Abstract
A fundamental goal of motor learning is to establish neural patterns that produce a desired behavioral outcome. It remains unclear how and when the nervous system solves this “credit–assignment” problem. Using neuroprosthetic learning where we could control the causal relationship between neurons and behavior, here we show that sleep–dependent processing is required for credit-assignment and the establishment of task-related functional connectivity reflecting the casual neuron-behavior relationship. Importantly, we found a strong link between the microstructure of sleep reactivations and credit assignment, with downscaling of non–causal activity. Strikingly, decoupling of spiking to slow–oscillations using optogenetic methods eliminated rescaling. Thus, our results suggest that coordinated firing during sleep plays an essential role in establishing sparse activation patterns that reflect the causal neuron–behavior relationship.
Introduction
Hallmarks of learning a new skill include a significant reduction of movement variability and a concomitant reduction in both the extent and variability of neural firing1–7. This process is associated with increasingly sparse task–related neural activation patterns5–8. A theoretical framework for the underlying computation is frequently labeled the “credit assignment problem”, i.e. determination of how a single neuron in a highly interconnected biological network causes a behavior9,10. Past work has suggested that a key goal of credit assignment is to select neural activity that truly reflects the causal neuron–behavior relationship8,11. However, it remains unknown how a complex and interconnected biological neural network can solve this computation.
We hypothesized that sleep–dependent reactivations may play an important role in network credit assignment. A large body of work indicates that sleep plays an important role in memory consolidation12–14. More specifically, reactivation of neural activity during sleep has been implicated in memory consolidation12,14–17. However, there has been great debate regarding the specific computational role of such reactivations12–14. Two commonly cited possibilities are that sleep–dependent reactivations lead to: (i) a general strengthening of functional connectivity, or (ii) a process of renormalization with both strengthening and weakening of functional connectivity12,14,18. In the case of renormalization, a theoretical prediction is that after a period of sleep, there may be rescaling of task-related activity (e.g. neural activations not causally linked to performance are selectively downscaled)18. Interestingly, such a process of rescaling of task–activations could be used for network credit assignment.
Here we used a neuroprosthetic–learning task, where the “decoder” and the causality of the neuron–behavior relationship are set by the experimenter8,11,19–24, to evaluate whether NREM sleep plays a role in credit assignment. Unlike natural motor behaviors, neuroprosthetic control offers a unique paradigm to study plasticity; a small set of neurons is chosen to causally control actuator movements (i.e. ‘direct’ neurons)8,19. In contrast, ‘indirect’ neurons show task–related activity even though they do not cause actuator movements8,11,25. Importantly, while past work has shown that learning proficient control through putative error–correction processes leads to increased activity of direct neurons and diminished activity of indirect neurons8,11,20,25,26, it remains unclear how and when this fundamental credit–assignment process is solved. Here we show that neural spiking triggered by slow–oscillations during sleep plays an essential role in credit assignment.
Results
Rescaling of Task Activity
In five rats implanted with microwire arrays in primary motor cortex (M1), we monitored sets of direct (TRD) and indirect (TRI) neurons during the initial learning (hereafter BMI1), during a period of sleep and subsequent task–performance upon awakening (hereafter BMI2). A linear decoder with randomized weights converted the firing rates of two randomly chosen TRD neurons into the angular velocity of the actuator. The decoder weights were held constant during the session to exclusively rely on neural learning. Notably, there are studies demonstrating that decoder adaptation can still induce long-term plasticity27. However, this was done in non-human primate models performing more complex tasks. In our experiments, animals trained to control the angular velocity of a feeding tube via modulation of neural activity. At the start of each trial, the angular position of the tube was set to 0° (Fig. 1a–b, P1). If the angular position of the tube was held for >300 ms at position P2 (90°), a defined amount of water was delivered (i.e. a successful trial); a trial was stopped if this was not achieved within 15 s. Over a typical 2–hour session, animals were able to learn the task. Consistent with past results23, after a period of NREM sleep, task performance improved at the start of BMI2 (also called BMI2Early; Fig. 1c, P < 0.05 for each of the 10 individual comparisons of BMI1Late and BMI2Early; overall paired t test, t9 = 7.62, *P < 10−4).
Figure 1. Rescaling of task activations after sleep.
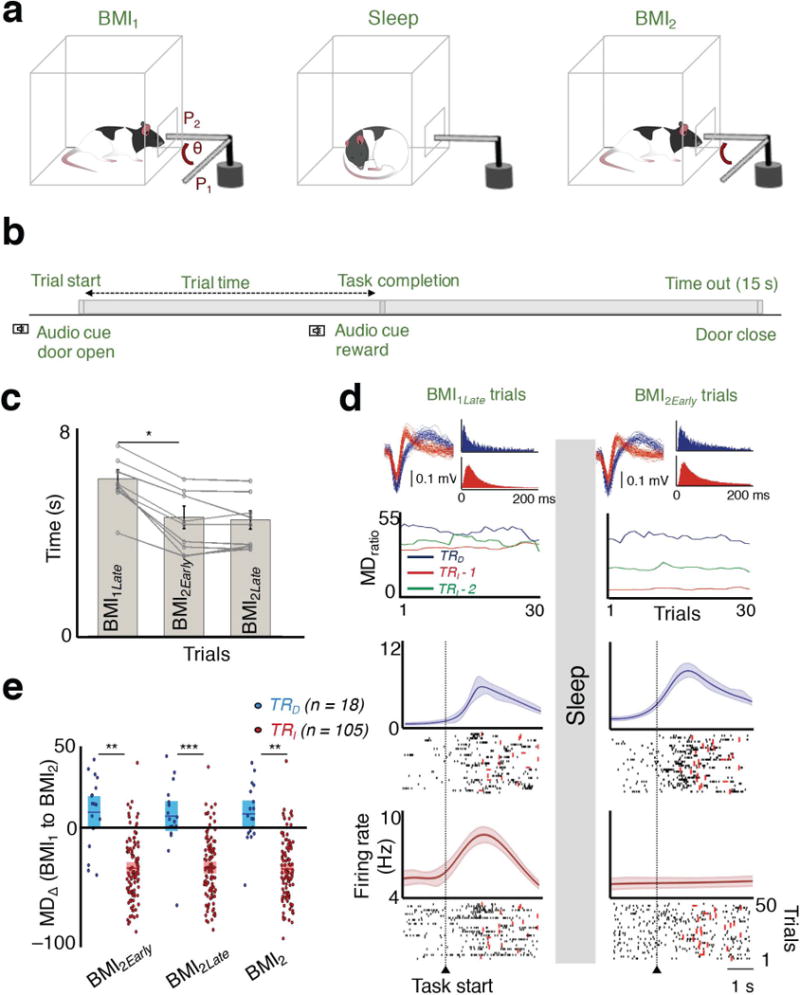
a, The practice sessions were separated by a block of sleep. Rats learned direct neural control of a feeding tube (θ = angular position). Successful trials required movement from P1 to P2 within 15 s. b, A typical trial structure is depicted. c, Comparison of trial times. A significant reduction in completion time was found between BMI1Late to BMI2Early (n = 10 sessions; paired t test, t9 = 7.62, *P < 10−4). d, At the top are the waveforms and inter-spike interval histograms of the neurons analyzed below (color-coded). Plot below shows the trend in the modulation depth ratio (MDratio) during BMI performance for three neurons before and after sleep. Another neuron whose waveform is not shown is depicted in green. Below are the peri–event histograms from BMI1Late and BMI2Early trials, respectively for the TRD and TRI neurons (in same color convention). Thick line represents mean; shaded area is the jackknife error. Below the PETHs are representative spike rasters from multiple trials. Red dot indicates task completion time for each trial. e, Average modulation depth change (MD∆) between BMI1 and BMI2 (mean in solid line ± s.e.m. in box; unpaired t tests; BMI1 and BMI2Early t121 = 6.79, **P < 10−9; BMI1 and BMI2Late t121 = 6.31, ***P < 10−8; BMI1 and BMI2 t121 = 6.96, **P < 10−9).
We next compared the activity of TRD and TRI neurons during task–performance immediately prior to and after sleep (i.e., intervening sleep or Sleeppost, duration: 36.94 ± 1.06 min, mean ± s.e.m., n = 10 sessions; paired t test of Sleeppre and Sleeppost durations: t9 = 0.056, P = 0.95). We specifically measured the change in the peak-firing rate during task performance relative to the baseline rate prior to the ‘GO’ cue (i.e. ‘modulation depth’ or MD). The majority of TRD cells increased their modulations (~67%), whereas a majority of TRI cells reduced their modulation (~90%). Strikingly, while TRD neurons experienced a slight but significant increase in modulation depth (7.39 ± 5.89 %, Wilcoxon signed-rank test, Z = −1.81, P = 0.03), there was a substantial net decrease in the MD of TRI neurons (–31.76 ± 2.18 %, paired t test, t104 = 14.58, P < 10−26) (Fig. 1, d–e). In addition, we found that the time spent in sleep predicted the extent of TRI downscaling (Spearman correlation, r = –0.71, P < 0.05).
