Abstract
Hypoxia is a potent microenvironmental factor that promotes tumor metastasis. Recent studies have revealed mechanisms by which hypoxia and activation of hypoxia inducible factor (HIF) dependent signaling promotes metastasis through the regulation of metabolic reprogramming, the stem cell phenotype, invasion, angiogenesis, immune suppression, the premetastatic niche, intravasation/extravasation, and resistance to apoptosis. These discoveries suggest novel paradigms in tumor metastasis and identify new opportunities for therapeutic intervention in the prevention and treatment of metastatic disease. Here we review the impact of hypoxia and hypoxic signaling pathways in tumor and stromal cells on each step of the metastatic cascade.
Hypoxic Signaling in the Primary Tumor Microenvironment
Metastasis is a complex and dynamic process that involves the ability of malignant cells to grow and survive within the primary tumor microenvironment, migrate and invade into the surrounding tissue stroma, intravasate and extravasate blood and lymphatic vessels, and colonize target organs [1]. In addition to the genetic and epigenetic events that occur during malignant tumor progression, the tumor microenviroment (TME) impacts tumor progression and metastasis. Stromal cells within the TME promote tumor progression and metastasis through a variety of mechanisms [2]. In addition hypoxia, or low oxygen tensions, is emerging as a key molecular feature of the TME that governs the metastatic potential of tumor and stromal cells.
Hypoxia is a prominent feature of the tumor microenvironment that develops from an imbalance between oxygen delivery and consumption. Oxygen delivery is impaired in solid tumors due to the abnormal vasculature that develops as a result of an imbalance between pro- and antiangiogenic signals. Additionally, oxygen consumption rates are high in proliferating tumor and infiltrating immune cells leading to hypoxic regions within the TME [3]. At the molecular level, hypoxia results in the stabilization of the hypoxia inducible transcription factors (HIF-1 and HIF-2) that help cells adapt to hypoxic stress by activating gene expression programs that control angiogenesis, glycolytic metabolism, invasion, migration, and erythropoiesis [4]. Clinically, HIF-1 and HIF-2 are associated with metastasis and poor patient survival in a variety of solid tumor types [5–9]. Moreover, HIF signaling is both necessary and sufficient to promote the metastatic potential of tumor cells, suggesting that HIF and its downstream targets may play an important role in metastatic progression [10–15]. Here we will review the current literature that highlight the diverse mechanisms by which hypoxic signaling promotes metastatic progression (Fig.1).
Figure 1. Hypoxia in the primary tumor promotes metastasis.
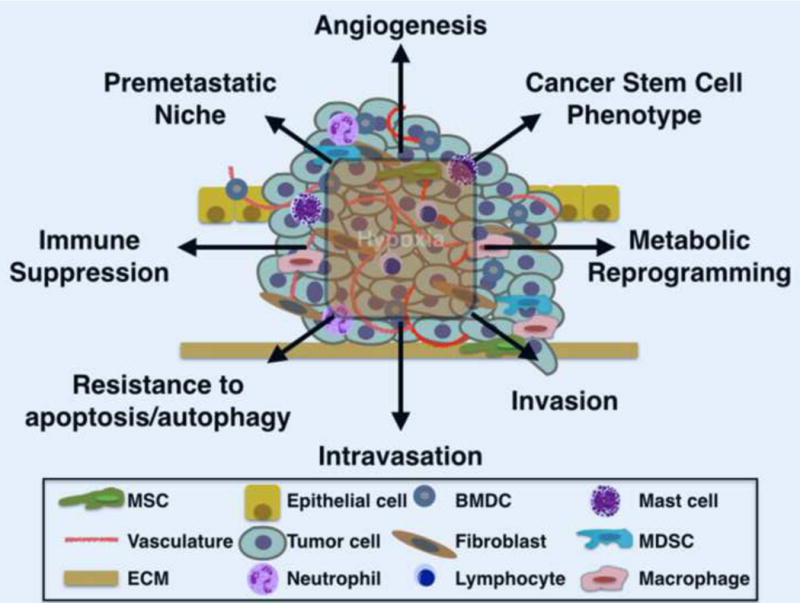
Hypoxia within the tumor microenvironment stabilizes the hypoxia inducible factors (HIF-1 and HIF-2) in tumor and stromal cells leading to the activation of target gene programs that facilitate metastasis.
Immune Evasion
Primary and metastatic tumors survive if they can evade immune attack. Recent clinical studies have highlighted the critical role of immune suppression in tumor control [16, 17]. However, these studies also reveal that tumor cells use a complex set of mechanisms that prevent the immune system from mounting an effective response, limiting the efficacy of single agent immunotherapies. Therefore, understanding the mechanisms that coordinate immunosuppression in the TME is an active area of investigation.
Hypoxia has been recently implicated as an important factor driving the immunosuppressive TME. In melanoma patients, the hypoxic gene signature is associated with innate tumor resistance to anti-PD-1 therapy [18]. In preclinical studies, exposure to respiratory hyperoxia (60% O2) leads to decreased intratumoral hypoxia, tumor regression, decreased metastasis, and prolonged survival. The antitumor effects of hyperoxia required the activities of endogenous T and NK cells suggesting an important role for the hypoxia in suppressing antitumor immune responses [19].
