Abstract
Objective
To understand the rate of genetic events in patients with autism spectrum disorder (ASD) who were exposed to assisted reproduction.
Design
Case control study using genetics data.
Setting
Twelve collaborating data collection sites across North America as part of the Simons Simplex Collection.
Patient(s)
2,760 children with ASD, for whom 1,994 had published copy number variation data and 424 had published gene mutation status available.
Intervention(s)
None.
Main Outcome Measure(s)
Rates of autism-associated genetic events in children with ASD conceived with assisted reproduction versus those conceived naturally.
Result(s)
No statistically significant differences in copy number variations or autism-associated gene-disrupting events were found when comparing ASD patients exposed to assisted reproduction with those not exposed to assisted reproduction.
Conclusion(s)
This is the first large genetic association to concurrently examine the genotype of individuals with ASD in relation to their exposure to ART versus natural conception, and it adds reassuring evidence to the argument that ART does not increase the risk of ASD.
Keywords: Assisted reproduction, assisted reproductive technology, autism, copy number variation
Autism spectrum disorder (ASD) is a neuropsychiatric condition that includes impairments in social interaction and communication as well as restricted or stereotyped behavior (1). The prevalence of ASD in U.S. children aged 6 to 17 years is estimated to be as high as 1 in 50 from recent data (2). The etiology of ASD is unknown; however, there is strong evidence of significant genetic influence, with hundreds of genes implicated, each of which generally conveys a slight increase in risk (3, 4). Autism risk is also elevated after environmental insult including in utero exposure to infections, toxins, and birth complications among others (5–7). More recently, epigenetic factors have been proposed as playing a significant role in the etiology of ASD, highlighting the contribution of both genetic and environmental factors (8, 9). Given the building evidence for the interplay of genetics and environment in the etiology of ASD, it is important to consider whether certain medical interventions may also play a role.
Assisted reproduction is a broad term referring to various interventions primarily used to treat infertility, including but not limited to fertility medications, in vitro fertilization (IVF), gamete intrafallopian transfer (GIFT), zygote intrafallopian transfer (ZIFT), and artificial insemination. Assisted reproductive technology (ART) as defined by the Centers for Disease Control and Prevention, is a more specific term, only including IVF, GIFT, and ZIFT. These three treatments alone accounted for 163,038 ART cycles resulting in 47,090 live births in 2011 (10).
As ART has become increasingly common, there have been concomitant concerns regarding the developmental outcomes of these pregnancies. Fortunately, major reviews and individual studies of ART procedures have been overall reassuring (11, 12). In general, neurodevelopmental outcomes of children conceived by ART seem to be comparable to outcomes in spontaneously conceived children (13). A number of studies have demonstrated that neither intracytoplasmic sperm injection (ICSI) or IVF are associated with cognitive disorders, developmental delays, or psychological/behavioral issues (14–21). Other studies have disputed the possibility of an association between IVF and a higher rate of karyotype abnormalities (22, 23).
Despite the positive outlook from these broad outcome measures, a number of specific differences have been observed between the outcomes of pregnancies conceived spontaneously versus those with ART. There have been suggestions that ART may affect genetic events that occur during ovulation or fertilization, such as with imprinted genes. Of the nine known imprinting syndromes, ART has been associated with two: Beckwith-Wiedemann syndrome and Silver-Russell syndrome (24). Moreover, it has been reported that the total risk of congenital malformations may be increased by about one-third through ART, with approximately a twofold increased risk of nervous systems defects (25). Pregnancies from IVF are also at higher risk for low birth weight and preterm birth compared with pregnancies conceived spontaneously (26–28). There are also other differences between IVF pregnancies and spontaneously conceived pregnancies, the most well known of which has been the increased multiple gestation rates (29). Specifically in regards to ICSI, there has been some recent evidence that ICSI is associated with mental retardation (30).
Various studies, primarily epidemiologic, have looked at the association between ASD and ART (30–35). Overall, the available data have not found a clear association between ASD and ART, but the results have not been entirely uniform. A 2009 meta-analysis looked at eight studies that examined possible relations between ART and ASD (35). The investigators noted methodological problems in the available studies as well as inconsistent results, and concluded that “possible associations with ASD need assessment in larger studies.” One recently published study concluded that ART was a risk factor for ASD (33), but another study claimed the opposite (34). However, the investigators in the latter study noted that the apparent increase in risk was due to confounding factors such as maternal age, educational level, parity, smoking, birth weight, and multiplicity. Another recent epidemiologic study documented similar findings, concluding that the risk between ASD and IVF disappeared once the analyses had been adjusted (31). It may be particularly important to adjust for parental characteristics because users of ART are more likely than fertile individuals both to be of increased age and to have chromosomal abnormalities (20). Furthermore, recent studies have shown that older paternal age is associated with autism-associated de novo copy number variation (CNV) (36, 37).