Changes in Functional Coupling During Sleep
We next compared the changes in functional connectivity in the recorded M1 neural ensembles during NREM sleep epochs prior to and after training. We specifically calculated the magnitude of spike–spike coherence (SSC) for TRD – TRD, and TRD – TRI, pairs both during the sleep that followed training (Sleeppost) and the sleep that preceded (Sleeppre). The SSC is a pair-wise measure of how phased locked two neurons are across of frequencies28. For TRD – TRI, pairs, the TRD neuron with stronger task-related modulation was chosen for SSC calculation relative to the other TRI neurons. We observed that the Sleeppost SSC curves for TRD – TRD unit pairs showed a significant increase in the 0.3 – 4 Hz band (Fig. 2a); this frequency band reflects slow-oscillatory activity during NREM sleep13,14. At the population level, these increases were greater for TRD – TRD pairs than TRD – TRI pairs (129.78 ± 10.29% increase for TRD − TRD pairs and 56.30 ± 4.73% increase for TRD – TRI pairs; unpaired t-test, t121 = 6.95, P < 10−7). We didn’t observe any significant differences near the spindle band (8–20 Hz) or ripple (100–300 Hz) frequency bands (data not shown). This indicates that the decoder coupled direct units (i.e. TRD – TRD) were significantly more likely to fire synchronously during slow-oscillations in relation to their coupling with indirect units (i.e. TRD – TRI) during Sleeppost. We also found that the firing rate of the neurons did not significantly change between the two epochs (mean firing rate for the two epochs: 6.54 ± 0.66 Hz to 6.62 ± 0.64 Hz, paired t tests, TRD neurons: t17 = −1.65, P = 0.11; TRI neurons: t104 = 0.049, P = 0.96). This may be consistent with a recent study regarding the firing changes in NREM29, where firing rate changes were evident during certain phases of sleep and with monitoring of the entire sleep period.
Figure 2. Changes in functional connectivity of direct neuronal pairs and reactivation microstructure.
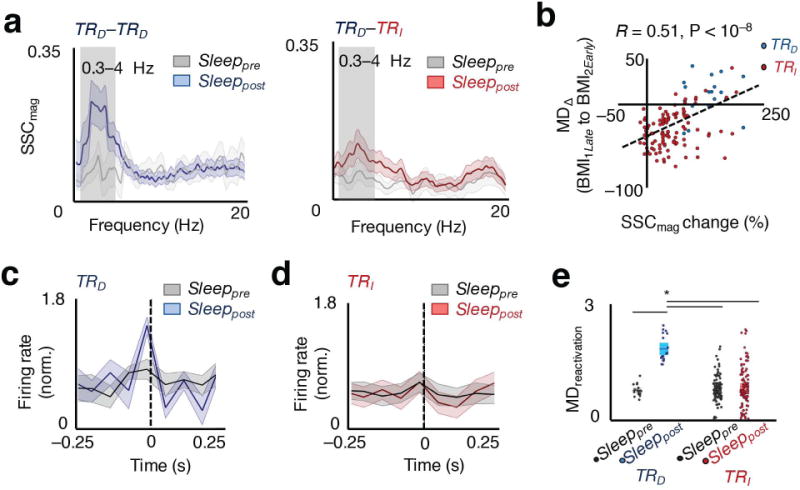
a, Example plot of SSC as a function of frequency during sleep prior to (Sleeppre) and after (Sleeppost for TRD – TRD; red for TRD – TRI pairs) skill acquisition. The lighter band is the jackknife error. The box highlights the 0.3 – 4 Hz band. b, Relationship between SSC change before and after learning, and change in task-related modulation after sleep, MDΔ (BMI1Late to BMI2Early), spearman correlation, r(123) = 0.51, P < 10−8. c, Average modulation depth during reactivations (MDreactivation, i.e. ratio of peak to tails) of TRD neurons from Sleeppre to Sleeppost. d, MDreactivation of TRI neurons from Sleeppre to Sleeppost. e, Average modulation depth during Sleeppre to Sleeppost reactivations for TRD and TRI neurons (mean in solid line ± s.e.m. in box, one-way ANOVA, F3,242 = 34.28, P < 10−17; significant post hoc t tests, *P < 0.05).
We next wondered whether individual pairwise changes in the post–learning functional connectivity could predict rescaling. As also indicated above, for each neuron we calculated a single SSC value by using a single TRD neuron as a “reference”. We thus examined if the specific changes in SSC could predict the MD changes for TRD and TRI units from BMI1 to BMI2 (Fig. 2b). Interestingly, we found that SSC changes were a strong predictor for rescaling (Pearson correlation, r = 0.51, P < 0.05), indicating that functional connectivity changes during sleep could account for our observed changes in task activations after sleep.
We also examined whether the precisely temporal pattern of spiking (i.e. “microstructure”) of sleep reactivations23,30,31 could also predict rescaling. In contrast to the general functional connectivity analysis, this approach is based on detection of temporally precise “reactivation events” that reflect the firing patterns that emerge with learning23,30,31. Importantly, our past work has shown that such reactivation events are also tightly related to slow oscillations23. We specifically used principal components analysis to create a template to reflect the ensemble activity that emerged with learning23,30,31. Subsequently, we evaluated the instantaneous reactivation strength during the two sleep epochs. We further measured the “microstructure” by binning the neural activity identified using reactivation analysis (i.e. using coarser time bins of 50 ms) with smaller time bins of 5 ms. In principle it is possible that the average microstructure of reactivations could resemble: (i) activity during BMI1, (ii) activity during BMI2, or (iii) evolve over time during sleep. Detailed analysis of the identified reactivation events indicated that there was no evolution of patterns in sleep (data not shown).
We next examined whether the microstructure of reactivation events more closely resembled task-activity during BMI1 or during BMI2. We thus examined the specific modulation of TRD and TRI neurons during the high percentile reactivation events (see Methods). We found that, at the population level, modulation of TRD neurons was significantly greater around the reactivation events than for TRI, thus resembling the task activations evident during BMI2. In other words, the identified reactivation events did not resemble BMI1 where there was similar modulation of TRD and TRI. Modulation of TRD neurons was also greater than in Sleeppre, while they remained unchanged for the TRI population from Sleeppre to Sleeppost (Fig. 2c–e; one way ANOVA, F3,242 = 34.28, P < 10−17). Such increased modulation was not apparent in randomly selected parts of Sleeppost (Supplementary Fig. 1; unpaired t test, t121 = −0.69, P = 0.49). Together, these results suggest that after learning, sleep reactivations demonstrated firing patterns that resembled, on average, the rescaled pattern. Interestingly, at the level of single neurons, the depth of modulation during reactivations (i.e. Fig. 2c–e) predicted how a neuron changed its task–related firing rate during BMI2 (i.e. significant relationship between lack of firing during reactivations and downscaling of task activity, linear regression, R2 = 0.17, P < 10−5, Supplementary Fig 2). Thus, we found that direct task related units fired more coherently during sleep, as indicated by the elevated SSC, as well as more robustly around reactivations, and their relative modulation depth were significantly greater than for indirect units during task performance in BMI2.
The Role of Reward
What determines the microstructure of reactivations? We first compared the differences between TRD and TRI firing during BMI1; it was difficult to distinguish the two populations based on the evolution of firing patterns locked to trial onset (Fig. 3). However, as recent studies suggest that neural activity linked to reward can be preferentially reactivated32–34, we also compared activity patterns locked to reward delivery. Notably, we found that it was substantially easier to distinguish the two populations in this “frame of reference”; TRD neurons showed a more robust and consistent modulation around reward (Fig. 3a). We quantified this by comparing the activity of pairs of neurons around task start and prior to reward. The peak modulation depth ratio for TRD neurons around task–start versus task–end was significantly different (respectively 16.20 ± 0.96 versus 26.25 ± 1.24, paired t-test, t17 = −6.81 P < 10−5). On the other hand, the modulation depth of TRI neurons did not significantly vary between the two frames of reference (13.84 ± 0.45 versus 12.86 ± 0.26 respectively, paired t-test, t104 = 1.95 P = 0.053).
Figure 3. Consistency of reward and frames of reference.
