Abstract
About 92.1 million Americans suffer from at least one type of cardiovascular disease. Worldwide, cardiovascular diseases are the number one cause of death (about 31% of all global deaths). Recent technological advancements in cardiac ultrasound imaging are expected to aid in the clinical diagnosis of many cardiovascular diseases. This article provides an overview of such recent technological advancements, specifically focusing on tissue Doppler imaging, strain imaging, contrast echocardiography, 3D echocardiography, point-of-care echocardiography, 3D volumetric flow assessments, and elastography. With these advancements ultrasound imaging is rapidly changing the domain of cardiac imaging. The advantages offered by ultrasound imaging include real-time imaging, imaging at patient bed-side, cost-effectiveness and ionizing-radiation-free imaging. Along with these advantages, the steps taken towards standardization of ultrasound based quantitative markers, reviewed here, will play a major role in addressing the healthcare burden associated with cardiovascular diseases.
Keywords: cardiac ultrasound, tissue Doppler imaging, strain imaging, contrast echocardiography, 3D echocardiography, point-of-care echocardiography, 3D volumetric flow assessments, cardiac elastography
Introduction
Confucius enunciated “I hear and I forget. I see and I remember…”. Interestingly, in cardiology, from utilizing sound for diagnosis based on auscultations [1] (from the Hippocratic period: 460 to 370 BC; still used in clinical practice today), techniques have evolved to translate ultrasound in to images for characterization of cardiac function. Medical use of ultrasound dates back to the 1940s with the use of ultrasound in cardiology being reported in the 1950s [2, 3]. Since then advancements in electronics and ultrasound transducers, coupled with signal and image processing algorithms have rapidly propelled the use of medical ultrasound with echocardiography regarded as one of cardiology’s 10 greatest discoveries of the 20th century [4].
Out of several imaging modalities available today for cardiac imaging, advantages associated with ultrasound include real-time imaging, imaging at patient bed-side (point of care), cost-effectiveness and ionizing-radiation-free imaging [5, 6]. The cost of other cardiac imaging modalities exceed that of 2D echocardiography by a factor of 3.1 to 14.0, whereas the cost of right and left heart catheterization, performed often to obtain diagnostic information, is greater by almost a factor of 20 [5]. As per the latest data from the American Heart Association, about 92.1 million Americans (more than 1 in 3 Americans) suffer from at least one type of cardiovascular disease [7] Worldwide, cardiovascular diseases are the number one cause of death (about 31% of all global deaths) [8]. Further, it is estimated that by 2030, the total annual cost associated with cardiovascular diseases in the United States will exceed $900 billion [7].
In briefly reviewing basic cardiac function, the heart is designed to respond to different loading (filling) conditions in the form of varying blood volumes and varying magnitudes of flow resistance. As a dynamic cyclic pump that is able to respond and adapt to varying flow requirements and pressure conditions, measuring the characteristics and functional parameters of the heart muscle becomes clinically relevant for assessing heart failure and myocardial ischemia (due to reduced blood flow to the heart). Abnormalities in the compliance of the heart muscle during the filling stage of the cardiac cycle (diastole) cause diastolic dysfunction and heart failure with preserved ejection fraction (HFpEF). Abnormalities in the pumping ability of the heart during the contraction of the heart muscle in the cardiac cycle (systole) causes systolic heart failure also known as heart failure with reduced ejection fraction (HFrEF).
Keeping in mind the advantages offered by ultrasound imaging and the healthcare burden of cardiovascular diseases, technological advancements have expanded the role of ultrasound in cardiac imaging. This review article will serve as an overview of some of these recent technological advancements in cardiac ultrasound including tissue Doppler imaging, strain imaging, contrast echocardiography, 3D echocardiography, point-of-care echocardiography, 3D volumetric flow assessments, and elastography.
Tissue Doppler Imaging (TDI)
For more than 50 years, since the first measurement of motion as well as flow in the heart was performed in Japan in the 1950’s by Satomura, the clinical use of ultrasound imaging has expanded dramatically [9, 10]. While ultrasound scanners have been detecting echoes scattered from blood based on the Doppler effect for most of that time [11], it was only in the late 1980’s that the concept of tissue Doppler imaging (TDI) for echocardiography (sometimes also referred to as Doppler myocardial imaging) emerged [12–14]. Conventional Doppler systems rely on high-pass filters to extract the high frequency, low amplitude signals caused by blood flow, but by inverting the signal processing TDI employs low-pass filters to isolate the low frequency, high amplitude signals associated with myocardial motion, in particular, the longitudinal component of the myocardial contraction [12, 13].
Tissue Doppler imaging measurements are performed using either the pulsed wave or the color coded modes. Pulsed wave TDI directly measures the instantaneous tissue velocity within a small (1-5 mm) sampling volume, while color coded TDI allows simultaneous interrogation of the entire color box (i.e., over a large region of interest; ROI), but necessitates post-processing to compensate for variations in the angle of interrogation across the color box in order to extract the mean tissue velocity (typically some 25% lower than the pulsed wave TDI values) [12, 13]. Both these modes rely on the pulsed Doppler principle but differ from one another based on the size of the region from which velocity measurements are performed, and how the resultant values are calculated and displayed. Consensus statements from a number of echocardiographic societies around the globe recommend quantitative TDI evaluations for assessment of systolic and diastolic left ventricular (LV) and right ventricular (RV) function, LV filling pressures and ventricular dyssynchrony, and for monitoring the treatment of patients with heart failure [15–18].
Over a cardiac cycle the pulsed wave TDI signal contains three peaks corresponding to the peak myocardial velocities during systole (s′ signifying myocardial contraction), early diastole (e′ signifying myocardial relaxation) and late diastole (a′ signifying active atrial contraction) (Fig. 1). Additionally, isovolumetric contraction and relaxation peaks can also be identified. Normal pulsed wave TDI values for s′, e′ and a′ can be found in the literature [12]. Quantitative TDI measurements can be used to characterize global and regional myocardial function and can provide prognostic markers for a number of cardiac diseases including coronary artery disease, heart failure and valvular heart diseases [12].
Figure 1.
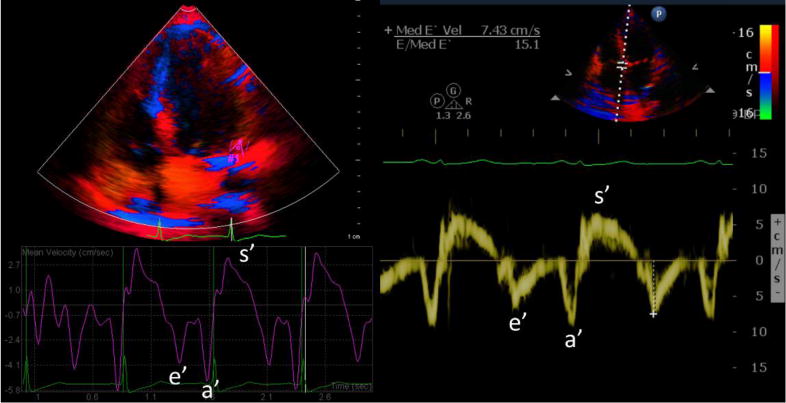
Color-coded tissue Doppler curves are displayed on the left after image post-processing. Pulsed-wave tissue Doppler curve is shown on the right with sample volume placed at the septal mitral annulus (e′=early diastolic velocity, a′=late diastolic velocity, and s′=systolic velocity; the peak after s′ and before e′ corresponds to the peak in the isovolumic relaxation phase, and the peak after a′ and before s′ corresponds to the peak in the isovolumic contraction phase).
The use of TDI measurements has also been evaluated for prognosis post cardiac resynchronization therapy (a pacemaker based therapy for resynchronizing ventricular contractions). A meta-analysis of 8 cardiac resynchronization therapy studies involving over 4,000 patients found that TDI had an 87–97% sensitivity and 55-100% specificity for differentiating between responders and non-responders [18]. However, these results were not confirmed by the prospective, multi-center PROSPECT trial, which resulted in sensitivities of 42-74% and specificities of 35-60% based on TDI assessments of responses to cardiac resynchronization therapy amongst 498 patients [19]. A meta-analysis of studies looking at detection of coronary artery disease concluded that TDI velocities provided significant separation amongst patients with and without coronary artery disease before and after stress tests [13]. However, while at rest these differences were expressed in the peak systolic velocity (i.e., s′ amplitude) and post stress differences appeared in the early diastolic velocity (i.e., e′ amplitude) [13]. Early diastolic velocities by TDI are also frequently used to estimate filling pressures; however, in a recent meta-analysis of 24 studies, Sharifov and colleagues found a poor to mediocre correlation of the TDI-based technique with invasively-determined LV filling pressures [20].
Limitations
With pulsed-wave TDI, only one sample volume can be interrogated at a time making the procedure time consuming. Although color coded TDI is faster as it allows multiple myocardial segments to be compared simultaneously, it requires post-processing to compensate for variations in the angle of interrogation and is much more susceptible to angular errors. TDI velocities acquired at angles greater than 20° (i.e., angle of incidence exceeding 20°) are not reliable [12]. Finally, the reproducibility of TDI measurements is relatively low and the absolute values are vendor specific [21, 22].
Strain Imaging
Strain imaging is a cardiac ultrasound technique to evaluate the myocardial deformation or the fractional change in the length of a myocardial segment. Interests in estimating parameters, specifically strain and elasticity, associated with myocardial deformation have evolved over the past several decades from techniques utilizing implanted cardiac markers to noninvasive imaging based strain estimation techniques using magnetic resonance imaging (MRI), computed tomography (CT) or ultrasound [23–26]. The interest in strain imaging stems from the ability to understand regional and global cardiac mechanics and to evaluate for early, subclinical LV dysfunction, which may not be adequately described by more standard parameters such as LV ejection fraction (EF). Myocardial deformation is a result of contraction and relaxation of the cardiac muscle fibers in the longitudinal, circumferential and radial directions during the cardiac cycle. Therefore, the measurements of myocardial deformations are typically made along these three axes, resulting in longitudinal strain, circumferential strain and radial strain, relative to a specific instant in the cardiac cycle [27]. Unlike myocardial velocity, strain is less susceptible to effects of tethering (i.e., influence of neighboring regions), cardiac translation, and loading conditions [26]. Two ultrasound based techniques used to estimate the strain associated with myocardial deformations are TDI, as discussed in the previous section, and speckle-tracking echocardiography (STE) [27, 28].
TDI based strain imaging
As previously mentioned, TDI depicts velocities within the myocardium as compared to conventional Doppler imaging that depicts velocities associated with the blood [29]. Strain rate which describes the rate of myocardial deformation can be derived from the ratio of the differences in these tissue velocities at two locations divided by the distance between the two locations. Myocardial strain (natural strain) is then calculated as the temporal integral of the TDI-derived strain rate; from this Lagrange strain can be found using the mathematical relationship (Lagrange strain = exp (natural strain) −1) [27]. Myocardial displacement can also be obtained by integrating a localized velocity profile obtained using TDI. TDI-based strain techniques are limited primarily by angle dependency and the requirement for high frame rates. In addition, radial and circumferential strain by TDI can only be assessed in a limited number of LV segments.
