Abstract
We report herein that the preparation of two novel discrete organoplatinum(II) metallacycles by means of coordination-driven self-assembly of a 90° organoplatinum(II) acceptor, cis-(PEt3)2Pt(OTf)2, with two donors, a pyridyl donor, 9,10-di(4-pyridylvinyl)anthracene (DPA), and one of two dicarboxylate ligands. The obtained two metallacycles display aggregation-induced emission (AIE) as well as solvatochromism. More interestingly, both metallacycles exhibit near-infrared fluorescent emission in the solid state, greatly different from the free ligand DPA. Furthermore, ammonia detection has been achieved by the observation of simple optical changes of the metallacycles’ thin films either in color or in fluorescence, thereby representing a promising platform for convenient biochemical analysis.
Graphical abstract
The continued development of luminescent materials is of great importance to both biology and materials science which demand constantly updated molecular architectures, synthetic methodologies and applied researches.1 Among these materials, near-infrared (NIR)-emitting species have attracted increasingly attention in optical imaging studies.2 It is because that light in NIR region (650–900 nm)3h,i does less harm to living cells, causes less absorption and scattering interference, and has stronger penetration into deep tissues with lowest autofluorescene background. Up to now, lots of near-infrared fluorescent dyes, for example, perylenediimide (PDI),3a cyanine,3b,c and BODIPY3d–f series of compounds, have been reported.3 However, many of them are often subjected to laborious covalent synthetic procedures and usually suffer from aggregation-caused quenching (ACQ) in concentrated solutions or in the solid state. Although aggregation-induced emission (AIE) molecules with NIR emission have attracted increasing attention, only a few works have been reported,4 wherein the corresponding AIE luminogens are often complicated and tedious chemical syntheses are needed.
It is well known that coordination-driven self-assembly via spontaneous formation of metal−ligand bonds has been seen as a powerful tool in constructing supramolecular coordination complexes (SCCs) with predictable and well-defined molecular structures.5 In particular, as developed by the Stang’ group, by making use of phosphine-capped Pt(II) as the square-planar metal acceptor and different kinds of organic ligands as donors, a series of 1D, 2D and 3D topological architectures have been built in nearly quantitative yields.5h By decorating the periphery and core of the precursors with various functional groups, discrete SCCs can be given some novel characters, such as fluorescence, amphiphilic behaviors, thermo-responsiveness, and so on.6 Recently, we locked tetraphenylethylene (TPE) cores into the discrete SCCs and obtained novel highly emissive metallacages both in dilute solutions and aggregated state, which are different from both ACQ and AIE phenomena.6a Although the above systems exhibited interesting photophysical properties in solutions, their performances in the solid state are underdeveloped. With the aim to integrate these two fascinating aspects into one material, we are interested in exploring metal-coordination chemistry to design and synthesize novel SCCs with special light-emitting properties in both states and attractive applications in terms of fluorescent detection.
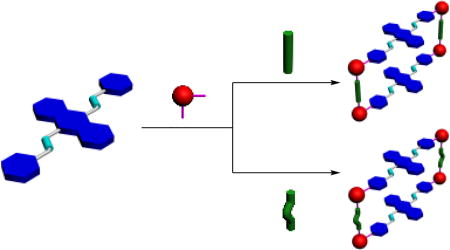
9,10-distyrylanthracene (DSA) derivatives, as a class of luminogens with AIE characteristics, have been widely used in bioimaging, cancer therapy, fluorescent probe, sensors, and so on.7 By replacing phenyl with pyridyl group in DSA, we gained organic pyridyl donor 3, 9,10-di(4-pyridylvinyl)anthracene (DPA), which is a well-designed and AIE-active ligand with suitable angle (180°) to coordinate with organoplatinum(II) acceptor.8 Herein, we report the construction of two rectangular metallacycles, 1 and 2, by means of coordination-driven self-assembly of 90° organoplatinum(II) acceptor 6 with two organic donors, pyridyl ligand 3 and dicarboxylate ligands 4 and 5, respectively. Both metallacycles emit fluorescence not only in dilute solutions but also in the aggregated state. Interestingly, near-infrared fluorescent emission behaviours were observed in their solid state. Moreover, Thin films made of 1 and 2 were demonstrated to be convenient test kits for detecting ammonia gas.
