 6 day trials.
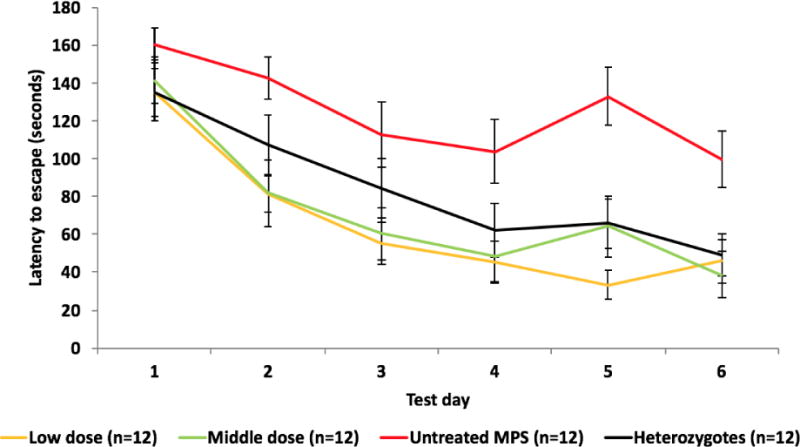
Cognitive performance was assessed using the Barnes maze test 4 days after the 8th injection. Data represent mean ± SEM of the time it took the animals to find the target escape hole (average of 4 trials each day) over 6 days of testing. Low dose group, 0.58 mg/kg; Middle dose group, 2 mg/kg.
3.5 Humoral immune responses in treated MPS I mice after 8 weekly infusions of IDUAL
Previous studies showed that human IDUA elicited a humoral immune response in MPS I animal models [15,18]. Strong adverse infusion reactions (transient immobility or lethargy) were found in some treated mice after the 3rd injection but were alleviated by injection of 2 mg/kg dexamethasone 1 hour prior to subsequent enzyme infusions. To assess the immune response elicited by IDUAL infusions, a series of ELISA experiments were conducted. No significant antibodies against RTB were detected (Figure 5A). However, antibodies against IDUAL were increased starting from Day 14 (Figure 5B). This coincides with the observation of adverse events following enzyme infusion. Antibodies against plant derived (Figure 5C) or mammalian derived IDUA (Figure 5D) were also detected with similar patterns of antibodies against IDUAL. Notably, there were no differences in antibody levels between the low dose and middle dose groups, indicating that humoral immune response is dependent on frequency instead of dose. Collectively, these results showed that antibodies against IDUAL were mainly targeting IDUA instead of RTB.
Figure 5. Antibody against IDUAL is mainly targeting IDUA instead of RTB lectin.
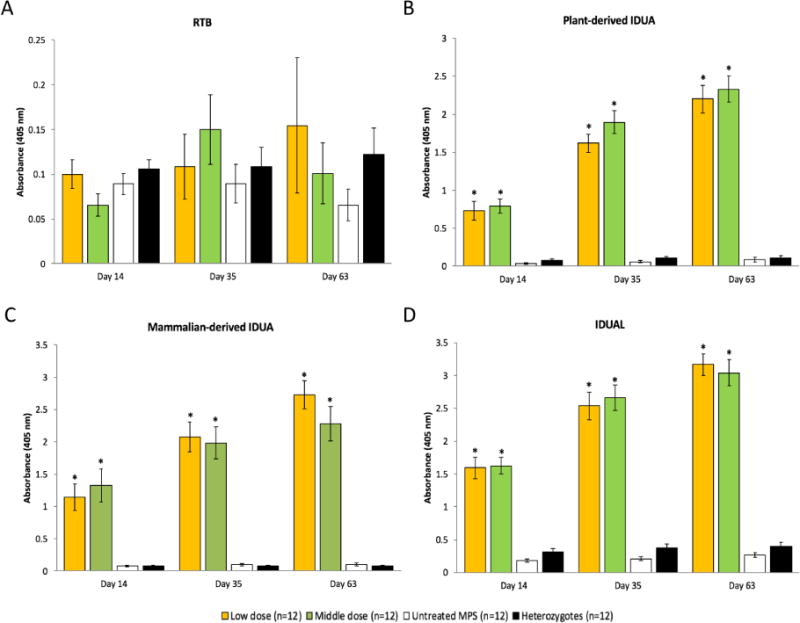
Antibody levels were assessed by ELISA by coating the plate with RTB lectin (A), IDUAL (B), plant derived IDUA (C) and mammalian derived IDUA (D). Low dose group, 0.58 mg/kg; Middle dose group, 2 mg/kg. Data are mean ± SEM. * indicates p<0.05 when comparing with untreated MPS I and heterozygotes group. The anti-RTB antibody and anti-IDUA antibody was included as positive controls. The negative controls showed the background level was approximately 0.15.
