Abstract
Accumulation of foam cells — macrophages with intracellular lipid droplets — in arterial walls is a hallmark of atherosclerosis. Bernelot Moens and colleagues report increases in circulating monocytes with intracellular lipid accumulation, associated CCR2 expression, and enhanced monocyte migration in patients with familial hypercholesterolaemia. These changes could be reversed by PCSK9-inhibitor treatment.
Hyperlipidaemia is a major risk factor for atherosclerotic cardiovascular disease (ASCVD). Patients with familial hypercholesterolaemia (FH) usually develop ASCVD at an early age and accumulate macrophages with intracellular lipid droplets (foam cells) in arteries, and also tendons, from childhood. The traditional paradigm for atherogenesis has focused on macrophage uptake of modified LDL and foam cell formation in arterial walls. Moreover, interactions between chemokines such as C-C motif chemokine 2 (CCL2, also known as MCP1) and chemokine receptors such as C-C chemokine receptor type 2 (CCR2; the receptor for CCL2) expressed on monocytes have important roles in atherogenesis mainly by mediating monocyte infiltration from the circulation into arterial walls1, where monocytes differentiate into macrophages, which take up modified LDL and then become lipid-laden foam cells.
In an article published in European Heart Journal, Bernelot Moens et al. report that, compared with healthy control individuals, patients with FH have higher CCR2 levels on circulating monocytes2. In these patients with FH, monocyte CCR2 levels were significantly correlated with plasma LDL-cholesterol levels and were positively associated with monocyte intracellular lipid accumulation. Importantly, these changes in monocyte phenotype correlated with enhanced monocyte migratory capacity ex vivo. Intriguingly, treatment with PCSK9 monoclonal antibodies (mAbs) in the patients with FH lowered plasma LDL-cholesterol levels by ~50% and reduced intracellular lipid accumulation and CCR2 levels in circulating monocytes, with reversal of the enhanced migratory capacity of monocytes. Given the role of monocytes, chemokines, and chemokine receptors in the development of atherosclerosis1, these findings are important in helping us to understand further the mechanisms for the early development of ASCVD in patients with FH.
PCSK9 mAb inhibitors are a new class of drugs that lower plasma LDL-cholesterol levels. Large clinical trials have shown that PCSK9 inhibitors combined with background maximally-tolerated statin therapy reduce the risk of cardiovascular events, particularly in patients who continue to have elevated LDL-cholesterol levels at baseline3,4. Although PCSK9 inhibitors do not reduce levels of C-reactive protein, the shift to a less atherogenic monocyte phenotype with PCSK9 mAb treatment reported by Bernelot Moens et al. provides evidence in humans that these therapies might alter the ‘inflammatory response’ in a beneficial way, consistent with evidence for the beneficial roles of this class of drugs in the treatment of ASCVD3,4.
Also important were the observations made by Bernelot Moens et al. that the number of circulating monocytes with intracellular lipid accumulation and monocyte migratory capacity was increased in patients with FH, and that intracellular lipid content was positively associated with monocyte levels of CCR2 in these patients2. In another publication, the same research group reported that monocytes from patients with familial dysbetalipoproteinaemia (FD) also had increased intracellular lipid accumulation and higher expression of surface β2-integrins, including integrin-αM (CD11b), integrin-αX (CD11c), and integrin-β2 (CD18)5. These observations support an important role of intracellular lipid accumulation in monocyte phenotypic changes in patients with FH or FD, and are consistent with previous reports from our laboratory and others showing emergence of monocytes with intracellular lipid droplets, which we termed ‘foamy monocytes’, and monocyte phenotypic changes including increased levels of integrin-αX in the circulation of humans and animals with hyperlipidaemia6–9. Foamy monocytes emerge early in the circulation of mice with hypercholesterolaemia6. In humans, even a single high-fat meal can increase lipid accumulation in circulating monocytes8,9. Importantly, foamy monocytes infiltrate into arterial walls and contribute to the development of atherosclerosis in mice with hypercholesterolaemia6. The observations made by Bernelot Moens et al. of circulating monocytes with intracellular lipid accumulation (‘foamy monocytes’), increased monocyte expression of chemokine receptors and adhesion molecules, and enhanced monocyte migration in patients with FH2 or FD5 also support an important role of these foamy monocytes in the development of ASCVD in humans with hyperlipidaemia — severe hyperlipidaemia in particular. Furthermore, the findings of this study suggest that monocyte lipid accumulation in patients with FH did not rely on the LDL receptor and could be reversed by PCSK9-inhibitor treatment2. Indeed, our previous report showed that foamy monocytes emerge in the circulation of mice deficient in the LDL receptor6, and that scavenger receptors such as platelet glycoprotein 4 (CD36), which was upregulated in monocytes from patients with FH2 and in mice with hyperlipidaemia6, might have important roles in monocyte uptake of cholesteryl-ester-rich lipoproteins and foamy monocyte formation in the circulation6.