STE based strain imaging
Two-dimensional STE relies on a semi-automated method to match speckle patterns in consecutive frames to estimate displacement of an ROI and therefore, provide an estimate of strain and strain rate (Fig. 2). This technique is, however, limited by the potential for through-plane speckle motion (i.e., speckle pattern movement with respect to the scan plane during a cardiac cycle; through-plane motion also affects TDI based measurements) and is therefore most reliable for longitudinal and circumferential (rather than radial) strain. The issue of through-plane motion can be overcome by 3D STE, which allows for the simultaneous measurement of global and regional longitudinal, circumferential, and radial strain. Three-dimensional STE utilizes specialized algorithms such as correlation analysis, block-matching (also utilized with 2D STE), elastic registration or model based approaches to quantify strain [30]. Both 2D and 3D STE, however, require image quality such that the morphological details of the myocardium can be tracked from one frame to the next. Lastly, 3D STE is limited at times by suboptimal temporal and spatial resolution (due to 3D acquisitions), but continued advancements in ultrasound transducer technology and beamforming are ongoing to overcome these issues.
Figure 2.
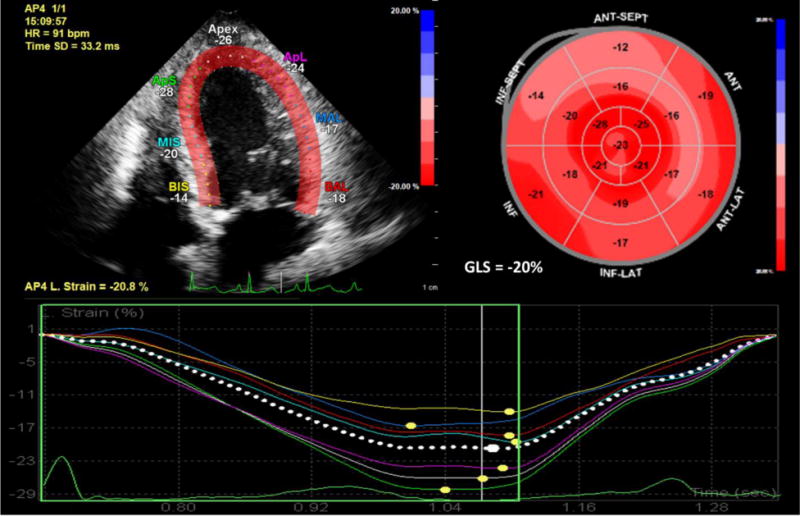
Longitudinal strain by two-dimensional speckle-tracking echocardiography is shown in the apical four chamber view in top left. Bull’s eye map showing individual longitudinal strain values for all myocardial segments with color overlay and global longitudinal strain is shown in the top right. Longitudinal strain curves for ventricular segments in the four chamber view are shown at the bottom.
Current Clinical Status of Strain Imaging
The time required for strain analysis and the lack of software and speckle-tracking algorithm standardization from the various vendors currently have prevented complete adaptation into routine clinical practice [30]. In fact, a meta-analysis of 2597 subjects from 24 studies demonstrated that normal values for global longitudinal, circumferential and radial strain varied significantly between studies citing vendor differences to be a source for these variations. In this study, the normal values for global longitudinal, circumferential and radial strain ranged from −22.1% to −15.9% (95% CI: −20.4% to −18.9%), −27.8% to −20.9% (95% CI −24.6% to −22.1%) and 35.1% to 59.0% (95% CI: 43.6% to 51.0%), respectively [31]. In an attempt to reduce inter-vendor variability, a consensus document was published in 2015 by members of the European Association of Cardiovascular Imaging, the American Society of Echocardiography and industry that standardized definitions and procedures for implementing STE [32]. Despite these ongoing issues, global longitudinal strain is emerging as an important parameter in clinical practice to determine etiology of cardiomyopathies, to aid with cardiac resynchronization therapy, and to track patients receiving potentially cardiotoxic chemotherapy [32, 33]. Continued efforts at standardization between different vendors and further clinical research may pave the way for more widespread adoption of strain imaging in echocardiography.
Contrast Echocardiography (CE)
While echocardiography is the most commonly used imaging modality for evaluating cardiac structure and function [34, 35], an estimated 20% of these studies may be suboptimal [36, 37]. Suboptimal image quality served as a catalyst for the development of commercial, microbubble-based, ultrasound contrast agents, which aid in the enhancement of the blood-pool tissue interface [38]. Although agitated saline has been used as a contrast agent for over 35 years, the microbubbles in agitated saline are too short-lived and too large to pass through the pulmonary capillary circuit thereby limiting their use [39]. As a result, commercial contrast agents were specifically engineered so the microbubbles were small enough (< 8 μm) to pass through the capillaries in the lungs to reach the left heart [39].
Compared to muscle, fluid, such as blood, typically appears anechoic (i.e., black) on an ultrasound image since red blood cells do not scatter ultrasound pulses as much as tissue. However, when ultrasound interacts with microbubbles, it causes them to oscillate (i.e., contract and expand), thereby increasing backscatter of the ultrasound beam [40]. The backscatter increases the echogenicity of the blood-filled region, making targets such as the LV walls easier to visualize (Fig. 3) [40]. Left ventricular opacification (LVO) for endocardial border detection (EBD) benefits from harmonic imaging with a low acoustic intensity to limit the possibility of causing adverse biological effects and improve the contrast-noise ratio [41]. Left ventricular opacification also enables more accurate quantitative evaluation of LV size and systolic function by assessment of chamber volumes and EF [42, 43]. The correlation and agreement of LV EF is significantly improved with the addition of contrast to echocardiography compared to MRI (from 0.6 without contrast to 0.8 with contrast; p < 0.05), which is considered the reference standard for LV EF assessment [42, 44]. Volume measurements with 2D and 3D CE agree moderately with MRI [45]. The reliability of LV function measurements is especially important for individuals requiring serial testing, such as those receiving potentially cardiotoxic chemotherapy [44, 46].
Figure 3.
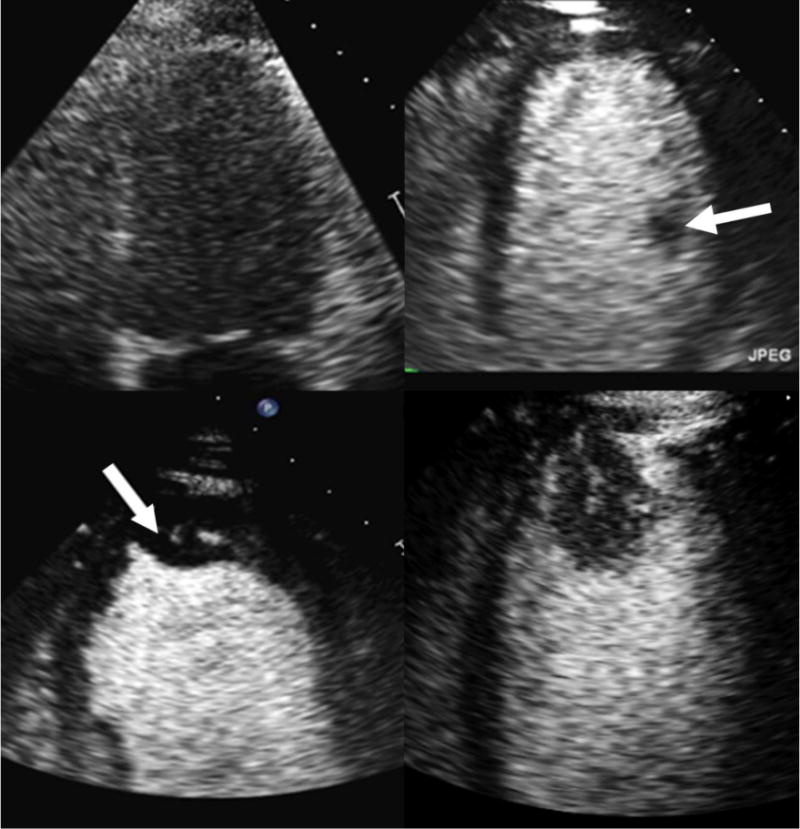
Endocardial borders are not well visualized without contrast (top left). Contrast microbubbles opacify the left ventricle improving delineation of endocardial borders (top right). A papillary muscle (white arrow), a normal intracardiac structure, is outlined by the contrast. Contrast enables clear visualization of an apical thrombus (arrow; bottom left) and apical tumor (bottom right). The presence of a vascular channel and enhancement with contrast help to differentiate apical tumor from apical thrombus.
Contrast agents also have a greater utility beyond EBD including assessment of structural cardiac abnormalities, evaluation of myocardial perfusion, and differentiation of intracardiac masses [46]. Because CE improves the ability to visualize the LV endocardium, a sonographer is able to create images that are not foreshortened and to better identify apical abnormalities such as apical-variant hypertrophic cardiomyopathy, apical aneurysms, and apical thrombi and tumors, which are often missed without contrast. Lastly, CE can also aid in the identification of LV non-compaction cardiomyopathy which is characterized by deep myocardial recesses and trabeculations, which results from the lack of normal compression of the layers of the myocardium [47].
Contrast echocardiography is also used with both pharmacologic and exercise stress echocardiography to improve EBD and facilitate evaluation of regional wall-motion before and after stress in order to exclude myocardial ischemia [48]. Although considered an off-label application, myocardial perfusion imaging (MPI) provides additional diagnostic and prognostic value to stress echocardiography, since it can help detect perfusion abnormalities in the sub-endocardium even when wall motion is still normal [49, 50]. The MPI technique uses short-high MI ultrasound pulses to destroy microbubbles within the heart muscle. The contrast is replenished in areas with normal perfusion, while replenishment is delayed or absent in regions with significant coronary obstruction [51]. MPI is also being used to help evaluate perfusion of the myocardium after infarction, to assess outcomes after coronary revascularization, and in those undergoing heart transplant [52–54].
Contrast echocardiography is highly sensitive (> 90%) for diagnosing intracardiac masses such as thrombi and tumors [46, 55]. Because a thrombus is an avascular collection of clotted blood; it will appear as a darkened area surrounded by contrast (Fig. 3) [46]. On the other hand, both benign and malignant masses are vascular to some degree [55]. In addition, if contrast perfusion in a mass occurs quickly, the probability is higher that it is malignant rather than benign (e.g., mass refilling velocity of 10.5 s−1 vs. 0.9 s−1 for a secondary malignant tumor vs. a myxoma, respectively) [55]. Lastly, contrast use during transesophageal echocardiography (TEE) prior to an electrical cardioversion can help differentiate thrombus in the left atrial appendage from other normal structures, reverberation artifact, and slow flow [46, 56].