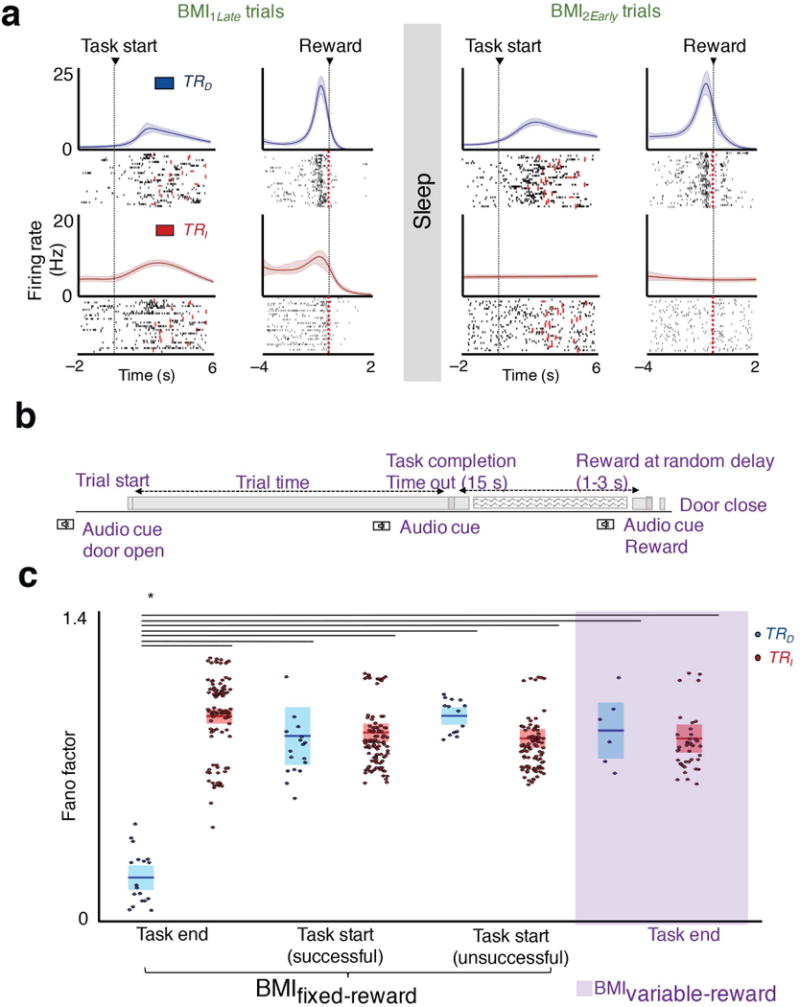
a, Neural firing centered to task start and task end/reward for the same session for regular BMI training (i.e. BMIfixed-reward). The lighter band is the jackknife error. b, Schematic of “variable-reward” BMI training. b, Schematic of variable-reward BMI trials. c, Average Fano factor of TRD and TRI neurons for the four sets of conditions, namely task-start (successful and unsuccessful trials are separately parsed) and task-end/reward frame in BMIfixed-reward, and task end in BMIvariable-reward (mean in solid line ± s.e.m. in box, task start and task end in BMIfixed-reward one-way ANOVA, F5,350 = 41.20, P < 10−32; task end in BMIfixed-reward and BMIvariable-reward one-way ANOVA, F3,166 = 83.86, P < 10−32, significant post hoc t tests, *P < 0.05).
In general, we also noted that there was an apparent reduction in the variability of firing patterns for TRD neurons as opposed to TRI neurons associated with task completion. We quantified changes using the Fano factor method35,36 (FF), which is a statistical measure of the trial-to-trial variability of neural firing. We found that TRD neurons had the lowest FF at task end, which coincided with reward (Fig 3c). These values were lesser than for task start of successful trials, and even lower than for task start of unsuccessful trials. Importantly, when we matched for firing rates between the two frames using a subset of the neurons, we still observed the same decline in FF for the TRD neurons in the task completion frame (TRD neurons’ FF : 0.37 ± 0.007 and 0.68 ± 0.016 for the task end and task start frame, TRI neurons’ FF : 0.71 ± 0.002 and 0.62 ± 0.002 for task end and task start respectively; one-way ANOVA, F5,350 = 41.20, P < 10−32). This suggested that the consistency of neural firing relative to reward may be an important determinant of rescaling.
To specifically dissociate task completion from reward, we performed ‘variable reward’ experiments (i.e. BMIvariable-reward) where we uncoupled task completion from reward (Fig. 3b). This is contrasted from experiments we have outlined above in which the reward was delivered at a fixed interval after task completion (i.e. BMIfixed-reward). More specifically, the water was delivered after a variable delay of 1–3 seconds after trial completion. While the animals could learn the task (30.62 ± 6.47% improvement from BMI1Early to BMI1Late; paired t-test, t3 = 4.46, P < 0.05), we did not observe significant performance gains from BMI1Late to BMI2Early as typically seen in BMIfixed-reward trials (Fig 1c). Interestingly, we also did not observe the rescaling effect; the change in modulation depth from BMI1Late to BMI2Early was 14.03 ± 7.89% and 3.35 ± 2.31% respectively for TRD and TR populations (paired t-test, t5 =−1.95, P = 0.10 for TRD, t40 = −1.46, P = 0.15 for TRI).
We then used these experiments to assess if our observed changes were truly related to reward or simply task completion. Interestingly, for BMIvariable-reward experiments, we no longer observed the reduction in FF for TRD neurons at task completion (one–way ANOVA, F3,166 = 83.86, P < 10−32, post-hoc t–test, P < 0.05; Fig. 3c). Moreover, they were indistinguishable from indirect neurons. Together, this data suggests that the lack of a temporally precise link between task completion and reward altered the differential modulation of the two populations previously seen. We then examined how the firing patterns of individual neurons changed for each of these two frames. We thus calculated the pairwise correlation between the sets of neurons during either trial start trial end. Consistent with our hypothesis, the correlated firing between pairs of TRD – TRD and TRD – TRI was significantly different for the reward–based frame for BMIfixed-reward relative to the BMIvariable-reward condition (i.e. ‘Pairwise Correlation’, Fig. 4a, one–way ANOVA, F7,304 = 8.36, P < 10−8, post-hoc t–test, P < 0.05).
Figure 4. Pairwise correlation of neural firing during task performance and reactivations during sleep.
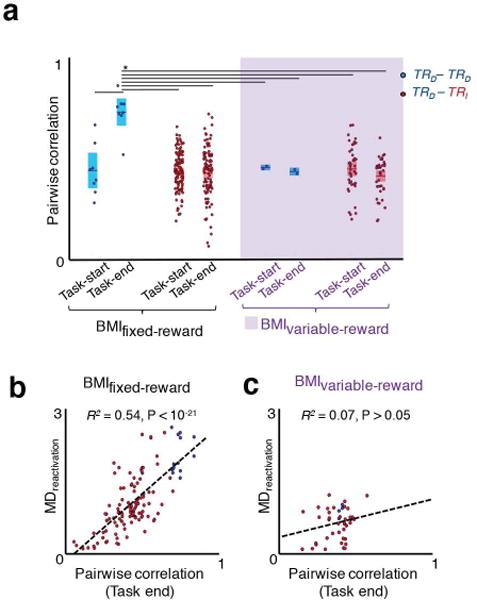
a, Pairwise correlation of neural firing for TRD – TRD and TRD – TRI pairs around task start and task end in BMIfixed-reward and BMIvariable-reward paradigms (mean in solid line ± s.e.m. in box; one-way ANOVA, F7,304 = 8.36, P < 10−8; significant post hoc t tests, *P < 0.05). b, Relationship of individual neural pairwise (i.e. at task end) and reactivation during sleep in BMIfixed-reward sessions (linear regression R2 = 0.54, P < 10−21; neural pairs are in same convention as Fig 4a). c, Relationship of individual neural pairwise correlations at task end and reactivation during sleep in BMIvariable-reward sessions (linear regression R2 = 0.07, P > 0.05; neural pairs are in same convention as Fig 4a).
What is the effect of reward on reactivations? Interestingly, we found that neural co-firing in the reward frame could strongly predict the microstructure of reactivations for the BMIfixed-reward experiments (Fig. 4b; R2 = 0.54, P < 10−21); this relationship was not significant relative to task start (spearman correlation, r = 0.12, P = 0.19), or for the BMIvariable-reward experiments (Fig 4c, R2 = 0.07, P > 0.05). Together, our results indicate that firing patterns found within reactivation events are most closely related to the consistency of neural firing relative to the time of reward.
Closed-Loop Inhibition of Spiking Activity During Slow Oscillations
We next used closed-loop optogenetic methods to evaluate the casual role of the changes in sleep37 functional connectivity in triggering both the offline performance gains and rescaling. We injected five rats with Jaws, a red–shifted halorhodopsin that is a potent silencer of neural activity38. After a period of several weeks, we performed a second surgery to implant microwire arrays attached to a cannula for fiber optic stimulation. The animals showed robust expression and ~60% neurons responded to optical stimulation by reducing firing (~43% average reduction, Fig. 5a–c). Using each animal as its own control, we compared the effects of either allowing normal sleep (n = 8 sessions; ‘OPTOOFF’) or conducting closed–loop perturbations (n = 11 sessions ; ‘OPTOUP’) to decouple spiking activity during UP states (i.e. activated states hallmarked by neural firing during NREM sleep; Fig. 5b)14,39. We considered each session from a given animal as an independent observation. Optogenetic inhibition during OPTOUP experiments was specifically triggered during slow-oscillations either by simple thresholding of filtered LFP during UP states (n = 8) or thresholding of power in the slow–wave band (n = 3; see Methods). For the OPTODOWN experiment, we exclusively used the filtered LFP to trigger the LED (Fig 5d). These experiments were randomly interleaved among the animals. For the optogenetic experiments, we selected TRD cells that responded to optical stimulation with reduced firing. Figure 5b and c show examples of a TRD neuron with normal firing during Sleeppre and suppressed firing during optogenetic stimulation linked to UP states (Sleeppost; population averages in Fig 5c). The stimulation pulses during OPTOUP and OPTODOWN experiments had similar incidences (Supplementary Fig 3a) and proportion compared to total time spent in sleep (Supplementary Fig 3b). All rats tolerated this manipulation without affecting total duration of sleep when compared with the OPTOOFF group (Supplementary Fig 4). Furthermore, there were no quantitative changes in sleep power across the three conditions (Fig. 5e, f; Fig 5f is a quantification of the 0.3–4 Hz band).
Figure 5. Optogenetic inhibition of neural activity during sleep.
