Abstract
There is a need for fast detection methods for the banned rodenticide tetramethylenedisulfotetramine (TETS), a highly potent blocker of the γ-aminobutyric acid (GABAA) receptors. General synthetic approach toward two groups of analogues was developed. Screening of the resulting library of compounds by FLIPR or whole-cell voltage-clamp revealed that despite the structural differences some of the TETS analogues retained GABAA receptor inhibition, however their potency was an order of magnitude lower. Antibodies raised in rabbits against some of the TETS analogues conjugated to protein recognized free TETS and will be used for the development of an immunoassay for TETS.
Keywords: poly-heterocycles, tetramine, cage convulsants, neurotoxicity, GABA, antibody-based assay
Introduction
TETS (tetramethylenedisulfotetramine, tetramine) is a highly lethal neurotoxic rodenticide. It is a non-competitive channel blocker of the γ-aminobutyric acid (GABAA) receptors that induces excessive excitation of the adult central nervous system (CNS). The LD50 in laboratory animals is 0.1 mg/kg, and 7–10 mg is considered to be a lethal dose for humans.[1] Despite being banned worldwide it is still available on the black market in China and other countries because of its ease of manufacture, profitability and effectiveness as a rodenticide.[2] The illegal use of TETS has led to multiple accidental human poisoning cases. Additionally, being water soluble, odorless and tasteless it has been often used in intentional poisonings and thus is considered as a potential threat agent.[3] More than three thousand poisonings between 2000 and 2012 in China have been associated with TETS.[3b]
China implemented multiple regulatory enforcement measures which had a positive impact on the frequency of such events but failed to completely remove TETS-containing products from the open market because of the lack of technical means to test for highly toxic rodenticides such as TETS.[2] Access to TETS in China and its illegal export to other countries necessitates better methods for its detection.[2] Current methods for the detection of TETS are mainly GC-MS based and thus require a laboratory setting, are laborious and expensive limiting their use. Immunoanalytical methods, on the other hand, are widely used for the detection of small molecules in different matrices[4] and have the advantage of being cheap, portable and high throughput. However, they require conjugation of the small-molecule analyte to a larger protein in order to generate an appropriate immune response and raise analyte-selective IgG antibodies.
Tetramine has a unique chemical structure including a rigid cage and multiple heteroatoms that may provide recognition points for antibodies. However, TETS lacks reactive functional groups that could be easily functionalized and used as the attachment points for the preparation of the immunizing and coating antigens. Therefore, haptens have to be synthesized de novo, not by modification of the target analyte (TETS) or its precursors as it is typically done for other analytes.[5] An additional challenge is to develop a synthetic route that would exclude production of free TETS as a by-product. These are probably the main reasons for the lack of an immunoassay for TETS to date. Interestingly, one monoclonal antibody (mAb) developed against cyclodiene pesticides, such as aldrin, was shown to cross-react with TETS.[6] However, due to significant structural differences between cyclodienes and TETS, the affinity of the monoclonal antibody (mAb) to TETS was low (IC50 = 3 µM or 0.72 µg/mL) and not suitable for analytical use. Thus, the development of structurally close TETS analogues possessing active functional groups will facilitate development of a cheap immunoanalytical method for its detection, and may be useful for regulatory and enforcement agencies charged with environmental, agricultural and homeland security. Additionally, such analogues could be used for the development of photoaffinity labels allowing identification of the TETS binding site, in-depth study of the mechanisms of its toxicity and evaluation of treatment options.
Therefore, in this work we developed a synthetic route to generate a library of TETS-like compounds. The potency of these compounds as excitotoxicants were assessed in bioassays with primary cultures of mouse hippocampal neurons and cultured cells expressing human GABAA receptors, and compared directly to TETS. The most promising analogues were conjugated to the carrier protein and injected in rabbits to produce polyclonal antibodies.
Results and Discussion
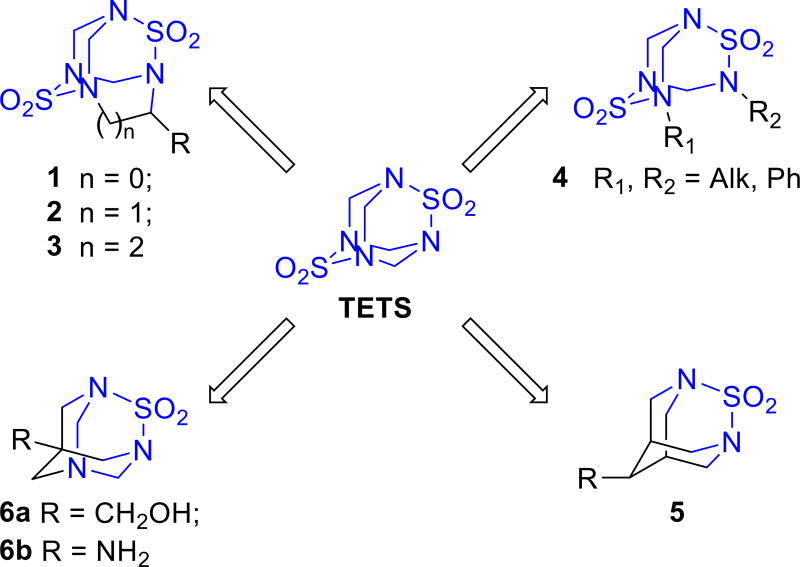
We designed four types of analogues with different degrees of similarity to TETS (Scheme 1). Although overall surface complementarity is considered to be an important determinant for antigen recognition, specific interactions like electrostatic and hydrogen bonding are frequently more critical determinants of antibody affinity.[7] It is therefore generally accepted that a good hapten will preserve the distinctive functional groups as well as the overall antigen structure.[8] Additionally, to ensure that distinctive functional groups remain well exposed and available for interaction with the antibody, the spacer arm should be as remote from them as possible.[8c] Following these considerations, TETS analogue 1 having a linker arm attached to one of the methylene bridges should be an ideal hapten because it preserves all the structural features of the parent compound like the adamantane structure and both sulfamide functions. Theoretically, its synthesis would involve co-condensation of formaldehyde and aldehyde with sulfamide resulting in formation of the mixture of TETS-like compounds including analogue 1 and TETS. Clearly, this approach would suffer from drawbacks such as poor yields and complicated chromatographic separation of the desired product. Most importantly, the possibility of formation of analogue 1 or its stability is doubtful. At least under standard reaction conditions, previous publications show that replacement of formaldehyde by more bulky aldehydes in the condensation reaction with sulfamide precludes the formation of the tricyclic core.[9] Thus either structural or functional group modifications were required during the process of hapten design.
