Abstract
BACKGROUND
The presence of brown adipose tissue (BAT) in humans and its potential role in human metabolism have been recently recognized. 18F-FDG PET/CT is the current standard imaging method to detect glucose uptake in metabolically active BAT after mild cold exposure. MRI has increasingly been exploited in characterization of BAT.
PURPOSE/HYPOTHESIS
The purpose is to implement quantitative Dixon MRI methods for BAT characterization at inactive and cold-activated states in normal weight, overweight, and obese subjects. The hypotheses are that MRI characteristics of BAT would differentiate between non-obese and obese subjects, and activation of BAT in response to thermal challenges that are detected by MRI would be correlated with BAT activity measured by PET/CT.
STUDY TYPE
This is a prospective study on human subjects.
POPULATION/SUBJECTS/PHANTOM/SPECIMEN/ANIMAL MODEL
Fifteen male subjects (20.7 ± 1.5 years old) including 6 normal weight, 5 overweight and 4 obese subjects participated the study.
FIELD STRENGTH/SEQUENCE ASSESSMENT
Multi-echo Dixon MRI sequence was performed on a 1.5 Tesla scanner. MR Imaging was acquired under thermoneutral, non-shivering thermogenesis and subsequent warm up conditions. Fat fraction (FF), R2* and the number of double bonds (ndb) were measured by solving an optimization problem that fits in- and out-of-phase MR signal intensities to the fat-water interference models. Imaging acquisition and post-processing were performed by two MRI physicists.
STATISTICAL TESTS
In each subject, Dixon MRI measurements of FF, R2* and ndb were calculated for each voxel within all BAT ROIs under each thermal condition. Mean FF, R2* and ndb were compared between non-obese (i.e. normal weight/overweight) and obese subjects using the two sample t-test. Receiver operating characteristic (ROC) analyses were performed to differentiate non-obese vs. obese subjects. BAT MRI measurement changes in response to thermal condition changes were compared with hypermetabolic BAT volume/activity measured by PET/CT using the Pearson’s correlation. In addition, BAT MRI measurements were compared with body adiposity using the Pearson’s correlation. A p-value (P) < 0.05 was considered statistically significant.
RESULTS
Obese subjects showed higher FF and lower R2* than non-obese subjects under all three thermal conditions (P < 0.01). ROC analyses demonstrated that FF and R2* were excellent predictors for the differentiation of non-obese from obese subjects (100% specificity and 100% sensitivity). FF changes under thermal challenges were correlated with hypermetabolic BAT volume (r = −0.55, P = 0.04 during activation, and r = 0.72, P = 0.003 during deactivation), and with BAT activity (r = 0.69, P = 0.006 during deactivation), as measured by PET/CT. FF and R2* under all three thermal conditions were highly correlated with body adiposity (P ≤ 0.002).
DATA CONCLUSION
MRI characteristics of BAT differentiated between non-obese and obese subjects in both inactivated and activated states. BAT activation detected by Dixon MRI in response to thermal challenges were correlated with glucose uptake of metabolically active BAT.
Keywords: MRI, PET/CT, Brown adipose tissue, Cold exposure, Obese
INTRODUCTION
Obesity has reached epidemic proportions in the United States and much of the developed world. More than one third of U.S. adults are obese and one third of children and adolescents are overweight or obese (1,2). The principle causes of overweight and obesity include energy imbalance due to dietary excess and sedentary lifestyles, though genetics also clearly plays a role.
In recent years, research has focused attention on the potential roles of adipose tissue in the pathogenesis and treatment of obesity and other related metabolic disorders. Adipose tissue in mammals consists of two functionally different types: white adipose tissue (WAT) and brown adipose tissue (BAT). BAT is the primary site of adaptive thermogenesis or the modulation of energy expenditure and heat generation during cold exposure and overfeeding, which has long been established in rodents (3). The presence of BAT in adult humans and its potential role in human metabolism were recently recognized. Published data indicates that whole-body energy expenditure is increased to a greater extent after cold exposure (cold-induced thermogenesis) and food intake (diet-induced thermogenesis) in individuals with active BAT shown on 18F-fluoro-2-deoxy-glucose (18F-FDG) positron emission tomography and computed tomography (PET/CT) images, than in those without active BAT (4–6). Increasing evidence suggests that human BAT may play a role in glucose metabolism and insulin sensitivity and may protect against obesity development (6–8).
Compared with white adipocytes, brown adipocytes consist of multilocular lipid droplets with abundant iron-containing mitochondria and cytoplasm. Depots of brown adipocytes also have a larger capillary network than white adipocytes due to their higher oxygen consumption and metabolic activity (9). The most pure BAT depots, as seen in the interscapular region in infants, have prenatal origins and are referred to as classical BAT (10). The supraclavicular fat depot (assumed to contain BAT) in adult humans, also known as beige or brite (brown-in-white) fat, contains a mixture of brown and white adipocytes (10–14).
18F-FDG PET/CT is a reference standard imaging method utilized to detect glucose uptake in metabolically active BAT, most commonly after mild cold exposure (15–17). However, large variations in BAT prevalence have been reported with 36 to 100% in lean and 20 to 50% in obese subjects, dependent upon subject characteristics (especially age), cooling procedures, and imaging protocols (18,19). Additional individual variation is likely due to the underestimation of BAT that is not fully activated, individual differences in FDG uptake by BAT due to competition from endogenous glucose (20), or individual variation in insulin sensitivity, which may also affect FDG uptake by BAT (21). Furthermore, PET/CT imaging is limited in characterization of BAT tissue fat composition. PET/CT also involves ionizing radiation, which limits its utility for longitudinal studies.
Given the lack of ionizing radiation, Magnetic Resonance Imaging (MRI) is more suitable than PET/CT for prospective and longitudinal imaging studies on healthy subjects. MRI can also characterize BAT regardless of its activation state. Chemical-shift Dixon MRI techniques have been recently exploited to measure the fat fraction (FF) and R2* of tissue. These parameters have been shown to be related to organ lipid and iron contents. Previous studies have demonstrated BAT has lower FF due to less lipid content and higher R2*, which is presumably due to a larger amount of iron-containing mitochondria, compared with WAT (22–24). In addition, more advanced fatty acid composition analyses using MRI indicated a lower degree of unsaturated triglycerides in BAT than WAT in rodent models (23,25,26). A recent studies quantified active and inactive BAT on co-registered PET/CT and MRI at both thermoneutral and cold exposure temperatures (27). A hybrid PET/MRI BAT study revealed that FF and T2* (i.e. reciprocal of R2*) of BAT during cold exposure and at thermoneutral temperature correlated inversely with FDG update, however cold exposure did not affect MRI measurements (28).
In this study, we proposed to implement quantitative Dixon MRI methods for BAT characterization at inactivated and activated states after cold exposure in young male subjects. Our hypotheses were that 1) normal weight, overweight, and obese subjects would display differential tissue properties and activation of BAT in response to thermal challenges, as detected by MRI, and 2) MRI parameter changes reflective of BAT activation would be correlated with BAT activity measured by 18F-FDG PET/CT.
