Abstract
Purpose
To examine the impact of a nurse-initiated tobacco cessation intervention focused on providing guideline-recommended care to hospitalized smokers.
Design
Pre-post quasi-experimental trial.
Setting
General medical units of four US Department of Veterans Affairs hospitals.
Subjects
898 adult Veteran smokers (503 and 395 were enrolled in the baseline and intervention periods, respectively).
Intervention
The intervention included academic detailing, adaptation of the computerized medical record, patient self-management support, and organizational support and feedback.
Measures
The primary outcome was self-reported 7-day point prevalence abstinence at six months.
Analysis
Tobacco use was compared for the pre-intervention and intervention periods with multivariable logistic regression using generalized estimating equations to account for clustering at the nurse level. Predictors of abstinence at six months were investigated with best subsets regression.
Results
Seven-day point prevalence abstinence during the intervention period did not differ significantly from the pre-intervention period at either three (adjusted odds ratio (AOR) and 95% confidence interval (CI95) = 0.78 [0.51–1.18]) or six months (AOR = 0.92; CI95 = 0.62–1.37). Predictors of abstinence included baseline self-efficacy for refraining from smoking when experiencing negative affect (p = 0.0004) and perceived likelihood of staying off cigarettes following discharge (p < 0.0001).
Conclusions
Tobacco use interventions in the VA inpatient setting likely require more substantial changes in clinician behavior and enhanced post-discharge follow-up to improve cessation outcomes.
Keywords: Smoking cessation, Tobacco use, Veterans, Hospitalization, Clinical practice guidelines
1. Introduction
1.1. Cigarette smoking in Veterans of military service
Cigarette smoking remains the leading preventable cause of morbidity and mortality in our society (Centers for Disease Control and Prevention [CDC], 2012). Although overall tobacco use has declined substantially over the past 50 years, the prevalence of smoking remains elevated in Veterans of the armed forces (Brown, 2010; Hoerster et al., 2012; Kramarow & Pastor, 2012). The excess burden attributable to tobacco use in this group is substantial, with health care costs to the Department of Veterans Affairs (VA) for tobacco-related medical care totaling several billion dollars annually (Barnett, Hamlett-Berry, Sung, & Max, 2015). Given that smokers are more likely to be hospitalized than non-smokers (Hanlon et al., 2007; Wilkins, Shields, & Rotermann, 2009), providing effective tobacco treatment to Veterans in the hospital setting is an important priority.
1.2. Treatment for cigarette smoking in the hospital setting
Compared to most primary care visits, the inpatient stay often provides an extended opportunity for clinicians to address smoking cessation (Kisuule, Necochea, Howe, & Wright, 2010; Rigotti, Clair, Munafò, & Stead, 2012). Smoke-free hospital policies allow smokers to refrain from smoking while they are physically removed from environmental triggers for tobacco use (Duffy, Reeves, Hermann, Karvonen, & Smith, 2008; Rigotti et al., 2012). Because of heightened health concerns brought about by acute illness and hospitalization, smokers also may feel increased vulnerability and may be especially receptive to smoking cessation advice (Grossman et al., 2012; Rigotti et al., 2012). Despite evidence that many hospitalized patients are interested in stopping smoking (Duffy et al., 2008; Katz, Goldberg, Smith, & Trick, 2008; Shah et al., 2010), the majority receive minimal or no assistance with quitting during their stay (Brown et al., 2004; Duffy et al., 2008).
Although the specific counseling components associated with the highest cessation rates for hospitalized smokers have not been adequately evaluated, available evidence does suggest that interventions involving a dedicated tobacco cessation specialist are associated with better treatment outcomes (France, Glasgow, & Marcus, 2001). Treatments that include more intensive inpatient counseling and sustained relapse prevention training following discharge also improve quit rates (France et al., 2001). In a systematic review of inpatient smoking cessation interventions, Rigotti et al. (2012) concluded that inpatient counseling combined with outpatient treatment lasting ≥1 month is effective for hospitalized smokers. Provision of nicotine replacement therapy (NRT) is also associated with improved cessation rates (France et al., 2001; Rigotti et al., 2012). Greater evidence is needed to determine the extent to which these findings translate into routine VA clinical practice, however, as most prior studies relied on research nurses or smoking cessation specialists rather than existing clinical staff to deliver the intervention (Rigotti, Munafo, & Stead, 2008). Furthermore, many hospitals lack the resources that would enable them to provide ongoing treatment support following discharge, potentially limiting the feasibility of this approach. Whether a brief intervention based on the Clinical Practice Guideline combined with ongoing counseling and support can improve long-term cessation rates remains to be determined (France et al., 2001).
1.3. Present study
We recently reported the impact of an enhanced academic detailing intervention involving face-to-face educational outreach regarding evidence-based tobacco cessation intervention strategies (Fiore et al., 2008), performance feedback, and the use of peer champions on nurses' delivery of guideline-recommended actions (based on the 5A's model) in four Veterans Administration hospitals (Katz, Holman, Johnson, et al., 2013; Katz et al., 2014). The primary aim of the present study was to determine the effectiveness of this nurse-initiated intervention with regard to cessation outcomes in hospitalized smokers.
2. Methods
2.1. Study design
Details regarding the study methodology and intervention have been reported elsewhere (Katz, Holman, Johnson, et al., 2013; Katz et al., 2014; Katz et al., 2009). Briefly, the study utilized a multi-site, prepost quasi-experimental design. Although a cluster randomized design in which hospitals are randomly assigned to intervention conditions would have arguably provided the highest degree of causal inference (without the risk of contamination inherent in studies involving randomization at the patient or nurse level), this design was not feasible due to budgetary constraints. During the pre-intervention period, inpatient nurses and physicians performed their usual duties without specific training in smoking cessation or use of the practice guideline. At the outset of the intervention period, study personnel trained unit nurses and physicians on how to implement the guideline. Each period lasted approximately eight months on average.
2.2. Participants
Participants included adult daily smokers (1+ cigarettes/day) aged ≥18 years admitted to a general medicine inpatient unit at one of four VA hospitals located in four states in the upper Midwest and Rocky Mountain regions (Denver, CO; Iowa City, IA; Minneapolis, MN; Omaha, NE). Sites included a total of nine medicine units (one to three per hospital) and averaged 2700 to 4000 general medicine admissions per year (additional information about participating hospitals provided in the Appendix A). Patients meeting the following criteria were excluded: hospitalized for <18 h, acute medical decompensation, altered mental status, unstable psychiatric disorder, dementia, communication barrier, pregnancy, terminal illness, and inability to be contacted by telephone. Research assistants (RAs) screened all admissions each weekday and on one weekend day. If preliminary eligibility criteria were met, the RA approached the patient to verify screening information, obtained informed consent, and administered the baseline interview. Enrollment occurred from 5/2009 through 12/2012. Informed consent was obtained from all participants in this study.
2.3. Intervention
The approach to guideline implementation was based on the Chronic Care Model (Wagner, Austin, & Von Korff, 1996) and incorporated several components that have been used successfully in prior interventions: enhanced academic detailing of staff nurses, adaptation of the electronic medical record, patient self-management, and organizational support and feedback. Each component is described below.
2.3.1. Enhanced academic detailing with staff nurses
Enhanced academic detailing (Sheffer et al., 2012) consisted of face-to-face training of inpatient registered nurses, performance feedback, and periodic check-ins with nurse managers and peer leaders, and was used to promote the 5A's framework. Personalized, on-site instruction was delivered to one or two unit nurses at a time during their assigned shift by a physician, nurse, or health psychologist on the research team. Training sessions lasted approximately 30 min. Nurses were also encouraged to complete a 30 minute online tutorial. In an effort to reach all nurses, training sessions were conducted during all shifts throughout the day and night. During this training, nurses were oriented to principles of stage-based cessation counseling and motivational interviewing (for example, “rolling with resistance”) (Rollnick, Mason, & Butler, 2000) and the use of NRT. To help ensure that treatment would be delivered even to patients hospitalized for a short period of time, nurses were instructed to deliver the intervention at the time of admission (or as soon as possible after the patient's acute medical condition had been stabilized). They were also encouraged, at their discretion, to reinforce the intervention content at subsequent contacts during the patient's stay. To increase practicability, nurses were taught how to deliver the 5A's in a succinct manner lasting <5 min. Although brief, similar interventions (based on the 5A's) as short as 3 min in duration have been associated with increased smoking cessation in primary care settings (Fiore et al., 2008). Further, the brief intervention was designed to be combined with more intensive post-discharge counseling through referral to the tobacco quitline for those interested in quitting smoking (see below). They were also taught how to use intervention tools that were adapted for the electronic medical record. These included a template that facilitated documentation of tobacco use assessment and counseling as well as links to treatment resources. Research team members also periodically checked in with nursing staff on the units to offer support and answer any questions they might have related to the intervention. Sites were provided with group feedback during training and again at the beginning, middle, and end of the intervention period to reinforce improvements in their performance of the 5A's as well as to draw their attention to any ongoing deficiencies in treatment delivery.