Scheme 1.
Design of compounds with functional determinants similar to TETS.
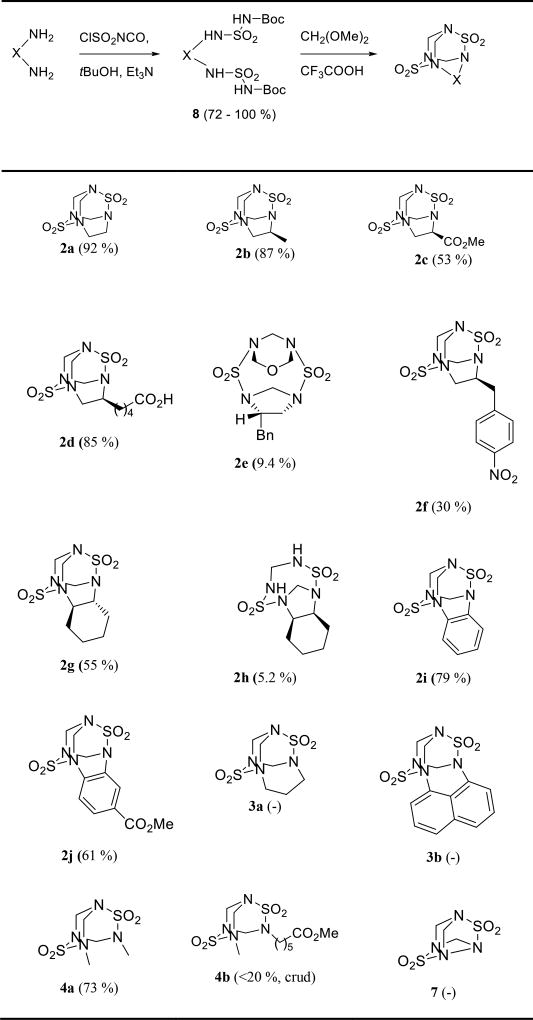
It was shown previously that monoalkyl or monoaryl sulfamides could be engaged in a similar reaction with formaldehyde as a parent nonsubstituted sulfamide giving TETS-like compounds 4 lacking one methylene bridge (Scheme 1).[9–10] Although synthetically very attractive this approach would result in haptens having two linker units that may cause complications during conjugation or negatively influence TETS recognition. This problem could be overcome by using asymmetrically substituted analogues 4 (Me = R1 ≠ R2). Unfortunately, condensation of equimolar amounts of N-methylsulfamide and methyl 6-(sulfamoylamino)hexanoate with polyformaldehyde not only gave poor yields of asymmetric analogue 4b (Table 1) but also its separation from the symmetrical analogues was complicated.
Table 1.
Synthesis of TETS analogues
Next, we studied whether compounds having two sulfamide functions connected through a variable-length linker undergo intramolecular condensation with formaldehyde to give tricyclic TETS like compounds. To answer this question disulfamides 8 had to be synthesized. Among multiple synthetic procedures available[11] we chose the method described by Masui et al.[12] because of its reported water tolerance and high product yields. Thus, reaction of the CSI (chlorosulfonyl isocyanate) with tert-butanol followed by the addition of the triethylamine and primary amine provided the Boc protected sulfamides 8 in good to excellent yields (Supporting Information, SI). The next steps called for cleavage of the Boc protecting group followed by condensation with formaldehyde. Since acidic media is necessary for both reactions these two steps were tested in a one-pot procedure. Since the bicyclic TETS analogue 4a is known, this condensation reaction was first tested with tert-butyl (N-methylsulfamoyl)carbamate 8k and produced the compound having identical physicochemical characteristics to the previously reported 4a in 73 % yield. Next this reaction was tested with a simple di-Boc protected disulfamide derived from easily available ethylene diamine. Reaction of di-tert-butyl (((2-(hydrosulfonylamino)ethyl)amino)sulfonyl)dicarbamate with dimethoxymethane in trifluoroacetic acid gave the TETS analogue 2a as a racemic mixture in 92 % yield (Table 1). To investigate the scope of this transformation and optimize reaction conditions a variety of differently substituted 1,2-diamines were tested. Since the choice of commercially available functionalized diamines is limited the substrate scope was first tested with most commonly available nonfunctionalized diamines followed by functionalized ones, which were obtained by multistep synthesis as described in the supporting information. Presence of aliphatic or carboxylate substituents on the ethylene bridge proved to be tolerable, however in this case the products were obtained as a mixture of two diastereomers (Table 1, 2b–d). Although, flash column chromatography has been tested as a purification technique, due to relatively low polarity and very poor solubility of 2 in non-polar solvents, use of this technique for purification was complicated. The major products were obtained in pure or almost pure form by recrystallization from methanol. Interestingly, introduction of a benzyl group on the ethylene bridge not only dramatically deteriorated the reaction yield but also resulted in formation of product 2e with an unexpected structure. Replacement of the benzyl group by p-nitrobenzyl resulted in formation of a mixture of at least 2 products which after recrystallization gave pure 2f in 30 % yield. Comparison of the NMR spectra of crude product, 2f and 2e revealed that chemical shifts of the minor product from the mixture were very similar to those of 2e and thus it likely had the same structure. Absence of the TETS-like product in the case of disulfamide 8e can be explained by intramolecular sulfamidoalkylation of the aromatic ring resulting in the formation of benzo-annelated side-products similar to a previous report.[9]
Disulfamide derived from (1R,2R)-cyclohexane-1,2-diamine also reacted with dimethoxymethane resulting in formation of TETS analogue 2g in 55 % yield as a single diastereoisomer. RS-cyclohexane-1,2-diamine, on the other hand, failed to give the desired tricyclic product probably because of considerable van der Waals repulsive forces between the tricyclic core and the axial hydrogens of the cyclohexane moiety which render this compound unstable. The only product which was obtained in only 5.2 % yield was the disulfamide 2h structurally related to 2e, but missing -CH2OCH2- bridge. Its structure was confirmed by spectroscopic methods and X-ray crystallography. Replacement of the ethylene bridge by phenylene was well tolerated (Table 1, 2i–j).
