Abstract
Syntheses of two siderophore-fluoroquinolone conjugates with a potential reduction triggered linker for drug release are described. The “trimethyl lock” based linker incorporated in the conjugates was designed to be activated by taking advantage of the reductive pathway of bacterial iron metabolism. Electrochemical and LC-MS studies indicated that the linker is thermodynamically reducible by common biological reductants and the expected lactonization proceeds rapidly with concomitant release of the drug. Antibacterial activity assays revealed that conjugates with the reduction-triggered linker were more potent than their counterparts with a stable linker, which suggests that drug release occurs inside bacterial cells.
Keywords: Siderophores, Antibiotics, “Trojan Horse”, Iron transport, Drug delivery, Drug release
Introduction
The ever-growing bacterial resistance to antibiotics is emerging as a serious problem that poses great threat to public health (Boucher et al. 2009; Spellberg et al. 2008). Not only is the situation worsened by the lack of new antibiotics being created, but the increasing use and misuse of existing antibiotics are also significant contributors that promote resistance (Levy and Marshall 2004; Projan 2003). Of several modes of resistance mechanisms, the inability of some antibiotics to enter the cell via size-restricted porins is especially significant (Fernandez and Hancock 2012; Pages et al. 2008). Thus, there is always a dire need for improved drug-delivery processes to circumvent the permeability problem.
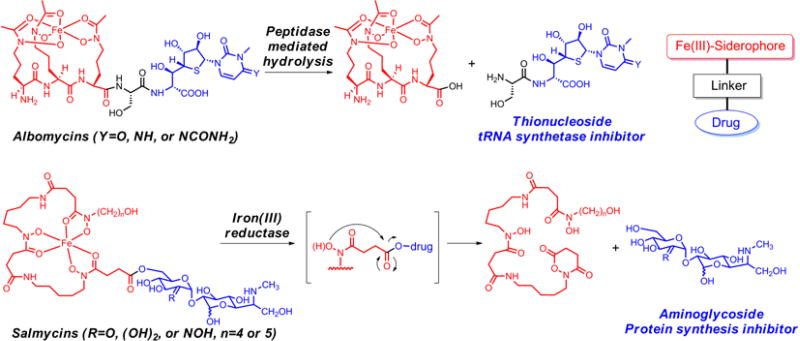
The idea of conjugation antibiotics to bacterial iron chelators, known as siderophores, to facilitate transport of antibiotics into the bacterial cell has attracted considerable attention (Ji et al. 2012a; Mislin and Schalk 2014; Möllmann et al. 2009; Page 2013; Zheng and Nolan 2014). Under iron-limited conditions, bacteria synthesize and secrete siderophores for extracellular solubilization of otherwise insoluble iron. Fe(III)-siderophore complexes are recognized by specific bacterial membrane receptors, and then translocated across the cell membrane in the presence of certain ATP-dependent active transporters. Once inside the cell, the iron is released from the Fe(III)-siderophore complex for further use, usually via a reductive mechanism, to give Fe(II), to which siderophores bind much more weakly (Harrington and Crumbliss 2009). Since bacterial iron acquisition largely relies on siderophore pathways, especially under iron-limited growth conditions during infection of a host, the use of siderophore-drug conjugates has become an attractive way to bypass bacterial permeability barriers and thus improve the efficacy of the drug and reduce the chances of resistance development. Several naturally occurring sideromycins, siderophore-antibiotics, such as albomycins (Braun et al. 1983; Braun et al. 2009) and salmycins (Vértesy et al. 1995; Wencewicz and Miller 2013; Wencewicz et al. 2009), have been proven to use specific iron transport systems to enter targeted bacteria, and consequently are very effective natural antibacterial agents (Figure 1). Extensive studies of albomycins have revealed that the drug component, a toxic thionucleoside, is enzymatically released after the conjugate is transported (Braun et al. 1983). Salmycins, albeit less studied, are proposed to release the constituent aminoglycoside antibiotic via an intramolecular cyclization process triggered by iron reduction (Roosenberg and Miller 2000; Wencewicz et al. 2013; Wencewicz et al. 2009). In both cases, an intracellular drug release process, initiated after active assimilation by bacteria, is believed to play a key role for the activity of the conjugates.
Figure 1.
General sideromycins (siderophore drug conjugates) and two naturally occurring examples, albomycin and salmycin.
Inspired by these natural examples, a series of synthetic siderophore-drug conjugates, properly designed to mimic natural siderophores, with potential drug release linkers have been reported. For example, pyoverdin and pyochelin, the two native siderophores of Pseudomonas aeruginosa, were conjugated to fluoroquinolones with both stable and hydrolysable methylenedioxy linkers to target the corresponding siderophore producing strain (Hennard et al. 2001; Noël et al. 2011; Rivault et al. 2007). Biological assays revealed that conjugates with a labile linker, that could induce release of the drug, displayed greatest activities. However, the methylenedioxy linker was non-specifically hydrolysable which could lead to premature release of the antibiotic in the extracellular media. Therefore, novel linkers with drug release function that could be selectively triggered inside microbial cells need to be further explored.