Perhaps most notably, a recent systematic review of many of the observational studies regarding ASD and ART concluded that there is no evidence that ART is significantly associated with ASD (38). Importantly, it should be pointed out that the findings tying ART to imprinting disorders seem particularly relevant in the context of ASD because imprinting disorders involve aberrant methylation (an epigenetic change in the quantity of methyl groups attached at certain sites in the genome) and aberrant methylation has been implicated in ASD (39). Finally, although there has been specific attention to ASD and ART, the broader question of whether assisted reproduction in general is associated with ASD has also been raised (32).
Although these studies have proven informative, no study to date has concurrently examined the genotype of individuals with ASD in relation to their exposure to ART versus natural conception. Given the concerns regarding the effect of ART on pregnancy outcomes, including genetic abnormalities, and in the context of the identified genetic contributions to ASD risk, it is important to look more specifically at the autism-associated genetic anomalies in ASD patients exposed to assisted reproduction. Therefore, we examined the relation between assisted reproduction and genetic mutations in children with ASD.
Materials and Methods
Participants were 2,760 4- to 18-year-old children with ASD and their families who previously had participated in the Simons Simplex Collection (SSC). The SSC, a project funded by the Simons Foundation Autism Research Initiative to identify de novo genetic variants related to ASD, includes 12 collaborating data collection sites across North America (for a description of the ascertainment, data collection, and validation procedures for the sample, see http://sfari.org) (40). Approval was obtained from each local institutional review board. All participants completed informed consent before participation in the study. As part of SSC study participation, experienced clinicians confirmed the ASD diagnosis using the Autism Diagnostic Interview, Autism Diagnostic Observation Schedule, and expert clinical judgment according to Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) criteria (41).
Information regarding assisted reproduction was collected through a clinician-administered, semistructured caregiver interview designed to gather data regarding early medical history for children and families participating in the SSC. Clinicians administering the interview were primarily specialists in mental health, not obstetrics. Specific to this study, caregivers were asked whether assisted reproduction had been used to initiate the pregnancy of the proband or siblings. If the caregiver responded yes, data regarding what type of assisted reproduction was collected. The options were oral fertility medications, injected fertility medications, artificial insemination, IVF, assisted hatching, frozen embryo, frozen eggs, sperm donor, egg donor, ICSI, GIFT, ZIFT, surrogacy, unknown, and other. Additionally, the maternal and paternal age at the time of conception was gathered.
Genotypic data regarding each participating family were collated from previously published studies identifying autism-associated CNV and putative causal ASD gene mutations (4, 42–44). For the purposes of this study, two categories of genetic data were examined, autism-associated CNV and autism-associated gene-disrupting events. Autism-associated gene-disrupting events were defined as gene-truncating loss-of-function mutations identified through exome sequencing.
In the sample of 2,760 SSC families with ASD, information regarding assisted reproduction history was available for 2,418 (87.6%) of the children with ASD. Of this subsample, 1,994 had published CNV information available, and 424 had published gene mutation status yielded through exome sequencing. Analyses were conducted with the subset of individuals with CNV and gene mutation data separately.
Genetic data was not available for all patients primarily due to the limits of the cost of the genetic analysis. The participants were selected for genetic analysis out of the larger pool by authors who had previously published their results with the SSC data set. These authors used a variety of criteria to select the patients they believed would most likely provide valuable genetic information. The criteria included low nonverbal intelligence, multiple unaffected siblings, and known multigenic CNV (43). Some patients were selected randomly as well.
A chi-square goodness of fit test was used to compare the rates of autism-associated genetic events in children with ASD conceived via assisted reproduction versus those conceived spontaneously. Analysis of variance (ANOVA) was used to examine relations between assisted reproduction use and genetic events (either CNV or gene mutation). Logistic regression was used to examine relations between genetic events and parental age.