2.3.2. Adaptation of the computerized information system
The nursing admission assessment in the electronic medical record was modified to include questions about tobacco use. It also included cues prompting nurses to complete the 5A's along with links to patient education materials and quitline referral forms. Finally, to facilitate prescribing of smoking cessation medications by ward physicians, computerized “quick orders” containing prefilled information regarding dosage, duration of use, and patient instructions were provided.
2.3.3. Patient self-management support
Self-help resources (smoking cessation brochure and motivational video), brief bedside counseling, pharmacotherapy, and proactive telephone counseling via a tobacco quitline (National Jewish Hospital, Denver, Colorado) were all offered to support patients' efforts to quit smoking. For patients who agreed to quitline counseling, nurses completed a referral form. These forms were later sent via fax to the tobacco quitline prior to patients' discharge from the hospital by a member of the research team. In an effort to reduce the likelihood of passive disenrollment after initial contact, we arranged a more aggressive call schedule compared to the quitline's usual follow-up protocol (involving up to eight follow-up telephone contacts for this study).
2.3.4. Organizational support and feedback
The purpose of the study and intervention components were explained to nursing leadership and union representatives from each hospital. A peer leader from each unit was identified to facilitate communication between the research team and nursing staff. Peer leaders also assisted their colleagues with patient counseling strategies and helped with intervention implementation and problem solving. Peer leaders at three sites also received additional training in brief cessation counseling (including role play and feedback from a standardized patient).
To assess delivery of the 5A's by nurses and physicians, participants were interviewed in person just prior to hospital discharge or via telephone within 48 h of discharge. As previously reported by Katz et al. (2014), results revealed significant improvements in nurse delivery of most of the recommended components of brief cessation counseling during the intervention period, compared to the pre-intervention period. Specifically, improvements were observed for: asking about smoking status (93% vs. 84%), assessing willingness to quit (66% vs. 56%), assisting with quitting (75% vs. 56%), and arranging follow-up (23% vs. 18%), respectively. Advice to quit, however, did not change significantly across study periods (55% vs. 49%). Examination of hospital pharmacy data revealed no significant differences in prescriptions for bupropion, varenicline or NRT across study periods (38% vs. 35%). Finally, although patients were more likely to be offered a quitline referral during the intervention period (12% vs. 6%), rates of referral (9% vs. 0%) and enrollment in quitline counseling remained low (Katz et al., 2014).
2.4. Follow-up
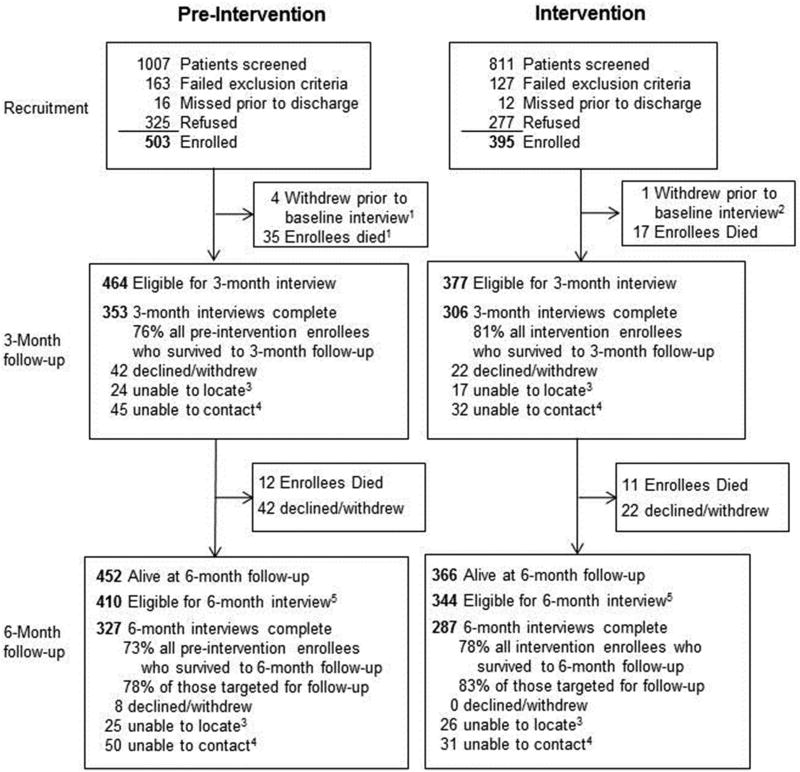
Three- and six-month follow-up phone interviews were conducted between 8/2009 and 6/2013 by an independent survey research organization (Iowa State University Survey and Behavioral Research Services, Ames, IA) with personnel who were blinded to treatment period. The telephone interviewers attempted to reach all enrolled patients at follow-up, except those who had died or had previously withdrawn from the study. Starting in 8/2011, a $10 incentive was offered upon completion of each follow-up assessment in an effort to enhance retention. Three-month follow-up rates were 76% and 81% for patients enrolled during the pre-intervention and intervention periods, respectively; corresponding rates for the six-month follow- up were 73% and 78%, respectively. Reasons for attrition (when available) for each period and follow-up interval are presented in Fig. 1. A sizeable number of participants died prior to three- (n = 52) and six- (n = 23) month follow-up, reflecting the compromised health status of study patients.
Fig. 1.
Study enrollment and follow-up.
2.5. Measures
2.5.1. Smoking cessation outcomes
The primary study outcome was self-reported 7-day point prevalence abstinence (PPA) at six-month follow-up. To qualify as abstinent, participants had to report no cigarette use within the past seven days. Secondary cessation outcomes included self-reported abstinence at three-months, 30-day point prevalence abstinence, and repeated PPA, which reflected whether participants were abstinent at both three and six months.
Participants who reported 7-day PPA at six months were asked to provide a saliva sample via a mailed collection kit for purposes of biochemical verification. Participants were also asked to complete a survey assessing tobacco and NRT use in the seven days preceding data collection. A $20 incentive was provided (not contingent upon results) to encourage return of the saliva sample. The samples were shipped to J2 Laboratories (Tucson, AZ) for analysis of cotinine (a metabolite of nicotine) (Society for Research on Nicotine & Tobacco [SRNT] Subcommittee on Biochemical Verification, 2002). A cut-off of <20 ng/ml was used to confirm abstinence from tobacco.
2.5.2. Secondary and exploratory outcomes
We assessed self-reported quit attempts lasting ≥24 h and daily cigarette consumption among those who were still smoking at three and six months. Readiness to quit smoking was measured using the Contemplation Ladder (Biener & Abrams, 1991), which consists of a single-item that asks participants to indicate their current readiness to quit smoking on a scale ranging from 0 (No thoughts of quitting) to 10 (Taking action to quit, such as cutting down or enrolling in a program). The Contemplation Ladder has demonstrated good predictive validity with regard to smoking cessation (Abrams, Herzog, Emmons, & Linnan, 2000). Self-efficacy related to smoking cessation was assessed using a revised 12-item version of the Smoking Self-Efficacy/Temptations Questionnaire (SSEQ) (Etter, Bergman, Humair, & Perneger, 2000). The SSEQ asks participants to indicate their confidence in their ability to refrain from smoking in various situations reflecting negative affect (6 items) and environmental cues (6 items) on a scale ranging from 1 (Not at all sure) to 5 (Absolutely sure). It has been found to have good internal consistency, test-retest reliability, and both construct and predictive validity (Etter et al., 2000).