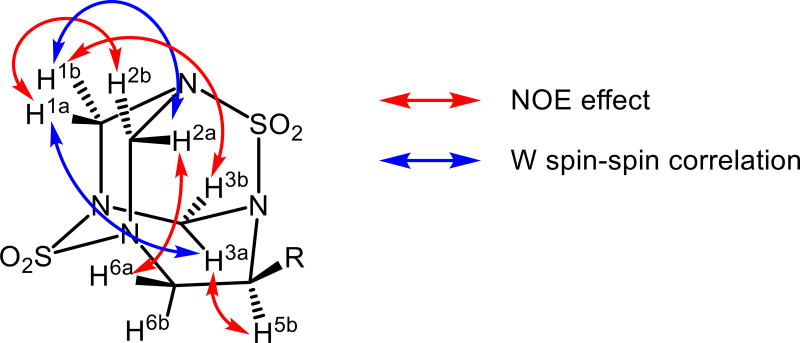
Studies of NOE and COSY spectra of analogues 2 revealed the following interesting features. A NOE effect was observed between spatially close pairs of hydrogens of the methylene bridges H1a-H2b and H1b-H3b, whereas spin-spin correlations in the COSY spectra were observed only for distant protons of the same methylene groups H1b-H2a and H1a-H3a (Figure 1). Modeling of the analogues 2 revealed that the systems H-C-N-C-H for which these spin-spin correlations were observed are almost flat and thus they could be attributed to long-range “W”-type correlations.[13] A NOE effect was also observed between spatially close protons of methylene and ethylene bridges H2a-H6a and H3a-H5b, and therefore could be used to confirm the relative stereochemistry in these analogues.
Figure 1.
Significant NOE and spin-spin correlations observed for 2.
Next, we tested if one of the methylene bridges in TETS could be replaced by propylene or if it could be completely deleted. For this purpose, we synthesized di-Boc protected disulfamides starting from 1,3-diaminopropane, 1,8-diaminonaphthalene and hydrazine, which were then reacted with dimethoxymethane in trifluoroacetic acid (Table 1, 3a, 3b, 7). All three reactions resulted in complex reaction mixtures showing no sign of the desired product by NMR spectroscopy or mass spectrometry. For 3a–b this result compares well with literature data showing that unlike ethylenediamine, 1,3-diaminopropane does not give a tricyclic condensation product with formaldehyde presumably because of steric factors.[14] However, it is likely that faster alternative condensation reactions compared to the formation of the eight-membered ring, required for construction of 3a–b skeleton, are responsible for absence of the desired product.
We also studied approaches to synthesize TETS analogues 5 and 6 which possess a lower degree of similarity to the parent molecule, but still have an adamantane-like structure and one of the two sulfamide functions preserved. Hapten 5 was very attractive from the synthetic point of view since precursors – bispidine derivatives have been described and are easily accessible, thus leaving solely the feasibility of sulfamide bridge formation uncertain. Thus, after the intermediates 10 and 11 were synthesized (Scheme 2) according to a literature procedure[15] the possibility of introduction of a sulfamide bridge was studied. For this transformation we first tested the reaction conditions employing sulfuryl chloride as sulfurylation reagent and triethylamine or pyridine as a base. In both cases, reactions resulted in complex mixtures as judged by NMR spectra. To overcome this problem a range of alternative sulfurylation reagents were tested, but none were successful at introducing the sulfamide bridge in bispidine derivatives 10, 11.
Scheme 2.
Synthesis of bispidine derivatives 10–11 and tentatives of their sulfurylation.