MATERIALS AND METHODS
Subjects
The study was HIPAA compliant and approved by the hospital’s Institutional Review Board for conduct of research on human subjects. Subjects provided written informed consent for participation. Normal weight with body mass index (BMI) of 18.5 to 24.9 kg/m2, overweight (BMI: 25.0 to 29.9 kg/m2) and obese (BMI: > 29.9 kg/m2) healthy male volunteers (18–24 years) were recruited during all seasons. The exclusion criteria included the following: presence of any active condition that affects energy metabolism, use of medications that affects brown fat activity, use of diabetes medication, hemoglobin A1c ≥ 7.0% or fasting plasma glucose >150 mg/dl, and contraindications to MRI. Since weight changes may affect BAT activity and metabolic markers (a secondary outcome of this study not reported here), subjects were required to be within 3% of their highest body weight and be weight stable (no weight change of > 3%) for the last 3 months (29).
Individualized Cooling Protocol
The individualized non-shivering thermogenesis (NST) condition was established to maximize the activation of BAT by a thermo-regulating machine (Criticool system and universal thermowrap, Mennen Medical Ltd, Rehovot, Israel). Subjects put on a thermowrap that was infused with water and connected to the temperature control system. The temperature of the MTRE system was initially set to 13°C to cool down the subject till the subject began shivering, and then the system temperature was increased by 1°C every 4 minutes until the subject stopped shivering. The mild cold temperature stabilized for 15 minutes after the subject stopped shivering was designated as the individual’s NST temperature.
Study Protocol
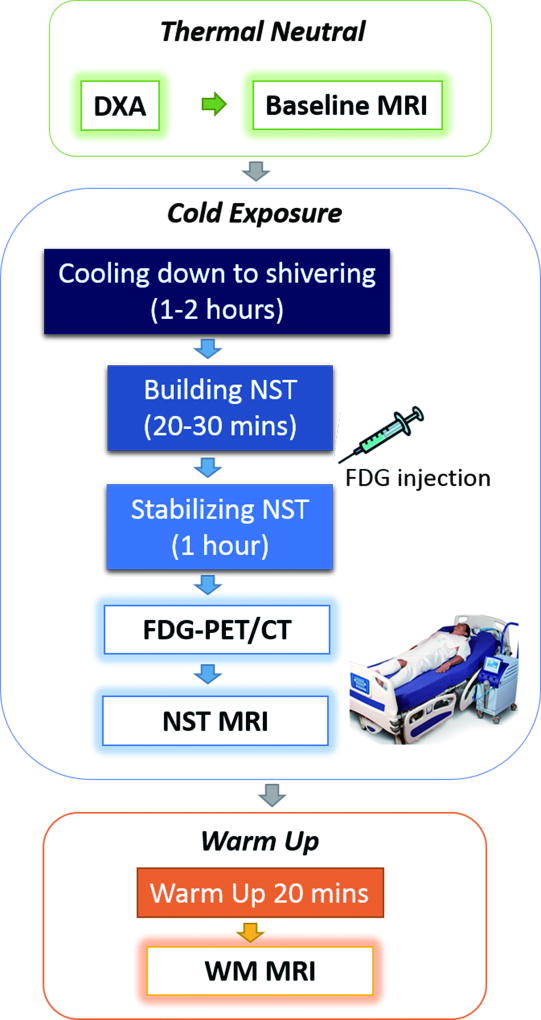
Subjects arrived having fasted for at least 5 hours and having abstained from alcohol, caffeine, energy drinks, and strenuous activity for 24 hours before the imaging study. Capillary blood glucose was checked by finger stick using a glucometer prior to imaging study to confirm a glucose level ≤ 150 mg/dl on that day. A dual-energy X-ray absorptiometry (DXA) whole body scan was first performed to measure body composition, followed by baseline pre-cold MRI at room temperature (21°C). Subjects then underwent the individualized cooling protocol to reach their individual NST state, followed by the 18F-FDG PET/CT (0.075mCi /kg with a maximum dose of 10.0 mCi) acquisition at NST. Subjects then underwent MRI at NST immediately after PET/CT, and again after warm-up (WM) to 30°C for 20 minutes, using the same thermo-regulating machine. Subjects kept the thermo-suit on during NST and WM MRIs. The study flowchart is shown in Fig. 1.
Figure 1.
Study workflow includes imaging procedures under thermoneutral baseline, non-shivering thermogenesis and warm up conditions. Individualized cooling protocols were implemented to activate the brown adipose tissues.
Imaging Acquisition
A DXA whole body scan (Lunar iDXA, GE Healthcare) was performed to measure the fat mass index (FMI) as fat mass/height2, percentage body fat (%fat) as the ratio of fat mass to total body mass, and the fat mass ratio (FMR) between trunk and total body. The PET/CT imaging (GE Discovery 690 VCT, GE Healthcare) started with the low-dose CT scan (120 kV, 30 mAs per slice, and 64×0.625 mm of collimation), followed by a PET scan from skull base to diaphragm in 2–3 bed positions. Fused PET and CT images were reconstructed in transverse, coronal and sagittal planes.
MR imaging was acquired on a 1.5T MRI scanner (Magnetom Aera, Siemens Medical Solutions, Erlangen, Germany), using a 20 channel head and neck coil. A three dimensional (3D) volumetric interpolated brain examination (VIBE) 8-point Dixon sequence was acquired in the coronal plane covering from clavicle bones to vertebral bodies of the cervical spine with the following parameters: field-of-view (FOV) = 280 × 400 mm2, slice thickness/gap = 4/0.5 mm, number of slices = 18–22, matrix = 224 × 316, repetition time (TR) = 20 ms, eight echo time (TE) = 2.3–18.4 ms with interval ΔTE = 2.3 ms, average =1, flip angle = 6° to reduce T1 effect, iPAT =2, bandwidth = 485 Hz/px, bipolar readout gradients, and no breath-hold was required. The same 3D–VIBE Dixon sequence was performed under all three thermal conditions: baseline, NST and WM.
Image Post-Processing
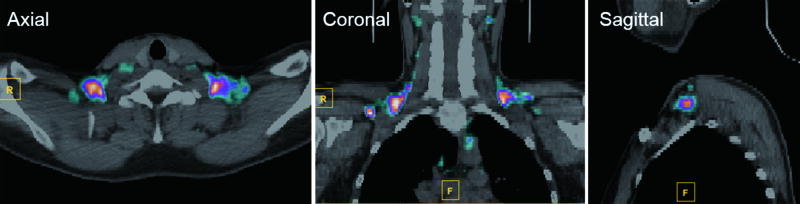
PET and CT images were analyzed using a MATLAB toolbox of Medical Image Reader and Viewer (30) that supports simultaneous PET/CT viewing and region-of-interest (ROI) measurements (MathWorks, Natick, MA, USA). Metabolically active BAT from neck to axillary areas were identified as increased FDG uptake with standardized uptake value (SUV) > 2.0 g/ml, and CT number between −200 and −10 Hounsfield unit (HU) indicating adipose tissues (29,31). By adjusting the SUV and CT number thresholds, ROIs of metabolically active BAT were manually defined on each axial slice of the fused PET/CT images (Fig. 2). The peak metabolic activity body weight adjusted maximal SUV (SUVmax, unit: g/ml) and the volume (ml) of BAT were measured. The total BAT activity (unit: kBq) was calculated as the product of the volumetric mean uptake (SUVmean, unit: kBq/ml) and the BAT volume.
Figure 2.
Metabolically active BAT from neck to axillary areas were identified as increased FDG uptake on the fused PET and CT images as SUV > 2.0 g/ml, and CT number between −200 and −10 HU.
