Abstract
Strategies aimed at modulating the gut microbiota by using live microbes range from single strains (probiotics or live biotherapeutics) to whole non-defined fecal transplants. Although often clinically efficacious, our understanding on how microbial-based strategies modulate gut microbiome composition and function is vastly incomplete. In this review, we present a framework based on ecological theory that provides mechanistic explanations for the findings obtained in studies that attempted to modulate the gut microbiota of humans and animals using live microbes. We argue that an ecological perspective grounded in theory is necessary to interpret, design, and predict the impact of microbiome-modulating strategies and thus advance our ability to develop novel and improved approaches with enhanced therapeutic efficiency.
Graphical abstract
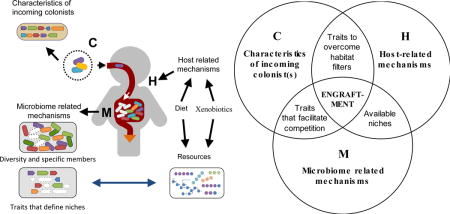
Using live microbes to modulate the gut microbiota
The gut microbiota is a critical determinant of human health by directly contributing to pathologies, influencing host predisposition to disease, and providing cues to maintain metabolic and immunological functions [1]. The crucial role of the microbiome in disease has been clearly proven in various animal models, providing a compelling case to design strategies that modulate gut microbiota composition and function. Such modulations can range from small temporary alterations in the compostion and/or metabolic output of the community to a more permanent and global transformation of microbiome structure. Although our mechanistic understanding on how the microbiota relates to human health is still in its infancy, and few cause-and-effect relationships have been established, microbiome-modulating strategies are increasingly aimed to redress dysbiotic patterns in both composition and function that are associated with disease [2].
One approach to modulate the gut microbiota is through the oral administration of live microorganisms [3*]. These strategies range from pure cultures of live microorganisms or consortia thereof, which are referred to as probiotics or live bio-therapeutics [4], to complex preparations of whole stool, such as Fecal Microbiota Transplants (FMT), or stool components (microbial cells, spores) [5]. Together, these strategies have been tested in a wide range of clinical contexts, with varying degree of success. Although various mechanisms have been established or suggested by which administered microbes exert their benefits, a modulation of gut microbiota composition and/or function is often one of the driving motivations to apply these approaches [3*]. Novel technologies based on next-generation sequencing now provide unprecedented insight into the effect of live microbes on the gut microbiome. There is substantial literature published describing the effects of probiotics on the gut microbiota composition, and although most strains show good survival during gastrointestinal passage and remain metabolically active, most human studies have shown extremely short persistence [6] and little effect on the composition of the resident microbiota [7]. FMTs appear to be much more successful in engrafting bacterial strains into an established gut microbiota [8*,9], but the reasons for these differences have been hardly studied and poorly understood. What the field currently lacks is a conceptual understanding of the effect of live bacteria on the gut microbiota and their potential to modulate the community.
Given that the digestive tract and its microbiota operate as a highly interactive and co-evolved ecosystem in which interactions among members and community characteristics are governed by the principles of community ecology [10], we argue that the modulation of gut microbiomes can only succeed when based on ecological and evolutionary criteria. The introduction of a microorganism into a gut ecosystem can be considered a biological invasion of non-native microbes into a highly adapted resident microbial community [11]. Here we apply concepts from invasion and general community ecology to develop a theoretical framework to understand the success of live microbes introduced into the digestive tract and their ecological impact on the resident microbial community. We then apply this framework to explain the findings that have been obtained with currently used microbiome-modulating interventions (probiotic strains and mixtures, synbiotics, and FMTs) in different contexts, and discuss its implications for the development of improved strategies and the open questions and challenges that remain.
An Ecological Framework
Biological invasions can be conceptualized as a multifaceted process that can be broken down into a series of at least four stages [12]. This framework, which has been recently extended to microbial invasions [13*], can be directly adopted towards microbiome-modulating strategies (Figure 1). Invasion stages are associated with barriers that must be overcome by the incoming microbe to allow colonization and an impact on community composition and/or function. From the incoming microbe’s perspective, the organism must be first introduced in an active form and in sufficient numbers (step 1), and secondly overcome the immediate habitat filters of the gastrointestinal tract to become established (step 2). Once established, the microbe must be able to gain access to resources under the competitive conditions of at least one site within the gastrointestinal tract to become metabolically active within the local community (step 3). If the local conditions allow the potential colonist to satisfy its minimum requirements so that replication is equal or greater to wash-out, the it has successfully occupied an ecological niche and persists, resulting in colonization [4,14]. Although colonization might not be necessary for ecological impact, it is still required that the invader is able to attain sufficient metabolic activity at a local site to engage in interactions with the resident members of the community (e.g. through competition, antagonism, or mutualism) that will ultimately cause changes to microbiota composition and/or function (step 4).
Figure 1.
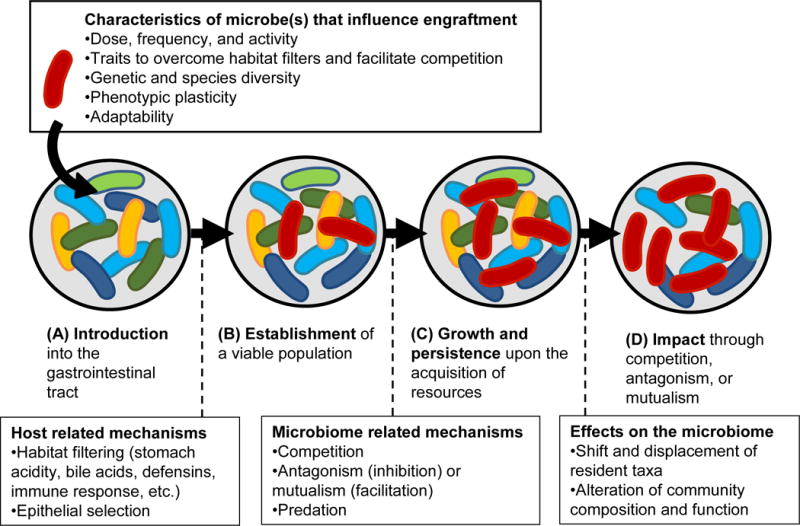
Successful invasion of a microorganism conceptualized as a 4-stage process. (A) The microbe needs to be introduced in sufficient numbers and in an active form, and possess the traits to withstand the pressures of the gut environment. (B) Habitat filters will select for microbes that possess the traits necessary to overcome them, while the host specifically selects for symbionts by a variety of mechanisms (glycans, epithelial capture, etc.). (C) The microbe needs to compete with resident members to access resources to grow and persist in an ecological niche. (D) Successful occupation of niches may result in metabolic activities and/or competitive or synergistic interactions that impacts the resident community’s composition and/or function. Adopted from Mallon et al. 2015 [13*].