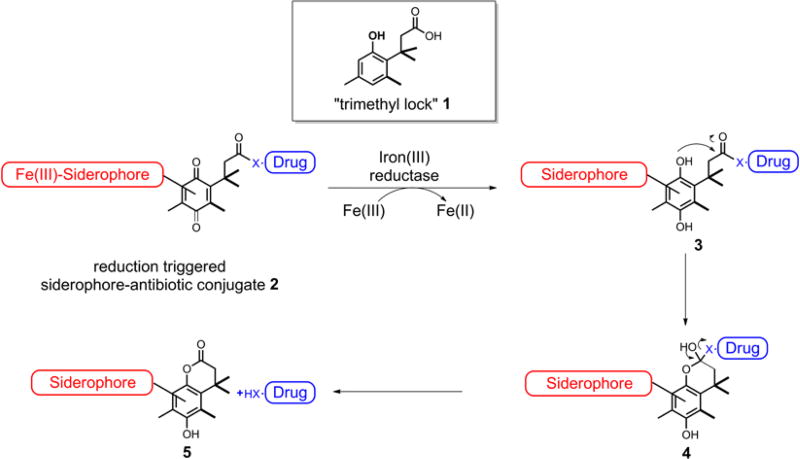
We recently reported use of a “trimethyl lock” induced lactonization triggered by the action of enzymes such as esterases/phosphatases for the drug release process in the design and syntheses of new siderophore-drug conjugates (Ji and Miller 2012). The chemical nature of the trimethyl lock system, 1, is an o-hydroxycinnamic acid derivative in which unfavorable steric interactions between three methyl groups encourage rapid lactonization to form a hydrocoumarin (Scheme 1) (Levine and Raines 2012). The esterases/phosphatases triggered linkers, similar to the pyoverdin/pyochelin-fluoroquinolone examples mentioned above, also have the disadvantage of being hydrolyzed by some extracellular hydrolases secreted into the culture medium by bacteria. In contrast to the extracellular esterases/phosphatases which are commonly seen, ferric reductases, the enzymes responsible for iron release from Fe(III)-siderophore complexes, are found predominantly inside bacterial cells with few exceptions (Schröder et al. 2003). Moreover, most ferric reductases lack substrate specificity (Miethke and Marahiel 2007; Schröder et al. 2003), suggesting that they may be suitable generalized targets for the development of drug release linkers incorporated in siderophore-drug conjugates. We therefore reasoned that the “trimethyl lock” 1 could provide an attractive reduction triggered linker which could release certain antibiotics in a controlled manner. In this approach, a quinone derivative was used as the precursor to the “trimethyl lock” structure, and the to-be-released drug was condensed with the carboxyl group. Upon active transport of the conjugate 2 into the microbe, it was expected that during the Fe(III) reduction process, the constituent quinone linker might also be reduced by hydride donors such as flavin or NAD(P)H used to reduce Fe(III) to Fe(II) for bacterial iron incorporation. The resulting hydroquinone 3 with the “trimethyl lock” structure only has a transient lifetime, and was anticipated to rapidly cyclize to form lactone 5 with concomitant release of the drug.
Scheme 1.
“trimethyl lock” derived siderophore-antibiotic conjugates.
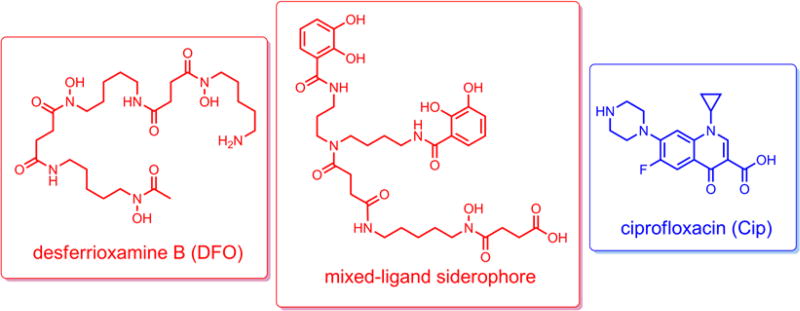
Two different siderophores, desferrioxamine B and a previously reported biscatecholate-monohydroxamate mixed-ligand siderophore (Scheme 2) were selected for appropriate modification to test our reduction triggered release design. Desferrioxamine B, a hydroxamate-containing siderophore, is utilized by a variety of Gram-positive and Gram-negative bacteria and some fungi (Müller and Raymond 1984). The artificial mixed-ligand siderophore containing both catecholate and hydroxamate ligands was designed to use multiple siderophore recognition processes to initiate active transport through the outer membrane of various Gram-negative species (Diarra et al. 1996; Ghosh et al. 1996; Wencewicz and Miller 2013). The drug linked to the siderophores was ciprofloxacin, a broad-spectrum fluoroquinolone antibiotic that targets bacterial DNA gyrases and topoisomerases (Mitscher 2005). Despite the aforementioned concerns about development of antibiotic resistance, ciprofloxacin is still active against many strains of pathogenic bacteria. However, previous attempts to demonstrate activity when conjugated to siderophores were unsuccessful apparently because, in contrast to β-lactam siderophore conjugates (Ji et al. 2012b; Möllmann et al. 2009; Zheng and Nolan 2014), attachment of the relatively large siderophore to ciprofloxacin interferes with target recognition of the modified fluoroquinolone (Wencewicz et al. 2009). Thus, herein we describe the syntheses of two siderophore-ciprofloxacin conjugates with and without the potential reduction triggered “trimethyl lock” linker, and a preliminary study of their biological activity. With the aim of assessing the feasibility of using the linker in siderophore-drug conjugates, electrochemical and LC-MS studies of linker activation are also reported. The results indicate that ciprofloxacin conjugates with the newly designed reduction triggered linker are notably more active than those without the releasable linker.
