Abstract
In many different human disorders, the cellular glycome is altered. An interesting but poorly understood alteration occurs in the mucin-type O-glycome, in which there is aberrant expression of the truncated O-glycans Tn (GalNAcα1-Ser/Thr) and its sialylated version sialyl-Tn (STn) (Neu5Acα2,6GalNAcα1-Ser/Thr). Both Tn and STn are tumor-associated carbohydrate antigens and tumor biomarkers, since they are not expressed normally and appear early in tumorigenesis. Moreover, their expression is strongly associated with poor prognosis and tumor metastasis. The Tn and STn antigens are also expressed in other human diseases and disorders, such as Tn syndrome and IgA nephropathy. The major pathological mechanism for expression of the Tn and STn antigens is compromised T-synthase activity, resulting from alteration of the X-linked gene that encodes for Cosmc, a molecular chaperone specifically required for the correct folding of T-synthase to form active enzyme. This review will summarize our current understanding of the Tn and STn antigens in terms of their biochemistry and role in pathology.
Keywords: Cancer, Disease, Glycosylation, IgA nephropathy, Tn antigen
1 Introduction
Glycosylation is one of the most abundant protein PTMs. The mucin-type O-glycans occurring on secreted and trans-membrane glycoproteins [1, 2] are a major category of glycosylation and serve important biological functions. Core 1 based O-glycans on glycoproteins play essential roles in immunity [3–10], leukocyte trafficking [3–6, 11], vascular biology [8, 12, 13], angiogenesis [12, 13], and lymphangiogenesis [14]; both core 1 and core 3 based O-glycans on glycoproteins and mucins protect gut epithelial cells from the microflora and extreme pH, and prevent tumorigenesis [15–18]. Many biologically important O-glycan structures have been identified, including sialyl core 1, sialyl Lewis x (SLeX) on core 2 O-glycans, sulfo-SLeX on extended core 1 O-glycans on leukocytes [3–6], and sulfo-LeX and SLeX on core 3 and core 4 O-glycans on gastrointestinal (GI) tract epithelium and secreted mucins [19, 20].
Mucin-type O-glycans are mainly synthesized by the sequential action of glycosyltransferases in the Golgi apparatus, although there are some reports of initiation of O-glycosylation in the ER in pathologic states [21]. Among these glycosyltransferases, core 1 β3-galactosyltransferase (T-synthase) is the key enzyme in forming the core 1 structure, which is the precursor for core 1 based O-glycans synthesized in all tissues. Interestingly, biosynthesis of active T-synthase requires an ER-localized molecular chaperone Cosmc that prevents the aggregation and subsequent proteasomal degradation of T-synthase [22–24]. The critical enzyme for core 3 based O-glycans synthesized in GI tract epithelia is core 3 β3-N-acetylglucosaminyltransferase (core 3 β3GnT, core 3 synthase) [25].
Under normal physiological conditions, the biosynthesis of O-glycans in vertebrates results in complex O-glycans extended beyond the core 1 disaccharide (Galβ1, 3GalNAc-α-Ser/Thr) in all cell types and the core 3 disaccharide (GlcNAcβ1,3GalNAc-α-Ser/Thr) in GI tract epithelial cells [1]. This indicates that Tn antigen is an immature structure that is typically modified or elongated with high efficiency. In pathological situations, these complex O-glycans are often structurally altered. The most common alteration is the truncation of the O-glycans to simply GalNAc-α-Ser/Thr (the Tn antigen) and its sialylated version, Neu5Acα2,6GalNAc-α-Ser/Thr (sialyl-Tn (STn)). For example, Tn and STn appear on blood cells of all linages in Tn syndrome, on IgA1 proteins in IgA nephropathy (IgAN), and on mucins and glycoproteins on tumor cells in many different cancers [1]. Various studies have also shown that Tn and STn antigens are associated with the pathogenesis of these diseases [1, 26–29]. Therefore, these aberrant O-glycans are considered human disease markers. This review will summarize our current understanding of the biochemistry of Tn and STn antigens with respect to how these aberrant O-glycans are expressed and their role in pathogenesis.
2 Mucin-type O-glycan biosynthetic pathways
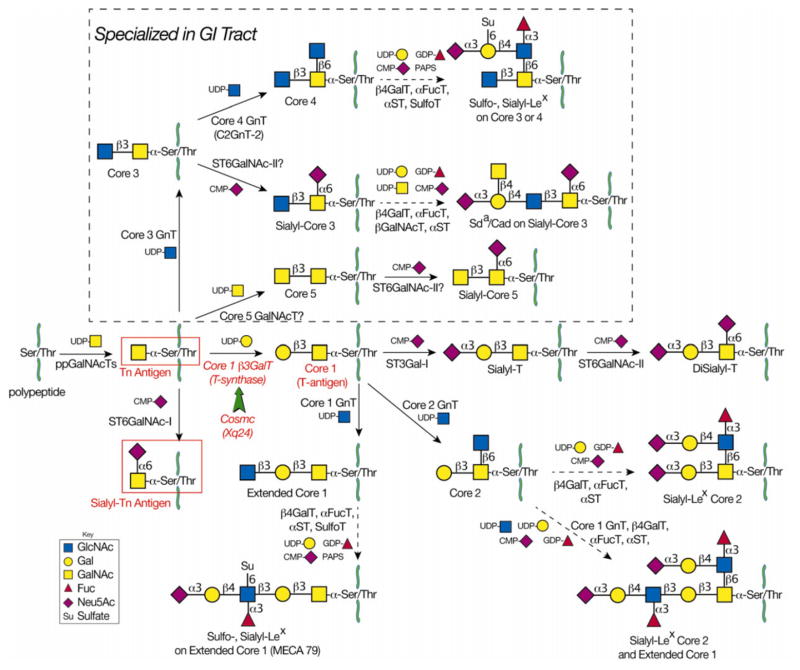
The biosynthesis of mucin-type O-glycans (or O-linked oligosaccharides), as summarized in Fig. 1, takes place mainly in the Golgi apparatus by sequential action of a set of glycosyltransferases. The initial step is the synthesis of glycopeptide by a polypeptide: α-N-acetylgalactosaminyl-transferase (pp-α-GalNAcT, ppGalNAcT) that transfers GalNAc from UDP-GalNAc to Ser/Thr residues of a polypeptide, forming GalNAc-α-Ser/Thr (the Tn antigen) [30]. There are 20 human genes encoding ppGalNAcTs, and while the enzymes have overlapping substrate specificities, each has its own distinct peptide preference as well. In normal conditions, the Tn antigen is always modified to form other structures by the sequential action of additional glycosyltransferases. The major and common pathway is through core 1 β3 galactosyltransferase (core 1 β3GalT, T-synthase), which transfers a Gal from UDP-Gal onto the Tn antigen to form Galβ1,3GalNAc-α-Ser/Thr, also called the core 1 structure or the T-antigen [31, 32]. This core 1 structure is usually further modified or extended by glycosyltransferases and sulfotransferases to form the complex O-glycans seen in most mucins, membrane glycoproteins, and secreted glycoproteins [33]. O-glycans play important roles in many biological processes, such as signal transduction, cell–cell interaction (selectins and their ligands), immunity, and angiogenesis [1]. The core 1 O-glycan is the most common core structure in normal glycoproteins of the endothelium and of hematopoietic cells, including erythrocytes and leukocytes [1, 34–36]. Epithelial cells of the GI tract also form core 1 structures, but can alternatively modify the Tn antigen to form the core 3 structure (GlcNAcβ1,3GalNAc-α-Ser/Thr) by the action of core 3 β 1,3 N-acetylglucosaminyltransferase (core 3 GnT, C3GnT, β3GnT-6). Similar to core 1, core 3 is further modified by many glycosyltransferases to generate core 3 based complex O-glycans. These extended structures include sulfo-Lex, SLex, or Sda on core 3 or core 4 O-glycans, all of which have been found on mucins such as MUC2 secreted from intestinal epithelial cells (IECs) [19, 20, 37]. There are many important unanswered questions about C3GnT, including its substrate specificity: both C3GnT and T-synthase share a common substrate (Tn antigen), but do their specificities coincide with, overlap, or complement one another? In addition to the core 1 and core 3 structures, Tn can be modified to form STn. Normally, however, CMP-Neu5Ac:GalNAc α2,6-sialyltransferase (ST6GalNAc-I) [38] has low activity and converts Tn antigen to STn antigen very inefficiently. Therefore, the STn antigen is hardly detectable in normal human tissues, although it can be found on bovine submaxillary mucin and ovine submaxillary mucin in submaxillary glands containing high levels of the ST6GalNAc-I [38, 39].
Figure 1.
The mucin-type O-glycosylation pathways. Mucin-type O-glycosylation begins in the Golgi when polypeptide GalNAc-transferases transfer GalNAc from UDP-Gal to Ser/Thr on a polypeptide chain to form Tn antigen (GalNAc-α-Ser/Thr). In nondiseased tissue, Tn antigen is further modified to form complex O-glycans. The T-synthase (Core 1 β3GalT) transfers Gal from UDP-Gal to GalNAc to form core 1 (T-antigen) in all cell types. Cosmc is the unique molecular chaperone for the T-synthase. Loss of Cosmc or T-synthase activity results in pathological expression of Tn antigen and sialyl-Tn (STn) antigen. The latter results from the actions of ST6GalNAc-I, which transfers Neu5Ac from CMP-Neu5Ac to the Tn antigen to form STn. Due to poor efficiency of ST6GalNAc-I, it is not likely that high expression of ST6GalNAc-I could outcompete functional T-synthase to result in pathologic STn expression. In GI epithelia, core 3 GnT transfers GlcNAc from UDP-GlcNAc to Tn antigen to form core 3. It is not known whether the T-synthase and core 3 GnT compete for the same substrates in GI epithelial cells. The T antigen is further modified to form extended core 1 structures. Similarly, core 3 is further modified to form extended core 3 structures, including sulfo-Lex, sialyl-Lex, or Sda on core 3 or core 4 O-glycans.
