CONSPECTUS
The mammalian brain is a phenomenal piece of “organic machinery” that has fascinated scientists and clinicians for centuries. The intricate network of tens of billions of neurons dispersed in a mixture of chemical and biochemical constituents gives rise to thoughts, feelings, memories, and life as we know it. In turn, subtle imbalances or damage to this system can cause severe complications in physical, motor, psychological, and cognitive function. Moreover, the inevitable loss of nerve tissue caused by degenerative diseases and traumatic injuries is particularly devastating because of the limited regenerative capabilities of the central nervous system (i.e., the brain and spinal cord).
Among current approaches, stem-cell-based regenerative medicine has shown the greatest promise toward repairing and regenerating destroyed neural tissue. However, establishing controlled and reliable methodologies to guide stem cell differentiation into specialized neural cells of interest (e.g., neurons and oligodendrocytes) has been a prevailing challenge in the field. In this Account, we summarize the nanotechnology-based approaches our group has recently developed to guide stem-cell-based neural regeneration. We focus on three overarching strategies that were adopted to selectively control this process.
First, soluble microenvironmental factors play a critical role in directing the fate of stem cells. Multiple factors have been developed in the form of small-molecule drugs, biochemical analogues, and DNA/RNA-based vectors to direct neural differentiation. However, the delivery of these factors with high transfection efficiency and minimal cytotoxicity has been challenging, especially to sensitive cell lines such as stem cells. In our first approach, we designed nanoparticle-based systems for the efficient delivery of such soluble factors to control neural differentiation. Our nanoparticles, comprising either organic or inorganic elements, were biocompatible and offered multifunctional capabilities such as imaging and delivery.
Moving from the soluble microenvironment in which cells are immersed to the underlying surface, cells can sense and consequently respond to the physical microenvironment in which they reside. For instance, changes in cell adhesion, shape, and spreading are key cellular responses to surface properties of the underlying substrate. In our second approach, we modulated the surface chemistry of two-dimensional substrates to control neural stem cell morphology and the resulting differentiation process. Patterned surfaces consisting of immobilized extracellular matrix (ECM) proteins and/or nanomaterials were generated and utilized to guide neuronal differentiation and polarization.
In our third approach, building on the above-mentioned approaches, we further tuned the cell−ECM interactions by introducing nanotopographical features in the form of nanoparticle films or nanofiber scaffolds. Besides providing a three-dimensional surface topography, our unique nanoscaffolds were observed to enhance gene delivery, facilitate axonal alignment, and selectively control differentiation into neural cell lines of interest. Overall, nanotechnology-based approaches offer the precise physicochemical control required to generate tools suitable for applications in neuroscience.
Graphical abstract
1. INTRODUCTION
Understanding how the central nervous system (CNS) works and developing therapies to repair this intricate system after it has been damaged have been long-lasting aspirations for scientists and clinicians. Malfunction in the CNS, which consists of the brain and spinal cord, tends to cause numerous complications, including physical, cognitive, and psychological impairment. Furthermore, the inevitable loss of nerve tissue caused by degenerative diseases (e.g., Parkinson’s disease) and traumatic injuries is particularly devastating because of the limited regenerative capabilities of the CNS. To this end, there is a clear unmet need for effective strategies to replace the destroyed neural tissue and attenuate the debilitating symptoms.
Considering the multifaceted complications incurred by damage to the CNS, stem-cell-based therapies have emerged as one of the most promising treatment options.1 While other treatments primarily aim to minimize damage from secondary injuries, stem cell therapies have been employed to promote regeneration and repair. Stem cell transplantation offers numerous benefits for facilitating CNS repair, including replacement of damaged cells, restoration of neuronal circuitry, reduction of inflammation/gliosis, and induction of axonal regeneration.2 Among the multiple types of stem cells currently available, neural stem cells (NSCs) have been widely studied as an essential source of engraftable cells for CNS therapies.3 Whether acquired endogenously from the CNS or derived from pluripotent stem cells, NSCs possess the ability to self-renew as well as differentiate into neural-restricted cell lineages.4 In particular, these multipotent NSCs can become one of three main cell types: neurons, astrocytes, or oligodendrocytes.3 Neurons are specialized signaling cells that transmit information throughout the nervous system in the form of electrical and chemical signals. On the other hand, glial cells are the supportive and most abundant cells of the nervous system, among which astrocytes maintain CNS homeostasis and oligodendrocytes provide insulation for optimal neuronal signaling.
From a therapeutic standpoint, the selective differentiation into neurons and oligodendrocytes has resulted in favorable outcomes for CNS repair.2 Numerous biochemical and genetic-based methodologies have been applied to both identify and optimize the factors critical for selectively guiding NSC differentiation.5–7 This Account describes the nanotechnology-based approaches that our group has recently developed to guide this process. We highlight three strategies that were adopted to selectively control neural differentiation: (i) delivery of soluble factors using multifunctional nanoparticles, (ii) control of stem cell morphology and behavior using surface patterning, and (iii) modulation of cell−extracellular matrix (ECM) interactions using nanoscaffolds.
2. DELIVERY OF SOLUBLE FACTORS USING MULTIFUNCTIONAL NANOPARTICLES
The conventional approach for controlling stem cell behavior and differentiation has involved adjusting the formulation of the cell medium with key chemical and biochemical constituents. For instance, a biochemical approach has been widely used to control stem cell differentiation, wherein small-molecule drugs are screened, isolated, and utilized to modulate specific signaling pathways within the cell.8 A significant advantage of using this approach is that small molecules provide temporal control over protein function by rapidly modulating single/multiple targets within a protein family.9 With recent advances in genetic engineering, gene delivery methods have also gained popularity for altering the expression and activity of specific cellular factors. RNA interference (RNAi) is one such example, wherein RNA molecules [e.g., small-interfering RNA (siRNA)] target the destruction of specific messenger RNA, which in turn silences or inhibits gene expression at the translational level.10 While these chemical and biochemical factors are becoming popular for directing the fate of stem cells, establishing effective nontoxic approaches to deliver small molecules and siRNA into these cells has been challenging. For example, a majority of small molecules are typically very hydrophobic and lack solubility in physiological solutions, which can greatly impair their delivery and efficacy.11 Additionally, conventional methods used to deliver siRNA into cells result in significant cytotoxicity and undesirable side effects.12 Moreover, for the successful application of the transfected stem cells, the cells must maintain their viability for an extended period of time after single or multiple transfections without affecting the intrinsic cellular functions. This presents a considerable challenge for achieving robust and reliable delivery of cargo into stem cells to control their differentiation into specific cell lineages. We have developed organic- and inorganic-nanomaterial-based delivery systems to address these limitations that are not only biocompatible—ensuring enhanced cell viability over extended time periods—but also offer multifunctional capabilities such as molecular imaging or simultaneous delivery of distinct factors.
