Abstract
Objective
In the current meta-analysis, we aimed to evaluate the diagnostic performance of hybrid cardiac imaging techniques compared with stand-alone coronary CT angiography (CCTA) for assessment of obstructive coronary artery disease (CAD).
Background
The usefulness of CCTA for detecting obstructive CAD remains suboptimal at present. Myocardial perfusion imaging (MPI) encompasses positron emission tomography, single photon-emission CT, and cardiac magnetic resonance imaging, which permit for the identification of myocardial perfusion defects to detect significant CAD. A hybrid approach comprising MPI and CCTA may improve diagnostic performance for detecting obstructive CAD.
Methods
PubMed and Web of Knowledge were searched for relevant publications between January 1, 2000 and December 31, 2015. Studies using CCTA and hybrid imaging for diagnosis of obstructive CAD (a luminal diameter reduction of >50% or >70% by invasive coronary angiography) were included. In total, 12 articles comprising 951 patients and 1,973 vessels were identified, and a meta-analysis was performed to determine pooled sensitivity, specificity, and summary receiver operating characteristic (sROC) curves.
Results
On a per-patient basis, the pooled sensitivity of hybrid imaging was comparable with CCTA (91% vs. 90%, p =0.28). Yet, specificity was higher for hybrid imaging versus CCTA (93% vs. 66%, p <0.001). On a per-vessel basis, sensitivity for hybrid imaging against CCTA was comparable (84% vs. 89%, p =0.29). Notably, hybrid imaging yielded a specificity of 95% vs. 83% for CCTA (p <0.001). sROC curves displayed improved discrimination for hybrid imaging beyond CCTA alone, on a per-vessel (AUC = 0.97 vs. 0.93, p =0.047) basis, however, not on a per-patient (AUC = 0.97 vs. 0.93, p =0.132) level, respectively.
Conclusion
Hybrid cardiac imaging demonstrated improved diagnostic specificity for detection of obstructive CAD compared with stand-alone CCTA, yet, improvement in overall diagnostic performance was relatively limited.
Keywords: Coronary computed tomography angiography, Coronary artery disease, Myocardial perfusion imaging
INTRODUCTION
Coronary CT angiography (CCTA) represents a non-invasive imaging modality that permits direct visualization of coronary artery disease (CAD). CCTA is a robust tool for identifying the presence or absence of CAD and provides a wealth of prognostic information (1). While numerous studies have confirmed the high negative predictive value (NPV) and sensitivity of CCTA, the positive predictive value (PPV) and specificity of this modality are typically lower (2,3). Specifically, an overestimation of stenosis by CCTA is often observed (4,5). In light of this, a CCTA-guided coronary stenosis strategy should perhaps be considered a suboptimal indicator of obstructive CAD.
Myocardial perfusion imaging (MPI) encompasses positron emission tomography (PET), single photon-emission CT (SPECT), and cardiac magnetic resonance imaging (CMR). These modalities identify stress-induced wall motion abnormalities or regional myocardial perfusion defects, and serve to identify individuals who may have flow-limiting coronary stenoses. A hybrid approach combining both modalities, MPI and CCTA, has the advantage of fusing the anatomic CCTA derived data with functional MPI perfusion data. In doing so, a hybrid approach has the potential to overcome the limitations of CCTA. One potential benefit of utilizing perfusion data is that it may assist in differentiating artifact-driven stenosis from true coronary luminal diameter narrowing, which may facilitate in diminishing the false-positive rate of CCTA. This dual-modality approach might therefore improve diagnostic performance for detecting obstructive CAD by overcoming many of the drawbacks relative to stand-alone CCTA.
To understand the clinical utility of this approach, we conducted a systematic literature review and meta-analysis to evaluate the diagnostic performance of hybrid cardiac imaging techniques in comparison with stand-alone CCTA for assessment of obstructive CAD as determined by invasive coronary angiography (ICA), a reference standard.
METHODS
Literature search
The electronic databases PubMed and ISI Web of Knowledge were systematically examined to locate relevant articles in English using predefined search criteria (Table 1). The search was confined to investigations that were published between January 1, 2000 and December 31, 2015. The following search terms were employed: positron emission tomography OR single photon emission computed tomography OR magnetic resonance imaging OR stress myocardial perfusion imaging OR functional AND (coronary computed tomography angiography OR anatomic) AND (combined or hybrid or comprehensive assessment) AND (diagnosis or detection) AND (coronary artery disease OR myocardial ischemia). Three investigators (A.R., D.H., and J.L.) independently scanned all manuscripts and performed data extraction. Abstracts were excluded because of insufficient data. All retrieved studies were examined and any potential overlapping data were omitted. Two independent reviewers (A.R. and D.H.) performed the final screening of reports for inclusion in the meta-analysis. In the event of any discord, a general consensus was met between reviewers after further extensive review of the full text articles.
Table 1.
