Abstract
Stem cells hold significant clinical potential to treat numerous debilitating diseases and injures that currently have no treatment plan. While several advances have been made in developing stem cell platforms and methods to induce their differentiation, there are two critical aspects need to be addressed: (1) efficient delivery of nucleic acids and small molecules for stem cell differentiation, and (2) effective, noninvasive, and real-time tracking of transplanted stem cells. To address this, there has been a trend of utilizing various types of nanoparticles to not only deliver biomolecules to targeted site but also track the location of transplanted stem cells in real time. Over the past decade, various types of nanoparticles, including magnetic nanoparticles, silica nanoparticles, quantum dots, and gold nanoparticles, have been developed to serve as vehicles for targeted biomolecule delivery. In addition of being biocompatible without causing adverse side effect to stem cells, these nanoparticles have unique chemical and physical properties that allow tracking and imaging in real time using different imaging instruments that are commonly found in hospitals. A summary of the landmark and progressive demonstrations that utilize nanoparticles for stem cell application is described.
INTRODUCTION
Since the last 15 years, there has been significant progress in the field of stem cell biology, and as a result, patients suffering from terminal diseases or traumatic injuries have new hope for a potential therapy.1 The field of stem cell biology and stem cell-based regenerative medicine has been rapidly advancing as a promising therapy to treat debilitating diseases and injuries caused by the loss of terminal cells.2 It is because stem cells are known for their potential to repair and/or replace damaged tissue.
Stem cells are undifferentiated and multipotent cells that can differentiate into specialized cells based on intrinsic or external cues that manipulate their genetic code.3 Differentiation of stem cells into specific lineages relies on expression patterns of specific genes. In normal human development, stem cell differentiation is innately guided by expression of intrinsic cues. But forced stem cell differentiation to selectively control fate requires external cues such as a specific microenvironment or delivery of differentiation-inducing factors.4–8
It has long been a vision for scientists to control stem cell behavior and fate as required for various clinical applications, and several methods to externally regulate stem cell fate have been developed. The long-term goal is to harvest stem cells from patients, and through the use of various external cues, to generate specialized cells for implantation back into the patients. Even though progress has been made, the use of conventional methods to induce differentiation, such as viral vectors, DNA plasmids, small molecules, and a combination of thereof, has specific limitations. Hence, researchers have been exploring alternatives, and as a result, the field of nanotechnology has significantly advanced for biological applications.
Nanotechnology has recently emerged as an exciting field of research involving the use of nanoscale materials for various applications including stem cell biology. Because of the extremely small scale of nanotechnology, ranging from 1 to 1000 nm, the potential of nanotechnology-based applications appears limitless. Researchers from multidisciplinary fields have integrated expertise from inorganic chemistry, organic chemistry, material science, engineering, and stem cell biology to develop various nanoplatforms and devices for manipulating stem cell behavior.9 In fact, over the past 10 years, the number of publications involving nanotechnology and stem cell biology has grown exponentially. This is because of the great potential that stems from its amazing intrinsic qualities and widespread application potential.
There are two primary modes through which nanotechnology can regulate stem cell fate: (1) fabrication of nanoscale surfaces to mimic the various natural three-dimensional (3D) microenvironment of cells and (2) delivery of nanoscale materials to selectively target intracellular pathways.9,10 The cellular microenvironment in the body is a 3D dynamic process that cannot be effectively replicated in the traditional cell culture dishes. However, various nanoscale scaffolds and nanopatterned substrates with variable surface roughness and porosity have been fabricated to more effectively replicate the in vivo niche.11 As a result, this not only provides insight into mechanistic studies to probe stem cell signaling pathways that induce differentiation but also a novel method to induce differentiation by mimicking the microenvironment.12 Moreover, in an alternative approach, researchers have developed nanomaterials that can be used as intracellular deliver vehicles to introduce specific small molecules and biomolecules into cells. The small molecules that are delivered can selectively activate and regulate specific signaling pathways in stem cells to induce targeted differentiation. These nanomaterials can be of different shapes, sizes, and compositions, and thus they can be tuned for specific applications.
While several types of nanomaterials have been developed as deliver agents, the most prominent and widely used are nanoparticles. Nanoparticles are small, spherical materials that can range in size from 2 to 500 nm, and the most widely used types of inorganic nanoparticles include magnetic nanoparticles (MNPs), gold nanoparticles, silica nanoparticles (SNPs), and quantum dots (QDs) (Figure 1). Each of these nanoparticles not only have the ability to carry specific small molecules into the cells but also have multifunctional properties such as contrast imaging, surface porosity, and magnetic capabilities for a synergistic effect to track and regulate stem cell behavior. Moreover, these nanoparticles are generally biocompatible with minimal side effect or cytotoxicity, thus allowing them to be used as safe delivery agents.13 Because certain compositions of gold nanoparticles and MNPs have been approved by the FDA for clinical applications, there is a surge of using these nanoparticles for stem cell differentiation with potential for translation into the clinic.14
FIGURE 1.
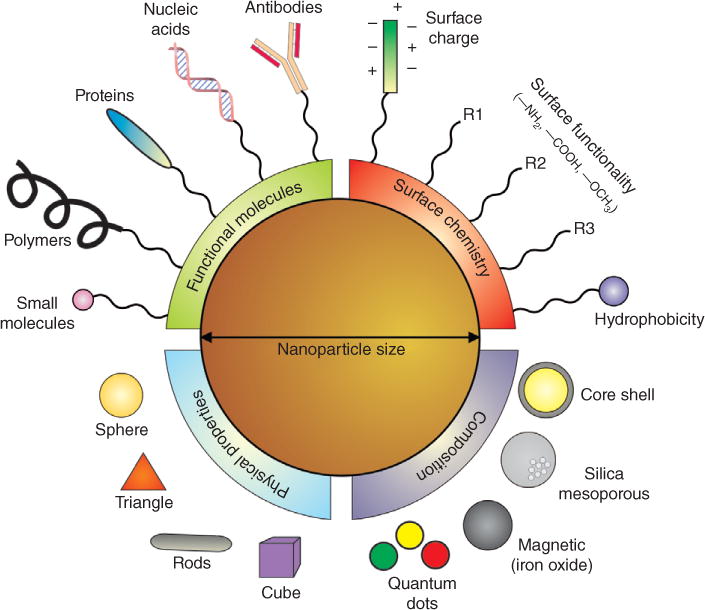
Designing nanoparticles for stem cell applications. The physical properties of nanoparticles can be selectively designed for specific applications based on the material composition and physical properties. Nanoparticles can be made functionally active depending on the surface chemistry and the functional biomolecules. Nanoparticles are highly tunable and have a modular chemistry, thus enabling their application for desired stem cell applications. (Reprinted with permission from Ref 15. Copyright 2011 Royal Society of Chemistry)
NANOPARTICLE UPTAKE MECHANISM
The first step for utilizing nanoparticle-based delivery of small molecules and exploiting their multifunctional properties for stem cell application involves efficient uptake of nanoparticles into the cell.15 Stem cells tightly regulate movement of cargo through the plasma membrane, and hence, a major challenge is the efficient intracellular uptake of nanoparticles. Because nanoparticles are comprised of inorganic compounds, the cell does not readily allow them to enter. However, the cell has an endocytosis mechanism to allow large substances and cargo, such as nanoparticles, to enter the cell.16 Endocytosis is an energy-dependent process by which cells engulf substances on the cell surface and shuttle them into the cytoplasm. Specifically, as the nanoparticles near the surface of the cells, they can bind to the cell surface receptors—if the nanoparticle is functionalized with such binding moieties—which signals the cell to change the conformation of the plasma membrane by forming a cavity through which the nanoparticles can enter. Then the plasma membrane cavity completely engulfs the nanoparticle and enters the cytoplasm. This process of endocytosis is ubiquitous for almost all adherent cell types. Endocytosis is divided into four categories with slightly different mechanisms to shuttle different-sized foreign substances into the cell: clathrin-mediated endocytosis, caveolae, micropinocytosis, and phagocytosis. Depending on the size and surface makers of the nanoparticle, one of these mechanisms allows nanoparticle uptake into the cell17,18 (Figure 2).
FIGURE 2.
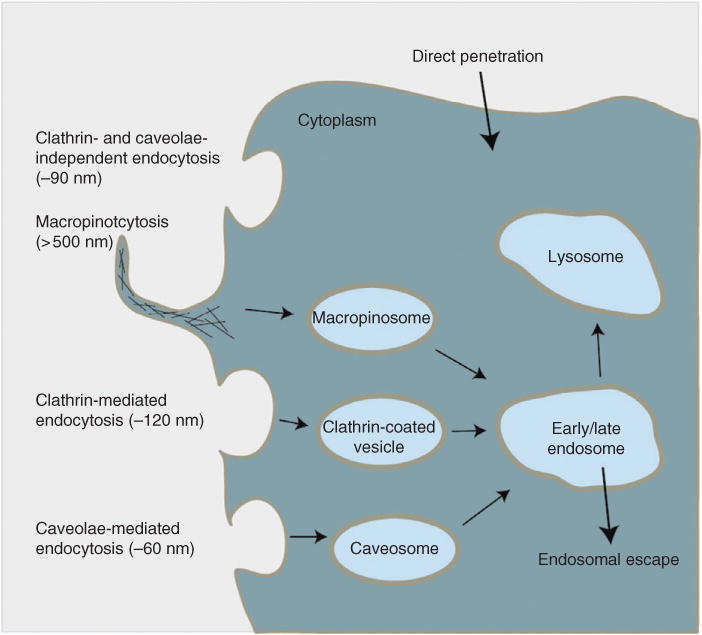
Cellular uptake mechanisms. Cells have various energy-dependent mechanisms for allowing extracellular cargo inside. Depending on the size of the cargo, processes such as caveolae mechanism are induced for small cargo, clathrin mechanism for intermediate cargo, and macropinocytosis for large cargo, are utilized by the cell. Because nanoparticles can vary in size ranging from 5 to 500 nm, different mechanisms are utilized by the cell to enable nanoparticle entry. (Reprinted with permission from Ref 17. Copyright 2014 American Chemical Society)
To facilitate the process of nanoparticle endocytosis, it is critical for nanoparticles to be functionalized with surface ligands that can bind to specific cell surface receptors. Cellular uptake dynamics for nanoparticle uptake is greatly dependent on the size of the nanoparticle and the peptides present on the surface.19 Cell-penetrating peptides are a class of peptides that are specifically designed and conjugated on nanoparticles to enable plasma membrane penetration.17,20 With the use of cell-penetrating peptides and nuclear localization signal peptides, it is possible to engineer nanoparticles to not only cross the plasma membrane but also the nuclear membrane to gain entry inside the nucleus.21,22 Moreover, the size of nanoparticles has been demonstrated to play a significant role in both plasma membrane and nuclear membrane uptake. Studies have shown that nanoparticles ranging from 10 to 100 nm can enter the cell, with an optimal nanoparticle diameter of 50 nm for maximum uptake.23 Therefore, depending on the application, the size of the nanoparticles must be optimized. Furthermore, the chemical properties of ligands present on the nanoparticle surface also play a critical role in cellular uptake. Properties such as solubility, pH, and hydrophobicity influence cellular uptake.24,25
When nanoparticles are incubated in biological fluid, such as cell culture media, proteins bind to the ligands on the nanoparticle surface to form what is called a protein corona, which can critically influence the interaction of nanoparticles with cells.26 The protein corona has been recently demonstrated to play a striking role in nanoparticle uptake.27 The concept of protein corona suggests that the mix of over 300 proteins, which are found in serum-containing culture media, rapidly adsorbs on the nanoparticle surface within a few minutes, and the interaction of these proteins with the cell surface enables uptake.28,29 Even though several membrane-penetrating peptides have been developed and demonstrated to be efficient, the formation of the protein corona and its exact mechanism, which is still unclear, may play a significant role.