2.1. Inorganic Nanomaterials
The ability to synthetically tune the properties of inorganic nanomaterials in terms of composition (e.g., noble metal, silica, magnetic) and surface functionality has facilitated their use in numerous bioapplications, including imaging, sensing, and drug/gene delivery.13 We designed multifunctional magnetic core−shell nanoparticles (MCNPs) for the facile transfection of siRNA into primary rat NSCs (rNSCs).14 MCNPs were synthesized by generation of a zinc-doped iron oxide (ZnFe2O4) core using a thermal decomposition method followed by deposition of a thin-layer Au shell. These core−shell MCNPs were further rendered water-soluble by ligand exchange and coated with polymeric dendrimers to aid the complexation and delivery of siRNA (Figure 1a,b). In this way, the magnetic core provides the ability to transfect the siRNA-coated ZnFe2O4@Au MCNPs using a magnetic field (Figure 1c) as well as to image the transfected rNSCs using magnetic resonance imaging (MRI). The outer gold shell further helps to make the MCNPs more biocompatible, permits facile surface functionalization, and enables the transfected rNSCs to be imaged using dark-field light scattering.
Figure 1.
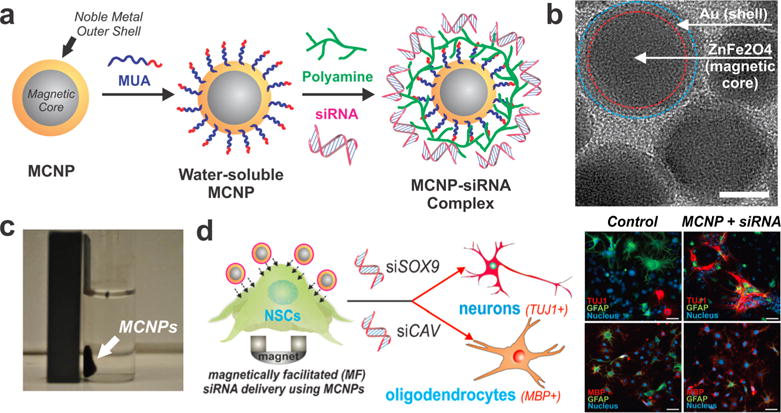
Magnetic core−shell nanoparticles (MCNPs) efficiently deliver siRNA into rNSCs. (a) MCNPs were functionalized to complex negatively charged siRNA. (b) Transmission electron microscopy image of MCNPs. Scale bar: 10 nm. (c) MCNPs dispersed in water are attracted to a magnet. (d) Magnetically facilitated delivery of siRNA to induce neural differentiation of rNSCs using MCNPs. Fluorescence images depict (top) neuronal and (bottom) oligodendrocyte differentiation after siSOX9 and siCAV delivery, respectively. Adapted with permission from ref 14. Copyright 2013 Wiley.
For gene knockdown studies, these multifunctional MCNPs were first used to silence green fluorescent protein (GFP) expressed within rNSCs using magnetofection. This was particularly advantageous compared with conventional transfection agents, as we observed a significant decrease in the intensity of GFP (∼60%). Furthermore, the minimal incubation time required to transfect rNSCs using magnetofection was found to be only 30 min, compared with the conventional 4−6 h without magnetofection. This resulted in enhanced cellular viability, which is extremely important because NSCs must survive and differentiate over a period of at least 7 days. Magnetofection was then used to deliver the MCNPs complexed to siRNA that promoted differentiation of rNSCs. In particular, siRNA targeting the inhibition of the transcription factor SOX9 (siSOX9) was delivered to enhance neuronal differentiation, and siRNA targeting the inhibition of caveolin-1 (siCAV) was delivered to enhance oligodendrocyte differentiation (Figure 1d). Thus, MCNPs are excellent multifunctional delivery vehicles that (1) retain their magnetic capabilities, (2) transfect NSCs with high efficiency in shorter incubation times, and (3) show negligible cytotoxicity. Such an approach can be useful when transitioning to in vivo studies, wherein stem cell differentiation can be controlled and the transplanted cells tracked using a single multifunctional platform.
2.2. Organic-Based Nanomaterials
In contrast to inorganic nanomaterials, organic-based nanomaterials are composed of elements generally found within the body (e.g., carbon, nitrogen, oxygen). As a result, such materials can be much more biocompatible and biodegradable right from the onset, making them attractive for biological studies. Among other applications, organic-based nanomaterials have been widely used to facilitate the delivery of therapeutics. Examples of such organic-based nanocarriers include liposomes, micelles, dendrimers, and polymeric nanoparticles.15 However, these systems have been primarily utilized to mediate drug/gene delivery for cancer therapies and treatments.16 We designed an organic-nanoparticle-based delivery system—DexAM—that solubilizes small molecules in physiological solutions and at the same time forms complexes with siRNA molecules for directing NSC differentiation.17 In this way, our system is a single delivery vehicle that provides (i) the ability to simultaneously deliver negatively charged nucleic acids and hydrophobic small molecules to achieve a synergistic enhancement in stem cell differentiation, (ii) high transfection efficiency, and (iii) minimal cytotoxicity, allowing stem cells to differentiate over longer periods. While such dual delivery platforms are widely prevalent for inducing apoptosis of cancer cells,18 DexAM was the first example demonstrating their application for stem cell differentiation.
DexAM is a β-cyclodextrin (β-CD)-modified dendritic polyamine construct that combines the unique properties of two distinct chemical moieties in a single vehicle (Figure 2a). First, the β-CD component, a cyclical sugar molecule containing a hydrophilic exterior and a hydrophobic interior, is a structure that is widely used in pharmaceutical applications to improve the solubility of hydrophobic compounds.19 Second, the dendritic polyamine backbone provides a positively charged surface that can be used to form cationic complexes with siRNA. This allows for effective intracellular delivery and endosomal escape within stem cells, ensuring maximum efficiency of knockdown. We first optimized the concentration of DexAM for siRNA delivery by delivering siRNA against green fluorescent protein (siGFP) in GFP-expressing rNSCs such that we could achieve maximum knockdown of GFP with minimal impact on cell viability. The DexAM construct was further utilized to enhance the neuronal differentiation of the rNSCs by codelivering the small molecule retinoic acid (RA) and siSOX9 (Figure 2b). We observed a synergistic effect on the neuronal differentiation of rNSCs, with a remarkable increase in the percentage of neurons from 25% (control) to 70% (simultaneous delivery) (Figure 2c,d). Previous studies have reported that overexpression of SOX9 prior to RA treatment counteracts the neuronal-promoting effects induced by RA.20 By simultaneously inhibiting SOX9 expression (with siSOX9) and delivering RA, we targeted distinct signaling pathways through distinct mechanisms of action to achieve enhancements in neuronal differentiation. We envision that organic-based constructs like DexAM have significant implications for utilization as delivery vehicles to control stem cell differentiation, as they can be multifunctional and biocompatible.