Scheme 2.
Structures of desferrioxamine B, biscatecholate-monohydroxamate mixed-ligand siderophore and ciprofloxacin.
Results and discussion
Design and syntheses of conjugates
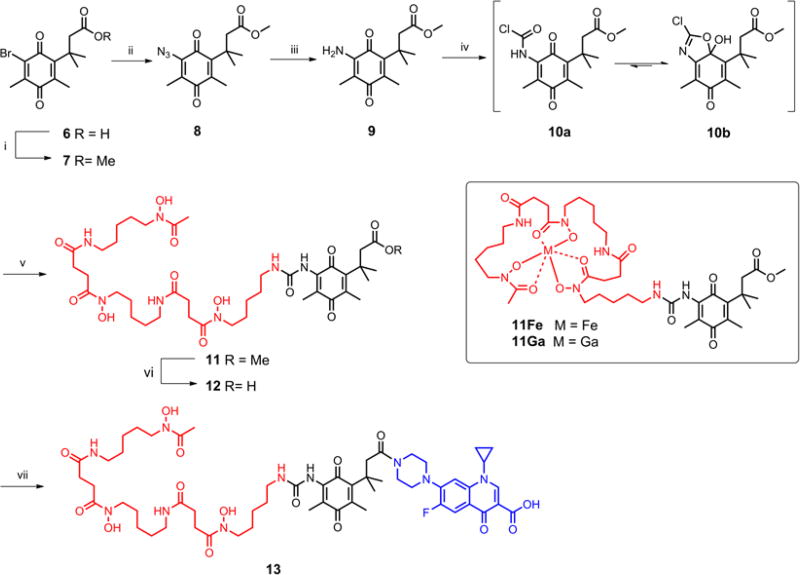
To initiate the syntheses of the conjugates, an effective linking strategy to attach siderophores to the reduction triggered linker had to be identified. We decided to incorporate a urea linkage because of its appreciable stability and synthetic accessibility. Thus, synthesis of the desferrioxamine conjugate (Scheme 3) started with bromo acid 6 which was conveniently converted to its methyl ester 7 using thionyl chloride in methanol. Nucleophilic substitution of 7 with NaN3 in aqueous methanol provided azide 8 in excellent yield. The azide was converted to vinylogous amide 9 in 66% yield by a triphenylphosphine-mediated reduction. Treatment of 9 with excess phosgene in toluene afforded an unstable intermediate with a proposed carbamoyl chloride structure 10a. Interestingly, proton and carbon NMR spectra suggested that the intermediate predominantly existed as the cyclic chloro oxazoline structure 10b, probably due to the electron deficiency of the quinone unit, which induces the intramolecular addition. Reaction of desferrioxamine with intermediate 10 in the presence of triethylamine gave ester 11 with the desired urea incorporated between the siderophore and the linker. Subsequent hydrolysis of the ester and coupling of the resulting acid 12 with ciprofloxacin using EDC and HOBt produced the desferrioxamine-ciprofloxacin conjugate 13 in moderate yield.
Scheme 3.
Synthesis of reduction triggered desferrioxamine-ciprofloxacin conjugate 13. Reagents and conditions: (i) SOCl2, MeOH, reflux, 77%; (ii) NaN3, MeOH-H2O, rt, 91%; (iii) 1. PPh3, CH2Cl2, rt; 2. AcOH, THF-H2O, reflux, 66%; (iv) 1. COCl2, toluene, rt; (v) desferrioxamine mesylate, Et3N, DMF, 0°C-rt, 74% for 2 steps; (vi) LiOH, MeOH-H2O, rt, 90%; (vii) ciprofloxacin, EDC, HOBt, Et3N, DMAP, DMF, 0°C-rt, 60%
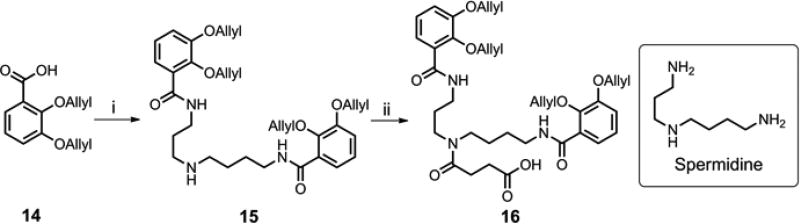
Synthesis of the mixed-ligand siderophore-ciprofloxacin conjugate, 23, started with preparation of the biscatechol fragment 16 (Scheme 4). Selective acylation of the primary amino groups of spermidine with 2,3-diallyloxybenzoic acid 14 was achieved by using N,N’-carbonyldiimidazole (CDI) as the activating reagent to give bis-acylated spermidine 15 in 87% yield. Treatment of amine 15 with succinic anhydride and a catalytic amount of DMAP generated acid 16 in 74% yield.
Scheme 4.