MAGNETIC NANOPARTICLES IN STEM CELL BIOLOGY
Magnetic nanoparticles (MNPs) are a class of nanoparticles, comprised of magnetic materials such as iron, cobalt, nickel, and zinc, which can be manipulated with a magnetic field.30 MNPs are spherical in shape and range in size from 10 to 100 nm, and their composition can have a mixture of various metals, such as Fe3O4, Fe2O3, NiFe2O4, and FeCo, that result in different magnetic properties.31 MNPs have desirable physiochemical properties, biological inertness, high stability in physiological conditions, and excellent magnetic properties that allow for noninvasive imaging and enhanced cellular uptake, thus establishing MNPs as excellent carriers of small molecules and biomolecules.32,33 The primary advantage of MNPs is their unique magnetic properties, and therefore, researchers have exploited these features by employing MNPs for three specific applications in stem cell biology: (1) enhanced delivery owing to magnetofection (magnet-facilitated delivery),34 (2) stem cell tracking using various imaging techniques,35,36 and (3) magnetically guiding stem cells to targeted sites in vivo.
Magnetically Facilitated Delivery for Enhanced Stem Cell Differentiation
Traditional methods to deliver MNPS typically require cell-penetrating peptides present on the nanoparticle surface or transfection reagents; but increasing the transfection efficiency so that more MNPs enter the cell without compromising viability is a challenge. The unique magnetic properties of MNP enable enhanced delivery owing to magnetofection, a technique that involves incubating MNPs in cell culture and placing a magnet underneath to generate a magnetic field that ‘pulls down’ the MNPs onto the cell surface.34,37 As a result, significantly more MNPs enter the cell and cell viability is not compromised through this process.
Recently, Lee and coworkers used a magnetofection-based approach to efficiently delivery MNPs into neural stem cells (NSCs) to induce neuronal differentiation.38 They used zinc-doped MNPs, ZnFe2O4, as a core and synthesized a gold shell to develop a magnetic core–shell nanoparticle (MCNP) (Figure 3(a)–(c)). The purpose of the outer gold shell is to increase biocompatibility, enable multiple biomolecules to anchor onto a single nanoparticle, and prevent free radical formation from the MNP core. Specifically, the MCNPs were functionalized with a linker molecule to increase water solubility. Then the polyamine was coated to make the surface positively charged and then a small-molecule nucleic acid called siRNA, which represses gene expression, was electrostatically conjugated on the surface (Figure 3(a)). First, delivery properties were tested using magnetofection, which revealed that green fluorescent protein (GFP)-labeled NSCs exposed to a magnet for just 30 min significantly increased the transfection efficiency. Moreover, magnetofection-based delivery of MCNP showed a remarkable downregulation in GFP when compared with MCNPs delivered using conventional methods (Figure 3(d)). Then the MCNP was utilized for the delivery of siRNA to control neuronal differentiation of NSCs, which revealed that NSCs can be selectively differentiated to either neurons or oligodendrocytes in an efficient and nontoxic manner (Figure 3(e) and (f)). Furthermore, the gold shell enabled dark-field imaging to confirm the presence of MCNPs inside the NSCs. This was the very first demonstration to show the utilization of MNPs for the delivery of small molecules into stem cells to induce differentiation.
FIGURE 3.
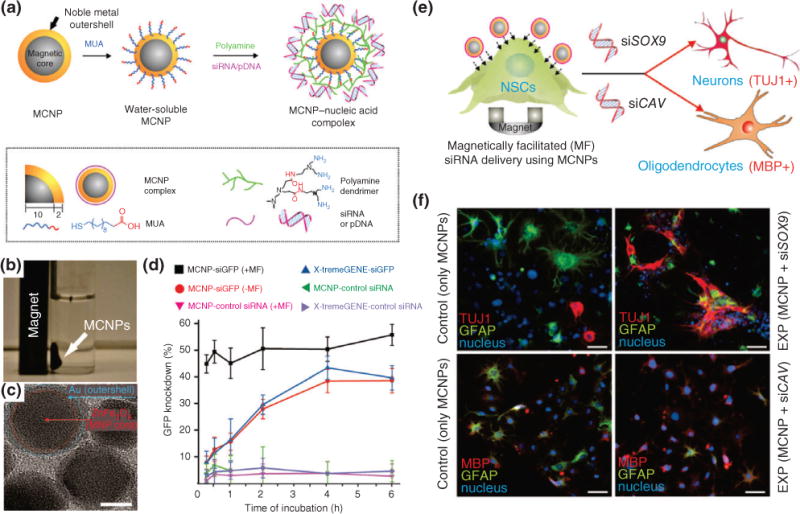
Magnetic core–shell nanoparticles (MCNPs) for stem cell differentiation and imaging. (a) Schematic of MCNPs functionalized with mercaptoundecanoic acid (MUA) followed by electrostatic conjugation of polyamide and nucleic acids for regulating gene expression in stem cells. (b) A representative image showing that MCNPs with a composition of ZnFe2O4 are attracted to a magnet. (c) TEM image of MCNPs (scale bar = 10 nm). (d) MCNPs were incubated in green fluorescent protein (GFP)-labeled rat neural stem cells (rNSCs) and exposed to magnetofection (MF). The resulting GFP knockdown was quantified and is directly correlated to the gene-regulating efficiency of the MCNPs. The greater the GFP knockdown, the greater its effect. (e) Schematic of rNSCs undergoing MF with MCNPs coated with nucleic acids targeting specific stem cell differentiation. (f) Immunofluorescence images showing the differentiation into neurospecific lineages with particular markers, TUJ1 (neuronal), GFAP (glial cells), and MBP (oligodendrocytes), based on the type of nucleic acid delivered. (Reprinted with permission from Ref 38. Copyright 2013 John Wiley and Sons)
MNPs for Stem Cell Labeling and Tracking
The second utilization of MNPs for stem cell-based application is stem cell tracking. The end goal for any stem cell-based research is successful transplantation into diseased or injured patients for regeneration. For this, one important criterion is to track the location of the cells after transplantation, ideally in a safe and noninvasive manner. For this purpose, MNPs can be used for stem cell-based therapies to track stem cell migration and localization in vivo because of their unique magnetic properties that enable imaging techniques such as magnetic resonance imaging (MRI).39 Among the available in vivo imaging techniques applicable for stem cell monitoring, MRI is particularly promising because it can provide high spatial resolution without compromising the patient’s care. Stem cells, especially, mesenchymal stem cells (MSCs), have been increasingly utilized for in vivo transplantation, and it is highly desired to track the location of these transplanted MSC in real time.40–42 For this purpose, MNPs can be loaded into MSCs before transplantation, and their location can be imaged in real time using MRI. For example, rat MSCs (rMSCs) secreting neurotrophic factors were labeled with MNPs and transplanted into Huntington’s disease rat models.43 After 18 days, the animals were sacrificed and their brains were imaged to access the migratory path of the transplanted cells. High-resolution 2D and 3D MRI revealed that the transplanted cells migrated along a distant route toward the lesioned site (Figure 4). This confirmed that MSCs can not only seek lesioned regions in vivo but also that MNP-labeled cells can be tracked via noninvasive MRI even 18 days post-transplantation. On the basis of this study, it is evident that MNPs are an invaluable tool for tracking stem cells.
FIGURE 4.
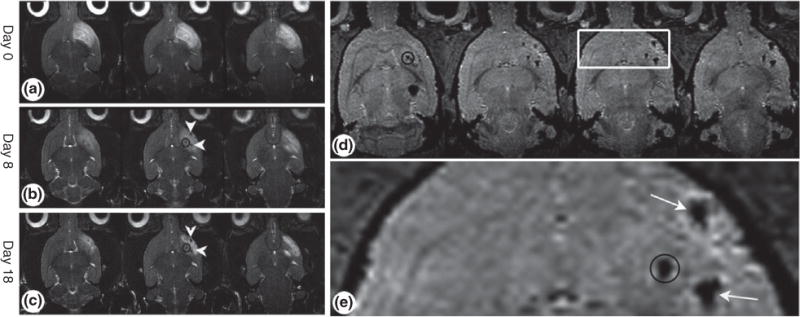
Tracking transplanted stem cells using magnetic resonance imaging (MRI) in vivo. Mesenchymal stem cells (MSCs) were loaded with magnetic nanoparticles (MNPs) and transplanted into rat brains. The loaded MSCs migrate toward the cortical lesion. (a–c) Time course of weighted MRI of rats that were induced with cortical damage. (d) Axial three-dimensional images showing accumulation of MSCs in the cortex and striatum. (e) Enlargement of the white box in (d). Throughout the time period, high-resolution MRI revealed that cells migrated along the distant route toward the lesion. The black circles represent the location of the induced lesion. White arrows point to MNP-loaded MSCs. (Reprinted with permission from Ref 43. Copyright 2008 John Wiley and Sons)
In another exciting application of MNPs for monitoring stem cell migration in vivo, NSCs were extracted from patients suffering from traumatic brain injury, and these NSCs were labeled with MNPs.44 Then, approximately 50,000 of these MNP-labeled NSCs were transplanted into the brain injury site. By utilizing MRI over a 10-week period, the progression and migration of the MNP-labeled NSCs was tracked, and reveled migration from the injection sites to white and gray matter. This phenomenon was not observed in patients who received unlabeled cells. Furthermore, it was confirmed that the magnetic signal was indeed from the MNP-labeled NSCs, and not macrophages that engulfed the NSCs, through double fluorescent imaging. It is evident that MNPs are great contrast agents for real-time imaging for stem cell therapies; however, there is one drawback that needs to be addressed. MNP-labeled cells transplanted into the body may not discriminate between labeled dead and live cells. Therefore, if the MNP-labeled cells die after being transplanted, the signal from the MNPs inside the cells will still persist. To test this effect, mice were transplanted with MNP-labeled MSCs and MNP-labeled dead MSCs in the spinal cord, and after 6 weeks, MRI detected a persistent signal from both conditions.45 This implies that even if cells can be tracked in vivo using MNPs and MRI, an alternative method such as fluorescence imaging has to be utilized to confirm the viability of transplanted cells.
