Abstract
Rationale
There is substantial state-to-state heterogeneity in tuberculosis (TB) in the United States; better understanding this heterogeneity can inform effective response to TB at the state level, the level at which most TB control efforts are coordinated.
Objectives
To characterize drivers of state-level heterogeneity in TB epidemiology in the four U.S. states that bear half the country’s TB burden: California, Florida, New York, and Texas.
Methods
We constructed an individual-based model of TB in the four U.S. states and calibrated the model to state-specific demographic and age- and nativity-stratified TB incidence data. We used the model to infer differences in natural history of TB and in future projections of TB.
Measurements and Main Results
We found that differences in both demographic makeup (particularly the size and composition of the foreign-born population) and TB transmission dynamics contribute to state-level differences in TB epidemiology. The projected median annual rate of decline in TB incidence in the next decade was substantially higher in Texas (3.3%; 95% range, −5.6 to 10.9) than in California (1.7%; 95% range, −3.8 to 7.1), Florida (1.5%; 95% range, −7.4 to 14), and New York (1.9%; 95% range, −6.4 to 9.8). All scenarios projected a flattening of the decline in TB incidence by 2025 without additional resources or interventions.
Conclusions
There is substantial state-level heterogeneity in TB epidemiology in the four states, which reflect both demographic factors and potential differences in the natural history of TB. These differences may inform resource allocation decisions in these states.
Keywords: tuberculosis, tuberculosis in the United States, mathematical modeling of tuberculosis, geographical heterogeneity in tuberculosis
Tuberculosis disease (TB) remains an important public health concern in the United States, with 9,557 reported cases in 2015 (1). TB incidence declined from 9.7 per 100,000 in 1993 to 3.0 in 2013 in the United States but remained essentially flat from 2013 to 2015 (1, 2). The leveling of TB incidence may represent statistical chance but may also reflect important underlying dynamics in the TB epidemic, such as the country’s changing demographic makeup or a plateau in current interventions’ achievable effect. Foreign-born individuals now account for two-thirds of new TB cases, with a TB incidence of 15.1 per 100,000/yr (2) and numbers that have increased from 7.9% of the U.S. population in 1990 to 13.2% in 2014 (3). As such, it remains uncertain whether declines in TB incidence will continue in the absence of additional interventions (4).
Importantly, the TB epidemic in the United States is not homogeneous. States differ in their demographic makeup (particularly the size and origin of their foreign-born populations) and historical rates of TB, which may reflect differences in ongoing transmission (5), latent TB infection (LTBI) prevalence, and differential reactivation probabilities (e.g., due to differences in underlying risk factors) (6) as well as funding and implementation of TB prevention and control efforts. Such differences may result in heterogeneous trajectories of TB incidence in the future as well as different ideal strategies for TB prevention and control at the state level. For example, contact investigation may have greater epidemiological impact in areas with more ongoing transmission, whereas preventive therapy may be more important in areas with higher population-level risks of reactivation. TB control activities are largely planned and implemented at the state level, with funding from federal and state governments in the United States, allowing for implementation of state-specific TB control strategies. As different states seek to implement the strategies that will be most effective in eliminating TB in their specific contexts, therefore, it will become increasingly important to account for such heterogeneities at the state level (4).
To illustrate the importance of these heterogeneities and assist in state-level decision-making, we constructed an individual-level model of TB, applied separately to the four states that report half of all incident TB cases in the United States: California, Florida, New York, and Texas. Our primary goals were (1) to characterize and quantify demographic and TB natural history mechanisms underlying state-level differences in TB epidemiology, and (2) to use this understanding to project state-specific TB incidence in the coming 10 years.
Methods
Overview
We developed an individual-based modeling framework structured to capture demographic and epidemiological processes generating underlying differences in TB epidemiology and applied this framework to four states: California, Florida, New York, and Texas. For each state, we investigated the demographic and epidemiologic parameters that best explained state-level TB incidence. Then, using state-specific best-fit models, we projected TB incidence through 2025.
Demographic and TB Data
We obtained demographic data on population sizes and age-specific U.S.- and foreign-born populations for the four states from the 5-year estimates of the American Community Surveys of 2005 and 2014 (7, 8). We obtained U.S. TB case report data from the National TB Surveillance System, stratified by state, nativity, and age for the 5-year periods 1993 to 1997, 2001 to 2005, and 2009 to 2013 using the Online Tuberculosis Information System data portal (9). We used these data to estimate the age- and state-specific TB incidence in the time periods 1993 to 1997, 2001 to 2005, and 2009 to 2013. These estimates served as calibration targets for the model, which aimed to capture both the historic trends in TB incidence and the distribution of TB by age and nativity. Due to the lack of American Community Surveys data for foreign-born 1993 to 1997 population estimates, we assumed that the proportion of the foreign-born population in each state was the same as in 2001 to 2005.
Modeling Framework
We used an individual-based framework to model demographic processes, differences in natural history, and transmission dynamics of TB. We modeled each state-level population as having a constant per capita birth rate and age-specific mortality rates on the basis of the Siler mortality model (10) (see online supplement). Each individual was modeled as dwelling in and transitioning between four TB categories: (1) uninfected, (2) LTBI, (3) active TB disease, and (4) successfully treated TB disease (Figure 1). We assumed the model population to mix homogeneously. Hence, the hazard of TB infection changes over time but does not differ by age or nativity. State-specific transmission rates (i.e., the average number of individuals infected by an infectious case per year) were allowed to decline exponentially over time (i.e., constant annual percentage decline), and this rate of decline was allowed to change in 1993, when more detailed data became available. Individuals with LTBI experience an ongoing risk of developing TB disease. We modeled reactivation rates by adapting a formula proposed by Vynnycky and Fine (11), which assumes an exponential decline in reactivation with time since infection, coupled with an increase in reactivation with age. Individuals with TB disease, on receiving proper diagnosis and successful treatment, were assumed to become immediately noninfectious. Individuals with TB disease (without treatment) were also subject to TB-related mortality. Reinfection of individuals previously treated or currently infected was also allowed.
