Key Points
Question
What are the associations of maternal obesity with fetal growth, and when do these associations start emerging?
Findings
In a longitudinal cohort study that included 468 obese and 2334 nonobese pregnant women, the femur length, humerus length, head circumference, and estimated weight of fetuses were significantly greater in the fetuses of obese women compared with fetuses of nonobese women. Differences commenced as early as 21 weeks’ gestation.
Meaning
Fetal growth differs in obese and nonobese pregnant women; the mechanisms of these findings and their long-term implications for child and adult health remain to be established.
This longitudinal cohort study characterizes the differences in fetal growth in cohorts of obese and nonobese pregnant women and identifies when anthropometric differences emerge.
Abstract
Importance
Despite the increasing prevalence of pregravid obesity, systematic evaluation of the association of maternal obesity with fetal growth trajectories is lacking.
Objective
To characterize differences in fetal growth trajectories between obese and nonobese pregnant women, and to identify the timing of any observed differences.
Design, Setting, and Participants
The Eunice Kennedy Shriver National Institute of Child Health and Human Development Fetal Growth Studies–Singletons study enrolled cohorts of pregnant women at 12 US health care institutions. Obese women (with prepregnancy body mass index > 30) and nonobese women (prepregnancy body mass indexes, 19-29.9) without major chronic diseases were recruited between 8 weeks and 0 days’ gestation and 13 weeks and 6 days’ gestation. A mixed longitudinal randomization scheme randomized participants into 1 of 4 schedules for 2-dimensional and 3-dimensional ultrasonograms to capture weekly fetal growth data throughout the remainder of their pregnancies.
Main Outcomes and Measures
On each ultrasonogram, fetal humerus length, femur length, biparietal diameter, head circumference, and abdominal circumference were measured. Fetal growth curves were estimated using linear mixed models with cubic splines. Median differences in the fetal measures at each gestational week of the obese and nonobese participants were examined using the likelihood ratio and Wald tests after adjustment for maternal characteristics.
Results
The study enrolled 468 obese and 2334 nonobese women between 8 weeks and 0 days’ gestation and 13 weeks and 6 days’ gestation. After a priori exclusion criteria, 443 obese and 2320 nonobese women composed the final cohort. Commencing at 21 weeks’ gestation, femur length and humerus length were significantly longer for fetuses of obese woman than those of nonobese women. Differences persisted in obese and nonobese groups through 38 weeks’ gestation (median femur length, 71.0 vs 70.2 mm; P = .01; median humerus length, 62.2 vs 61.6 mm; P = .03). Averaged across gestation, head circumference was significantly larger in fetuses of obese women than those of nonobese women (P = .02). Fetal abdominal circumference was not greater in the obese cohort than in the nonobese cohort but was significantly larger than in fetuses of normal-weight women (with body mass indexes between 19.0-24.9) commencing at 32 weeks (median, 282.1 vs 280.2 mm; P = .04). Starting from 30 weeks’ gestation, estimated fetal weight was significantly larger for the fetuses of obese women (median, 1512 g [95% CI, 1494-1530 g] vs 1492 g [95% CI, 1484-1499 g]) and the difference grew as gestational age increased. Birth weight was higher by almost 100 g in neonates born to obese women than to nonobese women (mean, 3373.2 vs 3279.5 g).
Conclusions and Relevance
As early as 32 weeks’ gestation, fetuses of obese women had higher weights than fetuses of nonobese women. The mechanisms and long-term health implications of these findings are not yet established.
Introduction
Obesity, including in women of reproductive age, has become a global epidemic. Data from the 2011/2012 National Health and Nutrition Examination Survey (NHANES) reported that 32% of reproductive-aged US women have a body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) of 30 or higher and are considered obese. Moreover, approximately 45% of US women were either overweight or obese (BMI 25 or higher) when becoming pregnant. Obese pregnant women are at higher risk for several common pregnancy complications, such as gestational diabetes, gestational hypertension, and preeclampsia. In addition, their fetuses are exposed to an unfavorable in utero metabolic environment. Emerging evidence from both animal and human experimental studies has demonstrated that increased levels of chronic inflammation, oxidative stress, insulin resistance, and glucose related to maternal obesity create an environment that can lead to altered fetal growth. Additional data from human epidemiologic studies corroborate that fetuses born to obese women are at greater risk for overgrowth, which may predispose them to obesity and cardiometabolic disorders later in life.
Most previous studies on maternal obesity and fetal growth were based on birth size and did not assess the longitudinal pattern of in utero fetal growth. Longitudinal and systematic evaluation of differences in growth trajectories across gestation by maternal obesity status, as measured by serial ultrasonography, is lacking. Moreover, it is unknown when differences in fetal growth might arise or persist. This information is particularly relevant in designing gestation-specific interventions that might reduce the effect of maternal obesity on fetal growth. Further, because obese women often experience coexisting morbidities such as diabetes and hypertension, it is important to determine whether the association of maternal obesity with fetal growth is independent of these comorbid conditions. In response to these critical data gaps, we sought to determine whether fetal growth differs by maternal prepregnancy obesity status by analyzing 2-dimensional serial ultrasonogramss taken throughout pregnancy from the cohort of women enrolled in the National Institute of Child Health and Human Development (NICHD) Fetal Growth Studies–Singletons. Moreover, we sought to identify the timing and duration of observed differences.