The supraclavicular adipose tissue areas with hyper-intensity on the in-phase, and hypo-intense on the out-of-phase Dixon VIBE magnitude images were identified as presumable BAT areas. Dixon MRI images were analyzed to measure the proton density fat fraction (FF), R2*, and fatty acid composition (FAC) of BAT, by solving an optimization problem that fits in- and out-of-phase signal intensities to the fat-water interference models. Complex signal fitting (Eq.1) was performed using the iterative graph cut algorithm proposed by Hernando et al (32) to resolve the ambiguity of whether a pixel is fat dominant or water dominant and avoid fat-water swaps. The algorithm determined the optimal R2* value for all possible field maps and then iteratively updated the field map, balancing fidelity to the data and a penalty for non-smooth field map estimates. After determining the optimal field map and R2* values, FF was determined in a separate step according to the variable projection (VARPRO) algorithm (33). In addition, when acquiring gradient echo images to perform fat-water separation, the signal at a voxel typically contains phase errors which are due to gradient delays and eddy currents. For a monopolar readout sequence with flyback gradients, these phase errors are constant and are effectively ignored during signal fitting. However, if bipolar readout gradients were used in the data acquisition presented in this article, it resulted in phase errors in the images which alternated in sign. Therefore, a phase error correction was applied to all data prior to signal fitting, with the phase errors estimated using Eq. 1.1 at each voxel (34).
| Eq. 1 |
| Eq. 1.1 |
Magnitude signal fitting was implemented using Matlab’s non-linear curve fitting function ‘lsqnonlin’ with the ‘trust-region-reflective’ optimization algorithm. For this function, initial values of FF and R2* were determined according to the results of the complex fitting. The allowable range for R2* was 0 to 100 s−1. The magnitude signal fitting model (Eq. 2) generated FFMag and R2*Mag values based on the multi-fat-peak model with prior knowledge of relative peak amplitudes.
| Eq. 2 |
FAC model (Eq.3) is based on the fact that double bonds in the fatty acid chains alter the number of hydrogens and therefore affect the MR signals. In FAC model, the relative amplitudes of fat peaks were expressed by the level of unsaturated triglycerides in terms of number of double bonds (ndb), number of methylene-interrupted double bonds (nmidb) and fatty acid chain length (cl) (23,25). To reduce the degree of freedom in nonlinear optimization, nmidb and cl were fixed in relation to ndb as the following: nmidb = 0.093 · ndb2 and cl = 16.8 + 0.25 · ndb. The initial value for ndb was 3, and the allowable range for ndb was between 1 and 6.
| Eq. 3 |
In Eq. 1–Eq. 3, S(TEi) is the signal intensity acquired at the ith TE, ρW and ρF are water and fat proton density, is the transverse relaxivity, Ψ in Eq. 1 is the field map arising from local magnetic field inhomogeneity, Np is the number of fat peaks, fn is the frequency shift of each fat peak relative to the water peak, and Cn (Eq.1 complex fitting and Eq.2 magnitude fitting) and αn (Eq.3 FAC model) are the normalized relative amplitude of the pth fat peak, which were established from the 1H-spectroscopic fat spectrum in rodent brown adipose tissues (23). Individual fat peak assignment and their relative magnitudes are listed in Table 1. Finally, FF was calculated as ρF/(ρW + ρF) derived from Eq.1–Eq.3.
Table 1.
Theoretical fat peak resonance frequency shift relative to the water peak with the relative magnitudes.
| Peak # |
Peak location (ppm) |
Frequency shift fn (ppm) |
Relative Magnitude Cn |
FAC model relative magnitude αn |
|---|---|---|---|---|
| 1 | 5.3 | −0.6 | 0.064 | 1 + 2 · ndb |
| Water | 4.7 | 0 | ||
| 2 | 4.2 | 0.5 | 0.039 | 4 |
| 3 | 2.75 | 1.95 | 0.012 | 2 · nmidb |
| 4 | 2.1 | 2.6 | 0.142 | 6 + 4 · (ndb – nmidb) |
| 5 | 1.3 | 3.4 | 0.653 | 6 + 6 (cl − 4) − ndb + 2 · nmidb |
| 6 | 0.9 | 3.8 | 0.089 | 9 |
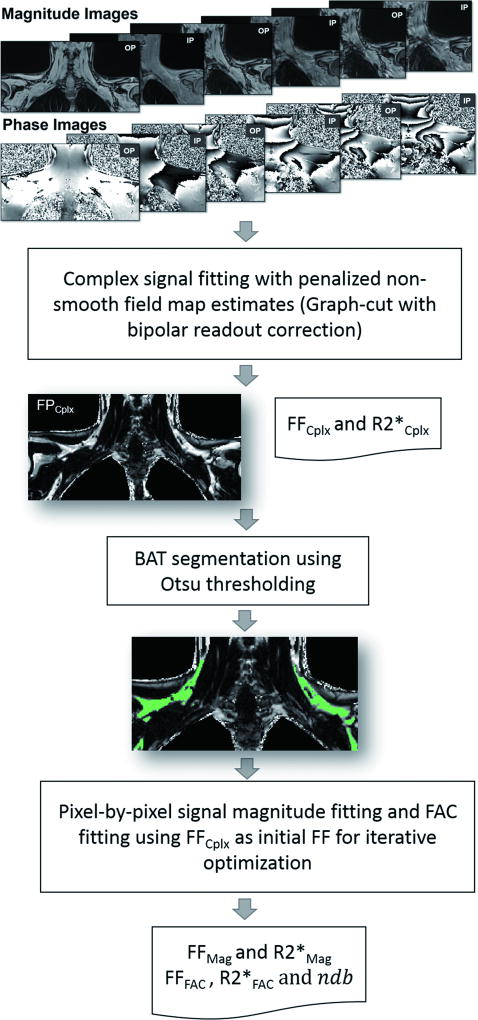
The diagram of MRI signal analyses are summarized in Fig. 3. Parametric FF maps were first reconstructed using the complex signal fitting model, where presumptive BAT depots in the supraclavicular fossae were identified by manually contouring the adipose tissue areas between the superior border of the fourth cervical vertebra and the inferior border of the glenoid fossae from superior to inferior, and between the coracoid processes from left to right. The Otsu thresholding segmentation method was then used to generate BAT ROIs by eliminating surrounding muscular tissues and vessels (35,36). Next, within the segmented BAT ROIs, pixel-by-pixel signal magnitude fitting models were applied to obtain the magnitude-based FF, R2* and FAC estimation, with the initialization of FF and R2* derived from the complex fitting model.
Figure 3.
The diagram of Dixon MRI signal analyses for calculations of proton density fat fraction (FF), R2* and fatty acid composition (FAC) of BAT. Complex signal fitting algorithm was first applied on the acquired eight echo in- and out-of-phase magnitude and phase images to derive the parametric maps of FFCplx and R2*Cplx. BAT ROIs (green areas) were defined in the FFCplx image by using the Otsu thresholding segmentation method to eliminate surrounding muscular and vessel areas. Pixel-by-pixel signal magnitude fitting algorithms were applied within the segmented BAT ROIs to derive FFMag and R2*Mag, and fatty acid composition measurements of FFFAC, R2*FAC and ndb.