There are multiple mechanisms by which the hypoxic TME inhibits antitumor immune responses. Hypoxia promotes resistance to cytotoxic CD8+ T cell (CTL) mediated killing. After antigen recognition, CTLs release cytotoxic granules containing granzymes and perforin to induce apoptosis of target cancer cells [20]. HIF signaling in cancer cells promotes resistance to lysis by activating anti-apoptotic responses and triggering autophagy [21–24]. Additionally, hypoxic tumor cells upregulate the expression of programmed death – ligand 1 (PD-L1) on cancer cells to increase apoptosis in CTLs [25]. Activation of HIF signaling also inhibits CTL function through the upregulation of CD39/CD73 enzymes by tumor cells that promote the accumulation of extracellular adenosine, a potent inhibitor of lymphocyte cytolytic function [26, 27]. Similar to CTLs, natural killer (NK) cell mediated lysis is inhibited by hypoxic tumor cells through the induction of autophagy [28, 29]. Moreover, hypoxic tumor cells evade macrophage phagocytosis through the upregulation of CD47, a cell surface protein that interacts with signal regulatory protein alpha (SRPα) on the surface of macrophages to block phagocytosis [30]. In addition to directly inhibiting T cell and macrophage antitumor responses, hypoxic cancer cells also secrete chemokines and cytokines to recruit immunosuppressive regulatory T cells (Tregs) and myeloid derived suppressor cells (MDSCs) to the tumor microenvironment [31].
Invasion
One of the first steps in the metastatic cascade is the local invasion of tumor cells from the primary tumor into the adjacent tissue parenchyma. Tumor cells utilize a variety of mechanisms to promote local invasion. They alter the expression of cell-cell and cell-extracellular matrix (ECM) adhesion molecules to enhance cell motility [32]. Tumor cells also upregulate the expression and secretion of extracellular proteases that breakdown the extracellular matrix and release growth factors that promote tumor invasion and growth. Additionally, invasive tumor cells recruit macrophages, fibroblasts, and mesenchymal stem cells (MSCs) to produce pro-migratory factors or deposit collagen networks for invasion [1].
Hypoxic signaling activates promigratory and invasive phenotypes of tumor cells through multiple mechanisms. HIF signaling can induce epithelial plasticity and a migratory phenotype through the direct and indirect regulation of the epithelial to mesenchymal (EMT) transcription factors Snail, Slug, Twist and Zeb1 [33]. Invasion of the surrounding extracellular matrix (ECM) can also be enhanced by HIF signaling through the upregulation and secretion of proteolytic enzymes such as matrix metalloproteinases (MMPs), cathepsins, lysyl oxidases, and prolyl-4-hydroxylses (P4H) to support the initial stages of metastasis by remodeling the ECM [34–36]. Recently, hypoxia has been shown to increase the secretion of tumor derived procollagen-lysine, 2-oxoglutarate 5-dioxygenase (PLOD2), a HIF target that hydroxylates lysine residues on collagen to promote the formation of mature collagen crosslinks, and facilitate metastasis in multiple tumor models [37, 38]. In addition to the direct affects on tumor cell invasion and migration, HIF signaling in tumor cells induces the expression of chemokines and cytokines that recruit macrophages and mesenchymal stem cells (MSCs) into the TME to support tumor cell invasion, migration, and metastasis [39, 40].
Cancer Stem Cell Phenotype
The tumor cell population within the primary tumor is heterogeneous. Only a small fraction of tumor cells, cancer stem cells (CSCs), have enhanced tumor-initiating potential, the ability to self-renew, and differentiate into multiple cell types [41]. It has been hypothesized that metastases are derived from primary CSCs that regain their regenerative potential at metastatic sites [42]. In support of this hypothesis, the expression of adult stem cell markers in patient primary tumors is associated with poor prognosis and metastasis [42]. Moreover, stem cell markers can be utilized to select for tumor cells with metastatic potential from cancer patient blood and primary tumor samples [42]. Recent studies have begun to define the niches and signaling pathways that select for and maintain the cancer stem cell phenotype.
Accumulating evidence suggests that hypoxia is an important microenvironmental factor that selects for the CSC phenotype. Hypoxia supports the generation and maintenance of the CSCs through the HIF-dependent activation of genes that drive self-renewal including OCT4, SOX2, NANOG, KLF4 and c-MYC [43, 44]. Additionally, activation of notch signaling by hypoxia induces stemness by promoting an dedifferentiated state in tumor cells [45]. In murine models of glioblastoma, HIF-1 and HIF-2 are required for glioma stem cell growth and survival in vivo [46]. Similarly, in the MMTV-PyMT murine model of breast cancer HIF-1 is an important factor driving the breast cancer stem cell phenotype, metastasis and survival [47].
Intravasation and Extravasation
HIF regulates tumor cell intravasation and extravasation from the vasculature. HIF activity in tumor cells results in the release of factors that modulate endothelial-endothelial cell and endothelial-tumor cell interactions. The upregulation of angiopoietin-like 4 (ANGPTL4) by HIF disrupts endothelial-endothelial cell interactions and allows for easy passage of tumor cells through blood vessels [13]. Simultaneously, HIF strengthens tumor cell-endothelial cell interactions through the activation of L1 cell adhesion molecule (L1CAM) [13]. Another mechanism by which HIF promotes tumor cell intravasation and extravasation is through the activation of genes, which control vascular permeability. Hypoxic induction of VEGF, angiopoietin 2, MMPs and UPAR cooperatively act to destabilize the vascular wall and allow for tumor cell entry [33].
In the endothelium, HIF signaling increases cell adhesion, coagulation, and endothelial permeability [48]. Recent studies demonstrated that lymphatic endothelial cells within premetastatic niches are conditioned by triple negative breast cancer cells to promote extravasation by recruiting tumor cells to these sites through CCL5 dependent mechanisms and by activating the expression of proangiogenic factors such as VEGF [49]. While the role of HIF-1 within lymphatic vessels remains to be determined, these studies suggest that HIF-1 may at least in part contribute to metastatic spread by modulating lymphatics.