2.5.3. Descriptive variables
Variables included for descriptive purposes and as model covariates included: 1) sociodemographics, 2) clinical characteristics including reasons for admission, symptoms of anxiety and depression (measured with the Hospital Anxiety and Depression Scale [HADS]; Zigmond & Snaith, 1983), self-reported alcohol use, and self-rated health; and 3) tobacco-related characteristics, including number of cigarettes smoked per day, nicotine dependence (assessed using the Fagerström Test for Nicotine Dependence [FTND] and the Heaviness of Smoking Index [HSI]; Heatherton, Kozlowski, Frecker, & Fagerström, 1991), nicotine withdrawal symptoms (using the Minnesota Nicotine Withdrawal Scale-Revised; Hughes, 2012; Hughes & Hatsukami, 1986), whether participants believed that they had a smoking-related medical condition, presence of a documented smoking-related illness, extent to which participants believed that quitting smoking would improve their health, self-reported abstinence from cigarettes during hospitalization, and perceived likelihood of staying off of cigarettes after hospital discharge.
2.6. Sample size calculations
Based on prior studies of smoking cessation among general medicine inpatients, we estimated a 7-day point prevalence abstinence rate of 12% at six-months during the pre-intervention phase (Katz et al., 2009). Using a target sample size of 500 participants in each phase (N =1000 total), we would have 87% power to detect a 7% absolute difference in 7-day point prevalence abstinence rates at six months (19% vs. 12%; risk ratio=1.58; odds ratio=1.72), which we considered a clinically meaningful difference.
2.7. Data analysis
2.7.1. Overview of data analytic approach and primary outcomes analysis
Differences in participant characteristics by study period were examined using independent samples t-tests, Wilcoxon rank-sum tests, and chi-square tests as appropriate. Self-reported tobacco use at three and six months was compared for the pre-intervention and intervention periods using multivariable logistic regression analyses. Analyses were conducted using generalized estimating equations (GEE) to account for clustering at the level of the admitting nurse. Covariates were chosen for inclusion in multivariable models based on two factors: 1) known associations with smoking outcomes, and 2) significant differences across study periods. We also estimated the impact of misreporting of smoking status by multiplying 7-day PPA at six months by biochemical confirmation rates.
2.7.2. Secondary analyses
In secondary analyses, any self-reported 24-hour quit attempt was modeled as a dichotomous outcome (1 + vs. none) using the same covariates described above for the primary outcome analyses. Continuous outcomes (daily cigarette consumption, readiness to quit smoking, and self-efficacy) were compared across study period using independent-samples t-tests. Self-efficacy was represented by two continuous variables based on subscale scores from the SSEQ reflecting self-efficacy in refraining from smoking in response to negative affect and environmental cues. Exploratory analyses were conducted to examine differences in intervention outcomes according to selected patient characteristics previously demonstrated to be related to quitting smoking. Specifically, interaction terms between study period and baseline smoking rate (<10 vs. 10+ cigarettes/day), depressive symptoms (HADS-D scores <8 vs. 8+), and anxiety (HADS-A scores <8 vs. 8+) were investigated using logistic regression.
2.7.3. Approaches to dealing with missing outcome data
For the primary outcome analyses, we computed the proportion of participants who were abstinent from smoking using the total number of subjects enrolled during each study period in the denominator; those with missing outcome data were assumed to be smoking (i.e., penalized imputation). Those who were deceased by the time of a given follow-up were excluded from the analysis (and from the denominator in calculating cessation rates) for that time point. Considering that a penalized imputation approach has the potential to produce biased estimates of treatment effect (Hedeker, Mermelstein, & Demirtas, 2007; Nelson, Partin, Fu, Joseph, & An, 2009), we also conducted sensitivity analyses using alternative strategies for handling missing outcome data (Katz, Holman, Nugent, et al., 2013; Rigotti et al., 2014). These included complete case analyses in which those participants with missing outcome data were excluded as well as multiple imputation (MI) of missing values. MI assumes that whether or not an outcome is missing does not depend on the value of that variable after controlling for observed variable values (i.e., outcomes are assumed to be missing at random). For the MI analyses, five data sets were generated using the chained equations method (White, Royston, & Wood, 2011). Parameter estimates were derived using the mi command in STATA, Version 12 (STATA Corp., College Station, TX).
2.7.4. Exploratory analyses of predictors of abstinence at six months
To identify baseline predictors of 7-day PPA at six-months, we used best subsets logistic regression (Hosmer, Jvanovic, & Lemeshow, 1989) based on the 586 participants with available data on both cessation outcomes and candidate predictors. The following five variables were initially forced into the model based on established associations in the literature with tobacco use and cessation: age (<50 years, 50–60 years, >60 years), education (≤12 years, 12 years, >12 years), nicotine dependence (HSI score modeled as continuous variable), depressive symptoms (<8 vs. ≥8), and smoking while in the hospital (yes vs. no) (Step 1). For the purpose of this analysis, the HSI was used rather than the FTND in order to minimize the number of participants who were dropped from the multivariable model on account of missing data. Collinearity was assessed by calculating correlations between individual candidate variables and examining variance inflation factors. Best subsets regression was subsequently used to determine the optimal combination of the remaining candidate variables after accounting for those entered in Step 1. Additional variables considered for inclusion in the model were: self-efficacy for resisting urges to smoke in response to environmental cues (modeled as a continuous variable), number of prior quit attempts lasting ≥24 h (0, 1, 2–5, >5), Contemplation Ladder score (0–4, 5–7, ≥8), whether or not the participant believed he or she had a smoking-related medical problem (yes vs. no), and the extent to which the participant believed that quitting smoking would improve his/her health (not at all, a little bit, somewhat, quite a bit, extremely so). The Bayesian Information Criterion (BIC) was used to identify the best fitting model from among all possible combinations of the candidate variables.
2.7.5. Exploratory analyses of associations between treatment delivery and cessation
Finally, in an effort to determine whether treatment delivery was associated with cessation outcomes, we examined relationships between patient-reported receipt of recommended counseling components and 7-day PPA at six months using GEE. A composite score representing the sum of each of the treatment components was modeled as both a continuous (range = 0 to 9) and categorical (0–3, 4–6, 7–9) variable. Each of the individual treatment components was also modeled separately to determine their relationship to six-month quit rates. Covariates were the same as those included in the primary outcomes analysis, with the exception of self-efficacy for staying quit following discharge from the hospital and self-efficacy for remaining abstinent when experiencing negative affect. Because these variables may partially reflect the impact of the intervention, they were not included in the model as covariates.
3. Results
3.1. Participant characteristics and follow-up
Participant enrollment and follow-up is presented in Fig. 1. A total of 503 and 395 participants were enrolled during the pre-intervention and intervention periods (61% and 59% of eligible patients, respectively). Participants averaged 59.1 (SD = 9.9) years of age and were predominantly male (96%) and white/non-Hispanic (87%). Median (IQR) cigarette consumption was 15 (10–20) cigarettes/day. Scores on the FTND (median [IQR] = 4 [3–6]) suggested moderate levels of addiction for the sample as a whole. Mean (SD) scores on the Contemplation Ladder were 6.8 (3.0), indicating that, on average, participants were thinking about quitting but were not quite ready to do so. Several statistically significant differences in participant characteristics were noted across study periods (Table 1). Specifically, participants enrolled in the pre-intervention period reported greater depressive symptoms, poorer health, greater alcohol use, more prior quit attempts, and greater self-efficacy for quitting smoking in the face of environmental factors.
Table 1.