Externally Guiding Transplanted Stem Cells to the Target Site In Vivo
Even after stem cells are transplanted in vivo, there is no guarantee that they migrate or localize to the area of the diseased or injured site. This limits the full potential of stem cell for regenerative medicine application in vivo. Hence, researchers are exploiting the magnetic properties of MNPs to selectively and exogenously guide transplanted stem cells to the lesioned site where stem cells are required. For example, in one recent study, MNPs were coated with a polyethylene glycol to increase biocompatibility and delivered to human MSCs (hMSCs).46 Then it was confirmed that these MNPs localize in the hMSC lysosomes and are not toxic to cells for a prolonged period. Then they tested the response of MNP-loaded hMSCs under both static and nonstatic conditions. Under static conditions, the MNP-loaded hMSCs were plated over an array of magnets for 4 h, which caused the cells to accumulate in the sites with the highest magnetic gradient (Figure 5(a)). Using mathematical models to mimic the bloodstream, the response of hMSCs was tested, and revealed that MNPs accumulated in the region of highest magnetic strength (Figure 5(b)). More interestingly, the MNP-loaded hMSCs were injected into the tail vein of mice, with a magnet placed on the proximal portion, which resulted in over a sixfold increase of accumulation of hMSCs in the tail (Figure 5(c)). This result has immense implications for in vivo experiments because transplanted cells loaded with MNPs can now be guided to the target site using external, noninvasive methods such as a magnet. Stem cell transplantation for regenerative medicine is a highly promising and pursued field of research, and MNPs provide an effective and noninvasive method to track their progression and precise location in vivo.
FIGURE 5.
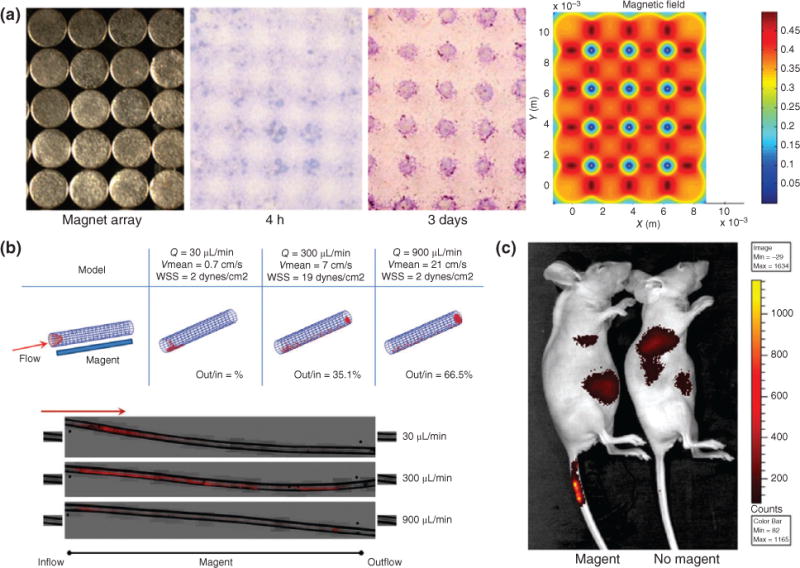
Guiding in vivo localization of magnetic nanoparticles (MNPs) using external magnets. (a) To demonstrate that MNPs are precisely controlled by the location of a magnetic field, a magnet array with spherical patterns was placed underneath a solution of MNPs, and resulted in MNPs localizing to locations of highest magnetic strength. (b) To simulate MNPs flowing in the bloodstream, a MNP solution passed through the tube with a magnet underneath and the localization of the MNP (red) is dictated by the flow rate of the solution. (c) MNPs were intravenously injected into the distal portion of the mouse tail vein while a magnet was placed at the injection site. High signal in the tail vein of the mice with the magnet confirms that localization of MNPs can be externally controlled by a magnet. (Reprinted with permission from Ref 46. Copyright 2013 John Wiley and Sons)
SILICA NANOPARTICLES IN STEM CELL BIOLOGY
Silica nanoparticles (SNPs) are a class of nanoparticles comprised of SiO2 and are used extensively for biomedical applications.47 Their inert properties, small and tunable diameters, and biofunctional capabilities make SNPs an attractive nanomaterial for biological applications. In fact, in 2011, an investigational new drug application for exploring SNPs for targeted molecular imaging was approved by the US Food and Drug Administration for an in-human clinical trial, thus highlighting SNPs as an effective platform with potential for clinical translation.48
SNPs can be categorized into two major categories: nonporous (solid) SNPs and mesoporous SNPs. Nonporous SNPs are solid, smooth nanoparticles and deliver biomolecule cargo through encapsulation within the SNP or through conjugation of biomolecules on the surface.49 On the other hand, mesoporous SNPs contain numerous pores (2–50 nm in size) on the surface that can hold biomolecule payloads for delivery.50 The pores are ‘capped’ with a gatekeeper molecule which functions to prevent the release of the payload until the gatekeeper molecule is degraded by intracellular enzymes or opened through external stimuli signals that alter the molecule conformation, thus allowing controlled release of biomolecule payload.50 The release profile of nonporous SNPs is controlled by the linker molecules or degradation of the silica matrix. Nonporous SNPs can be synthesized in various sizes and the pore size in mesoporous SNPs can be easily tuned based on the synthetic protocol. Both nonporous and mesoporous SNPs have found their niche in stem cell biology for various applications including stem cell differentiation, stem cell imaging, and in vivo real-time stem cell tracking.
Nonporous SNPs for Stem Cell Imaging and Differentiation
Nonporous SNPs have a great multifunctional surface that enables conjugation of active biomolecules for delivery into stem cells.51 As a result, SNPs have been demonstrated to deliver differentiation-specific molecules into stem cells for inducing differentiation and conversion into desired lineages. In one demonstration, SNPs were functionalized with insulin and delivered to rMSCs to induce adipogenic differentiation.52 Specifically, researchers first showed a systemic study confirming the biocompatibility of the SNPs with rMSCs that revealed high biocompatibility. Furthermore, high-resolution imaging showed that internalization of SNPs by rMSCs had no effect on the cellular structure of organelles. When the SNP–insulin conjugates were delivered to rMSCs, successful differentiation into adipogenic tissue was observed, thus demonstrating that the biological activity of insulin was not affected by conjugation to SNP. Hence, SNPs can be established as effective biocompatible carriers of molecules to induce stem cell differentiation. Furthermore, this result is in conjunction with other studies showing that SNPs are safe for prolonged internalization inside the cells.53,54
In addition to their multifunctional surface capable to delivering biomolecules into stem cells, SNPs have unique intrinsic properties that make them especially attractive for stem cell transplantation applications. SNPs can be detected and visualized using ultrasound owing to their high impedance mismatch.55 Ultrasound is a promising tool for stem cell therapy because of its high resolution, low cost, and high depth penetration. Moreover, ultrasound is readily available to clinicians and easy to use, thus making it applicable for stem cell tracking after implantation. One landmark demonstration using ultrasound and SNPs for live stem cell tracking showed the effectiveness of this technique, wherein researchers encapsulated 300 nm SNPs with a fluorescent dye and the element gadolinium to enhance MRI contrast and ultrasound imaging56 (Figure 6(a)). These SNPs were then delivered to hMSCs, and imaging showed intracellular aggregation of SNP, which actually enhanced the ultrasound signal without influencing cell behavior or metabolism. The SNP-loaded hMSCs made them applicable for cell sorting through their fluorescence signal. The SNP-loaded hMSCs were then transplanted into mouse model via injections, and after only 11 seconds, researchers were able to use ultrasound imaging to identify the exact location of cells and assess the possibility of a misinjection (Figure 6(b)). Furthermore, MRI was utilized after the ultrasound-guided delivery of SNP-loaded hMSCs into mouse cardiac tissue. Compared with traditional methods, the SNP loading increased the ultrasound and MRI contrast of labeled hMSCs by over 700 and 200%, respectively. Even after 13 days of implantation, the ultrasound signal and MRI contrast could still detect and identify the location of the transplanted hMSCs (Figure 6(c)). Lastly, researchers performed a series of experiments to evaluate the impact of SNPs on hMSCs, and found that all cellular functions, including proliferation, cytokine expression, and metabolic activity, were unaffected. Overall, the use of nonporous SNPs for stem cell tracking and stem cell differentiation is a highly promising area of research with potential for translation into the clinic.
FIGURE 6.
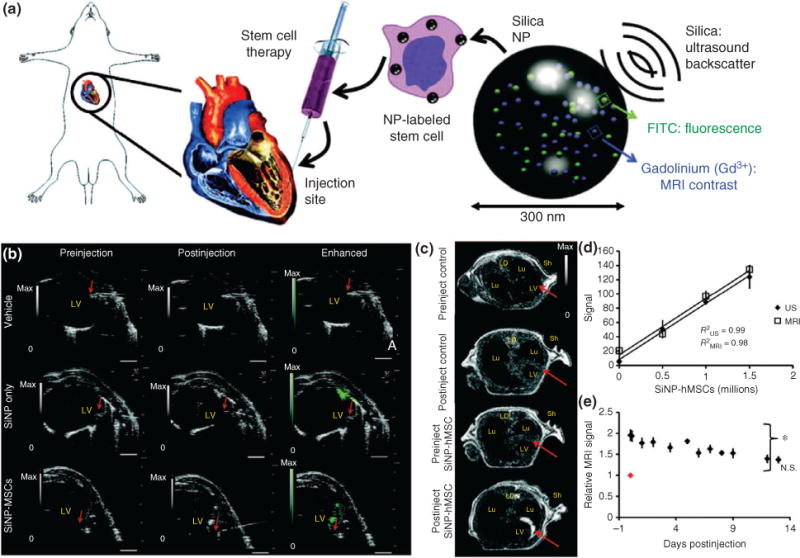
Silica nanoparticles (SNPs) for tracking stem cells in vivo using ultrasound. (a) Schematic of SNPs embedded with FITC for fluorescence imaging and gadolinium for enhancing MRI contrast delivered to mesenchymal stem cells (MSCs) and injected into rat heart tissue. (b) Ultrasound images of human MSCs (hMSCs) after intracardiac implantation in mice. The red arrow represents the bevel of the needle catheter. (c) MRI contract images show enhancement of SNP accumulation. (d) Quantification of the MRI and ultrasound (US) signal as a function of number of injected SNP-loaded hMSCs. (e) Animals injected (on day 0, red dot) with hMSCs were monitored sequentially for 12 days postinjection. (Reprinted with permission from Ref 56. Copyright 2013 The American Association for the Advancement of Science)
Mesoporous SNPs for Delivery of Differentiation-Specific Factors to Stem Cells
The second class of SNPs are mesoporous SNPs, which have pores on the surface in which biomolecules can be embedded and later released into the cell. Taking advantage of this unique feature, researchers demonstrated that differentiation-specific biomolecules can be loaded in the pores to induce stem cell differentiation.57 Bone morphogenetic proteins (BMPs) are factors that can induce osteogenic bone differentiation. Researchers embedded BMPs into mesoporous SNPs and delivered them into adipose-derived MSCs, with the goal that after the cells uptake the mesoporous SNPs, BMPs would be released from the pores to induce differentiation. Histological staining revealed successful osteogenic differentiation in a highly efficient manner. Comparatively, control studies with BMPs delivered without mesoporous SNPs showed minimal differentiation.