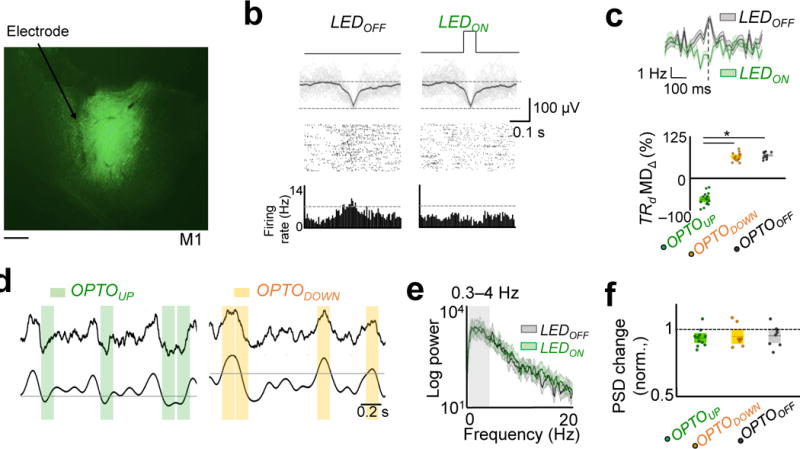
a, Fluorescence image of a coronal brain section showing neurons expressing Jaws (green) in M1. Scale bar is 500 μm. b, UP state triggered LED inhibition of a TRD cell in Sleeppost as compared to the activity of same cell in Sleeppre without stimulation. Rasters are shown along with raw traces of the local-field potential (LFPs) based on threshold crossing of the LFP. Dark line is the mean LFP. Bottom-most row shows histogram of firing activity. c, Top: Average modulation depth (MD) of a TRD cell in a representative OPTOUP experiment. Bottom: Average modulation depth (MD) of TRD cells around slow-oscillations in OPTOUP, OPTODOWN, and OPTOOFF experiments (mean in solid line ± s.e.m. in box, one-way ANOVA, F2,41 = 425.75, P < 10−27; significant post hoc t tests, *P < 0.05). d, Examples of the raw and filtered (0.3–4 Hz) traces and the stimulation period for respective OPTOUP and OPTODOWN experiments. e, Power spectrum of LFP from Sleeppre and Sleeppost in an OPTOUP experiments. The lighter band is the jackknife error. f, Power spectral changes (in 0.3 – 4 Hz) for OPTOUP, OPTODOWN, and OPTOOFF experiments (one-way ANOVA, F2,27 = 0.13, P = 0.87).
Interestingly, we observed significant worsening of performance only in the OPTOUP experiments (Fig. 6a–b). Figure 6a shows two examples of learning following pre- and post-sleep from two sessions in the same animal. Typically we observed a worsening of performance relative to the end of the previous session in OPTOUP experiments, but the performance level was still better than the earliest trials. This was not the case with respective OPTODOWN and OPTOOFF experiments. Together, these experiments suggest that decoupling of spiking during the UP states of slow-oscillations is sufficient to prevent offline gains. This also strongly suggested that such a process is activity-dependent and appeared to at least require the local firing of action potentials during sleep. Additionally, we also found that the performance worsening in BMI2 in the OPTOUP experiments was associated with increased firing variability of TRD neurons in both task-start and task-end frames of reference and was comparable to that of TRI neurons (TRD neurons Fano factor: 1.04 ± 0.04 and 1.11 ± 0.08 at task end and task start; TRI neurons Fano factor: 1.07 ± 0.017 and 1.09 ± 0.02 at task end and task start; one- way ANOVA, F3,220 = 0.44, P = 0.72; P > 0.05 for all post hoc multiple comparisons). This was not the case after robust learning sessions where TRD neurons were associated with a significant reduction in FF at task end (Fig 3c).
Figure 6. Optogenetic inhibition during UP states prevents consolidation.
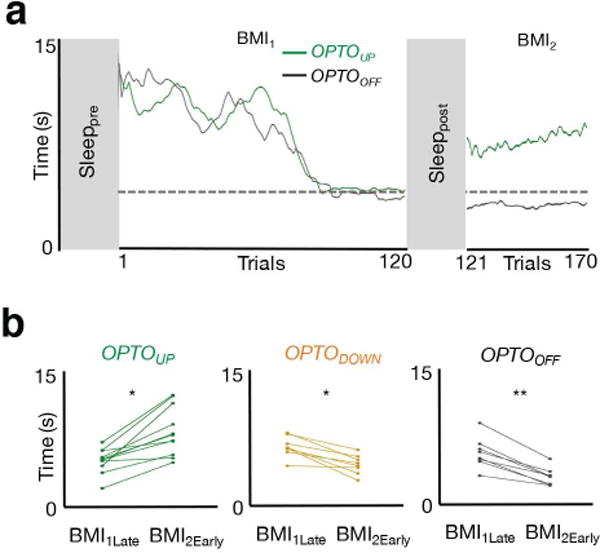
a, Learning curves from two BMI sessions in the same rat with and without optogenetic inhibition during sleep (i.e. OPTOUP and OPTOOFF sessions, respectively). b, Performance changes from BMI1Late to BMI2Early in each of the three respective conditions (OPTOUP sessions paired t test t10 = -5.52, *P < 10−3; OPTODOWN sessions paired t test t7 = 5.12, *P < 10−3; OPTOOFF sessions paired t test t7 = 7.73, **P < 10−4).
Optogenetic Inhibition and Rescaling
We next examined the extent of rescaling for the three experimental groups. Sessions with OPTOUP stimulation did not demonstrate rescaling of task activity in BMI2, whereas the OPTODOWN and OPTOOFF conditions resulted in the expected rescaling of TRI neurons as previously observed (Fig. 7a). Furthermore, we evaluated neural dynamics using spike-field coherence (SFC, see methods regarding equalizing the number of spikes); SFC was significantly reduced for TRI neurons from Sleeppre to Sleeppost in the OPTOUP group (Fig. 7b–c). Finally, we also assessed whether the extent of average SFC change (ΔSFCmag from Sleeppre to Sleeppost) of TRD neurons could predict the extent of rescaling of TRI neurons from BMI1 to BMI2 (MDΔ). Notably, we found a significant relationship between these changes in the SSC and the rescaling phenomenon (Fig. 7d; R2 = 0.66, P < 10−6). Together, these results suggest that our measured changes in sleep functional connectivity after learning may be required for the performance gains, the reduced variability of direct neurons and the rescaling of task related activity.
Figure 7. Optogenetic inhibition during UP states prevents rescaling of task activations.
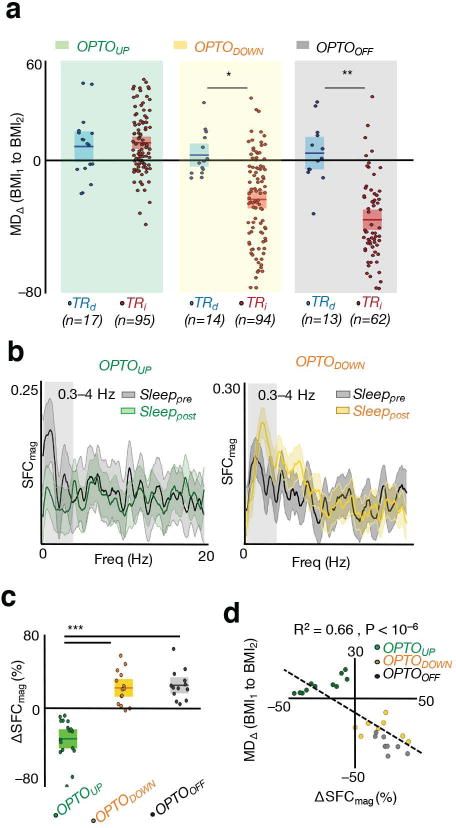
a, Rescaling of TRD and TRI neurons measured through modulation depth change (MD∆) from BMI1 and BMI2 in OPTOUP, OPTODOWN, and OPTOOFF experiments (mean in solid line ± s.e.m. in box; OPTOUP sessions unpaired t test t110 = −0.47, P = 0.64; OPTODOWN sessions unpaired t test t106 = 3.67, *P < 10−3; OPTOOFF sessions paired t test t73 = 5.52, **P < 10−6). b, Example plot of SFC as a function of frequency in Sleeppre and Sleeppost in OPTOUP and OPTODOWN experiment for two TRD neurons. The lighter band is the jackknife error. c, Averaged SFC changes from Sleeppre to Sleeppost for TRD neurons in OPTOUP, OPTODOWN, and OPTOOFF groups (mean in solid line ± s.e.m. in box, one-way ANOVA, F2,41 = 44.83, P < 10−10; significant post hoc t tests, ***P < 0.05). d, Averaged SFC changes for TRD cells versus averaged rescaling of TRI cells from BMI1 to BMI2 in OPTOUP, OPTODOWN, and OPTOOFF groups (linear regression R2 = 0.66, P < 10−6).
Discussion
In summary, we found striking evidence for rescaling of task–related neural activity after a period of NREM sleep. We specifically found that there was selective downscaling of TRI neural populations (i.e. non–causal) in comparison to TRD neurons (i.e. causal) during task performance after NREM sleep. Our results further revealed how individual TRD and TRI neurons might be chosen for downscaling; we found that patterns of activity during sleep were predictive of task–related rescaling. During task practice, activity patterns that were most consistently related to rewarded outcomes matched the “microstructure” of reactivations. A more gross measure of neural firing linked to slow-oscillatory activity (i.e. SSC in 0.3–4 Hz band) could also predict rescaling. Finally, we found that closed-loop optogenetic suppression of neural spiking during UP states prevented both performance gains and rescaling. Together, our results suggest that NREM sleep plays an essential role in determining task-related functional connectivity that reflects the causal neuron behavior relationship. A net result of this process is to assign network credit assignment and to create sparser patterns of task-related activity.