Synthesis of the protected biscatechol siderophore fragment 16. Reagents and conditions: (i) CDI, spermidine, CH2Cl2, 0°C-rt, 87%; (ii) succinic anhydride, DMAP, pyridine, 95°C, 74%
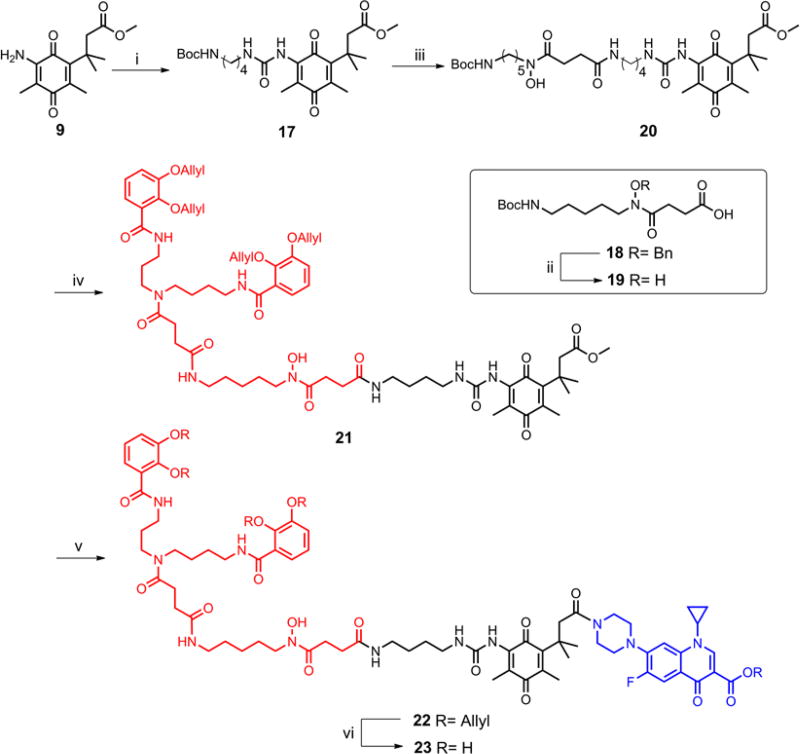
Since the carboxylic acid terminus in the mixed-ligand siderophore was not compatible with the chemistry of forming a urea linkage, an additional diamine spacer was needed. Therefore, using the same phosgene-based methodology, N-1-Boc-1,4-butanediamine was coupled with vinylogous amide 9 via a urea linkage, providing compound 17 in 91% yield (Scheme 5). After removal of the Boc protecting group from 17 with trifluoroacetic acid, the resulting amine was coupled with acid 19, the hydroxamate component of the mixed-ligand siderophore, to afford compound 20 in 50% yield. Again, the Boc protecting group in compound 20 was cleaved with TFA and the resulting amine was coupled with acid 16, to yield methyl ester 21. Subsequent hydrolysis of the ester and coupling of the resulting acid with O-allylciprofloxacin afforded the fully protected conjugate 22. Pd(0)-mediated global deallylation of 22 furnished the mixed-ligand siderophore-ciprofloxacin conjugate 23 in moderate yield.
Scheme 5.
Synthesis of mixed-ligand siderophore-ciprofloxacin conjugate 23. Reagents and conditions: (i) 1. COCl2, toluene, rt; 2. N-1-Boc-1,4-butanediamine, Et3N, CH2Cl2, 0°C-rt, 91%; (ii) H2, Pd/C, MeOH, rt, 99%; (iii) 1. TFA, CH2Cl2, 0°C-rt; 2. Acid 19, EDC, HOBt, Et3N, DMAP, CH2Cl2, 0°C-rt, 50%; (iv) 1. TFA, CH2Cl2, 0°C-rt; 2. Acid 16, EDC, HOBt, Et3N, DMAP, CH2Cl2, 0°C-rt, 43%; (v) 1. LiOH, MeOH-H2O, 0°C-rt; 2. O-Allylciprofloxacin, EDC, HOBt, Et3N, DMAP, CH2Cl2, 0°C-rt, 55%; (vi) Pd(PPh3)4, sodium benzenesulfinate, AcOH, THF-MeOH, rt, 48%.
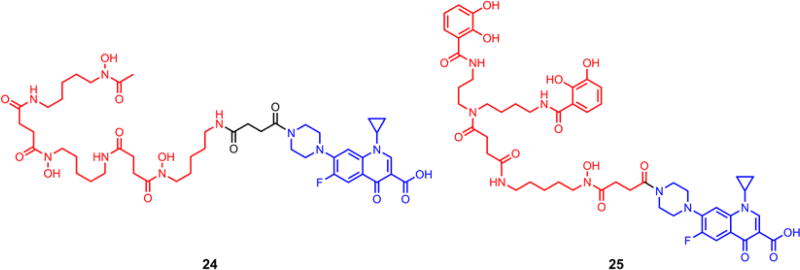
In parallel, we also synthesized desferrioxamine and mixed-ligand siderophore ciprofloxacin conjugates with stable succinic linkers as two controls to be included in the biological assays (Scheme 6).
Scheme 6.
Siderophore-ciprofloxacin conjugates (desferrioxamine 24 and mixed-ligand siderophore 25) with a stable linker.