Generally, stem cells are extremely sensitive to intracellular introduction of foreign inorganic matter, especially embryonic stem cells (ESCs). Just the presence of inorganic matter causes the stimulus of unwanted signaling pathways that can disrupt normal cells function and differentiation capacity. But a research group recently demonstrated that delivery of peptides using mesoporous SNPs does not have any adverse side effects on ESCs and, instead, promotes targeted differentiation.58 The mesoporous SNPs were loaded with two peptides, Cintrofin and Gliafin. Cintrofin has been shown to induce neuronal differentiation and promote survival, while Gliafin has been shown to promote neurite outgrowth. When these two peptides were loaded into mesoporous and delivered into ESC-derived motor neuron (MN) precursor cells, differentiation into MNs that exhibit neurite branching was observed. Moreover, these induced MNs displayed electrophysiological properties with a resting membrane potential close to the physiological range that would be driven to high spiking frequencies. Then, these ESCs were loaded with mesoporous SNPs and transplanted into mice models for in vivo differentiation and integration into the mice neural network. After 2 weeks, the condition with loaded mesoporous SNPs showed a neurite outgrowth and the volume of cells was almost 10 times larger than control conditions that lacked SNP and peptides. After 2 months, the condition with mesoporous SNP-loaded ESCs showed extensive neurite arborizations and expression of prominent markers such as choline acetyltransferase (ChAT), while control conditions showed minimal expression. Taken together, these results suggest that co-transplantation of ESCs loaded with mesoporous SNPs can increase transplant size, improve survival, and induce neurite outgrowth.
The development of induced pluripotent stem cells (iPSCs) as a robust source of embryonic-like cells, with the ability to differentiate into almost any cell type, has opened the door for stem cell therapies with potential clinical translation. The first ever demonstration of using SNPs with iPSCs was reported in 2013, wherein researchers evaluated the sensitivity of iPSCs to SNPs and the differentiation capacity of iPSCs transfected with DNA-loaded SNPs (Figure 7(a)).59 First, because iPSCs are difficult to transfect and are extremely sensitive to foreign inorganic matter, the uptake dynamics of mesoporous SNP was evaluated. Three types of mesoporous SNPs were tested, positively charged, negatively charged, and neutral, and the response to iPSCs was carefully observed and resulted in the identification that positively charged mesoporous SNPs were more efficiently internalized by the iPSCs (Figure 7(b)). Next, to ensure that the intracellular presence of mesoporous SNPs in iPSCs did not impair their function, metabolism, and differentiation capacity of iPSCs, various experiments were performed and showed that cell proliferation, pluripotency, and in vivo teratoma formation were not affected. In the final experiment, the mesoporous SNPs were loaded with an HNF3β-plasmid-DNA, which has been shown to induce hepatocyte differentiation. The iPSCs transfected with these mesoporous SNPs loaded with HNF3β exhibited successful differentiation into functional hepatocyte-like cells (Figure 7(c)). These results not only demonstrate the potential for stem cell labeling using mesoporous SNPs but also that they can act as efficient carriers of differentiation-specific biomolecules into sensitive cells such as iPSCs and induce their differentiation.
FIGURE 7.
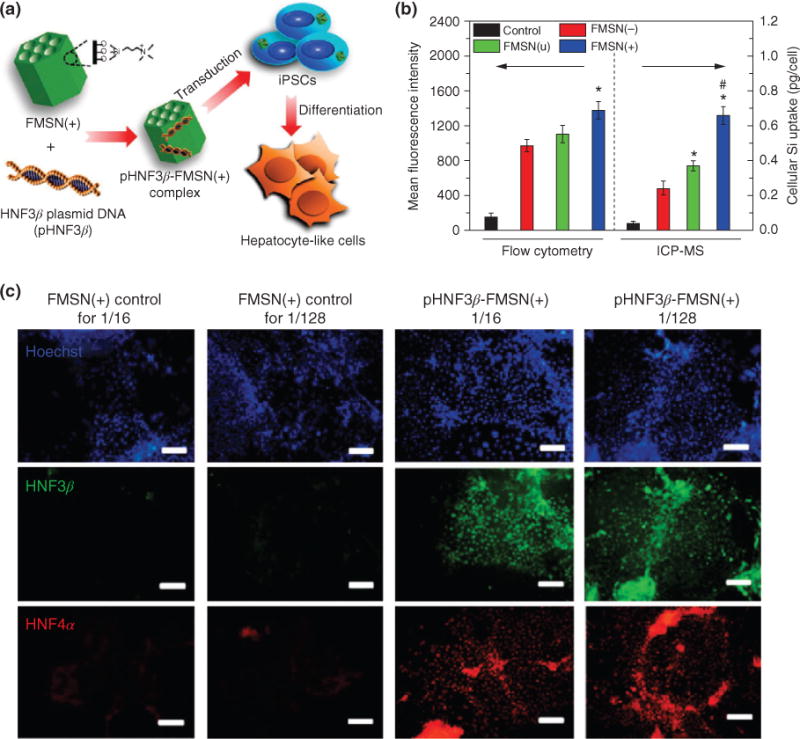
Mesoporous silica nanoparticles (SNPs) for cell labeling and differentiating induced pluripotent stem cells (iPSCs). (a) Positively charged mesoporous SNPs were functionalized with an HNF3β-plasmid-DNA (pHNF3β) and delivered to iPSCs. The treated iPSCs showed differentiation capacity and differentiated into hepatocyte-like cells with mature functions within 2 weeks. (b) Comparing uptake efficiency of different charged mesoporous SNPs shows that positively charged SNPs have greatest uptake. (c) Immunofluorescence analysis for the expression of hepatic markers HNF3β (green) and HNF4α (red) in iPSCs treated with loaded mesoporous SNPs. 1/16 and 1/128 refer to ratios of delivery (scale bar = 200 μm). (Reprinted with permission from Ref 59. Copyright 2013 American Chemical Society)
Hybrid Silica–MNPs Enhance Stem Cell Tracking
SNP and mesoporous SNPs have a unique chemical and synthetic property of being able to integrate with other types of metallic nanoparticle compositions such as gold and iron oxide.42 These hybrid SNPs are fabricated with a gold shell and a SNP core, or SNPs can act as a shell around magnetic iron oxide nanoparticle cores.60 The added benefit of these hybrid SNPs has a synergistic effect in terms of biomolecule loading and contract imaging. A landmark study demonstrated the feasibility of incorporating a magnetic iron oxide nanoparticle inside SNPs for synergistic stem cell labeling. These hybrid SNPs were 50 nm in diameter, and when they are delivered to hMSCs, sufficient MRI is achieved.61 Furthermore, when these loaded hMSCs are transplanted into mouse model, MRI can also detect their presence. Moreover, the differentiation capacity, proliferation, and viability of loaded hMSCs remain unaffected. Hybrid SNPs can have a big impact on translational medicine because of their biological inertness and synergistic modalities.
QUANTUM DOTS IN STEM CELL BIOLOGY
Quantum dots (QDs) are a class of multifunctional, fluorescent semiconductor nanoparticles that exhibit quantum mechanical properties of intrinsic emission profiles.62 QDs vary in size from 2 to 50 nm, and the emission properties of QDs are directly correlated to either the size or chemical composition (Figure 8(a)). QDs have a broad excitation spectra and a narrow emission spectra. The QDs emit certain wavelengths of fluorescent light, and thus are excellent nanomaterials for noninvasive in vivo imaging and stem cell tracking.63 Because QDs are excited by a single UV light source and can emit different wavelengths of fluorescent light, they are ideal tools for multiplex imaging.64 Moreover, QDs are extremely resistant to photobleaching, meaning that they retain emission intensity and brightness, even after long exposure times, and thus are excellent alternative to traditional molecular dyes to imaging applications. In recent years, the unique and photophysical properties have enabled researchers to broaden the application scope of QDs for stem cell applications because QDs have a multifunctional surface, which, in addition to fluorescent imaging, allows them to simultaneously deliver functional biomolecules. This combinatorial advantage of QDs has propelled them for stem cell applications.
FIGURE 8.
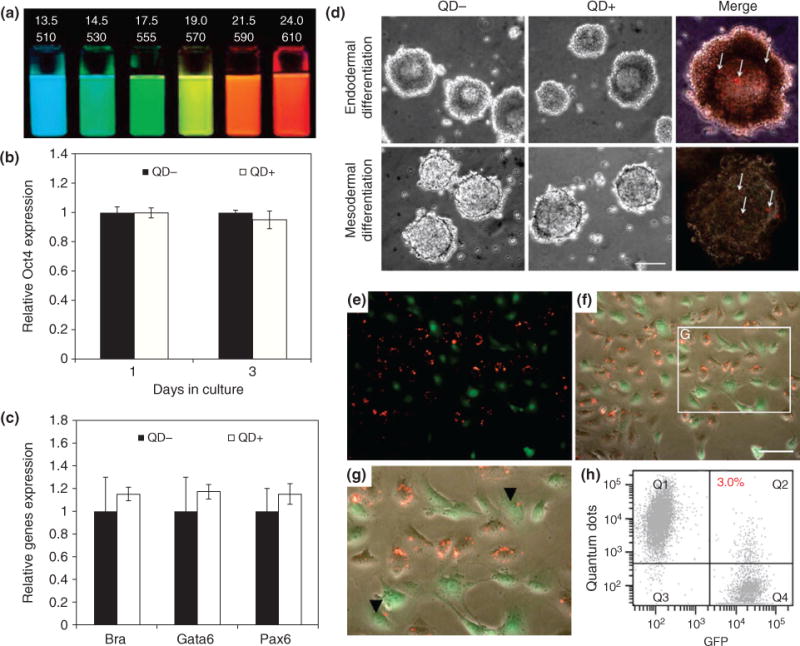
Fluorescent quantum dots (QDs) for stem cell labeling and tracking. (a) QDs of different diameters (top row, nm) and the respective emission wavelength (bottom row, nm) are tunable fluorescent probes. (b) QDs incubated in embryonic stem cells (ESCs) show that pluripotent makers such as Oct4 remain unaffected. (c) QD-treated ESCs can differentiate into three germ layers as evidenced by gene expression of each germ layer. (d) ESCs treated with QDs differentiate into germ layers after 4 days. (e–g) Co-culture of QDs-labeled kidney stem cells (KSCs) with green fluorescent protein (GFP)-labeled KSCs (green) confirms that internalized QDs (red) are not transferred to adjacent cells. (h) Flow cytometry confirms this result. (Reprinted with permission from Ref 66.)