Figure 2.
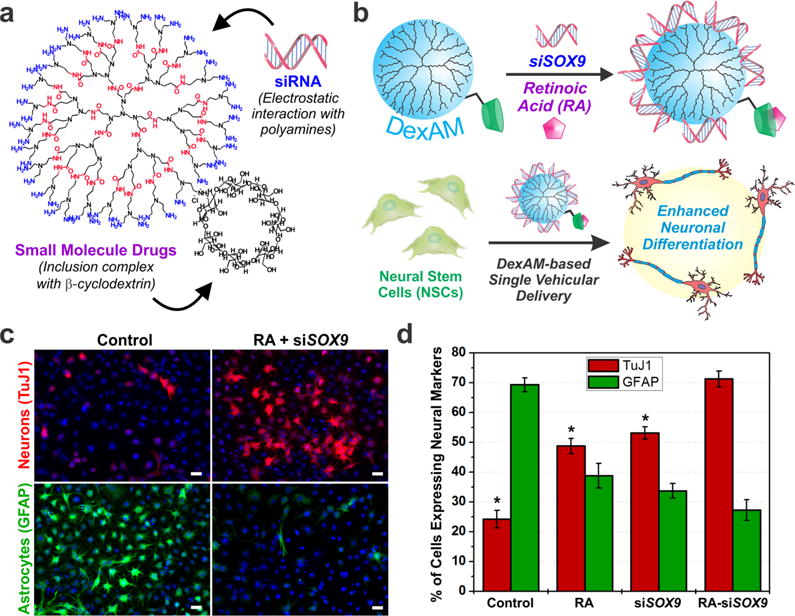
DexAM facilitates codelivery of siRNA and hydrophobic small molecules into rNSCs. (a) DexAM comprises a positively charged polyamine backbone to complex negatively charged siRNA and a β-cyclodextrin moiety to encapsulate small-molecule drugs. (b) Complexed DexAM constructs were delivered to rNSCs to enhance neuronal differentiation. (c) Fluorescence images of cells stained for the neuronal marker TuJ1 and the astrocytic marker GFAP. Scale bars: 20 μm. (d) Plot showing quantification of the stained cells. Statistical significance is compared to RA−siSOX9 treatment (*, p < 0.001). Adapted from ref 17. Copyright 2013 American Chemical Society.
3. CONTROLLING CELL MORPHOLOGY AND BEHAVIOR USING SURFACE PATTERNING
Besides the influence of factors delivered through the soluble microenvironment, understanding the role of the physical microenvironment in defining stem cell behavior has gained tremendous attention in the past decade. Stem cells are particularly sensitive to surface features, and therefore, researchers have selectively modified the surface chemistry of the underlying substrate to precisely regulate cell adhesion, spreading, shape, elongation, and consequently cell fate.21 In this regard, micro/nanopatterning techniques have gained popularity for investigating the impact of surface features on stem cell differentiation.22 Micro/nanopatterned surfaces are generally fabricated using soft lithographic techniques such as microcontact printing or hard lithographic methods such as photolithography.22 Various shapes—the most common ones being circles, squares, triangles, stripes, and grids—can be generated using these methods. Stem cells are then cultured and grown on these well-defined patterned two-dimensional (2D) surfaces, allowing the effects of spreading and shape on lineage commitment to be assessed. Previous studies have explored the differentiation of stem cells (e.g., ESCs, MSCs) on such patterned surfaces into a number of different cell fates, including osteocytes (bone), adipocytes (fat), chondrocytes (cartilage), and myocytes (muscle).22 Our group has focused on guiding the differentiation of NSCs into neuronal cell fate using patterned surfaces immobilized with ECM proteins and/ or specialized nanomaterials (e.g., carbon nanotubes, graphene oxide).
3.1. Patterning of Extracellular Matrix Proteins
The ECM provides both biochemical and biomechanical cues in the stem cell niche to mediate key cellular interactions such as adhesion, migration, proliferation, and differentiation.23 Our goal was to investigate the role and effect of pattern geometry and cell−cell interactions on NSC differentiation. To this end, we fabricated micropatterns of laminin, which is an essential ECM protein required for the adhesion and growth of NSCs.24 Micropatterns consisting of self-assembled monolayers (SAMs) of hydrocarbon ligands were first generated using microcontact printing (Figure 3a). The background, which is the area surrounding the patterned features, was then passivated using poly(ethylene glycol) molecules to prevent nonspecific attachment of proteins and cells. Thereafter, laminin was preferentially adsorbed on the SAMs and seeded with rNSCs on the square, stripe, and grid patterns, with each of the geometries controlling the extent of cell−cell interactions along with the cell−ECM interactions (Figure 3b). For instance, the square patterns isolated the rNSCs while the stripes led to one-way cell−cell interactions and the grids led to two-way cell−cell interactions. After about 1 week, we found that the differentiation of rNSCs was indeed determined by the pattern shape and geometry (Figure 3c). In particular, the rNSCs differentiated on the grid patterns (enabling the highest cell−cell interactions) showed a significantly higher percentage of differentiation into neurons compared with the rNSCs grown on the square patterns (offering minimal cell−cell interactions).
Figure 3.
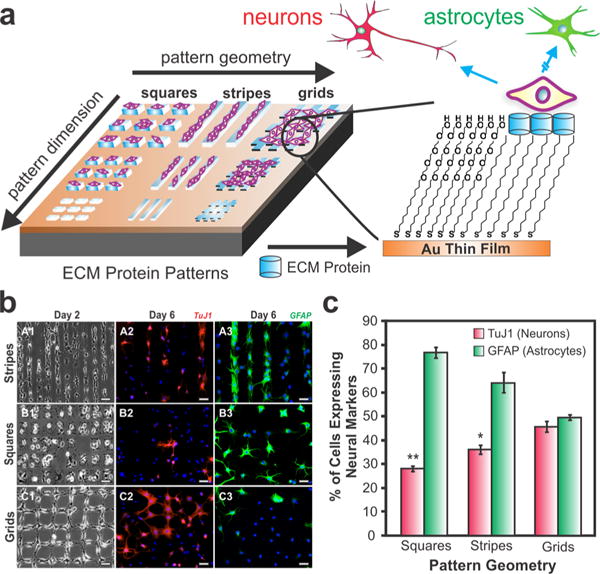
ECM protein patterns can guide neuronal differentiation of rNSCs. (a) Fabrication of ECM protein patterns of varying pattern geometries and dimensions to control rNSC differentiation. (b) Images showing rNSC growth at day 2 (A1-C1) and immunostaining for the neuronal marker TuJ1 (A2-C2) and the astrocytic marker GFAP (A3-C3) at day 6. Scale bars: 50 μm. (c) Plot showing quantification of the stained cells. Statistical significance is compared to grid patterns (*, p < 0.01; **, p < 0.001). Adapted with permission from ref 14. Copyright 2010 Wiley.