The outcome of each of these steps, and the factors that influence them, are strictly governed by ecological principles. The field of invasion ecology comprises numerous hypotheses designed to explain both the success and consequences of invasions. By integrating 29 hypotheses, Catford and colleagues postulated that invasion is a function of propagule pressure (P), the abiotic characteristics of the invaded ecosystem (A), and the ‘biotic’ characteristics of the invaded community and the invading organism (B) [12]. We have now adapted this framework towards microbiome-modulating strategies (Figure 2) by integrating specific ecological principles applicable to host-microbiome symbioses [10]. Given that the invaded ecosystem resides within a living host, we do not refer to its characteristics as ‘abiotic’ but instead ‘host-related’. In addition, we combine components of propagule pressure (such as the number of individuals introduced and the temporal frequency of the introduction) with the characteristics of the introduced organisms. We further apply principles of general community ecology (e.g. colonization history) and evolutionary biology (e.g. evolutionary history of colonists) (Figure 2). The resulting framework postulates that the successful establishment of microbes in the gastrointestinal tract is a function of the interplay between the characteristics of the potential colonist (C), host-related factors (H), and microbiome-related mechanisms (M). We discuss these three themes (Figure 2) and their key hypotheses (Table 1) below, and provide examples that demonstrate that these concepts are applicable to the gut microbiome.
Figure 2.
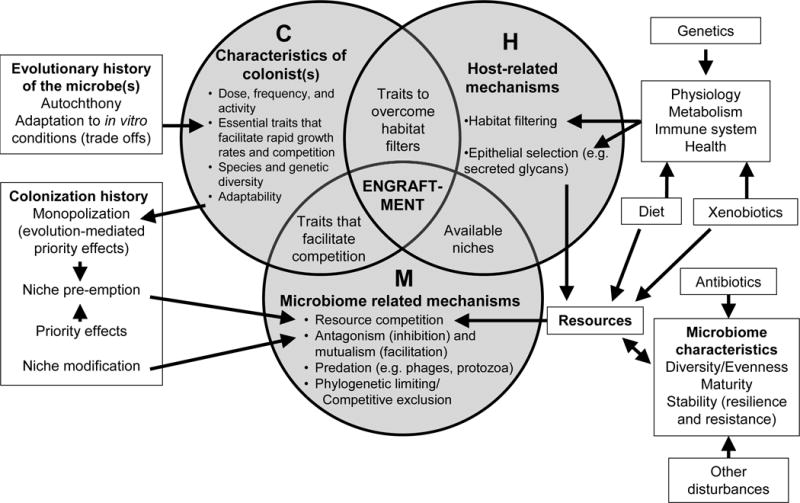
Ecological framework describing the characteristics, mechanisms and principles that influence colonization success of microorganisms used in microbiome-modulating strategies. (C) Characteristics of the potential colonists, dose, frequency of entry, activity, as well as the diversity of the propagule population increase the likelihood of colonization success. In addition, traits to overcome habitat filters, engage in symbiotic interactions with the host, and secure resources are essential for engraftment. (H) Host-related mechanisms select for microbes that possess traits to colonize the gut. Habitat filters such as bile acids, defensins, and immune responses select for organisms that possess the necessary adaptation. Host-derived glycans and the provision of adhesion sites can facilitate the establishment of microbes. (M) Microbiome-related mechanisms, mainly related to competition and microbe-microbe interactions define successful engraftment. All three components have to be compliant for engraftment to occur, and components of C, H, and M interact.
Table 1.
Invasion ecology hypotheses according to Catford and colleagues [12] that encompass ecological concepts relevant for microbiome-modulating strategies based on live microbes.