3 Molecular mechanisms for the expression of Tn and STn antigens
Although Tn and STn antigens have been found to be associated with pathological conditions for almost four decades, the mechanism that causes cells to express these antigens was poorly understood. The purification and cloning of the cDNA for T-synthase [31, 32], and especially the subsequent discovery of its specific molecular chaperone Cosmc [22], have led to a new era in the field. Mouse models have shown that deletion of Cosmc causes cell-surface expression of Tn and STn. Complete knockout of either T-synthase or Cosmc in mice, though lethal, results in uniform expression of Tn antigen throughout the entire embryo [12, 13]. Similarly, a tissue-specific Cosmc-KO shows Tn/STn expression in the targeted cell lineages [40]. Because they both modify Tn antigen, it was possible that C3GnT could compensate for loss of T-synthase activity in tissues where it is expressed. However, while knockout of C3GnT in mice only results in low expression of Tn antigen [17], IEC T-synthase knockout mice develop colitis and have massive Tn antigen expression in the IECs [18], indicating that C3GnT cannot compensate for T-synthase in these cells. In our preliminary studies, IEC-Cosmc-KO mice likewise express massive Tn/STn antigens in IECs.
Many groups have also demonstrated that spontaneous expression of Tn/STn on the cell surface results from mutations in Cosmc [2,22,23,41–43]. We also investigated the molecular mechanisms underlying Tn expression in Tn4 cells, a cell line generated from a male individual with Tn syndrome like disorder [43]. Tn4 cells have no detectable level of Cosmc transcript, but have a normal level of T-synthase transcript. We found that silencing of Cosmc in Tn4 cells is due to hyperme-thylation of the Cosmc promoter [44]. To date, alterations of Cosmc that result in a dysfunctional Cosmc include: (i) point mutations in the ORF [22,41,42], (ii) gene deletion (LOH) [41], and (iii) hypermethylation of the promoter for Cosmc [44]. These data support the mechanistic model (Fig. 2) in which the expression of Tn/STn antigens can arise from dysfunctional Cosmc [41]. To assess alterations of Cosmc in primary tumors, Yoo et al. [45] used PCR-SSCP on human breast and colon cancer samples but found no mutations in the Cosmc ORF. However, this approach ignores the potential for Tn expression to arise from mutations outside the ORF or from hypermethylation of the promoter region. Yoo et al. focused only on point mutations in the coding region of Cosmc and did not analyze samples for Tn antigen expression. In our preliminary studies of Cosmc in Tn/STn(+) colon tumors, we found instances of whole gene deletion and promoter region deletion. Thus, alterations in Cosmc may be the cause of Tn/STn expression even in cases when ORF mutations cannot be detected.
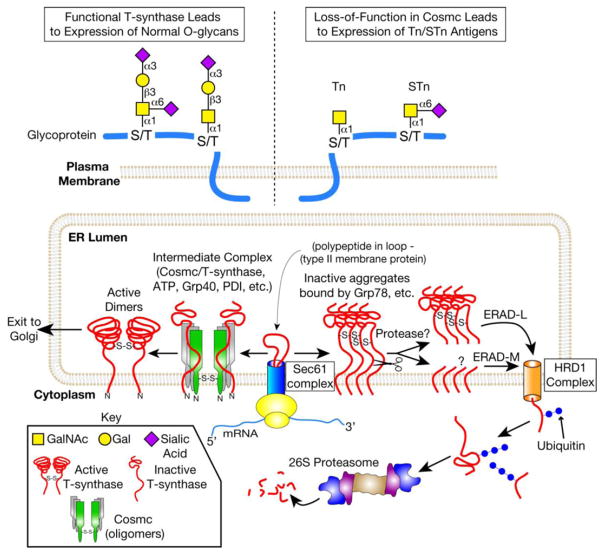
Figure 2.
The major molecular mechanism for the expression of Tn and STn antigens in cells lacking a functional Cosmc. Cosmc is the unique molecular chaperone for the T-synthase. Nascent T-synthase is translocated to the ER possibly via the Sec61 complex. Cosmc interacts cotranslationally with nascent, nonnative T-synthase to form active, dimeric T-synthase. The T-synthase is subsequently transported to the Golgi. In the Golgi, the T-synthase transfers Gal from UDP-Gal to Tn antigen to form the T antigen on polypeptide chains. Defective Cosmc due to genetic or epigenetic alterations in Cosmc (e.g. ORF mutations, promoter methylation, or loss of heterozygosity) results in aggregation and proteosomal degradation of the T-synthase. Mis-folded T-synthase interacts with Grp78, is cleaved in its lumenal domain by an unknown protease, and is retrotranslocated to the cytosol. In the cytosol, soluble T-synthase is polyubiquitinated and degraded by the 26S proteasome. Loss of T-synthase activity results in expression of Tn and sialylTn antigens, which are not present in normal, non-transformed tissue.
Recently, it was shown that Src kinase redistributes Golgi-localized ppGalNAc-Ts to the ER, and the authors speculated that Src-dependent relocation of ppGalNAc-Ts could play an important role in cancerous cellular transformation, including alteration of O-glycosylation [21]. Although this report showed the appearance of glycoproteins with Tn antigen in the ER, the authors did not report high levels of the Tn antigen on the cell surface. In our own studies, we have also seen accumulation of intracellular Tn expression in a few cases of colon cancer as well as in normal control tissues, but this did not correlate with elevated expression of either Tn or STn on the plasma membrane. These data suggest that activation of Src kinase may not be a major mechanism for the expression of Tn/STn on the cell surface.
How does Cosmc work as a molecular chaperone? During the biosynthesis of the T-synthase in the ER, Cosmc is predicted to bind to newly synthesized T-synthase and prevent its aggregation and subsequent degradation in the ER-associated degradation (ERAD) pathway. Based on our studies, a working model for how Cosmc functions in the ER to assist the folding of T-synthase is proposed in Fig. 2 [22, 24, 46]. In cells lacking functional Cosmc, T-synthase is synthesized but misfolded resulting in inactive aggregates. This misfolded T-synthase is retained in the ER probably through binding to Bip (Grp78) within the lumen of the ER, as demonstrated through copurification of Bip with misfolded T-synthase in LSC cells [24]. This misfolded T-synthase is then retrotranslo-cated to the cytosol, polyubiquitinated, and subsequently delivered to the 26S proteasome for degradation (Fig. 2). Since the lesion of T-synthase is located at its lumenal domain, this misfolded T-synthase most likely enters the ERAD-Lumenal (ERAD-L) pathway for its degradation. This ERAD-L pathway seems very efficient as there is no detectable T-synthase protein in mouse embryos with complete deletion of Cosmc [13]. The process of retrotranslocating inactive T-synthase from the ER to the cytoplasm, and then ubiquitinating it for targeted destruction is poorly understood; it may involve the HRD1 complex, which was recently shown to be the machinery for retrotranslocation of misfolded proteins for ERAD-L [47]. Interestingly, recombinant T-synthase expressed in LSC cells is proteolytically cleaved in the stem region by an unknown protease [24]. Inhibiting the proteasome in Cosmc-deficient cells leads to accumulation of inactive aggregates of full-length and partly degraded T-synthase protein in the ER lumen. Such observations raise additional questions. What is the cleavage site in the stem region of T-synthase? What protease is responsible for this cleavage? Is this cleavage necessary for the degradation of misfolded T-synthase? Are the misfolded lumenal and cytoplasmic/transmembrane domains of T-synthase independently degraded by ERAD-L and ERAD membrane/cytosolic (ERAD-M/C) pathways? Full understanding of these questions may reveal new machinery involved in the degradation of misfolded type-II transmembrane proteins.
In recent studies, it has been shown that recombinant T-synthase, when denatured by heat or treatment with guanidinium-HCl, can reacquire activity in vitro when incubated with recombinant Cosmc in the absence of other protein factors and independently of ATP binding or hydrolysis [48]. This is interesting since Cosmc was shown to bind ATP [24], suggesting that ATP may have some role in vivo in Cosmc interactions or functions. Clearly, there is much to be learned about the biological role of Cosmc and the need for a specific chaperone for the T-synthase.
3.1 Tn/STn antigens in Tn syndrome
Tn syndrome is a rare hematological disorder characterized by the expression of the Tn antigen on a subpopulation of blood cells in all lineages [26]. It was first described in a patient as a polyagglutinability syndrome of erythrocytes [49]. Clinically, patients with Tn syndrome usually appear healthy and do not require treatment. Laboratory tests may uncover moderate hemolytic anemia and reduced numbers of thrombocytes and leukocytes. The mechanisms leading to these symptoms appear to be multifactorial and are poorly understood.