The most impressive aspect of this study was that the rNSCs were differentiated using only basal medium with no additional differentiation-promoting exogenous factors added. This is in contrast to conventional approaches that generally employ small molecules (e.g., RA) or growth factors [e.g., brain-derived neurotrophic factor (BDNF)] to promote cell-type-specific differentiation. In turn, our results indicate the importance of cell shape and confinement in determining the fate of NSCs. In combination with previous findings on the influence of substrate elasticity on stem cell differentiation25,26 and biophysical cues on regulating epigenetic states and cell fate,27 the importance of integrating such physical cues to guide neural regeneration is promising.
3.2. Patterning of Nanomaterials
In addition to modulation of the cell morphology using combinatorial micropatterns, synthetic nanoscale materials offer unique surface properties that can influence cell behavior. Among others, carbon-based nanomaterials have become increasingly attractive for applications in neuroengineering because of their inherent mechanical, chemical, and electrical properties.28 Such properties have been observed to promote neuronal attachment/culture, neurite outgrowth, and electrical stimulation/recording, thus offering immense potential for enhanced neural interfaces.28 Our group has further explored the application of carbon-based nanomaterials to guide stem cell differentiation toward neuronal cell fates.
Given the significant role of patterning in controlling the differentiation process, we hypothesized that the fabrication of patterned geometries composed of such carbon-based nanomaterials may have a combined, synergistic impact on neuronal growth and differentiation. In one study, we utilized synergistic cues of ECM proteins and carbon nanotube (CNT) patterns to demonstrate polarization-controlled differentiation of fetal human NSCs (hNSCs).29 Laminin was selectively adsorbed on CNT micropatterns, wherein the laminin-coated CNTs were highly biocompatible and enabled the selective adhesion of hNSCs. Moreover, CNT patterns played a critical role in controlling the hNSC outgrowths during the growth and differentiation process as a result of cell−cell interactions and cytoskeletal tensions. Notably, the structural-polarization-controlled differentiation of individual hNSCs was achieved using CNT patterns comprising a square and a single line protruding from the square. After seeding, the hNSCs selectively adhered to the squares and grew along the lines protruding from the squares. The differentiated hNSCs extended their axons along the protruding line, allowing for controlled structural polarity on the CNT patterns.
In a recent study, we further extended this approach to another type of carbon-based nanomaterial known as graphene oxide (GO).30 As opposed to the tubular-shaped CNTs, GO is a single-atom-thick sheet that has a honeycomb carbon structure and is amphiphilic because of unique surface chemistries (i.e., −C−O−C−, −C−OH, −COOH).31 While previous studies explored stem cell culture/differentiation on bulk-coated GO surfaces, we utilized a microcontact printing approach to control the assembly of nanosized GO (NGO) into micropatterned geometries. Our methodology empowered us to generate combinatorial NGO patterns on multiple types of substrates, including gold-coated glass, cell culture dishes, and polymer substrates. Interestingly, the combinatorial NGO patterns of specific geometries permitted stem cell differentiation into specific cell lineages. In particular, we employed human adipose-derived mesenchymal stem cells (hADMSCs), which can differentiate into several lineages, including fat, bone, cartilage, and neural cells. Among the varying geometries that were utilized, hADMSCs adopted an elongated morphology on NGO line patterns, resulting in enhanced bone differentiation. In contrast, NGO grid patterns were observed to enhance hADMSC differentiation into neuronal cells (Figure 4a–c). The cells grown on the NGO grid patterns further showed large cellular extensions, indicative of neurite outgrowth (Figure 4d,e).
Figure 4.
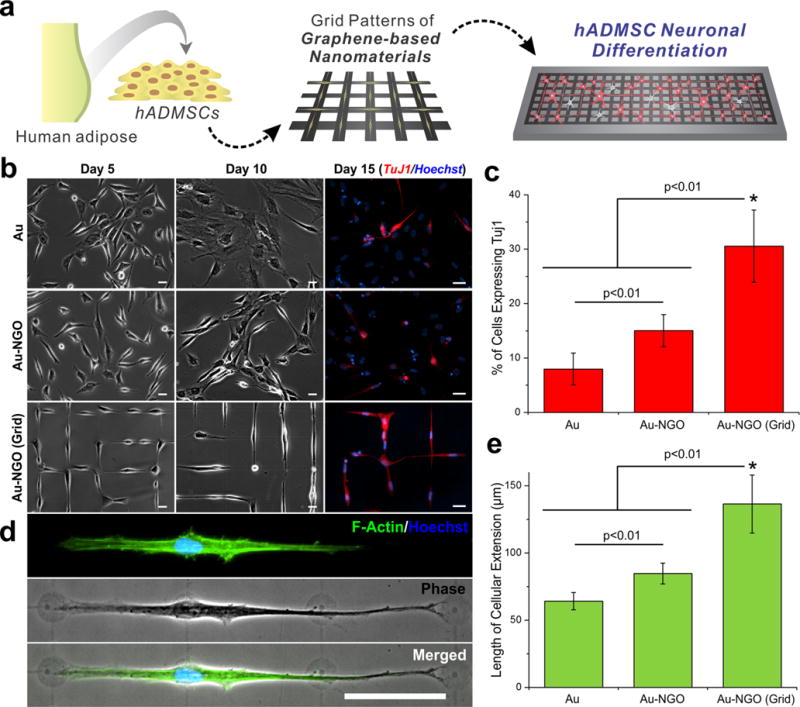
Patterned nanosized graphene oxide (NGO) induces neuronal differentiation of human adipose-derived mesenchymal stem cells (hADMSCs). (a) hADMSCs cultured on grid patterns of NGO facilitated differentiation into neuronal cells. (b) Images of hADMSCs grown on poly(L-lysine) (PLL)-coated Au (Au), NGO-coated Au (Au-NGO), and NGO grid-patterned substrates [Au-NGO (Grid)]. Scale bars: 20 μm. (c) Plot showing quantification of the percentage of cells expressing the neuronal marker TuJ1 (*, p < 0.01). (d) Images of cells stained for F-actin (green) and nucleus (blue) showing extensive cellular extension on NGO grid patterns. Scale bar: 50 μm. (e) Plot showing quantification of the length of cellular extension on various substrates (*, p < 0.01). Adapted from ref 30. Copyright 2015 American Chemical Society.
