Key Points
Question
Can cerebral embolic protection devices reduce the incidence of stroke after surgical aortic valve replacement?
Findings
In this randomized clinical trial that included 383 patients, there was no significant difference in freedom from central nervous system infarction between suction-based extraction and a standard aortic cannula (32.0% vs 33.3%, respectively) or between intra-aortic filtration and a standard aortic cannula (25.6% vs 32.4%, respectively).
Meaning
The incidence of central nervous system infarction after surgical aortic valve replacement was not altered by either of the 2 cerebral embolic protection devices.
Abstract
Importance
Stroke is a major complication of surgical aortic valve replacement (SAVR).
Objective
To determine the efficacy and adverse effects of cerebral embolic protection devices in reducing ischemic central nervous system (CNS) injury during SAVR.
Design, Setting, and Participants
A randomized clinical trial of patients with calcific aortic stenosis undergoing SAVR at 18 North American centers between March 2015 and July 2016. The end of follow-up was December 2016.
Interventions
Use of 1 of 2 cerebral embolic protection devices (n = 118 for suction-based extraction and n = 133 for intra-aortic filtration device) vs a standard aortic cannula (control; n = 132) at the time of SAVR.
Main Outcomes and Measures
The primary end point was freedom from clinical or radiographic CNS infarction at 7 days (± 3 days) after the procedure. Secondary end points included a composite of mortality, clinical ischemic stroke, and acute kidney injury within 30 days after surgery; delirium; mortality; serious adverse events; and neurocognition.
Results
Among 383 randomized patients (mean age, 73.9 years; 38.4% women; 368 [96.1%] completed the trial), the rate of freedom from CNS infarction at 7 days was 32.0% with suction-based extraction vs 33.3% with control (between-group difference, −1.3%; 95% CI, −13.8% to 11.2%) and 25.6% with intra-aortic filtration vs 32.4% with control (between-group difference, −6.9%; 95% CI, −17.9% to 4.2%). The 30-day composite end point was not significantly different between suction-based extraction and control (21.4% vs 24.2%, respectively; between-group difference, −2.8% [95% CI, −13.5% to 7.9%]) nor between intra-aortic filtration and control (33.3% vs 23.7%; between-group difference, 9.7% [95% CI, −1.2% to 20.5%]). There were no significant differences in mortality (3.4% for suction-based extraction vs 1.7% for control; and 2.3% for intra-aortic filtration vs 1.5% for control) or clinical stroke (5.1% for suction-based extraction vs 5.8% for control; and 8.3% for intra-aortic filtration vs 6.1% for control). Delirium at postoperative day 7 was 6.3% for suction-based extraction vs 15.3% for control (between-group difference, −9.1%; 95% CI, −17.1% to −1.0%) and 8.1% for intra-aortic filtration vs 15.6% for control (between-group difference, −7.4%; 95% CI, −15.5% to 0.6%). Mortality and overall serious adverse events at 90 days were not significantly different across groups. Patients in the intra-aortic filtration group vs patients in the control group experienced significantly more acute kidney injury events (14 vs 4, respectively; P = .02) and cardiac arrhythmias (57 vs 30; P = .004).
Conclusions and Relevance
Among patients undergoing SAVR, cerebral embolic protection devices compared with a standard aortic cannula did not significantly reduce the risk of CNS infarction at 7 days. Potential benefits for reduction in delirium, cognition, and symptomatic stroke merit larger trials with longer follow-up.
Trial Registration
clinicaltrials.gov Identifier: NCT02389894
This randomized clinical trial compares the efficacy and adverse effects of 2 cerebral embolic protection devices vs a shared control group in reducing ischemic central nervous system injury during surgical aortic valve replacement.
Introduction
The prevalence of aortic stenosis increases with aging and the expanding number of US patients requiring surgical aortic valve replacement (SAVR) or transcatheter aortic valve replacement (TAVR) is estimated to be 100 000 per year. Steady improvements in patient selection, surgical techniques, and perioperative management have improved survival and quality of life for these patients. However, concerns remain about the incidence of central nervous system (CNS) infarction, a serious complication of SAVR. Studies documented a high incidence (up to 60%) of radiographic brain infarcts on postoperative magnetic resonance imaging (MRI) scans, although the vast majority are subclinical. In a cohort study, 17% of patients undergoing SAVR (enrolled from 2008-2012) experienced clinical stroke, about 25% of which were moderate or severe. The effect of perioperative stroke on survival, quality of life, and cost is well established, whereas the effect of silent (nonsymptomatic) cerebral infarcts on these outcomes is unknown.
These concerns stimulated the development of cerebral embolic protection devices. Currently 2 devices are approved in the United States. The Embol-X (Edwards Lifesciences) intra-aortic filtration device has been shown to be safe and capture emboli (with a heparin-coated polyester mesh filter), but not reduce stroke in a mostly low-risk population undergoing coronary artery bypass graft surgery. The CardioGard (CardioGard) device extracts both particulate and gaseous emboli through suction-based extraction. A small trial of this device among patients undergoing SAVR reported a significant reduction in the total volume and number of radiographically detected brain lesions, but the effect on cognitive outcomes was not evaluated. More rigorous data are needed on the value of cerebral embolic protection devices in reducing ischemic CNS injury documented by clinical and radiographic means.
This trial evaluated the efficacy and adverse effects of these cerebral embolic protection devices among patients undergoing SAVR, which is a high-risk setting for CNS infarction. It included clinical, radiographic, and cognitive outcomes within 90 days.
Methods
We conducted a randomized clinical trial at 18 North American centers that compared each of 2 approved cerebral embolic protection devices vs a standard aortic cannula (control) in patients undergoing SAVR. This trial had a coordinating center, an events adjudication committee, and a data and safety monitoring board appointed by the National Institutes of Health (NIH) overseeing the trial. Participating institutional review boards approved the protocol and all patients gave written informed consent (the trial protocol appears in Supplement 1).
The overall aim of the trial was to establish the effectiveness of embolic protection devices vs standard of care. At the time the trial was initially designed, patients were to be randomized to the treatment group to receive the intra-aortic filtration device or to receive a standard aortic cannula in a 1:1 ratio. Within 6 weeks after initiating the trial, the suction-based extraction device became available, and patients were then to be randomized to receive the intra-aortic filtration device, the suction-based extraction device, or standard aortic cannula in a 1:1:1 ratio. The trial was not designed to directly compare devices, which would have required a substantially larger sample size.