Participant characteristics.
| Characteristic | Pre-intervention (N = 498)c |
Intervention (N = 394)d |
p value |
|---|---|---|---|
| Sociodemographics | |||
| Age, mean (SD) | 59.4 (9.7) | 58.8(10.2) | 0.42 |
| Gender, % male | 96 | 97 | 0.39 |
| Race, % nonwhite | 14 | 10 | 0.10 |
| Marital status (% married or living with companion) | 39 | 36 | 0.11 |
| Highest grade, median (IQR) | 13.1 (12.0–14.0) | 13.1 (12.0–14.0) | 0.72 |
| Clinical characteristics and self-rated health | |||
| Admission diagnosis, % | 0.55 | ||
| Cardiovascular system | 28 | 28 | |
| Respiratory system | 16 | 12 | |
| Digestive system | 12 | 14 | |
| Endocrine and metabolic diseases | 5 | 3 | |
| Hematologic and oncologic diseases | 6 | 5 | |
| Neurologic and psychiatric disorders | 4 | 4 | |
| Miscellaneous | 31 | 34 | |
| HADS-Da score, mean (SD) | 6.0 (4.1) | 5.3 (3.7) | 0.01 |
| Alcohol use in past 3 months (% yes) | 55 | 46 | 0.005 |
| Self-rated health (% excellent-very good) | 18 | 27 | 0.0007 |
| Tobacco-related characteristics | |||
| Cigarettes per day, median (IQR) | 15.0 (10.0–20.0) | 18.0 (8.5–20.0) | 0.22 |
| Fagerström Test for Nicotine Dependence, median (IQR) | 4.0 (3.0–6.0) | 5.0 (3.0–6.0) | 0.27 |
| Any smoking-related medical problem (%) | 70 | 65 | 0.07 |
| Do you believe that you currently have a smoking-related medical problem? (%) | 47 | 47 | 0.17 |
| Contemplation Ladder (0–10)b, mean (SD) | 6.8 (3.0) | 6.9 (3.1) | 0.63 |
| Self-efficacy - negative affect, mean (SD) | 17.1 (6.9) | 17.4 (7.7) | 0.53 |
| Self-efficacy - environmental cues, mean (SD) | 21.3 (11.5) | 19.0 (17.5) | 0.02 |
| Do you believe that quitting smoking would improve your health? (% at least “somewhat”) | 17 | 20 | 0.52 |
| Prior quit attempts (≥24 h), median (IQR) | 4.0 (1.0–10.0) | 3.0 (1.0–10.0) | 0.0001 |
| Smoked cigarettes while in hospital? (%) | 25 | 27 | 0.44 |
| Minnesota Nicotine Withdrawal Scale, median (IQR) | 11.0 (7.0–18.0) | 11.0 (5.0–18.0) | 0.26 |
| Likelihood of staying off cigarettes after hospital discharge (% reporting at least “somewhat likely”) | 43 | 46 | 0.38 |
Hospital Anxiety and Depression Scale (possible range = 0 to 21).
0 = “no thought of quitting”, 10 = “taking action to quit”.
Of 503 participants enrolled in the pre-intervention period, 5 withdrew, were discharged from the hospital, or died prior to completing the baseline assessment.
Of the 395 participants enrolled during the intervention period, 1 withdrew/was discharged from the hospital prior to completing the baseline assessment.
3.2. Cessation outcomes
There were no statistically significant differences in 7- or 30-day PPA rates at three or six months (Table 2). Assuming that participants with missing follow-up data were still smoking, 7-day PPA rates at three months were 15.5% and 12.7% for the pre-intervention and intervention periods, respectively (adjusted odds ratio [AOR]; 95% confidence interval [CI95] = 0.78; 0.51–1.18). At six months, 7-day PPA rates for those enrolled in the pre-intervention (15.3%) and intervention periods (14.5%) also did not differ significantly (AOR = 0.92; CI95: 0.62–1.37). Similarly, no statistically significant differences in 30-day PPA rates were noted between the two periods. The average intracluster correlation coefficients (ICC) at the nurse level for 7-day PPA at three- and six-months ranged from 0.0 to 0.1. Cessation outcomes for each of the study sites are provided in Supplemental Table 1. No significant treatment effects were observed at any of the individual study sites (all ps > 0.05).
Table 2.
Tobacco-related cessation outcomes.a
| Outcome | Pre-intervention | Intervention | Adjustedb OR (95% CI) |
|---|---|---|---|
| 3-Months, % | N = 464 | N = 377 | |
| 7-day PPA | 15.5 | 12.7 | 0.78 (0.51–1.18) |
| 30-day PPA | 11.0 | 8.8 | 0.78 (0.49–1.23) |
| Any 24-hour quit attempt | 55.6 | 54.2 | 1.00 (0.76–1.34) |
| Cigarettes per day, mean (SD)c | n = 284 12.6 (8.6) | n = 258 13.4 (8.7) | Diff: 0.14 (−1.46–1.74) |
| 6-Months, % | N = 452 | N = 366 | |
| 7-day PPA | 15.3 | 14.5 | 0.92 (0.62–1.37) |
| 30-day PPA | 10.8 | 9.8 | 0.89 (0.55–1.44) |
| Repeated PPA 7 days | 8.2 | 8.7 | 1.11 (0.66–1.86) |
| Repeated PPA 30 days | 5.8 | 6.3 | 1.14 (0.63–2.09) |
| Any 24-hour quit attempt | 66.4 | 65.3 | 1.01 (0.74–1.35) |
| Cigarettes per day, mean (SD)c | n = 260, 12.9 (11.4) | n = 234, 13.3 (8.6) | Diff: 0.39 (−1.46–2.25) |
PPA = point prevalence abstinence.
Based on penalized imputation in which participants with missing values at follow-up are assumed to be smoking. Participants who were deceased at the time of follow-up are not included.
Models include adjustment for the following covariates (obtained during the baseline interview): total number of quit attempts lasting ≥24 h, alcohol intake, self-rated health, and depressive symptoms as measured by the Hospital Anxiety and Depression Scale (<8 vs. 8+).
Based on complete case analysis.
The 122 participants reporting abstinence at six months (20% of those who completed follow-up) were asked to provide a saliva sample to biochemically verify their quit status. Collection kits were mailed to the 99 (81%) participants who agreed to the procedure. Of these, 55 samples (45% of those reporting abstinence) were returned. Seven samples could not be analyzed due to an insufficient or otherwise unusable specimen, resulting in 48 completed tests. Among the tests that were completed, 16 were considered uninterpretable because the participant reported using NRT in the prior seven days (because the alkaloid used for biochemical verification [cotinine] is a metabolite of nicotine and not specific to tobacco, any nicotine exposure [including medicinal nicotine] would generate a positive result). Of the 32 usable samples, 30 (94%) produced cotinine levels below 20 ng/ml, suggesting that self-reports were likely valid in most cases. Confirmation rates were similar for participants recruited during the pre-intervention (18/19 = 95%) and intervention (12/13 = 92%) periods. Applying these confirmation rates to self-reported quit rates results in corrected 7-day PPA rates of 14.5% (15.3 * 0.95) and 13.3% (14.5 * 0.92) for the pre-intervention and intervention periods, respectively.
3.3. Secondary outcomes
Approximately 55–65% of participants reported making at least one quit attempt at the three- and six-month follow-ups, with no statistically significant differences observed across period ((AOR = 1.00; CI95: 0.76–1.34) and (AOR = 1.01; CI95: 0.74–1.35) respectively). No significant differences in cigarette consumption or readiness to quit smoking were observed by period at either time point. An analysis of self-efficacy for maintaining abstinence at three months also revealed no significant differences by intervention period.
3.4. Subgroup analysis
Given that the intervention was not successful overall in enhancing cessation rates, we conducted post-hoc analyses to investigate whether participant characteristics moderated the treatment effect. Specifically, we found no significant differences in outcome by period according to baseline depressive symptoms, anxiety symptoms, or cigarette consumption.
3.5. Sensitivity analysis
Results of the sensitivity analysis using complete case and multiple imputation were generally consistent with the primary outcome analyses with one exception. In analyses based on multiple imputation, the odds of 7-day PPA at three months were marginally lower for the intervention compared to the pre-intervention period AOR = 0.66; CI95: 0.43–1.00. As with the penalized imputation analyses, no significant differences in 7-day PPA rates at six-month follow- up or repeated 7-day PPA were noted by period (see Supplemental Table 2).
3.6. Predictors of abstinence at 6 months
Among the variables entered in Step 1 of model-building, nicotine dependence was inversely associated with quitting, such that each unit increase in the HSI was associated with 0.87 lower odds of abstinence (CI95: 0.76–0.99) (Table 3). Conversely, not smoking during hospitalization was associated with greater odds of abstinence (AOR=2.79; CI95: 1.51–5.18). The BIC associated with the Step 1 model was 616 (c-statistic = 0.63).
Table 3.