Remarkably, the degree of differentiation on the NGO-patterned substrates was significantly higher than on both ECM-patterned and NGO-coated surfaces, suggesting that the combination of NGO and micropatterns was essential for promoting neurogenesis. We speculate that the morphological confinement of the cell to geometric orientations found in native tissue combined with the unique cell−substrate interactions offered by carbon-based nanomaterials may facilitate such synergistic outcomes. It is important to note that while the differentiated cells exhibit markers indicative of neuronal fate, determining whether these MSC-derived cells are in fact functional neurons would require further studies. Nevertheless, exploring the precise mechanism of action in such processes is an active area of current research.
4. MODULATION OF CELL−ECM INTERACTIONS USING NANOSCAFFOLDS
While the physical size of stem cells is in the micrometer range and can be modulated using surface patterning approaches as described earlier, they are also greatly influenced by topographical features in the nanometer range, which are orders of magnitude smaller than the cell itself. In fact, cells in the natural in vivo environment interact with extracellular components at the nanoscale, such as transmembrane adhesion molecules (e.g., integrins) binding to adjacent substrates via cell−substrate interactions.32 When the extracellular microenvironment is probed with membrane projections (e.g., filopodia), feedback mechanisms are generated in response to the underlying nanotopographical cues. This topography-dependent feedback generally leads to changes in cytoskeletal dynamics and intracellular signaling pathways that ultimately influence the cellular behavior and phenotype.33
Various forms of nanotopography, such as ridges, grooves, pillars, pits, and fibers, have previously been created and observed to alter stem cell adhesion, proliferation, and differentiation.33 However, while the response of MSCs to these nanotopographical features has been extensively investigated, studies of the response of NSCs are relatively limited.34 Our group has primarily focused on exploring the role of nanotopography in modulating NSC behavior and differentiation. By presenting NSCs with a variety of different nanotopographies in the form of nanoparticles and nanofibers, we harnessed distinct capabilities of our nanoscaffolds, including enhanced delivery of genetic factors, directed alignment of neural extensions, and/or selective neural differentiation.
4.1. Nanoparticle Films
The synthetic control over the physical and chemical properties of nanoparticles makes them particularly useful for biological applications. While the unique features of nanoparticles have made them especially popular for applications in drug/gene delivery, the physical size of nanoparticles (10−1000 nm) alone makes them attractive candidates for establishing nanotopography on cell culture surfaces. Nanoparticles can be assembled on substrates using a variety of methods, including drop-casting, spin-coating, Langmuir−Blodgett film formation, or centrifugation. Thereafter, the films can be further functionalized with biomolecules or other nanomaterials, thus presenting a unique interfacial surface for stem cell cultures.
In one study, we developed a nanotopography-based platform to deliver genetic material (e.g., siRNA) into rNSCs directly from the underlying surface in a process termed reverse uptake/transfection.35 This type of delivery is in contrast to solution-mediated delivery, or forward transfection, in which the siRNA is complexed with cationic lipids or polymers and then added to the medium above the cultured cells. Remarkably, our nanotopography-mediated reverse uptake (NanoRU) platform enabled the delivery of siRNA molecules in a nontoxic and highly effective manner without the need for exogenous cationic materials. NanoRU was fabricated by assembling monolayers of positively charged silica nanoparticles (SiNPs) on a bare glass substrate, whereby the films were further coated with negatively charged siRNA (to target specific genes for knockdown) and laminin (to facilitate rNSC attachment) (Figure 5a). In addition to being biocompatible, our platform permitted excellent rNSC attachment and spreading (Figure 5b).
Figure 5.
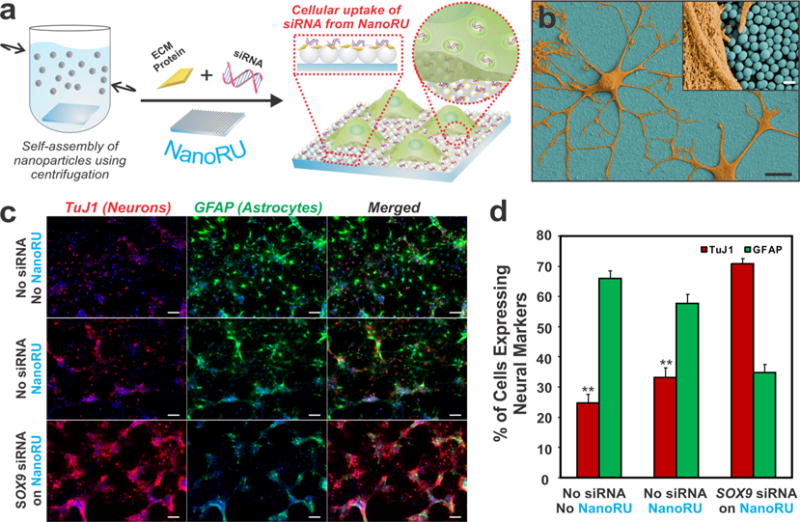
Nanotopographical features can mediate significant uptake of siRNA by rNSCs. (a) NanoRU facilitates cellular uptake of siRNA into rNSCs. (b) SEM image of rNSCs (orange) on NanoRU (blue). Scale bars: 10 μm and (inset) 500 nm. (c) Images stained for the neuronal marker TuJ1 (red) and the astrocytic marker GFAP (green) showing the extent of differentiation of rNSCs grown on bare glass with no NanoRU or siSOX9 coating (top), NanoRU without the siSOX9 coating (middle), and NanoRU with the siSOX9 coating (bottom). Scale bars: 50 μm. (d) Plot showing quantification of the percentage of cells expressing specific neural markers on various substrates (**, p < 0.001). Adapted with permission from ref 35. Copyright 2013 Nature Publishing Group.