Results
As shown in Table 1, in the sample of 1,994 individuals with ASD, 122 were the product of assisted reproduction. Demographics are not listed in the table but are as follows: 264 female, 1,730 male; 1,567 white, 84 Asian, 76 African American, 5 Native American, 157 more than one race, 90 other, 15 not specified. The percentage of autism-associated CNVs in ASD patients exposed to ART was 9.8%, compared with 10.3% in ASD patients not exposed to assisted reproduction, a nonsignificant difference [chi-square (1, N = 1994) = .02, P<.88]. Of the 424 patients with available gene mutation status, 24 were the result of assisted reproduction. The percentage of autism-associated gene-disrupting events in ASD patients exposed to assisted reproduction was 58.3%, compared with 51.3% in ASD patients not exposed to assisted reproduction, here again indicating no statistically significant differences in mutation rate [chi-square (1, N = 219)=.45, P<.50].
Table 1. Numbers and rates of genetic events.
| With event | Without event | Rate (%) | |
|---|---|---|---|
| Autism associated CNVs | |||
| Without assisted reproduction | 192 | 1,680 | 10.3 |
| With assisted reproduction | 12 | 110 | 9.8 |
| IVF | 2 | 24 | 7.7 |
| GIFT | 0 | 0 | NA |
| ZIFT | 0 | 0 | NA |
| ICSI | 0 | 4 | NA |
| Artificial insemination | 2 | 22 | 8.3 |
| Fertility medication only | 8 | 60 | 11.8 |
| Autism associated gene disrupting events | |||
| Without assisted reproduction | 205 | 195 | 51.3 |
| With assisted reproduction | 14 | 10 | 58.3 |
| IVF | 3 | 1 | 75.0 |
| GIFT | 0 | 0 | NA |
| ZIFT | 0 | 0 | NA |
| ICSI | 0 | 0 | NA |
| Artificial insemination | 3 | 1 | 75.0 |
| Fertility medication only | 8 | 8 | 50.0 |
Note: CNV = copy number variation; IVF = in vitro fertilization; GIFT = gamete intrafallopian transfer; NA = not applicable; ZIFT = zygote intrafallopian transfer; ICSI = intracytoplasmic sperm injection.
Despite the statistically nonsignificant results, given the 7% difference of autism-associated gene-disrupting events in ASD patients exposed to assisted reproduction versus ASD patients not exposed to assisted reproduction, a power analysis was run. Using G*Power 3.13 for a 1 degree of freedom test and assuming an effect size of w = 0.14 (as determined by the 7% difference in the observed sample) with alpha = 0.05 an observed power of 0.83 was calculated to detect this difference using the chi-square analysis. Thus, there was adequate power to have detected a significant difference had there been one.
Importantly, because assisted reproduction is a broad category of interventions, Table 1 also presents numbers of autism-associated CNVs and gene-disrupting events according to the following types of assisted reproduction: IVF (including with cryopreservation), GIFT, ZIFT, ICSI, artificial insemination, and fertility medication only. These numbers did not in general reach sufficient power for statistical analysis, but the genetic mutations appeared to be evenly distributed throughout the types of assisted reproduction.
Tables 2 and 3 present detailed information regarding all autism-associated genetic events identified in the ASD group exposed to assisted reproduction, along with descriptors such as parental ages, pregnancy number, and infertility reason. In Table 2, out of 12 patients with identified CNVs, 16 different regions were affected–none more than once–across 11 different chromosomes. In Table 3, out of 14 patients with genetic events, 23 genes were affected–none more than once–across 13 chromosomes.
Table 2. Specific copy number variation and subject descriptors.
| Subject ID a | Event | Region b | Inheritance | Paternal age (mo) | Maternal age (mo) | Pregnancy number | Multiple birth | Infertility reason |
|---|---|---|---|---|---|---|---|---|
| 12130.p1 | Dup | 5q22.2 | Paternal | 550 | 492 | 2 | Yes | Unexplained |
| Del | 8p22 | Maternal | ||||||
| 13335.p1 | Dup | 16p11.2 | De novo | 533 | 493 | 2 | Yes | Unexplained |
| Dup | 3q23 | Paternal | ||||||
| Del | 17p13.3 | Paternal | ||||||
| 11121.p1 | Dup | 7p22.1 | Paternal | 569 | 534 | 2 | No | Absent ovulation |
| 11154.p1 | Dup | 7q11.23 | De novo | 372 | 388 | 2 | No | Endometriosis |
| 11415.p1 | Dup | 3p21.31 | Paternal | 388 | 466 | 2 | No | Blocked fallopian tubes |
| 13784.p1 | Dup | 12p11.23 | De novo | 522 | 419 | 1 | No | Unexplained |
| 13494.p1 | Del | 10q22.2 | Maternal | 372 | 345 | 1 | No | Absent ovulation |
| Del | 18q23 | Paternal | ||||||
| 11660.p1 | Del | 2q13 | Maternal | 546 | 472 | 3 | No | Unexplained |
| 11184.p1 | Del | 16q23.2 | Paternal | 295 | 267 | 1 | No | Absent ovulation |
| 14074.p1 | Del | 15q13.1 | Paternal | 395 | 366 | 1 | No | Blocked fallopian tubes |
| 12132.p1 | Del | 15q24.1 | Paternal | 395 | 350 | 1 | No | Absent ovulation |
| 13215.p1 | Del | 16p13.11 | Paternal | 370 | 316 | 2 | No | Polycystic ovary syndrome |
Simons Simplex Collection (SSC) subject ID number.