Baseline predictors of 7-day point prevalence abstinence at 6-months (n= 586).
| Predictor | Step 1 model: adjusted OR (95% CI) |
Best fitting model: adjusted OR (95% CI) |
|---|---|---|
| Age | ||
| <50 years | 0.83 (0.41–1.67) | 0.86 (0.42–1.77) |
| 50–60 years | 1.02 (0.66–1.58) | 1.10 (0.70–1.74) |
| >60 years | 1.00 (Ref) | 1.00 (Ref) |
| Highest grade completed | ||
| ≤12 years | 0.83 (0.40–1.72) | 0.84 (0.40–1.78) |
| 12 years | 1.00 (0.65–1.55) | 1.08 (0.68–1.70) |
| >12 years | 1.00 (Ref) | 1.00 (Ref) |
| Heaviness of Smoking Index (HSI) | 0.87 (0.76–0.99) | 0.91 (0.79–1.04) |
| HADS-D score | ||
| <8 | 1.00 (Ref) | 1.00 (Ref) |
| ≥8 | 0.84 (0.52–1.36) | 1.05 (0.64–1.74) |
| Did participant smoke during hospitalization | ||
| No | 2.79 (1.51–5.18) | 1.85 (0.97–3.53) |
| Yes | 1.00 (Ref) | 1.00 (Ref) |
| Self-efficacy for remaining abstinent in response to negative affecta | – | 1.07 (1.03–1.10) |
| Perceived likelihood of staying off cigarettes after hospital dischargeb | – | 1.34 (1.16–1.55) |
Additional candidate variables considered for inclusion in the best subsets regression model: self-efficacy for resisting urges to smoke in response to environmental cues, number of prior quit attempts lasting ≥24 h, Contemplation Ladder score, whether or not the participant believes he or she has a smoking-related medical problem, and extent to which participant believes that quitting smoking would improve his/her health.
Represents change in odds of abstinence based on a 1-unit change in self-efficacy scale. Scale contains 6 items reflecting confidence in respondent's ability to refrain from smoking in different situations associated with negative affect, with response options ranging from 1 (not at all sure) to 6 (absolutely sure).
Coded as 1 (not at all likely), 2 (somewhat unlikely), 3 (neither likely nor unlikely), 4 (somewhat likely), or 5 (very likely).
Results of the best subsets regression analysis, which identified the combination of candidate variables that produced the best fitting model (in addition to those entered in Step 1 – i.e., age, education, nicotine dependence, depressive symptoms, whether the participant smoked during hospitalization), showed that higher baseline self-efficacy for refraining from smoking during periods of negative affect (AOR= 1.07; CI95: 1.03–1.10 for each unit increase in self-efficacy) and a greater perceived likelihood of staying off of cigarettes after discharge (AOR = 1.34; CI95: 1.16–1.55 for each unit increase in perceived likelihood of quitting) were both associated with greater odds of abstinence. The BIC (593) and c-statistic (0.72) were improved over the initial model.
3.7. Association between treatment delivery and cessation
Patient-reported receipt of counseling, measured as a composite score, was not associated with 7-day PPA at six months. In addition, none of the individual treatment components was significantly associated with smoking status when each was modeled separately.
4. Discussion
4.1. Smoking cessation outcomes
This study examined the impact of enhanced academic detailing to promote the implementation of clinical practice guidelines on tobacco use outcomes in hospitalized Veterans. Although rates of 7-day PPA (13–16%) were comparable to prior studies (Katz et al., 2009), no differences in cessation or other smoking-related outcomes were observed between study periods. Moreover, intervention effectiveness did not vary according to participant characteristics such as smoking rate or symptoms of depression or anxiety.
Hospital-based smoking cessation interventions for general medical inpatients have met with mixed results (Duffy et al., 2014; Gadomski, Gavett, Krupa, Tallman, & Jenkins, 2011; Hennrikus et al., 2005; Miller, Smith, DeBusk, Sobel, & Taylor, 1997; Reid et al., 2010; Stevens, Glasgow, Hollis, Lichtenstein, & Vogt, 1993; Stevens, Glasgow, Hollis, & Mount, 2000; Murray et al., 2013; Warner et al., 2016). In a systematic review, counseling interventions initiated during hospitalization and continued for at least one month after discharge were associated with increased odds of quitting (Rigotti et al., 2012). Considering that the quitline was the primary modality for delivering extended post-discharge counseling, the low rates of referral likely contributed to the intervention's limited impact on cessation outcomes in the current study. As previously reported (Katz et al., 2014), although the odds of receiving a quitline referral more than doubled during the intervention period, only 12% of patients reported having been offered a referral. Of the 36 (9%) participants who accepted a quitline referral during the intervention period, only 23 (64%) were successfully contacted by the quitline (Katz et al., 2014). Thus, the proportion of participants ultimately reached by quitline counseling was very small due to both low rates of referral and attrition across the phases of treatment initiation. These findings are similar to Sherman et al. (2016), who reported similar difficulties in reaching hospitalized patients for post-discharge telephone counseling, with only 51% receiving at least one call. It is also possible that referral to the quitline, although evidence-based (Stead, Hartmann-Boyce, Perera, & Lancaster, 2013) and cost-effective (Hollis et al., 2007), may not be the optimal treatment approach for all hospitalized Veterans.
The fact that prescription of smoking cessation medications did not increase during the intervention period also likely contributed to the null findings. Although patient-reported offers by nurses of NRT to help alleviate nicotine withdrawal during hospitalization increased from 23 to 54%, discussion of pharmacotherapy to assist with smoking cessation were relatively infrequent (24% across both study periods) (Katz et al., 2014). In addition, prior analysis of pharmacy data revealed no differences in the odds of being prescribed NRT, bupropion, or varenicline during the pre-intervention (35%) or intervention (38%) periods (Katz et al., 2014).
The short duration of nurse training may also have adversely affected delivery of the intervention components. Because training had to be conducted during nurses' shifts and around patient-care responsibilities, it was limited to approximately 30 min. Although nurses were also encouraged to complete an additional 30-minute on-line tutorial to supplement the in-person training, this was not mandatory. Despite statistically significant improvements in delivery of recommended counseling for four of the five A's (Ask, Assess, Assist, and Arrange follow-up) (Katz et al., 2014), there was still considerable room for improvement. More prolonged and intensive training with periodic refreshers may have led to better treatment fidelity and improved cessation rates. On the other hand, exploratory analyses suggest that receipt of treatment was not associated with a greater odds of quitting; however, it is important to recognize both the potential self-selection biases inherent in these analyses (e.g., patients' readiness to quit smoking likely influenced whether nurses delivered certain treatment components) and the fact that some counseling elements were contingent upon delivery of others.
Effective intervention approaches in primary care may not translate to the inpatient setting (Wolfenden, Campbell, Wiggers, Walsh, & Bailey, 2008). Although the reasons for this are difficult to determine with certainty, the fact that patients typically do not have established relationships with their inpatient clinicians (in contrast to those with their primary care providers) may have affected the nature of their interactions and patients' receptivity to the intervention. In addition, compared to the hospital setting, primary care may offer a more suitable environment for following up with patients and greater opportunities for reinforcing the intervention over time. Third, frequent handoffs and changes in clinical staff during the inpatient stay may have led to some discontinuity in care and diffusion of responsibility for delivering the intervention. Finally, although hospitalization is assumed to provide a teachable moment for treating tobacco use and dependence, the stress associated with their acute health condition may have adversely affected some patients'willingness to consider quitting. Results are also consistent with a larger substance abuse literature demonstrating that interventions involving screening and brief intervention that have been shown to be beneficial for risky alcohol use are associated with limited efficacy for other drug use disorders, particularly when implemented in general healthcare settings (Saitz, 2014). Collectively, these results suggest the need for alternative treatment approaches.
As suggested by An et al. (2006), more intensive interventions with large improvements in performance of the 5A's are likely necessary to significantly increase quit attempts and smoking cessation rates. To do so, however, can be challenging in the context of routine inpatient care, where competing demands and the impact on workflow among heavily taxed clinical staff limit the time available for cessation counseling (Katz, Holman, Johnson, et al., 2013). Stevens et al. (2000) concluded that cessation interventions in this setting should be delivered by dedicated professionals who are accountable for completing the intervention rather than existing clinical staff. More recently, Rigotti et al. (2014) found that a post-discharge intervention combining free medication and proactive automated interactive voice response telephone calls increased sustained abstinence relative to standard advice to obtain counseling and medication. This intervention, however, focused exclusively on smokers who already planned to quit; it is unclear whether such an approach would be effective in an unselected sample of hospitalized smokers (i.e., regardless of their readiness to quit).