Methods
Study Design
The NICHD Fetal Growth Studies–Singletons comprised 2 cohorts of pregnant women: those with singleton pregnancies and dichorionic twin pregnancies. Women in the singleton cohort (n = 2802) were aged 18 to 40 years, had a viable singleton pregnancy, and planned to deliver at participating hospitals; the group included 468 obese women (ie, those with a prepregnancy BMI of 30.0 to 44.9) and 2334 nonobese women (ie, those with a prepregnancy BMI of 19.0 to 29.9). Women were recruited from 12 US clinical sites between July 2009 and January 2013 and were followed up through delivery. After enrollment, 2585 women (92%) completed the protocol.
Both obese and nonobese pregnant women were excluded if they reported having major chronic conditions before pregnancy (autoimmune diseases, cancer, diabetes, chronic hypertension requiring 2 or more medications, renal disease requiring medical supervision, HIV/AIDS, or psychiatric disorders). In addition, nonobese women were excluded if they had epilepsy or seizures requiring medication, hematologic disorders, asthma, or thyroid disease. Both obese and nonobese women were excluded if they became pregnant with the use of ovulation stimulation drugs or assisted reproductive technology, or if they reported having smoked tobacco in the prior 6 months, having used illicit drugs in the past year, or having consumed 1 or more alcoholic drinks per day at the time of enrollment. In these analyses, 14 nonobese women and 25 obese women were identified as ineligible after enrollment and excluded from the final analytical population. Therefore, the final analytic study population included 443 obese and 2320 nonobese women.
Approval for human subjects research was obtained from the institutional review boards at all participating sites, and women gave informed consent before enrollment. Further details have been previously reported.
Exposure
Participants completed a screening and enrollment visit between 8 weeks and 0 days’ gestation and 13 weeks and 6 days’ gestation. During this visit, prepregnancy BMI was calculated based on self-reported weight and height. Self-reported weights and heights were highly correlated with the weights and heights subsequently measured by study personnel during the enrollment visit (correlation coefficient r = 0.97 for weight; r = 0.95 for height). Women were classified as obese, overweight, or normal weight if their prepregnancy BMI was 30.0 or greater, 25 to 29.99, or 18.5 to 24.9, respectively; both overweight and normal-weight women were considered nonobese.
Covariates
During the enrollment visit, research nurses interviewed women about diet and lifestyle; sociodemographic characteristics; and medical, reproductive, and pregnancy histories. In follow-up study visits, women were also interviewed about any changes in diet, lifestyle, and medical conditions. Maternal weight was measured at every postenrollment study visit via a standardized protocol.
Outcomes
Following the baseline enrollment visit and ultrasonography, women were randomized to 1 of the following 4 schedules for additional ultrasonographies: 16, 20, 24, 28, 32, and 35 weeks (schedule A); 18, 22, 26, 30, 34, and 36 weeks (schedule B); 20, 28, 32, 36, and 40 weeks (schedule C); and 22, 29, 33, 37, and 41 weeks (schedule D). By design, this mixed longitudinal randomization scheme captured fetal growth data for each week of pregnancy without exposing women to weekly ultrasonographic examinations. Study visits were scheduled within 1 week of the targeted gestational age to accommodate women’s availability.
At each ultrasonographic examination, fetal biometric measurements were performed following standard operating procedures and using identical equipment (Voluson E8; GE Healthcare). All measurements and images were captured in ViewPoint (GE Healthcare) and electronically transferred to the study’s imaging data coordination center. Longitudinal measurements were taken for humerus length, femur length, and biparietal diameter from the outer to the inner edge of the calvarial wall using the linear function and for head circumference and abdominal circumference using the ellipse function. The study’s central ultrasonographic unit credentialed all participating ultrasound technicians throughout the study. Furthermore, intraobserver correlations for all fetal growth ultrasonographic measurements were greater than 0.99 in both obese and nonobese women; no differences in the quality of data were found by maternal obesity.
Within 24 hours of delivery, neonatal anthropometric measurements, including birth weight and length, were completed. Newborns were classified as large for gestational age (LGA) if birth weight was greater than or equal to the 90th percentile for birth weight; small for gestational age if birth weight was less than the 10th percentile for birth weight, and macrosomic if birth weight was equal or greater than 4000 g. Estimated fetal weight (EFW) was calculated using the Hadlock formula, which incorporates head circumference, abdominal circumference, and femur length. Newborns were classified as having idiopathic fetal growth restriction if their EFW was less than 10% the EFW of the standard (nonobese) cohort in this study. The ratio of head circumference to abdominal circumference and femur length to abdominal circumference were calculated for examination of trends in growth patterns and body proportions over time.