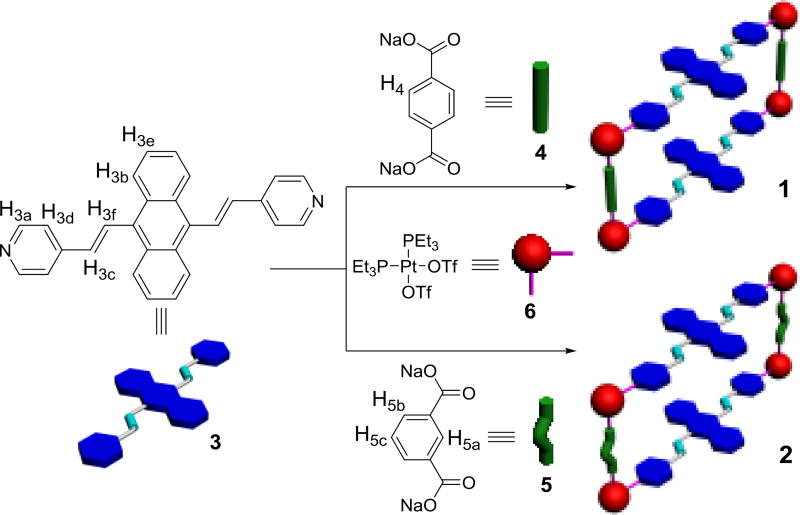
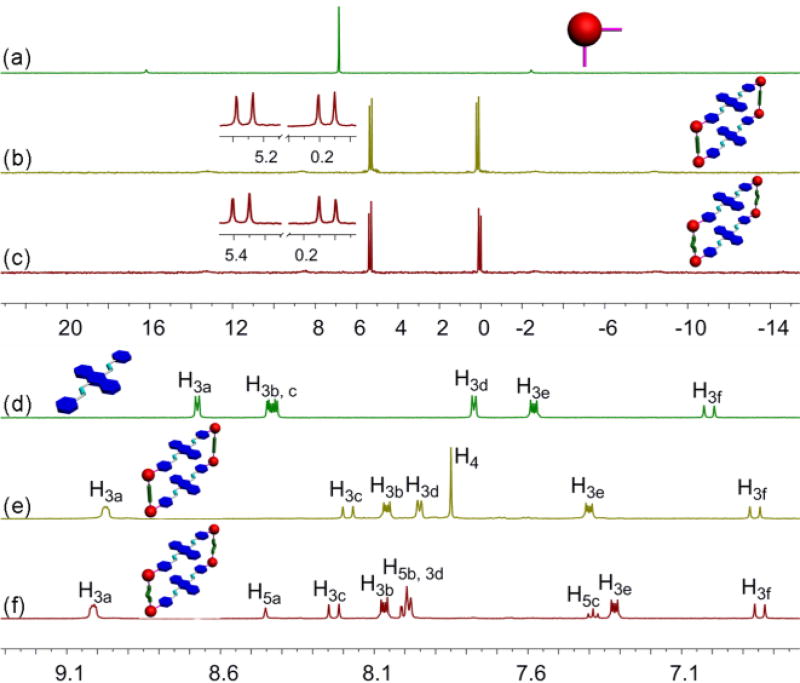
DPA ligand 3 was synthesized according to a reported method.9b As shown in Scheme 1, metallacycle 1 was prepared in one pot by the 2:2:4 stoichiometric combination of DPA 3, para-dicarboxylate ligand 4 and cis-(PEt3)2Pt(OTf)2 6 (OTf, OSO2CF3) in D2O/acetone-d6 (v/v = 8:1) at 50 °C for 12 hours and then acetone-d6 at room temperature for 8 hours. Similarly, by changing 4 to meta-dicarboxylate donor 5, we obtained analogous rectangle 2. The 31P{1H} and 1H NMR investigation of the resulting mixture indicated the formation of sole, discrete and highly symmetrical compounds. The 31P{1H} spectra of both 1 and 2 showed two coupled doublets of approximately equal intensity with concomitant 195Pt satellites due to the two distinct phosphorous environments, indicating the Pt(II) centres had a heteroligated N,O-coordination motif with one pyridyl and one carboxylate moiety per metal centre10 (Figure 1b,c). On the other hand, in the 1H NMR spectra of 1 and 2, signals corresponding to protons H3a and H3d on the pyridyl groups shifted downfield with respect to those of free ligand 3, in good agreement with the coordination of N-atoms to platinum centres (Figure 2e,f). Moreover, the formation of 1 and 2 was further proved by electrospray ionization time of flight mass spectrometry (ESI-TOF-MS). Peak at m/z = 990.29 for metallacycle 1 corresponding to the fragment of [M − 3OTf]3+ was found to support the intact and discrete [2+2+4] assembly (Figure S3). An almost same peak was observed to support the formation of the isomeric 2 (Figure S6). Both peaks were isotopically resolved and matched well with their calculated theoretical distributions.