4. Discussion
4.1 Benefits of plant-based bioproduction system
Plant-based bioproduction systems provides the advantage of reducing manufacturing costs and alleviating safety concerns that have affected mammalian-produced recombinant enzymes [12]. Utilizing carrot cells, Protalix produced a recombinant taliglurcerase, the enzyme deficient in Gaucher disease, for clinical use, which was approved by the FDA in 2012. This product, commercially known as Eleyso®, is not only less expensive than the mammalian-derived enzyme, but it does not show an increased immunogenicity compared with the mammalian-derived product [19]. In this study, a fusion protein composed of IDUA and RTB is produced through a plant-based production platform, utilizing transient expression in Nicotiana benthamiana, a close relative of the tobacco plant.
A previous study showed that RTB can elicit neutralizing IgG antibodies in mice [20]. However, the ELISA results from this study showed that antibodies against IDUAL were mainly targeting IDUA instead of RTB, indicating that RTB may not elicit additional humoral immune response. Moreover, antibody levels against plant derived IDUA were similar to that against mammalian derived IDUA, indicating that plant derived IDUA has similar epitopes as mammalian derived IDUA. In summary, these results demonstrated that IDUAL would not cause greater immunogenicity than mammalian derived IDUA.
4.2 Dose effects of IDUAL
In the initial dose-finding study, 3 different doses were tested by a single injection into MPS I mice. All three doses achieved significant increase in IDUA levels in visceral organs and plasma, in a dose-dependent manner. Both the middle and high dose groups achieved detectable IDUA levels in the brain cortex, which confirms the findings in a high dose ERT mouse study [15]. Interestingly, even the low dose group achieved detectable IDUA levels in the cerebellum, which has not been achieved by normal ERTs at the same dose. These results indicated that at the same dose used in clinical ERT, IDUAL has provided both systemic correction and potential neurological benefits.
In the long-term study, although the middle dose group achieved higher enzyme activity, there were no differences in the efficiency to clear GAG storage between these low and middle dose groups. More importantly, performances in Barnes maze tests were indistinguishable between these two groups. These results confirmed the finding that a small amount of IDUA activity can make a remarkable difference [21], and provided substantial evidence for choosing the optimal dose in future clinical trials.
4.3 Neurological improvements provided by RTB mediated delivery
Although the low dose group did not have statistically significant GAG reduction in the CNS, their performance in Barnes maze tests were significantly better than untreated MPS I mice. Previously, several studies identified elevation of GAG levels in MPS I mice [15, 22, 23], while some other studies could not [24]. Due to the relative low GAG levels in the CNS, current GAG assays may not be sensitive enough to consistently distinguish the tiny difference in a statistically significant manner. A previous study demonstrated that weekly high dose administration of IDUA in the same MPS I mouse model allowed for IDUA to cross the BBB, reducing GAG accumulation, and improving cognitive impairment [15]. Similar ‘high dose effects’ have been observed in MPS II [25], MPS IIIA [26], MPS VII [27], α-mannosidosis [28] and Krabbe disease [29]. It was speculated that high dose enzyme saturated the clearance receptors and prolonged its plasma half-life, leading to the enzyme delivery to adult brain via other mechanisms, e.g. fluid-phase pinocytosis [30]. In this study, IDUAL at the human clinical dose (0.58 mg/kg) successfully achieved neurological improvements, ruling out the possibility of the so-called ‘high dose effect’. These findings further highlighted the ability of RTB to facilitate delivery of IDUA into the CNS.
Supplementary Material
Figure S1. Treatment effects at 5 days after a single infusion of high dose IDUAL (5.8 mg/kg). (A) IDUA activities in cerebellum; (B) GAG levels in liver and spleen. Data are mean ± SEM. * indicates p<0.05 when comparing with untreated MPS I group.