Figure 1.

Schematic representation of the modeling framework. (A) Natural history of tuberculosis (TB) was captured in this framework by individual transitions between the four stages: uninfected, latent TB infection (LTBI), active TB, and after treatment. Individuals are born uninfected and acquire LTBI at a rate commensurate with the local force of infection. Individuals with LTBI reactivate to develop active TB disease, at a rate reflecting both age and time since infection. On receiving successful treatment, an individual progresses to the post-treatment stage. Both individuals with LTBI and treated individuals can be reinfected; individuals with previous history of TB are modeled to have partial protection against reinfection. (B) The reactivation rate among individuals with LTBI was modeled to decline continuously over time: the further away an individual is from the time of infection, the smaller the rate. Reactivation rates are also assumed to increase with age (not shown in this illustration; see online supplement for details). (C) Immigration was modeled as importation of individuals from one of the eight regions as described in METHODS. The region-specific size of the immigrant population varied by state. Individuals arriving to the United States were apportioned as uninfected, LTBI, or TB at arrival according to the TB prevalence in the region of origin.
Immigration and Importation of TB
Immigration was modeled explicitly as a continuous rate of influx into the population, the rate of which was calibrated to the size of the foreign-born population in each state. To capture heterogeneities in global TB incidence, we modeled eight countries/regions of origin: (1) Mexico, (2) Latin America (excluding Mexico), (3) China, (4) India, (5) Asia (excluding China and India), (6) Africa, (7) Europe, and (8) others. Each individual was probabilistically assigned a TB status on arrival depending on age and nativity, under the assumption that the individual experienced a constant hazard of TB infection (consistent with the prevalence in the region of origin; see Table E2 in the online supplement) from birth until arrival in the United States. The detailed models for immigration and importation of TB along with estimated age- and region-specific LTBI prevalence among foreign-born individuals are provided in the online supplement. We calibrated immigration rates from each region to fit the marginal distribution of the foreign-born population by age and region of origin (7, 8). The model-based simulations and demographic data are compared in Figure 2.
Figure 2.
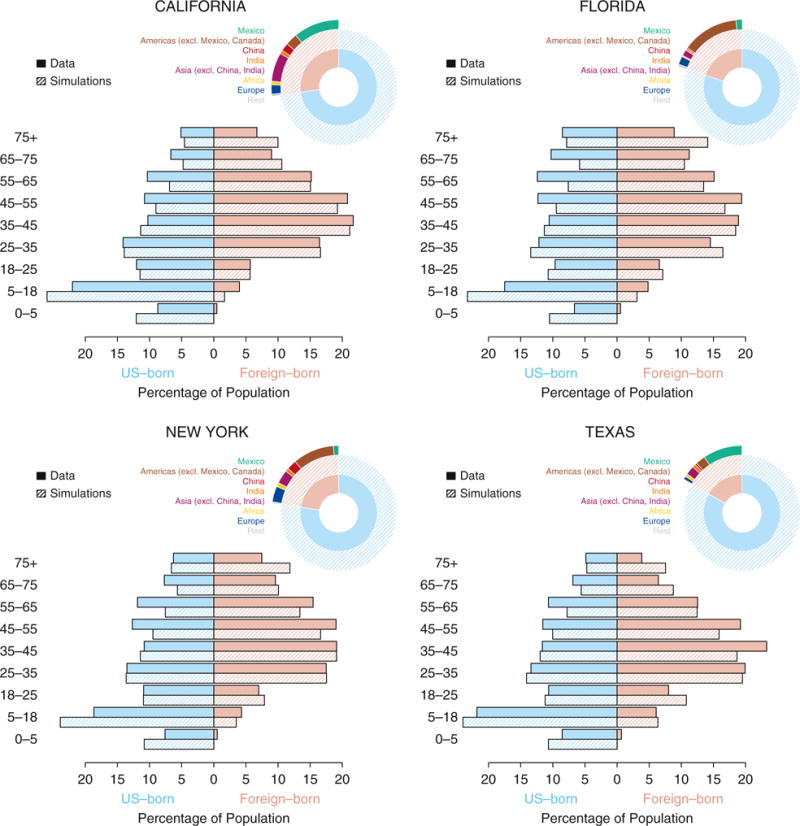
Comparisons of state-level demographic data and model simulations. For each of the four states (California, Florida, New York, and Texas), the pie charts show the size of the population that are U.S. (in light blue) and foreign (in pink) born, and their further subdivisions by eight regions: (1) Mexico, (2) Americas excluding Mexico and Canada, (3) China, (4) India, (5) Asia excluding China and India, (6) Africa, (7) Europe, and (8) rest of the world. The horizontal bars show the distribution of the population (light blue showing U.S. born, and pink showing foreign born) by age categories indicated on the side. The data (solid bars) are obtained from 5-year estimates (2009–2014) of the American Community Survey. Shown in hatched bars are the distributions in simulations of the calibrated models for each of the states.