Electrochemical and LC-MS studies
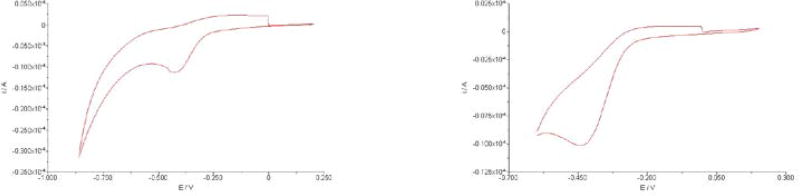
Since our intent was to have the quinone “trimethyl lock” linker in conjugates 13 and 23 activated by reduction, determination of the reduction potential of the quinone “trimethyl lock” linker was important. Two model compounds 11Fe and 11Ga were synthesized from compound 11 with the corresponding metal salts and subjected to cyclic voltammetry studies. The voltammograms of both complexes (Figure 2) showed only a single irreversible reduction peak for each, consistent with the literature report for other quinone “trimethyl lock” analogs (Mendoza et al. 2012; Ong et al. 2008). At pH 7, 11Fe and 11Ga displayed identical reduction peak potentials (Ep = −412 mV vs. SCE or −168 mV vs. NHE), demonstrating that they are thermodynamically reducible by common biological reductants such as NAD(P)H (Harrington and Crumbliss 2009). Complex 11Ga, in which the metal center is redox-inert within the experimental potential window, has the same reduction potential as 11Fe, suggesting that the measured potential is solely attributed to quinone reduction. The absence of an Fe(III) reduction wave of 11Fe also indicated that the reduction of the quinone unit is thermodynamically easier than reduction of the siderophore-chelated Fe(III).
Figure 2.
Cyclic voltammograms of 11Fe (left, 2 mM) and 11Ga (right, 2 mM). Conditions: platinum working electrode; platinum wire auxiliary electrode; saturated calomel electrode (SCE) reference electrode; 0.1 M KCl as the background electrolyte in 0.05 M phosphate buffer pH 7.0 at ambient temperature; scan rate = 100 mV s−1.
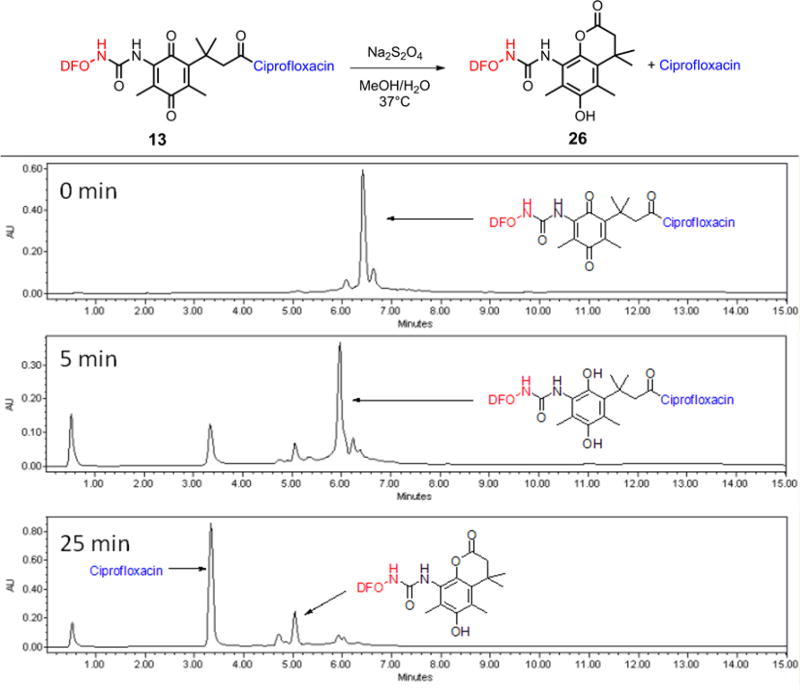
In order to examine whether the quinone “trimethyl lock” in the synthesized conjugates would induce release of the antibiotic under mild reduction conditions, conjugate 13 was treated with a ~20-fold excess of sodium dithionite (Na2S2O4) and the mixture was incubated at 37 °C. The reaction progress was monitored by LC-MS (Figure 3). HPLC chromatography and ESI-MS analyses showed that, after 5 min, the peak of the conjugate [(M+H)+=1152.37] was largely diminished while the hydroquinone intermediate [(M+H)+=1154.22], was the predominant species. Meanwhile, as proposed, the lactone 26 [(M+H)+=822.85], and the released ciprofloxacin [(M+H)+=332.18] also started to appear and complete release of the ciprofloxacin was achieved after 25 min. This result clearly showed that ciprofloxacin could be cleaved within a short time range after the linker is activated by reduction. The combined results from the electrochemical and chemical reduction studies demonstrated the feasibility of using the quinone “trimethyl lock” as a reduction triggered drug release linker in siderophore-antibiotic conjugates.
Figure 3.
LC-MS study of the chemical reduction of conjugate 13
Biological studies
The siderophore-ciprofloxacin conjugates 13 and 23 were evaluated for their ability to inhibit the growth of bacteria using standard agar diffusion assays. Representative Gram-positive strains included Bacillus subtilis, Staphylococcus aureus and Micrococcus luteus. The Gram-negative strains studied included Pseudomonas aeruginosa (a wild type K799/wt and a penetration mutant strain K799/61), Escherichia coli, and Acinetobacter baumannii. The parent drug ciprofloxacin and conjugates 24 and 25 each with a stable succinic linker were also included in the assay as controls.