| Concept | Relevant hypotheses | Relevant processes | Examples | Selected references |
|---|---|---|---|---|
| Microbial traits are important to overcome habitat filtering for survival. | ‘Sampling’ and ‘ideal weed’ hypotheses. | C, with interactions with H | Bacteria that are used in traditional yoghurt fermentations show low survival during passage of the human gut. | [47] |
| Genetic characteristics, origin, and life history of the invading species are important as they determine relevant traits to outcompete indigenous species. Characteristics like autochthony, high genotypic and phenotypic plasticity, rapid growth, and high and early fecundity and fertility are important. | ‘Sampling’ and ‘ideal weed’ hypotheses. | C, with interactions with M | ‘Allochthonous’ bacterial strains used in probiotics and fermented foods show no ability to persist in the human gut, while some strains of B. longum, which are core members of the human gut microbiome, show higher level of persistence. FMTs of human fecal origin show a substantial level of engraftment. | [6,8*,19*,48,49] |
| Joint evolutionary history enhances traits that facilitate persistence. | ‘Ideal weed’, ‘evolution of competitive ability’, and ‘adaptation’ hypotheses. | C, with interactions with M | Strains of L. reuteri from rodents and chickens outcompete strains from other hosts in their respective hosts and possess specific traits that facilitate colonization. Might explain the success of B. longum subsp. longum strains in the gut of humans. | [23,24,50] |
| Genetic diversity of the incoming microbes increases chance of successful invasions (i.e. higher likelihood for species that are competitive and resilient to enemies to be present in the invading species pool). Beneficial pre-exiting interactions between introduced microbes can facilitate invasion by synthrophy or altering ecosystem characteristics. | ‘Ideal weed’, ‘Propagule pressure’, ‘global competition’, ‘increased susceptibility’, and ‘Invasional meltdown | C, with interactions with M | FMTs, who have a high level of diversity, have shown that a subset of species engraft even if the recipient microbiome is not perturbed. | [8*,9] |
| Phages that are introduced with microbes (e.g. FMTs) reduce fitness of indigenous species in the recipient microbiome. | ‘Enemy of my enemy’ hypothesis | C, with interactions with M | Phage populations change in recipients of FMT, with correlates with engraftment, suggesting that donor-derived phages reduce competition by targeting members of the recipient’s microbiota. | [9,27*] |
| Habitat filters select for invaders that can only be successful if adapted to the conditions of the ecosystem. They lead to trait underdispersion and phylogenetic clustering. | ‘Habitat filtering’ hypothesis | H, with interactions with C (traits to overcome HF) | The bacterial community within the mammalian gut microbiota shows an extreme case of phylogenetic underdisperson with only 5–7 bacterial phyla being reliably represented of the hundreds of bacterial phyla in the environment. Both pathogens and commensals showed increased colonization of the murine gut when related species were present (the “like will to like” rule). Higher likelihood of engraftment of strains after FMT if the species are already present. | [8*,28,51] |
| Spare resources unused by the resident community provide an opportunity for invasion. Communities with higher diversity and evenness are more resilient to invasions as they exploit resources more efficiently [13*], while perturbations of the community free resources and generate ‘opportunity windows’ for invasions. | ‘Empty niche’, ‘opportunity windows’, ‘Increased resource availability’, ‘priority effects’, and ‘disturbance’ hypotheses. | M, with interactions with C (traits that facilitate access to resources) | Germ-free and antibiotic treated animals allow colonization even of non-adapted microbes as there is no or reduced competition for resources. Addition of a non-digestible carbohydrate facilitates the establishment of Bifidobacterium adolescentis in the rat gut. Competition within Bacteroides strains in gnotobiotic mice is driven by competition for specific carbohydrates. Early in live microbiota of babies that are breast fed is distinct from those that are formula fed. Breast milk promotescolonization of Bifidobacterium strains that specifically utilize milk oligosaccharides. | [19*,22,39,52–55] |
| Invasion can occur if invaders are functionally distinct from species in the resident community so that they differ in their respective niches and competition is avoided. This process causestrait/phylogenetic overdispersion. | ‘Empty niche’, ‘limiting similarity’, and ‘competitive exclusion’ hypotheses. | M, with interactions with C (traits that allow niche partitioning) | B. longum subs p. longum can engraft in a subset of subjects whose microbiome lacks carbohydrate-utilization-genes of the species. | [19*] |
| Competition is more severe if invader is closely related to resident species. | ‚Phylogenetic limiting similarity’ and ‘Darwin’s Neutralization’ hypotheses. | M, with interactions with C (taxonomy of the invader) | The species B. longum is underrepresented in the baseline microbiome of individuals that permit stable engraftment of B. longum subsp. longum AH1206. Colonization of Bacteroides strains in gnotobiotic mice was prevented only by members of the same species. | [19*,55] |
Characteristics of the potential colonist (C)
According to the propagule pressure hypothesis, successful invasions require a sufficient number of individuals to enter the ecosystem [12], which relates to the cell numbers (or dose) of the treatment and frequency with which they are applied. Once introduced, the microbes need to possess traits to overcome the habitat filters and compete for available resources while avoiding predators. Microbe(s) are more likely to possess such traits if they share an evolutionary association with the gastrointestinal tract and, if host specific, a particular host species [15], and if not extensively adapted to ‘in vitro’ conditions, which could lead to evolutionary ‘trade-offs’ that reduce fitness in the gut.
Most species used as probiotics survive passage through the intestinal tract. Persistence of these bacteria, however, is for the most part short-term, even for probiotic strains isolated from the human gut [6]. A likely reason for this finding is that most commercial strains belong to species (e.g. Lactobacillus species and Bifidobacterium animalis subp. lactis) that are allochthonous to the human gastrointestinal tract, and therefore lack the required traits to successfully colonize gut ecosystems [16,17]. In contrast, B. longum subsp. longum AH1206, which is likely autochthonous to the human gut as it belong to a species of the human core gut microbiome [18], can be stably established in a subset of humans for at least 6 months [19*]. One characteristic of autochthony is a joint evolutionary history with the host [20]. Several Bifidobacterium species have a demonstrated joint relationship with humans [21], providing a potential explanation for the long-term persistence of B. longum subsp. longum in humans [19*]. The importance of the evolutionary history of the incoming microbes is supported by the fact that microbiomes originating from mice outcompete those originating from other hosts [22]. In addition, strains of L. reuteri can only efficiently colonize Lactobacillus-free mice if they originate from rodents but not from other hosts [23]. This greater ecological fitness of rodent strains is associated with traits that have specifically evolved to overcome habitat filters, for example, genes to overcome rapid flow of the digesta (through adherence) [24] and acid resistance [25].
Several hypotheses in invasion ecology underscore the importance of genotypic and phenotypic diversity and plasticity of the incoming species pool (Table 1). The higher the number of different genotypes that are introduced, the higher the chance that some organisms will have the right adaptations to be successful, and genotypic and phenotypic plasticity enable rapid adaptation to the new environment [12,13*]. By design, probiotics, even when used as mixtures, have a lower genetic diversity than FMTs. Recent studies have shown that a surprisingly large subset of strains engraft after FMT, even if the micobiome of the recipient is not severely perturbed [8*,9].
Invasion ecology further predicts an important role of ‘enemies’ for successful colonization (Table 1). Given the dominant role of bacteriophages in shaping the composition of bacterial populations in the gut [26], it is likely that resident strains display at least some level of resistance against indigenous phages. After an FMT, the recipient microbiome becomes subjected to donor-derived phages to which they were not previously exposed to and that have the potential to inhibit or kill resident bacteria, opening niches for incoming microbes. In fact, Zuo and co-workers showed that the treatment response in FMT was associated with a high colonization level of donor-derived Caudovirales taxa that may have played a role in the success of the treatment [27*]. Future research is warranted to test the importance of the ‘enemy of my enemy’ hypothesis in gut microbiome modulation and specifically determine if phages contribute to engraftment during FMTs.