4.2. Predictors of abstinence
Our predictive model identifies patients who may benefit from additional encouragement and support for quitting smoking during and after hospitalization. Consistent with prior studies (Dornelas, Sampson, Gray, Waters, & Thompson, 2000; Gwaltney, Metrik, Kahler, & Shiffman, 2009; Schnoll et al., 2011; Warner et al., 2016), self-efficacy for quitting smoking, as operationalized both by self-rated ability to refrain from smoking while experiencing negative affect as well as perceived likelihood of staying off of cigarettes following hospital discharge, was associated with abstinence at six month follow-up. These findings suggest that interventions aimed at enhancing patients' confidence in their ability to quit smoking and that encourage abstinence during hospitalization may increase quit attempts and cessation. Support for this approach comes from prior outpatient trials in which increases in self-efficacy during treatment have been associated with improved cessation rates (Hendricks, Delucchi, & Hall, 2010; Schnoll et al., 2011). Prior to application in the field, our findings should be validated in an independent sample of hospitalized smokers.
4.3. Directions for future research
Future efforts to increase smoking cessation among inpatients may benefit from improved coordination with primary care to facilitate continued intervention following hospital discharge. Designating dedicated staff with expertise in smoking cessation to deliver treatment may also improve outcomes in the hospital setting (France et al., 2001). In addition, although the nurse counseling intervention did include brief motivational enhancement strategies for those not ready to quit smoking, a more intensive treatment approach and routine monitoring of treatment fidelity may have yielded more promising results. Post-discharge follow-up counseling delivered by hospital volunteers is another promising strategy that merits additional consideration in this patient population (Duffy et al., 2014).
4.4. Strengths and limitations
Strengths of the study included the integration of the intervention into routine practice using existing clinical staff. In addition, whereas many interventions target only patients who plan to quit smoking, we enrolled cigarette smokers regardless of their intentions to quit. While this approach likely reduced overall cessation rates, it also helped to ensure that larger proportions of smokers received guideline-recommended treatment. Another strength of this study was the involvement of multiple inpatient units across four VA medical centers.
Several study limitations are noteworthy. First, although the quasi-experimental pre-post design allowed us to examine the impact of the intervention on cessation outcomes using the pre-implementation period for comparison, the lack of random assignment does limit causal inference. Although we considered cluster randomization by hospital, such an approach was not feasible due to cost and budgetary constraints. Second, enrollment was less than intended on account of lower than expected recruitment at one of the sites during the intervention period. While this reduced statistical power, the observed effect sizes suggest that an inadequate sample was not responsible for the lack of significant treatment effects. Third, despite considerable efforts to reach participants for assessments, there was non-trivial loss to follow-up. Nevertheless, the proportion of subjects who completed follow-up compares favorably to another recent trial involving hospitalized Veterans (Duffy et al., 2014). Fourth, only a minority of self-reported quitters provided a usable saliva sample for biochemical confirmation. Further, the sample collection was not overseen by a third party to ensure that it actually came from the study participant. A prior study of smoking cessation among hospitalized Veterans reported high misclassification rates (21%) among self-reported quitters (Noonan, Jiang, & Duffy, 2013). It is possible that those who falsely reported abstinence were less likely to return their samples, which could have biased results. There was no evidence, however, of differential misreporting of abstinence between study periods. Fifth, some of the participants' responses on the baseline interview may have been influenced by any brief cessation counseling that they received at the time of admission. Sixth, the study sample was predominantly male and Caucasian, which limits the generalizability of our findings with regard to female and non-white Veterans. Seventh, for practical reasons, nurse training was limited in duration and intensity. Evidence suggests that training in motivational interviewing should incorporate ongoing coaching and feedback to enable clinicians to develop and maintain the requisite skills, knowledge, and confidence (Fu et al., 2015; Miller, Yahne, Moyers, Martinez, & Pirritano, 2004). Thus, the training may not have provided sufficient opportunity for nurses to become proficient in motivational enhancement and other counseling skills. Finally, although we collected reports from participants regarding nurse and physician delivery of the 5A's (Katz et al., 2014), we do not have information regarding the quality of treatment received or other indicators of treatment fidelity. In addition, we do not have data regarding whether participants used the written self-help materials or watched the motivational video. Such information would aid in the interpretation of study results and in the design of future hospital-based interventions.
5. Conclusions
In summary, a nurse-initiated intervention for hospitalized smokers that focused on implementation of clinical practice guidelines was not associated with improved cessation outcomes, despite significant improvements in recommended counseling behaviors. Future smoking cessation interventions in the inpatient setting may benefit by designating dedicated staff with expertise in tobacco cessation counseling to ensure that treatments can be fully delivered as intended both during hospitalization and following discharge. Ideally, such an approach would also involve coordination with patients' primary care providers so that treatment strategies can be aligned and integrated with ongoing outpatient care (Naylor & Keating, 2008). Future research should also examine the clinical effectiveness of brief motivational interventions to enhance self-efficacy and readiness to quit among hospitalized smokers.
Supplementary Material
Acknowledgments
Funding
This study was funded by Department of Veterans Affairs, Office of Research and Development and Health Services Research and Development (IIR 07-113).
Appendix A
Description of VA study hospitals
| Site 1 | Site 2 | Site 3 | Site 4 | |
|---|---|---|---|---|
| Enrollment period | ||||
| Pre-intervention | 5/2009–12/2009 | 10/2010–4/2011 | 11/2010–8/2011 | 1/2011–12/2011 |
| Post-intervention | 4/2010–12/2010 | 11/2011–9/2012 | 1/2012–8/2012 | 1/2012–12/2012 |
| Patient characteristics | ||||
| Age, mean | 62.9 | 65.1 | 62.7 | 60.4 |
| Gender, % male | 97 | 96 | 95 | 94 |
| Race, % white | 97 | 80 | 85 | 86 |
| Income ($), mean | 21,349 | 26,287 | 20,500 | 19,414 |
| General medical wards | ||||
| Annual number of admissions, n | 2988 | 4388 | 2701 | 2806 |
| Total number of nursing staff | 42 | 72 | 60 | 52 |
| % Registered nurses (RN)a | 67 | 68 | 73 | 58 |
| Average daily census (across wards), n | 29 | 62 | 33 | 42 |
| Average nursing hours per patient daya | 6.2 | 7.13 | 8.31 | 7.34 |
| Smoking cessation servicesb | ||||
| Responsible division | Mental health | Mental health | Mental health | Primary care |
| No. of consults per month | 50 | 60 | 65 | 30 |
| How often do new patients start program? | 1/month | 1/week | NS | 1/week |
| No. of individual counseling sessions per typical course of therapy | 3 | NS | 2 | 4 |
| Program includes group counseling | Y | Y | N | Y |
| Can patients receive pharmacotherapy without enrollment in smoking cessation program? | Y | Y | N | Y |
| Any use of telemedicine to provide cessation therapy? | Y | N | Y | N |
Source: VHA Support Service Center. http://vssc.med.va.gov (accessed 6/13/07).
Nurse staffing for Iowa City was estimated from local data.
Source: Office of the Assistant Deputy Undersecretary for Health for Policy and Planning. Veterans Health Administration. Smoking and Tobacco Use Cessation Report 2005. http://vaww.va.gov/haig/smoking/STUC_2005.pdf (last accessed 12/1/06).
Appendix B
Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.jsat.2017.03.015.
Footnotes
Trial registration: ClinicalTrials.gov identifier number NCT00816036.
Competing interests
None of the authors of the manuscript have any competing interests to declare.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the Institutional Review Boards at all participating sites.
Informed consent
All individuals taking part in the study provided written consent for participation prior to data collection.