In an initial test, we employed siRNA targeting GFP for knockdown and observed a size-dependent knockdown of GFP in the rNSCs, with 100 nm SiNPs showing the highest knockdown and 700 nm SiNPs showing the lowest knockdown. After further refinement, NanoRU was utilized to knock down the expression of SOX9 using siSOX9, which resulted in significantly enhanced neuronal differentiation of the rNSCs up to 70% (Figure 5c,d). Upon further investigation of the mechanism of the nanotopography-based delivery, we confirmed that NanoRU delivers only the siRNA (through caveolae-mediated endocytosis) in rNSCs and not the SiNPs. This attribute of NanoRU makes it very advantageous because extraneous delivery materials are not taken up by the rNSCs, hence minimizing the concerns associated with cytotoxicity. In this way, we uncovered a novel role of nanotopography in mediating the delivery of genetic material.
While differentiation into neuronal cells is vital, the alignment and specific guidance of neurons is one of the critical factors that can lead to enhanced therapeutic effects after nerve damage. In fact, when the nerve gaps resulting from injuries are too large, the distal and proximal ends of the damaged nerves are not able to communicate effectively, thus hindering the regeneration process. It is therefore extremely critical to provide an engineered microenvironment to precisely control the axonal alignment and growth of NSC-derived neurons for the development of highly effective treatments for nerve injuries. We discovered that simply coating GO on the SiNP films led to enhanced neuronal differentiation of hNSCs and the subsequent alignment of axons.36 We investigated the role of the underlying nanotopography and found that the SiNPs led to a remarkable increase in neuronal differentiation and an increase in the length of axons compared with the other controls. The graphene-hybrid structures were also biocompatible, supporting the growth and differentiation of hNSCs for over 3 weeks. Although we are further investigating the mechanism responsible for the axonal alignment, we believe that our results using nanoparticle films as nanotopographical cues can advance the field of neural tissue engineering.
4.2. Nanofiber Scaffolds
Nanofibers represent an enhanced three-dimensional (3D) cell culture platform relative to other types of nanotopographical surfaces. Synthetic nonwoven nanofiber scaffolds tend to mimic in vivo environments, with structural and mechanical similarity to native proteins (e.g., collagen, elastin), making them useful for translational tissue engineering applications.37 Researchers have developed a number of methodologies to synthesize nanofibers, including self-assembly of amphiphilic peptides, phase separation, and electrospinning.22 The electrospinning method, wherein insoluble nanofibers are generated from a viscous polymer solution by applying a high voltage difference, is especially robust and scalable.38 Electrospun nanofibers have been found to provide an ideal topography for fabricating nerve guidance conduits, directing neurite outgrowth/regeneration, and studying oligodendrocyte-mediated myelination.38
While the physical features of nanofiber scaffolds have made them valuable for neural engineering, we were interested in further modifying the nanofiber surface to create an enhanced scaffold for NSC culture. In this regard, graphene-based nanomaterials offer unique properties that are permissive for cell culture, including flexibility to coat irregular surfaces, high adsorption of proteins and cellular constituents, and facile surface modification to impart aqueous solubility.31 As described earlier, our group and others have observed remarkable advantages of using graphene-based nanomaterials such as GO for NSC culture. However, the majority of such work for stem cell culture has been performed on glass substrates, which has limited clinical relevance because of challenges in transplantation.
By combining the distinct properties of GO and nanofiber scaffolds, we designed a unique hybrid scaffold that we used to study rNSC behavior.39 Interestingly, rNSCs grown on these graphene−nanofiber hybrid scaffolds exhibited extensive branching of cell processes compared with nanofiber scaffolds alone, a feature that is characteristic of oligodendrocytes (Figure 6a).40 We systematically investigated this finding by varying the amount of GO coating on the nanofiber surface (Figure 6b). Upon assessing the gene expression of key markers indicative of neural differentiation, we observed a GO-concentration-dependent trend wherein coating with a higher concentration of GO was seen to promote differentiation in oligodendrocytes (Figure 6c). It is worth noting that there was a simultaneous decrease in astrocytic expression along with the upregulation of more than 10 oligodendrocyte markers, indicating the selective preference of rNSC differentiation toward oligodendrocytes on the hybrid scaffolds. While our preliminary studies indicate the possible role of the integrin signaling pathway in this process, determining the mechanism of action using such materials is an active area of research. Nevertheless, in view of the fact that differentiation inducers (e.g., growth factors, viral vectors) were not introduced into the culture medium, our results on selective guiding of stem cell differentiation merely by changing the properties of the underlying biomaterial scaffold are profound for advancing tissue engineering approaches.
Figure 6.
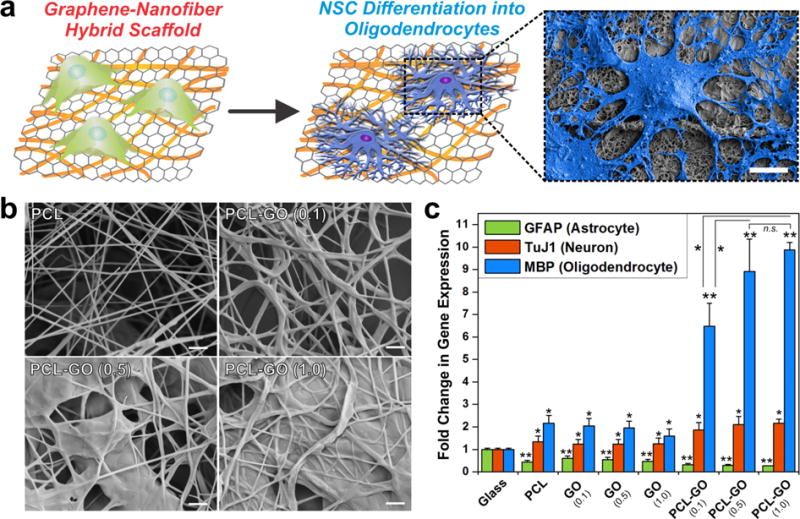
Graphene-coated polymeric nanofibers dramatically enhance oligodendroglial differentiation of rNSCs. (a) Polymeric nanofibers [composed of polycaprolactone (PCL)] coated with graphene oxide (GO) and seeded with rNSCs show enhanced differentiation into oligodendrocytes. The SEM image at the right shows the morphology of cells grown on graphene−nanofiber hybrid scaffolds. Scale bar: 10 μm. (b) SEM images of PCL nanofibers coated with GO solutions of varying concentrations: 0.0 mg/mL (PCL), 0.1 mg/mL [PCL-GO (0.1)], 0.5 mg/mL [PCL-GO (0.5)], and 1.0 mg/mL [PCL-GO (1.0)]. Scale bars: 1 μm. (c) Quantitative PCR of rNSCs grown on various substrates. The plot shows the fold change in gene expression of markers indicative of neurons (TuJ1), astrocytes (GFAP), and oligodendrocytes (MBP). The gene expression is relative to GAPDH and normalized to the conventional PLL-coated glass control (*, p < 0.05; **, p < 0.01; n.s., no significance). Adapted with permission from ref 39. Copyright 2014 Wiley.