Statistical Analysis
Characteristics of study participants were presented with means with standard deviation (SD) for continuous variables and as percentages for categorical variables. Differences in characteristics between obese and nonobese women were assessed by t test for continuous variables and by χ2 test for categorical variables. All serial ultrasonographic data were used to estimate individual fetal parameters, ratios of head circumference to abdominal circumference, ratios of femur length to abdominal circumference, and EFW by gestational age. Ultrasonographic measurements (biparietal diameter, head circumference, abdominal circumference, humerus length, and femur length), ratio of head circumference to abdominal circumference, ratio of femur length to abdominal circumference, and EFW were log-transformed to stabilize variances across gestational age and to improve normal approximations for the error structures used in statistical analysis.
The primary analysis compared fetal growth trajectories by maternal obesity status regardless of pregnancy complications or neonatal conditions. For modeling growth trajectories among obese women, we used linear mixed models with a cubic spline mean structure and a random-effects structure that included linear, quadratic, and cubic random effects and an intercept term for the individual fetus. A polynomial random-effects structure was chosen to provide flexibility in modeling between variation in the fetal growth trajectories of individual participants. Linear mixed models were also used to test between obese and nonobese groups for overall differences in fetal growth trajectories in EFW and other anthropometric variables. These used likelihood ratio tests of interaction terms between spline mean structure terms and obese-nonobese indicator variables. For the cubic spline mean structure, 3-knot points (25th, 50th, and 75th percentiles) were chosen at gestational ages that evenly split the distributions.
For an overall comparison of fetal growth trajectories between obese and nonobese women, the nonobese women were weighted to have the same distribution of race/ethnicity as in the obese cohort. For anthropometric parameters that were significantly different between obese and nonobese cohorts, we further tested for week-specific differences using Wald tests computed at each gestational age. These tests were conducted on the estimated curves with and without adjustments for the following maternal characteristics: age, race/ethnicity, parity, full-time employment/student status (yes/no), marital status (married/cohabitating vs single), health insurance (private/managed care vs Medicaid/other), annual income (less than $29 999, $30 000-$49 999, $50 000-$74 999, $75 000-$99 999, and more than $100 000), education (less than high school, high school, some college, undergraduate degree, and postgraduate degree) and infant sex (male or female). When performing covariate-adjusted tests for week-specific differences in fetal growth curves, we used a procedure of multiple imputation (with 20 imputations) to account for missing covariate data. Similar analysis methods were applied in the secondary analyses, which tested the difference in fetal growth measures between obese and normal-weight women (ie, those with BMIs between 18.5 and 24.9).
To assess the robustness of the findings, we performed a sensitivity analysis limited to fetuses from women without major obstetric complications associated with abnormal fetal growth, such as gestational diabetes, gestational hypertension, preeclampsia, and preterm delivery. By design, nonobese women were excluded post hoc from the analysis if they had additional chronic medical conditions (epilepsy or seizures requiring medication, hematologic disorders, asthma, or thyroid disease); the exclusion was not applied to obese women. We conducted a sensitivity analysis by excluding 16 obese women with the medical conditions that had been exclusion criteria for the nonobese cohort.
All analyses were implemented using SAS, version 9.4 (SAS Institute Inc) or R, version 3.1.2 (R Development Core Team). A 2-tailed P value less than .05 defined significance.
Results
In general, obese women had lower educational attainment and were more likely to be single, unemployed, and residing in households in lower income brackets than nonobese women (Table 1). The height of obese women was similar to that of nonobese women (mean, 162.8 cm vs 162.5 cm). Moreover, obese women were more likely to develop gravid diseases, such as gestational diabetes (8.4% vs 3.7%; P < .001), gestational hypertension (11.3% vs 3.0%; P < .001), and preeclampsia (7.4% vs 3.4%; P < .001), and to have a cesarean delivery than nonobese women (34.1% vs 25.2%; P < .001). Although the mean gestational age at delivery for obese women was similar to that of nonobese women (39.0 weeks vs 39.2 weeks; P = .29), infants born to obese women had heavier birth weights by almost 100 g (mean, 3373 vs 3279 g; P < .001) and were more likely to be LGA (11.7% vs 8.3%, P = .03). No significant differences were observed based on obese status for male sex, fetal growth restriction, or small-for-gestational age status.
Table 1. Characteristics of Study Participants by Prepregnancy Obesity Status.