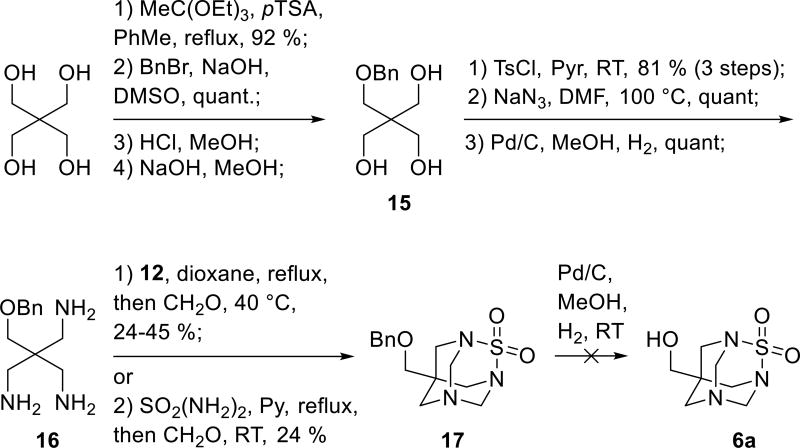
In parallel to our studies toward the TETS analogue 5 we also explored approaches aiming at synthesis of TETS analogues 6. Among the multitude of possible radicals R in the TETS analogue with general formula 6 (Scheme 1), we chose hydroxymethylene (HOCH2−) and amino groups because of the availability of precursors pentaerythritol and Tris base. Synthesis of compound 6a commenced by preparation of the known triamine 16[16] (Scheme 3). Briefly, pentaerythritol was first converted into monobenzyl derivative 15 via orthoester protection, alkylation and deprotection sequence. Tosylation of derivative 15 followed by nucleophilic substitution with azide ion and palladium catalyzed hydrogenation gave triamine 16. Transformation of 16 into the adamantane-like sulfamide 17 was achieved in 24 – 45 % yield by its treatment with an equimolar amount of catechol sulfate under reflux followed by addition of formaldehyde. Alternatively, refluxing triamine 16 with sulfamide in pyridine followed by the addition of formaldehyde also gave the tricyclic sulfamide 17, but in lower yield. The next step was benzyl deprotection using hydrogen and palladium on carbon. However, despite all efforts, the hydrogenation either did not proceed or was not chemoselective as concomitant hydrogenative cleavage of the C-N bond at the methylene bridge was occurring.
Scheme 3.
First synthetic approach toward hapten 6a.
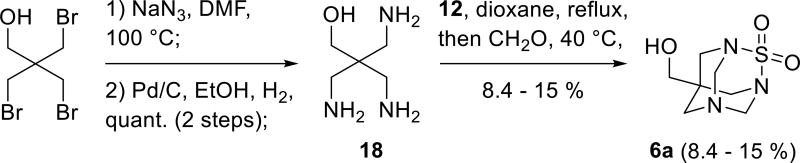
Elimination of the deprotection step was envisioned to avoid this problem and to considerably shorten the synthetic pathway. Thus, the unprotected triamino alcohol 18, an analogue of 16, was prepared in two steps from commercial pentaerythritol tribromide by nucleophilic substitution with azide ion followed by palladium catalyzed hydrogenation[17] (Scheme 4). The resulting triamine 18 was transformed into TETS analogue 6a by treatment with catechol sulfate followed by addition of formaldehyde. The yield of 6a (8.4 – 15 %) was considerably lower than the yield for 17. This might be attributed to poor solubility of the reaction intermediates obtained from 18 in dioxane (gummy substance was observed in the reaction mixture) leading to higher amounts of polymeric sulfamides.
Scheme 4.
Synthesis of the hapten 6a.
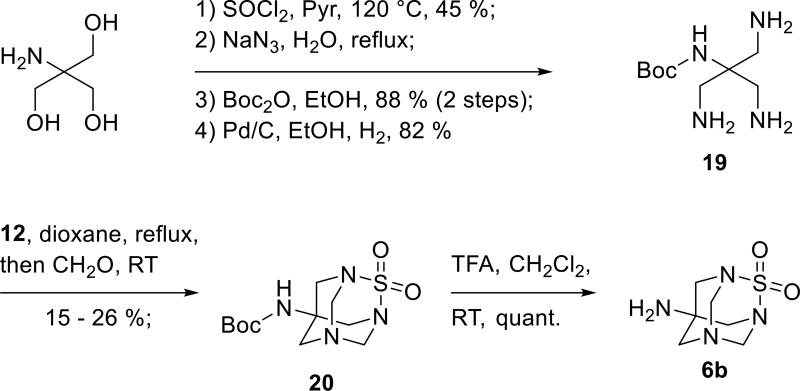
Synthesis of TETS analogue 6b started from Tris base, which was first transformed into trichloride followed by the azidation and protection of the amine function by a Boc group to give intermediate triazide (Scheme 5).[18] Palladium catalyzed hydrogenation of this triazide gave monoprotected tetraamine 19[19] that was reacted with catechol sulfate in refluxing dioxane followed by reaction with formaldehyde at RT. Treatment of the resulting intermediate 20 with trifluoroacetic acid liberated the free TETS analogue 6b in quantitative yield. All products and intermediates were characterized by NMR and other spectroscopic methods. Additionally, the identities of compounds 2e, 2g – 2j, 4a, 17 and 20 were proven by X-ray crystallography (see SI).
Scheme 5.
Synthesis of the hapten 6b.