Contrast echocardiography can also help to transform 90% of suboptimal studies into diagnostic exams, which may shorten time to diagnosis thereby significantly affecting patient management and care [37, 57]. It has been estimated that alterations in patient management, such as modifying drug regimens or performing additional tests/procedures, can be avoided in more than a third of patients with the use of CE; this translates to a cost savings of at least $122 per patient [57]. In summary, CE is a cost-effective, reliable, and efficient method to improve suboptimal images, decrease reader variability, and increase the accuracy of diagnosis, and, therefore, has become an established tool in many cardiac ultrasound laboratories.
3D Echocardiography
Three-dimensional (3D) echocardiography represents a major advance in the field of cardiac ultrasound and has become an established tool for the evaluation of cardiac structure and function. Attempts at 3D echocardiography with mechanically driven scanning techniques originated as early as 1974 [58], but these techniques were cumbersome, produced only static images, and were utilized primarily for research purposes [59]. ‘Real-time 3D echocardiography’ (in which the transducer remains stationary) became possible in the early 1990s with the development of matrix-array transducers (which contain up to 3,000 piezoelectric elements) and advances in parallel processing technology [59]. While 3D echocardiography is limited by lower spatial and temporal resolution compared to 2D echocardiography and 3D-specific artifacts, the technique allows cardiac structures and pathology to be viewed from unique, “life-like” perspectives, which would otherwise not be possible.
3D Echocardiography Acquisition and Display Techniques
With current real-time 3D echocardiography systems, a pyramidal or volumetric dataset is acquired with the use of a 3D-capable matrix array transthoracic or TEE probe. The size of the volume is denoted by the lateral (azimuth) and elevation planes and depth of the acquisition. The varying acquisition modes are the ‘live’ or narrow-angle mode (approximately 30 × 60 degrees), zoom or magnification mode (approximately 30 × 30 degrees), and the ‘full-volume’ or wide-angle mode (approximately 90 × 90 degrees) [59, 60]. Three-dimensional echocardiography can also be integrated with color flow Doppler in all three acquisition modes but the flow measurements are limited by the lower volume imaging rates. Due to the decreased temporal resolution of 3D images with increasing volume size and/or the addition of color Doppler, temporal resolution can be improved (>30 Hz) with electrocardiographically gated ‘multi-beat’ mode whereby multiple sub-volumes from several cardiac cycles are joined together to create a single volumetric dataset [60]. This technique improves temporal resolution, but at the cost of the “stitch artifact,” which is due to improper co-registration of sub-volumes caused by respiratory artifacts or irregular heart rhythms [60].
Three-dimensional echocardiography images can be displayed in multiple formats after image acquisition has been performed (Fig. 4). The most commonly used display method is the ‘volume-rendering’ whereby individual voxels are enhanced to produce depth perception for the viewer. These images can be cropped and oriented into views that best show the ROI and their spatial relationship to surrounding structures. ‘Multiplanar reconstruction” is a tomographic imaging technique whereby multiple 2D views (sagittal, coronal, and transverse planes) are created from the volumetric dataset and visualized simultaneously. Lastly, a ‘surface rendering’ is a computer-generated solid or wire-frame image of the surface of a 3D structure, which is usually created with manual or semi-automated tracing of a structure such as the LV or the mitral valve [60]. These last views are generally utilized for advanced quantitative analysis of cardiac structures.
Figure 4. 3D Echocardiographic Images.
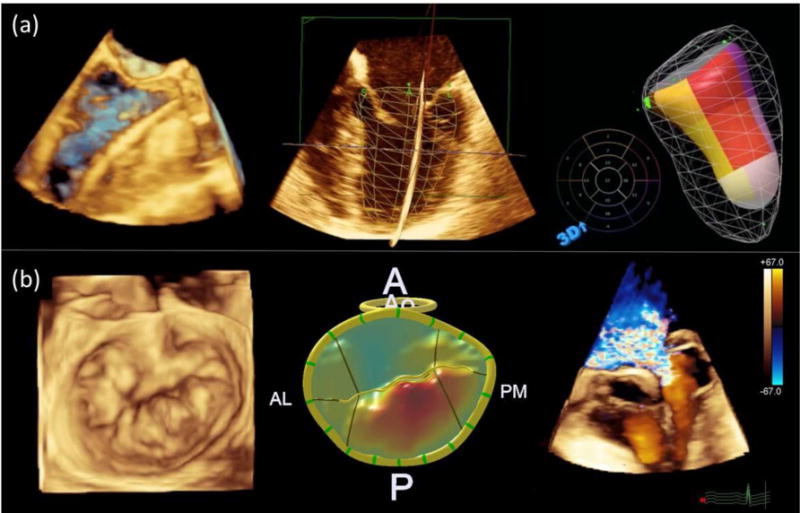
Panel a (top): Volume rendering showing wire in left ventricle during transcatheter aortic valve replacement (left); wire-frame surface rendering superimposed over multiplanar two-dimensional views of left ventricle (middle); and color-coded surface rendering of left ventricle with each color representing a single ventricular segment (right). Panel b (bottom): Volume rendering of mitral valve depicting posterior-leaflet mitral valve prolapse (left); surface rendering of mitral valve with color gradations representing degree of prolapse relative to annular plane (middle); and volume rendering with color Doppler depicting severe mitral regurgitation due to mitral valve prolapse (right).
Important Applications of 3D Echocardiography
Quantitative volumetric assessment of the LV including calculation of LV EF with 3D echocardiography is currently recommended over 2D echocardiography when feasible [60, 61]. Three-dimensional echocardiography overcomes limitations of 2D tomographic imaging (e.g., LV foreshortening and geometric assumptions regarding LV shape) [60]. Moreover, in a large meta-analysis of 23 studies (involving 1,638 echocardiograms), comparing volumes measured with both 2D and 3D echocardiography with volumes measured using cardiac MRI, the pooled biases for LV volumes were significantly lower for 3D (end diastolic volume: −19.1 ± 34.2 ml and end systolic volume: −10.1 ± 29.7 ml) than for 2D echocardiography (end diastolic volume: −48.2 ± 55.9 ml and end systolic volume: −27.7 ± 45.7 ml) [62]. A newer, fully ‘automated adaptive analytics algorithm’ for 3D analysis of the LV has recently been developed, which utilizes knowledge-based identification of global LV shape and chamber orientation followed by patient-specific adaptation to detect LV endocardial surfaces [63]. This algorithm and other such automated analyses have decreased total 3D analysis time as compared to manual 3D echocardiography methods [63, 64].
Three-dimensional echocardiography has revolutionized the assessment of valvular heart diseases. With 3D transesophageal echocardiography, the mitral valve can be visualized en face in the “surgeon’s view” as visualized in the operating room at the time of mitral valve repair [60]. Three-dimensional echocardiography has been used for identification of prolapsing mitral valve segments (based on 3D renderings of the mitral valve [65–67], for evaluating mitral and aortic regurgitation [68, 69] and for evaluation of mitral valve stenosis [70].
Three-dimensional echocardiography has emerged as an important tool in the pre-procedure planning and intra-procedure guidance of catheter-based structural heart disease procedures. Prior to transcatheter aortic valve replacement, 3D echocardiography can provide an important assessment of the aortic annulus and aortic root complex to determine the size and final positioning of the valve prosthesis [60, 71]. Atrial septal defect size is more accurately determined in 3D, since the presence of residual shunting after percutaneous closure is strongly associated with underestimation of defect size by 2D echocardiography [72]. Lastly, 3D echocardiography has become essential for determining patient suitability for valve repair, and for guiding percutaneous cardiac interventions including mitral valve repair [60, 65], closure of paravalvular prosthetic valve defects [60, 73], and left atrial appendage closure [60].
Point of Care Ultrasound
Point-of-care ultrasound (POCUS) is performed where a patient is being imaged or treated at the bedside, in an emergency department (ED), or even outside of a hospital rather than in an ultrasound lab [74]. Point-of-care ultrasound has been used for over two decades for clinical decision making and in the management of both the adult and pediatric patients [75, 76]. However, the advent of smaller and more portable technology has increased the availability and use of POCUS, which has led to multiple new applications of the diagnostic method [77]. For many physicians, including those in emergency medicine and critical care, POCUS is quickly becoming an integral tool for patient management decisions.
Kimura has provided an exhaustive review of POCUS drawing comparisons between traditional cardiac physical examination and describing the advantages of POCUS as a limited but informative ultrasound examination; the economic, quality of care and medico-legal considerations of POCUS are also discussed [78]. Following his review of 52 articles, Kimura concludes that POCUS augments physical exam findings and clinical assessment in cases of LV systolic dysfunction, left atrial enlargement, pulmonary edema or interstitial disease and pleural effusion, whereas expert ultrasound practice may be required in the assessments of right ventricular enlargement or pulmonary hypertension, valve regurgitation and severe aortic stenosis [78]. Moreover, based on a wide range of users (from medical students to cardiologists), the addition of POCUS increased the accuracy of clinical findings [78, 79]. For example, in a multicenter study of 443 patients referred for bedside consultation, a pocket size echocardiography device provided significantly higher diagnostic value (sensitivity: 88%; specificity: 86%) as compared to physical examination alone (sensitivity: 75%; specificity: 62%) and physical examination combined with electrocardiography (sensitivity: 80%; specificity: 67%) [79].
Fox et al. showed that the use of POCUS may be advantageous in the screening of certain clinical conditions in a dedicated patient population, e.g., hypertrophic cardiomyopathy in young athletes, since such conditions may not be readily identified by physical examination alone [80]. In a prospective study involving 78 patients (in the ED and intensive care unit) POCUS was useful in providing confirmation of correct supra-diaphragmatic central venous catheter placement (sensitivity: 86.8% and specificity: 100%) in less time (median time: 16 minutes) relative to chest radiography (median time: 32 minutes) [81]. In another study of 2683 patients presenting with dyspnea and admitted after ED evaluation, the performance of POCUS and standard ED workup for diagnosis of clinical conditions were compared [82]. While there were no statistically significant differences in the accuracy of POCUS and standard ED workup for the diagnosis of most clinical conditions, the time taken to arrive at the diagnosis by POCUS (24±10 minutes) was significantly lower (p = 0.025) than that for the standard ED workup (186±72 minutes) [82]. Dedicated POCUS protocols, therefore, have the potential to enhance the efficiency of current workflow and to better stratify patients needing additional evaluations. In another observational study from 20 hospitals in the United States and Canada POCUS was performed at the beginning and end of advanced cardiac life support in 793 patients suffering from out-of-hospital or in-ED cardiac arrest with pulseless electrical activity or asystole [83]. Results showed that cardiac activity identified by POCUS was the variable best associated with survival to hospital admission; POCUS also helped to identify findings that corresponded to non-advanced cardiac life support interventions [83].