| Characteristic | No. (%) | P Value | |
|---|---|---|---|
| Obese Womena | Nonobese Womenb | ||
| Maternal | |||
| Sample size, No. | 443 | 2320 | .11 |
| Age, mean (SD), y | 27.8 (5.6) | 28.2 (5.5) | <.001 |
| Race/ethnicity | |||
| Non-Hispanic white | 123 (37.8) | 610 (26.3) | |
| Non-Hispanic black | 165 (36.3) | 605 (26.1) | |
| Hispanic | 150 (33.9) | 646 (27.8) | |
| Asian/Pacific Islander | 5 (1.1) | 459 (19.8) | |
| Prepregnancy weight, mean (SD), kg | 91.5 (13.0) | 62.5 (9.6) | <.001 |
| Height, mean (SD), cm | 162.8 (6.9) | 162.5 (7.1) | .46 |
| Prepregnancy BMI, mean (SD)a | 34.5 (3.9) | 23.6 (3.1) | <.001 |
| Parity | <.001 | ||
| 0 | 158 (35.6) | 1143 (49.3) | |
| 1 | 145 (32.4) | 788 (34.0) | |
| 2 | 79 (18.1) | 277 (11.9) | |
| ≥3 | 81 (13.8) | 62 (4.8) | |
| Never married | 125 (28.2) | 498 (21.5) | <.001 |
| Education | <.001 | ||
| <High school | 70 (15.8) | 252 (10.9) | |
| High school/GED | 107 (24.2) | 401 (17.3) | |
| Some college/associate degree | 154 (35.8) | 677 (29.2) | |
| College undergraduate degree | 77 (17.7) | 562 (24.2) | |
| Postgraduate degree | 35 (8.2) | 427 (18.4) | |
| Private health insurance | 209 (51.0) | 1239 (57.6) | .03 |
| Pregnancy complications | <.001 | ||
| Preeclampsia | 33 (7.4) | 78 (3.4) | <.001 |
| Gestational hypertension | 50 (11.3) | 70 (3.0) | <.001 |
| Gestational diabetes | 37 (8.4) | 85 (3.7) | <.001 |
| Placenta abruption | 5 (1.1) | 29 (1.3) | .83 |
| Cesarean delivery | 151 (34.1) | 585 (25.2) | <.001 |
| Neonatal | |||
| Gestational age at delivery, mean (SD), wk | 39.0 (2.3) | 39.2 (1.8) | .29 |
| Birth weight, mean (SD), g | 3373.2 (484.4) | 3279.5 (466.7) | <.001 |
| Male | 204 (47.7) | 1104 (51.9) | .13 |
| Macrosomiab | 35 (7.9) | 118 (5.1) | .04 |
| Large for gestational age | 47 (11.7) | 176 (8.3) | .03 |
| Small for gestational age | 26 (6.5) | 190 (9.0) | .10 |
| IUGR | 5 (1.1) | 38 (1.8) | .43 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); GED, General Educational Development; IUGR, idiopathic intrauterine growth restriction: estimated fetal weight <10% of normal.
As estimated from self-reported pregravid weight and height at enrollment; obese women have BMI of 30 or more; nonobese women have BMIs of 19 to 29.99.
Macrosomia: birth weight ≥4000 g.
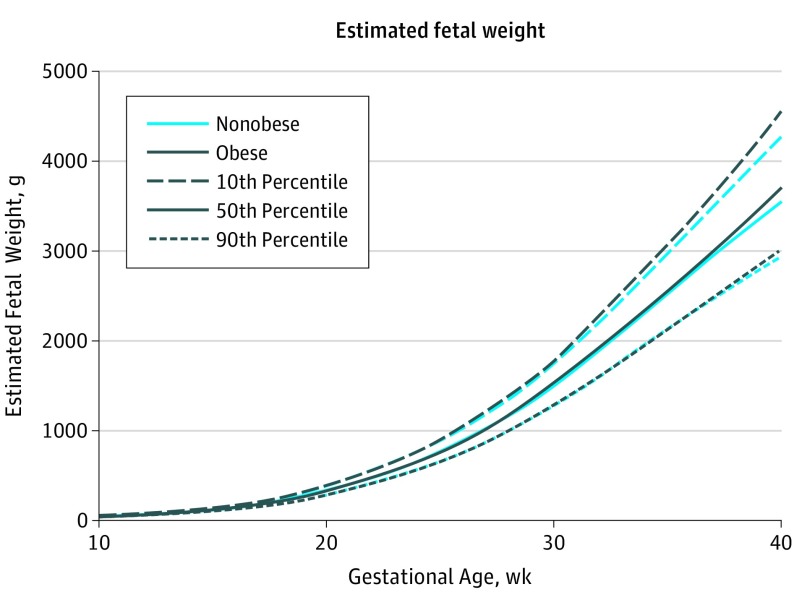
Fetal growth curves for EFW for obese and nonobese women, including the 10th, 50th, and 90th percentiles, are presented in Figure 1 and illustrate important differences. Corresponding EFWs by maternal adiposity status (ie, obese, all nonobese women, overweight women, and normal-weight women) are provided in Table 2. Starting from 30 weeks’ gestation through 38 weeks’ gestation (at which point a large percentage of the women had delivered), the EFW of fetuses born to obese women were significantly and progressively larger than those of nonobese women. Specifically, at 30 weeks, the median EFW for fetuses of obese women was 20 g heavier than for fetuses of all nonobese women (1512 vs 1492 g; P = .04) and 28 g heavier than the fetuses of normal-weight women (1512 vs 1484 g; P = .05). At 38 weeks’ gestation, this difference between obese and nonobese cohorts increased to 71 g (3217 vs 3146 g; P = .01) at the median and to 98 g (3217 vs 3119 g; P < .001) between obese and normal-weight women.
Figure 1. Distribution of Estimated Fetal Weight by Gestational Age and Prepregnancy Obesity Status.
Estimated fetal weight for singletons by prepregnancy obesity status, as estimated from linear mixed models with log-transformed outcomes and cubic splines.