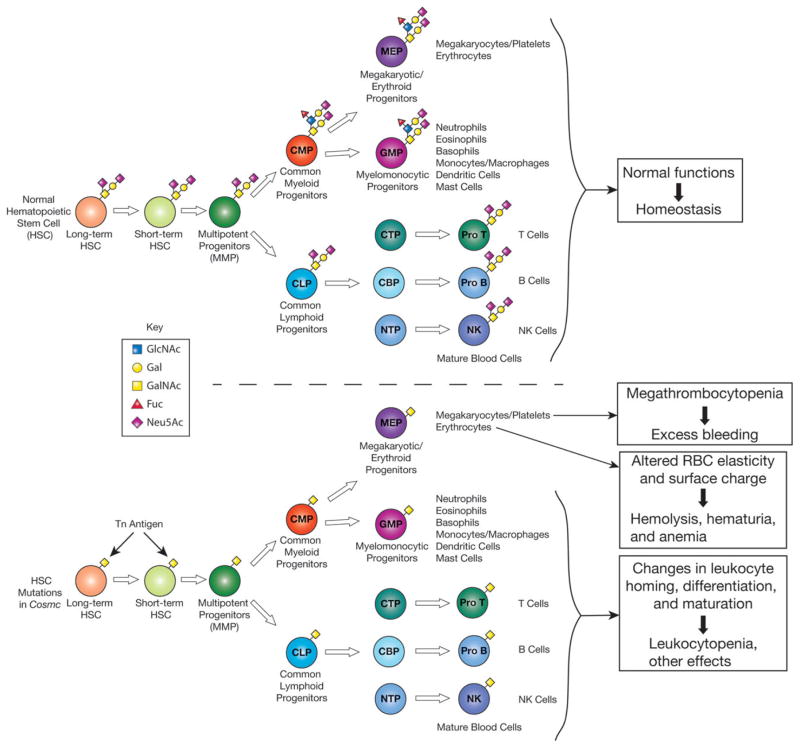
Studies have shown that the erythrocyte and leukocyte glycoproteins of Tn syndrome patients have reduced galactose and sialic acid content, which is linked to the defect in activity of T-synthase leading to exposure of GalNAc-α1-O-Ser/Thr (Tn antigen). Although an early study suggested that this might be due to hypermethylation of the promoter region of T-synthase, the underlying genetic changes in patient cells were instead shown to be due to acquired somatic mutations in Cosmc or hypermethylation of the promoter region of Cosmc. These mutations introduce an ORF shift and/or premature stop codon, while promoter hypermethylation blocks transcription completely, resulting in total or severe loss of chaperone function and hence T-synthase activity. All mutations in Cosmc identified in the blood of patients with Tn syndrome have been summarized in a recent review [1]. Because Tn syndrome arises from an acquired somatic alteration in Cosmc in an early blood progenitor, circulating cells contain both Tn-positive and Tn-negative populations, with deficient and functional Cosmc, respectively (Fig. 3). One possible cause of the hemolytic anemia, reduced thrombocyte count, and bleeding disorder that occasionally develop in patients with Tn syndrome is autoantibodies directed against the Tn antigen. These antibodies may be of the IgM cold agglutinin type as shown for autoantibodies against the carbohydrate I antigen present on adult erythrocytes. Another possible mechanism of pathology is dysfunction of major glycoprotein(s) on the platelets or leukocytes. Glycoproteins play critical roles in the biogenesis and function of these cells, and altered O-glycosylation may compromise the glycoproteins’ functions (Fig. 3). The recent discovery [40] that endothelial and hematopoietic cell Cosmc-KO mice develop megathrombocytopenia and bleeding disorder strongly suggests that thrombocytopenia and bleeding in Tn-syndrome patients are mainly caused by the impaired biogenesis and function of platelets lacking functional Cosmc.
Figure 3.
Tn and STn antigens on blood cells in Tn syndrome. Tn syndrome is a rare autoimmune disorder in which populations of blood cells from all lineages express Tn antigen. Genetic/epigenetic alterations in Cosmc in an early blood progenitor result in Tn expression and associated pathology, including hemolytic anemia, thrombocytopenia, and bleeding disorders. Pathology is thought to be due to formation of anti-Tn IgM antibodies and/or dysfunction of O-glycosylated proteins found on blood cells. A targeted deletion of Cosmc in murine endothelial/hematopoietic cells was recently observed to result in macrothrombocytopenia, prolonged tail-bleeding times, and dysregulation of multiple platelet integrins.
3.2 Tn/STn antigens in IgAN
IgAN, also called Berger’s disease, was first described by Dr. Jean Berger in 1968 [50]. More than four decades later, IgAN is the most common primary glomerulonephritis worldwide [51–53] and leads to terminal renal failure in 20–40% of patients over 20–25 years [54]. The majority of primary IgAN cases are sporadic, with only a minority of patients appearing within family clusters. To date, no causal gene has been identified [55–59] and additional physiological and environmental factors appear to be required for clinical manifestation of the disease. IgAN is characterized by deposition of IgA1 in the mesangium. Diagnosis of IgAN is currently based on the clinical symptoms of glomerulonephritis, such as hematuria and proteinuria; definitive diagnosis is by renal biopsy and histological evidence for IgA deposits [60]. These deposits elicit glomerular inflammation leading to progressive renal injury.
Human IgA1 is an O-glycosylated immunoglobulin with nine potential O-glycosylation sites in the hinge region (HR), up to six of which are occupied normally by the mono- and disialylated core 1 O-glycans [60–63]. Many studies suggest that patients with IgAN have O-glycans in the IgA1 HR with a deficiency of galactose and concomitant expression of Tn or STn, which may be responsible for initiating the pathology [64–69]. The expression of Tn and STn antigens on IgA1 in IgAN may be due to a B-cell restricted reduction in T-synthase activity [70].
Whether Cosmc and T-synthase play a role in the pathogenesis of IgAN is controversial. Several studies suggested that the transcript levels of Cosmc and/or T-synthase are reduced in the B cells of patients with IgAN [71–75]. Suzuki et al. [67] reported that IgA1-secreting cell lines from patients with IgAN produce aberrantly glycosylated IgA1 due to lower transcripts of both Cosmc and T-synthase in conjunction with upregulated ST6GalNAc-II. Others linked IgAN to polymorphisms in Cosmc and T-synthase [76, 77], while one report concluded that there is no mutation in Cosmc in patients with IgAN [78]. A key problem in clarifying the role of Cosmc/T-synthase in IgAN is that only a minor fraction of plasma cells secrete the IgA1 involved in this disease. Identification and isolation of this population of plasma cells is extremely difficult, but is crucial to unraveling the role that Cosmc and/or T-synthase may play in IgAN.
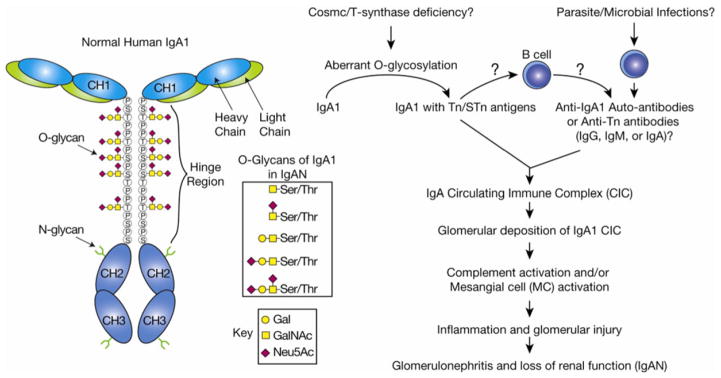
The abnormal glycosylation of the IgA1 HR probably contributes to the pathogenesis of IgAN (Fig. 4). Tn antigen in the HR can be recognized and bound by naturally occurring IgA or IgG antibodies resulting in the formation of circulating immune complexes [27,28,52,60,66,68,79], yet how these anti-Tn antibodies are raised in patients is unknown. Some proposed that these anti-Tn antibodies might be generated during infections with parasites and other pathogens that carry Tn antigens on their glycoproteins [80,81], as discussed below. Therefore, the propensity of an individual to develop autoimmune antiglycan antibodies may represent a cofactor in disease manifestation [51]. Alternatively, the aberrantly glycosylated HR may render IgA1 molecules prone to self-aggregation and formation of macromolecular complexes by a nonimmunological mechanism. The macromolecular IgA1 complexes may escape hepatic clearance because they are too large to pass through endothelial fenestrae to reach the hepatocytes [82]. Instead, they are shunted to the renal circulation where endothelial fenestrae overlying the glomerular mesangia are larger. The mesangial cells bind high molecular weight IgA1 with high affinity by a yet unknown mechanism [83]. The activation of mesangial cells by IgA1 immune complexes and the concomitant deposition of complement component 3 (C3) are considered the initiating events in the pathogenesis of IgAN. Clearly, IgAN is a complex disease, and many factors contribute to its immunopathogenesis and outcome. Fully understanding the molecular mechanism of aberrant O-glycosylation in the HR of IgA1 should aid in the development of noninvasive diagnostic techniques and novel therapeutic approaches.
Figure 4.
Tn and STn antigens on IgA1 in IgAN. IgA nephropathy (IgAN) is the most common glomerulonephritis and results in renal failure in 20–40% of patients over 25 years of age. Deposition of IgA1 in the glomerular mesangium is thought to drive the disease. Reduced galactose and increased Tn/STn have been observed on IgA1 isolated from renal biopsies of patients. Normal O-glycans in the IgA1 hinge region contain mono- or disialylated core 1 structures. Although the role of Cosmc/T-synthase in IgAN is controversial, a reduction in T-synthase transcript and activity has been observed in B cells isolated from IgAN patients. It is thought that Tn/STn expression could lead to IgA1 aggregation by anti-Tn/STn antibodies or nonimmunologic methods, e.g. by increasing the propensity of IgA1 to self-aggregate. Circulating IgA1 immune complexes deposit in the glomerular mesangium, activating mesangial cells and the complement cascade, which leads to glomerulonephritis and loss of renal function.
4 Tn and STn antigens are tumor biomarkers
Tn antigen (GalNAcα1-Ser/Thr) was first observed on human tumor cells in 1969 based on binding of the snail lectin Helix pomatia agglutinin (HPA), which specifically recognizes terminal α-linked GalNAc [84]. Springer et al. reported that Tn antigen was present at high levels in 90% of breast carcinomas. Cumulative studies showed that 70–90% of cancers of the colon, lung, bladder, cervix, ovary, stomach, and prostate express the Tn antigen. In contrast, little or no expression was observed in normal adult tissues. In many cancers, including cervical cancer, lung adenocarcinomas, colorectal carcinomas [85], breast carcinomas, and gastric carcinomas, Tn antigen expression correlates with metastatic potential and poor prognosis.