Monocytes are heterogeneous cell populations and are commonly classified in humans into three subsets (classical, intermediate, and nonclassical). Most previous studies indicated that levels of intermediate and/or nonclassical monocytes were increased in hyperlipidaemia and associated with ASCVD. Bernelot Moens et al. reported that the above phenotypic changes occurred mainly in classical monocytes, and that the ratios of each monocyte subset did not change in patients with FH or FD compared with healthy controls2,5. By contrast, Christensen et al. showed that, compared with healthy controls, children with FH, particularly those with low HDL-cholesterol levels, had higher circulating intermediate and nonclassical monocytes and lower levels of classical monocytes10. From the articles by Bernelot Moens et al., we note that the researchers followed the common gating strategy for flow cytometric analyses of monocytes (with low side scatter pattern) in patients with FH or FD2,5. However, from our previous studies, mainly in mouse models of hypercholesterolaemia, we realized that the flow cytometry gating strategy had to be revised in order to examine foamy monocytes, particularly monocytes with abundant lipid droplets, which displayed dramatic elevations in granularity with high side scatter pattern and, therefore, appeared in granulocyte regions6,7. Moreover, foamy monocytes had different phenotypes from nonfoamy monocytes in the same animals6,7. Therefore, it would be ideal to examine whether foamy monocytes that shifted their patterns to granulocyte regions were also present in the samples from these patients with FH or FD, and if so, to identify what the phenotypes and functions of these monocytes with elevated side scatter pattern are in these patients.
In summary, in addition to the current paradigm for atherogenesis with a focus on macrophage uptake of modified LDL and foam cell formation in arterial walls, reports2,5–9 from Bernelot Moens et al., our group, and others support a novel model of atherogenesis, in which hyperlipidaemia — severe hyperlipidaemia in particular — causes circulating monocytes to take up lipoprotein particles, leading to increased intracellular lipid accumulation and foamy monocyte formation in the circulation. Intracellular lipid accumulation in circulating monocytes might induce monocyte phenotypic changes that enhance monocyte migration from the circulation into arterial walls, accelerate monocyte differentiation into foamy macrophages in arterial walls, and, therefore, contribute to the development of atherosclerosis. Treatment with lipid-lowering drugs such as PCSK9 inhibitors reduces monocyte levels of intracellular lipids and improves monocyte phenotypes with decreased monocyte migration and might thereby prevent progression and enhance regression of atherosclerosis (FIG. 1). Further studies are needed to determine, particularly in patients with hyperlipidaemia, whether foamy monocytes have unique phenotypes compared with monocytes with little or no lipid accumulation from the same individual, whether foamy monocytes can help to identify individuals at high risk of cardiovascular disease who might benefit from lipid-lowering therapy, and whether therapies that target foamy monocytes and ‘improve’ monocyte phenotypes are associated with clinical ASCVD benefits.
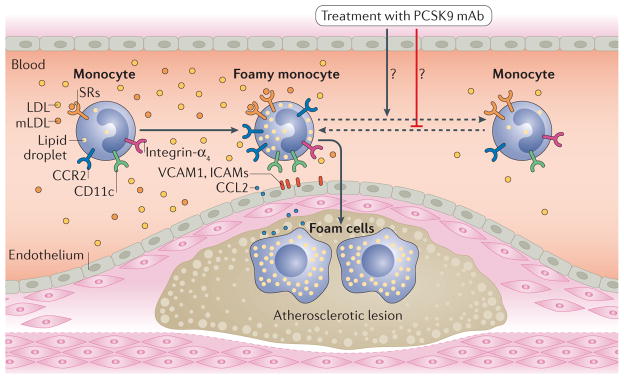
Figure 1. Monocyte phenotypes in patients with familial hypercholesterolaemia (FH) and effects of PCSK9 inhibitors.