Statistical Analysis
In each subject, Dixon MRI measurements of FF, R2* and ndb were calculated for each voxel within all BAT ROIs. Bad voxels with any measurement out of the range of boundary constrains were excluded from the final analyses. The range of boundary was 0.01 < FF < 0.99, 10 s−1 < R2* < 100 s−1, and 1 < ndb < 6.
Model Comparison
FFCplx and R2*Cplx derived from the complex signal model, FFMag and R2*Mag derived from the magnitude signal model, and FFFAC and R2*FAC derived from the FAC model were compared using the Pearson’s correlation and concordance correlation to evaluate the agreement among the three models.
MRI Changes in Response to Thermal Challenges
In each subject, the mean values of FF, R2*and ndb were calculated under baseline, NST and WM conditions. Mean FF, R2* and ndb were compared between non-obese (i.e. normal weight/overweight) and obese subjects using the two sample t-test. Receiver operating characteristic (ROC) analyses were performed to differentiate non-obese vs. obese subjects. ROC curves were generated based on logistic regression models with obese status as the outcome. The optimal threshold was chosen to maximize the sensitivity plus specificity, i.e., to minimize the distance of the curve from the upper left corner of the ROC space. In addition, a linear mixed model was used to test whether the thermal modifications of FF, R2* and ndb were significantly different between non-obese and obese subjects.
Correlation between MRI and PET Measurements
FF, R2* and ndb changes in response to thermal condition changes (i.e. the difference between baseline and NST conditions, and between NST and WM conditions) were compared with SUVmax, BAT volume, and total BAT activity using the Pearson’s correlation.
Correlation between BAT Imaging and Anthropometric Measurements
Image characteristics of BAT measured by MRI (FF, R2* and ndb) and by PET/CT (SUVmax, BAT volume and total BAT activity) were compared with DXA body adiposity measurements (FMI, %fat, and FMR), and BMI using the Pearson’s correlation.
All statistical analyses were performed in SAS 9.4 (SAS Institute, Cary, NC), and a p-value (P) < 0.05 was considered statistically significant.
RESULTS
A total 15 eligible male subjects (20.7 ± 1.5 years old) participated in this study, including 6 normal weight subjects with BMI range 20.6–24.7 kg/m2, 5 overweight subjects with BMI range 25.6–29.4 kg/m2, and 4 obese subjects with BMI range 31.3–35.9 kg/m2.
18F-FDG PET/CT Imaging Findings
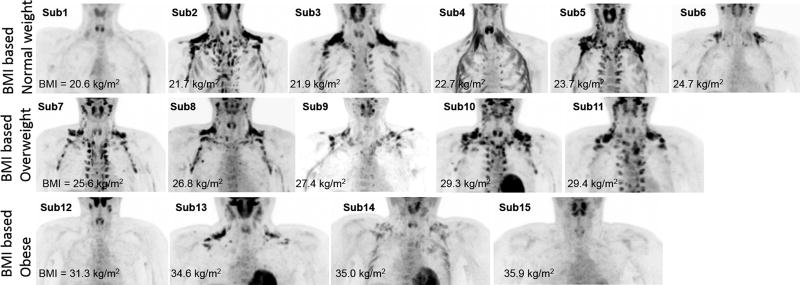
Extensive metabolically active BAT was detected in 11 out of 15 subjects (Fig.4). The other one normal weight and one obese subjects showed very small focus of hypermetabolic brown fat (volume = 0.24 in subject 1 and 0.09 ml in subject 12), and one other obese subject demonstrated no active brown fat (volume = 0 ml in subject 15). Another normal weight subject (subject 4) showed obvious FDG uptake located predominately within multiple muscle groups including the sternocleidomastoid and scalene muscles, therefore this subject was excluded from PET/CT analyses. There was a large variance in FDG uptake between individuals with SUVmax=14.3 ± 8.0 g/ml, volume = 33.5 ± 31.8 ml, and total activity = 260.9 ± 256.7 kBq in all subjects excluding subject 4. There was no significant correlation (P > 0.05)) between PET/CT BAT measurements (i.e. SUVmax, BAT volume and total BAT activity) and BMI and DXA measurements (i.e. FMI, %fat and FMR).
Figure 4.
A large variance of FDG uptake after cold exposure was demonstrated in normal weight (first row), over-weight (second row), and obese subjects (third row) as shown in the maximum-intensity-projection (MIP) PET images. In normal weight subject 4, a small amount of metabolically active brown fat in the supraclavicular fossa was detected, however, prominent metabolic activity in the neck bilaterally and paralleling the ribs appeared to be located within the muscles, and likely represented predominately muscular metabolic activity.
MRI Findings
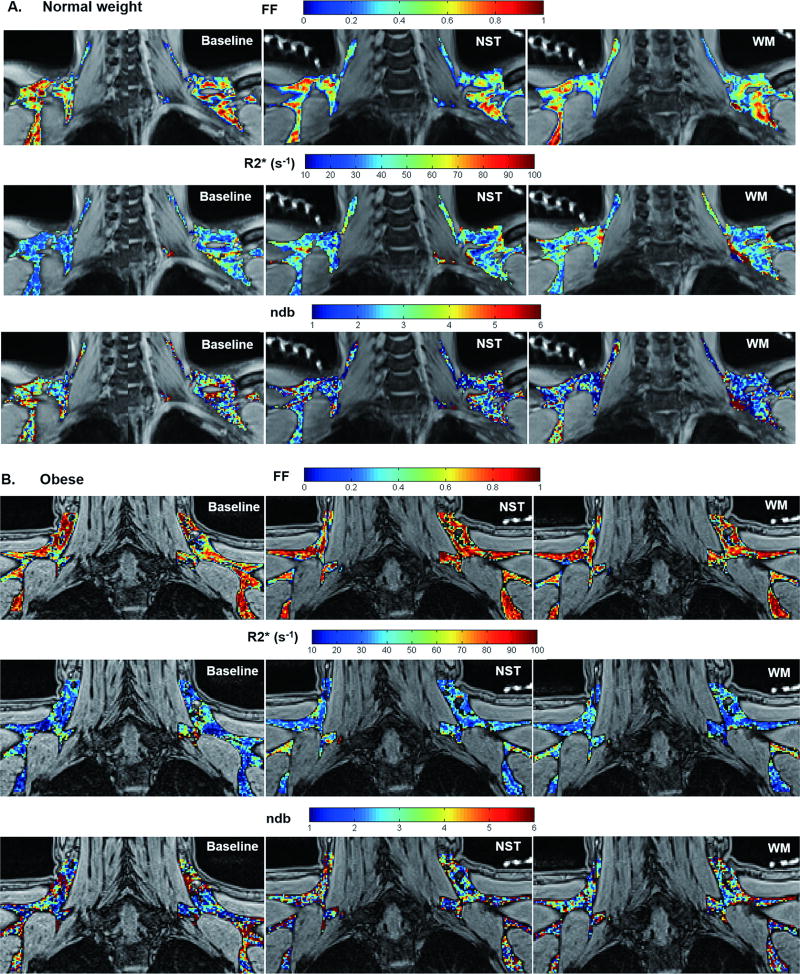
Supraclavicular fat pads were clearly identified in MR images in all subjects. Parametric maps of FFCplx, R2*Cplx and ndb of BAT areas under each thermal condition (i.e. baseline, NST and WM) were shown in a representative normal-weight (Fig. 5A) and an obese subject (Fig. 5B). Adipose tissue heterogeneity was shown within BAT ROIs on each parametric map.