These data are from previously published data (42).
Table 3. Specific genetic events and subject descriptors.
| Subject IDa | Chromosome | Gene b,c | Paternal age (mo)d | Maternal age (mo) d | Pregnancy number | Multiple birth | Infertility reason |
|---|---|---|---|---|---|---|---|
| 11184.p1 | 19 | HDGFRP2 | 431 | 324 | 1 | No | Absent ovulation |
| 11246.p1 | 2 | ARMC9 | 421 | 375 | 1 | No | Male factor |
| 11610.p1 | 2 5 | HDLBP DNAH5 | 371 | 332 | 4 | No | Unexplained |
| 11660.p1 | 1, 19 | NTNG1, CACNA1A | 546 | 472 | 3 | No | Unexplained |
| 11828.p1 | 7 | TRRAP | 381 | 371 | 1 | Yes | Unexplained |
| 12130.p1 | 20 | ADNP | 550 | 492 | 2 | Yes | Unexplained |
| 12603.p1 | 1, 1, 12 | DDI2 L1TD1 C12orf72 | 448 | 453 | 2 | Yes | Unexplained |
| 13335.p1 | 11,19 | ACP2 ZNF420 | 533 | 493 | 2 | Yes | Unexplained |
| 13494.p1 | 10 | NOLC1 | 372 | 345 | 1 | No | Absent ovulation |
| 13742.p1 | 12, 17 | CELA1 MYH10 | 348 | 344 | 2 | No | Absent ovulation |
| 12375.p1 | 9 | C9orf144B | 566 | 388 | 4 | No | Endometriosis |
| 13000.p1 | 6 | SLC17A3 | 358 | 332 | 2 | No | Unexplained |
| 13739.p1 | 11, 11 | CD151 MARK2 | 458 | 455 | 4 | Yes | Low hormone level |
| 13392.p1 | 15, 17, 17 | ITGA11 SLFN5 TUBG1 | 362 | 366 | 2 | No | Unexplained |
Results from the ANOVA in the subsample of individuals with CNV status indicated that assisted reproduction usage was associated with increased paternal (P<.000017) and maternal (P<.000014) age. Results from the ANOVA in the subsample of individuals with gene mutation status indicated a somewhat different pattern. No relation between assisted reproduction usage and paternal age was observed (P=.13) in this subsample, but a trend was observed suggesting that mothers of children with autism-associated gene-disrupting events using assisted reproduction were older than mothers not using assisted reproduction (P=.06).
Results from the logistic regression indicated that CNV status was not associated with paternal (P=.97) or maternal age (P=.73). However, results from the logistic regression in the subsample of individuals with gene mutation status indicated a different pattern. Although paternal age again did not reach statistical significance (P=.56), mothers of children with autism-associated gene-disrupting events were older than mothers of children without autism-associated gene-disrupting events (P=.03). This association is illustrated in Figure 1, with use of assisted reproduction included in the figure so as to demonstrate the impact of maternal age regardless of assisted reproduction.
Figure 1.