Colonization of Target Organs
The “seed and soil” hypothesis was first proposed by Paget in 1889 [50], who documented that the distribution of distant metastases is not random and cannot be completely explained by relative blood supply. Rather, Paget proposed that certain organs are predisposed for metastatic colonization [50]. There is now a large body of clinical evidence demonstrating that, in fact, the distribution of tumor metastases is not random. For example, prostate cancer often metastasizes to the bones, colon cancer has propensity to metastasize to the liver, and the primary site for ovarian metastasis is the peritoneal cavity [1]. Experimental evidence also supports this concept. Minn and colleagues were among the first to experimentally demonstrate that there are particular requirements for circulating tumor cells to colonize specific organs. They observed that individual cells from the pleural effusion of a breast cancer patient metastasized to specific organs, such as the bone, lung, or adrenal medulla [51]. Recent studies have identified hypoxia and HIF signaling as important factors governing tissue-specific metastasis.
Liver
The liver is a common site for metastasis of gastrointestinal tumor cells that travel through the portal circulation. Additionally, liver metastases are commonly found in patients with breast, lung, and ocular melanoma cancers. The hepatic vasculature is highly permeable suggesting that cellular adaptation to the liver microenvironment is a key barrier to the successful colonization of disseminated tumor cells in the liver [1][52].
Recent studies implicated hypoxia and metabolic stress as a barrier to successful metastatic colonization in the liver. The liver microenvironment contains areas of hypoxia that demarcate metabolic zones. For example, metabolically active hepatocytes are located in the periportal region of the liver where they display high rates of oxygen consumption. In contrast, perivenous hepatocytes are located within hypoxic regions of the liver and generate ATP through glycolysis [53, 54]. Using HIF-1 transcriptional luciferase reporter mice, Loo et al. showed that colon cancer cells experience hypoxia early after metastatic dissemination. Moreover, they discovered that hypoxic colon cancer cells in the liver were dependent upon creatine kinase brain (CKB), an enzyme that catalyzes the phosphorylation of creatine using extracellular ATP as a phosphate source [55][56]. These observations suggest that colon cancer cells upregulate CKB to adapt to metabolic stress induced by acute hypoxia during the early stages of dissemination in the liver [55]. Similarly, disseminated breast cancer cells metabolically adapt to the liver microenvironment through HIF-1-dependent mechanisms. Dupuy et al. reported that breast cancer cells that metastasize to the liver have a glycolytic phenotype in contrast to cells that metastasize to the lung or bone and that exhibit a phenotype of oxidative phosphorylation [57]. In these breast-to-liver tumor cells, HIF-1 is hyperactivated and contributes to glycolytic reprogramming through its downstream target pyruvate dehydrogenase kinase 1 (PDK1) [57]. Together, these findings suggest that the liver microenvironment selects for disseminated tumor cells that have the ability to metabolically adapt to hypoxic stress (Fig. 2).
Figure 2. Mechanisms by which hypoxic signaling promotes metastasis to the liver.
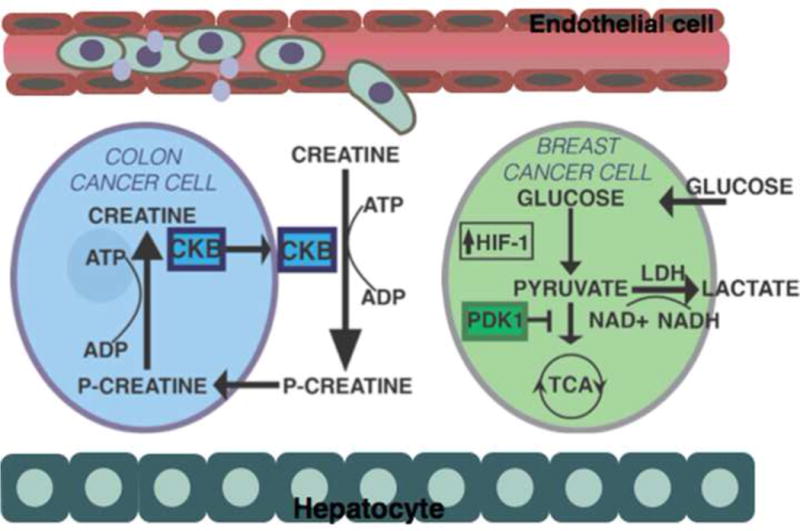
Hypoxia is a significant barrier for metastatic colonization in the liver. Disseminated colon cancer cells are dependent upon the expression of creatine kinase B (CKB) to adapt to metabolic stress associated with acute hypoxia. CKB catalyzes the phosphorylation of creatine using extracellular ATP. This allows tumor cells to generate ATP from extracellular sources of phospho-creatine during metabolic stress. Disseminated breast cancer cells also metabolically adapt to the hypoxic liver microenvironment through HIF-1 dependent upregulation of pyruvate dehydrogenase kinase 1 (PDK1). PDK1 expression promotes the generation of ATP through glycolysis by antagonizing the function of pyruvate dehydrogenase, a rate-limiting enzyme for pyruvate conversion to acetyl-coenzyme A and entry into the tricarboxylic acid cycle (TCA).
Lung
Pulmonary metastases are frequently observed in patients with sarcoma, breast, melanoma, gastrointestinal, and kidney cancers. Because cardiac output from the pulmonary artery circulates through the lungs, a high incidence of pulmonary metastases in cancer patients can be expected on the basis of blood flow alone. However, in contrast to the liver and bone, lung capillaries are lined with endothelial cells and are surrounded by a basement membrane and adjacent alveolar cells [58]. Therefore, extravasation and survival within the lung parenchyma are thought to be significant barriers to lung colonization.