Figure 5.
Parametric maps of FFCplx, R2*Cplx and ndb of BAT in a representative normal weight (A) and obese subject (B) acquired at baseline, NST and WM conditions demonstrated MRI measurement changes in response to thermal challenges. Note that the baseline images in (A) were stitched from two adjacent slices to better match the BAT ROIs in the NST and WM images. The bright signals in the background of NST and WM images were from the water-filled tubing connected to the thermos-suit.
Model Comparison
FFCplx and R2*Cplx derived from the complex signal model, FFMag and R2*Mag derived from the magnitude signal model, and FFFAC and R2*FAC derived from the FAC model were highly correlated and concordant (Table 2). With the high agreement between the three models, we chose FFCplx and R2*Cplx derived from the complex signal model and ndb derived from the FAC model for further analyses.
Table 2.
Pearson correlation and concordance calculation between model-based MRI measurements of FF and R2*.
| Pearson’s correlation coefficient (r) |
Concordance correlation coefficient |
|
|---|---|---|
| FFCplx vs FFMag | 0.921 | 0.916 |
| FFCplx vs FFFAC | 0.947 | 0.942 |
| FFMag vs FFFAC | 0.893 | 0.890 |
| R2*Cplx vs R2*Mag | 0.960 | 0.927 |
| R2*Cplx vs R2*FAC | 0.952 | 0.910 |
| R2*Mag vs R2*FAC | 0.976 | 0.975 |
MRI Changes in Response to Thermal Challenges
Mean and standard deviation of FFCplx, R2*Cplx and ndb for all imaging voxels in all subjects under three thermal conditions were summarized in Table 3. Obese subjects showed higher FFCplx and lower R2*Cplx than non-obese subjects under all three thermal conditions with all P < 0.01. However, there was no significant difference in ndb between non-obese and obese groups.
Table 3.
Mean and standard deviation (STD) of FFCplx, R2*Cplx and ndb for all imaging pixels in all, non-obese and obese subjects under three thermal conditions.
| Mean ± STD | All (N=15) | Non-obese (Normal/Overweight, N=11) |
Obese (N =4) |
p-value (P) non-obese vs. obese |
|
|---|---|---|---|---|---|
| FFCplx | baseline | 0.74±0.10 | 0.70±0.08 | 0.85±0.01 | 0.004* |
| NST | 0.71±0.11 | 0.67±0.08 | 0.84±0.04 | 0.001* | |
| WM | 0.71±0.11 | 0.67±0.09 | 0.83±0.04 | 0.005* | |
| R2*Cplx (s−1) | baseline | 42.19±4.43 | 43.94±3.82 | 37.37±0.91 | 0.005* |
| NST | 43.02±5.49 | 45.62±3.58 | 35.85±2.17 | 0.0002* | |
| WM | 43.50±5.96 | 46.12±4.47 | 36.29±2.16 | 0.001* | |
| ndb | baseline | 3.06±0.12 | 3.05±0.13 | 3.08±0.09 | 0.65 |
| NST | 2.96±0.14 | 2.94±0.15 | 3.01±0.11 | 0.42 | |
| WM | 2.94±0.16 | 2.93±0.18 | 2.97±0.11 | 0.66 | |
Statistically significant (P < 0.05)
ROC analyses demonstrated that FFCplx and R2*Cplx were excellent predictors for the differentiation of non-obese from obese subjects, especially FFCplx at baseline (cutoff FFCplx = 0.83) and R2*Cplx at NST (cutoff R2*Cplx = 38.3 s−1) and R2*Cplx at WM (cutoff R2*Cplx = 38.2 s−1) all provided 100% specificity and 100% sensitivity. ndb acquired at NST provided 73% sensitivity and 75% specificity to differentiate non-obese from obese subjects with a cutoff of 3.53.
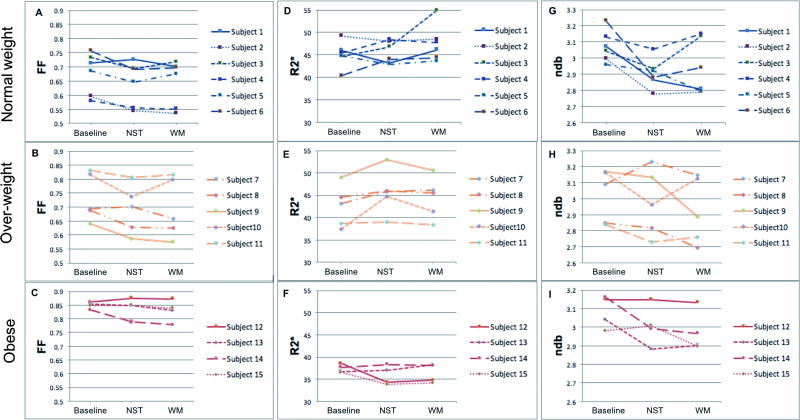
Changes of FFCplx, R2*Cplx and ndb in response to thermal challenges are shown in Fig. 6.
Fat fraction changes are demonstrated in Fig. 6A–C. At NST condition, FF decreased in 83% (5/6) normal weight subjects, 80% (4/5) over-weight subjects, and 50% (2/4) obese subjects compared with baseline. Both obese subjects (subject 13 and 14) having decreased FFCplx at NST demonstrated some degree of active brown fat most prominent in the supraclavicular and infraclavicular regions on their FDG-PET images, whereas the other two obese subjects (subject 13 and 15) did not show demonstrable FDG uptake in BAT on PET imaging. Comparably, the only normal-weight subject (subject 1) with increased FFCplx at NST showed no significant hypermetabolic brown fat on his PET images. However, the direction and degree of FF changes at WM condition varied across subjects in the non-obese group. Within 9 normal weight and overweight subjects having decreased FFCplx at NST, 5 of them showed the trend of FFCplx recovery at WM towards the baseline level, whereas the other 4 showed continued decreases in FFCplx at WM. The two obese subjects (subject 13 and 14) with decreased FFCplx at NST had continued FFCplx decrease at WM, whereas the other two obese subjects with increased FFCplx at NST had either stable or increased FFCplx at WM.
Changes in R2* are shown in Fig. 6D–F. At NST condition, R2* increased in 50% (3/6) normal weight subjects, 100% (5/5) overweight subjects, and 50% (2/4) of obese subjects from baseline. The two obese subjects (subject 13 and 14) with active brown fat on their PET imaging demonstrated increased R2*Cplx at NST, whereas the other two obese subjects lacked demonstrable active brown fat on PET had decreased R2*Cplx at NST. At WM condition, the direction and degree of R2* changes varied across subjects with the majority having a recovery trend towards the baseline level.
Changes in ndb are demonstrated in Fig. 6G–I. At NST condition, ndb decreased in 100% (6/6) normal weight subjects, 80% (4/5) overweight subjects, and 50% (2/4) of obese subjects compared with baseline. Consistent with FF and R2* observations, the two obese subjects (subject 13 and 14) with active brown fat on their PET images demonstrated decreased ndb at NST, whereas the other two obese subject lacking active brown fat on PET showed increased or unchanged ndb at NST. At WM condition, the direction and degree of ndb changes varied across all subjects.
Figure 6.