Development of immune response in rabbits
Analogues 2d, 2j, 2k (nitro group reduced to amine in 2f), 6a and 6b were selected from our library for animal immunization. These compounds represented the diversity of the synthesized library and possessed functional groups that could be used for chemical linking to the carrier protein. Thyroglobulin was chosen as a carrier protein for immunization because of its high immunogenicity and ease of use. Bovine serum albumin and conalbumin were used as carrier proteins in the preparation of coating antigens. For the conjugation 6a and 6b were first reacted with succinic anhydride to give corresponding monoester and monoamide respectively. The resulting monosuccinates, 2d and 2j were then directly conjugated to the carrier protein via carboxylic acid function using the standard activated ester method. Amine 2k was conjugated to the carrier protein by using either diazotization or glutaraldehyde methods (see SI).[20]
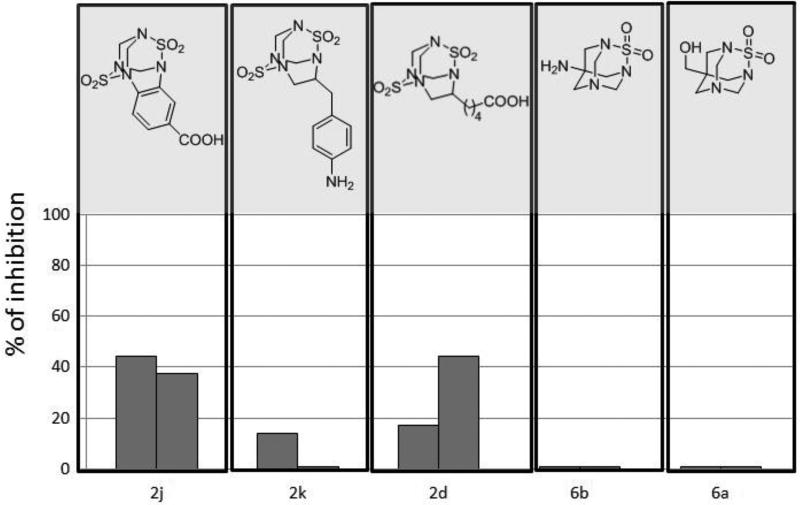
After immunization of rabbits, serum from the final bleed was analyzed in a competitive ELISA format with a concentration of TETS of 5 mg/L. The aim of this experiment was to study if synthetic analogues used for immunization could elicit an immune response and result in antibodies that recognized TETS. Figure 2 demonstrates that sera from rabbits immunized with haptens 2j, 2k and 2d recognized the corresponding haptens and its binding was to some extent inhibited by TETS. By contrast, even though sera obtained from rabbits immunized with haptens 6b and 6a still recognized the corresponding immunizing haptens, their binding was not altered by TETS. These data suggest that either haptens 6b and 6a are fairly distinct from the TETS structure and thus sera do not recognize the analyte or the affinity of the developed antibodies is much greater for the haptens and thus the concentration of TETS tested is not high enough to produce a visible inhibition effect. Here we present promising preliminary data for immunoassay development from rabbit sera immunized with haptens 2. Further evaluation of developed antibodies against TETS is a subject of a separate study.
Figure 2.
Inhibition of antibody binding on homologous coating antigen in the presence of TETS at 5 mg/L. Each bar represent serum from individual animal.
TETS analogues as tools for biological applications
The history of terror acts involving chemical agents[21] has raised concern about banned substances with high toxicity, including TETS. For instance, within the U.S. Department of Health and Human Services, the NIH is making a significant effort to pursue the development of new and improved medical countermeasures designed to prevent, diagnose, and/or treat the pathology caused by TETS.[22] To develop a successful treatment approach for the poisoning it is important to understand the mechanism of action of the agent. So far, TETS has only been identified to block the GABA receptor chloride channel (GABAAR), but the binding site remains unidentified. Knowledge of structure-activity relationship of TETS and analogues may prove to be useful in this regard, for example by helping design photoaffinity probes.
Effects of TETS and TETS analogs on Ca2+ Oscillations in Primary Cultured Hippocampal Neurons
For initial screening for neuroactive analogues we used a FLIPR bioassay detecting Ca2+ oscillations in cultured hippocampal neurons. Cultured hippocampal neurons (13–28 days in vitro (DIV)) display a balance of glutamatergic (excitatory) and GABAergic (inhibitory) signaling, which results in spontaneous synchronous Ca2+ oscillations of approximately 10 second duration. Application of GABAA receptor (GABAAR) antagonists such as picrotoxin, bicuculin and TETS, result in an increase in the amplitude of intracellular calcium peaks as revealed through a Ca2+ indicator, such as Fluo-4. Therefore, the FLIPR assay allows sensitive detection and high throughput screening of neuroactive compounds. Recordings of individual somas reveal that these oscillations are synchronous across a field of view, making them appropriate to study with lower spatial resolution, as the average signal from a large portion of a well in a 96-well plate. As with all primary neuronal cultures, there are culture-to-culture variations in the precise balance of types of neurons, but with so many wells from one culture, the complete dose-response curve for TETS can be obtained from one plate in one run with 5 duplicate wells with FLIPR.[23]