Recognizing the potential role of POCUS in cases of hypotension and cardiac arrest, the members of the Ultrasound Special Interest Group of the International Federation for Emergency Medicine presented a consensus statement in 2016 based on prospectively collected disease incidence data which described two protocols for the use of sonography in hypotension and cardiac arrest (SHoC) [84]. Both SHoC protocols prescribe a hierarchial scanning methodology comprising core, supplementary and additional views based on the “4F” approach – fluid, form, function, filling [84]. For both the SHoC protocols, subxiphoid or parasternal long axis cardiac view initiates the scanning hierarchy for clinical diagnosis [84]. Given this international consensus statement, the role of POCUS on clinical diagnosis and subsequent management of patients presenting in the ED with hypotension or cardiac arrest is likely to increase. Knowledge of the hemodynamic status of critical care patients is essential; therefore, it is not surprising that the most common application of POCUS in the intensive care units is to assess the heart [85]. Focused cardiac ultrasound (FoCUS) is a simplified approach to addressing a specific clinical question in a particular scenario, but as with all POCUS applications, the scope of what can or should be assessed is limited [86, 87]. However, the FoCUS scan is a rapid way of obtaining information about the heart (i.e., wall contraction, chamber dilatation, valve motion), which can be used to help determine the cause of a patient’s condition [87].
While POCUS is a tool that should be used for screening and as an adjunct to assessment, it should not be considered a replacement for other diagnostic methods [88, 89]. Because POCUS is not a comprehensive exam, all existing pathology may not be detected or fully examined, but the findings from the test can expedite clinical care [90].
3D Volumetric Flow Assessment
Assessment of cardiac blood volume is clinically useful for the diagnosis and management of a variety of clinical malignances. The estimation of volumetric flow using Doppler-based spatial velocities multiplied by the luminal cross sectional area is well established. Validation of this approach has been performed in in vitro flow phantoms by Hoyt and colleagues [91]. In this study, three individual operators used five separate ultrasound scanners to estimate flow volume in a phantom over a range of 100 to 1000 ml/min. The study demonstrated excellent correlation between controlled flow rates and ultrasound-derived flow measurements for all five systems (r2 > 99.1%), indicating this approach is accurate in scenarios where the lumen geometry is homogenous and properly visualized [91]. Clinically, this approach for volumetric flow assessment has been applied to the quantification of blood volumes in a variety of vascular structures in both adults and fetuses [92–94]. Despite this acceptance, numerous issues arise with this approach including heterogeneous flow, irregular lumen geometries, and the pulsatile nature of flow over the cardiac cycle [95]. These limitations become increasingly arduous when computing volumetric flow within the heart. However, recent advances in the design of real-time volumetric transducers (i.e. 4D imaging) combined with improved velocity estimation algorithms using Doppler or speckle tracking approaches now hold promise for improving the estimation of volumetric blood flow across the cardiac system.
The implementation of Doppler using 4D probes for the quantification of blood flow has been reported over the last 10 years [96–98]. Kripfgans and colleagues used this approach using a mechanically controlled 3D probe and a custom flow phantom, showing strong overall accuracy (+/− 15% or actual flow values) [97]. Importantly, it was also demonstrated that this accuracy was independent of Doppler angle and could be translated to in vivo applications (the group later showed a correlation of r2=0.95 between 4D ultrasound flow measurements and invasive blood flow meters in the femoral arteries of canines) [96, 97]. In a similar study, Forsberg et al. investigated a 4D Doppler system with semi-automatic flow estimation software [98]. Again using an in vitro flow phantom, blood-mimicking fluid was used to generate pulsatile flow at rates of 60-600 ml/min. The volumetric Doppler system showed excellent correlation with flow-meter measurements (r2 > 0.99) [98]. In vivo validation was then performed in the distal aorta of 6 rabbits, again showing excellent correlation (r2 = 0.86) between the 4D Doppler system measurements and an invasive flowmeter [98]. This approach was then translated to a pilot clinical trial in order to use 4D Doppler flow estimation for the assessment of carotid stenosis [99]. In a prospective trial, semi-automated volumetric Doppler estimates were obtained in 59 patients referred for clinical carotid ultrasounds. Strong agreement was found between the volumetric Doppler measurements and internal carotid artery peak systolic velocity (interclass correlation coefficient of 0.83) and between volumetric Doppler and ratio of internal carotid to common carotid peak systolic velocity ratio (interclass correlation coefficient of 0.65) [99]. Hence, these approaches are believed to provide a reasonable alternative to 2D volumetric blood flow measurements, with less angle dependency, less operator dependence, and greater reproducibility.
Recent developments in ultrasound imaging technology have led to the development of high frame rate ultrasound imaging with frame rates exceeding 20,000 frames/second [100]. Imaging at these high frame rates allow for new areas of imaging research, including plane wave imaging with ultrafast Doppler and high resolution vector flow imaging [101]. These advancements have proven useful for cardiac applications and have recently been used for complete visualization of Doppler blood flow over the entire heart at ultrafast frame rates [101], evaluation of cardiac electrophysiology [102], strain imaging [103], and the quantification of blood velocities using vector projections by tracking speckle movement [104]. For example, Van Cauwenberge et al. recently used ultrafast imaging with 2D speckle tracking to estimate intraventricular blood flow in neonates [105]. The group found good overall performance of this technique (underestimation and angular deviation of 28% and 13.5° during systole and 16% and 10.5° during diastole), but noted that out of plane vector motion compromised overall findings. Despite these limitations, 2D ultrafast imaging systems are now commercially available and their adoption for cardiac applications is expected to continue to grow.
Ultrafast ultrasound imaging has also been implemented on 4D probes using the matrix array transducers described above. Potential advantages of this implementation include limiting angle dependency, reducing out of plane motion, and providing a complete volumetric representation of both flow and cardiac structure. Provost and colleagues developed a custom 32 × 32 matrix array probe capable of performing 3D shear wave imaging, 3D ultrafast Doppler imaging, and a combination of 3D tissue and flow Doppler imaging at volume acquisition rates of thousands of volumes/second [101]. This custom platform was then used for cardiac imaging to track blood flow over the course of a single cardiac cycle, and also to investigate the motion of red blood cells at the carotid bifurcation [101]. Similarly, this group implemented 4D ultrafast imaging on a 1024 channel matrix array to obtain volumes at over 4000 volumes/second and validate their velocity-derived volumetric flow rates both in vitro and in vivo [106]. In a flow phantom, the group found less than 5% error in estimated flow volume, but these errors increased at higher flow rates and velocities (18.3% at 490 ml/min and 1.3 m/s). Finally, the group also performed preliminary in vivo imaging, quantifying the total volume flow over a single cardiac cycle and also demonstrating an ability to visualize 3D vortices [106]. While these approaches are still in their infancy, the ability to quantify blood flow using commercially available, volumetric transducers with either Doppler or ultrafast imaging is expected to improve clinicians’ ability to estimate volumetric cardiac blood flow in the near future.
Elastography
This section discusses three elastography techniques, acoustic radiation force impulse (ARFI) imaging, shear wave elasticity imaging (SWEI) and supersonic shear imaging (SSI) that all have the potential to significantly enhance the clinical evaluation of the heart. These three techniques use acoustic radiation force to mechanically stimulate tissue and monitor the response [107, 108]. Acoustic radiation force is one of the non-thermally mediated bioeffects of ultrasound, which is generated by a change in the density of energy and momentum of the propagating waves due to the absorption, reflection and scattering from inclusions or from spatial variations in propagation velocity [109]. ARFI imaging is considered a qualitative elastography technique, as it can provide relative measures of tissue stiffness, while SWEI is considered a quantitative elastography technique that can provide absolute measures of tissue stiffness [109, 110]. The third imaging technique, SSI, is also quantitative, as it can map the stiffness of soft tissue characterized by the Young modulus defined by the slope of the stress/strain curve [108].
There are several fundamental differences between these three imaging modalities that are comprehensively reviewed by others [108, 111], and describing these differences in detail is outside the scope of our current review. Briefly, ARFI uses a radiation force induced by a focused ultrasound beam to create a two-dimensional stiffness map by recording elasticity information from the axis of the pushing beam [111]. Unlike ARFI, that does not use shear waves, SWEI and SSI generate shear waves and aim to measure the velocity of shear wave propagation in tissue using different approaches. SWEI is based on a conventional imaging approach, using a single pushing beam to generate shear waves which are tracked at multiple off-axis lateral locations, distributed throughout the field of view with known separation distance from the pushing beam [111]. SSI is based on the use of ultrafast ultrasound imaging to track the shear wave. Unlike SWEI which can be implemented on existing clinical systems, SSI is a technique that requires a specialized ultrasound scanner (e.g., SuperSonic Imaging’s Aixplorer®) that creates a near plane wave supersonic shear front by rapidly successively focusing the supersonic push at multiple axial depths and then monitoring the shear wave propagation off-axis at multiple locations [111]. This technique uses ultrafast imaging (frame rate of 10,000 images per second) to measure the propagation speed of shear waves acoustically induced in tissue, allowing for the assessment of absolute tissue stiffness [108, 112].
Myocardial stiffness is considered to be an important component in the pathophysiology of diastolic dysfunction [113] and in HFpEF [114]. Myocardial stiffness is also an important parameter in clinically diagnosing hypertrophy (myocardial thickening) caused by high blood pressure [115] and dilated cardiomyopathy [116, 117]. Current methods directed at quantifying cardiac function using measures of the dynamic material properties of the myocardium, such as invasive cardiac chamber cavity pressure measurements and ultrasound-based strain and Doppler imaging are indirect measures of material properties of the myocardium and are typically load dependent [110, 118, 119]. However, ARFI imaging, SWEI and SSI are able to directly assess the mechanical properties of tissue [111, 112, 120, 121].
Vejdani-Jahromi et al. demonstrated that SWEI assessment of a Langendorff perfused rabbit heart was able to measure the relaxation time constant (τ), an important assessment of diastolic dysfunction and the shear modulus of stiffness of the myocardium, providing a systolic/diastolic stiffness ratio that provides a direct measure of load-independent myocardial stiffness [110]. In addition, they demonstrated that ARFI-based relative stiffness measurements correlate well with SWEI absolute stiffness measurements [110]. Hsu and colleagues demonstrated that ARFI imaging with an intracardiac probe positioned in the right atrium was able to monitor radiofrequency ablation better than standard B-mode images, as ARFI imaging was able to delineate variations of tissue stiffness within the area of myocardium at the ablation site, monitoring the extent of ablated tissue and differentiating it from the surrounding healthy tissue [122, 123]. Song et al. have investigated the impact of myocardial anisotrophy (muscle fiber orientation changes through the thickness of the wall) on shear wave speed in both a finite element model and when imaging different left ventricular wall segments from different transthoracic views in healthy children [124, 125]. In addition, this group recently systematically evaluated possible transthoracic echocardiographic views for cardiac shear wave elastography in both healthy children and adults [124, 126].