Model Simulations
Each simulation consisted of two phases: (1) a “burn-in” phase (pre-1993), in which the model was run for 100 years with a decline in TB transmission rate to ensure a declining trend of TB transmission during this era and to reproduce observed trends in age-specific LTBI prevalence, and (2) the calibration phase (1993–2013), in which we fit a different annual decline in the TB transmission rate to recapitulate recent TB incidence trends.
Model Fitting
We used a likelihood-based method to calibrate each state-specific model to available data. We used a binomial likelihood function, fitting data to state- and age-specific TB incidence among U.S.- and foreign-born individuals, in three time periods: 1993 to 1997, 2001 to 2005, and 2009 to 2013. For each state, we drew 200,000 different parameter combinations using Latin Hypercube sampling (12, 13) from the parameter ranges provided in Table E1. The single combination that yielded the highest likelihood (i.e., best fit to the data) was taken as the maximum likelihood estimate (MLE), which was then used as the baseline simulation for each state and compared with the MLE models of other states. Data and simulations from the MLE models are compared in Figure 3 and Figure E1.
Figure 3.
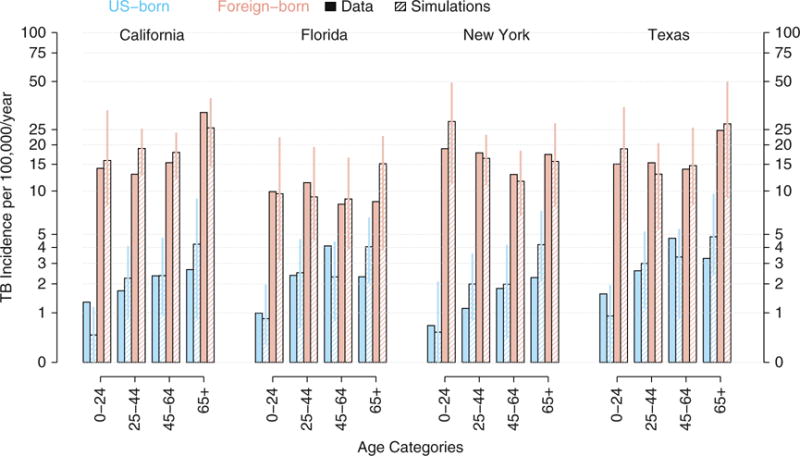
Reported and model-based estimates of annual tuberculosis (TB) incidence from 2009 to 2013, by age and origin (U.S. vs. foreign born) for each of the four states. The bar charts show TB incidence in California, Florida, New York, and Texas (from left to right), for the 5-year time period spanning 2009 to 2013. TB incidence is categorized by age in four categories (labeled at the bottom) and country of origin (U.S. born in light blue and foreign born in pink). The solid bars show reported data: TB case report data were obtained from Online Tuberculosis Information System (OTIS) data repository (9), and data on demographics were obtained from the American Community Survey (8). The hatched bars show model-based estimates: shown are medians (and 95% range) in 100 replicated simulations of the model, with maximum-likelihood estimates for each of the four states.
Natural History Parameter Estimation
We used the MLE model in each state to infer two key parameters associated with the natural history of TB, namely the historical rate of decline in TB transmission rate and the reactivation rates among adults with LTBI. Estimates for each parameter value were achieved by constructing a likelihood profile that maximized the likelihood function over all parameters while holding the parameter being profiled constant. The 95% confidence interval (CI) was estimated for each parameter by finding the range of parameter values whose likelihood estimates were within 1.92 log-likelihood units, on the basis of univariate confidence limits using the χ2 distribution with 1 degree of freedom (14).
Model Projections
We generated MLE model projections for 12 years, from 2014 to 2025, to validate the models against existing data (2014–2015) and project future trends. The projections were based on continuation of current immigration trends (including both the rates of immigration and the composition of persons arriving in the United States by nativity) and continuing decline in transmission rates as estimated to be occurring currently. We conducted 100 replicated simulations of the MLE model and estimated the median and 95% range of projected epidemiological parameters from the simulations.
Results
State-Level Demography
Between 2009 and 2014, foreign-born individuals composed 27.1% of the population in California, 22.6% in New York, 20.0% in Florida, and 16.8% in Texas. The corresponding regions of origin differed substantially across the states (Figure 2). Mexico—with TB prevalence of 42 per 100,000 persons in 2000 (15)—contributed a large percentage of the foreign born in Texas (56.1%) and California (40.8%) but not in Florida (7.1%) or New York (5.6%). Asian countries (TB prevalence in 2000 of 170 per 100,000 in China, 438 in India, and between 425-515 elsewhere [15]) accounted for 37.8% of the foreign born in California, 28.3% in New York, 20.2% in Texas, and only 10.6% in Florida. Latin America (excluding Mexico), with TB prevalence between 59 and 138 per 100,000 in 2000 (15), represented 68.0% of the foreign born in Florida (primarily from Cuba) and 43.8% in New York but only small percentages in California and Texas.
TB Incidence
Reported mean annual TB incidence between 2009 and 2013 was 5.9 per 100,000 persons in California, 3.8 in Florida, 4.6 in New York, and 4.7 in Texas. In comparison, the median (and 95% range) value across model simulations for the mean annual TB incidence over the same time period was 6.7 (5.3–8.0) in California, 3.9 (2.7–5.2) in Florida, 5.1 (3.9–6.5) in New York, and 4.7 (3.6–6.5) in Texas (Figure 3). Among U.S.-born individuals, reported annual TB incidence was relatively higher in Texas (2.7) compared with New York (1.3), California (1.8), and Florida (2.3). Corresponding model estimates were 2.4 (1.5–3.8) in Texas, compared with 1.9 (1.1–2.8) in New York, 1.8 (1–2.7) in California, and 2.2 (1.5–3.1) in Florida. Among foreign born individuals, reported TB incidence was lowest in Florida (9.5, vs. 15.8–16.9 in the other three states); this trend was also reflected in model estimates (11.1 in Florida; 95% range, 6.27–14.1; vs. 16.3–19.6 in the other three states). Comparisons of age-specific TB incidence among U.S.- and foreign-born persons between 2009 and 2013 are shown in Figure 3.