QDs for ESCs Labeling
The most prominent stem cells with the highest in vivo potential are ESCs because of their wide differentiation capacity. Therefore, the number of stem cell transplantation studies involving ESCs has been increasing. One important criteria of implantation is to track the location of transplanted ESCs. For this purpose, QDs have emerged as promising probes of stem cell labeling and imaging. But before utilizing QDs for stem cell application, it is critical to evaluate the effect and influence that QDs may have on stem cells, because stem cell are generally sensitive to foreign matter and can alter their metabolism which can adversely impact their differentiation capacity. Furthermore, traditional QDs are synthesized using toxic elements such as cadmium (Cd)- and selenide (Se)-based core, which can damage the cell even at low concentrations. However, the use of a ZnS shell prevents the release of the toxic Cd elements, thereby circumventing the cytotoxicity issue.65 Therefore, it is important to test the effects of QDs on stem cells. For this purpose, researchers tested the behavior and pluripotent characteristics of ESCs and kidney stem cells when delivered with QDs.66 Results indicate that the native pluripotency markers of ESCs such as Oct4, the proliferation rate, and the viability of ESCs are unchanged by the presence of QDs (Figure 8(b)). The differentiation capacity was also not influenced as QD-labeled ESCs were able to effectively differentiate into the three germ layers, endoderm, ectoderm, and mesoderm, with the genetic expression of lineage-specific being identical to differentiated ESCs lacking QDs (Figure 8(c) and (d)). Then, to confirm that the labeled ESCs did not excrete the QDs, which could potentially be uptaken by adjacent cells, fluorescent imaging revealed that ESCs do not excrete the QDs and that transfer of QDs in co-cultures was minimal (Figure 8(e)–(g)). Finally, it was determined that even if the ESCs die in culture, the QDs within are also not readily uptaken by adjacent cells. Hence, on the basis of these results, we can deduce that QDs are relatively safe for stem cell applications with minimal side effects.
In another demonstration, researchers sought to evaluate the effect of QDs on ESC behaviors and the feasibility of using QD-labeled ESCs for transplantation studies.67 To ensure that the pluripotency properties of ESCs were not affected by the presence of QDs, they delivered the QDs to ESCs and tested pluripotency markers such as Oct4, and found that expression of these markers remained unchanged. Thus, QDs were established as safe agents for further ESC studies. Then, six different types of QDs were delivered to ESCs, and these QD-labeled ESCs were injected into the backs of nude mice (Figure 9(a)). Fluorescent imaging revealed that using just a single UV light source, the emission from the six QD-labeled ESCs can be individually detected simultaneously. Finally, to test the prospects of utilizing QDs for long-term stem cell tracking, different amounts of QD-labeled ESCs were injected into nude mice and successive imaging was performed for several weeks, which showed that QDs emit a strong and detectable signal even 14 days postinjection (Figure 9(b) and (c)). This study opened the door for the use of QDs in stem cell research. For example, in another demonstration, bone-derived stem cells (BDSCs) were labeled with QDs and were injected into the retina to repair retinal injury.68 After transplantation, the location of the injected stem cells could be tracked using fluorescent imaging.
FIGURE 9.
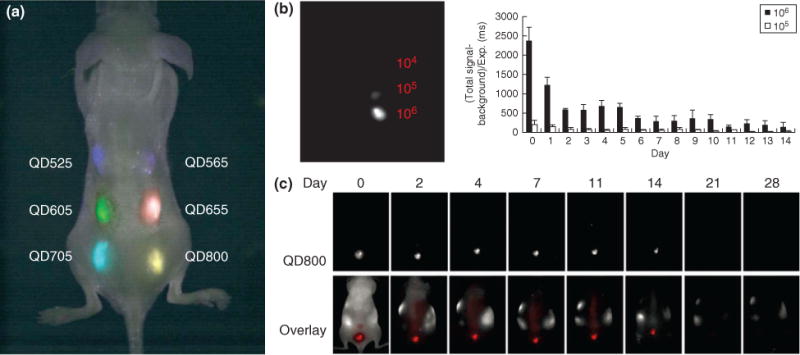
Embryonic stem cells (ESCs) loaded with quantum dots (QDs) can be simultaneously imaged. (a) ESCs were labeled with six different QDs and injected subcutaneously onto the backs of nude mice. These labeled ESCs could be imaged with good contrast with a single excitation wavelength. (b) Different number of QD-labeled ESCs, 104, 105, and 106, were injected into nude mice and signal was quantified and revealed that the signal of ESCs is proportional to number of cells injected. (c) To evaluate the clearance properties of labeled ESCs, mice were injected with QD-labeled ESCs and longitudinal imaging revealed that the QD signal can be detected up to 14 days. (Reprinted with permission from Ref 67. Copyright 2007 BioMed Central)
Nontoxic Alternative QDs for Stem Cell Biology
Combining different types of nanoparticles is an interesting subset of research because it includes the advantageous features from both nanoparticles on a single construct. Furthermore, it is desirable to coat QDs with a specific type of nanomaterial to prevent leeching of the toxic CdSe core. For this purpose, a recent demonstration showed that QDs can be coated with SNPs and still retain its fluorescence properties.69 A strong intracellular fluorescence signal confirmed that the QDs were effectively uptaken and were able to emit its signal. Moreover, it was determined that a 4-h incubation time is the optimal time for maximal uptake of QDs in stem cells.
Another class of recently developed QDs is called graphene quantum dots (GQDs), which are comprised of nanosized graphene sheets and have intrinsic fluorescent properties.70 Graphene is a lattice of sp2-carbon sheets that has attracted significant attention owing to its unique properties and vast potential for application in almost every field of research. When these graphene sheets are layered together, nanosized GNPs can be created. The most interesting feature of GQDs is their ability to fluoresce under a UV light source. Taking advantage of this, researchers recently developed a facile approach to synthesize large quantities of GQDs that are suitable for stem cell applications.71 Specifically, GQDs were used to label various types of cells including neurosphere cells, pancreas progenitor cells, and cardiac progenitor cells, in an effective manner without any cytotoxicity.
Lastly, conventional QDs are comprised of CdSe, which is quite toxic cells, and hence they are capped with an inert ZnS shell to prevent leaching of Cd or Se into the cells. However, it would be highly desirable to replace these toxic elements altogether and use inert elements while preserving the fluorescence properties of QDs. To this end, researchers have developed a unique and facile method to synthesize QDs comprised of nontoxic elements using a sonochemical approach.72 Specifically, QDs comprised of zinc, indium, silver, and sulfur (ZAIS-QDs) have been developed, which were demonstrated to be nontoxic to stem cells (Figure 10(a)). Moreover, the most attractive feature of ZAIS-QDs is that the emission profile can be tuned based exclusively on the composition of these starting elements (Figure 10(b)). Hence, a large-scale library of ZAIS-QDs was readily synthesized and subsequently transfected into hMSCs with high viability, and showed efficient uptake with a strong fluorescent signal (Figure 10(c) and (d)). Moreover, the ZAIS-QDs were functionalized with a nucleic acid for simultaneous gene regulation, thus demonstrating the multifaceted properties of QDs.
FIGURE 10.
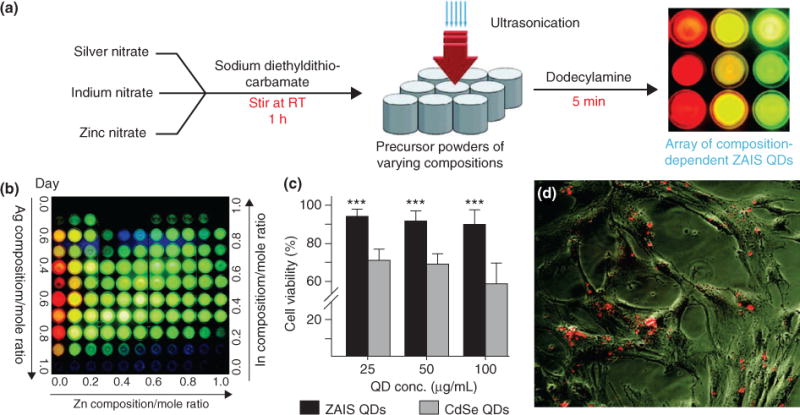
Nontoxic quantum dots (QDs) for stem cell imaging and delivery of nucleic acids. (a) Novel synthesis of ZAIS (Zn, Ag, In, and S) QDs using a sonochemical approach to tune emission properties based on the ratio of starting elements. (b) A library of ZAIS QDs synthesized by varying elemental composition. (c) Cell viability of novel ZAIS QDs compared to conventional CdSe QDs shows that ZAIS QDs are nontoxic to human mesenchymal stem cells (hMSCs). (d) ZAIS QDs are effectively internalized by hMSCs and show a strong fluorescence signal, thus making ZAIS QDs suitable for stem cell tracking applications. (Reprinted with permission from Ref 72. Copyright 2012 John Wiley and Sons)
NANOPARTICLE ARRAYS FOR STEM CELL BIOLOGY
The ability to use physical cues such as nanotopo-graphical cues, substrate patterns, and extracellular matrix (ECM) geometries to control stem cell fate is a highly promising area of research.11,73,74 While most techniques discussed thus far have focused on the delivery of nanoparticles, there is another application of nanoparticles involving surface topography. Instead of conjugating biomolecules onto nanoparticles for a forward transfection, a recently developed method involves fabricating an array of nanoparticles and then culturing stem cells directly on these nanoparticles. The physical topographical cues and the microsurface created by the nanoparticles can act to deliver biomolecules and to induce targeted differentiation and behavioral changes of stem cells.75
Nanoparticle Arrays to Deliver Nucleic Acids for Stem Cell Differentiation
In a recent landmark demonstration, researchers utilized a nanoparticle array to deliver hard-to-transfect biomolecules such as small interfering RNA (siRNA), which effectively regulate gene expression, into NSCs to induce neuronal differentiation.76 SNP sizes varying from 100 to 700 nm in diameter were assembled on a glass substrate using a facile centrifugation method to fabricate the nanotopography-mediated reverse uptake (NanoRU) platform (Figure 11(a)). Then, siRNA molecules were electrostatically conjugated on NanoRU. When NSCs were seeded on these substrates, NSCs readily attached to the surface and extended their axons (Figure 11(b)). To test the efficiency, GFP-labeled NSCs were seeded on NanoRU that was conjugated with siRNA specific for GFP. The results showed a remarkable trend of GFP knockdown as the size of the substrate nanoparticles became smaller, with 100 nm SNPs having the greatest GFP knockdown (Figure 11(c)). Thereafter, siRNA specific for the Sox9 gene, which is responsible for regulating neuronal differentiation, was conjugated on NanoRU and NSCs were seeded on top. The NSCs readily took up the siRNA, and after just 7 days, successful neuronal differentiation was induced (Figure 11(d) and (e)). The NanoRU platform was then compared to traditional methods of siRNA delivery such as commercially available reagents including Lipofectamine. NanoRU was more efficient and resulted in a significantly higher cell viability. Overall, the researchers concluded that based exclusively on the nanotopography created by the nanoparticle array, nucleic acids could easy be transfected into stem cells to regulate their differentiation.