Host related mechanisms (H)
There are virtually hundreds of host-related factors that constitute habitat filters and/or specifically select for the microbes that are most fitted, and their discussion is beyond the scope of this review. However, a few concepts are important to highlight (see also Table 1). First, habitat filters are influenced by the host’s physiology, metabolism, and immune system, which are in themselves influenced by the host’s genetics, health status, environment, and diet. Second, they lead to the selection of microbes with common traits, which therefore results in trait underdispersion and, in most cases, phylogenetic clustering [12]. By being dominated by only five bacterial phyla out of the hundreds of phyla found in terrestrial and aquatic ecosystems, the gut microbiota is phylogenetically underdispersed, showing the importance of strict selection through habitat filters [10]. In addition, habitat filtering is likely a key mechanism that underlies the “like will to like” rule, meaning there is a higher success of engraftment of incoming strains (both as single organisms or in FMTs) when related species are already present [8*,28]. The host also selects for specific co-evolved symbionts through the provision of adhesion sites [24] and resources in the form of secreted glycans (including mucus and milk glycans) [29]. These resources, together with those provided through the host’s diet, are key components of the available niches for which the incoming microbes have to compete for.
Microbiome related mechanisms (M)
Competition for resources is a key mechanism that determines species coexistence [12] and thus the success of colonization (Table 1). Communities with higher diversity and evenness are considered more resilient to invasions as they exploit resources more efficiently [13*], while perturbations of the community frees resources and generates ‘opportunity windows’ for invasions [12,30]. Accordingly, FMTs have a much higher degree of engraftment in patients with Clostridium difficile diarrhea whose microbiome is severely perturbed [31] compared to patients with metabolic syndrome [8*].
There are several additional hypotheses that concern resource availability but focus on specific members within a community. Niche-differentiation provides a ‘stabilizing’ effect that decreases negative interactions between the invader and the resident species [32]. In other words, the chance of invasion increases if the invader is functionally distinct from the species present in the recipient community by avoiding competition for resources; this is referred to as ‘limiting similarity’ (LS) [12]. Since closely related strains are on average functionally more similar, competition is generally more severe between them (an observation already noted by Charles Darwin in The origin of Species) [33]. These concepts appear to apply to the stable establishment of B. longum subsp. longum in the human gut, as the microbiome of subjects permissive to colonization (‘persisters’) had significant lower abundance of the species B. longum as well as of genes involved in carbohydrate utilization [19*]. Interestingly, some ‘persisters’ with high levels of B. longum still lacked functional genes, suggesting that although the ‘limiting similarity’ hypothesis applies, the functions that determine invasion were not phylogenetically conserved in all subjects. Overall, LS leads to opposing phylogenetic patterns when compared to habitat filtering, favoring different species with limited overlap in their niches [34]. Which of the two processes ultimately dominates in determining which species coexist and assemble within gut microbial ecosystems is likely to be context and taxon dependent, with higher selective pressure (for example during inflammation) favoring habitat filtering over competition for resources.
Other M-related mechanisms are antagonism (e.g. through bacteroicins), mutualism (facilitation), and predation (bacteriophages and protozoa). Probiotics could fail to engraft due to bacteriophages and/or bacteriocins present in the ecosystem to which the strain is susceptible to, but these topics have, to our knowledge, not been systematically studied.
Interactions between C, H, and M
The themes discussed above do not act in isolation (Figure 2). Incoming microbes have the potential to induce host responses (immune reactions, glycan formation and composition, etc.) that alter habitat filters [35]. In addition, once established and metabolically active, microbes alter niches for others and themselves [36]. Microbes can then continue to adapt to the dynamic environment that emerges through microbe-microbe and microbe-host interactions [10]. In fact, ‘adaptability’ of the incoming microbe is considered an important characteristic according to the ‘evolution of improved competitive ability’ hypothesis [12], but this has hardly been considered to date.
This evolutionary perspective on invasions underscores the importance of the timing of colonization, which can have a major effect on the interactions between C and M through ecoevolutionary feedback [37,38]. A colonist can gain an advantage over competing species if introduced before the latter’s arrival. Through priority effects, species that arrive early can reduce the amount of resources available for later arrivers, decreasing their competitive fitness [37]. In addition, early arrivers can increase their own fitness relative to later arrivers through adaptations [38]. Both mechanisms will enhance colonization of microbes that are introduced before competitors arrive, and apply during early microbiome assembly in infancy, but also after perturbations of already assembled microbiomes (e.g. after antibiotics) [37–39].
Ecological impact
The objective of microbiome-modulating strategies is a targeted alteration of the composition and/or function of the resident gut microbiota. For this outcome to happen, C, H, and M (Figure 2) have to be accommodating if not favorable for colonists to occupy niches (Step C in Figure 1) long enough to initiate metabolic activities that allow them to engage in competitive, antagonistic, or symbiotic interactions with other community members that would alter their abundance (Step D in Figure 2). The literature is often prone to unrealistic expectations on what current probiotics can achieve in this respect. Several studies claim that allochthonous microbes with marginal persistence can induce substantial changes to gut microbiota composition that, in some cases, even persist long after the strains has been washed out [40,41]. However, the mechanisms by which such dramatic shifts would occur have not been identified yet, and many studies do not control for confounders (compositional changes during storage of fecal samples, clustering by sequence run, cage effects in animal studies, etc.) that might be responsible for the findings. In fact, most well-controlled studies have shown that allochthonous strains have no impact on microbiome composition [40,42,43].
In Table 2, we summarize the characteristics of currently available microbial-based strategies for microbiome modulation, there impact on the ecosystem, their limitations, and how they might be improved. We argue that because of colonization resistance of gut ecosystems, and their homeostatic and resilient nature [44], it is not surprising that allochthonous organisms do not exert major effects on the gut microbiome. Lacking the necessary traits to efficiently compete for resources (step 3 in Figure 1), allochthonous strains are unlikely to attain sufficient metabolic activity to compete or antagonize well adapted members of the resident microbiota. In contrast, autochthonous microbes (either single organism, mixtures, or FMTs) can be introduced into the gut if a niche is available [19*] or if introduced strains have sufficient fitness to compete with resident species [8*]. This can be exploited to specifically reintroduce species that went ‘missing’ after antibiotic treatment or reestablish diversity in disturbed microbiomes, allowing both fine-tuning and community restoration (Table 2). If the incoming strains are antagonistic or mutualistic towards resident members in the ecosystem, specific alterations within the overall community could be the result.