Highlights.
These results for the first time demonstrate the efficacy of RTB as a novel carrier to deliver lysosomal enzyme to multiple tissues including the central nervous system. After an 8-week enzyme replacement therapy of RTB:IDUA fusion protein, significant IDUA enzyme activity and storage reduction was observed in MPS I mice. Treated MPS I mice also showed significant improvement in neurocognitive testing. This RTB-mediated delivery may have significant implications for therapeutic protein delivery impacting a broad spectrum of lysosomal, and potentially neurological diseases.
Acknowledgments
The investigators thank BioStrategies LC (State University, AR) for IDUAL protein and funding under an NIH SBIR grant 5R43NS086326-02. Dr. Li Ou is a fellow of the Lysosomal Disease Network (U54NS065768). The Lysosomal Disease Network is a part of the Rare Diseases Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), and NCATS. This consortium is funded through a collaboration between NCATS, the National Institute of Neurological Disorders and Stroke (NINDS), and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wraith JE, et al. Enzyme replacement therapy for mucopolysaccharidosis I: a randomized, double-blinded, placebo-controlled, multinational study of recombinant human alpha-L-iduronidase (laronidase) J Pediatr. 2004;144(5):581–8. doi: 10.1016/j.jpeds.2004.01.046. [DOI] [PubMed] [Google Scholar]
- 2.Sly WS. Enzyme replacement therapy: From concept to clinical practice. Acta Paediatr Suppl. 2002;91(439):71–78. doi: 10.1111/j.1651-2227.2002.tb03115.x. [DOI] [PubMed] [Google Scholar]
- 3.Boado RJ, et al. Reversal of lysosomal storage in brain of adult MPS-I mice with intravenous Trojan horse-iduronidase fusion protein. Mol Pharm. 2011;8(4):1342–50. doi: 10.1021/mp200136x. [DOI] [PubMed] [Google Scholar]
- 4.Wang D, et al. Engineering a lysosomal enzyme with a derivative of receptor-binding domain of apoE enables delivery across the blood-brain barrier. Proc Natl Acad Sci USA. 2013;110(8):2999–3004. doi: 10.1073/pnas.1222742110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boado RJ, et al. Insulin receptor antibody-iduronate 2-sulfatase fusion protein: pharmacokinetics, anti-drug antibody, and safety pharmacology in Rhesus monkeys. Biotechnol Bioeng. 2014;111(11):2317–25. doi: 10.1002/bit.25289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandvig K, et al. Lipid requirements for entry of protein toxins into cells. Prog Lipid Res. 2014;54:1–13. doi: 10.1016/j.plipres.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Sandvig K, et al. Clathrin-independent endocytosis: mechanisms and function. Curr Opin Cell Biol. 2011;23(4):413–420. doi: 10.1016/j.ceb.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Audi J, et al. Ricin poisoning: a comprehensive review. JAMA. 2005;294(18):2342–2351. doi: 10.1001/jama.294.18.2342. [DOI] [PubMed] [Google Scholar]
- 9.Bell CL, et al. Identification of the galactose binding domain of the adeno-associated virus serotype 9 capsid. J Virol. 2012;86(13):7326–7333. doi: 10.1128/JVI.00448-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thorne RG, et al. Quantitative analysis of the olfactory pathway for drug delivery to the brain. Brain research. 1995;692(1-2):278–282. doi: 10.1016/0006-8993(95)00637-6. [DOI] [PubMed] [Google Scholar]
- 11.Acosta W, et al. RTB Lectin: a novel receptor-independent delivery system for lysosomal enzyme replacement therapies. Sci Rep. 2015;5:14144. doi: 10.1038/srep14144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du H, et al. Wolman disease/cholesteryl ester storage disease: efficacy of plant-produced human lysosomal acid lipase in mice. J Lipid Res. 2008;49(8):1646–1657. doi: 10.1194/jlr.M700482-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohmi K, et al. Activated microglia in cortex of mouse models of mucopolysaccharidoses I and IIIB. Proc Natl Acad Sci USA. 2003;100:1902–1907. doi: 10.1073/pnas.252784899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ou L, et al. Standardization of α-L-iduronidase enzyme assay with Michaelis-Menten kinetics. Mol Genet Metab. 2014;111(2):113–115. doi: 10.1016/j.ymgme.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ou L, et al. High-dose enzyme replacement therapy in murine Hurler syndrome. Mol Genet Metab. 2014;111(2):116–122. doi: 10.1016/j.ymgme.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitley CB, et al. Diagnostic test for mucopolysaccharidosis. I. Direct method for quantifying excessive urinary glycosaminoglycan excretion. Clin Chem. 1989;35(3):374–379. [PubMed] [Google Scholar]