The models fit age- and nativity-specific reported TB incidence rates well from 2009 to 2013 (Figure 3, hatched vs. solid bars). This was also generally true for comparisons in 1993 to 1997 and 2001 to 2005 (Figure E2), with a few exceptions, such as underestimation of TB incidence among the foreign born in 1993 to 1997 in California (Figure E2, top left). Between 1993 and 2013 (Table 1), the model estimated an annual decline in TB incidence of 4.7% (95% range, 1.9–7.7%) in California (actual decline, 5.3%), 6.1% (2.5–9.9%) in Florida (actual decline, 6.3%), 7.0% (4.1–10.3%) in New York (actual decline, 7.8%) and 5.8% (2.3–9.3%) in Texas (actual decline, 5.2%). The models were generally more accurate in reflecting recent data (2009–2013) than older data (1993-1997 and 2001–2005) (Figure 4).
Table 1.
Annual Tuberculosis Incidence and Annual Percentage Declines, Comparing Reported Data with Model Simulations
| California | Florida | New York | Texas | |
|---|---|---|---|---|
| 1993, annual TB incidence per 100,000 persons | ||||
| Data | 16.4 | 11.8 | 21 | 13 |
| Model | 15.4 (9.9 to 20.4) | 11.9 (7.0 to 17.4) | 19.1 (13.7 to 27.1) | 13.4 (9.5 to 19.7) |
| 2013, annual TB incidence per 100,000 persons | ||||
| Data | 5.7 | 3.3 | 4.4 | 4.6 |
| Model | 6.0 (3.8 to 9.0) | 3.3 (1.9 to 5.7) | 4.8 (2.9 to 7.3) | 4.2 (2.5 to 6.8) |
| 1993–2013, annual decline in TB incidence, % | ||||
| Data | 5.3% | 6.3% | 7.8% | 5.2% |
| Model | 4.7% (1.9 to 7.7) | 6.1% (2.5 to 9.9) | 7.0% (4.1 to 10.3) | 5.8% (2.3 to 9.3) |
| 2025, annual TB incidence per 100,000 persons | ||||
| Model | 5.2 (2.9 to 7.1) | 2.5 (0.9 to 4.5) | 3.6 (1.8 to 5.5) | 2.9 (1.3 to 5.5) |
| 2015–2025, annual decline in TB incidence, % | ||||
| Model | 1.7% (−3.8* to 7.1) | 1.5% (−7.4* to 14.0) | 1.9% (−6.4* to 9.8) | 3.3% (−5.6* to 10.9) |
Definition of abbreviation: TB = tuberculosis.
Model estimates are based on the maximum likelihood estimate model and are presented as median (2.5 to 97.5 percentile) estimates.
Negative values indicate increases.
Figure 4.
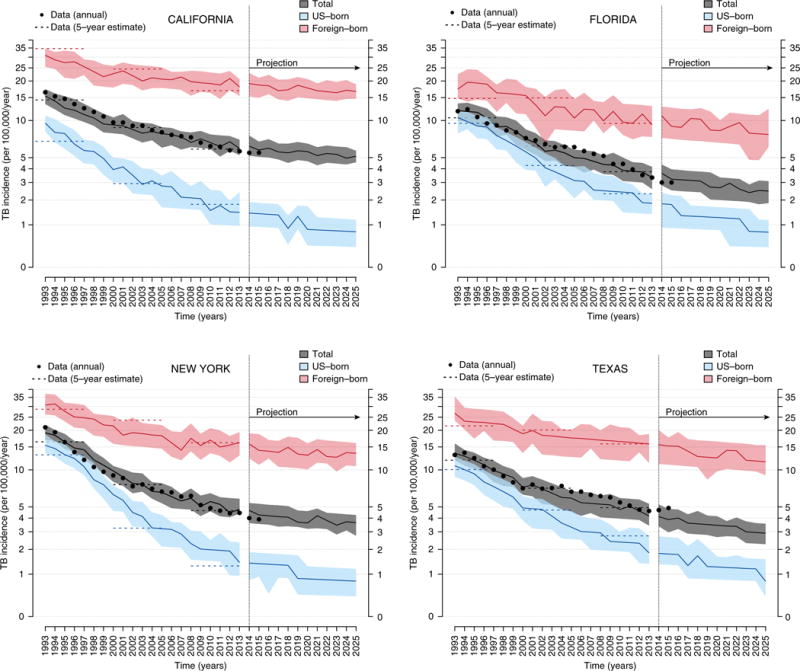
Model-based simulations and projections of trends in tuberculosis (TB) incidence in four states. Shown are model-based simulations in trends of TB incidence between 1993 and 2013 followed by projections up to 2025 for each of four states: California (top left), Florida (top right), New York (bottom left), and Texas (bottom right). The simulations are based on the state-specific maximum likelihood estimate (MLE) models, and projections are the continuation of model simulations (with continued decline in the transmission rates at the post-1993 estimate). For each panel, shown are medians (solid lines) and interquartile range (shaded area) of 100 replicate simulations of the MLE models. Shown in black dots are data for annual TB incidence between 1993 and 2015. Shown in dashed lines is the estimated mean annual TB incidence (based on data) in each state in three 5-year periods of 1993 to 1997, 2001 to 2005, and 2009 to 2013. The foreign-born population is represented in pink, U.S.-born population in light blue, and the total population in gray.