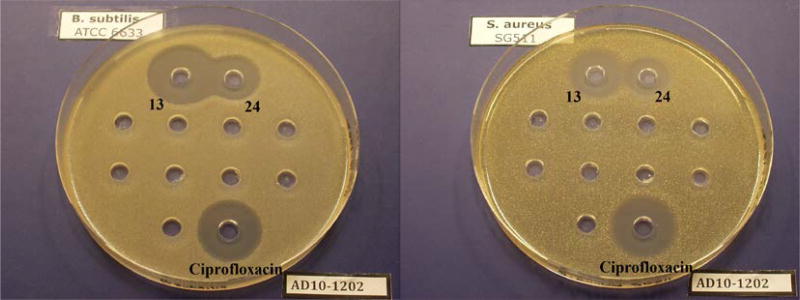
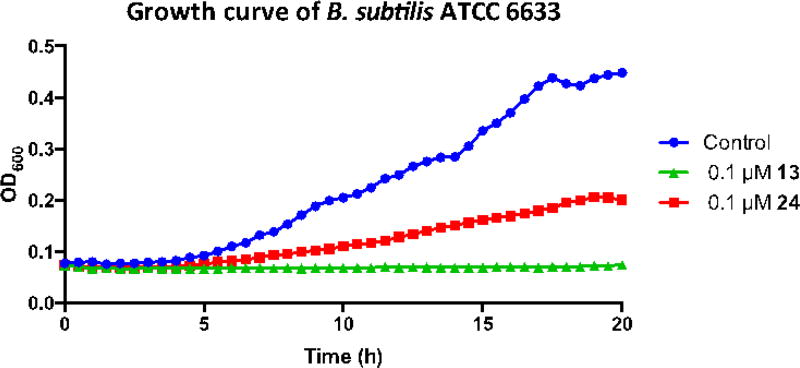
The results of assays for antibacterial activity of the four conjugates are summarized in Table 1. At the same concentration (0.2 mM), conjugates with the quinone “trimethyl lock” linker (13 and 23) were more active than their counterparts (24 and 25, respectively) with a non-releasable succinic linker in all cases, as indicated by the larger inhibition zones (Figure 4). A further kinetic study also showed that conjugate 13 with the reductive sensitive linker was more potent than 24 without the reducible linker at the same concentration (0.1 µM) in inhibiting growth of B. subtilis (Figure 5). It is reasonable to assume that, if recognized by the microbial siderophore receptor, 13 and 24, 23 and 25 are transported at similar rates, as they possess similar molecular shapes and topology around the iron coordination site. The results therefore suggest that the “trimethyl lock” facilitated drug release process occurred after the conjugate was transported. Also notable is that mixed-ligand conjugates 23 and 25 showed a narrower spectrum of activity (Gram-negative) relative to the desferrioxamine conjugates, which is consistent with previous observations and suggests bacterial outer membrane recognition selectivity as expected (Diarra et al. 1996; Ghosh et al. 1996; Wencewicz and Miller 2013). Although the anticipated drug release process might happen, all four conjugates are less active relative to the parent drug ciprofloxacin. This is possibly because 1) bacterial recognition of the chemically modified siderophore is affected; 2) activation efficiency of the linker is low inside the bacteria. Further investigation by attaching siderophores with better outer membrane receptor recognition to the linker could provide conjugates with enhanced activities. An artificial triscatecholate sideophore with linker attachment site remote from the site of iron binding is a promising candidate (Ji et al. 2012b). Improvement of the linker’s activation efficiency might be achieved by introducing electron-withdrawing groups to the quinone moiety (e.g. replacing -CH3 with -CF3) to further increase its electron deficiency although it could be more synthetically challenging.
Table 1.
Diameter of growth inhibition zones (mm) in the agar diffusion antibacterial susceptibility assay.
| Compds | Gram-positive bacteria | Gram-negative bacteria | |||||
|---|---|---|---|---|---|---|---|
| B. subtilis | S. aureus | M. luteus | P. aeruginosa | E. coli | A. baumannii | ||
|
| |||||||
| ATCC 6633 |
SG 511 | ATCC 10240 | K799/wt | K799/61 | X580 | ATCC 17961a | |
| Ciprofloxacin | 28c | 28b | 12Pb | 21c | 24/32pc | 31d | 15pb |
| 13 | 34 | 25 | 14 | 27/31P | 31/46P | 34/38P | NT |
| 24 | 26 | 22 | 0 | 18P | 19/26P | 32/39P | NT |
| 23 | 20/26p | 13P | 0 | 19 | 19/26p | 27 | 20 |
| 25 | 0 | 0 | 0 | 14 | 0 | 21 | 18 |
p, partially clear inhibition zone/colonies in the inhibition zone; P, unclear inhibition zone/many colonies in the inhibition zone; NT, not tested
Exactly 50 µL of a 0.2 mM solution of each compound dissolved in 1:9 DMSO/MeOH was filled in 9 mm wells in agar media (Mueller Hinton II). Inhibition zones read after incubation at 37 °C for 24 h.
A. baumannii was tested in the presence of 100 µM of 2,2’-bipyridine.
Ciprofloxacin was tested at (b) 5 µg/mL, (c) 1.66 µg/mL, (d) 0.33 µg/mL in H2O.