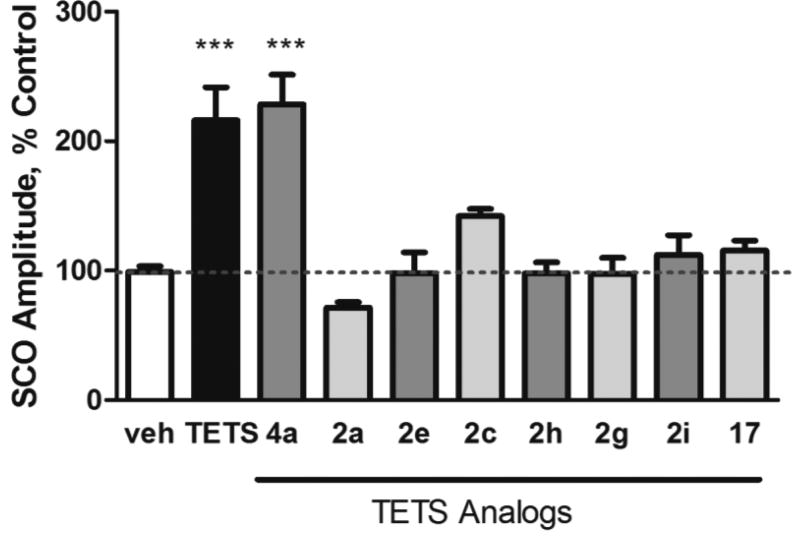
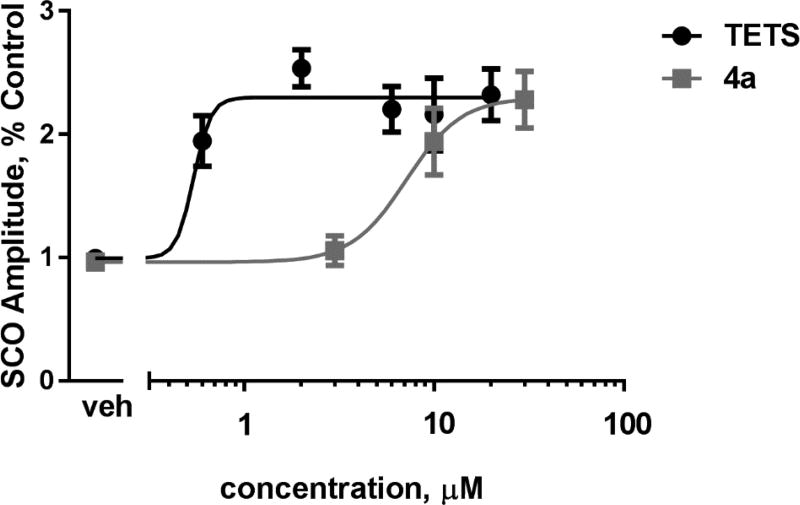
Addition of vehicle (0.03% dimethyl sulfoxide [DMSO]) had no significant effect on properties of the synchronous Ca2+ oscillations (SCO). By contrast, TETS at 10 µM significantly increased the amplitude and decreased the frequency of the SCOs.[23] Eight analogs of TETS were tested at 30 µM, and compared to TETS (Figure 3). Analog 4a produced a similar effect to TETS, with an increased SCO amplitude and lower spike frequency. The traces from this analog were indistinguishable from TETS (Figure S1). None of the other analogs tested had a significant difference in the SCO amplitude from vehicle. The TETS analog 4a was tested at 3 concentrations: 3, 10 and 30 µM and compared to TETS at concentrations from 0.06 µM to 20 µM (Figure 4). The EC50 value for 4a was found to be 7.15 µM (95% CI, 3.13 to 16.35 µM). Our use of TETS in this study was to compare on each plate, the amplitude increase of each analog with a maximal TETS response. An earlier study,[23] used a wider range of TETS concentrations on identically prepared cultures with the same procedures as this study, in the same lab, found the EC50 of TETS on amplitude of SCO to be 1.8 µM (95% CI: 1.12 to 2.80 µM). Our study used a narrower range of TETS, and indicated a lower EC50 of 0.5 µM, but did not include measurements at the lower concentrations of TETS needed to state the EC50 with confidence. Using both values, our conclusion is that TETS is at least 4 times more potent than the most effective TETS analog tested (4a), whereas the maximal response of the two is the same (efficacy).
Figure 3.
Screening for neuroactive TETS analogues with the FLIPR bioassay in cultured hippocampal neurons. The SCO amplitude after the addition of TETS at 10 µM or TETS analogs at 30 µM, compared to the addition of 0.03% DMSO vehicle control. The mean amplitude for each well after the addition is compared to the mean amplitude for that well before the addition, then normalized to the vehicle response. n = 7 or 8 for TETS and analogues; n = 12 for vehicle. ANOVA comparison with vehicle control, *** indicates p < 0.0001, all other differences were not significant from vehicle. Bars indicate SEM.
Figure 4.
TETS analog 4a has lower potency but similar efficacy to TETS. n = 4 for each group, bars indicate SEM.
Inhibition of GABAA currents by TETS and its analogs
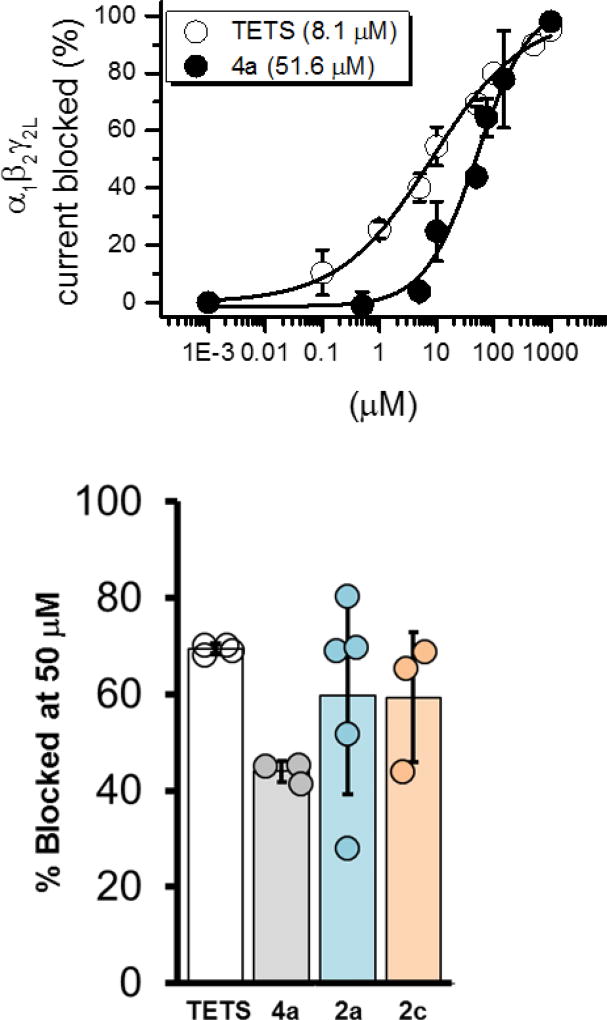
TETS causes neuronal hyperexcitability by competitively binding to GABAA receptors and reducing the hyperpolarizing chloride currents. To evaluate further neuromodulating properties and to determine the potency of the TETS analogs in inhibiting GABA-induced currents, the effect of the TETS analogs on currents produced by cultured cells expressing α1β2γ2L GABAA receptors were measured using whole-cell voltage-clamp and compared against current inhibition by TETS. Despite being a low-throughput technique, manual patch-clamping directly measures the activity of the compound on GABAAR current. Additionally, in contrast to cultured hippocampal neurons, we controlled the receptor subunits being expressed in our heterologous cells and thus the exact identity of the GABAA receptors being tested is always known. In this report, we chose the most abundant subunit combination in the mammalian CNS (α1β2γ2L) to determine the potency of TETS and its analogues. In contrast to TETS (IC50 = 7.9 ± 2.6 µM, n = 12), TETS analog 4a, identified as the most active analog in the SCO assays, only exhibited modest activity (IC50 = 48.0 ± 13.2 µM, n = 10) (Figure 5). Two additional analogs, 2a and 2c, which also showed some activity in the FLIPR assays, were determined to have comparable inhibitory effect on GABA-induced currents as 4a at the 50 µM test concentration (4a 43.9 ± 2.1%, n = 3; 2a 59.8 ± 20.5%, n = 5; 2c 59.4 ± 12.6%, n = 3). None of the tested analogs were more potent than TETS (69.4±1.0%, n = 4) on our receptors of choice.