Hypoxia and HIF signaling play a central role in breast-to-lung metastasis. Breast cancer patients at high risk of developing lung metastasis can be identified using a hypoxia response signature set of 45 genes [59]. Preclinical studies using human breast cancer cells (MDA-MB-231) as well as genetic mouse models of breast cancer (mouse mammary tumor virus (MMTV) polyoma middle T (PyMT) demonstrated that HIF-1 and HIF -2 drive the metastatic potential of breast cancer cells to the lung in vivo [13, 14, 59, 60]. There are multiple mechanisms by which this occurs. HIF signaling in primary breast cancer cells stimulates the secretion of proteins including lysyl oxidase (LOX) and LOX like proteins (LOXL2 and LOXL4) to prime the lung microenvironment for colonization [12, 61]. LOX, LOXL2, and LOXL4 remodel extracellular matrix proteins, such as collagen, to recruit bone marrow derived cells (BMDCs) into the lung [12, 62]. Once in the lung, BMDCs produce chemokines that recruit disseminated tumor cells and promote tumor extravasation [63, 64]. HIF signaling also directly facilitates tumor extravasation to the lung through the upregulation of factors that promote tumor-endothelial cell adhesions or decrease endothelial cell-cell adhesion [13]. These studies suggest that inhibition of HIF and/or key target genes may be important in the clinical management of lung metastasis (Fig. 3).
Figure 3. Mechanisms by which hypoxic signaling promotes metastasis to the lung.
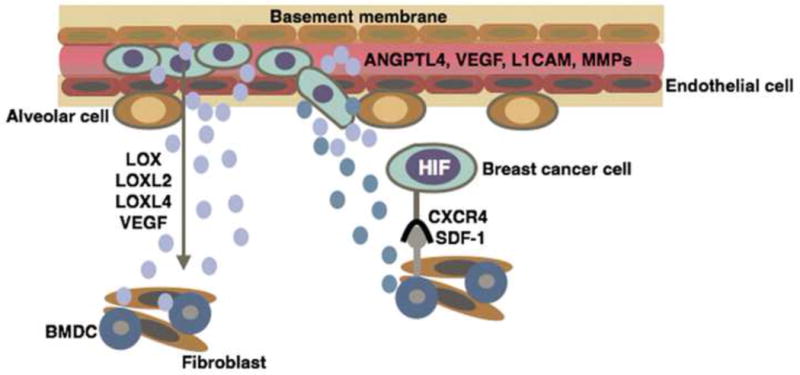
Hypoxic signaling promotes extravasation in the lung by upregulating the expression and secretion of proteins including lysyl oxidase (LOX), LOX like proteins (LOXL2 and LOXL4), and vascular endothelial growth factor (VEGF). LOX, LOXL2, and LOXL4 remodel extracellular matrix proteins, such as collagen, to recruit bone marrow derived cells (BMDCs) into the lung. BMDCs prime the lung microenvironment for metastatic colonization by producing chemokines such as stromal derived factor-1 (SDF-1) that recruit CXCR4 expressing tumor cells to the lung. HIF signaling also directly promotes tumor extravasation in the lung through the upregulation of factors that promote tumor-endothelial cell adhesions (L1 cell adhesion molecule, L1CAM) and decrease endothelial cell – cell adhesion (angiopoietin-like 4, ANGPTL4).
Bone
Bone metastases are commonly found in patients with breast, prostate, lung, thyroid, melanoma and kidney cancers. In fact, in breast and prostate cancers, the bone is often the first site of distant metastasis [65]. Bone metastases are classified as either osteoblastic (bone forming) or osteolytic (destructive to bone) based on the type of radiographic lesions that they induce. They uncouple the homeostatic balance between bone formation and bone destruction to support bone colonization [66].
Hypoxia and HIF signaling are implicated in the regulation of bone metastasis. The hypoxic gene signature is associated with bone metastasis over lymphatic, lung, or liver metastasis in breast cancer patients [67, 68]. Preclinical studies demonstrated that HIF-1 is both necessary and sufficient for MDA-MB-231 cells to metastasize to the bone [11, 59, 69]. There are multiple mechanisms by which HIF signaling may promote osteotropism. HIF activation promotes the secretion of parathyroid hormone related protein (PTHrP) and LOX that precondition the bone marrow microenvironment for breast cancer colonization [68, 70]. Breast tumor derived PTHrP promotes bone resorption and tumor growth within the bone microenvironment [71]. Similarly, tumor-derived LOX promotes the formation of osteolytic lesions and enhances circulating tumor cell colonization [68]. HIF-mediated upregulation of the chemokine receptor C-X-C motif receptor-4 (CXCR4) in breast tumor cells may also promote homing of breast tumor cells to the bone marrow, where the CXCR4 ligand stromal-derived factor-1 (SDF-1) is highly expressed [72–74]. Together these data suggest that targeting HIF-1 directly or key downstream HIF targets, such as PTHrP or LOX, may be efficacious in the clinical management of metastatic bone disease (Fig.4).
Figure 4. Mechanisms by which hypoxic signaling promotes metastasis to the bone.
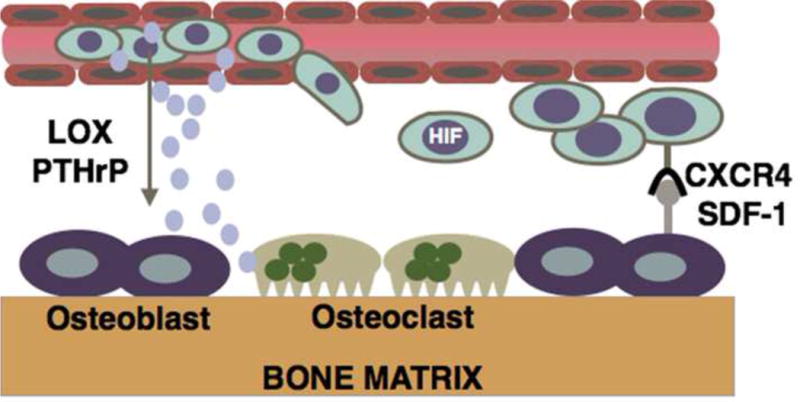
Tumor cells secrete factors such as lysyl oxidase (LOX) and parathyroid related protein (PTHrP) into the circulation where they precondition the bone marrow microenvironment for metastatic colonization. PTHrP and LOX modulate the activities of osteoblasts and osteoclasts to promote bone resorption and tumor growth. Additionally, HIF mediated upregulation of the chemokine receptor C-X-C motif receptor -4 (CXCR4) may promote breast tumor cell homing to the bone marrow microenvironment where the expression of its ligand, stromal derived factor-1 (SDF-1) is highly expressed by osteoblasts and other cells within the bone marrow microenvironment.