State-Level Differences in Natural History of TB
We used the model to infer state-level differences in the natural history of TB by estimating two major contributors to incidence, namely: (1) the annual decline in transmission rates, and (2) reactivation rates among adult individuals with remote LTBI. We estimated that TB transmission rates have been declining in all states but at higher rates in California (11%/yr; 95% CI, 5–13%) and New York (10%/yr; 95% CI, 9–15%) compared with Florida (6.8%/yr; 95% CI, 0–9%) and Texas (5.5%/yr; 95% CI, 3–13%) (Figure 5). These declines in transmission rates and the state-level differences are also reflected in the trends of estimated proportions of TB cases due to recent transmission (Figure E4): the percentage of TB cases due to recent transmission was estimated in 2013 to be between 10 and 20% in California and New York, compared with around 30% in Florida. In Texas, the decline in recent transmission was low (4.5%/yr), with a 95% CI that included zero (0–16%). In addition, we estimated the reactivation rates among adults with remote LTBI in California (0.046 per 100 person-years), Florida (0.037), and New York (0.036) to be lower than in Texas (0.058), although the CIs for all state-specific estimates overlapped (Figure 6).
Figure 5.
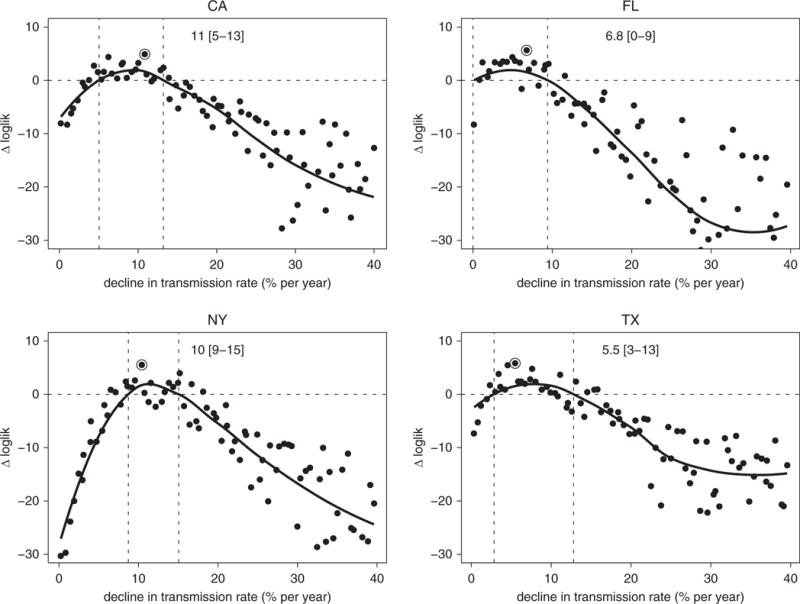
Estimated annual declines in tuberculosis transmission rates. Likelihood profiles (shown in a log scale as a difference with Δloglik = 0 representing the estimated 95% threshold) for the annual rates of decline in the transmission rate (in %/yr) in the four states: California (top left), Florida (top right), New York (bottom left), and Texas (bottom right). Each point on the profile represents the log-likelihood of each estimate (on the y-axis) maximized over all parameters with the decline in transmission held at the level on the x-axis. The point marked with an open circle shows the maximum likelihood estimate (MLE), and the two dashed vertical lines show the estimated 95% confidence interval (smoothed estimate of log-likelihood no lower than 1.92 less than the MLE). loglik = log-likelihood.
Figure 6.
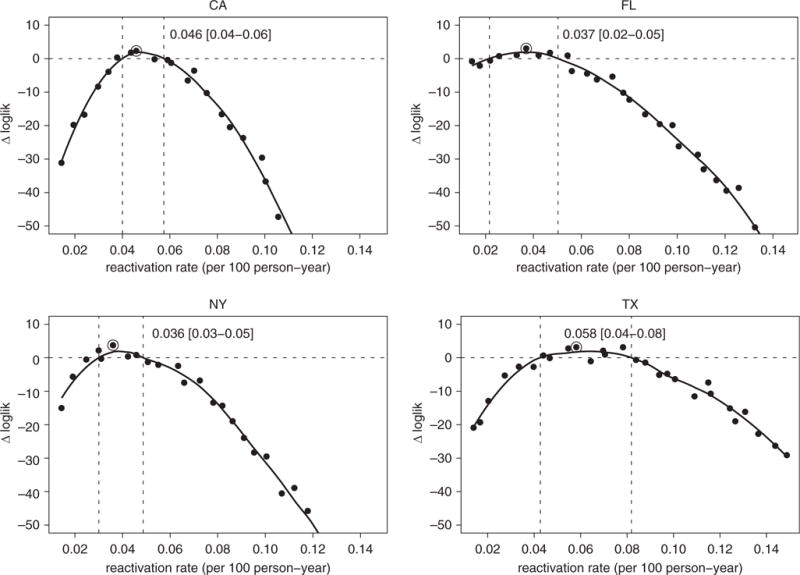
Estimated annual declines in tuberculosis reactivation rates. Likelihood profiles (shown in a log scale as a difference with Δloglik = 0 representing the estimated 95% threshold) for reactivation rate in the four states: California (top left), Florida (top right), New York (bottom left), and Texas (bottom right). The point marked with an open circle shows the maximum likelihood estimate, and the two dashed vertical lines show the estimated 95% confidence interval. loglik = log-likelihood.