Scheme 1.
Cartoon Representation of the Formation of metallacycles 1 and 2.
Figure 1.
Partial 31P{1H} (a–c) and 1H NMR (d–f) spectra (acetone-d6, 293 K) of building blocks 3 (d) and 6 (a) and of metallacycles 1 (b, e) and 2 (c, f).
Figure 2.
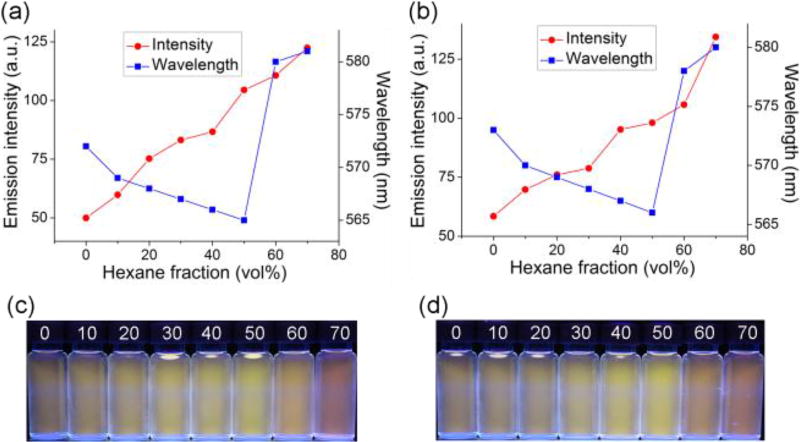
Dependence of maximum emission intensity and wavelength of metallacycles 1 (a) and 2 (b) on the composition of hexane in acetone/hexane mixtures (λex = 425 nm, c = 10.0 µM). The corresponding images of 1 (c) and 2 (d) solutions under UV lamp.
As AIE-active DPA 3 was locked into the rigid, discrete metallacycles successfully, we next investigated if the self-assembled SCCs were still AIE-active or not. Therefore, fluorescence spectra of both metallacycles in acetone with varying contents of hexane were recorded. When the hexane fraction reached more than 70%, compounds 1 and 2 started to precipitate from the mixed solvents. So, we chose the hexane fractions from 0 to 70% of acetone/hexane solutions of 1 or 2 to study the light-emitting properties. In pure acetone, 1 showed a pale orange fluorescence and its emission spectrum was centred at 576 nm. When the hexane content increased gradually from 0 to 50%, its emission maximum showed a little blue shift about 6 nm (Figure 2a). As the fraction of hexane continued to increase to 70%, the emission maximum of 1 experienced a red shift (16 nm) (Figure 2a), accompanying the emission colour change from yellow to orange red (Figure 2c). Meanwhile, during the process of hexane addition, the emission intensity of 1 gradually increased (Figure 2a), so did the corresponding quantum yields (from 6.7% to 19.4%) (Figure S9a). Furthermore, dynamic light scattering (DLS) was employed to study the aggregate formation of 1 in mixed acetone/hexane solvents with different hexane contents. As shown in Figure S10a, with the hexane fraction increasing from 10% to 30% to 40%, the obtained DLS results were 12 nm, 103 nm and 230 nm, respectively, indicating a process of gradual aggregation. Thus, metallacycle 1 was still AIE-active just like the free ligand 3. Likewise, rectangle 2 in acetone/hexane solutions exhibited similar AIE-active performances, such as the augment of fluorescent intensities (Figure 2b) and ΦF values (Figure S9b) along with gradual addition of hexanes.