FIGURE 11.
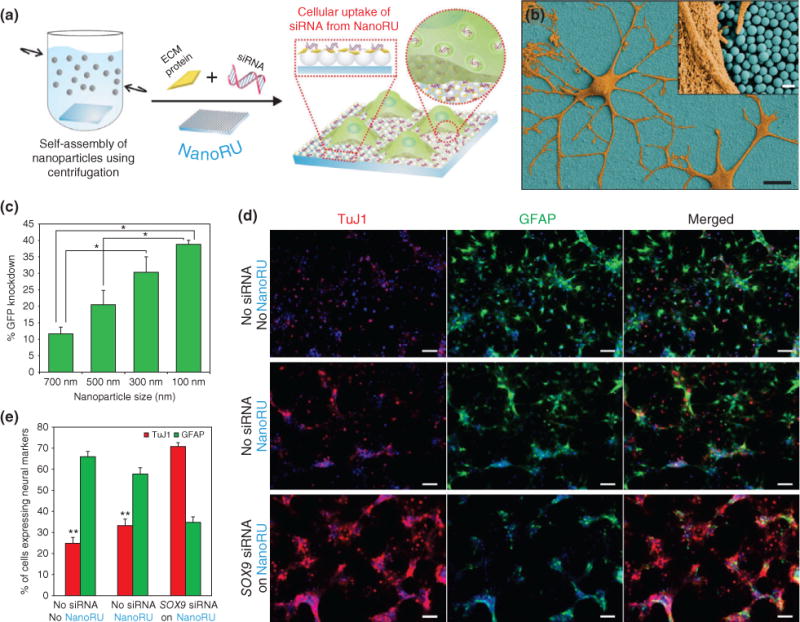
Nanotopography-mediated platform to deliver nucleic acids for stem cell differentiation. (a) Silica nanoparticles (SNPs) are assembled on a film and coated with extracellular matrix (ECM) proteins and nucleic acids (siRNA) to develop the nanotopography-mediated reverse uptake (NanoRU) platform. Neural stem cells (NSCs) cultured on this platform uptake the siRNA, which induces their differentiation into neurons. (b) Scanning electron microscopy image of neurons (brown) with extended axons cultured on NanoRU wherein the SNPs (blue) are visible. (c) Depending on the size of the SNPs on the surface, the uptake rate of siRNA is affected, which is reflected by the difference in green fluorescent protein (GFP) knockdown. Nanoparticle with 100 nm diameter showed the highest efficiency. (d) Fluorescence images showing the differentiation of NSCs into neurons using the NanoRU platform, and (e) the expression of the specific markers, Tuj1 (neuronal) and GFAP (glial cells), was quantified to reveal that NanoRU is an effective platform to induce stem cell differentiation. (Reprinted with permission from Ref 76. Copyright 2013 Nature Publishing Group)
Nanoparticle Arrays to Regulate Neuronal Behaviors of Stem Cells
In another study, the same authors modified the nanotopography by incorporating a sheet of graphene oxide on the surface of NanoRU to differentiate neural progenitor stem cells (hNPSCs)77 (Figure 12(a)). Remarkably, just owing to the influence of the surface, the axons of the hNPSCs started to align (Figure 12(b)–(d)). The degree of alignment and efficiency of neuronal differentiation were the highest when hNPSCs were seeded on a surface containing both SNPs and graphene oxide (Figure 12(e)). Furthermore, the results were reproduced on a flexible polymer, which can potentially be used for in vivo applications (Figure 12(f)). Results show a similar trend of axonal alignment and increase differentiation. Conventional nanoparticle-based methods directly conjugate biomolecules on nanoparticle surfaces for forward delivery, but the method of generating a biocompatible platform for stem cells to grow is a promising approach because biomolecules are readily uptaken with a synergistic effect of nanotopographical cues to guide stem cell differentiation.
FIGURE 12.
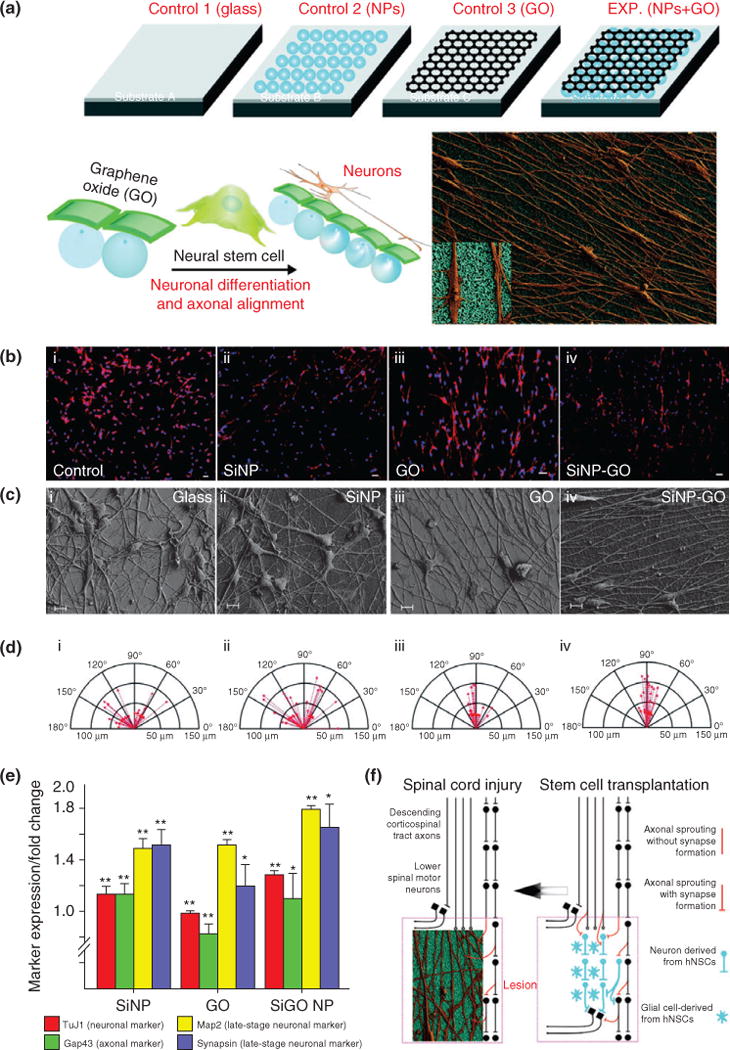
Axonal alignment and enhanced neural stem cell (NSC) differentiation on graphene–nanoparticle hybrid substrates. (a) Schematic diagram depicting the influence of nanoparticle (NP) monolayers coated with graphene oxide (GO) on the alignment of axons extending from human NSCs and their differentiation into neurons. (b–d) Aligned growth and extension of axons from differentiated hNSCs, and the compass plots showing the variation in the angle of orientation and length of axons. (e) Quantitative gene expression results for early- and late-stage neuronal markers expressed by the hNSCs differentiated on the different substrates, with the nanoparticle-hybrid condition having the maximal influence on expression of neuron-specific genes. (f) Scheme depicting the significance of alignment and growth of axons from differentiating hNSCs. The hNSCs that can be transplanted into the injured region (lesion) of a spinal cord differentiate into neurons and glial cells (image on right). The axons from the neurons (derived from hNSCs) if aligned can hasten the recovery process. Our SiNP–GO hybrid structures can provide the ideal microenvironment to align axons that could potentially improve communication leading to rapid recovery of the injured spinal cord (image on left). (Reprinted with permission from Ref 77. Copyright 2013 John Wiley and Sons)
CONCLUSIONS AND FUTURE OUTLOOK
Nanoparticles represent a powerful and innovate class of materials for applications in stem cell biology ranging from small molecule delivery, imaging, and in vivo tracking. The field of nanotechnology and nanomedicine has made significant strides over the past decade, and now, several promising approaches are reaching in vivo and clinical therapies. In order to effectively use nanoparticles for stem cell applications, there are three requirements: high biocompatibility, a multifunctional surface to enable conjugation of biomolecules, and properties to enable noninvasive imaging. To this end, many different types of nanoparticle have been developed for stem cell applications including MNPs, SNPs, QDs, and hybrid nanoparticles that integrate multiple nanoparticles together. Each of these nanoparticles has its own unique properties in terms of size, composition, biocompatibility, imaging capabilities, and possible side effects.
A majority of applications utilizing nanoparticles involve stem cell labeling and stem cell tracking in vivo. This is because nanoparticles have excellent intrinsic chemical and physical properties that allow efficient imaging after transplantation without any adverse side effects on cells or patients. Perhaps, the greatest feature that enables this is their small size, which allows them to remain inside the cells without causing any cellular damage. Furthermore, there is another scope of application of nanoparticles that involves delivery of nucleic acids and small molecules, which are otherwise difficult to deliver, into stem cells for differentiation. This opens up the door to test almost any type of molecule, regardless if its physical and chemical properties are not suitable for physiological environments. Furthermore, targeted delivery into specific intracellular organelles is achievable by adding targeting peptides on the nanoparticle surface. With all these advantages of nanoparticles, stem cell biologists and clinicians have the ability to choose the proper nanoparticle for their specific application.
The major obstacle that is currently hindering widespread use of nanoparticle for clinical stem cell use is the possibility of nanoparticles accumulating in organs and the possible side effects. Even though the nanoparticles may be biocompatible, the effect of accumulating nanoparticles in specific organs after transplanting nanoparticles-loaded stem cells needs to be addressed. With the recent development of hybrid nanoparticles that have a synergistic and combinatorial effect in terms on delivery and imaging, the development of safer nanoparticles appears promising. The future of utilizing nanoparticles for stem cell-based applications and clinical therapies is very bright. With a greater push for stem cell therapies to treat numerous debilitating diseases and injuries, more resources are now geared toward developing translational nanoparticle-based platforms for stem cell medicine.
Acknowledgments
KiBum Lee acknowledges the NIH Director’s Innovator Award (1DP20D006462-01), NIH R21 (1R21NS085569-01), New Jersey Commission on Spinal Cord (CSR13ERG005), NSF CHE-1429062, CBET-1236508, and Busch Biomedical Grant Program for funding.