Concluding Remarks
Hypoxia is an important microenvironmental factor that contributes to metastatic tumor progression at the primary and distant tissue sites. Modulation of the HIF signaling pathway in tumor cells has began to illuminate the diverse mechanisms by which HIF signaling promotes metastatic progression. However, important questions still remain in the field (Outstanding Questions Box).
Outstanding Questions Box.
What is the role of hypoxia and HIF signaling in tumor dormancy?
Are there HIF independent effects of hypoxia on metastasis?
How does hypoxia-mediated immune suppression affect the premetastatic niche?
How does HIF signaling couple metastasis with immune suppression?
What is the role of stromal HIF signaling in metastatic progression?
Tumor cells communicate with a variety of stromal cells including fibroblasts, bone marrow derived cells, immune, vascular, and epithelial cells to facilitate metastasis [32]. The majority of work in the field has focused on the role of hypoxic signaling in tumor cells and its impact on tumor stromal crosstalk during metastatic progression. Recent studies indicate that HIF signaling can also be activated within tumor stromal cells through a variety of mechanisms including hypoxia and secreted factors produced by tumor cells [40, 75]. However, the role of HIF signaling within tumor stromal cell populations remains largely unexplored. Studies with macrophage specific inactivation of HIF-1 or HIF-2 have revealed an important role for HIF signaling in mediating the protumorigenic properties of macrophages within multiple tumor models [76] [77]. Recent studies also have demonstrated that HIF signaling mediates bidirectional signaling between breast cancer cells and MSCs to promote metastasis [40]. In contrast, fibroblast specific inactivation of HIF-1 accelerated tumor growth in a murine model of breast cancer [78]. Further knowledge regarding of the role of HIF signaling within individual stromal cell populations is needed to understand how to best target this pathway for cancer therapy.
One of the rate limiting steps in metastasis is the transition from distant tissue infiltration to overt colonization. This process requires tumor cells to adapt to tissue specific barriers to colonization and exploit tissue specific survival signals [1]. Disseminated tumor cells frequently infiltrate tissues with hypoxic niches such as the liver and bone [1, 54, 79]. Although the role of hypoxia and HIF signaling in tumor dormancy has not been directly explored, recent studies have demonstrated that hypoxia is a key barrier to the successful metastatic colonization of breast and colon cancer cells in the liver [55, 57]. Moreover, hematopoietic stem cells reside within hypoxic niches in the bone marrow and utilize HIF signaling to maintain a quiescent state [80–83]. Disseminated tumor cells localize to HSC niches in the bone marrow [84]. Therefore, it is tempting to speculate that hypoxia may also influence the tumor dormancy in the bone marrow microenvironment.
Another critical step in metastasis is the ability of tumor cells to evade immune attack. There is growing interest in elucidating the molecular mechanisms by which tumor cells couple metastasis with immune evasion [85, 86]. Hypoxic tumor cells upregulate the expression of the EMT transcription factors including Zeb1 and Snail [33]. Interestingly, clinical studies have shown a correlation between the expression of EMT transcription factors and the expression of immunosuppressive checkpoint inhibitor and infiltrating regulatory T cells [87–89]. Moreover, preclinical studies showed that Snail-induced EMT accelerates metastasis not only through enhanced invasion but also by promoting immunosuppression through induction of Tregs [90]. Zeb1 also promotes metastasis and immunosuppression by inhibiting CD8+ T cell activity through the regulation of PD-L1 expression in tumors [88]. These findings raise the intriguing possibility that HIF signaling may couple metastasis and immunoresistance through the regulation of Snail and Zeb1.
Hypoxia and HIF signaling promotes multiple steps within the metastatic cascade. Therefore, there are a variety of HIF inhibitors and other agents that target the hypoxic signaling pathway are in preclinical and clinical development for the treatment of cancer [91]. Given that HIF signaling impacts the behavior of tumor and stromal cells in the TME, it will be important to evaluate the efficacy of these agents in immune competent preclinical models and in clinical studies to understand the impact of HIF inhibition of on the TME and tumor progression. Additionally, the evaluation of HIF inhibitors in combination with antiangiogenic or immunotherapies that target other components of the TME may help to overcome resistance to targeted therapy.
Trends Box.
-
–
Tumor metastasis relies on interactions between tumor cells and microenvironmental factors present within the primary tumor, vasculature, and distant tissue.
-
–
Hypoxia is a key microenvironmental factor that influences the behavior of tumor and stromal cells to support metastasis.
-
–
Clinically, hypoxia and the hypoxia inducible transcription factors (HIF-1 and HIF-2) are associated with metastasis and poor patient survival.
-
–
Hypoxic signaling promotes the early stages of metastasis associated with tumor cell invasion, migration, and intravasation as well as the late stages of metastasis where immune evasion, extravasation, metabolic adaptation, and tumor-stromal interactions at the distant tissue serve as barriers to successful metastatic colonization.
-
–
Hypoxic signaling promotes the formation of a premetastatic niche that is essential for tumor colonization at the distant site.