Projections of Future TB Incidence
Between 2015 and 2025, the projected median annual rates of decline in overall TB incidence were 1.7% per year (95% range, 3.8% increase to 7.1%) in California, 1.5% (95% range, 7.4% increase to 14%) in Florida, 1.9% (95% range, 6.4% increase to 9.8%) in New York, and 3.3% (95% range, 5.6% increase to 10.9%) in Texas. The projected future rates of decline were substantially smaller than historical annual declines (reported above and in Table 1), although the confidence intervals did include sustained declines for all states. The rates of decline among U.S.-born persons were projected to be almost half those observed during the past 2 decades in California, Florida, and New York, whereas in Texas the projected decline at 6.5% per year was close to past levels. These projections were reasonably robust to uncertainty in parameter estimates (Figure E5).
Discussion
These individual-based models of TB transmission in the four states contributing more than half of U.S. incident TB highlight important differences in state-level TB dynamics. Specifically, differences in demography (especially of the foreign-born population) may contribute to much of the state-level heterogeneity in observed TB incidence. However, state-level differences in TB dynamics and natural history also play an important role, generating heterogeneities in the projected trajectories of TB incidence. Importantly, this model predicts a flattening of the decline in TB incidence in the coming decade in all four states unless additional resources or interventions are devoted to TB control. These findings illustrate the existence of state-level heterogeneity in TB dynamics and may help to frame plans to achieve TB elimination at the state level.
This modeling work illustrates that the natural history of TB may differ on the state level, perhaps reflecting the dynamics of migration and history of TB in each state. For example, we estimated that TB transmission was declining slowly, and reactivation remaining high, in Texas relative to other states. This may reflect that the majority of TB in Texas occurs in the Mexican-born population, which may spend relatively more ongoing time in their country of origin (i.e., with greater TB exposure) than persons from other countries who have traveled farther distances and subsequently do not visit those countries often. By contrast, the decline in TB transmission was most prominent in New York, which may reflect the large drop in TB incidence (particularly among U.S.-born individuals through concerted TB control efforts) between 1993 and 2013 after the surge in TB incidence associated with HIV in the late 1980s (16). Large declines in transmission rates over the last 2 decades, ironically, lead to lower projected declines in TB incidence in the future, as there is not much ongoing transmission to halt, and the vast majority of TB incidence begins to reflect reactivation of LTBI among persons (mostly foreign born) who were likely infected in their countries of origin.
Our estimates of recent TB transmission within U.S. borders (between about 15% of all TB cases in California to 30% in Florida and Texas; Figure E4) are in line with genotype-based estimates of the fraction of TB cases that reflect recent transmission (as opposed to reactivation), which are as low as 15% (17). Our inferred reactivation rates in individuals with remote LTBI (0.03–0.06 per 100 person-years; Figure 6) are in line with findings of persistent reactivation among long-term immigrants to the United States (18, 19) and similar to some published estimates of reactivation risk (20), although lower than others (6).
Although a number of influential modeling works have elucidated various important facets of TB epidemiology (21–24), only a few have focused on the role of immigration (4, 25–27). These latter studies have also highlighted some of the challenges associated with achieving persistent declines in TB incidence and reaching elimination goals in settings where a significant fraction of the TB cases are a result of reactivation of latent infection and imported into the population via immigration. Our model differs from these previous models in that we use an individual-based framework and evaluate state-level heterogeneities in TB transmission. Our model structure allows incorporation of novel details, including the demographic composition of a foreign-born population by age and region of origin, declines in reactivation rates from time of infection, and use of a likelihood-based approach to draw inference on the values of natural history parameters from a population perspective. In the same vein, this flexible structure also allows for incorporating targeted interventions (such as providing LTBI testing and treatment to key populations on the basis of age, nativity, or other risk factors) at the state level in the future. Finally, because our approach relies on applying a uniform model structure and model calibration protocols across the states (including data, which are available for all 50 states), this approach can be readily applied to other states to project TB incidence for subpopulations in their states as well as plan for and implement interventions to accelerate TB elimination. In the future, by incorporating more detailed data on TB in high-risk populations, state-level TB control efforts, and immigration patterns, this model can be used to evaluate key TB interventions—including enhanced contact investigation and targeted testing and treatment of high-risk populations—at the state level. Costs can also be incorporated to evaluate budget impact and cost effectiveness.
As with any modeling study, we made several simplifying assumptions. First, in the absence of data to inform quantitative estimates, we assumed no difference between foreign-born and U.S.-born individuals in terms of TB transmission rates, respiratory mixing patterns, natural history of TB (6), or treatment-seeking behavior. Importantly, sensitivity analyses (Figures E6–E8) suggest that most of our primary results are not drastically affected by heterogeneous mixing (e.g., closer mixing of the foreign born with each other than with the U.S. born). We assumed that immigrating foreign-born individuals are representative of the population of the region where they are born, in terms of LTBI prevalence. However, documented immigrants often have substantially lower TB risk than individuals in their home countries (18), whereas refugees and undocumented individuals may have higher prevalence of TB or LTBI. We also did not model secular trends in reactivation rates; although there may have been decreases in reactivation rates over longer time periods (20), these trends may be less important over the 30-year calibration and projection period in this model. Our model did not capture several factors that can drive population heterogeneity in the risk of TB, such as the prevalence of HIV, drug-resistant TB, homelessness, and incarceration. Although on a population level none of these factors individually is more than 10% prevalent among patients with TB nationwide, the combination of these factors may influence trends in TB incidence. The model also did not account for within-state geographic heterogeneity, which is difficult to uniformly incorporate using a common structure for all states. Finally, in making future projections, we did not account for state-specific accelerations in TB programmatic efforts to target, test, and treat populations at risk for TB or LTBI or for potential future demographic changes, such as an increase in the size of the foreign-born population or potential decline in TB prevalence globally (28). As such, these projections should be taken with caution, illustrating only the likely future trends in TB incidence if demographics and global TB burden remain relatively constant.