Study and test characteristics
| Author, year | Test characteristics |
Patients (N) |
Vessels (N) |
Age ± SD, (range) |
Male, n (%) |
Criteria for performing hybrid imaging |
Reference standard |
Time interval |
QCA Threshold |
|---|---|---|---|---|---|---|---|---|---|
| Thomassen, 2013 (15) | PET/64-slice MDCT | 44 | 176 | 66 ± 9 | 23 (52) | Patients with suspected CAD | QCA | 1 Day | ≥50% |
| Groothuis, 2013 (21) | CMR/64-slice MDCT | 88 | 56 ± 10 | 96 (49) | Patients with suspected CAD | QCA ± FFR | 2 Months | QCA >70% or FFR ≤0.75 if 30–70% diameter stenosis, or QCA >50% if FFR was not available | |
| Schaap, 2013 (35) | SPECT/64-slice MDCT | 98 | 63 ± 10 | 67 (68) | Patients with suspected CAD | QCA + FFR | 14 Days | QCA ≥50% FFR <0.80 | |
| Li, 2013 (12) | SPECT/64-slice MDCT | 54 | 216 | 57 ± 9 | 36 (67) | Patients with suspected or known CAD | QCA + MPI | 30 Days | ≥50% |
| Danad, 2013 (16) | PET/64-slice MDCT | 120 | 360 | 61 ± 10 | 77 (64) | Patients with suspected CAD | QCA ± FFR | 70 Days | QCA ≥50% FFR ≤0.80 |
| Kadokami, 2011 (13) | SPECT/64-slice MDCT | 49 | 145 | 70 ± 8 | 35 (71) | Patients with suspected or known CAD | QCA + MPI | 3 Months | ≥50% |
| Kajander, 2010 (17) | PET/64-slice MDCT | 107 | 416 | 63.6 ± 7 | 64 (60) | Patients with suspected CAD | QCA + FFR | 2 Weeks | QCA ≥50% FFR ≤0.80 |
| Winther, 2015 (36) | SPECT/dual-source | 138 | 54 (22–72) | 94 (68) | Pre-renal transplant cardiac evaluation | QCA | 34 Days | ≥50% | |
| Scheffel, 2010 (18) | CMR/dual-source | 43 | 129 | 63 ± 9 | 34 (79) | Patients with suspected or known CAD | QCA | 20 Days | >50% |
| Donati, 2010 (19) | CMR/dual-source | 47 | 141 | 64 ± 9 | 38 (81) | Patients with suspected or known CAD | QCA | 8 Days | >50% |
| Sato, 2010 (14) | SPECT/64-slice MDCT | 130 | 390 | 67 ± 11 | 91 (70) | Patients with suspected CAD | QCA | 1 Month | ≥50% |
| Groves, 2009 (2) | PET/64-slice MDCT | 33 | 62 (47–74) | 28 (85) | Patients with suspected CAD | QCA | ≥50% |
Abbreviations: SPECT = single photon-emission computed tomography; PET = positron emission tomography; CMR = cardiac magnetic resonance imaging; MDCT = multi-detector computed tomography; CAD = coronary artery disease; MPI = myocardial perfusion imaging; QCA = quantitative coronary analysis; FFR = fractional flow reserve.
Study eligibility
The inclusion criteria for studies in the analysis were: 1) symptomatic patients with suspected CAD who underwent both CCTA and MPI, utilizing hybrid approach; 2) ICA with quantitative coronary analysis (QCA) that served as the reference standard for obstructive CAD with at least 50% luminal diameter reduction; and 3) absolute numbers of false positives/negatives and true positives/negatives employing both CCTA and hybrid cardiac imaging approaches that were reported in the article, or wherein sufficient data was available so that a 2×2 contingency table of results could be constructed. Studies were excluded from this meta-analysis if <64-slice CT scanner was utilized. All patients in the selected studies for this meta-analysis underwent both noninvasive anatomic and functional imaging, regardless of the CCTA findings.
Data collection
For the current meta-analysis, three independent authors (A.R., D.H., and J.L.) initially performed all data extraction, with subsequent verification independently performed by two of the authors (A.R. and D.H.). The following data were collected for each eligible investigation: year of publication; patient demographics; type of hybrid cardiac imaging methods; number of patients and vessels; criteria for hybrid imaging; and the QCA threshold used to describe obstructive CAD. The reference standard for the current meta-analysis was obstructive CAD with a diameter reduction of >50% or >70%, as defined by ICA with QCA analysis. For the meta-analysis, absolute numbers of true and false positive, and true and false negative results were extracted from the articles, or otherwise calculated from data provided in these articles. The findings were then summarized in a 2×2 contingency table. The selected articles were also evaluated for included references so as to ensure the complete inclusion of all studies. The methodological quality of the selected studies was independently assessed by two of the authors (A.R. and D.H.) according to the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) scale (6). Any discrepancies in quality assessment were resolved by consensus discussion.
Data analysis
Pooled measures for sensitivity, specificity, likelihood ratios, diagnostic odds ratio (DOR), as well as area under the receiver operating characteristic curves (AUC) along with their 95% confidence intervals (95% CIs) were calculated using DerSimonian Lair methodology (7). Calculation of DOR in this meta-analysis permitted the opportunity to test the discriminatory ability of both hybrid cardiac imaging and stand-alone CCTA for detecting obstructive and non-obstructive CAD. Specifically, a DOR of 1 indicates that the test has no discriminative power and a higher DOR is associated with improved diagnostic accuracy. The pooled diagnostic data were presented in test summary receiver-operator curves (sROC), that were reconstructed using Moses-Shapiro-Littenberg methodology and based on the pooled DOR of each index test (8). The Deeks’ method was employed to test for possible publication bias (9). A Cochran Q statistic and the I2 index were also used to test for any heterogeneity between the included studies. A substantial I2 index indicates heterogeneity beyond sampling variation. The heterogeneity was defined as low, moderate, and high by I2 = 25–50%, 50–75%, and >75%, respectively (10). Analyses were performed using STATA version 14 (StataCorp LP, College Station, TX, USA) and Meta-DiSc 1.4 (11). p-values less than 0.05 were considered statistically significant.
RESULTS
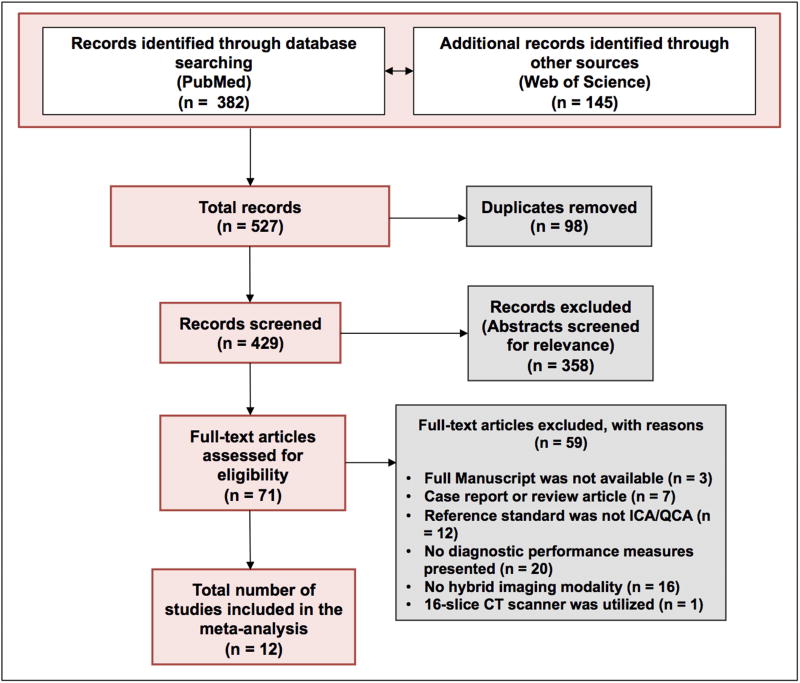
A systematic search revealed 527 potentially relevant articles. After removing 98 duplicates, 429 articles were screened by title and abstract. Of these, 71 articles were read full-text. Finally, a total of 12 studies met the inclusion criteria and were included in the meta-analysis. A flow chart of the search and selection process of the articles is shown in Figure 1.