Acknowledgments
This work was supported by NIH Grants CA-198291, CA-67166 and CA-197713, the Silicon Valley Foundation, the Sydney Frank Foundation and the Kimmelman Fund (AJG); and the Department of Defense Ovarian Cancer Research Academy OC140611 (EBR). This work was supported in part by the GI-CoRE (GSQ) at Hokkaido University, founded by the Ministry of Education, Culture, Sports, Science and Technology (MEXT). We apologize to those colleagues whose work we could not cite due to space constraints.
References
- 1.Obenauf AC, Massague J. Surviving at a distance: organ specific metastasis. Trends in cancer. 2015;1:76–91. doi: 10.1016/j.trecan.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 3.Brown JM, Giaccia AJ. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer research. 1998;58:1408–1416. [PubMed] [Google Scholar]
- 4.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin Y, et al. Clinicopathological characteristics of gynecological cancer associated with hypoxia-inducible factor 1alpha expression: a meta-analysis including 6,612 subjects. PloS one. 2015;10:e0127229. doi: 10.1371/journal.pone.0127229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsuo Y, et al. Hypoxia inducible factor-1 alpha plays a pivotal role in hepatic metastasis of pancreatic cancer: an immunohistochemical study. Journal of hepato-biliary-pancreatic sciences. 2014;21:105–112. doi: 10.1002/jhbp.6. [DOI] [PubMed] [Google Scholar]
- 7.Ping W, et al. Clinicopathological and prognostic significance of hypoxia-inducible factor-1alpha in esophageal squamous cell carcinoma: a meta-analysis. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2014;35:4401–4409. doi: 10.1007/s13277-013-1579-0. [DOI] [PubMed] [Google Scholar]
- 8.Luan Y, et al. Clinicopathological and prognostic significance of HIF-1alpha and HIF-2alpha expression in small cell lung cancer. Pathology, research and practice. 2013;209:184–189. doi: 10.1016/j.prp.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 9.Wang HX, et al. HIF-2alpha as a prognostic marker for breast cancer progression and patient survival. Genetics and molecular research: GMR. 2014;13:2817–2826. doi: 10.4238/2014.January.22.6. [DOI] [PubMed] [Google Scholar]
- 10.Yang MH, et al. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nature cell biology. 2008;10:295–305. doi: 10.1038/ncb1691. [DOI] [PubMed] [Google Scholar]
- 11.Hiraga T, et al. Hypoxia and hypoxia-inducible factor-1 expression enhance osteolytic bone metastases of breast cancer. Cancer research. 2007;67:4157–4163. doi: 10.1158/0008-5472.CAN-06-2355. [DOI] [PubMed] [Google Scholar]
- 12.Wong CC, et al. Hypoxia-inducible factor 1 is a master regulator of breast cancer metastatic niche formation. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:16369–16374. doi: 10.1073/pnas.1113483108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H, et al. HIF-1-dependent expression of angiopoietin-like 4 and L1CAM mediates vascular metastasis of hypoxic breast cancer cells to the lungs. Oncogene. 2012;31:1757–1770. doi: 10.1038/onc.2011.365. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Liao D, et al. Hypoxia-inducible factor-1alpha is a key regulator of metastasis in a transgenic model of cancer initiation and progression. Cancer research. 2007;67:563–572. doi: 10.1158/0008-5472.CAN-06-2701. [DOI] [PubMed] [Google Scholar]
- 15.Hanna SC, et al. HIF1alpha and HIF2alpha independently activate SRC to promote melanoma metastases. The Journal of clinical investigation. 2013;123:2078–2093. doi: 10.1172/JCI66715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Twyman-Saint Victor C, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boutros C, et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nature reviews Clinical oncology. 2016 doi: 10.1038/nrclinonc.2016.58. [DOI] [PubMed] [Google Scholar]
- 18.Hugo W, et al. Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell. 2016;165:35–44. doi: 10.1016/j.cell.2016.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hatfield SM, et al. Immunological mechanisms of the antitumor effects of supplemental oxygenation. Science translational medicine. 2015;7:277ra230. doi: 10.1126/scitranslmed.aaa1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harty JT, et al. CD8+ T cell effector mechanisms in resistance to infection. Annual review of immunology. 2000;18:275–308. doi: 10.1146/annurev.immunol.18.1.275. [DOI] [PubMed] [Google Scholar]
- 21.Lee YH, et al. Gain of HIF-1alpha under normoxia in cancer mediates immune adaptation through the AKT/ERK and VEGFA axes. Clinical cancer research: an official journal of the American Association for Cancer Research. 2015;21:1438–1446. doi: 10.1158/1078-0432.CCR-14-1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noman MZ, et al. Hypoxia-inducible miR-210 regulates the susceptibility of tumor cells to lysis by cytotoxic T cells. Cancer research. 2012;72:4629–4641. doi: 10.1158/0008-5472.CAN-12-1383. [DOI] [PubMed] [Google Scholar]
- 23.Noman MZ, et al. The cooperative induction of hypoxia-inducible factor-1 alpha and STAT3 during hypoxia induced an impairment of tumor susceptibility to CTL-mediated cell lysis. Journal of immunology. 2009;182:3510–3521. doi: 10.4049/jimmunol.0800854. [DOI] [PubMed] [Google Scholar]
- 24.Noman MZ, et al. Blocking hypoxia-induced autophagy in tumors restores cytotoxic T-cell activity and promotes regression. Cancer research. 2011;71:5976–5986. doi: 10.1158/0008-5472.CAN-11-1094. [DOI] [PubMed] [Google Scholar]
- 25.Barsoum IB, et al. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer research. 2014;74:665–674. doi: 10.1158/0008-5472.CAN-13-0992. [DOI] [PubMed] [Google Scholar]
- 26.Hatfield SM, et al. Systemic oxygenation weakens the hypoxia and hypoxia inducible factor 1alpha-dependent and extracellular adenosine-mediated tumor protection. Journal of molecular medicine. 2014;92:1283–1292. doi: 10.1007/s00109-014-1189-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sitkovsky MV, et al. Hypoxia-adenosinergic immunosuppression: tumor protection by T regulatory cells and cancerous tissue hypoxia. Clinical cancer research: an official journal of the American Association for Cancer Research. 2008;14:5947–5952. doi: 10.1158/1078-0432.CCR-08-0229. [DOI] [PubMed] [Google Scholar]