References
- Abrams DB, Herzog TA, Emmons KM, Linnan L. Stages of change versus addiction: A replication and extension. Nicotine & Tobacco Research. 2000;2:223–229. doi: 10.1080/14622200050147484. http://dx.doi.org/10.1080/14622200050147484. [DOI] [PubMed] [Google Scholar]
- An LC, Zhu SH, Nelson DB, Arikian NJ, Nugent S, Partin MR, et al. Benefits of telephone care over primary care for smoking cessation: A randomized trial. Archives of Internal Medicine. 2006;166:536–542. doi: 10.1001/archinte.166.5.536. http://dx.doi.org/10.1001/archinte.166.5.536. [DOI] [PubMed] [Google Scholar]
- Barnett PG, Hamlett-Berry K, Sung HY, Max W. Health care expenditures attributable to smoking in military veterans. Nicotine & Tobacco Research. 2015;17:586–591. doi: 10.1093/ntr/ntu187. http://dx.doi.org/10.1093/ntr/ntu187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biener L, Abrams DB. The contemplation ladder: Validation of a measure of readiness to consider smoking cessation. Health Psychology. 1991;10:360–365. doi: 10.1037//0278-6133.10.5.360. http://dx.doi.org/10.1037/0278-6133.10.5.360. [DOI] [PubMed] [Google Scholar]
- Brown DW. Smoking prevalence among US veterans. Journal of General Internal Medicine. 2010;25:147–149. doi: 10.1007/s11606-009-1160-0. http://dx.doi.org/10.1007/s11606-009-1160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DW, Croft JB, Schenck AP, Malarcher AM, Giles WH, Simpson RJ. Inpatient smoking-cessation counseling and all-cause mortality among the elderly. American Journal of Preventive Medicine. 2004;26:112–118. doi: 10.1016/j.amepre.2003.10.004. http://dx.doi.org/10.1016/j.amepre.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Current cigarette smoking among adults – United States, 2011. Morbidity and Mortality Weekly Report. 2012;61:889–894. [PubMed] [Google Scholar]
- Dornelas EA, Sampson RA, Gray JF, Waters D, Thompson PD. A randomized controlled trial of smoking cessation counseling after myocardial infarction. Preventive Medicine. 2000;30:261–268. doi: 10.1006/pmed.2000.0644. http://dx.doi.org/10.1006/pmed.2000.0644. [DOI] [PubMed] [Google Scholar]
- Duffy SA, Reeves P, Hermann C, Karvonen C, Smith P. In-hospital smoking cessation programs: What do VA patients and staff want and need? Applied Nursing Research. 2008;21:199–206. doi: 10.1016/j.apnr.2006.11.002. http://dx.doi.org/10.1016/j.apnr.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Duffy SA, Ronis DL, Karvonen-Gutierrez CA, Ewing LA, Dalack GW, Smith PM, et al. Effectiveness of the tobacco tactics program in the Department of Veterans Affairs. Annals of Behavioral Medicine. 2014;48:265–274. doi: 10.1007/s12160-014-9605-z. http://dx.doi.org/10.1016/j.apnu.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Etter JF, Bergman MM, Humair JP, Perneger TV. Development and validation of a scale measuring self-efficacy of current and former smokers. Addiction. 2000;95:901–913. doi: 10.1046/j.1360-0443.2000.9569017.x. http://dx.doi.org/10.1046/j.1360-0443.2000.9569017.x. [DOI] [PubMed] [Google Scholar]
- Fiore FC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Treating tobacco use and dependence: 2008 update. Clinical practice guideline. Rockville, MD: U.S. Department of Health and Human Services Public Health Service; 2008. [Google Scholar]
- France EK, Glasgow RE, Marcus AC. Smoking cessation interventions among hospitalized patients: What have we learned? Preventive Medicine. 2001;32:376–388. doi: 10.1006/pmed.2000.0824. http://dx.doi.org/10.1006/pmed.2000.0824. [DOI] [PubMed] [Google Scholar]
- Fu SS, Roth C, Battaglia CT, Nelson DB, Farmer MM, Do T, Zillich AJ. Training primary care providers in motivational interviewing: A comparison of two models. Patient Education and Counseling. 2015;98:61–68. doi: 10.1016/j.pec.2014.10.007. Dx.doi.org/10.1016/j.pec.2014.10.007. [DOI] [PubMed] [Google Scholar]
- Gadomski AM, Gavett J, Krupa N, Tallman N, Jenkins P. Effectiveness of an inpatient smoking cessation program. Journal of Hospital Medicine. 2011;6:E1–E8. doi: 10.1002/jhm.641. http://dx.doi.org/10.1002/jhm.641. [DOI] [PubMed] [Google Scholar]
- Grossman E, Shelley D, Braithwaite RS, Lobach I, Goffin A, Rogers E, Sherman S. Effectiveness of smoking-cessation interventions for urban hospital patients: Study protocol for a randomized controlled trial. Trials. 2012;13:126. doi: 10.1186/1745-6215-13-126. http://dx.doi.org/10.1186/1745-6215-13-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwaltney CJ, Metrik J, Kahler CW, Shiffman S. Self-efficacy and smoking cessation: A meta-analysis. Psychology of Addictive Behaviors. 2009;23:56–66. doi: 10.1037/a0013529. http://dx.doi.org/10.1037/a0013529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon P, Lawder R, Elders A, Clark D, Walsh D, Whyte B, Sutton M. An analysis of the link between behavioural, biological and social risk factors and subsequent hospital admission in Scotland. Journal of Public Health. 2007;29:405–412. doi: 10.1093/pubmed/fdm062. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström test for nicotine dependence: A revision of the Fagerström tolerance questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hedeker D, Mermelstein RJ, Demirtas H. Analysis of binary outcomes with missing data: Missing = smoking, last observation carried forward, and a little multiple imputation. Addiction. 2007;102:1564–1573. doi: 10.1111/j.1360-0443.2007.01946.x. http://dx.doi.org/10.1111/j.1360-0443.2007.01946.x. [DOI] [PubMed] [Google Scholar]
- Hendricks PS, Delucchi KL, Hall SM. Mechanisms of change in extended cognitive behavioral treatment for tobacco dependence. Drug and Alcohol Dependence. 2010;109:114–119. doi: 10.1016/j.drugalcdep.2009.12.021. http://dx.doi.org/10.1016/j.drugalcdep.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennrikus DJ, Lando HA, McCarty MC, Klevan D, Holtan N, Huebsch JA, et al. The TEAM project: The effectiveness of smoking cessation intervention with hospital patients. Preventive Medicine. 2005;40:249–258. doi: 10.1016/j.ypmed.2004.05.030. http://dx.doi.org/10.1016/j.ypmed.2004.05.030. [DOI] [PubMed] [Google Scholar]
- Hoerster KD, Lehavot K, Simpson T, McFall M, Reiber G, Nelson KM. Health and health behavior differences: U.S. military, veteran, and civilian men. American Journal of Preventive Medicine. 2012;43:483–489. doi: 10.1016/j.amepre.2012.07.029. http://dx.doi.org/10.1016/j.amepre.2012.07.029. [DOI] [PubMed] [Google Scholar]
- Hollis JF, McAfee TA, Fellows JL, Zbikowski SM, Stark M, Riedlinger K. The effectiveness and cost effectiveness of telephone counseling and the nicotine patch in a state tobacco quitline. Tobacco Control. 2007;16(Suppl. 1):i53–i59. doi: 10.1136/tc.2006.019794. http://dx.doi.org/10.1136/tc.2006.019794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosmer DW, Jvanovic B, Lemeshow S. Best subsets logistic regression. Biometrics. 1989;45:1265–1270. http://dx.doi.org/10.2307/2531779. [Google Scholar]
- Hughes JR. Background on the Minnesota Nicotine Withdrawal Scale – Revised (MNWS-R) [Accessed May 27, 2014];2012 Retrieve from http://www.uvm.edu/~hbpl/minnesota/minnesota/2012/Background_8_2012.pdf.