Similar to ARFI imaging and SWEI, SSI is a fairly new ultrasound-based diagnostic technique that relies on the use of acoustic waves remotely inducing a radiation force on tissue within the body. Supersonic Shear Imaging has shown the potential to provide clinically relevant information about tissue material properties that cannot be assessed currently without the use of radiopharmaceuticals and expensive imaging equipment. Pernot et al. recently demonstrated that SSI evaluation of passive diastolic myocardial stiffness can differentiate areas of the heart tissue that have been only transiently injured (stunned myocardium) and remain viable with the possibility of functional recovery versus tissue that has been irreversibly damaged (infarcted myocardium) in an ovine model [118]. Differentiating stunned myocardium (viable) versus infarcted myocardium (dead tissue) has been historically evaluated using radiopharmacologic agents such as Thallium-201 and single-photon emission computed tomography (SPECT) scans, and more recently using other radioactive isotopes and positron emission tomography (PET) scans. If SSI proves to be reliable and effective in identifying stunned versus infarcted myocardial tissue in the clinical setting, this technique could help identify patients that would benefit from cardiac interventions, such as coronary stent placement and coronary artery bypass, while decreasing exposure to radioactive agents used in current myocardial viability imaging.
Much work is still needed to assess which acoustic radiation force excitation methods will be clinically useful for cardiac evaluations in humans. Investigations thus far demonstrate that several elastography techniques have the potential to provide clinically meaningful information regarding myocardial stiffness in cardiac disease.
Conclusion
With the advancements summarized in the above review related to tissue Doppler imaging, strain imaging, contrast echocardiography, 3D echocardiography, point-of-care echocardiography, 3D volumetric flow assessments, and elastography, ultrasound imaging is rapidly changing the domain of cardiac imaging. The advantages offered by ultrasound imaging (with respect to real-time imaging, imaging at patient bedside, cost-effectiveness and ionizing-radiation-free alternative) coupled with steps towards standardization of ultrasound based quantitative markers will play a major role in addressing the healthcare burden associated with cardiovascular diseases.
Supplementary Material
Highlights.
Recent technological advancements in the field of cardiac ultrasound are reviewed
Contrast echocardiography has become an established practice in cardiac ultrasound
Standardization will translate these advancements for clinical cardiac imaging
Acknowledgments
This work was supported in part by the National Institutes of Health (R21HL130899, 2016) and the American Heart Association (15SDG25740015, 2015).
Appendix A: List of Abbreviations
- ARFI
acoustic radiation force impulse
- CE
contrast echocardiography
- CT
computed tomography
- EBD
endocardial border definition
- ED
emergency department
- EF
ejection fraction
- FoCUS
focused cardiac ultrasound
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- ICU
intensive care unit
- MI
mechanical index
- MPI
myocardial perfusion imaging
- MR(I)
magnetic resonance (imaging)
- LV
left ventricle/ventricular
- LVO
Left ventricular opacification
- PET
positron emission tomography
- POCUS
point-of-care ultrasound
- RV
right ventricle/ventricular
- SHOC
sonography in hypotension and cardiac arrest
- SWEI
shear wave elasticity imaging
- SWI
shear wave imaging
- SPECT
single-photon emission computed tomography
- STE
speckle-tracking echocardiography
- TDI
tissue Doppler imaging
- TEE
transesophageal echocardiography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hanna IR, Silverman ME. A history of cardiac auscultation and some of its contributors. Am J Cardiol. 2002;90:259–267. doi: 10.1016/s0002-9149(02)02465-7. [DOI] [PubMed] [Google Scholar]
- 2.Meyer RA. History of ultrasound in cardiology. J Ultrasound Med. 2004;23:1–11. doi: 10.7863/jum.2004.23.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Roelandt JR. Seeing the invisible: a short history of cardiac ultrasound. Eur J Echocardiogr. 2000;1:8–11. doi: 10.1053/euje.2000.0006. [DOI] [PubMed] [Google Scholar]
- 4.Mehta NJ, Khan IA. Cardiology’s 10 greatest discoveries of the 20th century. Tex Heart Inst J. 2002;29:164–171. [PMC free article] [PubMed] [Google Scholar]
- 5.Picano E. Economic and biological costs of cardiac imaging. Cardiovasc Ultrasound. 2005;3:13. doi: 10.1186/1476-7120-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winkelmann JW, Block RJ, Feinstein SB. Usefulness of echo enhancement in stress echocardiography. In: Nanda NC, Schlief R, Goldberg BB, editors. Advances in echo imaging using contrast enhancement. Kluwer Academic Publishers; 1997. pp. 361–371. [Google Scholar]
- 7.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, C American Heart Association Statistics, S. Stroke Statistics Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Organization WH. Cardiovascular diseases (CVDs) Fact Sheet. 2016 [Google Scholar]
- 9.Satomura S. A study on examining the heart with ultrasonics: I. Principle; II. Instrument. Japanese Circulation Journal. 1956;20:227. [Google Scholar]
- 10.White DN. Neurosonology pioneers. Ultrasound Med Biol. 1988;14:541–561. doi: 10.1016/0301-5629(88)90121-4. [DOI] [PubMed] [Google Scholar]
- 11.Wells PN. Ultrasonic colour flow imaging. Phys Med Biol. 1994;39:2113–2145. doi: 10.1088/0031-9155/39/12/001. [DOI] [PubMed] [Google Scholar]
- 12.Kadappu KK, Thomas L. Tissue Doppler imaging in echocardiography: value and limitations. Heart Lung Circ. 2015;24:224–233. doi: 10.1016/j.hlc.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal R, Gosain P, Kirkpatrick JN, Alyousef T, Doukky R, Singh G, Umscheid CA. Tissue Doppler imaging for diagnosis of coronary artery disease: a systematic review and meta-analysis. Cardiovasc Ultrasound. 2012;10:47. doi: 10.1186/1476-7120-10-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isaaz K, Thompson A, Ethevenot G, Cloez JL, Brembilla B, Pernot C. Doppler echocardiographic measurement of low velocity motion of the left ventricular posterior wall. Am J Cardiol. 1989;64:66–75. doi: 10.1016/0002-9149(89)90655-3. [DOI] [PubMed] [Google Scholar]
- 15.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelisa A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009;10:165–193. doi: 10.1093/ejechocard/jep007. [DOI] [PubMed] [Google Scholar]
- 16.Mor-Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G, Galderisi M, Marwick T, Nagueh SF, Sengupta PP, Sicari R, Smiseth OA, Smulevitz B, Takeuchi M, Thomas JD, Vannan M, Voigt JU, Zamorano JL. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. J Am Soc Echocardiogr. 2011;24:277–313. doi: 10.1016/j.echo.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 17.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. quiz 786-688. [DOI] [PubMed] [Google Scholar]
- 18.Bax JJ, Abraham T, Barold SS, Breithardt OA, Fung JW, Garrigue S, Gorcsan J, 3rd, Hayes DL, Kass DA, Knuuti J, Leclercq C, Linde C, Mark DB, Monaghan MJ, Nihoyannopoulos P, Schalij MJ, Stellbrink C, Yu CM. Cardiac resynchronization therapy: Part 1–issues before device implantation. J Am Coll Cardiol. 2005;46:2153–2167. doi: 10.1016/j.jacc.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 19.Chung ES, Leon AR, Tavazzi L, Sun JP, Nihoyannopoulos P, Merlino J, Abraham WT, Ghio S, Leclercq C, Bax JJ, Yu CM, Gorcsan J, 3rd, St John Sutton M, De Sutter J, Murillo J. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation. 2008;117:2608–2616. doi: 10.1161/CIRCULATIONAHA.107.743120. [DOI] [PubMed] [Google Scholar]
- 20.Sharifov OF, Schiros CG, Aban I, Denney TS, Gupta H. Diagnostic Accuracy of Tissue Doppler Index E/e’ for Evaluating Left Ventricular Filling Pressure and Diastolic Dysfunction/Heart Failure With Preserved Ejection Fraction: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.115.002530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Denes M, Farkas K, Erdei T, Lengyel M. Comparison of tissue Doppler velocities obtained by different types of echocardiography systems: are they compatible? Echocardiography. 2010;27:230–235. doi: 10.1111/j.1540-8175.2009.01018.x. [DOI] [PubMed] [Google Scholar]
- 22.Abe K, Yuda S, Sato Y, Yasui K, Nishi R, Hanada K, Hashimoto N, Kawamukai M, Kouzu H, Muranaka A, Hashimoto A, Tsuchihashi K, Watanabe N, Miura T. Intervendor Variabilities of Left and Right Ventricular Myocardial Velocities among Three Tissue Doppler Echocardiography Systems. Echocardiography. 2015;32:1790–1801. doi: 10.1111/echo.12962. [DOI] [PubMed] [Google Scholar]
- 23.Waldman LK, Fung YC, Covell JW. Transmural myocardial deformation in the canine left ventricle. Normal in vivo three-dimensional finite strains. Circ Res. 1985;57:152–163. doi: 10.1161/01.res.57.1.152. [DOI] [PubMed] [Google Scholar]
- 24.Mirsky I, Parmley WW. Assessment of passive elastic stiffness for isolated heart muscle and the intact heart. Circ Res. 1973;33:233–243. doi: 10.1161/01.res.33.2.233. [DOI] [PubMed] [Google Scholar]
- 25.Tee M, Noble JA, Bluemke DA. Imaging techniques for cardiac strain and deformation: comparison of echocardiography, cardiac magnetic resonance and cardiac computed tomography. Expert Rev Cardiovasc Ther. 2013;11:221–231. doi: 10.1586/erc.12.182. [DOI] [PubMed] [Google Scholar]
- 26.Urheim S, Edvardsen T, Torp H, Angelsen B, Smiseth OA. Myocardial strain by Doppler echocardiography. Validation of a new method to quantify regional myocardial function. Circulation. 2000;102:1158–1164. doi: 10.1161/01.cir.102.10.1158. [DOI] [PubMed] [Google Scholar]