Patients, Interventions, and End Points
The trial randomized patients aged 60 years or older undergoing SAVR for aortic stenosis with minimal or no deficits within 7 days of randomization according to the preoperative scores on the NIH Stroke Scale (NIHSS; score ≤1) and the modified Rankin Scale (score ≤2). Key exclusion criteria included clinical stroke during the 3 months prior to randomization; cardiac catheterization; cerebral or aortic arch angiography within 3 days of planned SAVR; and active endocarditis. Patients were randomized immediately after sternotomy and stratified by center and procedure (isolated aortic valve replacement vs aortic valve replacement and coronary artery bypass graft with or without mitral valve repair). The intra-aortic filtration device uses a heparin-coated polyester mesh filter. Filter size was determined by measuring the size of the distal ascending aorta (using computed tomography or an intraoperative measurement). The suction-based extraction device has a suction sideport located posterior to the main port of an aortic perfusion cannula. In the control group, a standard aortic perfusion cannula was used (eAppendix 1 in Supplement 2). Patients underwent SAVR between March 2015 and July 2016. The end of follow-up was December 2016.
Patients were assessed at baseline and at postoperative days 1, 3, 7, 30, and 90; the investigators were blinded to the end point data. The primary end point was freedom from clinical or radiographic CNS infarction at 7 days (± 3 days) after the procedure. Radiographic CNS infarcts were identified using a diffusion-weighted 1.5-T or 3.0-T MRI scanner.
Imaging-based stroke ascertainment was supplemented by serial neurological assessment using the NIHSS (score at postoperative days 1, 3, and 7) and reporting of ischemic stroke adverse events detected during hospitalization. All MRIs were read by a core laboratory and stroke events with clinical findings (NIHSS score ≥2) were adjudicated by an events adjudication committee composed of vascular neurologists.
The composite secondary end point was mortality, clinical ischemic stroke (including newly MRI-detected CNS infarcts associated with focal findings by the NIHSS before postoperative day 7), or acute kidney injury within 30 days after surgery. Other secondary end points included volume and number of radiographic brain lesions; delirium (assessed using the 3-minute Diagnostic Interview for Confusion Assessment Method [CAM] or CAM for use in patients in the intensive care unit) at baseline and at postoperative days 1, 3, and 7; modified Rankin Scale score; Barthel Index score; Geriatric Depression Scale score; survival; serious adverse events; and hospital readmissions within 90 days.
Quality of life (physical and mental subscales from the 12-Item Short-Form Health Survey) and cognition in 6 domains (verbal memory, visual memory, executive functioning, visuospatial or constructional praxis, attention, and information processing speed) were assessed at 90 days. Additional details regarding the definition and assessment of outcomes appear in eAppendix 2 in Supplement 2.
Statistical Analysis
Patients were randomized with equal allocation to 1 of 2 cerebral embolic protection device groups or to the control group. Random permuted block sizes of 3, 6, and 9 were used. The randomization sequence was generated by a trial statistician and randomization assignment was controlled centrally through a web-based data collection system and performed in the operating room. A sample size of 165 patients in each group ensured that each comparison had a power of approximately 90% to detect a between-group difference of 17.5% from an assumed control rate of 50% in the incidence of postoperative CNS infarcts, which corresponds to a 35% reduction in risk.
A single interim analysis was prespecified and performed and was based on group sequential monitoring with Lan-DeMets efficacy boundaries defined by an O’Brien-Fleming spending function. The consideration of halting for futility was prespecified if conditional power was below 20% (eAppendix 3 in Supplement 2). Intention-to-treat χ2 tests performed at the nominal .05 level (2-sided) were used to test hypotheses about differences between the intervention devices and the control intervention.
No adjustment was made to the type I error rate because these 2 planned comparisons are separate comparisons of each device group vs a shared control. Based on the recommendation of the data and safety monitoring board at the interim analysis, randomization but not follow-up was halted due to low conditional power of observing any between-group differences for the primary end point. At that point, 383 patients had been randomized.
The primary end point analysis used an iterative hot-deck multiple imputation approach, assuming a nonignorable missing data mechanism (eAppendix 3 in Supplement 2). The proportion of patients who experienced the composite clinical end point was compared using χ2 tests. The volume of brain infarcts was compared by permutation test.
Negative binomial models were used to analyze the number of brain infarcts (instead of protocol-defined zero-inflated Poisson regression models) because model fit was better. All-cause mortality was analyzed using the log-rank test and differences in adverse event rates were tested using Poisson regression. The incidence of delirium at postoperative days 1, 3, and 7 were compared using χ2 tests and the trajectory of delirium incidence over time was compared using generalized estimating equation logistic regression models.
The decline in scores for cognitive domains (decrease of 0.5 SD at 90 days) was compared between groups using logistic regression models, adjusting for baseline scores. Quality of life was assessed using t tests. Depression (presence vs absence), modified Rankin Scale scores (>2), and Barthel Index scores (≤80) were assessed using χ2 tests. All statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc).
Results
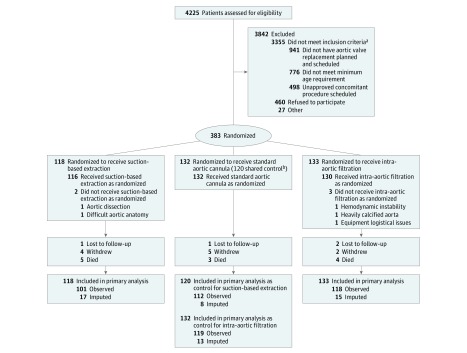
A total of 870 patients were screened and found eligible (Figure 1). Among 383 patients (mean age, 73.9 years; 38.4% women; 368 [96.1%] completed the trial), 118 were randomized to the suction-based extraction group, 133 to the intra-aortic filtration group, and 132 to the control group. Of the 132 patients randomized to the control group, the first 12 served as controls for the intra-aortic filtration group only because the suction-based extraction device was not yet clinically available and the other 120 served as controls for both the suction-based extraction group and the intra-aortic filtration group.
Figure 1. Flow of Patients Through the Cerebral Embolic Protection Device Trial.
aThese are the top 3 reasons for not meeting inclusion criteria. There were 1140 patients who were excluded for other reasons.
bThe first 12 patients randomized to the control group served as controls for the intra-aortic filtration group only and the other 120 patients served as controls for both the suction-based extraction group and the intra-aortic filtration group.
The randomized groups had similar baseline characteristics (Table 1 and eTable 1 in Supplement 2). Preoperatively, 36.3% of patients randomized to the suction-based extraction group had severe cognitive impairment (≥2 SDs below the mean of an age-standardized population) in 1 or more of the domains specified in Table 1 vs 25.7% of patients in the control group and 29.8% of patients in the intra-aortic filtration group vs 25.8% of patients in the control group.