5. CONCLUSIONS AND FUTURE OUTLOOK
In this Account, we have highlighted how nanotechnology-based approaches can be tailored to control stem-cell-based neural regeneration. By transitioning from the soluble micro-environment to 2D micropatterned surfaces to 3D nanotopographical scaffolds, our findings demonstrate the ability to intervene at multiple levels of scale to achieve remarkable control and functional outcomes. With the increasing interest in developing innovative technologies to advance the neuroscience frontier, nanoinspired tools and strategies are expected to play a pivotal role in this undertaking.
Although these approaches show great promise, there are numerous ongoing challenges. One of the biggest concerns with using new materials for biological applications is cytotoxicity. Since many of the materials and approaches described above are relatively recent findings (e.g., graphene), there are limited studies outlining detailed analyses of long-term viability, especially using in vivo models. Depending on the size, composition, concentration, and surface functionalization, different materials can pose diverse implications at the cellular, tissue, and systems level.
Another area of focus is finding the right balance of microenvironmental factors to acquire pure populations of differentiated cells. The lack of complete control over the differentiation process tends to result in heterogeneous cell populations, which requires sorting and can limit application at large scales. To this end, determining very specific features of the microenvironment that can reliably guide well-defined stem cell differentiation would be advantageous. For instance, is there a universal microenvironmental feature that can promote NSC differentiation into specialized subtype-specific neurons (e.g., dopaminergic) with high efficiency? Can physical cues be used to replace, or at least complement, the conventional use of soluble cues and gene vectors?
Lastly, the development of platforms that better mimic the native 3D microenvironment of the CNS is formidable. The capability to fabricate such systems in the lab (e.g., organ-on-a-chip) would permit more accurate models for in vitro testing. At the same time, it would enable the development of improved nanointerfaces to integrate with neural systems and modulate neural activity.41 Overall, we anticipate that the nanotechnology-based toolkit will prompt innovative solutions for the complex problems in neuroscience.
Acknowledgments
We acknowledge financial support from the National Institute of Health (NIH) Director’s New Innovator Award (1DP20D006462-01), the National Institute of Neurological Disorders and Stroke (NINDS) (1R21NS085569-02), the New Jersey Commission on Spinal Cord Research (Grant CSR13ERG005), and the Rutgers IAMDN. S. Shah also acknowledges NSF DGE 0801620, Integrative Graduate Education and Research Traineeship (IGERT) on the Integrated Science and Engineering of Stem Cells.
Biographies
Shreyas Shah received his Ph.D. in Chemistry and Chemical Biology from Rutgers University (New Brunswick, NJ). His doctoral research focused on developing nanomaterial-based 2D/3D scaffolds for neural regeneration, neural drug delivery, and neuromodulation. He is currently building a new lab/program in Physiological Communications at Bell Labs located in Murray Hill, NJ.
Aniruddh Solanki received his Ph.D. in Chemistry and Chemical Biology from Rutgers University (New Brunswick, NJ). He is currently pursuing his postdoctoral research at Brigham and Women’s Hospital and the Harvard Medical School.
Ki-Bum Lee is an Associate Professor of Chemistry and Chemical Biology at Rutgers University (New Brunswick, NJ). His research interest is to develop and integrate nanotechnologies and chemical functional genomics to modulate signaling pathways in cells toward specific cell lineages or behaviors.
Footnotes
Notes
The authors declare no competing financial interest.
References
- 1.Lindvall O, Kokaia Z. Stem cells for the treatment of neurological disorders. Nature. 2006;441:1094–1096. doi: 10.1038/nature04960. [DOI] [PubMed] [Google Scholar]
- 2.Aboody K, Capela A, Niazi N, Stern JH, Temple S. Translating Stem Cell Studies to the Clinic for CNS Repair: Current State of the Art and the Need for a Rosetta Stone. Neuron. 2011;70:597–613. doi: 10.1016/j.neuron.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Martino G, Pluchino S. The therapeutic potential of neural stem cells. Nat Rev Neurosci. 2006;7:395–406. doi: 10.1038/nrn1908. [DOI] [PubMed] [Google Scholar]
- 4.Trueman RC, Klein A, Lindgren HS, Lelos MJ, Dunnett SB. Repair of the CNS using endogenous and transplanted neural stem cells. Curr. Top Behav Neurosci. 2012;15:357–398. doi: 10.1007/7854_2012_223. [DOI] [PubMed] [Google Scholar]
- 5.Kirkeby A, Grealish S, Wolf DA, Nelander J, Wood J, Lundblad M, Lindvall O, Parmar M. Generation of Regionally Specified Neural Progenitors and Functional Neurons from Human Embryonic Stem Cells under Defined Conditions. Cell Rep. 2012;1:703–714. doi: 10.1016/j.celrep.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Kriks S, Shim J-W, Piao J, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A, Yang L, Beal MF, Surmeier DJ, Kordower JH, Tabar V, Studer L. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature. 2011;480:547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang N, Zuchero JB, Ahlenius H, Marro S, Ng YH, Vierbuchen T, Hawkins JS, Geissler R, Barres BA, Wernig M. Generation of oligodendroglial cells by direct lineage conversion. Nat Biotechnol. 2013;31:434–439. doi: 10.1038/nbt.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lyssiotis CA, Lairson LL, Boitano AE, Wurdak H, Zhu S, Schultz PG. Chemical control of stem cell fate and developmental potential. Angew Chem, Int Ed. 2011;50:200–242. doi: 10.1002/anie.201004284. [DOI] [PubMed] [Google Scholar]
- 9.Xu Y, Shi Y, Ding S. A chemical approach to stem-cell biology and regenerative medicine. Nature. 2008;453:338–344. doi: 10.1038/nature07042. [DOI] [PubMed] [Google Scholar]
- 10.Wilson RC, Doudna JA. Molecular Mechanisms of RNA Interference. Annu Rev Biophys. 2013;42:217–239. doi: 10.1146/annurev-biophys-083012-130404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jorgensen WL, Duffy EM. Prediction of drug solubility from structure. Adv Drug Delivery Rev. 2002;54:355–366. doi: 10.1016/s0169-409x(02)00008-x. [DOI] [PubMed] [Google Scholar]