Plot of the mean values of FF (A–C), R2* (D–F) and ndb (G–I) across all pixels within segmented BAT ROIs in MRI images for each subject (red group: obese, orange group: overweight, blue group: normalweight). These plots demonstrated the changes in BAT properties upon (NST) and post activation (WM) compared with baseline thermoneutral condition.
To further assess the correlation of FF and R2* values with obesity and thermal challenges, the linear mixed model demonstrated that FF values of obese patients were significant higher than those of non-obese subjects (P = 0.001), and the FF values of all subjects acquired at NST and WM were significantly lower than those acquired at baseline (P = 0.003). Also, R2* of obese subjects were significantly lower than that of non-obese subjects (P < 0.0001). However, the overall changes of FF and R2* in response to thermal condition changes were not significantly different between the obese and non-obese subjects (P =0.16 for FF, and P =0.10 for R2*).
Correlation between MRI and Measures of Adiposity
FFCplx and R2*Cplx under all three thermal conditions were highly correlated with BMI, FMI, %fat, and FMR (Table 4). ndb was not correlated with any of the measures of adiposity.
Table 4.
Pearson correlation between MRI measurements (FFCplx and R2*Cplx) and measures of adiposity under three thermal conditions. All correlations are statistically significant with P < 0.05.
| Pearson Correlation Coefficient r (P) |
BMI | FMI | %fat | FMR | |
|---|---|---|---|---|---|
| FFCplx | baseline | 0.78 (0.0006) | 0.84 (< 0.0001) | 0.85 (< 0.0001) | 0.90 (< 0.0001) |
| NST | 0.74 (0.0015) | 0.82 (0.0002) | 0.82 (0.0002) | 0.86 (< 0.0001) | |
| WM | 0.72 (0.0027) | 0.80 (0.0003) | 0.82 (0.0002) | 0.88 (< 0.0001) | |
| R2*Cplx (s−1) | baseline | −0.81 (0.0002) | −0.83 (0.0001) | −0.80 (0.0003) | −0.81 (0.0003) |
| NST | −0.72 (0.002) | −0.82 (0.0002) | −0.80 (0.0003) | −0.78 (0.0006) | |
| WM | −0.82 (0.0002) | −0.83 (0.0001) | −0.78 (0.0006) | −0.73 (0.002) | |
Correlation between MRI and 18F-FDG PET/CT Measurements
There were no correlations between MRI measurements and PET/CT measurements of BAT under individual thermal conditions. Nonetheless, FFCplx changes secondary to thermal challenges, which are reflective of the activation level of BAT, were correlated with PET measurements of BAT. FFCplx changes from baseline to NST due to BAT activation were negatively correlated with hypermetabolic BAT volume measured by FDG PET/CT (Pearson correlation coefficient r = −0.55, P = 0.04). In addition, FFCplx changes during post-activation from NST to WM were positively correlated with hypermetabolic BAT volume (r = 0.72, P = 0.003) and total BAT activity (r = 0.69, P = 0.006) measured by FDG PET/CT. However, R2*Cplx and ndb changes with thermal challenges were not correlated with PET measurements.
DISCUSSION
This study demonstrated that 1) MRI characterization of BAT can differentiate between non-obese and obese young male subjects, 2) fat fraction and R2* measurements of BAT depots are highly correlated with measures of adiposity, and 3) fat fraction changes within BAT in response to thermal challenges are correlated with BAT volume and activity as measured by 18F-FDG PET/CT.
We implemented quantitative Dixon MRI methods for BAT characterization at both inactivated and activated states after cold exposure. To reach the maximal BAT activation, we used an individualized cooling protocol to achieve NST instead of a less effective general cooling room protocol. Importantly, in addition to imaging BAT depots at baseline and NST, our protocol also included a third MRI scan at the warm-up condition to investigate BAT property recovery at the post-activated state. To implement the quantification of FF of BAT, it is challenging to determine whether water or fat is dominant when there are similar amounts of fat and water protons (i.e. FF ~50%) contributing to the MRI signal in each voxel. Accurate quantification of FF slightly higher or lower than 50% is critical to detect the minor FF changes secondary to thermal changes. Our study advanced the knowledge of techniques by implementing different fat quantification models of complex fitting, magnitude fitting and fatty acid composition estimation, and demonstrated that three signal fitting models were highly consistent in terms of the calculations of FF and R2*.
Brown adipocytes contain multilocular lipid droplets, and the size of the lipid droplets depends on the tissue’s activation level (37,38). Our results demonstrated that obese subjects had higher FF than non-obese subjects under all three thermal conditions, and BAT FF at baseline (i.e. inactivated status) can differentiate obese and non-obese subjects based on the ROC analysis. When BAT was metabolically active under non-shivering thermogenesis conditions, combustion of the fatty acids causes the depletion of lipids within adipocytes resulting in a lower FF (39,40). We demonstrated that FF decreased in the majority of non-obese subjects, as well as in the two obese subjects who had FDG-avid BAT on PET/CT. Based on our results that FF changes from baseline to NST and from NST to WM were correlated with the hypermetabolic BAT volume/activity detected by FDG PET/CT, we believe that FF changes under thermal challenges are related with the activation status of BAT, although it is not yet clear if the increasing FF during WM is due to the fat replenishment. Continuous measurements of FF during a longer exposure to the warm up phase can be done in the future study to investigate more stable restore of FF during BAT de-activation.
Brown adipocytes have abundant iron-containing mitochondria leading to shortened T2* (i.e. higher R2*). Our results demonstrated that obese subjects had lower R2* than non-obese subjects under all three thermal conditions, and BAT R2* at either NST (i.e. activated status) or WM condition (i.e. post-activated status) can differentiate obese and non-obese subject based on the ROC analysis. Acute R2* changes in response to thermal challenges may attribute to the increased oxygen consumption in brown adipocytes that contain a dense capillary network (9). Upon activation, increased metabolic activity of BAT induces more oxygen consumption, which in turn increases blood perfusion to supply oxygen to the tissues. As a result, T2* relaxation time changes during the hemodynamic process depending on whether oxygen consumption or blood perfusion is dominant. Increasing oxygen consumption leads to a higher level of deoxyhemoglobin in the blood, resulting in decreased T2* (i.e. increased R2*). Nonetheless, blood flow brings with it more oxyhemoglobin, leading to a decrease in regional deoxyhemoglobin and thus an increase in T2* (i.e. decreased R2*). When the higher demand of oxygen consumption exceeds blood oxygen supply, T2* would decrease (i.e. R2* increases), otherwise T2* would increase (i.e. R2* decreases). It has been reported that the increase in blood flow is accompanied by 10-fold increase in BAT oxygen consumption (41). A murine study found that BAT activation with norepinephrine led to increasing oxygen consumption that was not sufficiently compensated by the increased blood flow, and therefore T2/T2* was shortened (42). In a human study, cold-activated BAT showed T2*-weighted signal fluctuations temporally coincident with the cold stimulus, however, the sign of the signal change was uncertain (43). We demonstrated that R2* increased at NST in 8 out of 11 non-obese subjects and the two obese subjects with FDG-avid brown fat, suggesting significant BAT oxygen consumption upon activation. In contrast, R2* decreased in the other two obese subjects and one normal-weight subject (subject 1), all of whom were lacking BAT activity on their FDG PET images. Interestingly, the other two non-obese subjects (subject 2 and subject 5) showing decreased R2* demonstrated the highest BAT activity among all normal weight and overweight subjects, suggesting that a significant increase in blood flow and oxyhemoglobin may outweigh the oxygen consumption by BAT. In addition, the uncertainty of R2* changes could also be due to local tissue temperature variations during the thermogenesis process in BAT. The frequency shift between water and fat peaks may change due to local temperature change, which may lead to over- or under-estimation of R2* if the temperature-induced frequency shift is not corrected in the signal model.