Our modeling was also limited by unavailability of data. State-level demographic data obtained from the American Community Survey were only available starting in 2005; hence, we were not able to capture historical trends in immigration with similar quality. Other data that may also improve future modeling efforts include state-level prevalence estimates of LTBI (currently available only at the national level [29]), LTBI prevalence among those born in key countries, state-level estimates of the proportion of incident TB reflecting recent transmission, and state-level data on TB control and care (e.g., LTBI testing and treatment and contact investigation).
In summary, this modeling work reveals important differences in the population-level dynamics of TB at the state level. These differences reflect both demographic factors (particularly the historical size and makeup of the foreign-born population) and potential differences in the natural history of TB, as reflected in the rates of transmission and reactivation. Our findings illustrate that the mechanisms underlying TB epidemiology, as well as the future trajectory of TB in the absence of additional intervention, may differ substantially from one state to the next. Ultimately, if TB is to be eliminated in the United States, a one-size-fits-all approach is unlikely to be effective.
Supplementary Material
At a Glance Commentary.
Scientific Knowledge on the Subject
Model-based understanding of tuberculosis (TB) epidemiological dynamics is limited; previous works have not accounted for (1) trends in mechanistic drivers (e.g., immigration, and reactivation rates), and (2) geographical heterogeneity in potential drivers of TB, such as demographic makeup and historical TB dynamics. Better understanding the heterogeneity (along with the drivers) can inform effective state-level responses to TB.
What This Study Adds to the Field
We constructed an individual-based model of TB in the four U.S. states that bear half of the country’s TB burden—California, Florida, New York, and Texas—and calibrated the model to state-specific demographic, and age- and nativity-stratified TB incidence data. We used the model to infer differences in natural history of TB and in future projections of TB. We find that there is substantial state-level heterogeneity in TB in the United States, which reflect both demographic factors and potential differences in the natural history of TB. These heterogeneities can affect projections of TB incidence and the epidemiological impact of TB interventions.
Acknowledgments
The authors thank Dr. Jennifer Flood and Dr. Pennan Barry of the California Department of Public Health, TB Control Branch, and Dr. Margaret Oxtoby of the New York Department of Health for their input.
Supported by the U.S. Centers for Disease Control and Prevention, National Center for HIV, Viral Hepatitis, STD, and TB Prevention Epidemiologic and Economic Modeling Agreement #5U38PS004646. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
ORCID ID: 0000-0002-6106-6834 (S.S.).
Author Contributions: Conceived and designed the study: S.S. and D.W.D.; developed and coded the model and conducted model simulations and analyses: S.S.; wrote the first draft: S.S. All authors contributed to the interpretation of the results, revised the manuscript for content, and approved the final version.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Centers for Disease Control and Prevention. Reported tuberculosis in the United States, 2015. Atlanta, GA: U.S. Department of Health and Human Services, CDC; 2016. [Google Scholar]
- 2.Salinas JL, Mindra G, Haddad MB, Pratt R, Price SF, Langer AJ. Leveling of tuberculosis incidence - United States, 2013-2015. MMWR Morb Mortal Wkly Rep. 2016;65:273–278. doi: 10.15585/mmwr.mm6511a2. [DOI] [PubMed] [Google Scholar]
- 3.Pew Research Center. Statistical portrait of the foreign-born population in the United States. 2016 Apr 19; [accessed 2017 Aug 2]. Available from: http://www.pewhispanic.org/2016/04/19/statistical-portrait-of-the-foreign-born-population-in-the-united-states-trends/
- 4.Hill AN, Becerra J, Castro KG. Modelling tuberculosis trends in the USA. Epidemiol Infect. 2012;140:1862–1872. doi: 10.1017/S095026881100286X. [DOI] [PubMed] [Google Scholar]
- 5.Moonan PK, Ghosh S, Oeltmann JE, Kammerer JS, Cowan LS, Navin TR. Using genotyping and geospatial scanning to estimate recent Mycobaterium tuberculosis transmission, United States. Emerg Infect Dis. 2012;18:458–465. doi: 10.3201/eid1803.111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shea KM, Kammerer JS, Winston CA, Navin TR, Horsburgh CR., Jr Estimated rate of reactivation of latent tuberculosis infection in the United States, overall and by population subgroup. Am J Epidemiol. 2014;179:216–225. doi: 10.1093/aje/kwt246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.United States Census Bureau. American community survey, 2005: |American community survey 5-year estimate. [accessed 2015 Dec 25]. Available from: https://factfinder.census.gov/faces/nav/jsf/pages/searchresults.xhtml.
- 8.United States Census Bureau. American community survey, 2014: American community survey 5-year estimate. [accessed 2015 Dec 25]. Available from: https://factfinder.census.gov/faces/nav/jsf/pages/searchresults.xhtml.