Table 1. Baseline and Operative Characteristics.
| Suction-Based Extraction (n = 118) |
Intra-Aortic Filtration (n = 133) |
Controla (n = 132) |
|
|---|---|---|---|
| Baseline Characteristics | |||
| Male sex, No. (%) | 69 (58.5) | 81 (60.9) | 86 (65.2) |
| White race, No. (%) | 108 (91.5) | 126 (94.7) | 118 (89.4) |
| Age, mean (SD), y | 74.6 (6.8) | 73.6 (6.6) | 73.6 (6.7) |
| Medical and surgical history, No. (%) | |||
| Diabetes | 48 (40.7) | 36 (27.1) | 37 (28.0) |
| Renal insufficiency | 15 (12.7) | 18 (13.5) | 14 (10.6) |
| Myocardial infarction | 16 (13.6) | 15 (11.3) | 10 (7.6) |
| Atrial fibrillation | 14 (11.9) | 13 (9.8) | 16 (12.1) |
| Stroke or transient ischemic attack | 16 (13.6) | 11 (8.3) | 8 (6.1) |
| 12-Item Short-Form Health Survey score, mean (SD)b | |||
| Physical health composite | 41.4 (10.6) | 40.1 (11.0) | 40.2 (11.2) |
| Mental health composite | 53.2 (9.3) | 52.9 (9.6) | 52.9 (9.4) |
| Severe cognitive impairment, No./total (%)c | |||
| Verbal memory | 16/114 (14.0) | 19/127 (15.0) | 16/128 (12.5) |
| Executive function | 18/98 (18.4) | 21/119 (17.6) | 18/117 (15.4) |
| Auditory-verbal simple attention | 5/115 (4.3) | 4/127 (3.1) | 6/128 (4.7) |
| Visuomotor or information processing speed | 9/109 (8.3) | 11/123 (8.9) | 9/125 (7.2) |
| ≥1 Deficit | 37/102 (36.3) | 36/121 (29.8) | 31/120 (25.8) |
| White matter lesion volume, median (IQR), mm3 | 4592 (2433-8377) | 6303 (2686-10 027) | 4704 (2265-9776) |
| Large cortical lesions, No./total (%)d | 1/96 (1.0) | 2/109 (1.8) | 2/114 (1.8) |
| Maximum atheroma grade, mean (SD)e | 2.5 (0.7) | 2.3 (0.7) | 2.3 (0.6) |
| Operative Characteristics | |||
| Type of surgical procedure, No. (%) | |||
| Isolated aortic valve replacement | 67 (56.8) | 77 (57.9) | 80 (60.6) |
| Aortic valve replacement and CABG | 50 (42.4) | 51 (38.3) | 52 (39.4) |
| Aortic valve replacement and mitral valve repair with or without CABG | 1 (0.8) | 5 (3.8) | 0 |
| Concomitant procedures, No. (%)f | 17 (14.4) | 23 (17.3) | 20 (15.2) |
| Duration of cardiopulmonary bypass, mean (SD), min | 104.9 (39.6) | 109.1 (42.4) | 101.7 (39.8) |
| Debris captured in filter, No./total (%) | 79/106 (74.5) | 115/116 (99.1) | |
| Type of debris captured, No./total (%) | |||
| Calcification | 30/106 (28.3) | 23/116 (19.8) | |
| Valve tissue, arterial wall, or both | 53/106 (50.0) | 113/116 (97.4) | |
| Platelet-rich thrombus | 55/106 (51.9) | 39/116 (33.6) | |
| Other | 8/106 (7.5) | 13/116 (11.2) | |
Abbreviations: CABG, coronary artery bypass graft; IQR, interquartile range.
The first 12 patients randomized to the control group served as controls for the intra-aortic filtration group only and the other 120 patients served as controls for both the suction-based extraction group and the intra-aortic filtration group (eTable 1 in Supplement 2).
A higher score indicates a better health state. Values are normed as t scores (mean, 50 [SD, 10]).
Defined as 2 SD or greater below the mean in an age-standardized population. Cognition was measured using the revised Hopkins Verbal Learning Test, the Controlled Oral Word Association Test, parts A and B of the Trail Making Test, the Medical College of Georgia Complex Figures, and the third revision of the Wechsler Adult Intelligence Scale.
Defined as having a chronic infarct involving cortical gray matter with a maximum diameter greater than or equal to 4.0 cm.
Examined in the ascending aorta and the aortic arch and graded according to the grading scale by Katz (range, 1-5; 1 indicates normal to mild intimal thickening and 5 indicates any thickness with a mobile component).
The most common were annular enlargement for valve placement, ascending aorta repair or replacement, and left atrial appendage ligation.
Most patients underwent either isolated SAVR (58%) or a combined SAVR and coronary artery bypass graft procedure (41%). Cardiopulmonary bypass times were similar in both device groups and in the control group. Three patients randomized to intra-aortic filtration and 2 patients randomized to suction-based extraction did not receive the designated device due to anatomic constraints and hemodynamic instability. Embolic debris was captured in 99% of patients in the intra-aortic filtration group and in 75% of patients in the suction-based extraction group (Table 1).
Freedom from CNS infarction at postoperative day 7 (using imputation for missing data) was not significantly different between the suction-based extraction group and the control group (32.0% vs 33.3%, respectively; between-group difference, −1.3% [95% CI, −13.8% to 11.2%]) nor between the intra-aortic filtration group and the control group (25.6% vs 32.4%, respectively; between-group difference, −6.9% [95% CI, −17.9% to 4.2%]). Sensitivity analyses assuming different missing data mechanisms appear in eTable 2 in Supplement 2. Among patients who met the primary end point, the majority did not show clinical evidence of stroke (91% in suction-based extraction group vs 90% in control group; and 87% in intra-aortic filtration group vs 90% in control group). End point analyses stratified by procedure and site are provided in the eTable 3 in Supplement 2.
The proportion of patients in the suction-based extraction device group (with or without an MRI) who had clinically apparent stroke was 5.1% vs 5.8% among patients in the control group (between-group difference, −0.7%; 95% CI, −6.5% to 5.1%). The proportion of patients in the intra-aortic filtration group with stroke was 8.3% vs 6.1% among patients in the control group (between-group difference, 2.2%; 95% CI, −4.1% to 8.4%). Seventy-six percent of clinically apparent strokes were detected by postoperative day 3, and there were fewer cases of severe stroke among patients who received a cerebral embolic protection device within this 3-day window in a post hoc analysis (Table 2).
Table 2. Clinical End Points and Serious Adverse Events.