Table 2.
Current microbial-based strategies to modulate gut microbiomes and their ecological characteristics.
| Strategies | Relevant characteristics | What can they do | Limitations | How can they be improved | Open questions |
|---|---|---|---|---|---|
| Probiotic/live bio-therapeutic with allochthonous strains | Most survive passage through the GIT tract. Might still be metabolically active for short periods of time. | Although research is inconclusive, effects on gut microbiota composition and function are likely marginal. Can still directly impact the host, e.g. by producing bioactive compounds (if sufficiently active) and through immune modulation. | Originate from extra-intestinal habitats and do therefore lack the necessary traits to be competitive in the gut. | Potential for genetic modification to produce therapeutic compounds [3*]. Non-persistence is an advantage for genetically modified strains for bio-confinement†. | Can allochthones organisms produce enough recombinant products at intestinal sites of interest? |
| Probiotic/live bio-therapeutic with autochthonous strains | Possess traits for both survival and colonization of the gastrointestinal tract | Can engraft into the microbiome if niche is available or if resident member is outcompeted. | Genetic diversity is low. Strains were often selected based on in vitro criteria and were propagated over many generations in artificial conditions (e.g. laboratory), likely leading to ‘trade offs’. | Production of probiotic products should be performed to maximize activity and minimize lagphases in the gut, and to avoid ‘trade offs’. Combine with subtractive therapies (antibiotics, phages). Administered early in life†. | Is persistence of probiotics linked to bacteriophages in the resident microbiome? What genes within probiotic strains determine persistence? Can treatments be personalized? |
| Consortia of autochthonous strains | Higher diversity in traits for both survival and colonization | Genetic diversity is higher than when using single strains alone. If introduced strains come from the same community, facilitative syntrophic interactions between them are likely. | Key members of the intact community are likely to be missing. Inhibition between member strains. | Production of consortia should maximize activity and minimize lagphases in the gut. Composition should be formulated based on target disease. | How does genetic diversity contribute to persistence? Can treatments be personalized? |
| Synbiotics | Probiotic strain(s) combined with a prebiotic substrate. | Provides resources for the incoming microbe, leading to a relaxation of competition. | The resident microbiota competes with the incoming probiotic for the resource. | Synbiotic combinations have to be designed based on strict ecological criOrchidsOteria and focus to identify strain/prebiotic combinations that work under the constrains of the gut ecosystem. | Can synbiotics be formulated to improve persistence and efficacy of probiotics? |
| Fecal Microbiota Transplant (FMT) | Complex mixture of microbes generated from fecal sample(s) of healthy human donor(s) | Introduction of an entire community allows complex networks to be established in the recipient. Phages are present that might contribute to engraftment. High genetic diversity increases chance of engraftment. | Safety and regulatory concerns. Difficult to do, although capsules have been developed. | Combine with subtractive and/or modulatory (substrates) approaches to increase engraftment. Determine differences in donor and recipient microbiomes that determine efficacy. | How efficient are they for diseases other than CI. difficile diarrhea? What are the ecological principles that govern engraftment? What are the exact roles of components (phages, metabolites, etc.)? |
| Subtractive approaches followed by the bio-therapy. | Use of antimicrobial substances or replicating entities (bacteriophages) to remove native bacteria. | Can decrease competitors and open niches for the incoming microbes [39]. Priority effects facilitate engraftment of microbes introduced after subtractive intervention. | Open niches could be exploited by opportunistic native members or pathogenic microbes. Safety and regulatory concerns. | Improve specificity and use targeted instead of broad-spectrum approaches, paired with a consortium of strains that can occupy niches that become vacant. | Would the incorporation of phages and mobile genetic elements increase the risk of native populations to acquire resistance? Are personalized strategies necessary? |
Probiotic or live bio-therapeutic strains should be selected based on well-controlled clinical trials, and traits that facilitate their technological applicability (good growth, etc.) should only be considered when the clinical effects are sustained.
From an ecological perspective, it will probably prove difficult to reconfigure communities that are not severely perturbed (e.g. to achieve a healthier state), as this would require the replacement of keystone species. If keystone species were to be replaced by other microbes through competitive exclusion, the incoming microbes would have to occupy the same niches, which would mean that the community would likely maintain the same functions. However, the incoming strains could possess additional traits, which could alter ecosystem functionality as a whole. A more complete reconfiguration of an unperturbed community will probably require the removal of a large part of the original members through subtractive strategies [3*]. In addition, if the dysbiosis to be corrected is the result of a pathology, habitat filtering will ultimately select for similar microbiome patterns once the treatment is stopped. Future research should be devoted on the identification of mechanisms that could restructure host associated microbiomes, also considering prospective strategies that focus on early windows of microbiome assembly.
Implications and challenges
It is important to point out that neither colonization nor microbiome modulation per se are necessarily requirements for microbial-based therapeutics to exert benefits, as they might arise through immune modulation or other direct effects on the host. However, engraftment may increase efficacy, especially if health effects rely on the microbes to be metabolically active in the gut [3*]. The holistic ecological framework described here can aid in the interpretation of the effects of the currently used microbiome-modulating strategies, and further advance the field by providing a basis for the development of novel or improved approaches. An ecological perspective is therefore relevant for all aspects of designing a microbiome-modulating strategy, from strain selection, industrialization (biotechnology), formulation, application and dosage, to their safety assessment and regulation [45].