- 12.Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discovery. 2009;8:129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xue X, Wang F, Liu X. Emerging functional nanomaterials for therapeutics. J Mater Chem. 2011;21:13107–13127. [Google Scholar]
- 14.Shah B, Yin PT, Ghoshal S, Lee KB. Multimodal magnetic core-shell nanoparticles for effective stem-cell differentiation and imaging. Angew Chem, Int Ed. 2013;52:6190–6195. doi: 10.1002/anie.201302245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitragotri S, Burke PA, Langer R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat Rev Drug Discovery. 2014;13:655–672. doi: 10.1038/nrd4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chauhan VP, Jain RK. Strategies for advancing cancer nanomedicine. Nat Mater. 2013;12:958–962. doi: 10.1038/nmat3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah S, Solanki A, Sasmal PK, Lee K-B. Single vehicular delivery of siRNA and small molecules to control stem cell differentiation. J Am Chem Soc. 2013;135:15682–15685. doi: 10.1021/ja4071738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim C, Shah BP, Subramaniam P, Lee K-B. Synergistic Induction of Apoptosis in Brain Cancer Cells by Targeted Codelivery of siRNA and Anticancer Drugs. Mol Pharmaceutics. 2011;8:1955–1961. doi: 10.1021/mp100460h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brewster ME, Loftsson T. Cyclodextrins as pharmaceutical solubilizers. Adv Drug Delivery Rev. 2007;59:645–666. doi: 10.1016/j.addr.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 20.Stolt CC, Lommes P, Sock E, Chaboissier M-C, Schedl A, Wegner M. The Sox9 transcription factor determines glial fate choice in the developing spinal cord. Genes Dev. 2003;17:1677–1689. doi: 10.1101/gad.259003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy WL, McDevitt TC, Engler AJ. Materials as stem cell regulators. Nat Mater. 2014;13:547–557. doi: 10.1038/nmat3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higuchi A, Ling QD, Chang Y, Hsu ST, Umezawa A. Physical Cues of Biomaterials Guide Stem Cell Differentiation Fate. Chem Rev. 2013;113:3297–3328. doi: 10.1021/cr300426x. [DOI] [PubMed] [Google Scholar]
- 23.Gattazzo F, Urciuolo A, Bonaldo P. Extracellular matrix: a dynamic microenvironment for stem cell niche. Biochim Biophys Acta, Gen Subj. 2014;1840:2506–2519. doi: 10.1016/j.bbagen.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solanki A, Shah S, Memoli KA, Park SY, Hong S, Lee KB. Controlling differentiation of neural stem cells using extracellular matrix protein patterns. Small. 2010;6:2509–2513. doi: 10.1002/smll.201001341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 26.Wen JH, Vincent LG, Fuhrmann A, Choi YS, Hribar KC, Taylor-Weiner H, Chen S, Engler AJ. Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nat Mater. 2014;13:979–987. doi: 10.1038/nmat4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Downing TL, Soto J, Morez C, Houssin T, Fritz A, Yuan F, Chu J, Patel S, Schaffer DV, Li S. Biophysical regulation of epigenetic state and cell reprogramming. Nat Mater. 2013;12:1154–1162. doi: 10.1038/nmat3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monaco AM, Giugliano M. Carbon-based smart nanomaterials in biomedicine and neuroengineering. Beilstein J Nanotechnol. 2014;5:1849–1863. doi: 10.3762/bjnano.5.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park SY, Choi DS, Jin HJ, Park J, Byun KE, Lee KB, Hong S. Polarization-Controlled Differentiation of Human Neural Stem Cells Using Synergistic Cues from the Patterns of Carbon Nanotube Monolayer Coating. ACS Nano. 2011;5:4704–4711. doi: 10.1021/nn2006128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim T-H, Shah S, Yang L, Yin PT, Hossain MK, Conley B, Choi J-W, Lee K-B. Controlling Differentiation of Adipose-Derived Stem Cells Using Combinatorial Graphene Hybrid-Pattern Arrays. ACS Nano. 2015;9:3780–3790. doi: 10.1021/nn5066028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yin PT, Shah S, Chhowalla M, Lee K-B. Design, Synthesis, and Characterization of Graphene−Nanoparticle Hybrid Materials for Bioapplications. Chem Rev. 2015;115:2483–2531. doi: 10.1021/cr500537t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brizzi MF, Tarone G, Defilippi P. Extracellular matrix, integrins, and growth factors as tailors of the stem cell niche. Curr Opin Cell Biol. 2012;24:645–651. doi: 10.1016/j.ceb.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Dalby MJ, Gadegaard N, Oreffo ROC. Harnessing nanotopography and integrin-matrix interactions to influence stem cell fate. Nat Mater. 2014;13:558–569. doi: 10.1038/nmat3980. [DOI] [PubMed] [Google Scholar]
- 34.Tsimbouri P, Gadegaard N, Burgess K, White K, Reynolds P, Herzyk P, Oreffo R, Dalby MJ. Nanotopographical effects on mesenchymal stem cell morphology and phenotype. J Cell Biochem. 2014;115:380–390. doi: 10.1002/jcb.24673. [DOI] [PubMed] [Google Scholar]
- 35.Solanki A, Shah S, Yin PT, Lee K-B. Nanotopography-mediated reverse uptake for siRNA delivery into neural stem cells to enhance neuronal differentiation. Sci Rep. 2013;3:1553. doi: 10.1038/srep01553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Solanki A, Chueng S-TD, Yin PT, Kappera R, Chhowalla M, Lee K-B. Axonal alignment and enhanced neuronal differentiation of neural stem cells on graphene-nanoparticle hybrid structures. Adv Mater. 2013;25:5477–5482. doi: 10.1002/adma.201302219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X, Ding B, Li B. Biomimetic electrospun nanofibrous structures for tissue engineering. Mater Today. 2013;16:229–241. doi: 10.1016/j.mattod.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xie J, MacEwan MR, Schwartz AG, Xia Y. Electrospun nanofibers for neural tissue engineering. Nanoscale. 2010;2:35–44. doi: 10.1039/b9nr00243j. [DOI] [PubMed] [Google Scholar]
- 39.Shah S, Yin PT, Uehara TM, Chueng S-TD, Yang L, Lee K-B. Guiding Stem Cell Differentiation into Oligodendrocytes Using Graphene-Nanofiber Hybrid Scaffolds. Adv Mater. 2014;26:3673–3680. doi: 10.1002/adma.201400523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001;81:871–927. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- 41.Shah S, Liu J-J, Pasquale N, Lai J, McGowan H, Pang ZP, Lee K-B. Hybrid upconversion nanomaterials for optogenetic neuronal control. Nanoscale. 2015;7:16571–16577. doi: 10.1039/c5nr03411f. [DOI] [PMC free article] [PubMed] [Google Scholar]