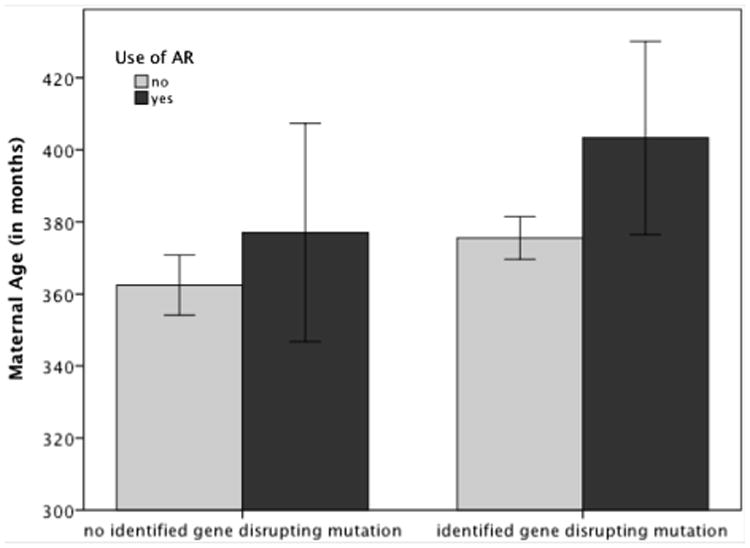
Impact of maternal age (error bars represent 95% confidence interval).
Discussion
Our study is the first to examine the genotype of patients with ASD who were conceived by assisted reproduction versus a control group in a relatively large sample. The purpose of this study was to examine the relations between assisted reproduction and genetic abnormalities in patients with ASD. Our results did not show an increased rate of autism-associated CNVs or autism-associated gene-disrupting events in children with ASD who were conceived by assisted reproduction versus children with ASD who had no contact with assisted reproduction. Further, autism-associated CNVs and autism-associated gene-disrupting events did not appear overrepresented within any particular subtype of assisted reproduction. Additionally, CNVs and gene mutations were seen across a wide variety of genomic loci.
If assisted reproduction was a significant independent risk factor for autism-associated genetic events, it would be expected that there would be a higher rate of these events in the group of children with ASD who were exposed to assisted reproduction. Additionally, it might be expected that if assisted reproduction leads to genetic events, there would be a specific type or pattern of genetic event that was seen. Neither was the case.
Increased paternal and maternal age was statistically significantly associated with use of assisted reproduction in the CNV group. In the subsample of individuals with gene mutation status, maternal age trended toward a significant association with use of assisted reproduction and was found to be a potential contributor. This variable reached statistical significance when comparing maternal age and presence of autism-associated gene-disrupting events in the ASD group exposed to assisted reproduction. As alluded to previously, it is possible that the association between assisted reproduction and ASD found in other reports is related to characteristics of the parents using assisted reproduction that increases ASD risk and not assisted reproduction itself. Specific to this report, it is possible that parental age–not assisted reproduction–is associated with an increased risk of autism-associated gene disruptions.
It was somewhat unexpected to see maternal age associated with gene mutations, given the already mentioned studies linking paternal age to autism-associated de novo CNV. However, the population studied in this report is more likely to have maternal age as a primary driver of the use of assisted reproduction. Indeed, the results above showed that in the group with gene mutation status no relation between assisted reproduction usage and paternal age was seen but a trend was observed suggesting that mothers using assisted reproduction were older than mothers not using assisted reproduction.
There are limitations to the conclusions that can be reached from these data. It is possible that some patients with ASD and assisted reproduction may not have developed ASD or any type of autism-associated genetic event had they not been exposed to assisted reproduction. Phrased differently, it is possible that ASD is an oligogenic disorder and that the group exposed to assisted reproduction will not have a different genotype than the group not exposed to assisted reproduction, but that in the group exposed to assisted reproduction it was assisted reproduction that caused the last mutation necessary to reach a pathological threshold. With that in mind, it would have been helpful to have a typically developing control group in this study. Additionally, although all currently known autism-associated genetic events were considered, there are likely to be more of these events discovered. Furthermore, although the study was sufficiently powered to look at assisted reproduction as a broad category of risk, it was not sufficiently powered to look at any subtype of assisted reproduction. With that acknowledged, as presented in Table 1, no type of assisted reproduction appeared grossly different from another in terms of CNV or mutation rates. Finally, this retrospective study did not incorporate medical records to verify parent report, which would have been ideal.
This study adds valuable information because it is the first to compare the genotype of patients with ASD who have also been exposed to assisted reproduction with patients with ASD who have not been exposed to assisted reproduction. In doing so, no association was found between rates of autism-associated genetic events and assisted reproduction, and no a major genotypic difference were found between patients with ASD exposed to assisted reproduction as compared to patients with ASD not exposed to assisted reproduction.