- 28.Baginska J, et al. Granzyme B degradation by autophagy decreases tumor cell susceptibility to natural killer-mediated lysis under hypoxia. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:17450–17455. doi: 10.1073/pnas.1304790110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Messai Y, et al. ITPR1 protects renal cancer cells against natural killer cells by inducing autophagy. Cancer research. 2014;74:6820–6832. doi: 10.1158/0008-5472.CAN-14-0303. [DOI] [PubMed] [Google Scholar]
- 30.Zhang H, et al. HIF-1 regulates CD47 expression in breast cancer cells to promote evasion of phagocytosis and maintenance of cancer stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:E6215–6223. doi: 10.1073/pnas.1520032112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palazon A, et al. HIF transcription factors, inflammation, and immunity. Immunity. 2014;41:518–528. doi: 10.1016/j.immuni.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 33.De Bock K, et al. Antiangiogenic therapy, hypoxia, and metastasis: risky liaisons, or not? Nat Rev Clin Oncol. 2011;8:393–404. doi: 10.1038/nrclinonc.2011.83. [DOI] [PubMed] [Google Scholar]
- 34.Semenza GL. The hypoxic tumor microenvironment: A driving force for breast cancer progression. Biochimica et biophysica acta. 2016;1863:382–391. doi: 10.1016/j.bbamcr.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krishnamachary B, et al. Regulation of colon carcinoma cell invasion by hypoxia-inducible factor 1. Cancer research. 2003;63:1138–1143. [PubMed] [Google Scholar]
- 36.Sudhan DR, Siemann DW. Cathepsin L inhibition by the small molecule KGP94 suppresses tumor microenvironment enhanced metastasis associated cell functions of prostate and breast cancer cells. Clinical & experimental metastasis. 2013;30:891–902. doi: 10.1007/s10585-013-9590-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eisinger-Mathason TS, et al. Hypoxia-dependent modification of collagen networks promotes sarcoma metastasis. Cancer discovery. 2013;3:1190–1205. doi: 10.1158/2159-8290.CD-13-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilkes DM, et al. Procollagen lysyl hydroxylase 2 is essential for hypoxia-induced breast cancer metastasis. Molecular cancer research : MCR. 2013;11:456–466. doi: 10.1158/1541-7786.MCR-12-0629. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Chaturvedi P, et al. Hypoxia-inducible factor-dependent signaling between triple-negative breast cancer cells and mesenchymal stem cells promotes macrophage recruitment. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E2120–2129. doi: 10.1073/pnas.1406655111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaturvedi P, et al. Hypoxia-inducible factor-dependent breast cancer-mesenchymal stem cell bidirectional signaling promotes metastasis. The Journal of clinical investigation. 2013;123:189–205. doi: 10.1172/JCI64993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Plaks V, et al. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell stem cell. 2015;16:225–238. doi: 10.1016/j.stem.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oskarsson T, et al. Metastatic stem cells: sources, niches, and vital pathways. Cell stem cell. 2014;14:306–321. doi: 10.1016/j.stem.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang C, et al. Hypoxia induces the breast cancer stem cell phenotype by HIF-dependent and ALKBH5-mediated m6A-demethylation of NANOG mRNA. Proceedings of the National Academy of Sciences of the United States of America. 2016 doi: 10.1073/pnas.1602883113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barnhart BC, Simon MC. Metastasis and stem cell pathways. Cancer metastasis reviews. 2007;26:261–271. doi: 10.1007/s10555-007-9053-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell. 2007;129:465–472. doi: 10.1016/j.cell.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Z, et al. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer cell. 2009;15:501–513. doi: 10.1016/j.ccr.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwab LP, et al. Hypoxia-inducible factor 1alpha promotes primary tumor growth and tumor-initiating cell activity in breast cancer. Breast cancer research: BCR. 2012;14:R6. doi: 10.1186/bcr3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Evans CE, et al. HIF-mediated endothelial response during cancer progression. International journal of hematology. 2012;95:471–477. doi: 10.1007/s12185-012-1072-3. [DOI] [PubMed] [Google Scholar]
- 49.Lee E, et al. Breast cancer cells condition lymphatic endothelial cells within pre-metastatic niches to promote metastasis. Nature communications. 2014;5:4715. doi: 10.1038/ncomms5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paget S. The distribution of secondary growths in cancer of the breast. Cancer metastasis reviews. 1989;8:98–101. 1889. [PubMed] [Google Scholar]
- 51.Minn AJ, et al. Distinct organ-specific metastatic potential of individual breast cancer cells and primary tumors. J Clin Invest. 2005;115:44–55. doi: 10.1172/JCI22320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chambers AF, et al. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 53.Jungermann K. Metabolic zonation of liver parenchyma. Seminars in liver disease. 1988;8:329–341. doi: 10.1055/s-2008-1040554. [DOI] [PubMed] [Google Scholar]
- 54.Jungermann K, Kietzmann T. Oxygen: modulator of metabolic zonation and disease of the liver. Hepatology. 2000;31:255–260. doi: 10.1002/hep.510310201. [DOI] [PubMed] [Google Scholar]
- 55.Loo JM, et al. Extracellular metabolic energetics can promote cancer progression. Cell. 2015;160:393–406. doi: 10.1016/j.cell.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiological reviews. 2000;80:1107–1213. doi: 10.1152/physrev.2000.80.3.1107. [DOI] [PubMed] [Google Scholar]
- 57.Dupuy F, et al. PDK1-Dependent Metabolic Reprogramming Dictates Metastatic Potential in Breast Cancer. Cell metabolism. 2015;22:577–589. doi: 10.1016/j.cmet.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 58.Nguyen DX, et al. Metastasis: from dissemination to organ-specific colonization. Nature reviews Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 59.Lu X, et al. In vivo dynamics and distinct functions of hypoxia in primary tumor growth and organotropic metastasis of breast cancer. Cancer research. 2010;70:3905–3914. doi: 10.1158/0008-5472.CAN-09-3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goto Y, et al. UCHL1 provides diagnostic and antimetastatic strategies due to its deubiquitinating effect on HIF-1alpha. Nature communications. 2015;6:6153. doi: 10.1038/ncomms7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Erler JT, et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 62.Erler JT, et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaplan RN, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gao D, et al. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319:195–198. doi: 10.1126/science.1150224. [DOI] [PubMed] [Google Scholar]