Rescaling and Sleep-Dependent Memory Processing
Two commonly cited possibilities for the role of sleep in memory consolidation are: (i) a general strengthening of synaptic connectivity, or (ii) a process of renormalization with net weakening of synaptic connectivity12,14,18. In the former, sleep is noted to have an active role in strengthening memories through enhanced local and distant connectivity, thus resulting in systems consolidation. In contrast, in the latter, renormalization of synaptic strengths is believed to restore synaptic homeostasis and thereby benefit memory functions. It is worth noting that both processes could occur but may operate over distinct timescales during long periods of sleep14. For example, recent evidence suggests that sleep is important both for pruning and growth of new spines40–42. Functionally, this could account for both the increases and decreases in neural firing after sleep29. Interestingly, a theoretical prediction is that synaptic renormalization may lead to rescaling of activity18; to our knowledge there is no direct evidence. For natural learning, assessment of task-dependent renormalization is likely to be difficult given that the causality of neural activity to behavior is largely still unknown.
Neuroprosthetic learning allows us to readily distinguish neural activity that is causal for actuator movements (i.e. TRD) versus activity that is non-causal. Using this task, we found evidence of rescaling of task activity; specifically, that the task-related modulation of causal neurons were slightly but significantly enhanced, while non-causal neurons showed selective downscaling of task-related modulation. While our specific experiments do not allow us to make conclusions regarding changes in synaptic strength, they do reveal that sleep-dependent processing can rescale task-dependent activations. At the very least, our results suggest that sleep-dependent processing does not exclusively strengthen functional connectivity as assessed by task-related neural firing. Moreover, given that we also found a small but significant improvement in task performance as well as increased modulation of direct task-neurons we cannot not exclude that a strengthening process may also simultaneously occur. Interestingly, our experiments using optogenetic suppression of spiking during the UP states suggests that our observed rescaling is driven by an activity-dependent process. Thus, our results also suggest that reactivations during sleep may be involved in a process of rescaling of task activity; this notion is also broadly in line with predictions that renormalization may rely upon the synchronous activity evident during slow oscillations 18.
Neuroprosthetic Memory Consolidation and Slow Oscillations
Our closed-loop optogenetic manipulation was triggered by phases of slow-oscillations during sleep. We found that while suppressing neural spiking during UP state (Fig 5b–d) perturbed sleep-dependent effects, similar perturbations in the DOWN state did not have detectable effects. This suggests that the spontaneous reactivation of both task and non-task related neurons during UP states are required for sleep-dependent gains. Importantly, our intervention did not appear to grossly affect sleep duration or the power-spectrum of sleep. However, it is still possible that other known processes that are linked to slow-oscillations might play a role. For example, it is known that spindles are associated with activity during UP states13,14. While we did not detect gross changes in power, it is still possible that disruption of spiking during slow-oscillations could affect spindles. Moreover, there is also a known link between cortical slow-oscillations and hippocampal ripples13,14. Future work can elucidate how other processes might contribute to consolidation after learning.
Our results further suggest that both performance gains and rescaling are regulated by spiking activity linked to slow-oscillations. More specifically, NREM sleep appears to have a three-fold effect on neural activity and performance. Firstly, there was a significant effect of enhanced performance. Secondly, there was a slight but significant increase in the modulation depth of TRD units. Finally, there was downscaling of TRI activity. The latter two appear to be related to a rescaling effect in which the two populations are differentially modified. Our OPTOUP intervention affected both performance gains and the rescaling effect. Interestingly, while it might seem that the modulation depth of TRD units was still increased, we observed a significant increase in task-related variability for TRD. Such enhanced variability may reflect poor consolidation of task activity patterns and underlie the degradation of performance after the OPTOUP intervention. It can be likened to ‘erosion’ of memory where rats forgot the neural activity pattern in BMI1 and had to relearn the task again. Together, this suggests that rescaling of the two neural populations may occur simultaneously during UP states.
Interestingly, the SSC analysis in Figure 2 suggests that the precise relationship between rescaling and SSC may be complex. There are at least three possibilities for why we measured a general increase in SSC in the setting of a largely selective enhancement of direct neurons. Firstly, it is possible that there is an elevated threshold for plasticity. In other words, the intercept of our linear regression line suggests that the zero crossing (i.e. threshold for enhancement) is for values greater than a zero change in SSC. Alternatively, it is possible that the general increase in SSC represents active processing of both populations during slow-oscillations. In this view, the system might actively sample both weak and strong functional connectivity in order to ultimately determine credit assignment. Such active sampling would appear to result in a general increase in SSC. It is also worth noting that for hippocampal replay, there may be dissociation between the external experience and internal processing43. Thus, it is also possible that the elevated SSC represents a schema for internal representation that is not strictly related to the actual awake experience.
Our results might also suggest that both performance gains and rescaling are optimized by the same mechanisms. However, it is still possible, that there is differential regulation of these two aspects of task performance. In both rodent and non-human primate models of neuroprosthetic learning, there is a dissociation between performance gains and rescaling8,23. For example, at the end of a typical practice session there were performance gains in the absence of rescaling (i.e. firing of non-causal activity). Similarly, past work in non-human primates has indicated that rescaling can take days to occur even in the presence of performance gains; the task used was substantially more complex than for rodents. This suggests that performance gains do not absolutely require rescaling. In our experiments, however, we found that sleep-dependent performance gains and rescaling were evident after a period of sleep. Moreover, disruption of spiking linked to slow-oscillations resulted in both degradation of performance and rescaling. This suggests that sleep-dependent processing co-regulates both processes. However, given that sleep is a collection of heterogeneous and non-stationary phenomena12,14, it is still quite possible that these two aspects can be dissociated. For example, our optogenetic intervention did not specifically examine the role of spindle activity that is coincident with slow-oscillations (i.e. as opposed to all spiking linked to it). Future work can help determine if performance gains and rescaling are always co-regulated during sleep.
Role of Reactivation in Credit Assignment
Our analysis specifically identified that timing of task activity relative to reward may determine credit assignment. Especially during “early learning”, co-firing of direct and indirect neurons occured over multiple seconds. It is likely that the animals were exploring patterns of neural activity that could successfully complete the task. Notably, traditional task-related PETHs for neuroprosthetic performance are calculated based on trial start; this is also typical for natural learning31,35. However, based on the extensive history on the role of reward in learning32–34, we also examined PETHs that were associated with task end and reward delivery. Interestingly, the frame relative to reward was the most predictive of rescaling and sleep-related reactivations. We also found that by perturbing the link between reward and task completion (i.e. the “variable reward” experiments in Fig 3,4) we no longer observed these phenomena. Together, these results are consistent with the growing notion that the patterns and extent of reward shapes learning and offline processing10,44.
What might be a computational role for our observed rescaling of cortical activity and its association with reward? In general, reward–related reactivation may be a broad mechanism to learn and remember experiences that lead to successful outcomes32–34,45. More specifically, the observed optimization of functional connectivity during sleep may provide important insight into the biological implementation of reinforcement learning (RL), a widely studied theoretical and experimental model for reward-based learning10,44. In RL, there is a noted tradeoff between “exploration” (i.e. gather new knowledge) versus “exploitation” (i.e. optimize decisions based on current knowledge)46; it remains unclear how this is precisely achieved in biological systems. Our data suggests that sleep–dependent processing can allow for more targeted exploration based on knowledge accumulated regarding reward–related neural firing during awake behaviors. Sleep may thus allow further exploration of the statistics of the causal relation of neural activity to successful outcomes. The net result is the establishment of neural activity patterns that appear to reflect the causal neuron-behavior relationship.
Methods
Animals/Surgery
Experiments were approved by the Institutional Animal Care and Use Committee at the San Francisco VA Medical Center. We used a total of ten adult Long–Evans male rats (n = 5 were used for optogenetic experiments). No statistical methods were used to pre-determine sample sizes but our sample sizes are similar to those reported in previous publications23,31. Animals were kept under controlled temperature and a 12–hour light: 12–hour dark cycle with lights on at 06:00 AM. Probes were implanted during a recovery surgery performed under isofluorane (1–3%) anesthesia. Atropine sulfate was also administered prior to anesthesia (0.02 mg/kg b.w.) The post–operative recovery regimen included administration of buprenorphine at 0.02 mg/kg b.w and meloxicam at 0.2 mg/kg b.w. Dexamethasone at 0.5 mg/kg b.w. and Trimethoprim sulfadiazine at 15 mg/kg b.w. were also administered post–operatively for five days. We used 32–channel microwire arrays; arrays were lowered down to 1400–1800 µm in the primary motor cortex (M1) in the upper limb area (1–3 mm anterior to bregma and 2–4 mm lateral from midline). The reference wire was wrapped around a screw inserted in the midline over the cerebellum. Final localization of depth was based on quality of recordings across the array at the time of implantation. All animals were allowed to recover for 1–week prior to start of experiments. Data collection and analysis were not performed blind to the conditions of the experiments.