Figure 1. Flow chart describing the literature search and selection algorithm.
Abbreviations: ICA = invasive coronary angiography; QCA = quantitative coronary analysis.
Baseline characteristics of each study are listed in Table 1. Of a total 951 patients, 739 (72%) were male and the mean age within studies ranged from 54–70 years. A total of 1,973 vessels were included in the current meta-analysis. Each study used at least 64-slice multi-detector CT or dual-source CT scanners. Three articles did not report per-patient diagnostic performance of MPI (12–14). Per-vessel results of CCTA as well as the hybrid approach were only available in 8 articles (12–19), and per-vessel results of MPI were only reported in 6 articles (14–19).
Quality assessment and publication bias
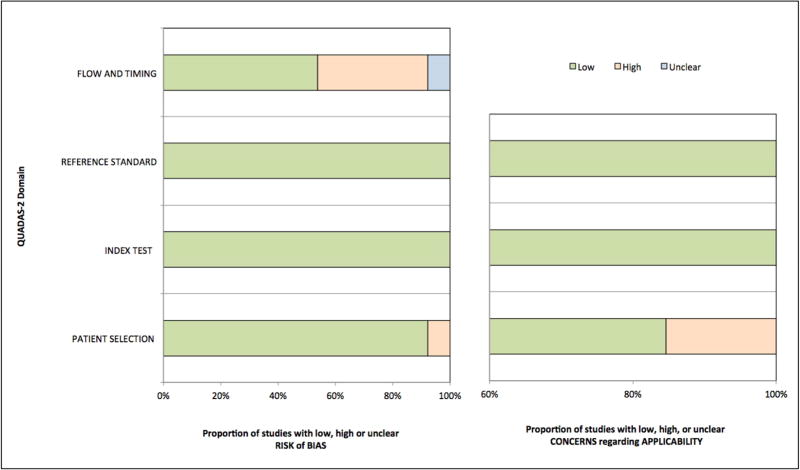
The methodological quality of the included studies as assessed by QUADAS-2 score was generally good, although the quality for flow and timing was substantially poor, indicating a potential risk of introduced bias (Figure 2). A summary of the QUADAS-2 quality scores for each study is shown in Table 2. Using Deeks’ test, there was no evidence of publication bias on both a per-patient and per-vessel level when utilizing the hybrid and stand-alone CCTA approaches (p >0.05 for all; Supplementary Figure 1A and 1B).
Figure 2. Quality assessment of included studies by QUADAS-2 revised criteria.
Abbreviations: QUADAS-2, Quality Assessment of Diagnostic Accuracy Studies.
Table 2.
Quality assessment of the studies included in the meta-analysis using the QUADAS-2 tool
| Study | RISK OF BIAS | APPLICABILITY CONCERNS | |||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| PATIENT SELECTION |
INDEX TEST |
REFERENCE STANDARD |
FLOW AND TIMING |
PATIENT SELECTION |
INDEX TEST |
REFERENCE STANDARD |
|
| Thomassen, 2013 (15) | Low | Low | Low | Low | Low | Low | Low |
| Groothuis, 2013 (21) | Low | Low | Low | High | Low | Low | Low |
| Schaap, 2013 (35) | Low | Low | Low | High | Low | Low | Low |
| Li, 2013 (12) | Low | Low | Low | Low | Low | Low | Low |
| Danad, 2012 (16) | Low | Low | Low | Low | High | Low | Low |
| Kadokami, 2011 (13) | Low | Low | Low | Low | Low | Low | Low |
| Kajander, 2010 (17) | Low | Low | Low | Low | Low | Low | Low |
| Winther, 2014 (36) | High | Low | Low | High | High | Low | Low |
| Scheffel, 2010 (18) | Low | Low | Low | Low | Low | Low | Low |
| Donati, 2010 (19) | Low | Low | Low | High | Low | Low | Low |
| Sato, 2010 (14) | Low | Low | Low | Low | Low | Low | Low |
| Groves, 2009 (2) | Low | Low | Low | Unclear | Low | Low | Low |
Diagnostic performance of CCTA and MPI for assessment of obstructive CAD
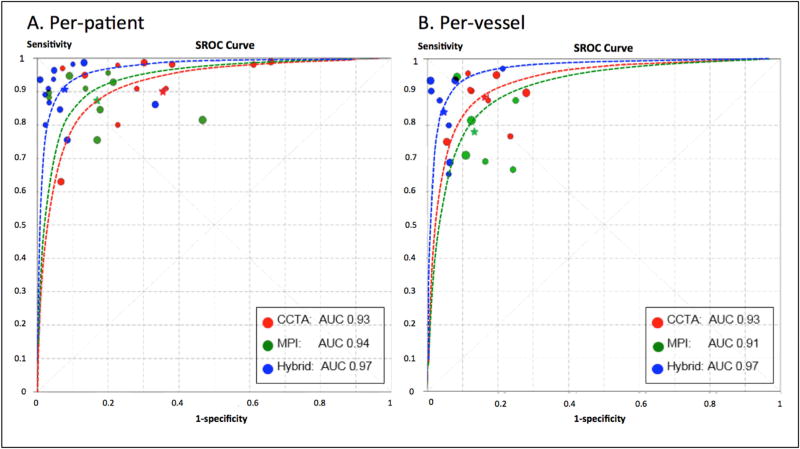
At the per-patient level, CCTA and MPI displayed comparable sensitivity (p =0.35) (Table 3). However, CCTA showed lower specificity (66%) for predicting obstructive CAD compared with MPI (83%) (p <0.001). At the per-vessel level, CCTA exhibited somewhat improved specificity, and results were similar compared with the MPI approach (p =0.02) (Table 3). Sensitivity was higher for stand-alone CCTA (89%) at the per-vessel level when compared with MPI alone (78%) (p <0.001). On both a per-patient and per-vessel level, sROC curves indicated that the discriminatory power did not differ statistically between CCTA and MPI approaches (p-value for difference: >0.05, Figure 3).