- 27.Smiseth OA, Torp H, Opdahl A, Haugaa KH, Urheim S. Myocardial strain imaging: how useful is it in clinical decision making? Eur Heart J. 2016;37:1196–1207. doi: 10.1093/eurheartj/ehv529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abraham TP, Dimaano VL, Liang HY. Role of tissue Doppler and strain echocardiography in current clinical practice. Circulation. 2007;116:2597–2609. doi: 10.1161/CIRCULATIONAHA.106.647172. [DOI] [PubMed] [Google Scholar]
- 29.McDicken WN, Sutherland GR, Moran CM, Gordon LN. Colour Doppler velocity imaging of the myocardium. Ultrasound Med Biol. 1992;18:651–654. doi: 10.1016/0301-5629(92)90080-t. [DOI] [PubMed] [Google Scholar]
- 30.Jasaityte R, Heyde B, D’Hooge J. Current state of three-dimensional myocardial strain estimation using echocardiography. J Am Soc Echocardiogr. 2013;26:15–28. doi: 10.1016/j.echo.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Yingchoncharoen T, Agarwal S, Popovic ZB, Marwick TH. Normal ranges of left ventricular strain: a meta-analysis. J Am Soc Echocardiogr. 2013;26:185–191. doi: 10.1016/j.echo.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 32.Voigt JU, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann R, Pedri S, Ito Y, Abe Y, Metz S, Song JH, Hamilton J, Sengupta PP, Kolias TJ, d’Hooge J, Aurigemma GP, Thomas JD, Badano LP. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. J Am Soc Echocardiogr. 2015;28:183–193. doi: 10.1016/j.echo.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Mondillo S, Galderisi M, Mele D, Cameli M, Lomoriello VS, Zaca V, Ballo P, D’Andrea A, Muraru D, Losi M, Agricola E, D’Errico A, Buralli S, Sciomer S, Nistri S, Badano L, C. Echocardiography Study Group Of The Italian Society Of Speckle-tracking echocardiography: a new technique for assessing myocardial function. J Ultrasound Med. 2011;30:71–83. doi: 10.7863/jum.2011.30.1.71. [DOI] [PubMed] [Google Scholar]
- 34.Glassy MS, Groves EM. Analysis of the quantitative improvements in resting echocardiographic image sharpness through the use of contrast enhanced echocardiography. Int J Cardiovasc Imaging. 2014;30:867–873. doi: 10.1007/s10554-014-0401-4. [DOI] [PubMed] [Google Scholar]
- 35.Larsson MK, Da Silva C, Gunyeli E, Ilami AA, Szummer K, Winter R, Bjallmark A. The potential clinical value of contrast-enhanced echocardiography beyond current recommendations. Cardiovasc Ultrasound. 2016;14:2. doi: 10.1186/s12947-015-0045-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Senior R, Becher H, Monaghan M, Agati L, Zamorano J, Vanoverschelde JL, Nihoyannopoulos P. Contrast echocardiography: evidence-based recommendations by European Association of Echocardiography. Eur J Echocardiogr. 2009;10:194–212. doi: 10.1093/ejechocard/jep005. [DOI] [PubMed] [Google Scholar]
- 37.Wei K. Contrast echocardiography: applications and limitations. Cardiol Rev. 2012;20:25–32. doi: 10.1097/CRD.0b013e318234218f. [DOI] [PubMed] [Google Scholar]
- 38.Appis AW, Tracy MJ, Feinstein SB. Update on the safety and efficacy of commercial ultrasound contrast agents in cardiac applications. Echo Res Pract. 2015;2:R55–62. doi: 10.1530/ERP-15-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Orde S, McLean A. Bedside myocardial perfusion assessment with contrast echocardiography. Crit Care. 2016;20:58. doi: 10.1186/s13054-016-1215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cosyns B, Roossens B, Hernot S, El Haddad P, Lignian H, Pierard L, Lancellotti P. Use of contrast echocardiography in intensive care and at the emergency room. Curr Cardiol Rev. 2011;7:157–162. doi: 10.2174/157340311798220467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mulvagh SL, Rakowski H, Vannan MA, Abdelmoneim SS, Becher H, Bierig SM, Burns PN, Castello R, Coon PD, Hagen ME, Jollis JG, Kimball TR, Kitzman DW, Kronzon I, Labovitz AJ, Lang RM, Mathew J, Moir WS, Nagueh SF, Pearlman AS, Perez JE, Porter TR, Rosenbloom J, Strachan GM, Thanigaraj S, Wei K, Woo A, Yu EH, Zoghbi WA, E. American Society of American Society of Echocardiography Consensus Statement on the Clinical Applications of Ultrasonic Contrast Agents in Echocardiography. J Am Soc Echocardiogr. 2008;21:1179–1201. doi: 10.1016/j.echo.2008.09.009. quiz 1281. [DOI] [PubMed] [Google Scholar]
- 42.Hoffmann R, von Bardeleben S, ten Cate F, Borges AC, Kasprzak J, Firschke C, Lafitte S, Al-Saadi N, Kuntz-Hehner S, Engelhardt M, Becher H, Vanoverschelde JL. Assessment of systolic left ventricular function: a multi-centre comparison of cineventriculography, cardiac magnetic resonance imaging, unenhanced and contrast-enhanced echocardiography. Eur Heart J. 2005;26:607–616. doi: 10.1093/eurheartj/ehi083. [DOI] [PubMed] [Google Scholar]
- 43.Jenkins C, Moir S, Chan J, Rakhit D, Haluska B, Marwick TH. Left ventricular volume measurement with echocardiography: a comparison of left ventricular opacification, three-dimensional echocardiography, or both with magnetic resonance imaging. Eur Heart J. 2009;30:98–106. doi: 10.1093/eurheartj/ehn484. [DOI] [PubMed] [Google Scholar]
- 44.Wood PW, Choy JB, Nanda NC, Becher H. Left ventricular ejection fraction and volumes: it depends on the imaging method. Echocardiography. 2014;31:87–100. doi: 10.1111/echo.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoffmann R, Barletta G, von Bardeleben S, Vanoverschelde JL, Kasprzak J, Greis C, Becher H. Analysis of left ventricular volumes and function: a multicenter comparison of cardiac magnetic resonance imaging, cine ventriculography, and unenhanced and contrast-enhanced two-dimensional and three-dimensional echocardiography. J Am Soc Echocardiogr. 2014;27:292–301. doi: 10.1016/j.echo.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 46.Porter TR, Abdelmoneim S, Belcik JT, McCulloch ML, Mulvagh SL, Olson JJ, Porcelli C, Tsutsui JM, Wei K. Guidelines for the cardiac sonographer in the performance of contrast echocardiography: a focused update from the American Society of Echocardiography. J Am Soc Echocardiogr. 2014;27:797–810. doi: 10.1016/j.echo.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 47.Lampropoulos KM, Dounis VG, Aggeli C, Iliopoulos TA, Stefanadis C. Contrast echocardiography: contribution to diagnosis of left ventricular non-compaction cardiomyopathy. Hellenic J Cardiol. 2011;52:265–272. [PubMed] [Google Scholar]
- 48.Marwick TH. Contrast stress echocardiography: completing the picture from image enhancement to improved accuracy and prognostic insight. Circulation. 2005;112:1382–1383. doi: 10.1161/CIRCULATIONAHA.105.566133. [DOI] [PubMed] [Google Scholar]
- 49.Gaibazzi N, Reverberi C, Lorenzoni V, Molinaro S, Porter TR. Prognostic value of high-dose dipyridamole stress myocardial contrast perfusion echocardiography. Circulation. 2012;126:1217–1224. doi: 10.1161/CIRCULATIONAHA.112.110031. [DOI] [PubMed] [Google Scholar]
- 50.Porter TR, Xie F. Contrast echocardiography: latest developments and clinical utility. Curr Cardiol Rep. 2015;17:569. doi: 10.1007/s11886-015-0569-9. [DOI] [PubMed] [Google Scholar]
- 51.Chong A, Haluska B, Wahi S. Clinical application and laboratory protocols for performing contrast echocardiography. Indian Heart J. 2013;65:337–346. doi: 10.1016/j.ihj.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rutz T, de Marchi SF, Roelli P, Gloekler S, Traupe T, Steck H, Eshtehardi P, Cook S, Vogel R, Mohacsi P, Seiler C. Quantitative myocardial contrast echocardiography: a new method for the non-invasive detection of chronic heart transplant rejection. Eur Heart J Cardiovasc Imaging. 2013;14:1187–1194. doi: 10.1093/ehjci/jet066. [DOI] [PubMed] [Google Scholar]
- 53.Du GQ, Xue JY, Guo Y, Chen S, Du P, Wu Y, Wang YH, Zong LQ, Tian JW. Measurement of myocardial perfusion and infarction size using computer-aided diagnosis system for myocardial contrast echocardiography. Ultrasound Med Biol. 2015;41:2466–2477. doi: 10.1016/j.ultrasmedbio.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 54.Cho JS, Her SH, Youn HJ, Kim CJ, Park MW, Kim GH, Chung WB, Park CS, Cho EJ, Kim MJ, Jung HO, Jeon HK. Usefulness of the parameters of quantitative myocardial perfusion contrast echocardiography in patients with chronic total occlusion and collateral flow. Echocardiography. 2015;32:475–482. doi: 10.1111/echo.12663. [DOI] [PubMed] [Google Scholar]
- 55.Uenishi EK, Caldas MA, Saroute AN, Tsutsui JM, Piotto GH, Falcao SN, Mathias W., Jr Contrast echocardiography for the evaluation of tumors and thrombi. Arq Bras Cardiol. 2008;91:e48–52. doi: 10.1590/s0066-782x2008001700015. [DOI] [PubMed] [Google Scholar]
- 56.Bernier M, Abdelmoneim SS, Stuart Moir W, Eifert Rain SS, Chandrasekaran K, Ammash NM, Mulvagh SL. CUTE-CV: a prospective study of enhanced left atrial appendage visualization with microbubble contrast agent use during transesophageal echocardiography guided cardioversion. Echocardiography. 2013;30:1091–1097. doi: 10.1111/echo.12240. [DOI] [PubMed] [Google Scholar]
- 57.Kurt M, Shaikh KA, Peterson L, Kurrelmeyer KM, Shah G, Nagueh SF, Fromm R, Quinones MA, Zoghbi WA. Impact of contrast echocardiography on evaluation of ventricular function and clinical management in a large prospective cohort. J Am Coll Cardiol. 2009;53:802–810. doi: 10.1016/j.jacc.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 58.Dekker DL, Piziali RL, Dong E., Jr A system for ultrasonically imaging the human heart in three dimensions. Comput Biomed Res. 1974;7:544–553. doi: 10.1016/0010-4809(74)90031-7. [DOI] [PubMed] [Google Scholar]