Conclusion
In this large sample, there was no increased rate of ASD-associated genetic events in ASD patients conceived by assisted reproduction compared with ASD patients conceived without assisted reproduction. Additionally, maternal age was identified as a potential contributor to ASD associated genetic events in the context of assisted reproduction. This study is the first on the subject to concurrently examine the genotype of individuals with ASD in relation to their exposure to assisted reproduction versus spontaneous conception and therefore adds valuable, reassuring evidence to the argument that assisted reproduction does not increase the risk of ASD.
Acknowledgments
Funded by the Simons Foundation Autism Research Initiative.
Footnotes
S.A. has nothing to disclose. J.W. has nothing to disclose. D.R. reports receiving royalties from WW Norton and Psychology Today. R.A. has nothing to disclose. R.B. has nothing to disclose.
References
- 1.Diagnostic and statistical manual of mental disorders. 5th. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 2.Blumberg S, Bramlett M, Kogan M, Schieve L, Jones J, Lu M. National Health Statistics Reports No 65. Hyattsville, MD: National Center for Health Statistics; 2013. Changes in prevalence of parent-reported autism spectrum disorder in school-aged U.S. children: 2007 to 2011–2012. [PubMed] [Google Scholar]
- 3.Betancur C. Etiological heterogeneity in autism spectrum disorders: more than 100 genetic and genomic disorders and still counting. Brain Res. 2011;1380:42–77. doi: 10.1016/j.brainres.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 4.O'Roak BJ, Vives L, Fu W, Egertson JD, Stanaway IB, Phelps IG, et al. Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science. 2012;338:1619–22. doi: 10.1126/science.1227764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landrigan PJ. What causes autism? Exploring the environmental contribution. Curr Opin Pediatr. 2010;22:219–25. doi: 10.1097/MOP.0b013e328336eb9a. [DOI] [PubMed] [Google Scholar]
- 6.Atladottir HO, Henriksen TB, Schendel DE, Parner ET. Autism after infection, febrile episodes, and antibiotic use during pregnancy: an exploratory study. Pediatrics. 2012;130:e1447–54. doi: 10.1542/peds.2012-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abel KM, Dalman C, Svensson AC, Susser E, Dal H, Idring S, et al. Deviance in fetal growth and risk of autism spectrum disorder. Am J Psychiatry. 2013;170:391–8. doi: 10.1176/appi.ajp.2012.12040543. [DOI] [PubMed] [Google Scholar]
- 8.Flashner BM, Russo ME, Boileau JE, Leong DW, Gallicano GI. Epigenetic factors and autism spectrum disorders. Neuromolecular Med. 2013;15:339–50. doi: 10.1007/s12017-013-8222-5. [DOI] [PubMed] [Google Scholar]
- 9.Millan MJ. An epigenetic framework for neurodevelopmental disorders: from pathogenesis to potential therapy. Neuropharmacology. 2013;68:2–82. doi: 10.1016/j.neuropharm.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. What is assisted reproductive technology? Available at: http://www.cdc.gov/art/. Updated April 4, 2013.
- 11.Allen VM, Wilson RD, Cheung A. Pregnancy outcomes after assisted reproductive technology. J Obstet Gynaecol Can. 2006;28:220–50. doi: 10.1016/S1701-2163(16)32112-0. [DOI] [PubMed] [Google Scholar]
- 12.Wilson CL, Fisher JR, Hammarberg K, Amor DJ, Halliday JL. Looking downstream: a review of the literature on physical and psychosocial health outcomes in adolescents and young adults who were conceived by ART. Hum Reprod. 2011;26:1209–19. doi: 10.1093/humrep/der041. [DOI] [PubMed] [Google Scholar]
- 13.Schieve LA, Rasmussen SA, Buck GM, Schendel DE, Reynolds MA, Wright VC. Are children born after assisted reproductive technology at increased risk for adverse health outcomes? Obstet Gynecol. 2004;103:1154–63. doi: 10.1097/01.AOG.0000124571.04890.67. [DOI] [PubMed] [Google Scholar]
- 14.Barnes J, Sutcliffe AG, Kristoffersen I, Loft A, Wennerholm U, Tarlatzis BC, et al. The influence of assisted reproduction on family functioning and children's socio-emotional development: results from a European study. Hum Reprod. 2004;19:1480–7. doi: 10.1093/humrep/deh239. [DOI] [PubMed] [Google Scholar]