There are many reports on the expression of Tn and STn in human colon cancer. Here, we summarize the main findings: (i) Tn and STn antigens are restricted to the primary/metastatic tumor, being absent from normal colon tissue. For example, Itzkowitz et al. [86] found that there was no expression of Tn and STn in cells of normal colonic mucosa; however, in colon cancers, the percentage of cases expressing each antigen were as follows: Tn (72–81%), STn (93–96%), and T (71%). Orntoft et al. [87] reported that Tn was not expressed in adult colorectal tissue but was accumulated in human colon carcinoma. (ii) The expression of Tn/STn is associated with poor prognosis, including metastasis of colon cancer. Many studies have concluded that HPA binding to colorectal carcinoma cells is an indicator of poor outcome. For example, Schumacher et al. [88] evaluated the binding of HPA to 130 colorectal carcinomas and found that the prognosis for the groups of patients whose colorectal cancer cells bound to HPA in tissue sections was almost as bad as those with Dukes’ Stage C disease. In a review, Mitchell [89] stated that HPA reactivity was equal or superior to other classical markers of the metastatic potential of human colon and breast cancer. When transplanted into severe combined immunodeficient mice, HPA-positive human breast and colon cancer cells metastasized while HPA-negative cancer cell lines in general did not [89]. Imada et al. [90] concluded that both STn expression and lymph node metastasis were important prognostic factors in patients with advanced colorectal carcinoma. (iii) Some studies showed that Tn/STn antigens are expressed at early stages of colon carcinogenesis. Wargovich et al. [91] found that carcinoembryonic antigen, E-cadherin, and STn antigen were elevated in aberrant crypt foci, which are the earliest recognizable histological precursor lesions for colon cancer. Yuan [92] discovered that both Tn and STn were expressed in cancer and premalignant lesion of colorectal tissues. Itzkowitz et al. [93] examined 103 colorectal polyps (79 adenomatous and 24 hyperplastic) for expression of Tn/STn antigens and found that Tn antigen was expressed by all of the polyps studied; STn, on the other hand, was expressed weakly by a few cells in 7 of 24 (29%) hyperplastic polyps. (iv) In a 1,2-dimethylhydrazine-induced rat colon carcinoma model, both Tn and STn were expressed by the first lesions detected following carcinogen administration and were constitutively expressed at higher levels during tumor development [94]. Furthermore, both Tn and STn appeared in the ascitic fluid of rats with colon cancer. These findings are consistent with Tn/STn antigens being biomarkers for the diagnosis, prognosis, and targeted therapy of human colon cancer. However, without precise molecular explanation for the abnormal expression of these tumor antigens, it is difficult to evaluate their role in human colon cancer development, progression, and metastasis. Understanding the molecular mechanism for Tn/STn expression in colon tumors will be extremely helpful in developing more specific and efficient diagnostic, prognostic, and therapeutic approaches against this disease.
The expression of the Tn and STn antigens in tumor cells may have broad biological consequences (Fig. 5). Many studies have shown a correlation between altered glycosylation and poor prognosis in terms of progression, invasion, and metastasis. Expression of truncated O-glycans also correlates with altered expression of cell surface mucins integrins, and can change the adhesive properties of cells. Cell adhesion molecules such as selectins, integrins, and extracellular matrix components are involved in the process of metastasis [95–106]. Interestingly, many of these are mucins or glycoproteins whose O-glycans may contribute to binding of these adhesion molecules to their ligands. Recently, Wagner et al. [107] observed that death-receptor O-glycosylation contributes to tumor cell sensitivity to the proapoptotic ligand Apo2L/TRAIL, indicating that altered O-glycosylation of tumor cells would provide an advantage by enabling escape from apoptotic signaling. In our preliminary studies, we have made the exciting observation that mutations in Cosmc and Tn/STn expression causes altered oligomerization and signaling of TRAIL receptors. In addition to changing the binding properties of glycoproteins, Tn antigen is itself directly recognized. Numerous studies have shown that the Tn antigen is bound by the C-type lectin macrophage galactose binding lectin (MGL), which is expressed by both dendritic cells and macrophages [108–111] and has been found in situ in colorectal tumors [110]. Thus, expression of the Tn antigen and its recognition by MGL may be involved in immune surveillance and tolerance [110, 112]. It would be highly informative to explore the relationship between Cosmc mutations, Tn antigen expression, and interactions with C-type lectins expressed by dendritic cells and macrophages. Modulation of the immune response can also occur at the level of the effector cells. Mucin-associated STn antigen can inhibit natural killer (NK)-cell-induced cytotoxicity directed against tumor cells [113]. In some tumors, mucin expression and altered glycosylation correlates with expression of galectins, such as galectin-3 [114], that may bind Tn antigen, contributing to metastatic extravasation and modulation of the adaptive immune response. Although these findings highlight various potential mechanisms, the actual benefit to a tumor cell expressing Tn and STn antigens is not well understood. Clearly, much remains to be explored to fully elucidate the consequences of Tn and STn antigen expression on tumor-derived glycoproteins, but mechanistic evidence is accumulating to support the hypothesis that abnormal expression of O-glycans is associated with altered cellular properties, metastatic potential, and immune susceptibility.
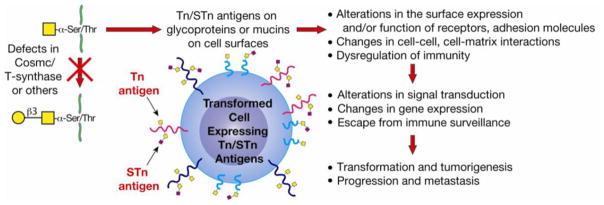
Figure 5.
Tn and STn antigens on tumor cells. Tn and STn antigens are tumor-associated carbohydrate antigens. They are not expressed on normal, nontransformed tissues. Defects in Cosmc or T-synthase can result in Tn/STn expression, although, to date, only defects in Cosmc have been observed in human tumors or cancer cell lines. Tn/STn expression is found on the majority of carcinomas and its expression correlates with progression of disease. However, the role for Tn/STn expression in tumorigenesis is unknown. Loss of O-glycosylation could lead to surface receptor dysregulation, changes in cell–cell and cell–matrix contacts, and/or immunoregulation. Subsequent changes in gene expression, signal transduction, and/or physicochemical interactions could facilitate tumor initiation, progression, and/or metastasis.
The molecular basis for Tn antigen expression by human tumor cells was recently shown to be dysfunctional Cosmc [41]. Somatic mutations in Cosmc or an absence of Cosmc transcript were the cause of Tn and STn expression in two specimens of human cervical carcinoma as well as several human tumor cell lines, including lymphoma-derived Jurkat, colorectal carcinoma-derived LSC and LS174T, and melanoma-derived LOX. Consistent with the findings in human tumors, mouse fibrosarcoma that formed spontaneously in aging mice also expressed Tn antigen due to deletion of 26 amino acids within the lumenal domain of Cosmc. The mouse neuroblastoma cell line Neuro-2a (also known as C1300) [42,115] is Tn positive and its Cosmc contains a G301T mutation resulting in a premature stop codon. A summary of mutations in Cosmc from tumor cells is provided in a recent review [1]. In addition, the finding that Tn4 B cells, a leukemia-like cell line generated from a Tn syndrome patient, lack Cosmc transcript due to hypermethylation of the Cosmc promoter suggests that epigenetic silencing of Cosmc may be an additional mechanism for expression of Tn/STn antigens in tumor cells. Thus, alteration of Cosmc is the major mechanism that induces expression of the Tn and STn antigens in human and animal tumors.
The characteristic expression of the Tn and STn antigens by human carcinomas, and their association with poor prognosis and metastasis, reinforces the conclusion that Tn/STn antigens are important biomarkers for human cancer. The finding that Cosmc dysfunction underlies this shared feature of many cancers strengthens the potential for targeting these antigens and/or the Cosmc gene for novel therapy of human neoplastic diseases.
5 Targeting Tn and STn in human tumors
The Tn antigen is a cryptic and nonphysiological glycan structure in humans, thus it is not surprising that it may be recognized as foreign by the immune system. The MGL expressed on myeloid antigen presenting cells is known to specifically bind terminal α- and β-linked GalNAc. Upon ligand binding, MGL rapidly internalizes and the endocytosed ligand is transported along the endosomal–lysosomal pathway for eventual presentation to MHC class II molecules. MGL can regulate Toll-like receptor signaling and thus influence the outcome of an immune response. Depending on additional signals to the dendritic cell, Tn binding may lead to either an active immune response or to an inhibited/tolerant response [116,117]. Surprisingly, small levels of anti-Tn antibody can be detected in the sera of most people [118–120]. The formation of such circulating anti-Tn antibodies was shown to be partly due to exposure to Enterobacteriaceae or other bacteria expressing the Tn antigen [80, 81]. The possibility that Tn expressing carcinomas could lead to increased production of anti-Tn antibodies has led to the evaluation of anti-Tn antibodies in sera as diagnostic and prognostic markers for tumors [121]. However, given the continued presence of Tn antigen positive blood cells in Tn syndrome and Tn-expressing tumor cells, immunosurveillance may allow tolerance to Tn antigen in some situations. If so, this might explain the ineffectiveness of Tn or STn antigen based anticancer vaccines.