Table 2. Geometric Mean and 95% Confidence Intervals for Estimated Fetal Weight (Grams) by Gestational Age and Maternal Prepregnancy Adiposity Status.
| Gestation Week | Estimated Fetal Weight, mean (95% CI), g | |||
|---|---|---|---|---|
| Obesea | Nonobese (All)b | Normal Weightc | Overweightd | |
| 11 | 43.9 (43.0-44.7) | 43.9 (43.6-44.2) | 43.9 (43.5-44.3) | 43.8 (43.2-44.5) |
| 12 | 54.8 (54.1-55.5) | 55.0 (54.7-55.3) | 55.1 (54.7-55.4) | 54.8 (54.3-55.4) |
| 13 | 68.9 (68.1-69.7) | 69.3 (68.9-69.6) | 69.4 (69.0-69.8) | 69.0 (68.4-69.6) |
| 14 | 87.0 (85.9-88.1) | 87.5 (87.1-88.0) | 87.7 (87.1-88.2) | 87.2 (86.4-88.1) |
| 15 | 110.1 (108.7-111.5) | 110.6 (110.0-111.2) | 110.7 (110.0-111.5) | 110.3 (109.3-111.4) |
| 16 | 139.0 (137.2-140.7) | 139.4 (138.7-140.2) | 139.6 (138.7-140.5) | 139.2 (137.9-140.5) |
| 17 | 174.6 (172.5-176.7) | 174.9 (174.0-175.8) | 175.0 (173.9-176.1) | 174.7 (173.1-176.2) |
| 18 | 217.6 (215.0-220.3) | 217.6 (216.5-218.7) | 217.7 (216.4-219.1) | 217.4 (215.5-219.3) |
| 19 | 268.3 (265.1-271.6) | 268.0 (266.6-269.4) | 268.2 (266.5-269.9) | 267.7 (265.3-270.1) |
| 20 | 327.1 (323.1-331.1) | 326.4 (324.8-328.1) | 326.7 (324.6-328.7) | 326.0 (323.1-328.9) |
| 21 | 394.4 (389.7-399.2) | 393.5 (391.5-395.5) | 393.7 (391.2-396.1) | 393.0 (389.6-396.5) |
| 22 | 471.1 (465.5-476.7) | 469.7 (467.3-472.1) | 469.8 (467.0-472.7) | 469.4 (465.4-473.5) |
| 23 | 557.6 (551.1-564.2) | 555.7 (552.9-558.5) | 555.7 (552.3-559.1) | 555.8 (551.0-560.6) |
| 24 | 654.7 (647.1-662.5) | 652.0 (648.8-655.3) | 651.7 (647.8-655.7) | 652.8 (647.2-658.4) |
| 25 | 763.1 (754.1-772.3) | 759.5 (755.6-763.3) | 758.6 (754.0-763.3) | 761.2 (754.6-767.9) |
| 26 | 883.7 (873.0-894.5) | 878.6 (874.1-883.2) | 877.1 (871.6-882.6) | 882.0 (874.1-889.9) |
| 27 | 1017.6 (1005.2-1030.2) | 1010.5 (1005.2-1015.9) | 1007.9 (1001.5-1014.3) | 1016.1 (1006.9-1025.4) |
| 28 | 1166.1 (1151.9-1180.6) | 1156.2 (1150.1-1162.3) | 1152.0 (1144.7-1159.3) | 1164.8 (1154.3-1175.4) |
| 29 | 1330.8 (1314.8-1347.1) | 1316.7 (1309.9-1323.6) | 1310.6 (1302.3-1318.9) | 1329.3 (1317.5-1341.3) |
| 30 | 1511.7 (1493.6-1530.0)e | 1492.1 (1484.4-1499.9) | 1483.7 (1474.4-1493.1) | 1509.4 (1496.0-1523.0) |
| 31 | 1707.3 (1686.4-1728.4)e | 1681.2 (1672.3-1690.2) | 1670.4 (1659.7-1681.1) | 1703.8 (1688.2-1719.5) |
| 32 | 1915.0 (1890.7-1939.7)e | 1882.1 (1871.8-1892.5) | 1868.7 (1856.4-1881.1) | 1910.1 (1892.1-1928.3) |
| 33 | 2131.1 (2103.2-2159.4)e | 2091.8 (2080.0-2103.6) | 2075.9 (2061.9-2090.0) | 2125.2 (2104.5-2146.0) |
| 34 | 2350.3 (2319.2-2381.7)e | 2306.3 (2293.2-2319.5) | 2288.3 (2272.7-2304.0) | 2344.5 (2321.4-2367.7) |
| 35 | 2566.9 (2532.7-2601.6)e | 2521.2 (2506.7-2535.8) | 2501.5 (2484.3-2518.8) | 2562.9 (2537.4-2588.6) |
| 36 | 2780.9 (2741.9-2820.5)e | 2733.7 (2717.3-2750.1) | 2712.4 (2693.0-2731.9) | 2778.4 (2749.4-2807.6) |
| 37 | 2995.9 (2951.3-3041.2)e | 2942.2 (2923.7-2960.8) | 2918.9 (2897.0-2941.0) | 2990.9 (2958.0-3024.2) |
| 38 | 3217.4 (3168.0-3267.6)e | 3145.7 (3125.4-3166.2) | 3119.2 (3095.1-3143.4) | 3201.4 (3165.0-3238.1) |
Abbreviation: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared).