Figure 5.
Effect of TETS and its analogs on GABA-induced currents. Top: Dose-response association curves showing percentage of the α1β2γ2L current blocked by increasing concentrations of either TETS or its analog 4a. Bottom: Percentage of current blocked by TETS and its analogs at test concentration of 50 µM. Percentage blocked for TETS is 69.4 ± 1.0% (n = 4), 4a is 43.9 ± 2.1% (n = 3), 2a is 59.8 ± 20.5% (n = 5) and 2c is 59.4 ± 12.6% (n = 3). Error bars indicate SD.
Thus, we have successfully synthesized and identified analogues of TETS that are active on GABAA receptors. Despite being several fold less potent then TETS in our electrophysiological studies, these analogues retained the functional groups required for binding and blocking α1β2γ2L GABAA receptors. Additionally, although TETS is a known GABAA receptor inhibitor, its exact binding site and selectivity for the various GABAA receptor subtypes have not been investigated. Thus, further testing of these analogues and TETS on additional GABAA receptor subtypes will provide the information on their exact potency and selectivity on the GABAA receptors. The fact that analogues and TETS exhibit a different potency ranking in the SCO and the patch-clamp experiments is probably caused by the fundamentally different nature of the two assays which in one case uses Ca2+ signaling as a downstream effect of GABAA receptor blockade while directly measuring blockade of GABA-induced chloride currents in the other. Another possibility could be that the TETS analogous exhibit differential selectivity for different GABAA receptor subtypes present in the hippocampal neurons used for the SCO experiments.
Conclusions
In summary we have developed a general synthetic approach toward two classes of tricyclic sulfamides structurally related to the neurotoxic TETS molecule. Bioactivity of some of the synthesized compounds was evaluated by studying their effects on synchronous calcium oscillations in cultured hippocampal neurons and on receptor currents in cells expressing α1β2γ2L GABAAR and the results were compared to those of the parent TETS compound. Although none of the tested TETS analogues was as potent as TETS, the potency of some was only an order of magnitude lower on α1β2γ2L GABAAR compared to TETS. In view of a recent burst of research interest in the sulfamide pharmacophore for the development of new medicines for a broad spectrum of pharmacological targets[24] the tricyclic sulfamides described here appear to be useful building blocks for the construction of new drug candidates. Six TETS analogues were used for conjugation to the carrier protein and rabbit immunization. Preliminary data suggest that the produced antibodies recognized TETS with sensitivities higher than 5 µg/mL. Use of these sera for the development of the first immunoassay for sensitive detection and quantification of TETS will be published in due course.
Supplementary Material
Acknowledgments
This work was supported by the Counter ACT Program, National Institutes of Health Office of the Director, and the National Institute of Neurological Disorders and Stroke, Grant Number U54 NS079202; and NIEHS, Superfund Research Program, P42 ES04699.
Footnotes
Supporting information for this article is given via a link at the end of the document.
References
- 1.International Program on Chemical Safety. http://www.inchem.org/documents/pims/chemical/pim982.htm#2.1.