Table 3.
Meta-analysis for the diagnostic performance of CCTA alone, MPI alone, and hybrid cardiac imaging
| N | Sensitivity (95% CI) |
Specificity (95% CI) |
Positive likelihood ratio (95% CI) |
Negative likelihood ratio (95% CI) |
Diagnostic odds ratio (95% CI) |
|
|---|---|---|---|---|---|---|
| Per-patient analysis | ||||||
| CCTA | 12 | 0.90 (0.87 – 0.92) | 0.66 (0.61 – 0.70) | 3.39 (2.22 – 5.17) | 0.06 (0.02 – 0.23) | 53.80 (28.61 – 101.19) |
| MPI | 9 | 0.87 (0.83 – 0.90) | 0.83 (0.78 – 0.87) | 5.02 (2.99 – 8.44) | 0.15 (0.09 – 0.25) | 39.55 (15.64 – 100.02) |
| Hybrid | 12 | 0.91 (0.88 – 0.93) | 0.93 (0.90 – 0.95) | 12.80 (6.56 – 24.96) | 0.11 (0.07 – 0.18) | 159.00 (57.42 – 440.25) |
| Per-vessel analysis | ||||||
| CCTA | 8 | 0.89 (0.86 – 0.91) | 0.83 (0.81 – 0.85) | 5.75 (4.00 – 8.26) | 0.14 (0.09 – 0.22) | 44.27 (25.39 – 77.20) |
| MPI | 6 | 0.78 (0.74 – 0.81) | 0.87 (0.84 – 0.89) | 5.24 (3.52 – 7.82) | 0.25 (0.16 – 0.39) | 22.59 (10.41 – 49.03) |
| Hybrid | 8 | 0.84 (0.81 – 0.87) | 0.95 (0.94 – 0.96) | 16.53 (9.14 – 29.90) | 0.14 (0.07 – 0.26) | 137.90 (53.93 – 352.66) |
Abbreviations: CCTA = coronary computed tomography angiography; MPI = myocardial perfusion imaging; Hybrid = hybrid cardiac imaging encompassing single photon-emission computed tomography, positron emission tomography, and cardiac magnetic resonance imaging; 95% CI = 95% confidence interval
Figure 3. Summary receiver-operator curves displaying the diagnostic performance of hybrid imaging techniques versus stand-alone coronary computed tomography angiography and myocardial perfusion imaging.
p-value difference: at per-patient level, CCTA versus hybrid, MPI versus hybrid, and CCTA versus MPI, all p >0.05; at per-vessel level, CCTA versus hybrid p =0.046, MPI versus hybrid and CCTA versus MPI, all p >0.05. Abbreviations: CCTA = coronary computed tomographic angiography; MPI = myocardial perfusion imaging; AUC = area under the receiver operating characteristic curve.
Diagnostic performance of hybrid and CCTA imaging methods for assessment of obstructive CAD
Pooled estimates of per-patient and per-vessel sensitivity, specificity, positive likelihood ratio (LR+), negative likelihood ratio (LR−), and DOR according to CCTA, MPI, and hybrid approaches are reported in Table 3. Forest plots for sensitivity and specificity on a per-patient level are reported in Supplementary Figure 2. On a per-patient level, sensitivity, LR−, and DOR of hybrid versus CCTA imaging techniques to detect obstructive CAD were 91%, 0.11, and 159.00, versus 90%, 0.06, and 53.80, respectively (p >0.05, for all). Hybrid imaging displayed a higher specificity (93%) and LR+ (12.80) when compared with stand-alone CCTA (66% and 3.39) (p <0.05, for all). At the patient level, sROC curves revealed that the hybrid imaging approach did not exhibit a larger AUC value when compared with stand-alone CCTA (0.97 versus 0.93, respectively; p-value for difference: 0.132; Figure 3).
Forest plots for sensitivity and specificity on a per-vessel level are reported in Supplementary Figure 3. On a per-vessel level, specificity (95%) and LR+ (16.53) were higher for the hybrid approach when compared with stand-alone CCTA (specificity: 83% and LR+: 5.75) (p <0.05, for all) (Table 3). Moreover, on a per-vessel basis, sROC curves showed a statistically significant and higher AUC value for the hybrid approach when compared with stand-alone CCTA (0.97 versus 0.93, respectively; p-value for difference: 0.047; Figure 3).
I2 index test indicated significant heterogeneity for sensitivity and specificity on both a per-patient and per-vessel level (Supplementary Figure 2 and 3, Supplementary Table 1). On a per-patient level, significant heterogeneity for sensitivity and specificity was observed for CCTA (I2 = 89% and 88%, respectively, p <0.001). On a per-vessel level, significant heterogeneity for specificity was observed for CCTA (I2 = 91%, p <0.001). The hybrid approach showed significant heterogeneity for sensitivity and specificity on a per-vessel level (I2 = 88% and 87%, respectively, p <0.001) (Supplementary Figure 2 and 3, Supplementary Table 1).