Can a tumor expressing Tn antigen be eliminated by passive immunization with anti-Tn and anti-STn antibodies? Based on in vitro and animal model studies, the approach is promising. One mAb, MLS128, directly inhibited in vitro growth of human colon and breast cancer cell lines [122]. Several others were not directly cytotoxic, but activated antibody-dependent cellular cytotoxicity both in vitro and in vivo. Of these, one resulted in the rejection of human tumor cells in a xenograft severe combined immunodeficient mouse model, while another induced macrophage and neutrophil-mediated rejection of murine breast tumor in syngeneic mice [123–125]. While each of these antibodies is promising, their fine specificity has not been carefully evaluated. It is unclear why one is directly cytotoxic while the others are not. It is likely that the context of Tn recognition, i.e. which Tn-bearing glycoprotein(s) or peptide sequence(s) are bound, underlies the distinction.
Human tumor-associated glycoprotein-72 is a mucin molecule expressed in colon, breast, pancreatic, ovarian, lung, and gastric cancers. The mAbs B72.3 and CC49 recognize epitopes of tumor-associated glycoprotein-72 containing STn and sialyl-T antigen (sialyl core 1), respectively. CC49 has been analyzed and a humanized antibody [126–128] is undergoing a clinical trial for use in radioimmunoguided surgery. Although some studies showed that the antibody distributed mainly in the xenograft human tumor in mice and in primary human colorectal carcinoma [129], sialylT is considered a normal O-glycan structure and reactivity to normal tissue is a concern. Certainly, more research is needed using Tn or STn antibodies in passive immunity to treat Tn-positive cancer. A major hurdle for these studies is the lack of specific, well-defined anti-Tn mAbs.
6 Conclusion
Mucin-type O-glycosylation is one of the most common protein PTMs and plays important roles in many biological processes. The Tn and STn antigens occur in human and animal pathologies and are recognized as disease markers. Importantly, it has been demonstrated that the aberrant expression of Tn and STn results primarily from dysfunctional Cosmc, which encodes a specific molecular chaperone necessary to prevent the aggregation and subsequent proteasomal degradation of T-synthase during biosynthesis. This important finding has created a new direction of research aimed at uncovering the genetic and potentially epigenetic regulation of protein O-glycosylation in biology and pathology. However, much remains to be learned about the biochemical details of why tumor cells express these antigens and the advantage to the tumor of aberrant O-glycosylation. Future studies need to further define those key O-glycosylated glycoproteins whose altered glycosylation lead to pathogenesis, tumorigenesis, progression, and metastasis. Such knowledge will be critical to understanding the full picture of Tn and STn tumor biology, and will be extremely beneficial both in defining the pathological consequences of Tn expression and in developing novel diagnostic and therapeutic strategies for cancer and other Tn-related disorders.
Acknowledgments
The work by the authors was supported by NIH Grant number R01DK80876 to T.J. for IgA nephropathy study, 2011-Georgia Cancer Coalition (GCC) Cancer Research Award to T.J., and NIH Grant number U01CA168930 from the NCI to R.D.C and T.J. for the NCI sponsored Alliance of Glycobiologists for Detection of Cancer.
Abbreviations
- ERAD
ER-associated degradation
- ERAD-L
ERAD-lumenal
- GI
gastrointestinal
- HPA
Helix pomatia agglutinin
- HR
hinge region
- IEC
intestinal epithelial cell
- IgAN
IgA nephropathy
- MGL
macrophage galactose binding lectin
- SLeX
sialyl Lewis x
- STn
sialyl-Tn
Footnotes
The authors have declared no conflict of interest.
References
- 1.Ju T, Otto VI, Cummings RD. The Tn antigen-structural simplicity and biological complexity. Angew Chem. 2011;50:1770–1791. doi: 10.1002/anie.201002313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steentoft C, Vakhrushev SY, Vester-Christensen MB, Schjoldager KT, et al. Mining the O-glycoproteome using zinc-finger nuclease-glycoengineered simple cell lines. Nat Methods. 2011;8:977–982. doi: 10.1038/nmeth.1731. [DOI] [PubMed] [Google Scholar]
- 3.Ellies LG, Tsuboi S, Petryniak B, Lowe JB, et al. Core 2 oligosaccharide biosynthesis distinguishes between se-lectin ligands essential for leukocyte homing and inflammation. Immunity. 1998;9:881–890. doi: 10.1016/s1074-7613(00)80653-6. [DOI] [PubMed] [Google Scholar]
- 4.Homeister JW, Thall AD, Petryniak B, Maly P, et al. The alpha(1,3)fucosyltransferases FucT-IV and FucT-VII exert collaborative control over selectin-dependent leukocyte recruitment and lymphocyte homing. Immunity. 2001;15:115–126. doi: 10.1016/s1074-7613(01)00166-2. [DOI] [PubMed] [Google Scholar]
- 5.Yeh JC, Hiraoka N, Petryniak B, Nakayama J, et al. Novel sulfated lymphocyte homing receptors and their control by a Core1 extension beta 1,3-N-acetylglucosaminyltransferase. Cell. 2001;105:957–969. doi: 10.1016/s0092-8674(01)00394-4. [DOI] [PubMed] [Google Scholar]
- 6.Wilkins PP, McEver RP, Cummings RD. Structures of the O-glycans on P-selectin glycoprotein ligand-1 from HL-60 cells. J Biol Chem. 1996;271:18732–18742. doi: 10.1074/jbc.271.31.18732. [DOI] [PubMed] [Google Scholar]
- 7.Yago T, Leppanen A, Carlyon JA, Akkoyunlu M, et al. Structurally distinct requirements for binding of P-selectin glycoprotein ligand-1 and sialyl Lewis x to Anaplasma phagocytophilum and P-selectin. J Biol Chem. 2003;278:37987–37997. doi: 10.1074/jbc.M305778200. [DOI] [PubMed] [Google Scholar]
- 8.Tenno M, Ohtsubo K, Hagen FK, Ditto D, et al. Initiation of protein O glycosylation by the polypeptide GalNAcT-1 in vascular biology and humoral immunity. Mol Cell Biol. 2007;27:8783–8796. doi: 10.1128/MCB.01204-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marth JD, Grewal PK. Mammalian glycosylation in immunity. Nature Rev Immunol. 2008;8:874–887. doi: 10.1038/nri2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daniels MA, Hogquist KA, Jameson SC. Sweet ‘n’ sour: the impact of differential glycosylation on T cell responses. Nat Immunol. 2002;3:903–910. doi: 10.1038/ni1002-903. [DOI] [PubMed] [Google Scholar]
- 11.Sperandio M. Selectins and glycosyltransferases in leukocyte rolling in vivo. FEBS J. 2006;273:4377–4389. doi: 10.1111/j.1742-4658.2006.05437.x. [DOI] [PubMed] [Google Scholar]
- 12.Xia L, Ju T, Westmuckett A, An G, et al. Defective angiogenesis and fatal embryonic hemorrhage in mice lacking core 1-derived O-glycans. J Cell Biol. 2004;164:451–459. doi: 10.1083/jcb.200311112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Ju T, Ding X, Xia B, et al. Cosmc is an essential chaperone for correct protein O-glycosylation. Proc Natl Acad Sci USA. 2010;107:9228–9233. doi: 10.1073/pnas.0914004107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu J, Gerhardt H, McDaniel JM, Xia B, et al. Endothelial cell O-glycan deficiency causes blood/lymphatic misconnections and consequent fatty liver disease in mice. J Clin Invest. 2008;118:3725–3737. doi: 10.1172/JCI36077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johansson ME, Larsson JM, Hansson GC. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4659–4665. doi: 10.1073/pnas.1006451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawashima H. Roles of the gel-forming MUC2 mucin and its O-glycosylation in the protection against colitis and colorectal cancer. Biol Pharm Bull. 2012;35:1637–1641. doi: 10.1248/bpb.b12-00412. [DOI] [PubMed] [Google Scholar]
- 17.An G, Wei B, Xia B, McDaniel JM, et al. Increased susceptibility to colitis and colorectal tumors in mice lacking core 3-derived O-glycans. J Exp Med. 2007;204:1417–1429. doi: 10.1084/jem.20061929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu J, Wei B, Wen T, Johansson ME, et al. Loss of intestinal core 1-derived O-glycans causes spontaneous colitis in mice. J Clin Invest. 2011;121:1657–1666. doi: 10.1172/JCI45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomsson KA, Holmen-Larsson JM, Angstrom J, Johansson ME, et al. Detailed O-glycomics of the Muc2 mucin from colon of wild-type, core 1- and core 3-transferase-deficient mice highlights differences compared with human MUC2. Glycobiology. 2012;22:1128–1139. doi: 10.1093/glycob/cws083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larsson JM, Karlsson H, Sjovall H, Hansson GC. A complex, but uniform O-glycosylation of the human MUC2 mucin from colonic biopsies analyzed by nanoLC/MSn. Glycobiology. 2009;19:756–766. doi: 10.1093/glycob/cwp048. [DOI] [PubMed] [Google Scholar]