A larger number of brown adipocytes contains a lower levels of unsaturated triglycerides and thus less number of double bonds. In a mouse model, a range of values of ndb (2.49–3.63) and nmidb (0.7–1.81) have been reported, depending on age (23). Our results demonstrated that ndb at NST (i.e. activated status) can differentiate obese and non-obese subjects based on the ROC analysis. Upon BAT activation, combustion of the fatty acids may further decrease unsaturated fatty acid synthesis (i.e. the triglyceride unsaturation level). Our results demonstrated ndb decreased at NST in 10 out of 11 non-obese subjects, and the two obese subjects with FDG-avid BAT.
MRI measurements were also compared with DXA and anthropometric measurements. FF and R2* were highly correlated with measures of adiposity, while not correlated with PET/CT BAT measurements, under any individual thermal condition. Of note, the dynamic changes of FF and R2* during activation and post-activation processes were correlated with BAT volume and activity measured by PET/CT, suggesting that MRI measurements in response to thermal challenges can detect the metabolic activity of BAT.
A previous study in nine subjects using a general cold-room cooling protocol reported a small FF decrease after cold exposure (82.8 ± 5.0% at baseline and 80.9 ± 6.1% after cold exposure), followed by a sustained low FF (80.9 ± 6.5%) after rewarming (44). A more recent 18FDG PET/MRI study in nine normal weight, three overweight and one obese subjects with both genders and a wide age range (19–55 years) (28) demonstrated that FF and R2* at room temperature and under cold exposure were inversely correlated with the glucose uptake measured by FDG PET, which differs from our results. Another recent PET/MR study reported there was no significant differences in FF and R2* between FDG-avid and non-avid supraclavicular BAT (45), which was consistent with our findings. However, both of these PET/MR studies reported that cold exposure did not significantly affect FF and/or T2*, which, in our opinion, could be due to the limitations in the quantification methods. The first study (28) utilized two-echo signal-intensity-based method, and the second study (45) utilized magnitude-based pixel-by-pixel signal fitting, which was especially challenging due to water and fat swaps in BAT that typically has a fat fraction around 50%. Overall, these studies included heterogeneous subject populations, which may confound the analyses of BAT properties and activities. Furthermore, neither of these studies investigated the differences in inactivated and activated BAT MRI characteristics between non-obese and obese subjects.
The limitation of our study includes that co-registration between PET and MRI was not performed to constrain FDG-avid active BAT areas in the corresponding MRI images. We anticipate to translate the MRI techniques developed in the present study to hybrid PET/MRI studies to further investigate the MRI features of FDG-avid and non-avid BAT. A recently published standardized FDG-PET/CT guideline should be followed to improve the consistency of BAT PET/CT study (29). Secondarily, our study has a small sample size. A future larger study will be performed based on the techniques that were developed and validated in this study, aiming to investigate the association between metabolically active BAT and adiposity, and to explore the potential for activation/recruitment of BAT as a treatment of obesity and related metabolic diseases. Lastly, given the multi-parametric MRI characterization of BAT developed in this study, clustering methods with joint multi-parametric evaluations are warranted for measuring the volume of active vs. inactive BAT on MRI (36).
In conclusion, Dixon MRI characterized BAT tissue properties for differentiation between non-obese and obese subjects at both inactivated and activated states. Based on our results, it is likely that BAT is more metabolically active if the following MRI changes are all observed at NST compared with baseline: FF decreases, R2* increases and ndb decreases. Moreover, Dixon MRI detected BAT activation level in response to thermal challenges, and this was well-correlated with glucose uptake of metabolically active BAT after cold exposure, as measured by 18F-FDG PET/CT. MRI measurements for characterization of BAT tissue properties and detection of BAT activity may help understand obesity development, and serve as an imaging biomarker to facilitate discovery of novel therapeutic targets for metabolic disease that activate or recruit brown or brite adipocytes.
Acknowledgments
Grant support: Grant Number 1R21DK103145-01 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA : the journal of the American Medical Association. 2012;307(5):483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA : the journal of the American Medical Association. 2012;307(5):491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 3.Kajimura S, Saito M. A new era in brown adipose tissue biology: molecular control of brown fat development and energy homeostasis. Annu Rev Physiol. 2014;76:225–249. doi: 10.1146/annurev-physiol-021113-170252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoneshiro T, Aita S, Matsushita M, et al. Brown adipose tissue, whole-body energy expenditure, and thermogenesis in healthy adult men. Obesity (Silver Spring, Md) 2011;19(1):13–16. doi: 10.1038/oby.2010.105. [DOI] [PubMed] [Google Scholar]
- 5.Chondronikola M, Volpi E, Borsheim E, et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes. 2014;63(12):4089–4099. doi: 10.2337/db14-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hibi M, Oishi S, Matsushita M, et al. Brown adipose tissue is involved in diet-induced thermogenesis and whole-body fat utilization in healthy humans. Int J Obes. 2016;40:1655–1661. doi: 10.1038/ijo.2016.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saito M, Okamatsu-Ogura Y, Matsushita M, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58(7):1526–1531. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stanford KI, Middelbeek RJW, Townsend KL, et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest. 2013;123(1):215–223. doi: 10.1172/JCI62308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fruhbeck G, Becerril S, Sainz N, Garrastachu P, Garcia-Velloso MJ. BAT: a new target for human obesity? Trends in pharmacological sciences. 2009;30(8):387–396. doi: 10.1016/j.tips.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Lidell ME, Betz MJ, Dahlqvist Leinhard O, et al. Evidence for two types of brown adipose tissue in humans. Nature medicine. 2013;19(5):631–634. doi: 10.1038/nm.3017. [DOI] [PubMed] [Google Scholar]
- 11.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, et al. Cold-activated brown adipose tissue in healthy men. The New England journal of medicine. 2009;360(15):1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 12.Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes & development. 2013;27(3):234–250. doi: 10.1101/gad.211649.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nature medicine. 2013;19(10):1252–1263. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 14.Giralt M, Villarroya F. White, brown, beige/brite: different adipose cells for different functions? Endocrinology. 2013;154(9):2992–3000. doi: 10.1210/en.2013-1403. [DOI] [PubMed] [Google Scholar]