Although most probiotic products are composed of allochthonous strains, the potential of using autochthonous members of the human microbiome to develop next-generation probiotics and bio-therapeutics is increasingly recognized [3*,4]. However, other factors that support biological invasions, such as genotypic diversity and adaptability of microbes, as well as their ‘early fecundity and fertility’ [12], have been hardly considered. The latter suggests that the approaches by which probiotic strains are maintained and produced (e.g. freeze drying) should be optimized so evolutionary ‘trade-offs’ in the organisms and lag phases in the gut are minimized. The importance of genetic diversity does not only provide an explanation for the success of FMTs, but suggests that probiotics should be rather applied as a consortium of strains if engraftment is the goal (Table 2). The central importance of resources opens up several opportunities to improve engraftment by pairing additive (probiotics, FMT), subtractive (antibiotics, bacteriophages), or modulatory (prebiotics) approaches [3*]. Finally, the importance of colonization history in invasion suggests that administration of microbes early in infancy or after subtractive approaches might enable long-term colonization, and could even change the trajectory of the assembly of the entire microbiome through priority effects and historical contingency, with longstanding effects [37–39].
An ecological perspective is further necessary for the exact prediction of the impact and consequences of strategies. The individualized nature of microbiomes make the response to modulations inherently subject-specific [19*,46]. Information on what drives this individuality can, once understood, be used to generate predictive models to personalize strategies. In addition, microbiome-modulating strategies constitute a challenge for regulatory agencies as they represent a novel paradigm in drug development [3*]. Stable alterations of the gut microbiome, and the genetic changes of the introduced organisms that are likely to occur during long-term colonization raise questions regarding the pharmacology, standardization, and control of such therapies. The full potential of microbiome-modulating strategies can only be realized if the regulatory framework considers their unique biological and ecological characteristics, which will require different avenues than the ones available for generic drugs or foods [45]. A dialog between regulators and researchers will be necessary to develop such frameworks, and these discussions will have to be informed by an understanding of the ecological effects of microbiome-modulating therapies.
Highlights.
Microbiome-modulation through live microbes has enormous potential to improve health
Recent studies have provided new insights into the impact of live microbes on gut microbiomes
Ecological theory can help reach a conceptual understanding on the impact of microbiome-modulating interventions
An ecological perspective will be essential to improve currently available strategies and develop novel ones
Acknowledgments
JW acknowledges support through the Campus Alberta Innovates Chair (CAIP) program and the National Institute of Health (NIH) (5R01GM099525-02). His program is currently supported by grants of the Canadian Institute of Health Research (CIHR), Natural Sciences and Engineering Research Council of Canada (NSERC), and the JPI HDHL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors are not aware of any possible conflict of interest
References
- 1.Young VB. The intestinal microbiota in health and disease. Curr Opin Gastroenterol. 2012;28:63–69. doi: 10.1097/MOG.0b013e32834d61e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker AW, Lawley TD. Therapeutic modulation of intestinal dysbiosis. Pharmacol Res. 2013;69:75–86. doi: 10.1016/j.phrs.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 3*.Mimee M, Citorik RJ, Lu TK. Microbiome therapeutics - Advances and challenges. Adv Drug Deliv Rev. 2016 doi: 10.1016/j.addr.2016.04.032. Review that discusses current subtractive and additive approaches to manipulate the microbiota and the challenges encountered by the scientific community in the development of microbiome therapeutics. Highlights the importance of understanding the ecological principles for a rational design of effective microbial-based therapeutics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Toole PW, Marchesi JR, Hill C. Next-generation probiotics: the spectrum from probiotics to live biotherapeutics. Nat Microbiol. 2017;2:17057. doi: 10.1038/nmicrobiol.2017.57. [DOI] [PubMed] [Google Scholar]
- 5.Olle B. Medicines from microbiota. Nat Biotechnol. 2013;31:309–15. doi: 10.1038/nbt.2548. [DOI] [PubMed] [Google Scholar]
- 6.Jacobsen CN, Nielsen VR, Hayford AE, Møller PL, Michaelsen KF, Pærregaard A, Sandström B, Tvede M, Jakobsen M. Screening of probiotic activities of forty-seven strains of Lactobacillus spp by in vitro techniques and evaluation of the colonization ability of five selected strains in humans. Appl Environ Microbiol. 1999;65:4949–4956. doi: 10.1128/aem.65.11.4949-4956.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kristensen NB, Bryrup T, Allin KH, Nielsen T, Hansen TH, Pedersen O. Alterations in fecal microbiota composition by probiotic supplementation in healthy adults : a systematic review of randomized controlled trials. Genome Med. 2016 doi: 10.1186/s13073-016-0300-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8*.Li SS, Zhu A, Benes V, Costea PI, Hercog R, Hildebrand F, Huerta-Cepas J, Nieuwdorp M, Salojärvi J, Voigt AY, et al. Durable coexistence of donor and recipient strains after fecal microbiota transplantation. Science. 2016;352:586–9. doi: 10.1126/science.aad8852. This study provides in detail insight into the impact of FMT on the recipient microbiome in individual suffering from metabolic syndrome by using a metagenomic analysis with strain level resolution. [DOI] [PubMed] [Google Scholar]