Viral injections
We used a red-shifted halorhodopsin, Jaws (AAV8-hSyn-Jaws-KGC-GFP-ER2, UNC Viral Core) for neural silencing in 5 rats for optogenetic experiments38. Viral injections were done at least 2.5 weeks prior to chronic microelectrode array implant surgeries. Rats were anesthetized, as stated before and body temperature was maintained at 37°C with a heating pad. Burr hole craniotomies were performed over injection sites, and the virus was injected using a Hamilton Syringe with 34G needle. 500nl injections (100 nl per min) were made into deep cortical layers (1.4 mm from surface of brain) at two sites in M1 (coordinates relative to bregma: posterior, 0.5 mm and lateral, 3.5 mm; and anterior, 1.5 mm and lateral, 3.5 mm). After the injections, the skin was sutured and the animals were allowed to recover with same regimen as stated above. Viral expression was confirmed with fluorescence imaging. Optogenetic inhibition significantly reduced firing in M1 neurons, with a reduction in 50–70% of recorded cells.
Electrophysiology
We recorded extracellular neural activity using tungsten microwire electrode arrays (MEAs, Tucker–Davis Technologies or TDT, FL). We recorded spike and LFP activity using a 128–channel TDT–RZ2 system (Tucker–Davies Technologies). Spike data was sampled at 24414 Hz and LFP data at 1018 Hz. ZIF–clip based analog headstages with a unity gain and high impedance (~1 GΩ) was used. Optogenetic experiments, including controls, were done with digital headstages primarily because of the ability to pass the optical fiber through the commutator. Only clearly identifiable units with good waveforms and high signal–to–noise were used. The remaining neural data was recorded for offline analysis. Behavior related timestamps (i.e. trial onset, trial completion) were sent to the RZ2 analog input channel using a digital board and synchronized to neural data. We initially used an online sorting program (SpikePac, TDT) for neuroprosthetic control. We then conducted offline sorting23.
Behavior
After recovery, animals were typically handled for several days prior to the start of experimental sessions. Animals acclimated to a custom plexiglass behavioral box (Fig. 1a) during this period. The box was equipped with a door at one end. Initially, water delivery from the actuator was not introduced and they were just acclimatized to the box. Towards the end of the acclimation period, the rats typically fell asleep while in the box. Animals were then water scheduled such that water (from the feeding tube illustrated in Fig. 1a) was available in a randomized fashion while in the behavioral box. We monitored body weights on a daily basis to ensure that the weight did not drop below 95% of the initial weight. Behavioral sessions were conducted in the morning, with second sessions conducted in the afternoon. We recorded neural data from the rats for 2 hours prior to start of BMI training (that comprised Sleeppre). The rats were then allowed to perform the task over a ~2–hour session (BMI1). Recorded neural data was entered in real–time from the TDT workstation to custom routines in Matlab. These then served as control signals for the angular velocity of the feeding tube. The rats typically performed ~180–200 trials per session. These sessions typically lasted from 90 to 120 minutes based on the rate of trial completion. Following this, we recorded neural data from animals for a 2–hour period (including Sleeppost). The animals then continued with another 90 to 120 minute training session (BMI2). Sorted units at the beginning of the recording were checked for maintenance throughout the second training session.
Neural control of the feeding tube
During the BMI training sessions, we typically randomly selected two well–isolated units as ‘direct’ and allowed their neural activity to control the angular velocity of the feeding tube. In two of the 10 sessions (i.e. from the 5 non-viral injected rats), there was only one neuron selected as the direct unit. The remaining neurons in all the experiments (i.e. indirect) were there recorded but not causally linked to actuator movements. We did not find any systematic differences in waveform shape (i.e. narrow vs. broad) or baseline firing rate for these two populations. These units maintained their stability throughout the recording as evidenced by stability of waveform shape and interspike–interval histograms. We binned the spiking activity into 100 ms bins. We then established a mean firing rate for each neuron over a 3–5 minute baseline period. During this period the animals were typically transitioning between walking, exploring and periods of rest.
The mean firing rate was then subtracted from its current firing rate at all times. The specific transform that we used was:
where θv was the angular velocity of the feeding tube, r1(i) and r2(i) were firing rates of the direct units. G1 and G2 were randomized coefficients that ranged from +1 to –1 and were held constant after initialization. C was a fixed constant that scaled the firing rates to arrive at a value for angular velocity. The animals were then allowed to control the feeding tube via modulation of neural activity. The tube started at the same position at the start of each trial (P1 in Fig. 1a,b). The calculated angular velocity was added to the previous angular position at each time step (100 ms). During each trial, the angular position could range from –45 to +180 degrees. If the tube stayed in the ‘target zone’ (P2 in Fig. 1a; spanned 10° area) for a period of 300 ms, a water reward was delivered. In the BMIvariable-reward experiments (n = 4 sessions in two rats), the rats correctly positioned the tube, but reward delivery (i.e. the water from the tube) was randomly delayed by a period ranging from 1–3 seconds. In contrast, the BMIfixed-reward (i.e. typical BMI session), the reward was delivered with a fixed delay of ~200 ms relative to task completion. In the beginning of a session, most rats were unsuccessful at bringing the feeding tube to position P2. Most rats steadily improved control and reduced the time to completion of the task during the first session. We obtained multiple learning sessions from each animal. These sessions were typically several days to 1 week apart to ensure that new units were recorded. Consistent with past studies, we also found that incorporation of new units into the control scheme required new learning8,23.
Closed-loop sleep experiments using optogenetics
Three types of experiments were conducted using the 5 JAWS injected animals, namely: (i) OPTOUP (n = 11); (ii) OPTODOWN (n = 8); and (iii) OPTOOFF (n = 8). These experiments were largely randomly interspersed among the animals. However, while the OPTODOWN were only conducted in 3 animals, these animals also contributed to the OPTOUP and OPTOOFF experiments. In general, we identified the phases of the LFP associated with ‘UP’ and ‘DOWN’ states based on the relationship of the neural spiking to the LFP. For example, as shown in Figure 5, the negativity in our LFP signals was associated with neural spiking and thus consistent with an UP state, which are natural states of increased activity during slow oscillations.
The closed-loop interventions were conducted by triggering the LED light based on real-time detection of cortical states. We used a custom script in the RPvdsEx Prgram (TDT) to identify slow oscillations in real-time during sleep blocks. In the OPTOUP experiments, we conducted two types of triggering (n = 3 power based; n = 8 filtering based). In both cases, the LED light was delivered during cortical ‘UP’ states by placing a manual threshold on filtered LFP trace; the manual threshold was selected visually to coincide with the respective phase on the slow oscillations as noted below. For the “power based” triggering, we used the following approach. The algorithm/workstation calculated the LFP power in the 0.1 – 4 Hz range and compared it to the threshold. Once the threshold was exceeded for >100 ms, LED illumination (625nm Fiber-Coupled LED (ThorLabs), with 200/400 μm diameter optic fibers (Doric Lenses) was triggered for 100 ms. For the ‘filtering based’ approach, we used a real-time implementation of a Butterworth filter to filter the raw LFP in a 0.1–4 Hz band (Figure 5d). The UP state was determined by setting a ‘negative’ threshold on the LFP (i.e. as displayed in the convention in Figure 5d). The LED was again triggered when it was respectively above/below this threshold. Notably, this type of stimulation was exclusive to the UP state. Because we did not observe any differences we combined both sets as the OPTOUP condition.
During OPTODOWN sessions, we directly placed a ‘positive’ threshold on the filtered LFP; thus the stimulation was triggered during threshold crossings of ‘DOWN’ (i.e. DOWN states with natural periods of quiescence during slow oscillations). These stimulations were also typically brief (i.e. 100 ms). A typical example is shown in Fig 5. Supplementary Fig 3 shows that total incidents of 100 ms stimulations were similar in both OPTOUP and OPTODOWN experiments, and the light was on for a similar proportion of time. Finally, a group of control experiments called OPTOOFF (i.e. where no stimulation was triggered) was also conducted in the JAWS injected rats. Durations of total pre and post sleep were similar in all 3 session types (Supplementary Fig 4). We also calculated LFP power and SFC changes for individual neurons in all 3 groups.
Data Analysis
Sessions and changes in performance
Analysis was performed in Matlab (Mathworks, Natick, MA) with custom–written routines. A total of 10 BMIfixed-reward training sessions recorded from 5 rats were used for our initial analysis. All of these sessions demonstrated ‘robust learning’ (i.e. > 3 SD drop in time to completion in the last 1/3 of trials or ‘late’ trials in comparison to the first 1/3 of trials or ‘early’ trials). These sessions were followed by a second training session (i.e. BMI2). In Fig. 1c we compared changes in task performance across sessions. Specifically, we compared the performance change between BMI1Late, BMI2Early and BMI2Late by calculating the mean and standard error of the time to completion during the last third trials in BMI1 and the first and last third trials BMI2 (Fig. 1c). We used a paired t–test to assess statistical significance.
Task–related activity
The distinction between TRD and TRI neurons was based on whether units were used for the direct neural control of the feeding tube. The change in modulation depth (MD∆) was calculated by comparing the peak activity around the task (in the 5 second window after the task start/4 sec prior to task-end/reward) over baseline firing activity (averaged activity of 4 seconds prior to task start) on the peri-event time histograms (PETH, bin length 50 ms). In other words, the MD∆ is a measure of the modulation of firing rate relative to the pre-task start baseline rate. Modulation of baseline firing activity after the ‘Go cue’ (task start) or prior to receipt of ‘reward’ (task end) was calculated and this was compared for TRD and TRI neurons from BMI1 to BMI2 (MD∆ change from BMI1 to BMI2). This was calculated across the last third of trials from BMI1 and first and last third of trials from BMI2 (BMI2Early and BMI2Late respectively). In a BMI session with approximately 200 trials, these values were averaged across ~65 trials. To ensure that any online training effects were not contributing to the observed reduction in MD∆ of TRI units, in a subset of these sessions we also averaged MD∆ for just 30 trials before and after; no significant differences were evident.