- Hughes JR, Hatsukami DK. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. http://dx.doi.org/10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Katz A, Goldberg D, Smith J, Trick WE. Tobacco, alcohol, and drug use among hospital patients: Concurrent use and willingness to quit. Journal of Hospital Medicine. 2008;3:369–375. doi: 10.1002/jhm.358. http://dx.doi.org/10.1002/jhm.358. [DOI] [PubMed] [Google Scholar]
- Katz D, Vander Weg M, Fu S, Prochazka A, Grant K, Buchanan L, et al. A before-after implementation trial of smoking cessation guidelines in hospitalized veterans. Implementation Science. 2009;10:58. doi: 10.1186/1748-5908-4-58. http://dx.doi.org/10.1186/1748-5908-4-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz D, Holman J, Johnson S, Hillis S, Fu S, Grant K, et al. Implementing smoking cessation guidelines for hospitalized veterans: Effects on nurse attitudes and performance. Journal of General Internal Medicine. 2013;28:1420–1429. doi: 10.1007/s11606-013-2464-7. http://dx.doi.org/10.1007/s11606-013-2464-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz DA, Holman JE, Nugent AS, Baker LJ, Johnson SR, Hillis SL, et al. The emergency department action in smoking cessation (EDASC) trial: Impact on cessation outcomes. Nicotine & Tobacco Research. 2013;15:1032–1043. doi: 10.1093/ntr/nts219. http://dx.doi.org/10.1093/ntr/nts219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz DA, Holman J, Hillis SL, Fu S, Grant K, Buchanan L, et al. Implementing best evidence in smoking cessation treatment for hospitalized veterans – Results from the VA-BEST trial. The Joint Commission Journal on Quality and Patient Safety. 2014;40:493–502. doi: 10.1016/s1553-7250(14)40064-3. [DOI] [PubMed] [Google Scholar]
- Kisuule F, Necochea A, Howe EE, Wright S. Utilizing audit and feedback to improve hospitalists' performance in tobacco dependence counseling. Nicotine & Tobacco Research. 2010;12:797–800. doi: 10.1093/ntr/ntq093. http://dx.doi.org/10.1093/ntr/ntq093. [DOI] [PubMed] [Google Scholar]
- Kramarow EA, Pastor PN. Current smoking among men aged 25–64 years, by age group and veteran status – National Health Interview Survey (NHIS), United States, 2007–2010. Morbidity and Mortality Weekly Report. 2012;61:929. [Google Scholar]
- Miller NH, Smith PM, DeBusk RF, Sobel DS, Taylor CB. Smoking cessation in hospitalized patients: Results of a randomized trial. Archives of Internal Medicine. 1997;157:409–415. http://dx.doi.org/10.1001/archinte.1997.00440250059007. [PubMed] [Google Scholar]
- Miller WR, Yahne CE, Moyers TB, Martinez J, Pirritano M. A randomized trial of methods to help clinicians learn motivational interviewing. Journal of Consulting and Clinical Psychology. 2004;72:1050–1062. doi: 10.1037/0022-006X.72.6.1050. http://dx.doi.org/10.1037/0022-006X.72.6.1050. [DOI] [PubMed] [Google Scholar]
- Murray RL, Leonardi-Bee J, Marsh J, Jayes L, Li J, Parrott S, Britton J. Systematic identification and treatment of smokers by hospital based cessation practitioners in a secondary care setting: Cluster randomised controlled trial. BMJ. 2013;347:f4004. doi: 10.1136/bmj.f4004. http://dx.doi.org/10.1136/bmj.f4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor M, Keating SA. Transitional care: Moving patients from one setting to another. American Journal of Nursing. 2008;108(9 Suppl):58–63. doi: 10.1097/01.NAJ.0000336420.34946.3a. http://dx.doi.org/10.1097/01.NAJ.0000336420.34946.3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DB, Partin MR, Fu SS, Joseph AM, An LC. Why assigning ongoing tobacco use is not necessarily a conservative approach to handling missing tobacco cessation outcomes. Nicotine & Tobacco Research. 2009;11:77–83. doi: 10.1093/ntr/ntn013. http://dx.doi.org/10.1093/ntr/ntn013. [DOI] [PubMed] [Google Scholar]
- Noonan D, Jiang Y, Duffy SA. Utility of biochemical verification of tobacco cessation in the Department of Veterans Affairs. Addictive Behaviors. 2013;38:1792–1795. doi: 10.1016/j.addbeh.2012.11.006. http://dx.doi.org/10.1016/j.addbeh.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid RD, Mullen KA, Slovinec D'Angelo ME, Aitken DA, Papadakis S, Haley PM, et al. Smoking cessation for hospitalized smokers: an evaluation of the “Ottawa Model”. Nicotine & Tobacco Research. 2010;12:11–18. doi: 10.1093/ntr/ntp165. http://dx.doi.org/10.1093/ntr/ntp165. [DOI] [PubMed] [Google Scholar]
- Rigotti NA, Munafo MR, Stead LF. Smoking cessation interventions for hospitalized smokers: A systematic review. Archives of Internal Medicine. 2008;168:1950–1960. doi: 10.1001/archinte.168.18.1950. http://dx.doi.org/10.1001/archinte.168.18.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigotti NA, Clair C, Munafò MR, Stead LF. Interventions for smoking cessation in hospitalized patients. Cochrane Database of Systematic Reviews. 2012;16:CD001837. doi: 10.1002/14651858.CD001837.pub3. http://dx.doi.org/10.1002/14651858.CD001837.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigotti NA, Regan S, Levy DE, Japuntich S, Chang Y, Park ER, et al. Sustained care intervention and postdischarge smoking cessation among hospitalized adults: A randomized clinical trial. JAMA. 2014;312:719–728. doi: 10.1001/jama.2014.9237. http://dx.doi.org/10.1001/jama.2014.9237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollnick S, Mason P, Butler C. Health behavior change: A guide for practitioners. New York, NY: Churchill Livingstone; 2000. [Google Scholar]
- Saitz R. Screening and brief intervention for unhealthy drug use: Little or no efficacy. Frontiers in Psychiatry. 2014;5:121. doi: 10.3389/fpsyt.2014.00121. http://dx.doi.org/10.3389/fpsyt.2014.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoll RA, Martinez E, Tatum KL, Glass M, Bernath A, Ferris D, et al. Increased self-efficacy to quit and perceived control over withdrawal symptoms predict smoking cessation following nicotine dependence treatment. Addictive Behaviors. 2011;36:144–147. doi: 10.1016/j.addbeh.2010.08.024. http://dx.doi.org/10.1016/j.addbeh.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah LM, King AC, Basu A, Krishnan JA, Borden WB, Meltzer D, et al. Effect of clinician advice and patient preparedness to quit on subsequent quit attempts in hospitalized smokers. Journal of Hospital Medicine. 2010;5:26–32. doi: 10.1002/jhm.536. http://dx.doi.org/10.1002/jhm.536. [DOI] [PubMed] [Google Scholar]
- Sheffer MA, Baker TB, Fraser DL, Adsit RT, McAfee TA, Fiore MC. Fax referrals, academic detailing, and tobacco quitline use: A randomized trial. American Journal of Preventive Medicine. 2012;42:21–28. doi: 10.1016/j.amepre.2011.08.028. http://dx.doi.org/10.1016/j.amepre.2011.08.028. [DOI] [PubMed] [Google Scholar]
- Sherman SE, Link AR, Rogers ES, Krebs P, Ladapo JA, Shelley DR, Grossman E. Smoking-cessation interventions for urban hospital patients: A randomized comparative effectiveness trial. American Journal of Preventive Medicine. 2016;51:566–577. doi: 10.1016/j.amepre.2016.06.023. http://dx.doi.org/10.1016/j.amepre.2016.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research. 2002;4:149–159. doi: 10.1080/14622200210123581. http://dx.doi.org/10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Stead LF, Hartmann-Boyce J, Perera R, Lancaster T. Telephone counseling for smoking cessation. Cochrane Database of Systematic Reviews. 2013;12:CD002850. doi: 10.1002/14651858.CD002850.pub3. http://dx.doi.org/10.1002/14651858. [DOI] [PubMed] [Google Scholar]
- Stevens VJ, Glasgow RE, Hollis JF, Lichtenstein E, Vogt TM. A smoking-cessation intervention for hospital patients. Medical Care. 1993;31:65–72. doi: 10.1097/00005650-199301000-00005. [DOI] [PubMed] [Google Scholar]
- Stevens VJ, Glasgow RE, Hollis JF, Mount K. Implementation and effectiveness of a smoking-cessation intervention for hospital patients. Medical Care. 2000;38:451–459. doi: 10.1097/00005650-200005000-00002. [DOI] [PubMed] [Google Scholar]
- Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Quarterly. 1996;74:511–544. http://dx.doi.org/10.2307/3350391. [PubMed] [Google Scholar]
- Warner DO, Nolan MB, Kadimpati S, Burke MV, Hanson AC, Schroeder DR. Quitline tobacco interventions in hospitalized patients: A randomized trial. American Journal of Preventive Medicine. 2016 doi: 10.1016/j.amepre.2016.03.005. http://dx.doi.org/10.1016/j.amepre.2016.03.005 (In press) [DOI] [PubMed]
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Statistics in Medicine. 2011;30:377–399. doi: 10.1002/sim.4067. http://dx.doi.org/10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- Wilkins K, Shields M, Rotermann M. Smokers' use of acute care hospitals – A prospective study. Health Reports. 2009;20:1–9. [PubMed] [Google Scholar]
- Wolfenden L, Campbell E, Wiggers J, Walsh RA, Bailey LJ. Helping hospital patients quit: What the evidence supports and what guidelines recommend. Preventive Medicine. 2008;46:346–357. doi: 10.1016/j.ypmed.2007.12.003. http://dx.doi.org/10.1016/j.ypmed.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. http://dx.doi.org/10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.