- 15.van der Lans AA, Wierts R, Vosselman MJ, Schrauwen P, Brans B, van Marken Lichtenbelt WD. Cold-activated brown adipose tissue in human adults: methodological issues. American journal of physiology Regulatory, integrative and comparative physiology. 2014;307(2):R103–113. doi: 10.1152/ajpregu.00021.2014. [DOI] [PubMed] [Google Scholar]
- 16.Virtanen KA, Lidell ME, Orava J, et al. Functional brown adipose tissue in healthy adults. The New England journal of medicine. 2009;360(15):1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 17.van der Lans AA, Hoeks J, Brans B, et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. The Journal of clinical investigation. 2013;123(8):3395–3403. doi: 10.1172/JCI68993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Lans A, Wierts R, Vosselman M, Schrauwen P, Brans B, van Marken Lichtenbelt W. Cold-activated brown adipose tissue in human adults: methodological issues. Am J Physiol Regul Integr Comp Physiol. 2014;307(2):R103–113. doi: 10.1152/ajpregu.00021.2014. [DOI] [PubMed] [Google Scholar]
- 19.Vijgen G, Bouvy N, Jaap Teule G, Brans B, Schrauwen P, Van Marken Lichtenbelt W. Brown adipose tissue in morbidly obese subjects. PloS ONE. 2011;6(2):e17247. doi: 10.1371/journal.pone.0017247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma SW, Foster DO. Uptake of glucose and release of fatty acids and glycerol by rat brown adipose tissue in vivo. Can J Physiol Pharmacol. 1986;64(5):609–614. doi: 10.1139/y86-101. [DOI] [PubMed] [Google Scholar]
- 21.Orava J, Nuutila P, Lidell ME, et al. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab. 2011;14(2):272–279. doi: 10.1016/j.cmet.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 22.Hu HH, Perkins TG, Chia JM, Gilsanz V. Characterization of human brown adipose tissue by chemical-shift water-fat MRI. AJR Am J Roentgenol. 2013;200(1):177–183. doi: 10.2214/AJR.12.8996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamilton G, Smith DL, Jr, Bydder M, Nayak KS, Hu HH. MR properties of brown and white adipose tissues. Journal of magnetic resonance imaging : JMRI. 2011;34(2):468–473. doi: 10.1002/jmri.22623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu HH, Hines CD, Smith DL, Jr, Reeder SB. Variations in T(2)* and fat content of murine brown and white adipose tissues by chemical-shift MRI. Magn Reson Imaging. 2012;30(3):323–329. doi: 10.1016/j.mri.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bydder M, Girard O, Hamilton G. Mapping the double bonds in triglycerides. Magnetic resonance imaging. 2011;29(8):1041–1046. doi: 10.1016/j.mri.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Peterson P, Mansson S. Simultaneous quantification of fat content and fatty acid composition using MR imaging. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2013;69(3):688–697. doi: 10.1002/mrm.24297. [DOI] [PubMed] [Google Scholar]
- 27.Gifford A, Towse TF, Walker RC, Avison MJ, Welch EB. Characterizing active and inactive brown adipose tissue in adult humans using PET-CT and MR imaging. Am J Physiol Endocrinol Metab. 2016;311(1):E95–E104. doi: 10.1152/ajpendo.00482.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holstila M, Pesola M, Saari T, et al. MR signal-fat-fraction analysis and T2* weighted imaging measure BAT reliably on humans without cold exposure. Metabolism. 2017;70:23–30. doi: 10.1016/j.metabol.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Chen KY, Cypess AM, Laughlin MR, et al. Brown Adipose Reporting Criteria in Imaging STudies (BARCIST 1.0): Recommendations for Standardized FDG-PET/CT Experiments in Humans. Cell Metab. 2016;24(2):210–222. doi: 10.1016/j.cmet.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schaefferkoetter J. https://wwwmathworkscom/matlabcentral/fileexchange/53745-medical-image-reader-and-viewer.
- 31.Gifford A, Towse TF, Walker RC, Avison MJ, Welch EB. Human brown adipose tissue depots automatically segmented by positron emission tomography/computed tomography and registered magnetic resonance images. J Vis Exp. 2015;(96) doi: 10.3791/52415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hernando D, Kellman P, Haldar JP, Liang ZP. Robust water/fat separation in the presence of large field inhomogeneities using a graph cut algorithm. Magn Reson Med. 2010;63(1):79–90. doi: 10.1002/mrm.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hernando D, Haldar JP, Sutton BP, Ma J, Kellman P, Liang ZP. Joint estimation of water/fat images and field inhomogeneity map. Magn Reson Med. 2008;59(3):571–580. doi: 10.1002/mrm.21522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peterson P, Mansson S. Fat quantification using multiecho sequences with bipolar gradients: investigation of accuracy and noise performance. Magn Reson Med. 2014;71(1):219–229. doi: 10.1002/mrm.24657. [DOI] [PubMed] [Google Scholar]
- 35.Otsu N. A threshold selection method from gray-level histograms. IEEE Trans Syst Man Cybernet. 1979;9:62–66. [Google Scholar]
- 36.Hui SC, Ko JK, Zhang T, et al. Quantification of brown and white adipose tissue based on Gaussian mixture model using water-fat and T2* MRI in adolescents. J Magn Reson Imaging. 2017 doi: 10.1002/jmri.25632. [DOI] [PubMed] [Google Scholar]
- 37.Heaton JM. The distribution of brown adipose tissue in the human. Journal of anatomy. 1972;112(Pt 1):35–39. [PMC free article] [PubMed] [Google Scholar]
- 38.Tanuma Y, Tamamoto M, Ito T, Yokochi C. The occurrence of brown adipose tissue in perirenal fat in Japanese. Archivum histologicum Japonicum = Nihon soshikigaku kiroku. 1975;38(1):43–70. doi: 10.1679/aohc1950.38.43. [DOI] [PubMed] [Google Scholar]
- 39.Hirvonen J, Elfving R. Depletion of fats from the brown adipose tissue cells of rats dead from cold exposure. Z Rechtsmed. 1973;72(1):50–55. doi: 10.1007/BF02076848. [DOI] [PubMed] [Google Scholar]
- 40.van Dam AD, Boon MR, Berbee JF, Rensen PC, van Harmelen V. Targeting white, brown and perivascular adipose tissue in atherosclerosis development. Eur J Pharmacol. 2017 doi: 10.1016/j.ejphar.2017.03.051. [DOI] [PubMed] [Google Scholar]
- 41.Matthias A, Ohlson KB, Fredriksson JM, Jacobsson A, Nedergaard J, Cannon B. Thermogenic responses in brown fat cells are fully UCP1-dependent. UCP2 or UCP3 do not substitute for UCP1 in adrenergically or fatty scid-induced thermogenesis. The Journal of biological chemistry. 2000;275(33):25073–25081. doi: 10.1074/jbc.M000547200. [DOI] [PubMed] [Google Scholar]
- 42.Khanna A, Branca RT. Detecting brown adipose tissue activity with BOLD MRI in mice. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2012;68(4):1285–1290. doi: 10.1002/mrm.24118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Rooijen BD, van der Lans AA, Brans B, et al. Imaging Cold-Activated Brown Adipose Tissue Using Dynamic T2*-Weighted Magnetic Resonance Imaging and 2-Deoxy-2-[18F]fluoro-D-glucose Positron Emission Tomography. Investigative radiology. 2013;48(10):708–714. doi: 10.1097/RLI.0b013e31829363b8. [DOI] [PubMed] [Google Scholar]
- 44.Lundstrom E, Strand R, Johansson L, Bergsten P, Ahlstrom H, Kullberg J. Magnetic resonance imaging cooling-reheating protocol indicates decreased fat fraction via lipid consumption in suspected brown adipose tissue. PloS one. 2015;10(4):e0126705. doi: 10.1371/journal.pone.0126705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCallister A, Zhang L, Burant A, Katz L, Branca RT. A pilot study on the correlation between fat fraction values and glucose uptake values in supraclavicular fat by simultaneous PET/MRI. Magn Reson Med. 2017 doi: 10.1002/mrm.26589. [DOI] [PMC free article] [PubMed] [Google Scholar]