- 59.Hung J, Lang R, Flachskampf F, Shernan SK, McCulloch ML, Adams DB, Thomas J, Vannan M, Ryan T. Ase, 3D echocardiography: a review of the current status and future directions. J Am Soc Echocardiogr. 2007;20:213–233. doi: 10.1016/j.echo.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 60.Lang RM, Badano LP, Tsang W, Adams DH, Agricola E, Buck T, Faletra FF, Franke A, Hung J, de Isla LP, Kamp O, Kasprzak JD, Lancellotti P, Marwick TH, McCulloch ML, Monaghan MJ, Nihoyannopoulos P, Pandian NG, Pellikka PA, Pepi M, Roberson DA, Shernan SK, Shirali GS, Sugeng L, Ten Cate FJ, Vannan MA, Zamorano JL, Zoghbi WA, E. American Society of. E. European Association of EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. J Am Soc Echocardiogr. 2012;25:3–46. doi: 10.1016/j.echo.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 61.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39 e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 62.Dorosz JL, Lezotte DC, Weitzenkamp DA, Allen LA, Salcedo EE. Performance of 3-dimensional echocardiography in measuring left ventricular volumes and ejection fraction: a systematic review and meta-analysis. J Am Coll Cardiol. 2012;59:1799–1808. doi: 10.1016/j.jacc.2012.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsang W, Salgo IS, Medvedofsky D, Takeuchi M, Prater D, Weinert L, Yamat M, Mor-Avi V, Patel AR, Lang RM. Transthoracic 3D Echocardiographic Left Heart Chamber Quantification Using an Automated Adaptive Analytics Algorithm. JACC Cardiovasc Imaging. 2016;9:769–782. doi: 10.1016/j.jcmg.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 64.Otani K, Nakazono A, Salgo IS, Lang RM, Takeuchi M. Three-Dimensional Echocardiographic Assessment of Left Heart Chamber Size and Function with Fully Automated Quantification Software in Patients with Atrial Fibrillation. J Am Soc Echocardiogr. 2016;29:955–965. doi: 10.1016/j.echo.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 65.Tsang W, Lang RM. Three-dimensional echocardiography is essential for intraoperative assessment of mitral regurgitation. Circulation. 2013;128:643–652. doi: 10.1161/CIRCULATIONAHA.112.120501. discussion 652. [DOI] [PubMed] [Google Scholar]
- 66.Tsang W, Weinert L, Sugeng L, Chandra S, Ahmad H, Spencer K, Mor-Avi V, Lang RM. The value of three-dimensional echocardiography derived mitral valve parametric maps and the role of experience in the diagnosis of pathology. J Am Soc Echocardiogr. 2011;24:860–867. doi: 10.1016/j.echo.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 67.Ben Zekry S, Nagueh SF, Little SH, Quinones MA, McCulloch ML, Karanbir S, Herrera EL, Lawrie GM, Zoghbi WA. Comparative accuracy of two- and three-dimensional transthoracic and transesophageal echocardiography in identifying mitral valve pathology in patients undergoing mitral valve repair: initial observations. J Am Soc Echocardiogr. 2011;24:1079–1085. doi: 10.1016/j.echo.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 68.Kahlert P, Plicht B, Schenk IM, Janosi RA, Erbel R, Buck T. Direct assessment of size and shape of noncircular vena contracta area in functional versus organic mitral regurgitation using real-time three-dimensional echocardiography. J Am Soc Echocardiogr. 2008;21:912–921. doi: 10.1016/j.echo.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 69.Buck T, Plicht B. Real-Time Three-Dimensional Echocardiographic Assessment of Severity of Mitral Regurgitation Using Proximal Isovelocity Surface Area and Vena Contracta Area Method. Lessons We Learned and Clinical Implications. Curr Cardiovasc Imaging Rep. 2015;8:38. doi: 10.1007/s12410-015-9356-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schlosshan D, Aggarwal G, Mathur G, Allan R, Cranney G. Real-time 3D transesophageal echocardiography for the evaluation of rheumatic mitral stenosis. JACC Cardiovasc Imaging. 2011;4:580–588. doi: 10.1016/j.jcmg.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 71.Smith LA, Dworakowski R, Bhan A, Delithanasis I, Hancock J, Maccarthy PA, Wendler O, Thomas MR, Monaghan MJ. Real-time three-dimensional transesophageal echocardiography adds value to transcatheter aortic valve implantation. J Am Soc Echocardiogr. 2013;26:359–369. doi: 10.1016/j.echo.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 72.Johri AM, Witzke C, Solis J, Palacios IF, Inglessis I, Picard MH, Passeri JJ. Real-time three-dimensional transesophageal echocardiography in patients with secundum atrial septal defects: outcomes following transcatheter closure. J Am Soc Echocardiogr. 2011;24:431–437. doi: 10.1016/j.echo.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 73.Hagler DJ, Cabalka AK, Sorajja P, Cetta F, Mankad SV, Bruce CJ, Sinak LJ, Chandrasekaran K, Rihal CS. Assessment of percutaneous catheter treatment of paravalvular prosthetic regurgitation. JACC Cardiovasc Imaging. 2010;3:88–91. doi: 10.1016/j.jcmg.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 74.Junker R, Schlebusch H, Luppa PB. Point-of-care testing in hospitals and primary care. Dtsch Arztebl Int. 2010;107:561–567. doi: 10.3238/arztebl.2010.0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Killu K, Coba V, Mendez M, Reddy S, Adrzejewski T, Huang Y, Ede J, Horst M. Model Point-of-Care Ultrasound Curriculum in an Intensive Care Unit Fellowship Program and Its Impact on Patient Management. Crit Care Res Pract. 2014;2014:934796. doi: 10.1155/2014/934796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marin JR, Lewiss RE, C.o.P.E.M. American Academy of Pediatrics. A.o.E.U. Society for Academic Emergency Medicine. P.E.M.C. American College of Emergency Physicians. U. World Interactive Network Focused on Critical Point-of-care ultrasonography by pediatric emergency medicine physicians. Pediatrics. 2015;135:e1113–1122. [Google Scholar]
- 77.Solomon SD, Saldana F. Point-of-care ultrasound in medical education–stop listening and look. N Engl J Med. 2014;370:1083–1085. doi: 10.1056/NEJMp1311944. [DOI] [PubMed] [Google Scholar]
- 78.Kimura BJ. Point-of-care cardiac ultrasound techniques in the physical examination: better at the bedside. Heart. 2017 doi: 10.1136/heartjnl-2016-309915. [DOI] [PubMed] [Google Scholar]
- 79.Di Bello V, La Carrubba S, Conte L, Fabiani I, Posteraro A, Antonini-Canterin F, Barletta V, Nicastro I, Mariotti E, Severino S, Caso P, Benedetto F, Savino K, Carerj S. Siec, Incremental Value of Pocket-Sized Echocardiography in Addition to Physical Examination during Inpatient Cardiology Evaluation: A Multicenter Italian Study (SIEC) Echocardiography. 2015;32:1463–1470. doi: 10.1111/echo.12910. [DOI] [PubMed] [Google Scholar]
- 80.Fox JC, Lahham S, Maldonado G, Klaus S, Aish B, Sylwanowicz LV, Yanuck J, Wilson SP, Shieh M, Anderson CL, English C, Mayer R, Mohan UR. Hypertrophic Cardiomyopathy in Youth Athletes: Successful Screening With Point-of-Care Ultrasound by Medical Students. J Ultrasound Med. 2017 doi: 10.7863/ultra.16.06044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wilson SP, Assaf S, Lahham S, Subeh M, Chiem A, Anderson C, Shwe S, Nguyen R, Fox JC. Simplified point-of-care ultrasound protocol to confirm central venous catheter placement: A prospective study. World J Emerg Med. 2017;8:25–28. doi: 10.5847/wjem.j.1920-8642.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zanobetti M, Scorpiniti M, Gigli C, Nazerian P, Vanni S, Innocenti F, Stefanone VT, Savinelli C, Coppa A, Bigiarini S, Caldi F, Tassinari I, Conti A, Grifoni S, Pini R. Point-of-care ultrasonography for evaluation of acute dyspnea in the emergency department. Chest. 2017 doi: 10.1016/j.chest.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 83.Gaspari R, Weekes A, Adhikari S, Noble VE, Nomura JT, Theodoro D, Woo M, Atkinson P, Blehar D, Brown SM, Caffery T, Douglass E, Fraser J, Haines C, Lam S, Lanspa M, Lewis M, Liebmann O, Limkakeng A, Lopez F, Platz E, Mendoza M, Minnigan H, Moore C, Novik J, Rang L, Scruggs W, Raio C. Emergency department point-of-care ultrasound in out-of-hospital and in-ED cardiac arrest. Resuscitation. 2016;109:33–39. doi: 10.1016/j.resuscitation.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 84.Atkinson P, Bowra J, Milne J, Lewis D, Lambert M, Jarman B, V EN, Lamprecht H, Harris T, Connolly J. International Federation for Emergency Medicine Consensus Statement: Sonography in hypotension and cardiac arrest (SHoC): An international consensus on the use of point of care ultrasound for undifferentiated hypotension and during cardiac arrest. CJEM. 2016:1–12. doi: 10.1017/cem.2016.394. [DOI] [PubMed] [Google Scholar]
- 85.Zieleskiewicz L, Muller L, Lakhal K, Meresse Z, Arbelot C, Bertrand PM, Bouhemad B, Cholley B, Demory D, Duperret S, Duranteau J, Guervilly C, Hammad E, Ichai C, Jaber S, Langeron O, Lefrant JY, Mahjoub Y, Maury E, Meaudre E, Michel F, Muller M, Nafati C, Perbet S, Quintard H, Riu B, Vigne C, Chaumoitre K, Antonini F, Allaouchiche B, Martin C, Constantin JM, De Backer D, Leone M, Car’Echo, N. AzuRea Collaborative Point-of-care ultrasound in intensive care units: assessment of 1073 procedures in a multicentric, prospective, observational study. Intensive Care Med. 2015;41:1638–1647. doi: 10.1007/s00134-015-3952-5. [DOI] [PubMed] [Google Scholar]
- 86.Spencer KT, Kimura BJ, Korcarz CE, Pellikka PA, Rahko PS, Siegel RJ. Focused cardiac ultrasound: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2013;26:567–581. doi: 10.1016/j.echo.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 87.Via G, Hussain A, Wells M, Reardon R, ElBarbary M, Noble VE, Tsung JW, Neskovic AN, Price S, Oren-Grinberg A, Liteplo A, Cordioli R, Naqvi N, Rola P, Poelaert J, Gulic TG, Sloth E, Labovitz A, Kimura B, Breitkreutz R, Masani N, Bowra J, Talmor D, Guarracino F, Goudie A, Xiaoting W, Chawla R, Galderisi M, Blaivas M, Petrovic T, Storti E, Neri L, Melniker L, U. International Liaison Committee on Focused Cardiac. U. International Conference on Focused Cardiac International evidence-based recommendations for focused cardiac ultrasound. J Am Soc Echocardiogr. 2014;27:683 e681–683 e633. doi: 10.1016/j.echo.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 88.Montoya J, Stawicki SP, Evans DC, Bahner DP, Sparks S, Sharpe RP, Cipolla J. From FAST to E-FAST: an overview of the evolution of ultrasound-based traumatic injury assessment. Eur J Trauma Emerg Surg. 2016;42:119–126. doi: 10.1007/s00068-015-0512-1. [DOI] [PubMed] [Google Scholar]
- 89.Schoch ML, Du Toit D, Marticorena RM, Sinclair PM. Utilising point-of-care ultrasound for vascular access in haemodialysis. Renal Society of Australasia Journal. 2015;11:78–82. [Google Scholar]