Diagnostic performance of hybrid SPECT/CCTA, PET/CCTA, and CMR/CCTA imaging modalities for assessment of obstructive CAD
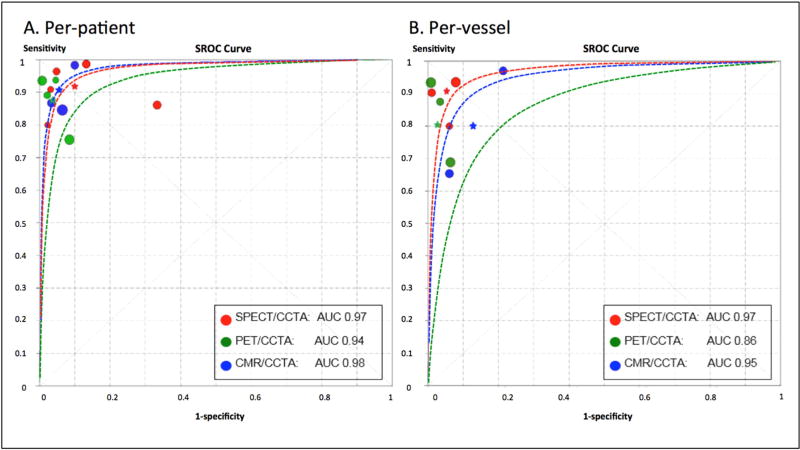
Overall, hybrid SPECT/CCTA demonstrated the highest sensitivity at both a per-patient (92%) and per-vessel (91%) level for assessment of obstructive CAD as compared with PET/CCTA (87% and 81%, respectively) (p <0.05, for all) (Table 4). However, specificity was higher for PET/CCTA as compared with SPECT/CCTA. The specificity for PET/CCTA was 96% at the per-patient level, and 97% at the per-vessel level, as compared to SPECT/CCTA (90% and 95%, respectively) (p <0.05, for all). Despite this, there was no difference in the sROC curves among the various hybrid imaging modalities on either a per-patient or per-vessel level (p-values for difference >0.05 for all, Figure 4).
Table 4.
Meta-analysis for the diagnostic performance of various hybrid cardiac imaging modalities
| N | Sensitivity (95% CI) |
Specificity (95% CI) |
Positive likelihood ratio (95% CI) |
Negative likelihood ratio (95% CI) |
Diagnostic odds ratio (95% CI) |
|
|---|---|---|---|---|---|---|
| Per-patient analysis | ||||||
| SPECT/CCTA | 5 | 0.92 (0.88 – 0.95) | 0.90 (0.85 – 0.93) | 10.38 (3.60 – 29.94) | 0.08 (0.03 – 0.27) | 158.16 (21.11 –1185.00) |
| PET/CCTA | 4 | 0.87 (0.80 – 0.92) | 0.96 (0.92 – 0.99) | 22.12 (5.20 – 94.00) | 0.12 (0.05 – 0.29) | 213.68 (25.94 – 1760.10) |
| CMR/CCTA | 3 | 0.91 (0.83 – 0.96) | 0.94 (0.88 – 0.98) | 12.86 (5.90 – 28.02) | 0.13 (0.07 – 0.26) | 120.36 (35.42 – 408.98) |
| Per-vessel analysis | ||||||
| SPECT/CCTA | 3 | 0.91 (0.86 – 0.95) | 0.95 (0.93 – 0.96) | 18.51 (8.01 – 42.76) | 0.11 (0.05 – 0.24) | 174.33 (52.59 – 577.89) |
| PET/CCTA | 3 | 0.81 (0.75 – 0.86) | 0.97 (0.95 – 0.98) | 28.42 (7.68 – 105.17) | 0.15 (0.04 – 0.51) | 202.03 (19.51 – 2091.8) |
| CMR/CCTA | 2 | 0.80 (0.73 – 0.87) | 0.87 (0.79 – 0.92) | 6.37 (2.69 – 15.07) | 0.13 (0.01 – 2.07) | 53.95 (13.48 – 215.83) |
Abbreviations: SPECT = single photon-emission computed tomography; CCTA = coronary computed tomography angiography; PET = positron emission tomography; CMR = cardiac magnetic resonance imaging; 95% CI = 95% confidence interval
Figure 4. Summary receiver-operator curves displaying the diagnostic performance of hybrid imaging techniques.
p-value difference: at both per-patient and per-vessel level, SPECT/CCTA versus PET/CCTA, PET/CCTA versus CMR/CCTA, and SPECT/CCTA versus CMR/CCTA, all p >0.05. Abbreviations: SPECT = single photon-emission computed tomography; PET = positron emission tomography; CMR = cardiac magnetic resonance imaging; CCTA = coronary computed tomographic angiography; AUC = area under the receiver operating characteristic curve.
DISCUSSION
In the current meta-analysis, we investigated the diagnostic performance of hybrid MPI/CCTA cardiac imaging compared with stand-alone CCTA for identifying obstructive CAD in patients who underwent both anatomic and functional testing. Overall, hybrid imaging techniques outperformed stand-alone CCTA with superior specificity and LR+. Moreover, at a per-vessel level, hybrid versus stand-alone CCTA imaging demonstrated improved discrimination based on sROC curves for identifying obstructive CAD.
The current meta-analysis revealed that, at a per-patient level, even though pooled sensitivity was comparable for hybrid cardiac imaging modalities versus stand-alone CCTA, pooled specificity and LR+ were considerably higher for hybrid imaging as compared with stand-alone CCTA. While the sROC curves demonstrated a trend towards improved discrimination for hybrid imaging for identifying obstructive CAD as compared with stand-alone CCTA, the difference was not statistically significant, which is most likely due to the relatively low per-patient sample size. Similarly, at a per-vessel level, pooled sensitivity was comparable for hybrid versus stand-alone CCTA imaging. However, both pooled specificity and LR+ were appreciably higher for hybrid versus stand-alone CCTA imaging at a per-vessel level. Likewise, at a per-vessel level, sROC curves for the hybrid imaging approach displayed a significantly higher discriminatory ability for detecting CAD when compared with the stand-alone CCTA approach.
Prior individual studies have shown that hybrid cardiac imaging techniques have yielded superior diagnostic performance for detecting obstructive CAD, along with additional information regarding hemodynamically significant coronary lesions when compared with those of stand-alone CCTA (12,13,16,17,20). The largest study included in this meta-analysis demonstrated that hybrid imaging significantly improved specificity and overall accuracy (95% and 91%, respectively) for the detection of obstructive CAD compared with CCTA alone (39% and 57%, respectively, p <0.001) (21). Although we did not evaluate clinical outcomes in this study, our overall findings are also in keeping with seminal data from invasive studies such as the multicenter FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) study which demonstrated that simultaneous assessment of anatomic and physiological coronary lesions by ICA and fractional flow reserve (FFR) resulted in improved clinical outcomes over anatomic analysis alone (22). The current meta-analysis represents the first systematic aggregation of hybrid non-invasive cardiac imaging using MPS and CCTA to our knowledge. As such, it extends the findings of smaller studies, which may be limited by factors such as small sample size or single-center design, and broadens generalizability.