In patients with FH, hepatic clearance of LDLs is impaired, LDL levels are elevated, and LDLs can be modified in blood. Modified LDLs (mLDLs) are then taken up by circulating monocytes, possibly via scavenger receptors (SRs), such as platelet glycoprotein 4 (CD36) and macrophage scavenger receptor type 1, leading to lipid accumulation within monocytes and formation of foamy monocytes in the circulation. Lipid accumulation in monocytes can change the monocyte phenotype leading to upregulation of C-C chemokine receptor type 2 (CCR2) and SRs, and also of adhesion molecules such as integrin-αX (CD11c). In patients with FH, foamy monocytes with upregulation of CCR2 and adhesion molecules show enhanced migration capacity and might, therefore, accelerate development of atherosclerosis by infiltrating into arterial walls and becoming foam cells. Treatment with PCSK9 monoclonal antibodies (mAbs) in patients with FH significantly lowers LDL levels and also reduces monocyte levels of intracellular lipids and CCR2, with reductions in monocyte migration capacity, and might, therefore, prevent progression and enhance regression of atherosclerosis. CCL2, C-C motif chemokine 2; ICAM, intercellular adhesion molecule; VCAM1, vascular cell adhesion protein 1.
Acknowledgments
The authors are supported by NIH grant R01 HL098839, AHA award AHA16GRNT30410012, and American Diabetes Association award 1-17-IBS-082. We thank Zeqin Lian (Baylor College of Medicine, Houston, Texas, USA) for helpful input, and Kerrie Jara (Baylor College of Medicine, Houston, Texas, USA) for editorial assistance.
Footnotes
Competing interests statement
C.M.B. has received grant/research support from and is a consultant for Amgen, Regeneron, and Sanofi-Synthelabo. H.W. declares no competing interests.
References
- 1.Rahman MS, Murphy AJ, Woollard KJ. Effects of dyslipidaemia on monocyte production and function in cardiovascular disease. Nat Rev Cardiol. 2017 doi: 10.1038/nrcardio.2017.34. http://dx.doi.org/10.1038/nrcardio.2017.34. [DOI] [PubMed]
- 2.Bernelot Moens SJ, et al. PCSK9 monoclonal antibodies reverse the pro-inflammatory profile of monocytes in familial hypercholesterolaemia. Eur Heart J. 2017 doi: 10.1093/eurheartj/ehx002. http://dx.doi.org/10.1093/eurheartj/ehx002. [DOI] [PubMed]
- 3.Sabatine MS, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017 doi: 10.1056/NEJMoa1615664. http://dx.doi.org/10.1056/NEJMoa1615664. [DOI] [PubMed]
- 4.Ridker PM, et al. Cardiovascular efficacy and safety of bococizumab in high-risk patients. N Engl J Med. 2017;376:1527–1539. doi: 10.1056/NEJMoa1701488. [DOI] [PubMed] [Google Scholar]
- 5.Bernelot Moens SJ, et al. Remnant cholesterol elicits arterial wall inflammation and a multilevel cellular immune response in humans. Arterioscler Thromb Vasc Biol. 2017 doi: 10.1161/ATVBAHA.116.308834. https://doi.org/10.1161/ATVBAHA.116.308834. [DOI] [PubMed]
- 6.Xu L, et al. Foamy monocytes form early and contribute to nascent atherosclerosis in mice with hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2015;35:1787–1797. doi: 10.1161/ATVBAHA.115.305609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu H, et al. Functional role of CD11c+ monocytes in atherogenesis associated with hypercholesterolemia. Circulation. 2009;119:2708–2717. doi: 10.1161/CIRCULATIONAHA.108.823740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan IM, et al. Postprandial monocyte activation in individuals with metabolic syndrome. J Clin Endocrinol Metab. 2016;101:4195–4204. doi: 10.1210/jc.2016-2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varela LM, et al. A high-fat meal promotes lipid-load and apolipoprotein B-48 receptor transcriptional activity in circulating monocytes. Am J Clin Nutr. 2011;93:918–925. doi: 10.3945/ajcn.110.007765. [DOI] [PubMed] [Google Scholar]
- 10.Christensen JJ, et al. Altered leukocyte distribution under hypercholesterolemia: a cross-sectional study in children with familial hypercholesterolemia. Atherosclerosis. 2017;256:67–74. doi: 10.1016/j.atherosclerosis.2016.11.031. [DOI] [PubMed] [Google Scholar]