Obese women: BMI, 30 or more.
Nonobese women: BMI, 19-29.9.
Normal-weight women: BMI, less than 25.
Overweight women: BMI, 25-29.9.
Indicates a statistically significant mean difference in estimated fetal weight values between obese vs nonobese groups and between obese vs normal weight groups (P < .05).
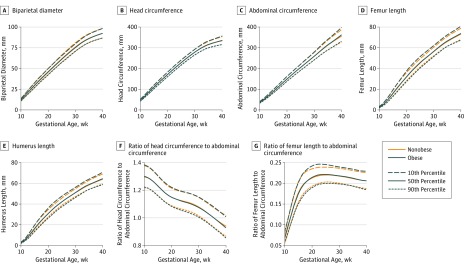
The curves for individual fetal anthropometric measurements are presented in Figure 2. Corresponding results are provided in eTables 1-7 in the Supplement. The fetuses of obese women had longer femur and humerus lengths, larger head circumferences, and larger femur length to abdominal circumference ratios than the fetuses of nonobese women (P < .05). More specifically, commencing at 21 weeks’ gestation, both femur and humerus lengths were longer in the fetuses of obese women than in those of nonobese women, even after covariate adjustment. Such differences persisted through 38 weeks’ gestation (median, 71.3 vs 70.2 mm for femur length and 62.2 vs 61.6 mm for humerus length). Head circumference was also larger in the fetuses of obese woman than the fetuses of nonobese women, most significantly between 33 and 35 weeks. The fetuses of obese women had greater femur length to abdominal circumference ratios than did fetuses of nonobese women, although significant differences were restricted to time spans in early pregnancy (at 11 weeks), midpregnancy (19 to 26 weeks) and late pregnancy (31 to 35 weeks). Overall, mean abdominal circumference did not differ significantly between obese and nonobese cohorts. However, when being compared with normal-weight women, average fetal abdominal circumference was significantly larger for obese women, starting from 32 weeks’ gestation (median, 282.1 vs 280.2 mm) through 38 weeks’ gestation (median, 341.9 vs 338.3 mm). Overall fetal biparietal diameter was not statistically different in fetuses of obese women compared with those of either nonobese or normal-weight women.
Figure 2. Distribution of Fetal Anthropometric Measurements by Gestational Age and Obesity Status.
Estimated fetal anthropometric parameters for obese and nonobese women, as estimated from linear mixed models with log-transformed outcomes and cubic splines.
To evaluate whether the association of obesity with fetal growth was independent of gravid complications particularly related to fetal growth (gestational diabetes, gestational hypertension, and preeclampsia), we restricted our analyses to women without these conditions. The results observed were similar. For instance, humerus length remained significantly longer in fetuses of obese women compared with nonobese women commencing at 20 weeks’ gestation even after covariate adjustment. Such differences persisted through 40 weeks’ gestation (median, 64.7 vs 64.2 mm for humerus length; adjusted global test P = .003). Similarly, from 24 weeks’ gestation through 39 weeks’ gestation, femur length was significantly longer in fetuses of obese women compared with nonobese women (median, 72.5 vs 71.9 mm for femur length at 39 weeks’ gestation; adjusted global test P = .04).
We also investigated whether there was a sex-specific association between maternal obesity and fetal growth by stratifying the cohort by fetal sex and performing tests of interaction by model fitting. No statistically significant interaction was observed.
Discussion
In this prospective longitudinal study, we observed significant differences in fetal growth between obese and nonobese pregnant women without preexisting major chronic conditions. To our knowledge, our study is the first to demonstrate that femur and humerus lengths in the fetuses of obese women were significantly longer than in the fetuses of nonobese women, starting from 21 weeks’ gestation, extending through the rest of pregnancy, and persisting even after adjustments for sociodemographic differences between obese and nonobese cohorts. Furthermore, the differences in femur and humerus lengths were not accounted for by an increased risk of common obesity-associated gravid diseases. Overall, head circumference was also greater in fetuses of obese women, with the difference being significant between 33 and 35 weeks. Abdominal circumference of the fetuses of obese women didn’t differ significantly from those of nonobese women, but this measurement was significantly greater than normal-weight women starting from 32 weeks’ gestation onward. Overall, EFW among obese women was significantly and progressively larger starting at 30 weeks’ gestation, and the difference became larger with increasing gestational age. At delivery, neonates of obese women were significantly heavier and more likely to be LGA.
Our findings of significantly larger EFWs for fetuses of obese women compared with nonobese women are generally consistent with findings from the Generation R Study. In this study conducted in the Netherlands, prepregnancy BMI was significantly and positively related to estimated fetal weight from midpregnancy onward. Our finding that a significant elevated risk of LGA is associated with maternal obesity is generally in line with findings from additional previous studies. However, previous research on maternal obesity and fetal growth has relied mainly on birth weight. Data on the relation between other fetus anthropometric measures and maternal adiposity were not reported in the Generation R study, and studies with longitudinal ultrasonographic measures of fetal growth are scant.