- 17.Laoharawee K, et al. Prevention of neurocognitive deficiency in mucopolysaccharidosis type II mice by CNS-directed, AAV9-mediated iduronate sulfatase gene transfer. Hum Gene Ther. 2017 doi: 10.1089/hum.2016.184. [DOI] [PubMed] [Google Scholar]
- 18.Dickson P, et al. Immune tolerance improves the efficacy of enzyme replacement therapy in canine mucopolysaccharidosis I. J Clin Invest. 2008;118(8):2868–2876. doi: 10.1172/JCI34676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aviezer D, et al. A plant-derived recombinant human glucocerebrosidase enzyme–a preclinical and phase I investigation. PLoS One. 2009;4(3):e4792. doi: 10.1371/journal.pone.0004792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yermakova A, et al. Protective immunity to ricin toxin conferred by antibodies against the toxin’s binding subunit (RTB) Vaccine. 2011;29(45):7925–7935. doi: 10.1016/j.vaccine.2011.08.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guffon N, et al. Follow-up of nine patients with Hurler syndrome after bone marrow transplantation. J Pediatr. 1998;133(1):119–25. doi: 10.1016/s0022-3476(98)70201-x. [DOI] [PubMed] [Google Scholar]
- 22.Wolf DA, et al. Direct gene transfer to the CNS prevents emergence of neurologic disease in a murine model of mucopolysaccharidosis type I. Neurobiol Dis. 2011;43(1):123–133. doi: 10.1016/j.nbd.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ou L, et al. Elements of lentiviral vector design study toward gene therapy for treating mucopolysaccharidosis I. Mol Genet Metab Rep. 2016;8:93–97. doi: 10.1016/j.ymgmr.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma X, et al. Improvements in mucopolysaccharidosis I mice after adult retroviral vector-mediated gene therapy with immunomodulation. Mol Ther. 2007;15(5):889–902. doi: 10.1038/sj.mt.6300112. [DOI] [PubMed] [Google Scholar]
- 25.Polito VA, et al. Correction of CNS defects in the MPSII mouse model via systemic enzyme replacement therapy. Hum Mol Genet. 2010;19(24):4871–4885. doi: 10.1093/hmg/ddq420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rozaklis T, et al. Impact of high-dose, chemically modified sulfamidase on pathology in a murine model of MPS IIIA. Exp Neurol. 2011;230(1):123–130. doi: 10.1016/j.expneurol.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Vogler C, et al. Overcoming the blood-brain barrier with high-dose enzyme replacement therapy in murine mucopolysaccharidosis VII. Proc Natl Acad Sci USA. 2005;102(41):14777–82. doi: 10.1073/pnas.0506892102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roces DP, et al. Efficacy of enzyme replacement therapy in alpha-mannosidosis mice: a preclinical animal study. Hum Mol Genet. 2004;13(18):1979–1988. doi: 10.1093/hmg/ddh220. [DOI] [PubMed] [Google Scholar]
- 29.Lee WC, et al. Enzyme replacement therapy results in substantial improvements in early clinical phenotype in a mouse model of globoid cell leukodystrophy. FASEB J. 2005;19(11):1549–1551. doi: 10.1096/fj.05-3826fje. [DOI] [PubMed] [Google Scholar]
- 30.Sly WS, et al. Enzyme therapy in mannose receptor-null mucopolysaccharidosis VII mice defines roles for the mannose 6-phosphate and mannose receptors. Proc Natl Acad Sci USA. 2006;103:15172–15177. doi: 10.1073/pnas.0607053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Treatment effects at 5 days after a single infusion of high dose IDUAL (5.8 mg/kg). (A) IDUA activities in cerebellum; (B) GAG levels in liver and spleen. Data are mean ± SEM. * indicates p<0.05 when comparing with untreated MPS I group.