- 21.Gill DJ, Chia J, Senewiratne J, Bard F. Regulation of O-glycosylation through Golgi-to-ER relocation of initiation enzymes. J Cell Biol. 2010;189:843–858. doi: 10.1083/jcb.201003055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ju T, Cummings RD. A unique molecular chaperone Cosmc required for activity of the mammalian core 1 beta 3-galactosyltransferase. Proc Natl Acad Sci USA. 2002;99:16613–16618. doi: 10.1073/pnas.262438199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ju T, Cummings RD. Protein glycosylation: chaperone mutation in Tn syndrome. Nature. 2005;437:1252. doi: 10.1038/4371252a. [DOI] [PubMed] [Google Scholar]
- 24.Ju T, Aryal RP, Stowell CJ, Cummings RD. Regulation of protein O-glycosylation by the endoplasmic reticulumlocalized molecular chaperone Cosmc. J Cell Biol. 2008;182:531–542. doi: 10.1083/jcb.200711151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwai T, Inaba N, Naundorf A, Zhang Y, et al. Molecular cloning and characterization of a novel UDP-GlcNAc:GalNAc-peptide beta1,3-N-acetylglucosa-minyltransferase (beta 3Gn-T6), an enzyme synthesizing the core 3 structure of O-glycans. J Biol Chem. 2002;277:12802–12809. doi: 10.1074/jbc.M112457200. [DOI] [PubMed] [Google Scholar]
- 26.Berger EG. Tn-syndrome. Biochim Biophys Acta. 1999;1455:255–268. doi: 10.1016/s0925-4439(99)00069-1. [DOI] [PubMed] [Google Scholar]
- 27.Barratt J, Feehally J, Smith AC. Pathogenesis of IgA nephropathy. Semin Nephrol. 2004;24:197–217. doi: 10.1016/j.semnephrol.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Barratt J, Smith AC, Feehally J. The pathogenic role of IgA1 O-linked glycosylation in the pathogenesis of IgA nephropathy. Nephrology (Carlton) 2007;12:275–284. doi: 10.1111/j.1440-1797.2007.00797.x. [DOI] [PubMed] [Google Scholar]
- 29.Lisowska E. Tn antigens and their significance in oncology. Acta Biochim Pol. 1995;42:11–17. [PubMed] [Google Scholar]
- 30.Schjoldager KT, Clausen H. Site-specific protein O-glycosylation modulates proprotein processing – deciphering specific functions of the large polypeptide GalNAc-transferase gene family. Biochim Biophys Acta. 2012;1820:2079–2094. doi: 10.1016/j.bbagen.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Ju T, Brewer K, D’Souza A, Cummings RD, Canfield WM. Cloning and expression of human core 1 beta1,3-galactosyltransferase. J Biol Chem. 2002;277:178–186. doi: 10.1074/jbc.M109060200. [DOI] [PubMed] [Google Scholar]
- 32.Ju T, Cummings RD, Canfield WM. Purification, characterization, and subunit structure of rat core 1 Beta1,3-galactosyltransferase. J Biol Chem. 2002;277:169–177. doi: 10.1074/jbc.M109056200. [DOI] [PubMed] [Google Scholar]
- 33.Brockhausen I. Pathways of O-glycan biosynthesis in cancer cells. Biochim Biophys Acta. 1999;1473:67–95. doi: 10.1016/s0304-4165(99)00170-1. [DOI] [PubMed] [Google Scholar]
- 34.Lowe JB. Glycosylation in the control of selectin counter-receptor structure and function. Immunol Rev. 2002;186:19–36. doi: 10.1034/j.1600-065x.2002.18603.x. [DOI] [PubMed] [Google Scholar]
- 35.Carlow DA, Gossens K, Naus S, Veerman KM, et al. PSGL-1 function in immunity and steady state homeostasis. Immunol Rev. 2009;230:75–96. doi: 10.1111/j.1600-065X.2009.00797.x. [DOI] [PubMed] [Google Scholar]
- 36.Gahmberg CG, Autero M, Hermonen J. Major O-glycosylated sialoglycoproteins of human hematopoietic cells: differentiation antigens with poorly understood functions. J Cell Biochem. 1988;37:91–105. doi: 10.1002/jcb.240370109. [DOI] [PubMed] [Google Scholar]
- 37.Robbe-Masselot C, Maes E, Rousset M, Michalski JC, Capon C. Glycosylation of human fetal mucins: a similar repertoire of O-glycans along the intestinal tract. Glycoconj J. 2009;26:397–413. doi: 10.1007/s10719-008-9186-9. [DOI] [PubMed] [Google Scholar]
- 38.Ikehara Y, Kojima N, Kurosawa N, Kudo T, et al. Cloning and expression of a human gene encoding an N-acetylgalactosamine-alpha2,6-sialyltransferase (ST6GalNAc I): a candidate for synthesis of cancer-associated sialyl-Tn antigens. Glycobiology. 1999;9:1213–1224. doi: 10.1093/glycob/9.11.1213. [DOI] [PubMed] [Google Scholar]
- 39.Tsuji T, Osawa T. Carbohydrate structures of bovine submaxillary mucin. Carbohydr Res. 1986;151:391–402. doi: 10.1016/s0008-6215(00)90358-6. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Jobe SM, Ding X, Choo H, et al. Platelet biogenesis and functions require correct protein O-glycosylation. Proc Natl Acad Sci USA. 2012;109:16143–16148. doi: 10.1073/pnas.1208253109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ju T, Lanneau GS, Gautam T, Wang Y, et al. Human tumor antigens Tn and sialyl Tn arise from mutations in Cosmc. Cancer Res. 2008;68:1636–1646. doi: 10.1158/0008-5472.CAN-07-2345. [DOI] [PubMed] [Google Scholar]
- 42.Schietinger A, Philip M, Yoshida BA, Azadi P, et al. A mutant chaperone converts a wild-type protein into a tumor-specific antigen. Science. 2006;314:304–308. doi: 10.1126/science.1129200. [DOI] [PubMed] [Google Scholar]
- 43.Crew VK, Singleton BK, Green C, Parsons SF, et al. New mutations in C1GALT1C1 in individuals with Tn positive phenotype. Br J Haematol. 2008;142:657–667. doi: 10.1111/j.1365-2141.2008.07215.x. [DOI] [PubMed] [Google Scholar]
- 44.Mi R, Song L, Wang Y, Ding X, et al. Epigenetic silencing of the chaperone Cosmc in human leukocytes expressing tn antigen. J Biol Chem. 2012;287:41523–41533. doi: 10.1074/jbc.M112.371989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoo NJ, Kim MS, Lee SH. Absence of COSMC gene mutations in breast and colorectal carcinomas. APMIS. 2008;116:154–155. doi: 10.1111/j.1600-0463.2008.00965.x. [DOI] [PubMed] [Google Scholar]
- 46.Aryal RP, Ju T, Cummings RD. Tight complex formation between Cosmc chaperone and its specific client non-native T-synthase leads to enzyme activity and client-driven dissociation. J Biol Chem. 2012;287:15317–15329. doi: 10.1074/jbc.M111.312587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carvalho P, Stanley AM, Rapoport TA. Retrotranslocation of a misfolded luminal ER protein by the ubiquitinligase Hrd1p. Cell. 2010;143:579–591. doi: 10.1016/j.cell.2010.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aryal RP, Ju T, Cummings RD. The endoplasmic reticulum chaperone Cosmc directly promotes in vitro folding of T-synthase. J Biol Chem. 2010;285:2456–2462. doi: 10.1074/jbc.M109.065169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moreau R, Dausset J, Bernard J, Moullec J. Acquired hemolytic anemia with polyagglutinability of erythrocytes by a new factor present in normal blood. Bull Mem Soc Med Hop Paris. 1957;73:569–587. [PubMed] [Google Scholar]
- 50.Berger J, Hinglais N. Intercapillary deposits of IgA-IgG. J Urol Nephrol (Paris) 1968;74:694–695. [PubMed] [Google Scholar]
- 51.Kiryluk K, Julian BA, Wyatt RJ, Scolari F, et al. Genetic studies of IgA nephropathy: past, present, and future. Pediatr Nephrol. 2010;25:2257–2268. doi: 10.1007/s00467-010-1500-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu HH, Chu KH, Yang YH, Lee JH, et al. Genetics and immunopathogenesis of IgA nephropathy. Clin Rev Allergy Immunol. 2011;41:198–213. doi: 10.1007/s12016-010-8232-0. [DOI] [PubMed] [Google Scholar]
- 53.Levy M, Berger J. Worldwide perspective of IgA nephropathy. Am J Kidney Dis. 1988;12:340–347. doi: 10.1016/s0272-6386(88)80021-0. [DOI] [PubMed] [Google Scholar]
- 54.D’Amico G. Natural history of idiopathic IgA nephropathy: role of clinical and histological prognostic factors. Am J Kidney Dis. 2000;36:227–237. doi: 10.1053/ajkd.2000.8966. [DOI] [PubMed] [Google Scholar]
- 55.Paterson AD, Liu XQ, Wang K, Magistroni R, et al. Genome-wide linkage scan of a large family with IgA nephropathy localizes a novel susceptibility locus to chromosome 2q36. J Am Soc Nephrol. 2007;18:2408–2415. doi: 10.1681/ASN.2007020241. [DOI] [PubMed] [Google Scholar]
- 56.Gharavi AG, Yan Y, Scolari F, Schena FP, et al. IgA nephropathy, the most common cause of glomerulonephritis, is linked to 6q22–23. Nat Genet. 2000;26:354–357. doi: 10.1038/81677. [DOI] [PubMed] [Google Scholar]
- 57.Schena FP, Cerullo G, Torres DD, Zaza G, et al. Searching for IgA nephropathy candidate genes: genetic studies combined with high throughput innovative investigations. Contrib Nephrol. 2007;157:80–89. doi: 10.1159/000102308. [DOI] [PubMed] [Google Scholar]