- 9.Centers for Disease Control and Prevention. Online Tuberculosis Information System (OTIS), National Tuberculosis Surveillance System, United States, 1993–2013. Division of TB Elimination, CDC WONDER online database; 2015. Apr, [accessed 2017 Aug 2]. Available from: http://wonder.cdc.gov/tb-v2013.html. [Google Scholar]
- 10.Siler W. A competing-risk model for animal mortality. Ecology. 1979;60:750–757. [Google Scholar]
- 11.Vynnycky E, Fine PE. The natural history of tuberculosis: the implications of age-dependent risks of disease and the role of reinfection. Epidemiol Infect. 1997;119:183–201. doi: 10.1017/s0950268897007917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blower SM, Dowlatabadi H. Sensitivity and uncertainty analysis of complex models of disease transmission: an HIV model, as an example. Int Stat Rev. 1994;62:229–243. [Google Scholar]
- 13.Sanchez MA, Blower SM. Uncertainty and sensitivity analysis of the basic reproductive rate. Tuberculosis as an example. Am J Epidemiol. 1997;145:1127–1137. doi: 10.1093/oxfordjournals.aje.a009076. [DOI] [PubMed] [Google Scholar]
- 14.Kendall M, Stuart S. Inference and relationship. 4th. Vol. 2. London: Griffin; 1979. The advanced theory of statistics. [Google Scholar]
- 15.World Health Organization. Global tuberculosis report 2015. [accessed 2017 Aug 2]. Available from: http://apps.who.int/iris/bitstream/10665/191102/1/9789241565059_eng.pdf.
- 16.Frieden TR, Fujiwara PI, Washko RM, Hamburg MA. Tuberculosis in New York City: turning the tide. N Engl J Med. 1995;333:229–233. doi: 10.1056/NEJM199507273330406. [DOI] [PubMed] [Google Scholar]
- 17.France AM, Grant J, Kammerer JS, Navin TR. A field-validated approach using surveillance and genotyping data to estimate tuberculosis attributable to recent transmission in the United States. Am J Epidemiol. 2015;182:799–807. doi: 10.1093/aje/kwv121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walter ND, Painter J, Parker M, Lowenthal P, Flood J, Fu Y, Asis R, Reves R, Tuberculosis Epidemiologic Studies Consortium Persistent latent tuberculosis reactivation risk in United States immigrants. Am J Respir Crit Care Med. 2014;189:88–95. doi: 10.1164/rccm.201308-1480OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cain KP, Benoit SR, Winston CA, Mac Kenzie WR. Tuberculosis among foreign-born persons in the United States. JAMA. 2008;300:405–412. doi: 10.1001/jama.300.4.405. [DOI] [PubMed] [Google Scholar]
- 20.Horsburgh CR, Jr, O’Donnell M, Chamblee S, Moreland JL, Johnson J, Marsh BJ, Narita M, Johnson LS, von Reyn CF. Revisiting rates of reactivation tuberculosis: a population-based approach. Am J Respir Crit Care Med. 2010;182:420–425. doi: 10.1164/rccm.200909-1355OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blower SM, McLean AR, Porco TC, Small PM, Hopewell PC, Sanchez MA, Moss AR. The intrinsic transmission dynamics of tuberculosis epidemics. Nat Med. 1995;1:815–821. doi: 10.1038/nm0895-815. [DOI] [PubMed] [Google Scholar]
- 22.Dye C, Garnett GP, Sleeman K, Williams BG. Prospects for worldwide tuberculosis control under the WHO DOTS strategy: directly observed short-course therapy. Lancet. 1998;352:1886–1891. doi: 10.1016/s0140-6736(98)03199-7. [DOI] [PubMed] [Google Scholar]
- 23.Abu-Raddad LJ, Sabatelli L, Achterberg JT, Sugimoto JD, Longini IM, Jr, Dye C, Halloran ME. Epidemiological benefits of more-effective tuberculosis vaccines, drugs, and diagnostics. Proc Natl Acad Sci USA. 2009;106:13980–13985. doi: 10.1073/pnas.0901720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vynnycky E, Fine PEM. Interpreting the decline in tuberculosis: the role of secular trends in effective contact. Int J Epidemiol. 1999;28:327–334. doi: 10.1093/ije/28.2.327. [DOI] [PubMed] [Google Scholar]
- 25.Wolleswinkel-van B, Nagelkerke NJD, Broekmans JF, Borgdorff MW. The impact of immigration on the elimination of tuberculosis in The Netherlands: a model based approach. Int J Tuberc Lung Dis. 2002;6:130–136. [PubMed] [Google Scholar]
- 26.Jia Z-W, Tang G-Y, Jin Z, Dye C, Vlas SJ, Li X-W, Feng D, Fang LQ, Zhao WJ, Cao WC. Modeling the impact of immigration on the epidemiology of tuberculosis. Theor Popul Biol. 2008;73:437–448. doi: 10.1016/j.tpb.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Y, Khan K, Feng Z, Wu J. Projection of tuberculosis incidence with increasing immigration trends. J Theor Biol. 2008;254:215–228. doi: 10.1016/j.jtbi.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 28.Baker BJ, Winston CA, Liu Y, France AM, Cain KP. Abrupt decline in tuberculosis among foreign-born persons in the United States. Plos One. 2016;11:e0147353. doi: 10.1371/journal.pone.0147353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bennett DE, Courval JM, Onorato I, Agerton T, Gibson JD, Lambert L, McQuillan GM, Lewis B, Navin TR, Castro KG. Prevalence of tuberculosis infection in the United States population: the national health and nutrition examination survey, 1999-2000. Am J Respir Crit Care Med. 2008;177:348–355. doi: 10.1164/rccm.200701-057OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