The diagnostic performance of stand-alone CCTA is impeded by suboptimal image quality in cases with motion artifacts or heavy calcification. Indeed, prior data have demonstrated a relatively low PPV of CCTA in the evaluation of CAD (23). These findings are of practical clinical importance as it can lead to increased downstream testing and unnecessary invasive procedures such as ICA. ICA assessment carries an associated risk of complications and therapeutic interventions of non-ischemic coronary lesions, particularly in patients with low-to-intermediate pre-test likelihood of CAD (24,25). Stress MPI incorporating SPECT, PET, and CMR, provides additive diagnostic benefit by assessment of the functional significance of coronary stenoses. In pooled analyses, the sensitivity and specificity of MPI to diagnose obstructive CAD is typically between 85–90% and 70–75%, respectively (26–28). However, despite its high reported diagnostic performance, the real world accuracy of MPI is less sanguine. When corrected for referral bias (more positive studies undergo the gold standard test), the sensitivity and specificity for identifying individuals with obstructive CAD is approximately 65% and 67%, respectively (29). Numerous potential explanations exist to account for these findings, including patient motion artifacts, variable techniques for attenuation correction, true MPI abnormalities such as diffuse atherosclerosis, or variation by sites.
Given the aforementioned limitations of CCTA and MPI individually, hybrid imaging holds appeal as an efficient diagnostic strategy in the workup of suspected CAD. In fact, the current consensus recommendation of the Society of Cardiac Computed Tomography/American College of Radiology on CCTA reporting, CAD-RADS (Coronary Artery Disease Reporting and Data System), recommends physicians report “consider functional assessment” in patients with CAD-RADS category 3, or 50–69% stenosis (30). Although a cost-effectiveness analysis was outside the scope of the current study, routine implementation of a hybrid anatomic-functional approach would necessitate further study of its benefits, harms (including radiation doses), costs, and unintended consequences. While the addition of a second imaging exam would be expected to increase upfront costs, it must be balanced by consideration of its effect on downstream testing and procedures. Limited data suggest that hybrid SPECT/CCTA is associated with optimal resource utilization and improved selection for ICA and revascularization (31). Recognizing that the economic burden of hybrid imaging might be a significant practical barrier to implementation, further study might also focus on populations where it may be most clinically meaningful — in patients with intermediate stenosis or suspected microvascular dysfunction. In routine practice, a logical approach would be sequential testing after an initial equivocal test (32), or when questions remain about the presence of microvascular dysfunction.
The clinical utility of a hybrid imaging strategy is further supported by the recent multicenter EVINCI (EValuation of INtegrated Cardiac Imaging for the Detection and Characterization of Ischaemic Heart Disease) hybrid sub-study. In this study of 252 patients with suspected CAD, nearly 20% of patients undergoing hybrid imaging had a perfusion defect on MPS reassigned to a different coronary artery. Matched abnormal CCTA and MPS perfusion findings were associated with a relatively high rate of revascularization as compared with mismatched or discordant findings, emphasizing the clinical value of hybrid imaging (33). Further study of the clinical utility of a hybrid anatomic-functional approach might also consider the addition of newer CT applications such as non-invasive fractional flow reserve derived by cardiac CT (FFRCT) or atherosclerotic plaque features which could be performed without additional imaging requirements. In routine clinical practice however, access issues and financial barriers remain around FFRCT.
Understanding which particular method of hybrid cardiac imaging may provide the highest diagnostic performance for the assessment of obstructive CAD was beyond the scope of this study, given the limited number of studies included in this analysis. Hence, whether stand-alone CCTA provides improved diagnostic performance in combination with SPECT, PET, or CMR could not be adequately assessed based on the current study findings. Although specificity was highest for PET/CCTA when compared with SPECT/CCTA or CMR/CCTA on both per-patient and per-vessel levels, the sROC curves displayed the lowest discriminatory ability for PET/CCTA against other hybrid modalities for identifying obstructive CAD, with a statistically non-significant p-value for difference. Data from additional studies may elucidate the role of various imaging methods for detecting obstructive CAD (34).
This study is not without limitations. The reference standard was not invasive FFR as not all pooled studies in this investigation employed FFR as the reference standard. Consequently, due to the paucity of available data, QCA was employed as the reference standard in the current meta-analysis. Despite an observed three-fold difference in the DOR when utilizing a hybrid imaging approach rather than CCTA alone for assessing obstructive CAD, caution should be taken when interpreting these findings in light of the relatively few studies that were available for this meta-analysis – the latter likely being responsible for the relatively wide 95% CIs. The statistical non-significance of the hybrid imaging approach versus stand-alone CCTA at the per-patient and per-vessel levels may be attributed to the fact that not all included studies utilized invasive FFR as the reference standard. Last, there was substantially high heterogeneity for sensitivity and specificity between included studies. Furthermore, the source of heterogeneity was not identified in the current meta-analysis due to the limited number of studies across each imaging modality. Therefore, our overall conclusions are limited by high heterogeneity and should be interpreted with caution. Despite these limitations, efforts were made to select high-quality studies and the current meta-analysis represents the first synthesis of hybrid cardiac imaging. These findings warrant further validation in larger prospective studies.
CONCLUSION
The current meta-analysis suggests improved diagnostic specificity of hybrid cardiac imaging techniques for identifying obstructive CAD as compared with stand-alone CCTA, however, improvement in overall diagnostic performance was relatively limited.
Supplementary Material
At both per-patient and per-vessel level, all p >0.05. Abbreviations: CCTA = coronary computed tomographic angiography.
At both per-patient and per-vessel level, all p >0.05. Abbreviations: Hybrid = hybrid cardiac imaging modalities.
The imaging modalities include coronary computed tomography angiography, myocardial perfusion imaging, and hybrid cardiac imaging.
The imaging modalities include coronary computed tomography angiography, myocardial perfusion imaging, and hybrid cardiac imaging.
Clinical Perspectives.