- 9.Kang D, Adams JB, Gregory AC, Borody T, Chittick L, Fasano A, Khoruts A, Geis E, Maldonado J, Mcdonough-means S, et al. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome. 2017;5:1–16. doi: 10.1186/s40168-016-0225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walter J, Ley R. The human gut microbiome: ecology and recent evolutionary changes. Annu Rev Microbiol. 2011;65:411–29. doi: 10.1146/annurev-micro-090110-102830. [DOI] [PubMed] [Google Scholar]
- 11.Costello EK, Stagaman K, Dethlefsen L, Bohannan BJM. The application of ecological theory toward an understanding of the human microbiome. Science. 2012;336:1255–62. doi: 10.1126/science.1224203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Catford JA, Jansson R, Nilsson C. Reducing redundancy in invasion ecology by integrating hypotheses into a single theoretical framework. Divers Distrib. 2009;15:22–40. [Google Scholar]
- 13*.Mallon CA. Microbial Invasions: The Process, Patterns, and Mechanisms. Trends Microbiol. 2015;23:719–729. doi: 10.1016/j.tim.2015.07.013. Excellent review applying the concepts of invasion ecology to microbial ecosystem. [DOI] [PubMed] [Google Scholar]
- 14.Chase JM, Leibold MA. Ecological niches: Linking classical and contemporary approaches. The University of Chicago Press; 2003. [Google Scholar]
- 15.Duar RM, Lin XB, Zheng J, Martino ME, Grenier T, Pérez-Muñoz ME, Leulier F, Gänzle M, Walter J. Lifestyles in transition: evolution and natural history of the genus Lactobacillus. FEMS Microbiol Rev. 2017 doi: 10.1093/femsre/fux030. doi: https://doi.org/10.1093/femsre/fux030. [DOI] [PubMed]
- 16.Walter J. Ecological role of lactobacilli in the gastrointestinal tract: Implications for fundamental and biomedical research. Appl Environ Microbiol. 2008;74:4985–4996. doi: 10.1128/AEM.00753-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biavati B, Mattarelli P. Genus I Bifidobacterium. In: Whitman W, Goodfellow M, Kämpfer P, Busse H-J, Trujillo M, Ludwig W, Suzuki K, editors. Bergey’s Manual of Systematic Bacteriolog. Volume 5: The Actinobacteria. Springer; New York: 2012. pp. 171–206. [Google Scholar]
- 18.Schloissnig S, Arumugam M, Sunagawa S, Mitreva M, Tap J, Zhu A, Waller A, Mende DR, Kultima JR, Martin J, et al. Genomic variation landscape of the human gut microbiome. Nature. 2013;493:45–50. doi: 10.1038/nature11711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19*.Maldonado-Gomez MX, Martinez I, Bottacini F, O’Callaghan A, Ventura M, van Sinderen D, Hillmann B, Vangay P, Knights D, Hutkins RW, et al. Stable Engraftment of Bifidobacterium longum AH1206 in the Human Gut Depends on Individualized Features of the Resident Microbiome. Cell Host Microbe. 2016;20:515–526. doi: 10.1016/j.chom.2016.09.001. First study to show a stable engraftment of a probiotic strain for over 6 months in a subset of human subjects using methods that allow resolution at strain level. Using a metagenomic approach, the study further identified differences in microbiome of persisters and non-persisters to be related to researches that provide a niche opportunity for the strain. [DOI] [PubMed] [Google Scholar]
- 20.Savage DC. Microbial Ecology of the Gastrointestinal Tract. Annu Rev Microbiol. 1977;31:107–33. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- 21.Moeller AH, Caro-quintero A, Mjungu D, Georgiev AV, Lonsdorf EV, Muller MN, Pusey AE, Peeters M, Beatrice H. Cospeciation of gut microbiota with hominids. 2017;353:380–382. doi: 10.1126/science.aaf3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grimm V, Radulovic K, Riedel CU. Colonization of C57BL/6 mice by a potential probiotic Bifidobacterium bifidum strain under germ-free and specific pathogen-free conditions and during experimental colitis. PLoS One. 2015;10:1–14. doi: 10.1371/journal.pone.0139935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frese SA, Benson AK, Tannock GW, Loach DM, Kim J, Oh PL, Heng NCK, Patil PB, Juge N, Mackenzie DA, et al. The Evolution of Host Specialization in the Vertebrate Gut Symbiont. Lactobacillus reuteri. 2011;7 doi: 10.1371/journal.pgen.1001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frese SA, Mackenzie DA, Peterson DA, Schmaltz R, Fangman T, Zhou Y, Zhang C, Benson AK, Cody LA, Mulholland F, et al. Molecular Characterization of Host-Specific Biofilm Formation in a Vertebrate Gut Symbiont. 2013;9 doi: 10.1371/journal.pgen.1004057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krumbeck JA, Marsteller NL, Frese SA, Peterson DA, Ramer-tait AE, Hutkins RW, Walter J. Characterization of the ecological role of genes mediating acid resistance in Lactobacillus reuteri during colonization of the gastrointestinal tract. 2016;18:2172–2184. doi: 10.1111/1462-2920.13108. [DOI] [PubMed] [Google Scholar]
- 26.Mills S, Shanahan F, Stanton C, Hill C, Coffey A, Ross RP, Mills S, Shanahan F, Stanton C, Hill C, et al. Movers and shakers: Influence of bacteriophages in shaping the mammalian gut microbiota. Gut Microbes. 2013;4:4–16. doi: 10.4161/gmic.22371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zuo T, Wong SH, Lam K, Lui R, Cheung K, Tang W, Ching JYL, Chan PKS, Chan MCW, Wu JCY, et al. Bacteriophage transfer during faecal microbiota transplantation in Clostridium difficile infection is associated with treatment outcome. Gut. 2017 doi: 10.1136/gutjnl-2017-313952. [Epub ahead of print]. *Elegant study that suggests that bacteriophages might be responsible for some of the effects of FMTs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stecher B, Chaffron S, Käppeli R, Hapfelmeier S, Freedrich S, Weber TC, Kirundi J, Suar M, McCoy KD, von Mering C, et al. Like will to like: abundances of closely related species can predict susceptibility to intestinal colonization by pathogenic and commensal bacteria. PLoS Pathog. 2010;6:e1000711. doi: 10.1371/journal.ppat.1000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schluter J, Foster KR. The Evolution of Mutualism in Gut Microbiota Via Host Epithelial Selection. PLoS Biol. 2012;10:e1001424. doi: 10.1371/journal.pbio.1001424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shea K, Chesson P. Community ecology theory as a framework for biological invasions. Tree. 2002;17:170–176. [Google Scholar]