- 90.M. American Institute of Ultrasound in, P. American College of Emergency AIUM practice guideline for the performance of the focused assessment with sonography for trauma (FAST) examination. J Ultrasound Med. 2014;33:2047–2056. doi: 10.7863/ultra.33.11.2047. [DOI] [PubMed] [Google Scholar]
- 91.Hoyt K, Hester FA, Bell RL, Lockhart ME, Robbin ML. Accuracy of volumetric flow rate measurements: an in vitro study using modern ultrasound scanners. J Ultrasound Med. 2009;28:1511–1518. doi: 10.7863/jum.2009.28.11.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Oktar SO, Yucel C, Karaosmanoglu D, Akkan K, Ozdemir H, Tokgoz N, Tali T. Blood-flow volume quantification in internal carotid and vertebral arteries: comparison of 3 different ultrasound techniques with phase-contrast MR imaging. AJNR Am J Neuroradiol. 2006;27:363–369. [PMC free article] [PubMed] [Google Scholar]
- 93.Sauders JB, Wright N, Lewis KO. Measurement of human fetal blood flow. Br Med J. 1980;280:283–284. doi: 10.1136/bmj.280.6210.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vans DH, McDicken N. Doppler ultrasound: physics, instrumentation and signal processing. John Wiley and Sons; 2002. [Google Scholar]
- 95.Burns PN. Measuring volume flow with Doppler ultrasound-an old nut. Ultrasound Obstet Gynecol. 1992;2:238–241. doi: 10.1046/j.1469-0705.1992.02040237-2.x. [DOI] [PubMed] [Google Scholar]
- 96.Richards MS, Kripfgans OD, Rubin JM, Hall AL, Fowlkes JB. Mean volume flow estimation in pulsatile flow conditions. Ultrasound Med Biol. 2009;35:1880–1891. doi: 10.1016/j.ultrasmedbio.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kripfgans OD, Rubin JM, Hall AL, Gordon MB, Fowlkes JB. Measurement of volumetric flow. J Ultrasound Med. 2006;25:1305–1311. doi: 10.7863/jum.2006.25.10.1305. [DOI] [PubMed] [Google Scholar]
- 98.Forsberg F, Stein AD, Liu JB, Deng X, Ackerman W, Herzog D, Abend K, Needleman L. Validating volume flow measurements from a novel semiautomated four-dimensional Doppler ultrasound scanner. Acad Radiol. 2006;13:1204–1210. doi: 10.1016/j.acra.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 99.Forsberg F, Stein AD, Merton DA, Lipcan KJ, Herzog D, Parker L, Needleman L. Carotid stenosis assessed with a 4-dimensional semiautomated Doppler system. J Ultrasound Med. 2008;27:1337–1344. doi: 10.7863/jum.2008.27.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sandrin L, Catheline S, Tanter M, Hennequin X, Fink M. Time-resolved pulsed elastography with ultrafast ultrasonic imaging. Ultrason Imaging. 1999;21:259–272. doi: 10.1177/016173469902100402. [DOI] [PubMed] [Google Scholar]
- 101.Provost J, Papadacci C, Arango JE, Imbault M, Fink M, Gennisson JL, Tanter M, Pernot M. 3D ultrafast ultrasound imaging in vivo. Phys Med Biol. 2014;59:L1–L13. doi: 10.1088/0031-9155/59/19/L1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Provost J, Lee WN, Fujikura K, Konofagou EE. Imaging the electromechanical activity of the heart in vivo. Proc Natl Acad Sci U S A. 2011;108:8565–8570. doi: 10.1073/pnas.1011688108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Park S, Aglyamov SR, Scott WG, Emelianov SY. Strain imaging using conventional and ultrafast ultrasound imaging: numerical analysis. IEEE Trans Ultrason Ferroelectr Freq Control. 2007;54:987–995. doi: 10.1109/tuffc.2007.344. [DOI] [PubMed] [Google Scholar]
- 104.Udesen J, Gran F, Hansen KL, Jensen JA, Thomsen C, Nielsen MB. High frame-rate blood vector velocity imaging using plane waves: simulations and preliminary experiments. IEEE Trans Ultrason Ferroelectr Freq Control. 2008;55:1729–1743. doi: 10.1109/TUFFC.2008.858. [DOI] [PubMed] [Google Scholar]
- 105.Van Cauwenberge J, Lovstakken L, Fadnes S, Rodriguez-Morales A, Vierendeels J, Segers P, Swillens A. Assessing the Performance of Ultrafast Vector Flow Imaging in the Neonatal Heart via Multiphysics Modeling and In Vitro Experiments. IEEE Trans Ultrason Ferroelectr Freq Control. 2016;63:1772–1785. doi: 10.1109/TUFFC.2016.2596804. [DOI] [PubMed] [Google Scholar]
- 106.Correia M, Provost J, Tanter M, Pernot M. 4D ultrafast ultrasound flow imaging: in vivo quantification of arterial volumetric flow rate in a single heartbeat. Phys Med Biol. 2016;61:L48–L61. doi: 10.1088/0031-9155/61/23/L48. [DOI] [PubMed] [Google Scholar]
- 107.Nightingale K. Acoustic Radiation Force Impulse (ARFI) Imaging: a Review. Curr Med Imaging Rev. 2011;7:328–339. doi: 10.2174/157340511798038657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bercoff J, Tanter M, Fink M. Supersonic shear imaging: a new technique for soft tissue elasticity mapping. IEEE Trans Ultrason Ferroelectr Freq Control. 2004;51:396–409. doi: 10.1109/tuffc.2004.1295425. [DOI] [PubMed] [Google Scholar]
- 109.Nightingale K, McAleavey S, Trahey G. Shear-wave generation using acoustic radiation force: in vivo and ex vivo results. Ultrasound Med Biol. 2003;29:1715–1723. doi: 10.1016/j.ultrasmedbio.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 110.Vejdani-Jahromi M, Nagle M, Jiang Y, Trahey GE, Wolf PD. A Comparison of Acoustic Radiation Force-Derived Indices of Cardiac Function in the Langendorff Perfused Rabbit Heart. IEEE Trans Ultrason Ferroelectr Freq Control. 2016;63:1288–1295. doi: 10.1109/TUFFC.2016.2543026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Doherty JR, Trahey GE, Nightingale KR, Palmeri ML. Acoustic radiation force elasticity imaging in diagnostic ultrasound. IEEE Trans Ultrason Ferroelectr Freq Control. 2013;60:685–701. doi: 10.1109/TUFFC.2013.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pernot M, Couade M, Mateo P, Crozatier B, Fischmeister R, Tanter M. Real-time assessment of myocardial contractility using shear wave imaging. J Am Coll Cardiol. 2011;58:65–72. doi: 10.1016/j.jacc.2011.02.042. [DOI] [PubMed] [Google Scholar]
- 113.Kass DA, Bronzwaer JG, Paulus WJ. What mechanisms underlie diastolic dysfunction in heart failure? Circ Res. 2004;94:1533–1542. doi: 10.1161/01.RES.0000129254.25507.d6. [DOI] [PubMed] [Google Scholar]
- 114.Westermann D, Kasner M, Steendijk P, Spillmann F, Riad A, Weitmann K, Hoffmann W, Poller W, Pauschinger M, Schultheiss HP, Tschope C. Role of left ventricular stiffness in heart failure with normal ejection fraction. Circulation. 2008;117:2051–2060. doi: 10.1161/CIRCULATIONAHA.107.716886. [DOI] [PubMed] [Google Scholar]
- 115.Yamamoto K, Masuyama T, Sakata Y, Nishikawa N, Mano T, Yoshida J, Miwa T, Sugawara M, Yamaguchi Y, Ookawara T, Suzuki K, Hori M. Myocardial stiffness is determined by ventricular fibrosis, but not by compensatory or excessive hypertrophy in hypertensive heart. Cardiovasc Res. 2002;55:76–82. doi: 10.1016/s0008-6363(02)00341-3. [DOI] [PubMed] [Google Scholar]
- 116.Nagueh SF, Shah G, Wu Y, Torre-Amione G, King NM, Lahmers S, Witt CC, Becker K, Labeit S, Granzier HL. Altered titin expression, myocardial stiffness, and left ventricular function in patients with dilated cardiomyopathy. Circulation. 2004;110:155–162. doi: 10.1161/01.CIR.0000135591.37759.AF. [DOI] [PubMed] [Google Scholar]
- 117.Makarenko I, Opitz CA, Leake MC, Neagoe C, Kulke M, Gwathmey JK, del Monte F, Hajjar RJ, Linke WA. Passive stiffness changes caused by upregulation of compliant titin isoforms in human dilated cardiomyopathy hearts. Circ Res. 2004;95:708–716. doi: 10.1161/01.RES.0000143901.37063.2f. [DOI] [PubMed] [Google Scholar]
- 118.Pernot M, Lee WN, Bel A, Mateo P, Couade M, Tanter M, Crozatier B, Messas E. Shear Wave Imaging of Passive Diastolic Myocardial Stiffness: Stunned Versus Infarcted Myocardium. JACC Cardiovasc Imaging. 2016;9:1023–1030. doi: 10.1016/j.jcmg.2016.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pislaru C, Bruce CJ, Anagnostopoulos PC, Allen JL, Seward JB, Pellikka PA, Ritman EL, Greenleaf JF. Ultrasound strain imaging of altered myocardial stiffness: stunned versus infarcted reperfused myocardium. Circulation. 2004;109:2905–2910. doi: 10.1161/01.CIR.0000129311.73402.EF. [DOI] [PubMed] [Google Scholar]
- 120.Sarvazyan AP, Rudenko OV, Nyborg WL. Biomedical applications of radiation force of ultrasound: historical roots and physical basis. Ultrasound Med Biol. 2010;36:1379–1394. doi: 10.1016/j.ultrasmedbio.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 121.Sinkus R. Elasticity of the heart, problems and potentials. Current Cardiovascular Imaging. 2014;7:9288. [Google Scholar]
- 122.Hsu SJ, Bouchard RR, Dumont DM, Wolf PD, Trahey GE. In vivo assessment of myocardial stiffness with acoustic radiation force impulse imaging. Ultrasound Med Biol. 2007;33:1706–1719. doi: 10.1016/j.ultrasmedbio.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hsu SJ, Fahey BJ, Dumont DM, Wolf PD, Trahey GE. Challenges and implementation of radiation-force imaging with an intracardiac ultrasound transducer. IEEE Trans Ultrason Ferroelectr Freq Control. 2007;54:996–1009. doi: 10.1109/tuffc.2007.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Song P, Bi X, Mellema DC, Manduca A, Urban MW, Pellikka PA, Chen S, Greenleaf JF. Pediatric Cardiac Shear Wave Elastography for Quantitative Assessment of Myocardial Stiffness: A Pilot Study in Healthy Controls. Ultrasound Med Biol. 2016;42:1719–1729. doi: 10.1016/j.ultrasmedbio.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 125.Urban MW, Qiang B, Song P, Nenadic IZ, Chen S, Greenleaf JF. Investigation of the effects of myocardial anisotropy for shear wave elastography using impulsive force and harmonic vibration. Phys Med Biol. 2016;61:365–382. doi: 10.1088/0031-9155/61/1/365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Song P, Bi X, Mellema DC, Manduca A, Urban MW, Greenleaf JF, Chen S. Quantitative Assessment of Left Ventricular Diastolic Stiffness Using Cardiac Shear Wave Elastography: A Pilot Study. J Ultrasound Med. 2016;35:1419–1427. doi: 10.7863/ultra.15.08053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