Competency in medical knowledge
In the current meta-analysis, hybrid cardiac imaging using CCTA and MPI demonstrated superior specificity compared with stand-alone CCTA for identifying obstructive CAD at both the per-patient and per-vessel level. However, the sensitivity of a hybrid cardiac imaging approach was comparable to stand-alone CCTA.
Translational outlook
Additional studies are needed to determine the utility of hybrid cardiac imaging in situations where it could prove most clinically useful, its cost-effectiveness, as well as which particular combination of MPI and CCTA have the most favorable diagnostic performance.
Acknowledgments
Dr. Min serves as a consultant to HeartFlow, serves on the advisory board of Arineta, has ownership in MDDX and Autoplaq, and has a research agreement with GE Healthcare.
Financial disclosure
This study was funded by the National Institute of Health (Bethesda, MD, USA) under award numbers R01 HL111141 and R01 HL118019, and also supported, in part, by a generous gift from the Dalio Institute of Cardiovascular Imaging (New York, NY, USA) and the Michael Wolk Foundation (New York, NY, USA).
ABBREVIATIONS AND ACRONYMS
- CCTA
coronary CT angiography
- CAD
coronary artery disease
- NPV
negative predictive value
- PPV
positive predictive value
- MPI
myocardial perfusion imaging
- PET
positron emission tomography
- SPECT
single photon-emission CT
- CMR
cardiac magnetic resonance imaging
- QCA
quantitative coronary analysis
- QUADAS-2
Quality Assessment of Diagnostic Accuracy Studies
- DOR
diagnostic odds ratio
- sROC
summary receiver-operator curves
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
All other authors have no conflicts of interest to disclose.
References
- 1.Min JK, Shaw LJ, Berman DS. The present state of coronary computed tomography angiography a process in evolution. J Am Coll Cardiol. 2010;55:957–65. doi: 10.1016/j.jacc.2009.08.087. [DOI] [PubMed] [Google Scholar]
- 2.Groves AM, Speechly-Dick ME, Kayani I, et al. First experience of combined cardiac PET/64-detector CT angiography with invasive angiographic validation. Eur J Nucl Med Mol Imaging. 2009;36:2027–33. doi: 10.1007/s00259-009-1213-y. [DOI] [PubMed] [Google Scholar]
- 3.Meijboom WB, Meijs MF, Schuijf JD, et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol. 2008;52:2135–44. doi: 10.1016/j.jacc.2008.08.058. [DOI] [PubMed] [Google Scholar]
- 4.Meijboom WB, Van Mieghem CA, van Pelt N, et al. Comprehensive assessment of coronary artery stenoses: computed tomography coronary angiography versus conventional coronary angiography and correlation with fractional flow reserve in patients with stable angina. J Am Coll Cardiol. 2008;52:636–43. doi: 10.1016/j.jacc.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 5.Gaemperli O, Husmann L, Schepis T, et al. Coronary CT angiography and myocardial perfusion imaging to detect flow-limiting stenoses: a potential gatekeeper for coronary revascularization? Eur Heart J. 2009;30:2921–9. doi: 10.1093/eurheartj/ehp304. [DOI] [PubMed] [Google Scholar]
- 6.Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3:25. doi: 10.1186/1471-2288-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 8.Moses LE, Shapiro D, Littenberg B. Combining independent studies of a diagnostic test into a summary ROC curve: data-analytic approaches and some additional considerations. Stat Med. 1993;12:1293–316. doi: 10.1002/sim.4780121403. [DOI] [PubMed] [Google Scholar]
- 9.Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005;58:882–93. doi: 10.1016/j.jclinepi.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 10.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol. 2006;6:31. doi: 10.1186/1471-2288-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li JM, Shi RF, Zhang LR, Li T, Dong Z. Combined CT angiography and SPECT myocardial perfusion imaging for the detection of functionally relevant coronary stenoses. Mol Med Rep. 2013;7:1391–6. doi: 10.3892/mmr.2013.1354. [DOI] [PubMed] [Google Scholar]
- 13.Kadokami T, Ando S, Momii H, et al. Diagnostic performance of cardiac fusion images from myocardial perfusion imaging and multislice computed tomography coronary angiography for assessment of hemodynamically significant coronary artery lesions: an observational study. Nucl Med Commun. 2012;33:60–8. doi: 10.1097/MNM.0b013e32834d3bde. [DOI] [PubMed] [Google Scholar]
- 14.Sato A, Nozato T, Hikita H, et al. Incremental value of combining 64-slice computed tomography angiography with stress nuclear myocardial perfusion imaging to improve noninvasive detection of coronary artery disease. J Nucl Cardiol. 2010;17:19–26. doi: 10.1007/s12350-009-9150-5. [DOI] [PubMed] [Google Scholar]
- 15.Thomassen A, Petersen H, Diederichsen AC, et al. Hybrid CT angiography and quantitative 15O-water PET for assessment of coronary artery disease: comparison with quantitative coronary angiography. Eur J Nucl Med Mol Imaging. 2013;40:1894–904. doi: 10.1007/s00259-013-2519-3. [DOI] [PubMed] [Google Scholar]
- 16.Danad I, Raijmakers PG, Appelman YE, et al. Hybrid imaging using quantitative H215O PET and CT-based coronary angiography for the detection of coronary artery disease. J Nucl Med. 2013;54:55–63. doi: 10.2967/jnumed.112.104687. [DOI] [PubMed] [Google Scholar]
- 17.Kajander S, Joutsiniemi E, Saraste M, et al. Cardiac positron emission tomography/computed tomography imaging accurately detects anatomically and functionally significant coronary artery disease. Circulation. 2010;122:603–13. doi: 10.1161/CIRCULATIONAHA.109.915009. [DOI] [PubMed] [Google Scholar]
- 18.Scheffel H, Stolzmann P, Alkadhi H, et al. Low-dose CT and cardiac MR for the diagnosis of coronary artery disease: accuracy of single and combined approaches. Int J Cardiovasc Imaging. 2010;26:579–90. doi: 10.1007/s10554-010-9595-2. [DOI] [PubMed] [Google Scholar]