- 15.Papaligoura Z, Panopoulou-Maratou O, Solman M, Arvaniti K, Sarafidou J. Cognitive development of 12 month old Greek infants conceived after ICSI and the effects of the method on their parents. Hum Reprod. 2004;19:1488–93. doi: 10.1093/humrep/deh270. [DOI] [PubMed] [Google Scholar]
- 16.Sutcliffe AG, Taylor B, Saunders K, Thornton S, Lieberman BA, Grudzinskas JG. Outcome in the second year of life after in-vitro fertilisation by intracytoplasmic sperm injection: a UK case-control study. Lancet. 2001;35:2080–4. doi: 10.1016/s0140-6736(00)05180-1. [DOI] [PubMed] [Google Scholar]
- 17.Ponjaert-Kristoffersen I, Bonduelle M, Barnes J, Nekkebroeck J, Loft A, Wennerholm UB, et al. International collaborative study of intracytoplasmic sperm injection-conceived, in vitro fertilization-conceived, and naturally conceived 5-year-old child outcomes: cognitive and motor assessments. Pediatrics. 2005;115:e283–9. doi: 10.1542/peds.2004-1445. [DOI] [PubMed] [Google Scholar]
- 18.Leunens L, Celestin-Westreich S, Bonduelle M, Liebaers I, Ponjaert-Kristoffersen I. Follow-up of cognitive and motor development of 10-year-old singleton children born after ICSI compared with spontaneously conceived children. Hum Reprod. 2008;23:105–11. doi: 10.1093/humrep/dem257. [DOI] [PubMed] [Google Scholar]
- 19.Mains L, Zimmerman M, Blaine J, Stegmann B, Sparks A, Ansley T, et al. Achievement test performance in children conceived by IVF. Hum Reprod. 2010;25:2605–11. doi: 10.1093/humrep/deq218. [DOI] [PubMed] [Google Scholar]
- 20.Agarwal P, Loh SK, Lim SB, Sriram B, Daniel ML, Yeo SH, et al. Two-year neuro developmental outcome in children conceived by intracytoplasmic sperm injection: prospective cohort study. BJOG. 2005;112:1376–83. doi: 10.1111/j.1471-0528.2005.00663.x. [DOI] [PubMed] [Google Scholar]
- 21.Sutcliffe AG, Saunders K, McLachlan R, Taylor B, Edwards P, Grudzinskas G, et al. A retrospective case-control study of developmental and other outcomes in a cohort of Australian children conceived by intracytoplasmic sperm injection compared with a similar group in the United Kingdom. Fertil Steril. 2003;79:512–6. doi: 10.1016/s0015-0282(02)04701-5. [DOI] [PubMed] [Google Scholar]
- 22.Conway DA, Patel SS, Liem J, Fan KJ, Jalian R, Williams J, 3rd, et al. The risk of cytogenetic abnormalities in the late first trimester of pregnancies conceived through assisted reproduction. Fertil Steril. 2011;95:503–6. doi: 10.1016/j.fertnstert.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 23.Shevell T, Malone FD, Vidaver J, Porter TF, Luthy DA, Comstock CH, et al. Assisted reproductive technology and pregnancy outcome. Obstet Gynecol. 2005;106(5 Pt 1):1039–45. doi: 10.1097/01.AOG.0000183593.24583.7c. [DOI] [PubMed] [Google Scholar]
- 24.Vermeiden JPW, Bernardus RE. Are imprinting disorders more prevalent after human in vitro fertilization or intracytoplasmic sperm injection? Fertil Steril. 2013;99:642–51. doi: 10.1016/j.fertnstert.2013.01.125. [DOI] [PubMed] [Google Scholar]
- 25.Wen J, Jiang J, Ding C, Dai J, Liu Y, Xia Y, et al. Birth defects in children conceived by in vitro fertilization and intracytoplasmic sperm injection: a meta-analysis. Fertil Steril. 2012;97:1331–1337. e1–4. doi: 10.1016/j.fertnstert.2012.02.053. [DOI] [PubMed] [Google Scholar]
- 26.Wang YA, Sullivan EA, Black D, Dean J, Bryant J, Chapman M. Preterm birth and low birth weight after assisted reproductive technology-related pregnancy in Australia between 1996 and 2000. Fertil Steril. 2005;83:1650–8. doi: 10.1016/j.fertnstert.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 27.McGovern PG, Llorens AJ, Skurnick JH, Weiss G, Goldsmith LT. Increased risk of preterm birth in singleton pregnancies resulting from in vitro fertilization-embryo transfer or gamete intrafallopian transfer: a meta-analysis. Fertil Steril. 2004;82:1514–20. doi: 10.1016/j.fertnstert.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 28.Schieve LA, Meikle SF, Ferre C, Peterson HB, Jeng G, Wilcox LS. Low and very low birth weight in infants conceived with use of assisted reproductive technology. N Engl J Med. 2002;346:731–7. doi: 10.1056/NEJMoa010806. [DOI] [PubMed] [Google Scholar]