- 2.Croddy E. Arch Toxicol. 2004;78:1–6. doi: 10.1007/s00204-003-0509-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.a) Li JM, Gan J, Zeng TF, Sander JW, Zhou D. Neurotoxicology. 2012;33:207–211. doi: 10.1016/j.neuro.2011.10.008. [DOI] [PubMed] [Google Scholar]; b) Li Y, Gao YX, Yu XZ, Peng JM, Ma F, Nelson L. Bmc Public Health. 2014;14 doi: 10.1186/1471-2458-14-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.a) Goel P. African Journal of Biotechnology. 2013;12:7158–7167. [Google Scholar]; b) Sherry J. Chemosphere. 1997;34:1011–1025. doi: 10.1016/s0045-6535(97)00403-7. [DOI] [PubMed] [Google Scholar]; c) Anfossi L, Baggiani C, Giovannoli C, D'Arco G, Giraudi G. Anal Bioanal Chem. 2013;405:467–480. doi: 10.1007/s00216-012-6033-4. [DOI] [PubMed] [Google Scholar]; d) Kavanagh O, Elliott CT, Campbell K. Anal Bioanal Chem. 2015;407:2749–2770. doi: 10.1007/s00216-015-8502-z. [DOI] [PubMed] [Google Scholar]; e) Franek M, Hruska K. Vet Med-Czech. 2005;50:1–10. [Google Scholar]; f) Morozova VS, Levashova AI, Eremin SA. J Anal Chem+ 2005;60:202–217. [Google Scholar]
- 5.a) Vasylieva N, Ahn KC, Barnych B, Gee SJ, Hammock BD. Environmental science & technology. 2015;49:10038–10047. doi: 10.1021/acs.est.5b01005. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Franek M, Kolar V, Granatova M, Nevorankova Z. J Agr Food Chem. 1994;42:1369–1374. [Google Scholar]; c) Yang JY, Wang H, Jiang YM, Sun YM, Pan KT, Lei HT, Wu Q, Shen YD, Xiao ZL, Xu ZL. Molecules. 2008;13:871–881. doi: 10.3390/molecules13040871. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Lee JK, Ahn KC, Park OS, Kang SY, Hammock BD. J Agr Food Chem. 2001;49:2159–2167. doi: 10.1021/jf001140v. [DOI] [PubMed] [Google Scholar]
- 6.Esser T, Karu AE, Toia RF, Casida JE. Chemical research in toxicology. 1991;4:162–167. doi: 10.1021/tx00020a007. [DOI] [PubMed] [Google Scholar]
- 7.Janeway C, Travers P, Walport M, Shlomchik M. Immunobiology: The Immune System in Health and Disease. 5. Garland Science; New York: 2001. [Google Scholar]
- 8.a) Harrison RO, Goodrow MH, Gee SJ, Hammock BD. Acs Sym Ser. 1990;451:14–27. [Google Scholar]; b) Karu AE. In: Hazard assessment of chemicals. Saxena J, editor. Vol. 8. Taylor and Francis; 1984. pp. 232–243. [Google Scholar]; c) Tijssen P. In: Practice and Theory of Enzyme Immunoassays. Burdon RH, Knippenberg PH, editors. Vol. 15. Elsevier; Amsterdam, The Netherlands: 1985. [Google Scholar]
- 9.Lee CH, Kohn H. J Org Chem. 1990;55:6098–6104. [Google Scholar]
- 10.Dusemund J, Schurreit T. Arch Pharm. 1986;319:826–829. [Google Scholar]
- 11.a) Mcdermott SD, Spillane WJ. Org Prep Proced Int. 1984;16:49–77. [Google Scholar]; b) Nicolaou KC, Longbottom DA, Snyder SA, Nalbanadian AZ, Huang XH. Angew Chem Int Edit. 2002;41:3866–3870. doi: 10.1002/1521-3773(20021018)41:20<3866::AID-ANIE3866>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 12.Masui Y, Watanabe H, Masui T. Tetrahedron Lett. 2004;45:1853–1856. [Google Scholar]
- 13.Jackman LM, Sternhell S. Chapter 4-4. LONG-RANGE INTERPROTON COUPLING. In: Jackman LM, Sternhell S, editors. Applications of Nuclear Magnetic Resonance Spectroscopy in Organic Chemistry. Second. Vol. 10. Pergamon Press; 1969. [Google Scholar]
- 14.Murray-Rust P, Riddell FG. Revue canadienne de chimie. 1975;53:1933–1935. [Google Scholar]
- 15.Wang Z, Miller EJ, Scalia SJ. Org Lett. 2011;13:6540–6543. doi: 10.1021/ol202840s. [DOI] [PubMed] [Google Scholar]
- 16.Dunn TJ, Neumann WL, Rogic MM, Woulfe SR. J Org Chem. 1990;55:6368–6373. [Google Scholar]
- 17.Chugunov PA, Chinarev AA, Tuzikov AB, Formanovsky AA, Prokhorov VV, Gambaryan AS, Bovin NV. Mendeleev Commun. 2009;19:62–63. [Google Scholar]
- 18.a) Diaz DD, Punna S, Holzer P, Mcpherson AK, Sharpless KB, Fokin VV, Finn MG. J Polym Sci Pol Chem. 2004;42:4392–4403. [Google Scholar]; b) Martinu T, Dailey WP. J Org Chem. 2000;65:6784–6786. doi: 10.1021/jo000734u. [DOI] [PubMed] [Google Scholar]
- 19.Abdu-Allah HHM, Watanabe K, Daikoku S, Kanie O, Tsubata T, Ando H, Ishida H, Kiso M. Synthesis-Stuttgart. 2011:2968–2974. [Google Scholar]
- 20.Hermanson GT. Bioconjugate Techniques. Third. Academic Press; Boston: 2013. pp. i–iii. [Google Scholar]
- 21.Johnston R. 2015 http://www.johnstonsarchive.net/terrorism/chembioattacks.html.
- 22.a) CounterACT. https://www.ninds.nih.gov/Current-Research/Trans-Agency-Activities/CounterACT.; b) http://counteract.ucdavis.edu/
- 23.Cao Z, Hammock BD, McCoy M, Rogawski MA, Lein PJ, Pessah IN. Toxicological sciences : an official journal of the Society of Toxicology. 2012;130:362–372. doi: 10.1093/toxsci/kfs244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.a) Winum JY, Scozzafava A, Montero JL, Supuran CT. Med Res Rev. 2006;26:767–792. doi: 10.1002/med.20068. [DOI] [PubMed] [Google Scholar]; b) Reitz AB, Smith GR, Parker MH. Expert Opin Ther Pat. 2009;19:1449–1453. doi: 10.1517/13543770903185920. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.