| Suction-Based Extraction (n = 118)a |
Control (n = 120)b,c |
Absolute Difference (95% CI)d |
P Valuee |
Intra-Aortic Filtration (n = 133)f |
Control (n = 132)b,g |
Absolute Difference (95% CI)d |
P Valuee |
|
|---|---|---|---|---|---|---|---|---|
| Clinical End Points Within First 7 d, No./Total (%) | ||||||||
| Primary end pointh | ||||||||
| Imputed, % (95% CI) | 68.0 (59.1 to 76.8) | 66.7 (58.1 to 75.2) | 1.3 (−11.2 to 13.8) | .84 | 74.4 (66.4 to 82.5) | 67.6 (58.8 to 76.4) | 6.9 (−4.2 to 17.9) | .22 |
| Observed | 68/101 (67.3) | 73/112 (65.2) | 2.1 (−10.6 to 14.9) | .74 | 86/118 (72.9) | 78/119 (65.5) | 7.3 (−4.4 to 19.1) | .22 |
| Died within 7 di | 0/117 | 1/120 (0.8) | −0.8 (−2.5 to 0.8) | >.99 | 1/133 (0.8) | 1/131 (0.8) | 0 (−2.1 to 2.1) | >.99 |
| Radiographic infarctsj | 66/101 (65.3) | 71/111 (64.0) | 1.4 (−11.5 to 14.3) | .83 | 83/115 (72.2) | 76/118 (64.4) | 7.8 (−4.1 to 19.7) | .20 |
| Clinically apparent stroke within 7 di,k | 6/117 (5.1) | 7/120 (5.8) | −0.7 (−6.5 to 5.1) | .81 | 11/133 (8.3) | 8/131 (6.1) | 2.2 (−4.1 to 8.4) | .50 |
| Severe (NIHSS score >20) | 1/6 (16.7) | 3/7 (42.9) | 1/11 (9.1) | 3/8 (37.5) | ||||
| Moderate to severe (NIHSS score 16-20) | 0/6 | 0/7 | 0/11 | 0/8 | ||||
| Moderate (NIHSS score 5-15) | 2/6 (33.3) | 1/7 (14.3) | 5/11 (45.5) | 2/8 (25.0) | ||||
| Mild (NIHSS score 0-4) | 3/6 (50.0) | 3/7 (42.9) | 5/11 (45.5) | 3/8 (37.5) | ||||
| Clinically apparent stroke within 3 d | 4/6 (66.7) | 6/7 (85.7) | 8/11 (72.7) | 7/8 (87.5) | ||||
| Severe (NIHSS score >20) | 0/4 | 3/6 (50.0) | 1/8 (12.5) | 3/7 (42.9) | ||||
| Moderate to severe (NIHSS score 16-20) | 0/4 | 0/6 | 0/8 | 0/7 | ||||
| Moderate (NIHSS score 5-15) | 1/4 (25.0) | 1/6 (16.7) | 5/8 (62.5) | 2/7 (28.6) | ||||
| Mild (NIHSS score 0-4) | 3/4 (75.0) | 2/6 (33.3) | 2/8 (25.0) | 2/7 (28.6) | ||||
| Deliriuml | ||||||||
| Day 1 | 19/111 (17.1) | 16/110 (14.5) | 2.6 (−7.0 to 12.2) | .60 | 27/121 (22.3) | 18/119 (15.1) | 7.2 (−2.6 to 17.0) | .15 |
| Day 3 | 15/108 (13.9) | 13/113 (11.5) | 2.4 (−6.4 to 11.2) | .59 | 15/117 (12.8) | 18/124 (14.5) | −1.7 (−10.4 to 7.0) | .70 |
| Day 7 | 7/112 (6.3) | 17/111 (15.3) | −9.1 (−17.1 to −1.0) | .03 | 10/123 (8.1) | 19/122 (15.6) | −7.4 (−15.5 to 0.6) | .07 |
| Clinical End Points at 30 d, No./Total (%)i | ||||||||
| Compositem | 25/117 (21.4) | 29/120 (24.2) | −2.8 (−13.5 to 7.9) | .61 | 44/132 (33.3) | 31/131 (23.7) | 9.7 (−1.2 to 20.5) | .08 |
| Clinically apparent stroke | 6/117 (5.1) | 7/120 (5.8) | −0.7 (−6.5 to 5.1) | .81 | 11/132 (8.3) | 8/131 (6.1) | 2.2 (−4.0 to 8.5) | .49 |
| Died | 4/117 (3.4) | 2/120 (1.7) | 1.8 (−2.3 to 5.8) | .44 | 3/132 (2.3) | 2/131 (1.5) | 0.7 (−2.6 to 4.0) | >.99 |
| AKI (stage 1-3, serious, and nonserious) | 19/117 (16.2) | 24/120 (20.0) | −3.8 (−13.6 to 6.0) | .45 | 35/132 (26.5) | 25/131 (19.1) | 7.4 (−2.7 to 17.5) | .15 |
| Serious Adverse Events by 90 d, No. of Events (Rate/100 Patient-Months) | ||||||||
| AKIn | 3 (0.9) | 4 (1.2) | −0.3 (−1.8 to 1.3) | .74 | 14 (3.8) | 4 (1.1) | 2.7 (0.4 to 4.9) | .02 |
| Stage 1 | 1 (0.3) | 0 | 0.3 (−0.3 to 0.9) | .32 | 3 (0.8) | 0 | 0.8 (−0.1 to 1.7) | .08 |
| Stage 2 | 0 | 2 (0.6) | −0.6 (−1.4 to 0.2) | .16 | 4 (1.1) | 2 (0.5) | 0.5 (−0.8 to 1.8) | .42 |
| Stage 3 | 2 (0.6) | 2 (0.6) | 0 (−1.2 to 1.2) | .97 | 7 (1.9) | 2 (0.5) | 1.3 (−0.2 to 2.9) | .10 |
| Bleeding | 8 (2.5) | 6 (1.8) | 0.7 (−1.5 to 2.9) | .55 | 5 (1.3) | 6 (1.6) | −0.3 (−2.0 to 1.5) | .75 |
| Cardiac arrhythmias | 31 (9.5) | 25 (7.4) | 2.1 (−2.3 to 6.6) | .35 | 57 (15.3) | 30 (8.1) | 7.2 (2.3 to 12.1) | .004 |
| Cardiac arrest | 0 | 1 (0.3) | −0.3 (−0.9 to 0.3) | .32 | 6 (1.6) | 2 (0.5) | 1.1 (−0.4 to 2.6) | .16 |
| Sustained ventricular arrhythmia with defibrillation or cardioversion | 0 | 0 | 1 (0.3) | 0 | 0.3 (−0.3 to 0.8) | .32 | ||
| Sustained supraventricular arrhythmia with drug treatment or cardioversion | 25 (7.7) | 22 (6.5) | 1.2 (−2.9 to 5.2) | .58 | 43 (11.6) | 25 (6.8) | 4.8 (0.4 to 9.1) | .03 |
| Conduction abnormalities or sustained bradycardia with permanent pacemaker placement | 6 (1.8) | 2 (0.6) | 1.3 (−0.4 to 2.9) | .15 | 7 (1.9) | 3 (0.8) | 1.1 (−0.6 to 2.7) | .21 |
| Major infectiono | 10 (3.1) | 12 (3.6) | −0.5 (−3.3 to 2.3) | .73 | 15 (4.0) | 15 (4.1) | 0 (−2.9 to 2.9) | .98 |
| Myocardial infarction | 0 | 0 | 3 (0.8) | 0 | 0.8 (−0.1 to 1.7) | .08 | ||
| Neurological dysfunction | 4 (1.2) | 10 (3.0) | −1.7 (−3.9 to 0.5) | .12 | 7 (1.9) | 12 (3.3) | −1.4 (−3.7 to 0.9) | .24 |
| Transient ischemic attack | 0 | 1 (0.3) | −0.3 (−0.9 to 0.3) | .32 | 0 | 1 (0.3) | −0.3 (−0.8 to 0.3) | .32 |
| Ischemic stroke | 3 (0.9) | 3 (0.9) | 0 (−1.4 to 1.5) | .97 | 4 (1.1) | 4 (1.1) | 0 (−1.5 to 1.5) | .99 |
| Toxic metabolic encephalopathy | 1 (0.3) | 1 (0.3) | 0 (−0.8 to 0.8) | .98 | 2 (0.5) | 1 (0.3) | 0.3 (−0.6 to 1.2) | .57 |
| Seizure | 0 | 2 (0.6) | −0.6 (−1.4 to 0.2) | .16 | 0 | 3 (0.8) | −0.8 (−1.7 to 0.1) | .08 |
| Other | 0 | 3 (0.9) | −0.9 (−1.9 to 0.1) | .08 | 1 (0.3) | 3 (0.8) | −0.5 (−1.6 to 0.5) | .31 |