In addition, major chronic conditions, such as diabetes, that disproportionately affect obese women have not been fully considered in previous studies, making it hard to determine whether the elevated risks of LGA are related to these complications or to the excess maternal BMI per se. Because our cohort of obese women was free of many preexisting major chronic conditions, we were able to assess the associations specifically with obesity.
A potential mechanism for fetal growth might be related to the greater insulin resistance and fetal glucose exposure that is found in obese women. However, the precise underlying mechanisms remain unclear. The development of insulin resistance is a physiologic goal of pregnancy, because it provides nutrients to a growing fetus. Women who are obese at conception are more insulin resistant, which may lead to overnutrition of the fetus and overgrowth at birth. Maternal prepregnancy obesity not only reflects maternal insulin resistance but also other maternal characteristics, including maternal overnutrition, fat accumulation, and low-grade systemic inflammation. All these conditions either independently or in combination may lead to adverse programming effects in the fetuses.
The prospective longitudinal measurement of fetal growth and implementation of a standardized ultrasonography protocol to ensure high-quality measurements are major strengths of this study. Furthermore, the quality of ultrasonographic data was excellent and did not vary by maternal obesity status, because intraobserver correlations for all fetal growth measures were greater than 0.99 in both obese and nonobese women. Other strengths include weekly measurement of fetal growth across pregnancy, the a priori credentialing of ultrasonography technicians, and attention to covariate adjustment. In addition, this prospective cohort study was conducted in a multiracial cohort from across the United States who lacked major chronic conditions such as hypertension, diabetes, and cardiovascular disease. This aspect is essential to help tease apart the effect of these obesity-related chronic conditions from the impact of prepregnancy obesity alone.
Limitations
Some potential limitations of the present study merit discussions. The study methods may limit the generalizability of these findings to pregnant women without chronic conditions. As in other observational studies, measurement errors or residual confounding cannot be entirely eliminated. While prepregnancy obesity (BMI) was our primary exposure for analysis, it was self-reported by women on recruitment into the cohort; however, self-reported weight was highly correlated with measured maternal weight (r = 0.97) in this and in other US populations. Lastly, there were some differences in the exclusion criteria pertaining to maternal preexisting chronic conditions between the 2 cohorts, to ensure a sufficient number of obese women were included. This underscores the need for careful interpretation of the results. However, in sensitivity analyses that excluded obese women with chronic conditions, our findings remained the same.
Conclusions
In this prospective longitudinal study among US pregnant women, we observed that fetuses born to obese women had longer bone lengths and head circumference than those born to nonobese women. Such differences commenced as early as 21 weeks’ gestation and remained through delivery. Moreover, starting from 32 weeks’ gestation, fetuses had greater abdominal circumference than those of normal-weight women. The mechanisms for and long-term health implications of these differences remain unknown. Future work integrating measurements of neonatal body composition with long-term follow-up are warranted. Such data will also be relevant in informing the design of gestation-specific interventions for obese women to optimize fetal growth and health outcomes.
eTable 1. Geometric mean and 95% confidence intervals for Biparietal diameters (mm) by gestational age and maternal pre-pregnancy adiposity status, NICHD Fetal Growth Studies–Singletons.
eTable 2. Geometric mean and 95% confidence intervals for head circumference (mm) by gestational age and maternal pre-pregnancy adiposity status, NICHD Fetal Growth Studies–Singletons.
eTable 3. Geometric mean and 95% confidence intervals for abdominal circumference (mm) by gestational age and maternal pre-pregnancy adiposity status, NICHD Fetal Growth Studies–Singletons.
eTable 4. Geometric mean and 95% confidence intervals for femur length (mm) by gestational age and maternal pre-pregnancy adiposity status, NICHD Fetal Growth Studies–Singletons.
eTable 5. Geometric mean and 95% confidence intervals for humerus length (mm) by gestational age and maternal pre-pregnancy adiposity status, NICHD Fetal Growth Studies–Singletons.
eTable 6. Geometric mean and 95% confidence intervals for ratio: head circumference/abdominal circumference by gestational age and maternal pre-pregnancy adiposity status, NICHD Fetal Growth Studies–Singletons.