- 58.Bisceglia L, Cerullo G, Forabosco P, Torres DD, et al. Genetic heterogeneity in Italian families with IgA nephropathy: suggestive linkage for two novel IgA nephropathy loci. Am J Hum Genet. 2006;79:1130–1134. doi: 10.1086/510135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beerman I, Novak J, Wyatt RJ, Julian BA, Gharavi AG. The genetics of IgA nephropathy. Nat Clin Pract Nephrol. 2007;3:325–338. doi: 10.1038/ncpneph0492. [DOI] [PubMed] [Google Scholar]
- 60.Julian BA, Novak J. IgA nephropathy: an update. Curr Opin Nephrol Hypertens. 2004;13:171–179. doi: 10.1097/00041552-200403000-00005. [DOI] [PubMed] [Google Scholar]
- 61.Baenziger J, Kornfeld S. Structure of the carbohydrate units of IgA1 immunoglobulin. II. Structure of the O-glycosidically linked oligosaccharide units. J Biol Chem. 1974;249:7270–7281. [PubMed] [Google Scholar]
- 62.Mattu TS, Pleass RJ, Willis AC, Kilian M, et al. The glycosylation and structure of human serum IgA1, Fab, and Fc regions and the role of N-glycosylation on Fc alpha receptor interactions. J Biol Chem. 1998;273:2260–2272. doi: 10.1074/jbc.273.4.2260. [DOI] [PubMed] [Google Scholar]
- 63.Tarelli E, Smith AC, Hendry BM, Challacombe SJ, Pouria S. Human serum IgA1 is substituted with up to six O-glycans as shown by matrix assisted laser desorption ionisation time-of-flight mass spectrometry. Carbohydr Res. 2004;339:2329–2335. doi: 10.1016/j.carres.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 64.Giannakakis K, Feriozzi S, Perez M, Faraggiana T, Muda AO. Aberrantly glycosylated IgA1 in glomerular immune deposits of IgA nephropathy. J Am Soc Nephrol. 2007;18:3139–3146. doi: 10.1681/ASN.2007030259. [DOI] [PubMed] [Google Scholar]
- 65.Gharavi AG, Moldoveanu Z, Wyatt RJ, Barker CV, et al. Aberrant IgA1 glycosylation is inherited in familial and sporadic IgA nephropathy. J Am Soc Nephrol. 2008;19:1008–1014. doi: 10.1681/ASN.2007091052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mestecky J, Tomana M, Moldoveanu Z, Julian BA, et al. Role of aberrant glycosylation of IgA1 molecules in the pathogenesis of IgA nephropathy. Kidney Blood Press Res. 2008;31:29–37. doi: 10.1159/000112922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Suzuki H, Moldoveanu Z, Hall S, Brown R, et al. IgA1-secreting cell lines from patients with IgA nephropathy produce aberrantly glycosylated IgA1. J Clin Invest. 2008;118:629–639. doi: 10.1172/JCI33189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Novak J, Julian BA, Tomana M, Mestecky J. IgA glycosylation and IgA immune complexes in the pathogenesis of IgA nephropathy. Semin Nephrol. 2008;28:78–87. doi: 10.1016/j.semnephrol.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hiki Y. O-linked oligosaccharides of the IgA1 hinge region: roles of its aberrant structure in the occurrence and/or progression of IgA nephropathy. Clin Exp Nephrol. 2009;13:415–423. doi: 10.1007/s10157-009-0173-7. [DOI] [PubMed] [Google Scholar]
- 70.Allen AC, Topham PS, Harper SJ, Feehally J. Leucocyte beta 1,3 galactosyltransferase activity in IgA nephropathy. Nephrol Dial Transplant. 1997;12:701–706. doi: 10.1093/ndt/12.4.701. [DOI] [PubMed] [Google Scholar]
- 71.Qin W, Zhou Q, Yang LC, Li Z, et al. Peripheral B lymphocyte beta1,3-galactosyltransferase and chaperone expression in immunoglobulin A nephropathy. J Intern Med. 2005;258:467–477. doi: 10.1111/j.1365-2796.2005.01558.x. [DOI] [PubMed] [Google Scholar]
- 72.Qin W, Zhong X, Fan JM, Zhang YJ, et al. External suppression causes the low expression of the Cosmc gene in IgA nephropathy. Nephrol Dial Transplant. 2008;23:1608–1614. doi: 10.1093/ndt/gfm781. [DOI] [PubMed] [Google Scholar]
- 73.Inoue T, Sugiyama H, Hiki Y, Takiue K, et al. Differential expression of glycogenes in tonsillar B lymphocytes in association with proteinuria and renal dysfunction in IgA nephropathy. Clin Immunol. 2010;136:447–455. doi: 10.1016/j.clim.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 74.Yamada K, Kobayashi N, Ikeda T, Suzuki Y, et al. Down-regulation of core 1 beta1,3-galactosyltransferase and Cosmc by Th2 cytokine alters O-glycosylation of IgA1. Nephrol Dial Transplant. 2010;25:3890–3897. doi: 10.1093/ndt/gfq325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xie LS, Qin W, Fan JM, Huang J, et al. The role of C1GALT1C1 in lipopolysaccharide-induced IgA1 aberrant O-glycosylation in IgA nephropathy. Clin Invest Med. 2010;33:E5–E13. doi: 10.25011/cim.v33i1.11832. [DOI] [PubMed] [Google Scholar]
- 76.Li GS, Zhang H, Lv JC, Shen Y, Wang HY. Variants of C1GALT1 gene are associated with the genetic susceptibility to IgA nephropathy. Kidney Int. 2007;71:448–453. doi: 10.1038/sj.ki.5002088. [DOI] [PubMed] [Google Scholar]
- 77.Pirulli D, Crovella S, Ulivi S, Zadro C, et al. Genetic variant of C1GalT1 contributes to the susceptibility to IgA nephropathy. J Nephrol. 2009;22:152–159. [PubMed] [Google Scholar]
- 78.Malycha F, Eggermann T, Hristov M, Schena FP, et al. No evidence for a role of cosmc-chaperone mutations in European IgA nephropathy patients. Nephrol Dial Transplant. 2009;24:321–324. doi: 10.1093/ndt/gfn538. [DOI] [PubMed] [Google Scholar]
- 79.Barratt J, Feehally J. IgA nephropathy. J Am Soc Nephrol. 2005;16:2088–2097. doi: 10.1681/ASN.2005020134. [DOI] [PubMed] [Google Scholar]
- 80.Springer GF, Desai PR, Cantrell JL. A rodent carcinoma and its lipidic extracts possess human blood group Tn-, T-, N- and M-specificities. Naturwissenschaften. 1981;68:274–276. doi: 10.1007/BF01047339. [DOI] [PubMed] [Google Scholar]
- 81.Springer GF, Horton RE. Blood group isoantibody stimulation in man by feeding blood group-active bacteria. J Clin Invest. 1969;48:1280–1291. doi: 10.1172/JCI106094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Novak J, Julian BA, Tomana M, Mesteck J. Progress in molecular and genetic studies of IgA nephropathy. J Clin Immunol. 2001;21:310–327. doi: 10.1023/a:1012284402054. [DOI] [PubMed] [Google Scholar]
- 83.Leung JC, Tang SC, Chan DT, Lui SL, Lai KN. Increased sialylation of polymeric lambda-IgA1 in patients with IgA nephropathy. J Clin Lab Anal. 2002;16:11–19. doi: 10.1002/jcla.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Prokop O, Uhlenbruck G. N-acetyl-D-galactosamine in tumor cell membranes: demonstration by means of Helix agglutinins. Med Welt. 1969;46:2515–2519. [PubMed] [Google Scholar]
- 85.Konno A, Hoshino Y, Terashima S, Motoki R, Kawaguchi T. Carbohydrate expression profile of colorectal cancer cells is relevant to metastatic pattern and prognosis. Clin Exp Metastasis. 2002;19:61–70. doi: 10.1023/a:1013879702702. [DOI] [PubMed] [Google Scholar]
- 86.Itzkowitz SH, Yuan M, Montgomery CK, Kjeldsen T, et al. Expression of Tn, sialosyl-Tn, and T antigens in human colon cancer. Cancer Res. 1989;49:197–204. [PubMed] [Google Scholar]
- 87.Orntoft TF, Harving N, Langkilde NC. O-linked mucin-type glycoproteins in normal and malignant colon mucosa: lack of T-antigen expression and accumulation of Tn and sialosyl-Tn antigens in carcinomas. Int J Cancer. 1990;45:666–672. doi: 10.1002/ijc.2910450416. [DOI] [PubMed] [Google Scholar]
- 88.Schumacher U, Higgs D, Loizidou M, Pickering R, et al. Helix pomatia agglutinin binding is a useful prognostic indicator in colorectal carcinoma. Cancer. 1994;74:3104–3107. doi: 10.1002/1097-0142(19941215)74:12<3104::aid-cncr2820741207>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 89.Mitchell BS, Schumacher U. The use of the lectin Helix pomatia agglutinin (HPA) as a prognostic indicator and as a tool in cancer research. Histol Histopathol. 1999;14:217–226. doi: 10.14670/HH-14.217. [DOI] [PubMed] [Google Scholar]
- 90.Imada T, Rino Y, Hatori S, Takahashi M, et al. Sialyl Tn antigen expression is associated with the prognosis of patients with advanced colorectal cancer. Hepatogastroenterology. 1999;46:208–214. [PubMed] [Google Scholar]
- 91.Wargovich MJ, Chang P, Velasco M, Sinicrope F, et al. Expression of cellular adhesion proteins and abnormal glycoproteins in human aberrant crypt foci. Appl Immunohistochem Mol Morphol. 2004;12:350–355. doi: 10.1097/00129039-200412000-00011. [DOI] [PubMed] [Google Scholar]
- 92.Yuan M. The expression of Tn and S-Tn antigens in cancer pre-malignant lesion of colorectal tissues by enzyme immunohistochemical method. Zhonghua Bing Li Xue Za Zhi. 1989;18:211–213. [PubMed] [Google Scholar]