- 65.Johnson RW, et al. HIF targets in bone remodeling and metastatic disease. Pharmacology & therapeutics. 2015;150:169–177. doi: 10.1016/j.pharmthera.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Waning DL, Guise TA. Molecular mechanisms of bone metastasis and associated muscle weakness. Clinical cancer research: an official journal of the American Association for Cancer Research. 2014;20:3071–3077. doi: 10.1158/1078-0432.CCR-13-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Woelfle U, et al. Molecular signature associated with bone marrow micrometastasis in human breast cancer. Cancer research. 2003;63:5679–5684. [PubMed] [Google Scholar]
- 68.Cox TR, et al. The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase. Nature. 2015;522:106–110. doi: 10.1038/nature14492. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 69.Dunn LK, et al. Hypoxia and TGF-beta drive breast cancer bone metastases through parallel signaling pathways in tumor cells and the bone microenvironment. PloS one. 2009;4:e6896. doi: 10.1371/journal.pone.0006896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Manisterski M, et al. Hypoxia induces PTHrP gene transcription in human cancer cells through the HIF-2alpha. Cell cycle. 2010;9:3723–3729. [PubMed] [Google Scholar]
- 71.Guise TA, et al. Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. The Journal of clinical investigation. 1996;98:1544–1549. doi: 10.1172/JCI118947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Staller P, et al. Chemokine receptor CXCR4 downregulated by von Hippel-Lindau tumour suppressor pVHL. Nature. 2003;425:307–311. doi: 10.1038/nature01874. [DOI] [PubMed] [Google Scholar]
- 73.Muller A, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 74.Dar A, et al. Mutual, reciprocal SDF-1/CXCR4 interactions between hematopoietic and bone marrow stromal cells regulate human stem cell migration and development in NOD/SCID chimeric mice. Experimental hematology. 2006;34:967–975. doi: 10.1016/j.exphem.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 75.Facciabene A, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011;475:226–230. doi: 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]
- 76.Doedens AL, et al. Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer research. 2010;70:7465–7475. doi: 10.1158/0008-5472.CAN-10-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Imtiyaz HZ, et al. Hypoxia-inducible factor 2alpha regulates macrophage function in mouse models of acute and tumor inflammation. The Journal of clinical investigation. 2010;120:2699–2714. doi: 10.1172/JCI39506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim JW, et al. Loss of fibroblast HIF-1alpha accelerates tumorigenesis. Cancer research. 2012;72:3187–3195. doi: 10.1158/0008-5472.CAN-12-0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Spencer JA, et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature. 2014;508:269–273. doi: 10.1038/nature13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Takubo K, et al. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell stem cell. 2010;7:391–402. doi: 10.1016/j.stem.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 81.Miharada K, et al. Cripto regulates hematopoietic stem cells as a hypoxic-niche-related factor through cell surface receptor GRP78. Cell stem cell. 2011;9:330–344. doi: 10.1016/j.stem.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 82.Simsek T, et al. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell stem cell. 2010;7:380–390. doi: 10.1016/j.stem.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Krock BL, et al. The aryl hydrocarbon receptor nuclear translocator is an essential regulator of murine hematopoietic stem cell viability. Blood. 2015;125:3263–3272. doi: 10.1182/blood-2014-10-607267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shiozawa Y, et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. The Journal of clinical investigation. 2011;121:1298–1312. doi: 10.1172/JCI43414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Malladi S, et al. Metastatic Latency and Immune Evasion through Autocrine Inhibition of WNT. Cell. 2016;165:45–60. doi: 10.1016/j.cell.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Papaspyridonos M, et al. Id1 suppresses anti-tumour immune responses and promotes tumour progression by impairing myeloid cell maturation. Nature communications. 2015;6:6840. doi: 10.1038/ncomms7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lou Y, et al. Epithelial-mesenchymal transition is associated with a distinct tumor microenvironment including elevation of inflammatory signals and multiple immune checkpoints in lung adenocarcinoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2016 doi: 10.1158/1078-0432.CCR-15-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen L, et al. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nature communications. 2014;5:5241. doi: 10.1038/ncomms6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mak MP, et al. A Patient-Derived, Pan-Cancer EMT Signature Identifies Global Molecular Alterations and Immune Target Enrichment Following Epithelial-to-Mesenchymal Transition. Clinical cancer research: an official journal of the American Association for Cancer Research. 2016;22:609–620. doi: 10.1158/1078-0432.CCR-15-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kudo-Saito C, et al. Cancer metastasis is accelerated through immunosuppression during Snail-induced EMT of cancer cells. Cancer cell. 2009;15:195–206. doi: 10.1016/j.ccr.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 91.Onnis B, et al. Development of HIF-1 inhibitors for cancer therapy. Journal of cellular and molecular medicine. 2009;13:2780–2786. doi: 10.1111/j.1582-4934.2009.00876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