For Figures 1 and 3, PETH were smoothed using a Bayesian adaptive-regression spline algorithm, implemented within MATLAB using toolboxes downloaded at (http://www.cnbc.cmu.edu/~rkelly/code.html)31,47. The algorithm automatically optimized for the number and location of “knots” (i.e., regions in which a new local regression model improves the overall fit of the curve) was determined automatically using a Markov chain Monte Carlo implemented to optimize the Bayes Information Criteria and thereby, offered a better visualization of dynamic changes in the rate of change of spike trains. These curves were not used for other sets of analysis.
Identification of NREM oscillations
Identification of pre and post–NREM epochs was performed by combined visual assessment of presence of low–frequency, high amplitude slow–wave oscillations as well as a 3 SD threshold of the filtered data (0.3 – 4 Hz). If there was a sustained reduction > 1.5 seconds in the amplitude of the slow-wave activity below threshold during a continuous epoch we excluded these segments23,31.
Coherency measure
We used the Chronux toolbox to calculate the SSC (http://chronux.org/) 48. Its magnitude is a function of frequency and takes values between 0 and 1. For it’s calculation, the pre- and post-sleep were segmented into 20-s segments and then the coherency measured was averaged across segments. For the multitaper analysis, we used a time-bandwidth (TW) product of 10 with 19 tapers. To compare coherences across groups, a z score was calculated using the programs available in the Chronux Toolkit. Coherence between activity in two regions, Cxy was calculated and defined as
where Rxx and Ryy are the power spectra and Rxy is the cross-spectrum. More specifically, it is a pairwise measure of synchronized co-firing of neurons in a frequency dependent manner. For example, during NREM sleep, it can quantify synchronous co-firing relative to low frequency oscillation’s in the 0.3–4 Hz range. Our previous work has also shown that SSC values are related to the spike cross-correlogram measured during UP states23.
Spectral analysis were calculated in segmented NREM epochs and averaged across these epochs across animals. Mean coherence was calculated between 0.3 – 4 Hz. Significance testing on coherence estimates was performed on mean estimates between TRD – TRD and TRD – TRI pairs using unpaired t-tests. The task-related direct unit with the greatest depth modulation was used to calculate SSC for every other unit. Similarly, for SFC analysis in optogenetic experiments, mean power changes in the 0.3–4 Hz band were compared for OPTOUP; OPTODOWN and OPTOOFF experiments. We also equaled the number of spikes in pre- and post- sleep23,28 to account for the changes in firing rates; this was especially pertinent for the optogenetic intervention studies.
Ensemble activation analyses
To characterize ensemble reactivations following sleep, we performed an analysis that compared neural activity patterns during Sleep1 and Sleep2 with a template that was created during task execution in BMI1 23,30,31. We first computed a pairwise unit activity correlation matrix during BMI1 by concatenating binned spike trains (tbin = 50 ms) for each neuron across trials (0.5s prior to the onset of trial up to 5s after the onset of BMI task for each trial). This concatenated spike train was z-transformed, and then organized into a 2-D matrix organized by neurons (x) and time (B for number of time bins). From this spike count matrix, we calculated the correlation matrix (Ctask), and then calculated the eigenvector for the largest eigenvalue from this correlation matrix to study. This eigenvector was used as the ensemble template of activity, which was then projected back on to the neural activity trains from the same population of neurons during Sleep1 and Sleep2. This projection was a linear combination of Z-scored binned neural activity from the two blocks above, weighted by the PC ensemble (i.e., the eigenvector) calculated from the BMI1 matrix. This linear combination has been described as the “activation strength” of that particular ensemble. In this analysis we focused on the first eigenvector, as the first PC explained most task-related variance (see Supplementary Figure 5 for two examples).
Reactivation triggered peri-event time histogram (“microstructure” of reactivation)
We also constructed time histograms of single unit activity around reactivation events. We binned spike counts from 250 ms before and after ensemble reactivation events using a 5 ms bin size and calculated the mean/standard error of the binned neural firing. The reactivation events that were chosen for PETHs were those with a reactivation strength that was significantly greater than for the pre- sleep block. Usually top 10–20 percentile reactivation strengths from the post-sleep fulfilled this criterion. Once the PETHs were constructed, the modulation depth around reactivations (MDreactivation) was calculated by comparing the peak of firing during reactivation to the mean baseline firing (i.e. at the tails). t-test was performed to compare MDreactivation between TRD and TRI units, and also their levels in pre-sleep. We also checked for MDreactivation of TRD and TRI units at random low-percentile reactivation events and their MDreactivation was indistinguishable (Supplementary Fig 1).
Analyses of neural firing variability and neuronal pair correlations
The modulation characteristics of each neuron in the BMI task in the two frames of reference (namely, ‘task-start’ and ‘task-end’) were examined using the following: (1) Fano factor, which is a statistical measure of the dynamics of the firing rate of a cell35,36; and (2) Cross-correlation calculated between the rates of cell pairs. Fano factor, F is defined as follows:
where σ2 is the variance and μ is the mean of a spike count process (here in a 50 ms time window). μ was the average firing rate and was calculated as follows:
where C(n) is the spike counts in 50 ms time window and B is the total window sample number. Since, fano factor can be influenced by firing rate, we also compared fano factor in task start and task end frames of reference where the firing rates were similar and we still found similar trends. Cross-correlation, on the other hand, measured the similarity of two firing rate series (50 ms bins) as a function of the displacement of one relative to the other. This pairwise correlation of the neural activity was calculated for TRD – TRD and TRD – TRI neuronal pairs using Matlab’s xcorr function (Fig. 4). Time series of concatenated binned spike counts were created either around task start (first 1 sec) or around task end (from trial end to 1 sec prior). Statistical comparisons were performed using a repeated-measures ANOVA, followed by post-hoc t tests to identify specific time points that were significantly different.
Statistics
There were a total of 10 robust BMI learning sessions that we used (BMIfixed-reward) for analyzing the trends from BMI1 to BMI2. There were a total of 18 TRD and 105 TRI units in these experiments. There were also 4 BMIvariable-reward sessions where we had 6 TRD and 41 TRI neurons. Optogenetics experiments (in JAWS injected rats) had 11 sessions with OPTOUP stimulation (with 17 TRD and 95 TRI units), 8 sessions with OPTODOWN stimulation (with 14 TRD and 94 TRI units), and 8 sessions with OPTOOFF stimulation (with 13 TRD and 62 TRI units). We also recorded sleep prior to (Sleeppre) and after (Sleeppost) after BMI1. In all these experiments, we performed paired t-test to compare performance changes from BMI1 to BMI2; MD∆ change for TRD or TRI units from BMI1 to BMI2; MDreactivation change and firing rate changes for TRD and TRI units from Sleeppre to Sleeppost; SSCmag changes for TRD – TRD and TRD – TRI neuronal pairs from Sleeppre to Sleeppost (Fig. 1c, 6b). Data distribution was tested for normality and non-parametric test was substituted if needed (Wilcoxon signed rank test). Unpaired t–tests were also used for comparisons such as MDreactivation in TRD versus TRI units pools; MD∆ change for TRD versus TRI units from BMI1 and BMI2; and features of stimulation in OPTOUP and OPTODOWN experiments (Fig. 1e, 7a; Supplementary Fig. 1, 3). We also performed one–way ANOVA with multiple comparisons (test of homogeneity of variances was done) wherever significance assessment was required (Fig. 2e, 3c, 4a, 5c,f, and 7c; Supplementary Fig. 4). We also used linear regression or correlation to evaluate trends between MDreactivation versus MD∆ change from BMI1 and BMI2, or correlated firing around task start or task end; pairwise firing correlation of TRD – TRD and TRD – TRI neuronal pairs versus MDreactivation; between time spent in NREM sleep and MD∆ change from BMI1 and BMI2 for different units; and SSCmag changes for TRD – TRD and TRD – TRI neuronal pairs versus MD∆ change for TRD or TRI units from BMI1 to BMI2; and SFC changes in optogenetics experiments, versus MD∆ change (Fig. 2b, 4b,c 7d; Supplementary Fig. 2).
Supplementary Material
Acknowledgments
This work was supported by awards from the Department of Veterans Affairs, Veterans Health Administration (VA Merit: 1I01RX001640 to K. Ganguly, VA CDA 1IK2BX003308 to D. S. Ramanathan); the National Institute of Neurological Disorders and Stroke (1K99NS097620 to T. Gulati and 5K02NS093014 to K. Ganguly); the American Heart/Stroke Association (15POST25510020 to T. Gulati); the Burroughs Wellcome Fund (1009855 to K. Ganguly); and start-up funds from the SFVAMC, NCIRE and UCSF Department of Neurology (to K. Ganguly).
Footnotes
Accession Codes
All relevant data are available from authors
Data Availability Statement
The data that support the findings from this study are available from the corresponding author upon request.
Author Contributions
T. G. and K. G. conceived of the experiments. L.G. and T. G. performed surgical procedures and collected the data. A.B., D. S. R. and T.G. analyzed the data. T. G. and K. G. wrote the manuscript. L. G. and D. S. R. edited the manuscript.