eTable 7. Geometric mean and 95% confidence intervals for ratio: femur length/abdominal circumference by gestational age and maternal pre-pregnancy adiposity status, NICHD Fetal Growth Studies–Singletons.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity among adults: United States, 2011-2012. NCHS Data Brief. 2013;(131):1-8. [PubMed] [Google Scholar]
- 2.Gunderson EP. Childbearing and obesity in women: weight before, during, and after pregnancy. Obstet Gynecol Clin North Am. 2009;36(2):317-332, ix. ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelson SM, Matthews P, Poston L. Maternal metabolism and obesity: modifiable determinants of pregnancy outcome. Hum Reprod Update. 2010;16(3):255-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denison FC, Roberts KA, Barr SM, Norman JE. Obesity, pregnancy, inflammation, and vascular function. Reproduction. 2010;140(3):373-385. [DOI] [PubMed] [Google Scholar]
- 5.Challier JC, Basu S, Bintein T, et al. . Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta. 2008;29(3):274-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Radaelli T, Varastehpour A, Catalano P, Hauguel-de Mouzon S. Gestational diabetes induces placental genes for chronic stress and inflammatory pathways. Diabetes. 2003;52(12):2951-2958. [DOI] [PubMed] [Google Scholar]
- 7.Stewart FM, Freeman DJ, Ramsay JE, Greer IA, Caslake M, Ferrell WR. Longitudinal assessment of maternal endothelial function and markers of inflammation and placental function throughout pregnancy in lean and obese mothers. J Clin Endocrinol Metab. 2007;92(3):969-975. [DOI] [PubMed] [Google Scholar]
- 8.Gaillard R, Rifas-Shiman SL, Perng W, Oken E, Gillman MW. Maternal inflammation during pregnancy and childhood adiposity. Obesity (Silver Spring). 2016;24(6):1320-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Field CJ. Early risk determinants and later health outcomes: implications for research prioritization and the food supply. Summary of the workshop. Am J Clin Nutr. 2009;89(5):1533S-1539S. [DOI] [PubMed] [Google Scholar]
- 10.Buck Louis GM, Bloom MS, Gatto NM, Hogue CR, Westreich DJ, Zhang C. Epidemiology’s continuing contribution to public health: The power of “Then and Now”. Am J Epidemiol. 2015;181(8):e1-e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grantz KL, Grewal J, Albert PS, et al. . Dichorionic twin trajectories: the NICHD Fetal Growth Studies. Am J Obstet Gynecol. 2016;215(2):221.e1-221.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hediger ML, Fuchs KM, Grantz KL, et al. . Ultrasound quality assurance for singletons in the National Institute Of Child Health And Human Development fetal growth studies. J Ultrasound Med. 2016;35(8):1725-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duryea EL, Hawkins JS, McIntire DD, Casey BM, Leveno KJ. A revised birth weight reference for the United States. Obstet Gynecol. 2014;124(1):16-22. [DOI] [PubMed] [Google Scholar]
- 14.Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK. Estimation of fetal weight with the use of head, body, and femur measurements—a prospective study. Am J Obstet Gynecol. 1985;151(3):333-337. [DOI] [PubMed] [Google Scholar]
- 15.Pinheiro JC, Bates DM. Mixed-Effects Models in S and S-Plus. New York: Springer Science–Business Media New York; 2000. [Google Scholar]
- 16.Sterne JA, White IR, Carlin JB, et al. . Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ay L, Kruithof CJ, Bakker R, et al. . Maternal anthropometrics are associated with fetal size in different periods of pregnancy and at birth: the Generation R Study. BJOG. 2009;116(7):953-963. [DOI] [PubMed] [Google Scholar]
- 18.Cedergren MI. Maternal morbid obesity and the risk of adverse pregnancy outcome. Obstet Gynecol. 2004;103(2):219-224. [DOI] [PubMed] [Google Scholar]
- 19.Cnattingius S, Bergström R, Lipworth L, Kramer MS. Prepregnancy weight and the risk of adverse pregnancy outcomes. N Engl J Med. 1998;338(3):147-152. [DOI] [PubMed] [Google Scholar]
- 20.Sebire NJ, Jolly M, Harris JP, et al. . Maternal obesity and pregnancy outcome: a study of 287,213 pregnancies in London. Int J Obes Relat Metab Disord. 2001;25(8):1175-1182. [DOI] [PubMed] [Google Scholar]
- 21.Ehrenberg HM, Mercer BM, Catalano PM. The influence of obesity and diabetes on the prevalence of macrosomia. Am J Obstet Gynecol. 2004;191(3):964-968. [DOI] [PubMed] [Google Scholar]
- 22.Gaillard R. Maternal obesity during pregnancy and cardiovascular development and disease in the offspring. Eur J Epidemiol. 2015;30(11):1141-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1(6):466-473. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Geometric mean and 95% confidence intervals for Biparietal diameters (mm) by gestational age and maternal pre-pregnancy adiposity status, NICHD Fetal Growth Studies–Singletons.
eTable 2. Geometric mean and 95% confidence intervals for head circumference (mm) by gestational age and maternal pre-pregnancy adiposity status, NICHD Fetal Growth Studies–Singletons.
eTable 3. Geometric mean and 95% confidence intervals for abdominal circumference (mm) by gestational age and maternal pre-pregnancy adiposity status, NICHD Fetal Growth Studies–Singletons.
eTable 4. Geometric mean and 95% confidence intervals for femur length (mm) by gestational age and maternal pre-pregnancy adiposity status, NICHD Fetal Growth Studies–Singletons.
eTable 5. Geometric mean and 95% confidence intervals for humerus length (mm) by gestational age and maternal pre-pregnancy adiposity status, NICHD Fetal Growth Studies–Singletons.
eTable 6. Geometric mean and 95% confidence intervals for ratio: head circumference/abdominal circumference by gestational age and maternal pre-pregnancy adiposity status, NICHD Fetal Growth Studies–Singletons.
eTable 7. Geometric mean and 95% confidence intervals for ratio: femur length/abdominal circumference by gestational age and maternal pre-pregnancy adiposity status, NICHD Fetal Growth Studies–Singletons.