| Renal failure | 0 | 2 (0.6) | −0.6 (−1.4 to 0.2) | .16 | 1 (0.3) | 2 (0.5) | −0.3 (−1.2 to 0.6) | .56 |
| Respiratory failure | 3 (0.9) | 9 (2.7) | −1.8 (−3.8 to 0.3) | .09 | 8 (2.2) | 11 (3.0) | −0.8 (−3.1 to 1.5) | .48 |
| Heart failure | 4 (1.2) | 8 (2.4) | −1.1 (−3.2 to 0.9) | .27 | 2 (0.5) | 9 (2.4) | −1.9 (−3.7 to −0.1) | .03 |
| Venous thromboembolism eventp | 1 (0.3) | 2 (0.6) | −0.3 (−1.3 to 0.7) | .58 | 4 (1.1) | 2 (0.5) | 0.5 (−0.8 to 1.8) | .42 |
| All serious adverse events | 91 (28.0) | 112 (33.3) | −5.3 (−13.7 to 3.2) | .22 | 157 (42.2) | 126 (34.2) | 8.0 (−0.8 to 16.9) | .08 |
| Hospital readmissions | 24 (8.4) | 20 (6.8) | 1.7 (−2.8 to 6.1) | .47 | 30 (9.3) | 23 (7.1) | 2.2 (−2.3 to 6.6) | .34 |
| Quality of Life at 90 d | ||||||||
| 12-Item Short-Form Health Survey score, mean (95% CI)q | ||||||||
| Mental health composite | 55.4 (53.8 to 57.1) | 55.1 (53.5 to 56.6) | 0.4 (−1.8 to 2.6) | .74 | 55.2 (53.4 to 57.0) | 54.8 (53.4 to 56.3) | 0.3 (−2.0 to 2.7) | .77 |
| Physical health composite | 44.9 (43.2 to 46.5) | 44.7 (43.1 to 46.3) | 0.1 (−2.2 to 2.4) | .92 | 43.0 (41.1 to 45.0) | 44.2 (42.6 to 45.8) | −1.2 (−3.7 to 1.3) | .36 |
| Geriatric Depression Scale score >10, No./total (%)r | 5/104 (4.8) | 7/108 (6.5) | −1.7 (−7.9 to 4.5) | .60 | 13/122 (10.7) | 9/119 (7.6) | 3.1 (−4.2 to 10.3) | .40 |
| Functional Status at 90 d, No./Total (%) | ||||||||
| Decline in overall neurocognitions | 24/81 (29.6) | 27/86 (31.4) | 1.1 (0.5 to 2.2)d | .82 | 28/98 (28.6) | 31/96 (32.3) | 0.8 (0.4 to 1.5)d | .54 |
| Modified Rankin Scale score >2t | 7/110 (6.4) | 4/112 (3.6) | 2.8 (−2.9 to 8.5) | .34 | 5/127 (3.9) | 5/123 (4.1) | −0.1 (−5.0 to 4.7) | >.99 |
| Barthel Index ≤80u | 2/105 (1.9) | 3/109 (2.8) | −0.8 (−4.9 to 3.2) | >.99 | 2/123 (1.6) | 4/120 (3.3) | −1.7 (−5.6 to 2.2) | .44 |
| Mortality, No. | 5 | 3 | 1.7 (0.4 to 7.2)d | .45 | 4 | 3 | 1.3 (0.3 to 5.9)d | .71 |
Abbreviations: AKI, acute kidney injury; NIHSS, National Institutes of Health Stroke Scale.
The number of patient-months was 324.7 and there were 284.8 patient-months outside the hospital.
The first 12 patients randomized to the control group served as controls for the intra-aortic filtration group only and the other 120 patients served as controls for both the suction-based extraction group and the intra-aortic filtration group.
The number of patient-months was 336.3 and there were 295.6 patient-months outside the hospital.
For decline in overall neurocognition, the odds ratio (95% CI) is given because the analysis is based on a logistic model adjusting for baseline score. For mortality, the hazard ratio (95% CI) is given because the analysis was time to event.
Calculated using the χ2 test or the Fisher exact test for the clinical, modified Rankin Scale, Barthel Index, and Geriatric Depression Scale end points; using Poisson regression for serious adverse events and hospitalizations; using t tests for 12-Item Short-Form Health Survey scores; using a logistic regression model (adjusted for baseline score) for neurocognitive decline; and using the log-rank test for mortality.
The number of patient-months was 372.0 and there were 322.7 patient-months outside the hospital.
The number of patient-months was 368.9 and there were 322.8 patient-months outside the hospital.
Freedom from clinical or radiographic central nervous system infarction measured by diffusion-weighted magnetic resonance imaging (MRI) at 7 days (± 3 days) after the procedure. Deaths were counted as treatment failures. The denominator for the observed primary end point is the number of patients with a nonmissing MRI image or evidence of a clinical stroke or death before 7 days.
Two patients withdrew prior to day 7 and were not included in the denominators; 1 additional patient withdrew prior to day 30.
Of 334 patients, the sites used a diffusion-weighted 1.5-T MRI scanner for 210 (63%) and a diffusion-weighted 3-T MRI scanner for 124 (37%).
Five patients had evidence of a clinical infarction and either did not undergo diffusion-weighted MRI or had no lesions (2 in suction-based extraction group, 2 in intra-aortic filtration group, and 1 in the control group).
Measured by Confusion Assessment Method assessment at baseline and at days 1, 3, and 7.
Clinically apparent stroke, AKI, or death within 30 days of surgery.
The AKI stage definitions appear in eAppendix 2 in Supplement 2.
Defined as a new infection accompanied by pain, fever, drainage, or leukocytosis that is treated with an antimicrobial agent (nonprophylactic). A positive culture from the infected site or organ should be present unless strong clinical evidence indicates the need for treatment despite negative cultures.
Deep vein thrombosis, pulmonary embolism, or other.
A higher score indicates a better health state. Values are normed as t scores (mean, 50 [SD, 10]).
A score of 10 or less indicates no depression, whereas a score of 11 or greater indicates depression.