Competing Financial Interests Statement
None
References
- 1.Yin HH, et al. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nat Neurosci. 2009;12:333–341. doi: 10.1038/nn.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dayan E, Cohen LG. Neuroplasticity subserving motor skill learning. Neuron. 2011;72:443–454. doi: 10.1016/j.neuron.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tumer EC, Brainard MS. Performance variability enables adaptive plasticity of ‘crystallized’ adult birdsong. Nature. 2007;450:1240–1244. doi: 10.1038/nature06390. [DOI] [PubMed] [Google Scholar]
- 4.Shmuelof L, Krakauer JW. Are we ready for a natural history of motor learning? Neuron. 2011;72:469–476. doi: 10.1016/j.neuron.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peters AJ, Chen SX, Komiyama T. Emergence of reproducible spatiotemporal activity during motor learning. Nature. 2014;510:263–267. doi: 10.1038/nature13235. [DOI] [PubMed] [Google Scholar]
- 6.Ganguly K, Carmena JM. Emergence of a stable cortical map for neuroprosthetic control. PLoS Biol. 2009;7:e1000153. doi: 10.1371/journal.pbio.1000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huber D, et al. Multiple dynamic representations in the motor cortex during sensorimotor learning. Nature. 2012;484:473–478. doi: 10.1038/nature11039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ganguly K, Dimitrov DF, Wallis JD, Carmena JM. Reversible large-scale modification of cortical networks during neuroprosthetic control. Nat Neurosci. 2011;14:662–667. doi: 10.1038/nn.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abbott LF, DePasquale B, Memmesheimer RM. Building functional networks of spiking model neurons. Nat Neurosci. 2016;19:350–355. doi: 10.1038/nn.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee D, Seo H, Jung MW. Neural basis of reinforcement learning and decision making. Annu Rev Neurosci. 2012;35:287–308. doi: 10.1146/annurev-neuro-062111-150512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clancy KB, Koralek AC, Costa RM, Feldman DE, Carmena JM. Volitional modulation of optically recorded calcium signals during neuroprosthetic learning. Nat Neurosci. 2014 doi: 10.1038/nn.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tononi G, Cirelli C. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron. 2014;81:12–34. doi: 10.1016/j.neuron.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 14.Genzel L, Kroes MC, Dresler M, Battaglia FP. Light sleep versus slow wave sleep in memory consolidation: a question of global versus local processes? Trends Neurosci. 2014;37:10–19. doi: 10.1016/j.tins.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Cramer SC, et al. Motor cortex activation is preserved in patients with chronic hemiplegic stroke. Ann Neurol. 2002;52:607–616. doi: 10.1002/ana.10351. [DOI] [PubMed] [Google Scholar]
- 16.Marshall L, Born J. The contribution of sleep to hippocampus-dependent memory consolidation. Trends Cogn Sci. 2007;11:442–450. doi: 10.1016/j.tics.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science (80-) 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- 18.Nere A, Hashmi A, Cirelli C, Tononi G. Sleep-dependent synaptic down-selection (I): modeling the benefits of sleep on memory consolidation and integration. Front Neurol. 2013;4:143. doi: 10.3389/fneur.2013.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jarosiewicz B, et al. Functional network reorganization during learning in a brain-computer interface paradigm. Proc Natl Acad Sci U S A. 2008;105:19486–19491. doi: 10.1073/pnas.0808113105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koralek AC, Jin X, Long JD, 2nd, Costa RM, Carmena JM. Corticostriatal plasticity is necessary for learning intentional neuroprosthetic skills. Nature. 2012;483:331–335. doi: 10.1038/nature10845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor DM, Tillery SI, Schwartz AB. Direct cortical control of 3D neuroprosthetic devices. Science (80-) 2002;296:1829–1832. doi: 10.1126/science.1070291. [DOI] [PubMed] [Google Scholar]
- 22.Moritz CT, Perlmutter SI, Fetz EE. Direct control of paralysed muscles by cortical neurons. Nature. 2008;456:639–642. doi: 10.1038/nature07418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gulati T, Ramanathan DS, Wong CC, Ganguly K. Reactivation of emergent task-related ensembles during slow-wave sleep after neuroprosthetic learning. Nat Neurosci. 2014;17:1107–1113. doi: 10.1038/nn.3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gulati T, et al. Robust neuroprosthetic control from the stroke perilesional cortex. J Neurosci. 2015;35:8653–61. doi: 10.1523/JNEUROSCI.5007-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fetz EE. Volitional control of neural activity: implications for brain-computer interfaces. J Physiol. 2007;579:571–579. doi: 10.1113/jphysiol.2006.127142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koralek AC, Costa RM, Carmena JM. Temporally precise cell-specific coherence develops in corticostriatal networks during learning. Neuron. 2013;79:865–872. doi: 10.1016/j.neuron.2013.06.047. [DOI] [PubMed] [Google Scholar]
- 27.Orsborn AL, et al. Closed-loop decoder adaptation shapes neural plasticity for skillful neuroprosthetic control. Neuron. 2014;82:1380–1393. doi: 10.1016/j.neuron.2014.04.048. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell JF, Sundberg KA, Reynolds JH. Spatial attention decorrelates intrinsic activity fluctuations in macaque area V4. Neuron. 2009;63:879–888. doi: 10.1016/j.neuron.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watson BO, Levenstein D, Greene JP, Gelinas JN, Buzsaki G. Network Homeostasis and State Dynamics of Neocortical Sleep. Neuron. 2016;90:839–852. doi: 10.1016/j.neuron.2016.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peyrache A, Khamassi M, Benchenane K, Wiener SI, Battaglia FP. Replay of rule-learning related neural patterns in the prefrontal cortex during sleep. Nat Neurosci. 2009;12:919–926. doi: 10.1038/nn.2337. [DOI] [PubMed] [Google Scholar]
- 31.Ramanathan DS, Gulati T, Ganguly K. Sleep-Dependent Reactivation of Ensembles in Motor Cortex Promotes Skill Consolidation. PLoS Biol. 2015;13:e1002263. doi: 10.1371/journal.pbio.1002263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lansink CS, Goltstein PM, Lankelma JV, McNaughton BL, Pennartz CM. Hippocampus leads ventral striatum in replay of place-reward information. PLoS Biol. 2009;7:e1000173. doi: 10.1371/journal.pbio.1000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Lavilleon G, Lacroix MM, Rondi-Reig L, Benchenane K. Explicit memory creation during sleep demonstrates a causal role of place cells in navigation. Nat Neurosci. 2015;18:493–495. doi: 10.1038/nn.3970. [DOI] [PubMed] [Google Scholar]
- 34.Singer AC, Frank LM. Rewarded outcomes enhance reactivation of experience in the hippocampus. Neuron. 2009;64:910–921. doi: 10.1016/j.neuron.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Churchland MM, et al. Stimulus onset quenches neural variability: a widespread cortical phenomenon. Nat Neurosci. 2010;13:369–378. doi: 10.1038/nn.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song W, Giszter SF. Adaptation to a cortex-controlled robot attached at the pelvis and engaged during locomotion in rats. J Neurosci. 2011;31:3110–3128. doi: 10.1523/JNEUROSCI.2335-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyamoto D, et al. Top-down cortical input during NREM sleep consolidates perceptual memory. Science (80-) 2016;352:1315–1318. doi: 10.1126/science.aaf0902. [DOI] [PubMed] [Google Scholar]
- 38.Chuong AS, et al. Noninvasive optical inhibition with a red-shifted microbial rhodopsin. Nat Neurosci. 2014;17:1123–1129. doi: 10.1038/nn.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steriade M, Nunez A, Amzica F. A novel slow (< 1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci. 1993;13:3252–3265. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang G, et al. Sleep promotes branch-specific formation of dendritic spines after learning. Science (80-) 2014;344:1173–1178. doi: 10.1126/science.1249098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Vivo L, et al. Ultrastructural evidence for synaptic scaling across the wake/sleep cycle. Science (80-) 2017;355:507–510. doi: 10.1126/science.aah5982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maret S, Faraguna U, Nelson AB, Cirelli C, Tononi G. Sleep and waking modulate spine turnover in the adolescent mouse cortex. Nat Neurosci. 2011;14:1418–1420. doi: 10.1038/nn.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gupta AS, van der Meer MA, Touretzky DS, Redish AD. Hippocampal replay is not a simple function of experience. Neuron. 2010;65:695–705. doi: 10.1016/j.neuron.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Doherty JP, Cockburn J, Pauli WM. Learning, Reward, and Decision Making. Annu Rev Psychol. 2017;68:73–100. doi: 10.1146/annurev-psych-010416-044216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schultz W. Behavioral theories and the neurophysiology of reward. Annu Rev Psychol. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- 46.Ishii S, Yoshida W, Yoshimoto J. Control of exploitation-exploration meta-parameter in reinforcement learning. Neural Netw. 2002;15:665–687. doi: 10.1016/s0893-6080(02)00056-4. [DOI] [PubMed] [Google Scholar]
- 47.Wallstrom G, Liebner J, Kass RE. An Implementation of Bayesian Adaptive Regression Splines (BARS) in C with S and R Wrappers. J Stat Softw. 2008;26:1–21. [PMC free article] [PubMed] [Google Scholar]
- 48.Mitra P, Bokil H. Observed brain dynamics. Oxford University Press; 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