Figure 4.
Agar diffusion assay for conjugates 13 and 24 tested against B. subtilis ATCC 6633 (left) and S. aureus SG511 (right).
Figure 5.
Growth curve of B. subtilis in the presence of conjugates 13 and 24 under iron deficient conditions.
Conclusions
Two siderophore-ciprofloxacin conjugates with a potential reduction triggered linker for drug release were synthesized. The linker incorporated in the conjugates was designed to be activated by taking advantage of the reductive pathway of bacterial iron metabolism. Upon reduction, a rapid “trimethyl lock” facilitated lactonization was anticipated to release the antibiotic from the conjugate. Electrochemical studies indicated that the quinone moiety in the linker is thermodynamically reducible by common biological reductants and the expected lactonization is rapid and induces concomitant release of the drug as supported by LC-MS analysis. Furthermore, conjugates with the reduction triggered linker were more potent in antibacterial assays relative to their counterparts with the stable linker, which suggests the drug release occurred inside the bacterial cells. However, the activity of conjugates 13 and 23 was weaker than that of the parent drug ciprofloxacin, suggesting that either the siderophore recognition or linker activation efficiency or both factors require further optimization. Once optimized, the efficient synthetic strategy developed for attaching siderophores to the quinone “trimethyl lock” linker via a urea linkage can and will be used in the development of new generation of siderophore-drug conjugates with a drug release linker.
Supplementary Material
Acknowledgments
Partial funding for this work was provided by NIH grant AI054193. C. J. gratefully acknowledges the UND Chemistry-Biochemistry-Biology (CBBI) Interface Program funded by NIH (T32GM075762) for fellowship support. We gratefully acknowledge the use of the NMR facilities provided by the Lizzadro Magnetic Resonance Research Center at the University of Notre Dame (UND) under the direction of Dr. Jaroslav Zajicek and the mass spectrometry services provided by The UND Mass Spectrometry & Proteomics Facility (Mrs. N. Sevova, Dr. W. Boggess, and Dr. M. V. Joyce; supported by the National Science Foundation under CHE-0741793). We gratefully thank Mrs. Patricia A. Miller (UND) for antibacterial susceptibility testing.
Footnotes
Experimental procedures, copies of spectral data, and characterization data. This material is available free of charge via the internet.
References
- Boucher HW, et al. Bad bugs, no Drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- Braun V, Günthner K, Hantke K, Zimmermann L. Intracellular activation of albomycin in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1983;156:308–315. doi: 10.1128/jb.156.1.308-315.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V, Pramanik A, Gwinner T, Koberle M, Bohn E. Sideromycins: tools and antibiotics. Biometals. 2009;22:3–13. doi: 10.1007/s10534-008-9199-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diarra MS, et al. Species selectivity of new siderophore-drug conjugates that use specific iron uptake for entry into bacteria. Antimicrob Agents Chemother. 1996;40:2610–2617. doi: 10.1128/aac.40.11.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez L, Hancock REW. Adaptive and mutational resistance: role of porins and efflux pumps in drug resistance. Clin Microbiol Rev. 2012;25:661–681. doi: 10.1128/cmr.00043-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Ghosh M, Niu C, Malouin F, Möllmann U, Miller MJ. Iron transport-mediated drug delivery using mixed-ligand siderophore-β-lactam conjugates. Chem Biol. 1996;3:1011–1019. doi: 10.1016/s1074-5521(96)90167-2. [DOI] [PubMed] [Google Scholar]
- Harrington JM, Crumbliss AL. The redox hypothesis in siderophore-mediated iron uptake. Biometals. 2009;22:679–689. doi: 10.1007/s10534-009-9233-4. [DOI] [PubMed] [Google Scholar]
- Hennard C, Truong QC, Desnottes JF, Paris JM, Moreau NJ, Abdallah MA. Synthesis and activities of pyoverdin-quinolone adducts: a prospective approach to a specific therapy against Pseudomonas aeruginosa. J Med Chem. 2001;44:2139–2151. doi: 10.1021/jm990508g. [DOI] [PubMed] [Google Scholar]
- Ji C, Juárez-Hernández RE, Miller MJ. Exploiting bacterial iron acquisition: siderophore conjugates. Future Med Chem. 2012a;4:297–313. doi: 10.4155/fmc.11.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji C, Miller MJ. Chemical syntheses and in vitro antibacterial activity of two desferrioxamine B-ciprofloxacin conjugates with potential esterase and phosphatase triggered drug release linkers. Bioorg Med Chem. 2012;20:3828–3836. doi: 10.1016/j.bmc.2012.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji C, Miller PA, Miller MJ. Iron transport-mediated drug delivery: practical syntheses and in vitro antibacterial studies of tris-catecholate siderophore-aminopenicillin conjugates reveals selectively potent antipseudomonal activity. J Am Chem Soc. 2012b;134:9898–9901. doi: 10.1021/ja303446w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine MN, Raines RT. Trimethyl lock: a trigger for molecular release in chemistry, biology, and pharmacology. Chem Sci. 2012;3:2412–2420. doi: 10.1039/c2sc20536j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy SB, Marshall B. Antibacterial resistance worldwide: causes, challenges and responses. Nat Med. 2004;10:S122–S129. doi: 10.1038/nm1145. [DOI] [PubMed] [Google Scholar]