Defined as the number of patients whose z score (computed relative to the study population at baseline, adjusting for age, education, and sex) at day 90 had decreased by 0.5 SD relative to the baseline score.
A score of 3 to 5 corresponds to moderate disability; score of 6, death.
A score of 80 or less corresponds to mild to moderate or greater disability.
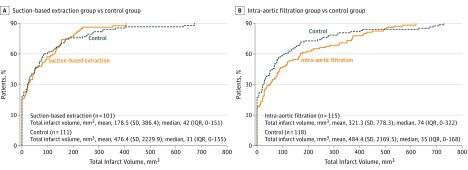
Patients frequently showed evidence of multiple new CNS infarcts. The number of infarcts was not significantly different between patients in the suction-based extraction group and the control group (mean, 2.4 vs 2.3, respectively; between-group difference, 0.1 [95% CI, −0.8 to 1.0]; P = .88), nor between patients in the intra-aortic filtration group and the control group (mean, 2.8 vs 2.7, respectively; between-group difference, 0.1 [95% CI, −0.8 to 1.1]; P = .78). Total lesion volume was not significantly different between patients in the suction-based extraction group and the control group (mean, 178.5 mm3 vs 476.4 mm3, respectively; between-group difference, −297.9 mm3 [95% CI, −741.3 to 145.6 mm3]; P = .28), nor between patients in the intra-aortic filtration group and the control group (mean, 321.3 mm3 vs 484.4 mm3, respectively; between-group difference, −163.1 mm3 [95% CI, −586.0 to 259.8 mm3]; P = .49).
Figure 2 depicts the cumulative distribution of total lesion volume up to the 90th percentile. Infarct volumes were larger in patients with a clinically apparent stroke (mean, 1688 mm3) than in those without a clinically apparent stroke (mean, 236 mm3) (between-group difference, 1451.9 mm3 [95% CI, 883.0-2020.8 mm3]; P = .001). Data on the risk of large volume infarcts appear in the eFigure in Supplement 2.
Figure 2. Distribution of Volume of Infarcted Brain Tissue by Randomization Group Observed on the Day 7 Diffusion-Weighted MRI Scan.
Panels A and B depict the cumulative distribution of observed infarct volume up to the 90th percentile for each treatment group compared with the control group. The y-axis gives the percentage of patients with an observed total infarct volume less than or equal to that of the corresponding infarct volume on the x-axis. IQR indicates interquartile range; MRI, magnetic resonance imaging.
The proportion of patients experiencing the composite end point of death, clinically apparent ischemic stroke, or acute kidney injury within 30 days of surgery was 21.4% in the suction-based extraction group vs 24.2% in the control group (between-group difference, −2.8%; 95% CI, −13.5% to 7.9%) and 33.3% in the intra-aortic filtration group vs 23.7% in the control group (between-group difference, 9.7%; 95% CI, −1.2% to 20.5%). The individual components of the composite 30-day end point were not significantly different across groups (Table 2).
Mortality, composite neurological events, and overall serious adverse events at 90 days were not significantly different across groups (Table 2). Patients in the intra-aortic filtration group vs patients in the control group experienced significantly more acute kidney injury events (14 vs 4, respectively; between-group difference, 2.7 [95% CI, 0.4-4.9]) and cardiac arrhythmias (57 vs 30, respectively; between-group difference, 7.2; 95% CI, 2.3-12.1).
The length of stay during the index hospitalization was not significantly different between patients in the suction-based extraction group and in the control group (mean, 9.8 days vs 10.3 days, respectively; between-group difference, −0.5 days [95% CI, −2.1 to 1.1 days]) nor was length of ICU stay (mean, 4.0 days vs 4.2 days, respectively; between-group difference, −0.1 days [95% CI, −1.0 to 0.7 days]). Similarly, length of stay during the index hospitalization was not significantly different between patients in the intra-aortic filtration group and in the control group (mean, 10.4 days vs 10.3 days, respectively; between-group difference, 0.1 days [95% CI, −1.5 to 1.7 days]), nor was length of ICU stay (mean, 4.5 days vs 4.1 days, respectively; between-group difference, 0.4 days [95% CI, −0.5 to 1.2 days]). Hospital readmission rates were not significantly different between both intervention groups and the control group.
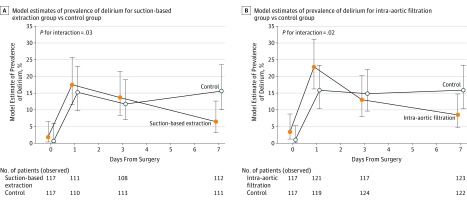
There were significant differences in the trajectories for delirium occurrence (baseline to day 7) among patients in the suction-based extraction group vs the control group (P = .03) and among patients in the intra-aortic filtration group vs the control group (P = .02; Figure 3). At postoperative day 7, 6.3% of patients in the suction-based extraction group experienced delirium vs 15.3% of patients in the control group (between-group difference, −9.1%; 95% CI, −17.1% to −1.0%) and 8.1% of patients in the intra-aortic filtration group vs 15.6% of patients in the control group (between-group difference, −7.4%; 95% CI, −15.5% to 0.6%).
Figure 3. Prevalence of Delirium Over Time.
Delirium was measured by Confusion Assessment Method assessment at baseline and at days 1, 3, and 7. The probability of delirium over time was modeled using generalized estimating equations. The interaction term between days from surgery and randomization group was statistically significant (P < .05), indicating that the incidence of delirium over time was significantly different between the treatment groups and the control group.
The 12-Item Short-Form Health Survey composite physical and mental health scores were not significantly different between the 2 device intervention groups and the control group (Table 2). Moreover, no differences were observed in the overall cognition scores for the 2 device intervention groups and the control group (Table 2). However, the decline in overall cognition scores was greater for patients experiencing clinically apparent stroke than for other patients (71.4% vs 28.0%; between-group difference, 43.5% [95% CI, 19.2%-67.7%]). With the exception of executive function, which showed less decline among patients in the intra-aortic filtration group vs patients in the control group, there were no differences among the neurocognitive domain comparisons (eTable 4 in Supplement 2).
Discussion
Despite the fact that debris was captured in most patients who received a cerebral embolic protection device, rates of clinical and radiographic infarction were not reduced. Nearly 69% of patients who underwent SAVR experienced clinical or radiographic stroke. However, the majority of these events was only detectable by postoperative diffusion-weighted MRI, with just 9% of patients exhibiting clinical findings. Neither the number of MRI lesions nor total lesion volume differed between patients receiving either of the cerebral embolic protection devices and the patients in the control group. However, the infarct volume pattern suggested a possible differential effect of devices compared with the control intervention, with larger volume infarcts more numerous in patients in the control group. This observation may be important because the risk of clinically evident stroke increases with infarct volume.