- 29.Adamson GD, de Mouzon J, Lancaster P, Nygren KG, Sullivan E, Zegers-Hochschild F. World collaborative report on in vitro fertilization, 2000. Fertil Steril. 2006;85:1586–622. doi: 10.1016/j.fertnstert.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 30.Sandin S, Nygren KG, Iliadou A, Hultman CM, Reichenberg A. Autism and mental retardation among offspring born after in vitro fertilization. JAMA. 2013;310:75–84. doi: 10.1001/jama.2013.7222. [DOI] [PubMed] [Google Scholar]
- 31.Lehti V, Brown AS, Gissler M, Rihko M, Suominen A, Sourander A. Autism spectrum disorders in IVF children: a national case-control study in Finland. Hum Reprod. 2013;28:812–8. doi: 10.1093/humrep/des430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lyall K, Pauls DL, Spiegelman D, Santangelo SL, Ascherio A. Fertility therapies, infertility and autism spectrum disorders in the Nurses' Health Study II. Paediatr Perinat Epidemiol. 2012;26:361–72. doi: 10.1111/j.1365-3016.2012.01294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zachor DA, Ben Itzchak E. Assisted reproductive technology and risk for autism spectrum disorder. Res Dev Disabil. 2011;32:2950–6. doi: 10.1016/j.ridd.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 34.Hvidtjørn D, Grove J, Schendel D, Schieve LA, Svaerke C, Ernst E, et al. Risk of autism spectrum disorders in children born after assisted conception: a population-based follow-up study. J Epidemiol Community Health. 2011;65:497–502. doi: 10.1136/jech.2009.093823. [DOI] [PubMed] [Google Scholar]
- 35.Hvidtjørn D, Schieve L, Schendel D, Jacobsson B, Svaerke C, Thorsen P. Cerebral palsy, autism spectrum disorders, and developmental delay in children born after assisted conception: a systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2009;163:72–83. doi: 10.1001/archpediatrics.2008.507. [DOI] [PubMed] [Google Scholar]
- 36.O′Roak BJ, Vives L, Girirajan S, Karakoc E, Krumm N, Coe BP, et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature. 2012;485:246–50. doi: 10.1038/nature10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kong A, Frigge ML, Masson G, Besenbacher S, Sulem P, Magnusson G, et al. Rate of de novo mutations and the importance of father's age to disease risk. Nature. 2012;488:471–5. doi: 10.1038/nature11396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Conti E, Mazzotti S, Calderoni S, Saviozzi I, Guzzetta A. Are children born after assisted reproductive technology at increased risk of autism spectrum disorders? A systematic review. Hum Reprod. 2013;28:3316–27. doi: 10.1093/humrep/det380. [DOI] [PubMed] [Google Scholar]
- 39.Melnyk S, Fuchs GJ, Schulz E, Lopez M, Kahler SG, Fussell JJ, et al. Metabolic imbalance associated with methylation dysregulation and oxidative damage in children with autism. J Autism Dev Disord. 2012;42:367–77. doi: 10.1007/s10803-011-1260-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fischbach GD, Lord C. The Simons Simplex Collection: a resource for identification of autism genetic risk factors. Neuron. 2010;68:192–5. doi: 10.1016/j.neuron.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 41.Diagnostic and statistical manual of mental disorders. IV-TR. Washington, DC: American Psychiatric Publishing; 2000. [Google Scholar]
- 42.Girirajan S, Rosenfeld JA, Coe BP, Parikh S, Friedman N, Goldstein A, et al. Phenotypic heterogeneity of genomic disorders and rare copy-number variants. N Engl J Med. 2012;367:1321–31. doi: 10.1056/NEJMoa1200395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanders SJ, Murtha MT, Gupta AR, Murdoch JD, Raubeson MJ, Willsey AJ, et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature. 2012;485:237–41. doi: 10.1038/nature10945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanders SJ, Ercan-Sencicek AG, Hus V, Luo R, Murtha MT, Moreno-De-Luca D, et al. Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron. 2011;70:863–85. doi: 10.1016/j.neuron.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