- Mendoza MF, Hollabaugh NM, Hettiarachchi SU, McCarley RL. Human NAD(P)H:quinone oxidoreductase Type I (hNQO1) activation of quinone propionic acid trigger groups. Biochemistry. 2012;51:8014–8026. doi: 10.1021/bi300760u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miethke M, Marahiel MA. Siderophore-based iron acquisition and pathogen control. Microbiol Mol Biol Rev. 2007;71:413–451. doi: 10.1128/mmbr.00012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mislin GLA, Schalk IJ. Siderophore-dependent iron uptake systems as gates for antibiotic Trojan horse strategies against Pseudomonas aeruginosa. Metallomics. 2014;6:408–420. doi: 10.1039/c3mt00359k. [DOI] [PubMed] [Google Scholar]
- Mitscher LA. Bacterial topoisomerase inhibitors: quinolone and pyridone antibacterial agents. Chem Rev. 2005;105:559–592. doi: 10.1021/cr030101q. [DOI] [PubMed] [Google Scholar]
- Möllmann U, Heinisch L, Bauernfeind A, Köhler T, Ankel-Fuchs D. Siderophores as drug delivery agents: application of the "Trojan Horse" strategy. Biometals. 2009;22:615–624. doi: 10.1007/s10534-009-9219-2. [DOI] [PubMed] [Google Scholar]
- Müller G, Raymond KN. Specificity and mechanism of ferrioxamine-mediated iron transport in Streptomyces pilosus. J Bacteriol. 1984;160:304–312. doi: 10.1128/jb.160.1.304-312.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noël S, Gasser V, Pesset B, Hoegy F, Rognan D, Schalk IJ, Mislin GLA. Synthesis and biological properties of conjugates between fluoroquinolones and a N3•-functionalized pyochelin. Org Biomol Chem. 2011;9:8288–8300. doi: 10.1039/c1ob06250f. [DOI] [PubMed] [Google Scholar]
- Ong W, Yang Y, Cruciano AC, McCarley RL. Redox-triggered contents release from liposomes. J Am Chem Soc. 2008;130:14739–14744. doi: 10.1021/ja8050469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page MGP. Siderophore conjugates. Ann NY Acad Sci. 2013;1277:115–126. doi: 10.1111/nyas.12024. [DOI] [PubMed] [Google Scholar]
- Pages JM, James CE, Winterhalter M. The porin and the permeating antibiotic: a selective diffusion barrier in Gram-negative bacteria. Nat Rev Microbiol. 2008;6:893– 903. doi: 10.1038/nrmicro1994. [DOI] [PubMed] [Google Scholar]
- Projan SJ. Why is big Pharma getting out of antibacterial drug discovery? Curr Opin Microbiol. 2003;6:427–430. doi: 10.1016/j.mib.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Rivault F, Liebert C, Burger A, Hoegy F, Abdallah MA, Schalk IJ, Mislin GLA. Synthesis of pyochelin-norfloxacin conjugates. Bioorg Med Chem Lett. 2007;17:640–644. doi: 10.1016/j.bmcl.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Roosenberg JM, Miller MJ. Total synthesis of the siderophore danoxamine. J Org Chem. 2000;65:4833–4838. doi: 10.1021/jo000050m. [DOI] [PubMed] [Google Scholar]
- Schröder I, Johnson E, de Vries S. Microbial ferric iron reductases. FEMS Microbiol Rev. 2003;27:427–447. doi: 10.1016/s0168-6445(03)00043-3. [DOI] [PubMed] [Google Scholar]
- Spellberg B, et al. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:155–164. doi: 10.1086/524891. [DOI] [PubMed] [Google Scholar]
- Vértesy L, Aretz W, Fehlhaber HW, Kogler H. Salmycin A–D, antibiotics from Streptomyces violaceus, DSM 8286, having a siderophore aminoglycoside structure. Helv Chim Acta. 1995;78:46–60. doi: 10.1002/hlca.19950780105. [DOI] [Google Scholar]
- Wencewicz TA, Long TE, Möllmann U, Miller MJ. Trihydroxamate siderophore–fluoroquinolone conjugates are selective sideromycin antibiotics that target Staphylococcus aureus. Bioconjugate Chem. 2013;24:473–486. doi: 10.1021/bc300610f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wencewicz TA, Miller MJ. Biscatecholate-monohydroxamate mixed ligand siderophore-carbacephalosporin conjugates are selective sideromycin antibiotics that target Acinetobacter baumannii. J Med Chem. 2013;56:4044–4052. doi: 10.1021/jm400265k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wencewicz TA, Möllmann U, Long TE, Miller MJ. Is drug release necessary for antimicrobial activity of siderophore-drug conjugates? Syntheses and biological studies of the naturally occurring salmycin "Trojan Horse" antibiotics and synthetic desferridanoxamine-antibiotic conjugates. Biometals. 2009;22:633–648. doi: 10.1007/s10534-009-9218-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng T, Nolan EM. Enterobactin-mediated delivery of β-lactam antibiotics enhances antibacterial activity against pathogenic Escherichia coli. J Am Chem Soc. 2014;136:9677–9691. doi: 10.1021/ja503911p. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.