Footnotes
Conflict of interest: The authors report no conflicts of interest.
References
- 1.Ilic D, Polak J. Stem cell based therapy-where are we going? Lancet. 2012;379:877–878. doi: 10.1016/S0140-6736(12)60155-X. [DOI] [PubMed] [Google Scholar]
- 2.Sterneckert JL, Reinhardt P, Scholer HR. Investigating human disease using stem cell models. Nat Rev Genet. 2014;15:625–639. doi: 10.1038/nrg3764. [DOI] [PubMed] [Google Scholar]
- 3.Tabar V, Studer L. Pluripotent stem cells in regenerative medicine: challenges and recent progress. Nat Rev Genet. 2014;15:82–92. doi: 10.1038/nrg3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah S, Solanki A, Sasmal PK, Lee K-B. Single vehicular delivery of siRNA and small molecules to control stem cell differentiation. J Am Chem Soc. 2013;135:15682–15685. doi: 10.1021/ja4071738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pang ZP, Yang N, Vierbuchen T, Ostermeier A, Fuentes DR, Yang TQ, Citri A, Sebastiano V, Marro S, Sudhof TC, et al. Induction of human neuronal cells by defined transcription factors. Nature. 2011;476:220–223. doi: 10.1038/nature10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caiazzo M, Dell’Anno MT, Dvoretskova E, Lazarevic D, Taverna S, Leo D, Sotnikova TD, Menegon A, Roncaglia P, Colciago G, et al. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 2011;476:224–227. doi: 10.1038/nature10284. [DOI] [PubMed] [Google Scholar]
- 7.Hou P, Li Y, Zhang X, Liu C, Guan J, Li H, Zhao T, Ye J, Yang W, Liu K, et al. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science. 2013;341:651–654. doi: 10.1126/science.1239278. [DOI] [PubMed] [Google Scholar]
- 8.Solanki A, Shah S, Memoli KA, Park SY, Hong S, Lee K-B. Controlling differentiation of neural stem cells using extracellular matrix protein patterns. Small. 2010;6:2509–2513. doi: 10.1002/smll.201001341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bressan E, Carraro A, Ferroni L, Gardin C, Sbricoli L, Guazzo R, Stellini E, Roman M, Pinton P, Sivolella S, et al. Nanotechnology to drive stem cell commitment. Nanomedicine (Lond) 2013;8:469–486. doi: 10.2217/nnm.13.12. [DOI] [PubMed] [Google Scholar]
- 10.King-Chuen W, Ching-Li T, Chi-Chang W, Feng-Chen K, Yuan-Kun T, Edmund CS, Yang-Kao W. Nanotechnology in the regulation of stem cell behavior. Sci Technol Adv Mater. 2013;14:054401. doi: 10.1088/1468-6996/14/5/054401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalby MJ, Gadegaard N, Oreffo ROC. Harnessing nanotopography and integrin-matrix interactions to influence stem cell fate. Nat Mater. 2014;13:558–569. doi: 10.1038/nmat3980. [DOI] [PubMed] [Google Scholar]
- 12.Kshitiz, Kim D-H, Beebe DJ, Levchenko A. Micro- and nanoengineering for stem cell biology: the promise with a caution. Trends Biotechnol. 2011;29:399–408. doi: 10.1016/j.tibtech.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel S, Jung D, Yin PT, Carlton P, Yamamoto M, Bando T, Sugiyama H, Lee K-B. NanoScript: a nanoparticle-based artificial transcription factor for effective gene regulation. ACS Nano. 2014;8:8959–8967. doi: 10.1021/nn501589f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung D, Minami I, Patel S, Lee J, Jiang B, Yuan Q, Li L, Kobayashi S, Chen Y, Lee K-B, et al. Incorporation of functionalized gold nanoparticles into nanofibers for enhanced attachment and differentiation of mammalian cells. J Nanobiotechnol. 2012;10:23. doi: 10.1186/1477-3155-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chou LY, Ming K, Chan WC. Strategies for the intracellular delivery of nanoparticles. Chem Soc Rev. 2011;40:233–245. doi: 10.1039/c0cs00003e. [DOI] [PubMed] [Google Scholar]
- 16.Cleal K, He L, Watson PD, Jones AT. Endocytosis, intracellular traffic and fate of cell penetrating peptide based conjugates and nanoparticles. Curr Pharm Des. 2013;19:2878–2894. doi: 10.2174/13816128113199990297. [DOI] [PubMed] [Google Scholar]
- 17.Copolovici DM, Langel K, Eriste E, Langel Ü. Cell-penetrating peptides: design, synthesis, and applications. ACS Nano. 2014;8:1972–1994. doi: 10.1021/nn4057269. [DOI] [PubMed] [Google Scholar]
- 18.Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 19.Oh E, Delehanty JB, Sapsford KE, Susumu K, Goswami R, Blanco-Canosa JB, Dawson PE, Granek J, Shoff M, Zhang Q, et al. Cellular uptake and fate of PEGylated gold nanoparticles is dependent on both cell-penetration peptides and particle size. ACS Nano. 2011;5:6434–6448. doi: 10.1021/nn201624c. [DOI] [PubMed] [Google Scholar]
- 20.Madani F, Lindberg S, Langel U, Futaki S, Graslund A. Mechanisms of cellular uptake of cell-penetrating peptides. J Biophys. 2011;2011:414729. doi: 10.1155/2011/414729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krpetić Ž, Saleemi S, Prior IA, Sée V, Qureshi R, Brust M. Negotiation of intracellular membrane barriers by TAT-modified gold nanoparticles. ACS Nano. 2011;5:5195–5201. doi: 10.1021/nn201369k. [DOI] [PubMed] [Google Scholar]
- 22.Tkachenko AG, Xie H, Coleman D, Glomm W, Ryan J, Anderson MF, Franzen S, Feldheim DL. Multifunctional gold nanoparticle−peptide complexes for nuclear targeting. J Am Chem Soc. 2003;125:4700–4701. doi: 10.1021/ja0296935. [DOI] [PubMed] [Google Scholar]
- 23.Chithrani BD, Ghazani AA, Chan WCW. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006;6:662–668. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 24.H-m D, Y-q M. Controlling cellular uptake of nanoparticles with pH-sensitive polymers. Sci Rep. 2013;3:2804. doi: 10.1038/srep02804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saha K, Kim ST, Yan B, Miranda OR, Alfonso FS, Shlosman D, Rotello VM. Surface functionality of nanoparticles determines cellular uptake mechanisms in mammalian cells. Small. 2013;9:300–305. doi: 10.1002/smll.201201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tenzer S, Docter D, Kuharev J, Musyanovych A, Fetz V, Hecht R, Schlenk F, Fischer D, Kiouptsi K, Reinhardt C, et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat Nano. 2013;8:772–781. doi: 10.1038/nnano.2013.181. [DOI] [PubMed] [Google Scholar]
- 27.Lundqvist M, Stigler J, Elia G, Lynch I, Cedervall T, Dawson KA. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc Natl Acad Sci USA. 2008;105:14265–14270. doi: 10.1073/pnas.0805135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lesniak A, Fenaroli F, Monopoli MP, Åberg C, Dawson KA, Salvati A. Effects of the presence or absence of a protein corona on silica nanoparticle uptake and impact on cells. ACS Nano. 2012;6:5845–5857. doi: 10.1021/nn300223w. [DOI] [PubMed] [Google Scholar]
- 29.Salvati A, Pitek AS, Monopoli MP, Prapainop K, Bombelli FB, Hristov DR, Kelly PM, Aberg C, Mahon E, Dawson KA. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat Nano. 2013;8:137–143. doi: 10.1038/nnano.2012.237. [DOI] [PubMed] [Google Scholar]
- 30.Lu AH, Salabas EL, Schuth F. Magnetic nanoparticles: synthesis, protection, functionalization, and application. Angew Chem Int Ed Engl. 2007;46:1222–1244. doi: 10.1002/anie.200602866. [DOI] [PubMed] [Google Scholar]
- 31.Reddy LH, Arias JL, Nicolas J, Couvreur P. Magnetic nanoparticles: design and characterization, toxicity and biocompatibility, pharmaceutical and biomedical applications. Chem Rev. 2012;112:5818–5878. doi: 10.1021/cr300068p. [DOI] [PubMed] [Google Scholar]
- 32.Colombo M, Carregal-Romero S, Casula MF, Gutierrez L, Morales MP, Bohm IB, Heverhagen JT, Prosperi D, Parak WJ. Biological applications of magnetic nanoparticles. Chem Soc Rev. 2012;41:4306–4334. doi: 10.1039/c2cs15337h. [DOI] [PubMed] [Google Scholar]
- 33.Yin PT, Shah BP, Lee K-B. Combined magnetic nanoparticle-based microRNA and hyperthermia therapy to enhance apoptosis in brain cancer cells. Small. 2014;10:4106–4112. doi: 10.1002/smll.201400963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laurentt N, Sapet C, Le Gourrierec L, Bertosio E, Zelphati O. Nucleic acid delivery using magnetic nanoparticles: the magnetofection technology. Ther Deliv. 2011;2:471–482. doi: 10.4155/tde.11.12. [DOI] [PubMed] [Google Scholar]
- 35.Lewin M, Carlesso N, Tung C-H, Tang X-W, Cory D, Scadden DT, Weissleder R. Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nat Biotechnol. 2000;18:410–414. doi: 10.1038/74464. [DOI] [PubMed] [Google Scholar]
- 36.Neoh KG, Kang ET. Surface modification of magnetic nanoparticles for stem cell labeling. Soft Matter. 2012;8:2057–2069. [Google Scholar]
- 37.Jenkins SI, Pickard MR, Granger N, Chari DM. Magnetic nanoparticle-mediated gene transfer to oligodendrocyte precursor cell transplant populations is enhanced by magnetofection strategies. ACS Nano. 2011;5:6527–6538. doi: 10.1021/nn2018717. [DOI] [PubMed] [Google Scholar]
- 38.Shah B, Yin PT, Ghoshal S, Lee K-B. Multimodal magnetic core–shell nanoparticles for effective stem-cell differentiation and imaging. Angew Chem Int Ed. 2013;52:6190–6195. doi: 10.1002/anie.201302245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang C, Neoh KG, Wang L, Kang E-T, Shuter B. Magnetic nanoparticles for magnetic resonance imaging: modulation of macrophage uptake by controlled PEGylation of the surface coating. J Mater Chem. 2010;20:8512–8520. [Google Scholar]