The reported incidence of newly detected clinical and radiographic infarcts after cardiac surgery has varied across observational studies likely in relation to cohort size, patient characteristics, approaches to clinical assessment, and imaging techniques. The volume of radiographic infarcts seen in this study (the majority of which were small with a median infarct volume of 48.7 mm3) is concordant with that reported in prior studies using MRI after patients underwent SAVR. Imaging studies in the general population have reported that clinically silent brain infarction (including microinfarction <3 mm) is associated with dementia, mortality, and risk of future stroke and death; however, it is not clear whether these same associations pertain to periprocedural infarcts that are clinically silent.
The proportion of patients with clinical stroke findings at day 7 (with or without MRI evidence of infarction) were not significantly different between patients in the suction-based extraction group and the control group (5.1% vs 5.8%, respectively) nor between patients in the intra-aortic filtration group and the control group (8.3% vs 6.1%, respectively). However, the majority of clinical stroke findings were detected by postoperative day 3, suggesting that many cases of perioperative stroke may be preventable with use of a cerebral embolic protection device.
In a post hoc analysis, there were numerically fewer patients with severe clinical stroke (NIHSS score >20) among patients receiving a cerebral embolic protection device compared with patients in the control group at days 0 through 3. Given that severity (defined by NIHSS score) at onset is one of the strongest predictors of long-term outcome after acute stroke, a reduction in the risk of severe stroke during the early perioperative period might be a tangible benefit of cerebral embolic protection device use during SAVR, and may justify a larger trial.
The rate of clinically apparent stroke (6.5%) in this trial is much higher than the 7-day stroke incidence of 1% to 3% reported in several prior studies, and is most likely attributable to active ascertainment with early repeated neurological assessments. However, there was a lower incidence of stroke with clinical findings than the 17% seen in a previously reported cohort study, perhaps related to a treatment shift from SAVR to TAVR among high-risk patients that occurred during the course of this trial. There was a large difference in this trial between observed cases of stroke during the course of clinical care and cases of stroke detected through protocol-specified, routine NIHSS screening, suggesting that many clinical events are undetected or underreported in practice.
Delirium (defined as a disturbance of consciousness and cognition that can develop acutely after surgery) is common and occurs in 11% to 46% of patients after surgery. Prior studies have shown that cerebral embolization is associated with delirium, a condition that is costly and associated with poor outcomes, including prolonged hospitalization, readmissions, long-term cognitive decline, and mortality. Longitudinally (over 7 days postoperatively), there was a significant difference between patients who received one of the device interventions and patients in the control group. At day 7 (when perioperative medications are less of a factor), fewer patients who received a device experienced in-hospital delirium than patients in the control group and the difference achieved statistical significance with the suction-based extraction device. This difference may be related to the fact that, in addition to particulate matter, the suction-based device also extracts gaseous microemboli, which have been shown to affect neuropsychological functioning early during the postoperative phase among patients undergoing cardiac surgery.
More than 25% of patients had severe cognitive impairment on baseline testing in 1 domain compared with age-adjusted norms, especially in verbal memory and executive functioning. These domains are associated with late-onset Alzheimer-related pathology and cerebrovascular disease. Similar findings have been reported with TAVR. Given the advanced age of patients with aortic stenosis and the increased incidence of cerebrovascular disease, higher rates of postoperative cognitive impairment in populations undergoing SAVR and TAVR is not unexpected. These findings underscore the importance of evaluating cognitive impairment during risk assessment given its association with postoperative delirium and late-onset dementia.
Baseline performance-adjusted cognitive outcomes at 90 days were not significantly different between the patients in the device groups and the control group with the exception of a reduced decline in executive functioning in the intra-aortic filtration group. This finding requires more investigation, but might relate to the lower incidence of delirium observed among patients in the device treatment groups compared with patients in the control group.
There was evidence of an increased incidence of acute kidney injury and a higher rate of cardiac arrhythmias among patients in the intra-aortic filtration group compared with the control group. These findings merit further investigation, but need to be interpreted in the context of multiple statistical comparisons for adverse events, which increases the likelihood of finding abnormalities due to random variation alone.
Limitations
This trial has several limitations. First, even though the trial subscribes to the latest imaging recommendations for assessing neurological outcomes in the postcardiac surgery setting, the significance of the many small and clinically silent lesions identified by diffusion-weighted MRI cannot be established. Second, although the optimal time frame to assess the effectiveness of an intraoperative cerebral embolic protection device is within the first few postoperative days, 7-day imaging is more feasible for patients undergoing cardiac surgery. As a result, this study may have captured radiographic and clinical CNS infarctions not related to intraoperative embolization (eg, from postoperative atrial fibrillation), thus overestimating the infarct burden, whereas smaller lesions may have normalized by 7 days, underestimating the infarct burden.
Third, although the NIHSS score has been validated as a measure of stroke severity, it does not inherently inform whether a clinical stroke has occurred. However, clinical stroke end point adjudication was performed by experienced vascular neurologists who reviewed trial data to determine whether an event occurred. Fourth, randomization was halted due to low conditional power for the primary end point, diminishing the power to detect differences for the secondary end points. In addition, the long-term effects on cognition may not have been fully evident within the 90-day follow-up of the trial. Fifth, the trial was not designed to directly compare devices, which would have required a substantially larger sample size.
Conclusions
Among patients undergoing SAVR, cerebral embolic protection devices compared with a standard aortic cannula did not significantly reduce the risk of CNS infarction at 7 days. Potential benefits for reduction in delirium, cognition, and symptomatic stroke merit larger trials with longer follow-up.
Trial protocol
eAppendix 1. Devices and their Deployment
eAppendix 2. Outcomes and Ascertainment
eAppendix 3. Statistical Information
eFigure. Relative Risk of "Large Volume" Infarcts for Each Treatment Device Compared to Control
eTable 1. Baseline and operative characteristics
eTable 2. Incidence of Clinical or Radiographic CNS Infarction at 7 Days Post procedure Assuming Various Missing Data Mechanisms
eTable 3. Unadjusted and Adjusted Odds Ratios of Clinical or Radiographic CNS Infarction at 7 Days Post procedure
eTable 4. Neurocognitive Decline at 90 Days by Domain
eReferences
References
- 1.Duke Clinical Research Institute Adult cardiac surgery database: executive summary 10 years. http://www.sts.org/sites/default/files/documents/2016Harvest2_ExecutiveSummary_new.pdf. Accessed July 10, 2017.