- 19.Donati OF, Scheffel H, Stolzmann P, et al. Combined cardiac CT and MRI for the comprehensive workup of hemodynamically relevant coronary stenoses. AJR Am J Roentgenol. 2010;194:920–6. doi: 10.2214/AJR.09.3225. [DOI] [PubMed] [Google Scholar]
- 20.Rispler S, Keidar Z, Ghersin E, et al. Integrated single-photon emission computed tomography and computed tomography coronary angiography for the assessment of hemodynamically significant coronary artery lesions. J Am Coll Cardiol. 2007;49:1059–67. doi: 10.1016/j.jacc.2006.10.069. [DOI] [PubMed] [Google Scholar]
- 21.Groothuis JG, Beek AM, Brinckman SL, et al. Combined non-invasive functional and anatomical diagnostic work-up in clinical practice: the magnetic resonance and computed tomography in suspected coronary artery disease (MARCC) study. Eur Heart J. 2013;34:1990–8. doi: 10.1093/eurheartj/eht077. [DOI] [PubMed] [Google Scholar]
- 22.Tonino PA, De Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213–24. doi: 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 23.Mollet NR, Cademartiri F, van Mieghem CA, et al. High-resolution spiral computed tomography coronary angiography in patients referred for diagnostic conventional coronary angiography. Circulation. 2005;112:2318–23. doi: 10.1161/CIRCULATIONAHA.105.533471. [DOI] [PubMed] [Google Scholar]
- 24.Redberg RF, Walsh J. Pay now, benefits may follow--the case of cardiac computed tomographic angiography. N Engl J Med. 2008;359:2309–11. doi: 10.1056/NEJMp0805920. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen LH, Ortner N, Norgaard BL, Achenbach S, Leipsic J, Abdulla J. The diagnostic accuracy and outcomes after coronary computed tomography angiography vs. conventional functional testing in patients with stable angina pectoris: a systematic review and meta-analysis. Eur Heart J Cardiovasc Imaging. 2014;15:961–71. doi: 10.1093/ehjci/jeu027. [DOI] [PubMed] [Google Scholar]
- 26.Klocke FJ, Baird MG, Lorell BH, et al. ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging) Circulation. 2003;108:1404–18. doi: 10.1161/01.CIR.0000080946.42225.4D. [DOI] [PubMed] [Google Scholar]
- 27.Kim C, Kwok YS, Heagerty P, Redberg R. Pharmacologic stress testing for coronary disease diagnosis: A meta-analysis. Am Heart J. 2001;142:934–44. doi: 10.1067/mhj.2001.119761. [DOI] [PubMed] [Google Scholar]
- 28.Fleischmann KE, Hunink MG, Kuntz KM, Douglas PS. Exercise echocardiography or exercise SPECT imaging? A meta-analysis of diagnostic test performance. J Nucl Cardiol. 2002;9:133–4. doi: 10.1067/mnc.2002.120681. [DOI] [PubMed] [Google Scholar]
- 29.Miller TD, Hodge DO, Christian TF, Milavetz JJ, Bailey KR, Gibbons RJ. Effects of adjustment for referral bias on the sensitivity and specificity of single photon emission computed tomography for the diagnosis of coronary artery disease. Am J Med. 2002;112:290–7. doi: 10.1016/s0002-9343(01)01111-1. [DOI] [PubMed] [Google Scholar]
- 30.Cury RC, Abbara S, Achenbach S, et al. CAD-RADS: Coronary Artery Disease - Reporting and Data System: An Expert Consensus Document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Radiology (ACR) and the North American Society for Cardiovascular Imaging (NASCI). Endorsed by the American College of Cardiology. J Am Coll Radiol. 2016;13:1458–1466. e9. doi: 10.1016/j.jacr.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 31.Pazhenkottil AP, Nkoulou RN, Ghadri JR, et al. Prognostic value of cardiac hybrid imaging integrating single-photon emission computed tomography with coronary computed tomography angiography. Eur Heart J. 2011;32:1465–71. doi: 10.1093/eurheartj/ehr047. [DOI] [PubMed] [Google Scholar]
- 32.Kaufmann PA, Buechel RR. Cardiac SPECT/CCTA hybrid imaging : One answer to two questions? Herz. 2016;41:391–7. doi: 10.1007/s00059-016-4438-0. [DOI] [PubMed] [Google Scholar]
- 33.Liga R, Vontobel J, Rovai D, et al. Multicentre multi-device hybrid imaging study of coronary artery disease: results from the EValuation of INtegrated Cardiac Imaging for the Detection and Characterization of Ischaemic Heart Disease (EVINCI) hybrid imaging population. Eur Heart J Cardiovasc Imaging. 2016;17:951–60. doi: 10.1093/ehjci/jew038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.VU University Medical Center. Prospective Comparison of Cardiac PET/CT, SPECT/CT Perfusion Imaging and CT Coronary Angiography With Invasive Coronary Angiography. Bethesda (MD): National Library of Medicine (US); 2000. [cited 2017 Jan 5]. ClinicalTrials.gov [Internet]. Available from: http://clinicaltrials.gov/show/NCT01521468 NLM Identifier: NCT 01521468. [Google Scholar]
- 35.Schaap J, Kauling RM, Boekholdt SM, et al. Incremental diagnostic accuracy of hybrid SPECT/CT coronary angiography in a population with an intermediate to high pre-test likelihood of coronary artery disease. Eur Heart J Cardiovasc Imaging. 2013;14:642–9. doi: 10.1093/ehjci/jes303. [DOI] [PubMed] [Google Scholar]
- 36.Winther S, Svensson M, Jorgensen HS, et al. Diagnostic Performance of Coronary CT Angiography and Myocardial Perfusion Imaging in Kidney Transplantation Candidates. JACC Cardiovasc Imaging. 2015;8:553–62. doi: 10.1016/j.jcmg.2014.12.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
At both per-patient and per-vessel level, all p >0.05. Abbreviations: CCTA = coronary computed tomographic angiography.
At both per-patient and per-vessel level, all p >0.05. Abbreviations: Hybrid = hybrid cardiac imaging modalities.
The imaging modalities include coronary computed tomography angiography, myocardial perfusion imaging, and hybrid cardiac imaging.
The imaging modalities include coronary computed tomography angiography, myocardial perfusion imaging, and hybrid cardiac imaging.