- 93.Itzkowitz SH, Bloom EJ, Lau TS, Kim YS. Mucin associated Tn and sialosyl-Tn antigen expression in colorectal polyps. Gut. 1992;33:518–523. doi: 10.1136/gut.33.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Berriel E, Hill M, Barcia JJ, Ubillos L, et al. Simple mucin-type cancer associated antigens are pre-cancerous biomarkers during 1,2-dimethylhydrazine-induced rat colon carcinogenesis. Oncol Rep. 2005;14:219–227. [PubMed] [Google Scholar]
- 95.Araki M, Araki K, Biancone L, Stamenkovic I, et al. The role of E-selectin for neutrophil activation and tumor metastasis in vivo. Leukemia. 1997;11(Suppl 3):209–212. [PubMed] [Google Scholar]
- 96.Izumi Y, Kawamura YJ, Irimura T. Carbohydrate antigens in carcinoma invasion and metastasis. Nippon Geka Gakkai Zasshi. 1996;97:140–144. [PubMed] [Google Scholar]
- 97.Kim YJ, Borsig L, Varki NM, Varki A. P-selectin deficiency attenuates tumor growth and metastasis. Proc Natl Acad Sci USA. 1998;95:9325–9330. doi: 10.1073/pnas.95.16.9325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Koukoulis GK, Patriarca C, Gould VE. Adhesion molecules and tumor metastasis. Hum Pathol. 1998;29:889–892. doi: 10.1016/s0046-8177(98)90191-5. [DOI] [PubMed] [Google Scholar]
- 99.Laidler P, Litynska A. Tumor cell N-glycans in metastasis. Acta Biochim Pol. 1997;44:343–357. [PubMed] [Google Scholar]
- 100.Matsusako T, Muramatsu H, Shirahama T, Muramatsu T, Ohi Y. A metastasis-associated antigen is present on a 60 kDa glycoprotein in transitional cell carcinoma of the human urinary bladder. Histochem J. 1992;24:805–810. doi: 10.1007/BF01046352. [DOI] [PubMed] [Google Scholar]
- 101.Matsushita Y, Kitajima S, Goto M, Tezuka Y, et al. Selectins induced by interleukin-1beta on the human liver endothelial cells act as ligands for sialyl Lewis X-expressing human colon cancer cell metastasis. Cancer Lett. 1998;133:151–160. doi: 10.1016/s0304-3835(98)00220-1. [DOI] [PubMed] [Google Scholar]
- 102.McEver RP. Selectin-carbohydrate interactions during inflammation and metastasis. Glycoconj J. 1997;14:585–591. doi: 10.1023/a:1018584425879. [DOI] [PubMed] [Google Scholar]
- 103.Meyer T, Hart IR. Mechanisms of tumour metastasis. Eur J Cancer. 1998;34:214–221. doi: 10.1016/s0959-8049(97)10129-0. [DOI] [PubMed] [Google Scholar]
- 104.Sass PM. The involvement of selectins in cell adhesion, tumor progression, and metastasis. Cancer Invest. 1998;16:322–328. doi: 10.3109/07357909809084652. [DOI] [PubMed] [Google Scholar]
- 105.Tang DG, Honn KV. Adhesion molecules and tumor metastasis: an update. Invasion Metastasis. 1994;14:109–122. [PubMed] [Google Scholar]
- 106.Yeatman TJ, Nicolson GL. Molecular basis of tumor progression: mechanisms of organ-specific tumor metastasis. Semin Surg Oncol. 1993;9:256–263. [PubMed] [Google Scholar]
- 107.Wagner KW, Punnoose EA, Januario T, Lawrence DA, et al. Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat Med. 2007;13:1070–1077. doi: 10.1038/nm1627. [DOI] [PubMed] [Google Scholar]
- 108.Higashi N, Fujioka K, Denda-Nagai K, Hashimoto S, et al. The macrophage C-type lectin specific for galactose/N-acetylgalactosamine is an endocytic receptor expressed on monocyte-derived immature dendritic cells. J Biol Chem. 2002;277:20686–20693. doi: 10.1074/jbc.M202104200. [DOI] [PubMed] [Google Scholar]
- 109.Iida S, Yamamoto K, Irimura T. Interaction of human macrophage C-type lectin with O-linked N-acetylgalactosamine residues on mucin glycopeptides. J Biol Chem. 1999;274:10697–10705. doi: 10.1074/jbc.274.16.10697. [DOI] [PubMed] [Google Scholar]
- 110.Saeland E, van Vliet SJ, Backstrom M, van den Berg VC, et al. The C-type lectin MGL expressed by dendritic cells detects glycan changes on MUC1 in colon carcinoma. Cancer Immunol Immunother. 2007;56:1225–1236. doi: 10.1007/s00262-006-0274-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.van Vliet SJ, van Liempt E, Saeland E, Aarnoudse CA, et al. Carbohydrate profiling reveals a distinctive role for the C-type lectin MGL in the recognition of helminth parasites and tumor antigens by dendritic cells. Int Immunol. 2005;17:661–669. doi: 10.1093/intimm/dxh246. [DOI] [PubMed] [Google Scholar]
- 112.Napoletano C, Rughetti A, Agervig Tarp MP, Coleman J, et al. Tumor-associated Tn-MUC1 glycoform is internalized through the macrophage galactose-type C-type lectin and delivered to the HLA class I and II compartments in dendritic cells. Cancer Res. 2007;67:8358–8367. doi: 10.1158/0008-5472.CAN-07-1035. [DOI] [PubMed] [Google Scholar]
- 113.Ogata S, Maimonis PJ, Itzkowitz SH. Mucins bearing the cancer-associated sialosyl-Tn antigen mediate inhibition of natural killer cell cytotoxicity. Cancer Res. 1992;52:4741–4746. [PubMed] [Google Scholar]
- 114.Byrd JC, Bresalier RS. Mucins and mucin binding proteins in colorectal cancer. Cancer Metastasis Rev. 2004;23:77–99. doi: 10.1023/a:1025815113599. [DOI] [PubMed] [Google Scholar]
- 115.Lundstrom M, Jeansson S, Olofsson S. Host cell-induced differences in the O-glycosylation of herpes simplex virus gC-1. II. Demonstration of cell-specific galactosyltransferase essential for formation of O-linked oligosaccharides. Virology. 1987;161:395–402. doi: 10.1016/0042-6822(87)90132-2. [DOI] [PubMed] [Google Scholar]
- 116.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annual Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 117.Geijtenbeek TB, van Vliet SJ, Engering A, t Hart BA, van Kooyk Y. Self- and nonself-recognition by C-type lectins on dendritic cells. Annu Rev Immunol. 2004;22:33–54. doi: 10.1146/annurev.immunol.22.012703.104558. [DOI] [PubMed] [Google Scholar]
- 118.Dausset J, Moullec J, Bernard J. Acquired hemolytic anemia with polyagglutinability of red blood cells due to a new factor present in normal human serum (Anti-Tn) Blood. 1959;14:1079–1093. [PubMed] [Google Scholar]
- 119.Springer GF, Desai PR, Murthy MS, Scanlon EF. Human carcinoma-associated precursor antigens of the NM blood group system. J Surg Oncol. 1979;11:95–106. doi: 10.1002/jso.2930110204. [DOI] [PubMed] [Google Scholar]
- 120.Friedenreich V. The Thomsen Hemagglutination Phenomenon. Vol. 1. Levin and Munskgaard; Copenhagen: 1930. [Google Scholar]
- 121.Wandall HH, Blixt O, Tarp MA, Pedersen JW, et al. Cancer biomarkers defined by autoantibody signatures to aberrant O-glycopeptide epitopes. Cancer Res. 2010;70:1306–1313. doi: 10.1158/0008-5472.CAN-09-2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zamri N, Masuda N, Oura F, Yajima Y, et al. Effects of two monoclonal antibodies, MLS128 against Tn-antigen and 1H7 against insulin-like growth factor-I receptor, on the growth of colon cancer cells. Biosci Trends. 2012;6:303–312. [PubMed] [Google Scholar]
- 123.Kubota T, Matsushita T, Niwa R, Kumagai I, Nakamura K. Novel anti-Tn single-chain Fv-Fc fusion proteins derived from immunized phage library and antibody Fc domain. Anticancer Res. 2010;30:3397–3405. [PubMed] [Google Scholar]
- 124.Ando H, Matsushita T, Wakitani M, Sato T, et al. Mouse-human chimeric anti-Tn IgG1 induced anti-tumor activity against Jurkat cells in vitro and in vivo. Biol Pharm Bull. 2008;31:1739–1744. doi: 10.1248/bpb.31.1739. [DOI] [PubMed] [Google Scholar]
- 125.Hubert P, Heitzmann A, Viel S, Nicolas A, et al. Antibody-dependent cell cytotoxicity synapses form in mice during tumor-specific antibody immunotherapy. Cancer Res. 2011;71:5134–5143. doi: 10.1158/0008-5472.CAN-10-4222. [DOI] [PubMed] [Google Scholar]
- 126.Agnese DM, Abdessalam SF, Burak WE, Jr, Arnold MW, et al. Pilot study using a humanized CC49 monoclonal antibody (HuCC49DeltaCH2) to localize recurrent colorectal carcinoma. Ann Surg Oncol. 2004;11:197–202. doi: 10.1245/aso.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 127.Rogers BE, Roberson PL, Shen S, Khazaeli MB, et al. Intraperitoneal radioimmunotherapy with a humanized anti-TAG-72 (CC49) antibody with a deleted CH2 region. Cancer Biother Radiopharm. 2005;20:502–513. doi: 10.1089/cbr.2005.20.502. [DOI] [PubMed] [Google Scholar]
- 128.Fang L, Holford NH, Hinkle G, Cao X, et al. Population pharmacokinetics of humanized monoclonal antibody HuCC49deltaCH2 and murine antibody CC49 in colorectal cancer patients. J Clin Pharmacol. 2007;47:227–237. doi: 10.1177/0091270006293758. [DOI] [PubMed] [Google Scholar]
- 129.Shen S, Forero A, Meredith RF, LoBuglio AF. Biodistribution and dosimetry of In-111/Y-90-HuCC49DeltaCh2 (IDEC-159) in patients with metastatic colorectal adenocarcinoma. Cancer Biother Radiopharm. 2011;26:127–133. doi: 10.1089/cbr.2010.0864. [DOI] [PubMed] [Google Scholar]