- 31.Fuentes S, Van Nood E, Tims S, Jong IH, Jf C, Keller JJ, Zoetendal EG, De Vos WM. Reset of a critically disturbed microbial ecosystem : faecal transplant in recurrent Clostridium difficile infection. ISME Journal. 2014;8:1621–1633. doi: 10.1038/ismej.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chesson P. Mechanisims of Maintenance of Species Diversity. Annu Rev Ecol Syst. 2000;31:343–66. [Google Scholar]
- 33.Darwin C. The Origin of Species, by Charles Darwin, A variorum text. University of Pennsylvania Press; 1859. [Google Scholar]
- 34.Mayfield MM, Levine JM. Opposing effects of competitive exclusion on the phylogenetic structure of communities. Ecol Lett. 2010;13:1085–93. doi: 10.1111/j.1461-0248.2010.01509.x. [DOI] [PubMed] [Google Scholar]
- 35.Hooper LV, Gordon JI. Commensal Host-Bacterial Relationships in the Gut. Science. 2001;292:1115–1119. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 36.Cavender-Bares J, Kozak KH, Fine PVA, Kembel SW. The merging of community ecology and phylogenetic biology. Ecol Lett. 2009;12:693–715. doi: 10.1111/j.1461-0248.2009.01314.x. [DOI] [PubMed] [Google Scholar]
- 37.Fukami T. Historical Contingency in Community Assembly: Integrating Niches, Species Pools, and Priority Effects. 2015;46:1–23. [Google Scholar]
- 38.De Meester L, Vanoverbeke J, Kilsdonk LJ, Urban MC. Evolving Perspectives on Monopolization and Priority Effects. Trends Ecol Evol. 2016;31:136–146. doi: 10.1016/j.tree.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 39.Staley C, Kaiser T, Beura LK, Hamilton MJ, Weingarden AR, Bobr A, Kang J, Masopust D, Sadowsky MJ, Khoruts A. Stable engraftment of human microbiota into mice with a single oral gavage following antibiotic conditioning. Microbiome. 2017;5:87. doi: 10.1186/s40168-017-0306-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Derrien M, van Hylckama Vlieg JET. Fate, activity, and impact of ingested bacteria within the human gut microbiota. Trends Microbiol. 2015;23:354–366. doi: 10.1016/j.tim.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 41.Zhang J, Wang L, Guo Z, Sun Z, Gesudu Q, Kwok L, Zhang H. 454 pyrosequencing reveals changes in the faecal microbiota of adults consuming Lactobacillus casei Zhang. FEMS Microbiol Ecol. 2014;88:612–622. doi: 10.1111/1574-6941.12328. [DOI] [PubMed] [Google Scholar]
- 42.McNulty NP, Yatsunenko T, Hsiao A, Faith JJ, Muegge BD, Goodman AL, Henrissat B, Oozeer R, Cools- S, Gobert G, et al. The impact of a consortium of fermented milk strains on the gut microbiome of gnotobiotic mice and monozygotic twins. Sci Transl Med. 2012;3:1–26. doi: 10.1126/scitranslmed.3002701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lahti L, Salonen A, Kekkonen RA. Associations between the human intestinal microbiota, Lactobacillus rhamnosus GG and serum lipids indicated by integrated analysis of high-throughput profiling data. PeerJ. 2013;1:e32. doi: 10.7717/peerj.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lawley TD, Walker AW. Intestinal colonization resistance. Immunology. 2013;138:1–11. doi: 10.1111/j.1365-2567.2012.03616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Green BJM, Barratt MJ, Kinch M, Gordon JI. Food and microbiota in the FDA regulatory framework. Science. 2017;357:39–40. doi: 10.1126/science.aan0836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang C, Derrien M, Levenez F, Brazeilles R, Ballal SA, Kim J, Degivry M, Quéré G, Garault P, van Hylckama Vlieg JET, et al. Ecological robustness of the gut microbiota in response to ingestion of transient food-borne microbes. ISME J. 2016;10:2235–2245. doi: 10.1038/ismej.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Del Campo R, Bravo D, Canto R, Ruiz-garbajosa P, Garcı R, Montesi-libois A, Yuste F, Abraira V, Baquero F. Scarce Evidence of Yogurt Lactic Acid Bacteria in Human Feces after Daily Yogurt Consumption by Healthy Volunteers. Appl Environ Microbiol. 2005;71:547–549. doi: 10.1128/AEM.71.1.547-549.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walter J. Ecological Role of Lactobacilli in the Gastrointestinal Tract: Implications for Fundamental and Biomedical Research. Appl Environ Microbiol. 2008;74:4985–4996. doi: 10.1128/AEM.00753-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fujiwara S, Seto Y, Kimura A, Hashiba H. Intestinal transit of an orally administered streptomycin-rifampicin-resistant variant of Bifidobacterium longum SBT2928: its long-term survival and effect on the intestinal microflora and metabolism. J Appl Microbiol. 2001;90:43–52. doi: 10.1046/j.1365-2672.2001.01205.x. [DOI] [PubMed] [Google Scholar]
- 50.Duar RM, Frese SA, Lin XB, Fernando SC, Burkey TE, Tasseva G, Peterson DA, Blom J, Wenzel CQ, Szymanski CM, et al. Experimental evaluation of host adaptation of Lactobacillus reuteri to different vertebrate species. Appl Environ Microbiol. 2017;83 doi: 10.1128/AEM.00132-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harmsen H, Wildeboer-Veloo A, Raangs G, Wagendorp A, Klijn N, Bindels J, Welling G. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. Pediatr Gastroenterol Nutr. 2000;30:61–7. doi: 10.1097/00005176-200001000-00019. [DOI] [PubMed] [Google Scholar]
- 53.Underwood MA, Kalanetra KM, Bokulich NA, Lewis ZT, Mirmiran M, Tancredi DJ, Mills DA. A comparison of two probiotic strains of bifidobacteria in premature Infants. J Pediatr. 2015;163 doi: 10.1016/j.jpeds.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krumbeck JA, Maldonado-Gomez MX, Martínez I, Frese SA, Burkey TE, Rasineni K, Ramer-Tait AE, Harris EN, Hutkins RW, Walter J. Introducing the concept of In Vivo Selection to identify bacterial stains with enhanced ecological performance when used in synbiotic applications. Appl Environ Microbiol. 2015;81:2455–2465. doi: 10.1128/AEM.03903-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee SM, Donaldson GP, Mikulski Z, Boyajian S, Ley K, Mazmanian SK. Bacterial colonization factors control specificity and stability of the gut microbiota. Nature. 2013;501:426–429. doi: 10.1038/nature12447. [DOI] [PMC free article] [PubMed] [Google Scholar]