- 2.Grover FL, Vemulapalli S, Carroll JD, et al. . 2016 Annual report of the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Ann Thorac Surg. 2017;103(3):1021-1035. [DOI] [PubMed] [Google Scholar]
- 3.Floyd TF, Shah PN, Price CC, et al. . Clinically silent cerebral ischemic events after cardiac surgery. Ann Thorac Surg. 2006;81(6):2160-2166. [DOI] [PubMed] [Google Scholar]
- 4.Alassar A, Soppa G, Edsell M, et al. . Incidence and mechanisms of cerebral ischemia after transcatheter aortic valve implantation compared with surgical aortic valve replacement. Ann Thorac Surg. 2015;99(3):802-808. [DOI] [PubMed] [Google Scholar]
- 5.Messé SR, Acker MA, Kasner SE, et al. . Stroke after aortic valve surgery. Circulation. 2014;129(22):2253-2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puskas JD, Winston AD, Wright CE, et al. . Stroke after coronary artery operation. Ann Thorac Surg. 2000;69(4):1053-1056. [DOI] [PubMed] [Google Scholar]
- 7.Salazar JD, Wityk RJ, Grega MA, et al. . Stroke after cardiac surgery: short- and long-term outcomes. Ann Thorac Surg. 2001;72(4):1195-1201. [DOI] [PubMed] [Google Scholar]
- 8.Banbury MK, Kouchoukos NT, Allen KB, et al. . Emboli capture using the Embol-X intraaortic filter in cardiac surgery. Ann Thorac Surg. 2003;76(2):508-515. [DOI] [PubMed] [Google Scholar]
- 9.Bolotin G, Huber CH, Shani L, et al. . Novel emboli protection system during cardiac surgery. Ann Thorac Surg. 2014;98(5):1627-1633. [DOI] [PubMed] [Google Scholar]
- 10.Sacco RL, Kasner SE, Broderick JP, et al. . An updated definition of stroke for the 21st century. Stroke. 2013;44(7):2064-2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeMets DL, Lan KKG. Interim analysis: the alpha spending function approach. Stat Med. 1994;13(13-14):1341-1352. [DOI] [PubMed] [Google Scholar]
- 12.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35(3):549-556. [PubMed] [Google Scholar]
- 13.Glas KE, Swaminathan M, Reeves ST, et al. . Guidelines for the performance of a comprehensive intraoperative epiaortic ultrasonographic examination. J Am Soc Echocardiogr. 2007;20(11):1227-1235. [DOI] [PubMed] [Google Scholar]
- 14.Stolz E, Gerriets T, Kluge A, et al. . Diffusion-weighted magnetic resonance imaging and neurobiochemical markers after aortic valve replacement. Stroke. 2004;35(4):888-892. [DOI] [PubMed] [Google Scholar]
- 15.Cook DJ, Huston J III, Trenerry MR, et al. . Postcardiac surgical cognitive impairment in the aged using diffusion-weighted magnetic resonance imaging. Ann Thorac Surg. 2007;83(4):1389-1395. [DOI] [PubMed] [Google Scholar]
- 16.Barber PA, Hach S, Tippett LJ, et al. . Cerebral ischemic lesions on diffusion-weighted imaging are associated with neurocognitive decline after cardiac surgery. Stroke. 2008;39(5):1427-1433. [DOI] [PubMed] [Google Scholar]
- 17.Patel N, Horsfield MA, Banahan C, et al. . Impact of perioperative infarcts after cardiac surgery. Stroke. 2015;46(3):680-686. [DOI] [PubMed] [Google Scholar]
- 18.Knipp SC, Matatko N, Schlamann M, et al. . Small ischemic brain lesions after cardiac valve replacement detected by diffusion-weighted magnetic resonance imaging. Eur J Cardiothorac Surg. 2005;28(1):88-96. [DOI] [PubMed] [Google Scholar]
- 19.Uddin A, Fairbairn TA, Djoukhader IK, et al. . Consequence of cerebral embolism after transcatheter aortic valve implantation compared with contemporary surgical aortic valve replacement. Circ Cardiovasc Interv. 2015;8(3):e001913. [DOI] [PubMed] [Google Scholar]
- 20.Vermeer SE, Hollander M, van Dijk EJ, et al. . Silent brain infarcts and white matter lesions increase stroke risk in the general population. Stroke. 2003;34(5):1126-1129. [DOI] [PubMed] [Google Scholar]
- 21.Patel N, Minhas JS, Chung EM. Risk factors associated with cognitive decline after cardiac surgery: a systematic review. Cardiovasc Psychiatry Neurol. 2015;2015:370612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel N, Minhas JS, Chung EM. The presence of new MRI lesions and cognitive decline after cardiac surgery. J Card Surg. 2015;30(11):808-812. [DOI] [PubMed] [Google Scholar]
- 23.Meller SM, Baumbach A, Brickman AM, Lansky AJ. Clinical implications for diffusion-weighted MRI brain lesions associated with transcatheter aortic valve replacement. Catheter Cardiovasc Interv. 2014;83(3):502-508. [DOI] [PubMed] [Google Scholar]
- 24.König IR, Ziegler A, Bluhmki E, et al. . Predicting long-term outcome after acute ischemic stroke. Stroke. 2008;39(6):1821-1826. [DOI] [PubMed] [Google Scholar]
- 25.Shahian DM, O’Brien SM, Filardo G, et al. . The Society of Thoracic Surgeons 2008 cardiac surgery risk models. Ann Thorac Surg. 2009;88(1)(suppl):S43-S62. [DOI] [PubMed] [Google Scholar]
- 26.O’Brien SM, Shahian DM, Filardo G, et al. . The Society of Thoracic Surgeons 2008 cardiac surgery risk models. Ann Thorac Surg. 2009;88(1)(suppl):S23-S42. [DOI] [PubMed] [Google Scholar]
- 27.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franco K, Litaker D, Locala J, Bronson D. The cost of delirium in the surgical patient. Psychosomatics. 2001;42(1):68-73. [DOI] [PubMed] [Google Scholar]
- 29.Saczynski JS, Marcantonio ER, Quach L, et al. . Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367(1):30-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gottesman RF, Grega MA, Bailey MM, et al. . Delirium after coronary artery bypass graft surgery and late mortality. Ann Neurol. 2010;67(3):338-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doganci S, Gunaydin S, Kocak OM, et al. . Impact of the intensity of microemboli on neurocognitive outcome following cardiopulmonary bypass. Perfusion. 2013;28(3):256-262. [DOI] [PubMed] [Google Scholar]
- 32.Kapadia SR, Kodali S, Makkar R, et al. . Protection against cerebral embolism during transcatheter aortic valve replacement. J Am Coll Cardiol. 2017;69(4):367-377. [DOI] [PubMed] [Google Scholar]
- 33.Lansky AJ, Messé SR, Brickman AM, et al. . Proposed standardized neurological endpoints for cardiovascular clinical trials. J Am Coll Cardiol. 2017;69(6):679-691. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eAppendix 1. Devices and their Deployment
eAppendix 2. Outcomes and Ascertainment
eAppendix 3. Statistical Information
eFigure. Relative Risk of "Large Volume" Infarcts for Each Treatment Device Compared to Control
eTable 1. Baseline and operative characteristics
eTable 2. Incidence of Clinical or Radiographic CNS Infarction at 7 Days Post procedure Assuming Various Missing Data Mechanisms
eTable 3. Unadjusted and Adjusted Odds Ratios of Clinical or Radiographic CNS Infarction at 7 Days Post procedure
eTable 4. Neurocognitive Decline at 90 Days by Domain
eReferences