- 40.Wei X, Yang X, Han Z-P, Qu F-F, Shao L, Shi Y-F. Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacol Sin. 2013;34:747–754. doi: 10.1038/aps.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruan J, Ji J, Song H, Qian Q, Wang K, Wang C, Cui D. Fluorescent magnetic nanoparticle-labeled mesenchymal stem cells for targeted imaging and hyperthermia therapy of in vivo gastric cancer. Nanoscale Res Lett. 2012;7:309. doi: 10.1186/1556-276X-7-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee JM, Kim BS, Lee H, Im GI. In vivo tracking of mesechymal stem cells using fluorescent nanoparticles in an osteochondral repair model. Mol Ther. 2012;20:1434–1442. doi: 10.1038/mt.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sadan O, Shemesh N, Barzilay R, Bahat-Stromza M, Melamed E, Cohen Y, Offen D. Migration of neurotrophic factors-secreting mesenchymal stem cells toward a quinolinic acid lesion as viewed by magnetic resonance imaging. Stem Cells. 2008;26:2542–2551. doi: 10.1634/stemcells.2008-0240. [DOI] [PubMed] [Google Scholar]
- 44.Zhu J, Zhou L, XingWu F. Tracking neural stem cells in patients with brain trauma. New Engl J Med. 2006;355:2376–2378. doi: 10.1056/NEJMc055304. [DOI] [PubMed] [Google Scholar]
- 45.Gonzalez-Lara LE, Xu X, Hofstetrova K, Pniak A, Chen Y, McFadden CD, Martinez-Santiesteban FM, Rutt BK, Brown A, Foster PJ. The use of cellular magnetic resonance imaging to track the fate of iron-labeled multipotent stromal cells after direct transplantation in a mouse model of spinal cord injury. Mol Imaging Biol. 2011;13:702–711. doi: 10.1007/s11307-010-0393-y. [DOI] [PubMed] [Google Scholar]
- 46.Landázuri N, Tong S, Suo J, Joseph G, Weiss D, Sutcliffe DJ, Giddens DP, Bao G, Taylor WR. Magnetic targeting of human mesenchymal stem cells with internalized superparamagnetic iron oxide nanoparticles. Small. 2013;9:4017–4026. doi: 10.1002/smll.201300570. [DOI] [PubMed] [Google Scholar]
- 47.Slowing II, Vivero-Escoto JL, Trewyn BG, Lin VSY. Mesoporous silica nanoparticles: structural design and applications. J Mater Chem. 2010;20:7924–7937. [Google Scholar]
- 48.Benezra M, Penate-Medina O, Zanzonico PB, Schaer D, Ow H, Burns A, DeStanchina E, Longo V, Herz E, Iyer S, et al. Multimodal silica nanoparticles are effective cancer-targeted probes in a model of human melanoma. J Clin Invest. 2011;121:2768–2780. doi: 10.1172/JCI45600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang L, Cheng J. Nonporous silica nanoparticles for nanomedicine application. Nano Today. 2013;8:290–312. doi: 10.1016/j.nantod.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang F, Li L, Chen D. Mesoporous silica nanoparticles: synthesis, biocompatibility and drug delivery. Adv Mater. 2012;24:1504–1534. doi: 10.1002/adma.201104763. [DOI] [PubMed] [Google Scholar]
- 51.Bharali DJ, Klejbor I, Stachowiak EK, Dutta P, Roy I, Kaur N, Bergey EJ, Prasad PN, Stachowiak MK. Organically modified silica nanoparticles: a nonviral vector for in vivo gene delivery and expression in the brain. Proc Natl Acad Sci USA. 2005;102:11539–11544. doi: 10.1073/pnas.0504926102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu D, He X, Wang K, He C, Shi H, Jian L. Biocompatible silica nanoparticles-insulin conjugates for mesenchymal stem cell adipogenic differentiation. Bioconjug Chem. 2010;21:1673–1684. doi: 10.1021/bc100177v. [DOI] [PubMed] [Google Scholar]
- 53.Gao Y, Wang Y, Fu A, Shi W, Yeo D, Luo KQ, Ow H, Xu C. Tracking mesenchymal stem cell tumor-homing using fluorescent silica nanoparticles. J Mater Chem B. 2015;3:1245–1253. doi: 10.1039/c4tb01452a. [DOI] [PubMed] [Google Scholar]
- 54.Hsiao J-K, Tsai C-P, Chung T-H, Hung Y, Yao M, Liu H-M, Mou C-Y, Yang C-S, Chen Y-C, Huang D-M. Mesoporous silica nanoparticles as a delivery system of gadolinium for effective human stem cell tracking. Small. 2008;4:1445–1452. doi: 10.1002/smll.200701316. [DOI] [PubMed] [Google Scholar]
- 55.Casciaro S, Conversano F, Ragusa A, Malvindi MA, Franchini R, Greco A, Pellegrino T, Gigli G. Optimal enhancement configuration of silica nanoparticles for ultrasound imaging and automatic detection at conventional diagnostic frequencies. Invest Radiol. 2010;45:715–724. doi: 10.1097/RLI.0b013e3181e6f42f. [DOI] [PubMed] [Google Scholar]
- 56.Jokerst JV, Khademi C, Gambhir SS. Intracellular aggregation of multimodal silica nanoparticles for ultrasound-guided stem cell implantation. Sci Transl Med. 2013;5:177ra135. doi: 10.1126/scitranslmed.3005228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Neumann A, Christel A, Kasper C, Behrens P. BMP2-loaded nanoporous silica nanoparticles promote osteogenic differentiation of human mesenchymal stem cells. RSC Adv. 2013;3:24222–24230. [Google Scholar]
- 58.Garcia-Bennett AE, Kozhevnikova M, Konig N, Zhou C, Leao R, Knopfel T, Pankratova S, Trolle C, Berezin V, Bock E, et al. Delivery of differentiation factors by mesoporous silica particles assists advanced differentiation of transplanted murine embryonic stem cells. Stem Cells Transl Med. 2013;2:906–915. [Google Scholar]
- 59.Chen W, Tsai P-H, Hung Y, Chiou S-H, Mou C-Y. Nonviral cell labeling and differentiation agent for induced pluripotent stem cells based on mesoporous silica nanoparticles. ACS Nano. 2013;7:8423–8440. doi: 10.1021/nn401418n. [DOI] [PubMed] [Google Scholar]
- 60.Chatterjee K, Sarkar S, Jagajjanani Rao K, Paria S. Core/shell nanoparticles in biomedical applications. Adv Colloid Interface Sci. 2014;209:8–39. doi: 10.1016/j.cis.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 61.Lu CW, Hung Y, Hsiao JK, Yao M, Chung TH, Lin YS, Wu SH, Hsu SC, Liu HM, Mou CY, et al. Bifunctional magnetic silica nanoparticles for highly efficient human stem cell labeling. Nano Lett. 2007;7:149–154. doi: 10.1021/nl0624263. [DOI] [PubMed] [Google Scholar]
- 62.Kramer IJ, Sargent EH. The architecture of colloidal quantum dot solar cells: materials to devices. Chem Rev. 2013;114:863–882. doi: 10.1021/cr400299t. [DOI] [PubMed] [Google Scholar]
- 63.Medintz IL, Uyeda HT, Goldman ER, Mattoussi H. Quantum dot bioconjugates for imaging, labelling and sensing. Nat Mater. 2005;4:435–446. doi: 10.1038/nmat1390. [DOI] [PubMed] [Google Scholar]
- 64.Bera D, Qian L, Tseng T-K, Holloway PH. Quantum dots and their multimodal applications: a review. Materials. 2010;3:2260–2345. [Google Scholar]
- 65.Jung J, Solanki A, Memoli KA, Kamei K-I, Kim H, Drahl MA, Williams LJ, Tseng H-R, Lee K. Selective inhibition of human brain tumor cells through multifunctional quantum-dot-based siRNA delivery. Angew Chem Int Ed. 2010;49:103–107. doi: 10.1002/anie.200905126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rak-Raszewska A, Marcello M, Kenny S, Edgar D, Sée V, Murray P. Quantum dots do not affect the behaviour of mouse embryonic stem cells and kidney stem cells and are suitable for short-term tracking. PLoS One. 2012;7:e32650. doi: 10.1371/journal.pone.0032650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lin S, Xie X, Patel M, Yang Y-H, Li Z, Cao F, Gheysens O, Zhang Y, Gambhir S, Rao J, et al. Quantum dot imaging for embryonic stem cells. BMC Biotechnol. 2007;7:67. doi: 10.1186/1472-6750-7-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang H-C, Brown J, Alayon H, Stuck BE. Transplantation of quantum dot-labelled bone marrow-derived stem cells into the vitreous of mice with laser-induced retinal injury: Survival, integration and differentiation. Vision Res. 2010;50:665–673. doi: 10.1016/j.visres.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 69.Vibin M, Vinayakan R, John A, Fernandez FB, Abraham A. Effective cellular internalization of silica-coated CdSe quantum dots for high contrast cancer imaging and labelling applications. Cancer Nanotechnol. 2014;5:1. doi: 10.1186/s12645-014-0001-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bacon M, Bradley SJ, Nann T. Graphene quantum dots. Part Part Syst Charact. 2014;31:415–428. [Google Scholar]
- 71.Zhang M, Bai L, Shang W, Xie W, Ma H, Fu Y, Fang D, Sun H, Fan L, Han M, et al. Facile synthesis of water-soluble, highly fluorescent graphene quantum dots as a robust biological label for stem cells. J Mater Chem. 2012;22:7461–7467. [Google Scholar]
- 72.Subramaniam P, Lee SJ, Shah S, Patel S, Starovoytov V, Lee K-B. Generation of a library of non-toxic quantum dots for cellular imaging and siRNA delivery. Adv Mater. 2012;24:4014–4019. doi: 10.1002/adma.201201019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bettinger CJ, Langer R, Borenstein JT. Engineering substrate topography at the micro- and nanoscale to control cell function. Angew Chem Int Ed. 2009;48:5406–5415. doi: 10.1002/anie.200805179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shah S, Yin PT, Uehara TM, Chueng S-TD, Yang L, Lee K-B. Guiding stem cell differentiation into oligodendrocytes using graphene-nanofiber hybrid scaffolds. Adv Mater. 2014;26:3673–3680. doi: 10.1002/adma.201400523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yim EKF, Darling EM, Kulangara K, Guilak F, Leong KW. Nanotopography-induced changes in focal adhesions, cytoskeletal organization, and mechanical properties of human mesenchymal stem cells. Biomaterials. 2010;31:1299–1306. doi: 10.1016/j.biomaterials.2009.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Solanki A, Shah S, Yin PT, Lee K-B. Nanotopography-mediated reverse uptake for siRNA delivery into neural stem cells to enhance neuronal differentiation. Sci Rep. 2013;3:1553. doi: 10.1038/srep01553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Solanki A, Chueng S-TD, Yin PT, Kappera R, Chhowalla M, Lee K-B. Axonal alignment and enhanced neuronal differentiation of neural stem cells on graphene-nanoparticle hybrid structures. Adv Mater. 2013;25:5477–5482. doi: 10.1002/adma.201302219. [DOI] [PMC free article] [PubMed] [Google Scholar]