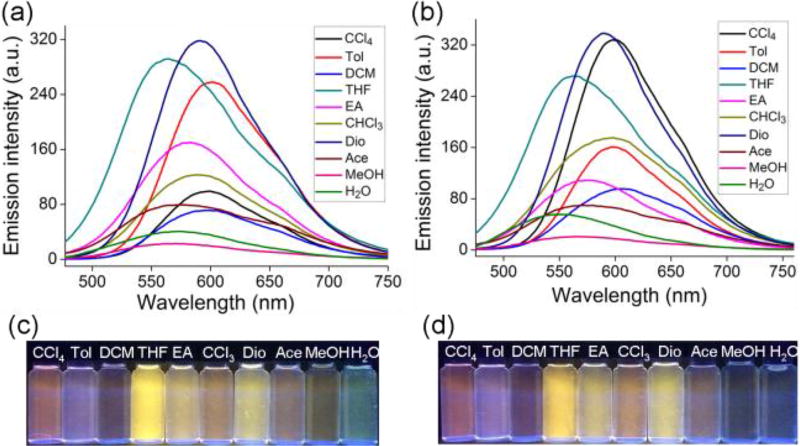
With these two AIE assemblies in hand, we then investigated their solvatochromism properties in different solvents. Due to the difference of the solvents polarities, the UV–vis absorption spectra of the two metallacycles underwent distinct changes, such as the intensities of the absorption bands and related wavelengths (Figure S11). Furthermore, the light-emitting properties of both metallacycles in different solvents were recorded by the emission spectroscopy. Upon increasing the solvent polarity, irregular and colourful changes happened. Comparatively, the emission intensity of 1 in certain solvents, such as toluene, THF and dioxane, was high, while in some polar solvents, like ethyl acetate (EA), methanol and water, its intensity descended (Figure 3a). Assembly 2 displayed similar optical performances except that the emission intensity of 2 in CCl4 solution was higher than that of 1 while lower in EA solution (Figure 3b), probably originating from the slight difference of the molecular structures between 1 and 2 (Figure S16). Under UV irradiation at 365 nm, both rectangles showed long-wavelength emission with varied colours in different solvents (Figure 3c,d). We supposed that the polarity and ability to dissolve 1 or 2 of different solvents may had a competitive relationship when these two factors had an impact on their optical properties at the same time.
Figure 3.
Fluorescence spectra of metallacycles 1 (a) and 2 (b) in different solvents (λex = 425 nm, c = 10.0 µM). Photographs of 1 (c) and 2 (d) under UV illumination in different solvents. Tol, toluene; DCM, dichloromethane; THF, tetrahydrofuran; EA, ethyl acetate; Dio, dioxane; Ace, acetone.
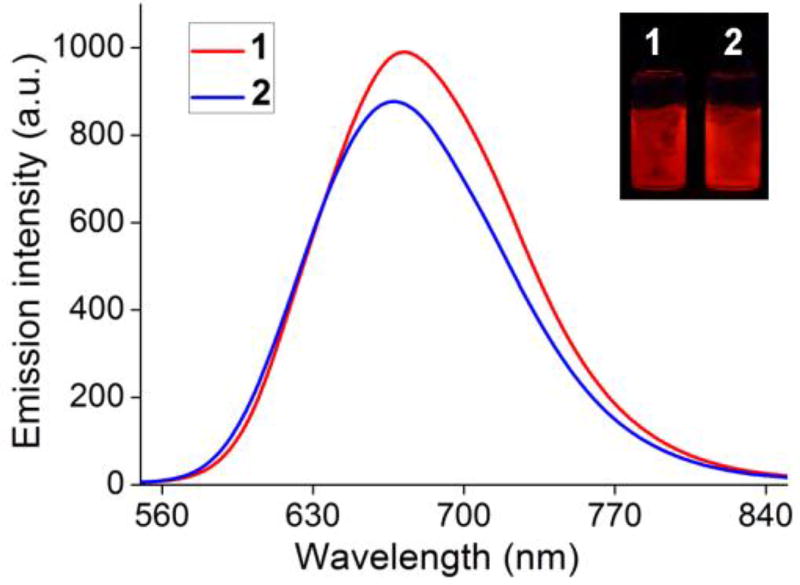
The interesting photophysics of 1 and 2 in solutions made us curious about their optical performance in the solid state or as films. After dried completely, products 1 and 2 became red solids, quite differing from the free ligand 3 which emitted a yellow light. Under a UV lamp at 365 nm, the building block 3 displayed green emission (Figure S15), while metallacycles 1 and 2 showed a bright red fluorescence (inset of Figure 4). For solid-state measurements, thin films of 1 and 2 on sheet glass were made. As shown in the emission spectra (Figure 4), both emitted in the near-infrared region with emission centres at 672 nm for 1 and 667 nm for 2. Given the observations above, we proposed a facile coordination-driven self-assembly strategy to design and construct NIR-emitting assemblies which provide an excellent complement to traditional NIR-emitting materials that usually need complicated molecular design and tedious organic synthesis.
Figure 4.
Fluorescence spectra of 1 and 2 in solid state at room temperature (λex = 425 nm). Inset: photos of 1 and 2 under a UV lamp at 365 nm.
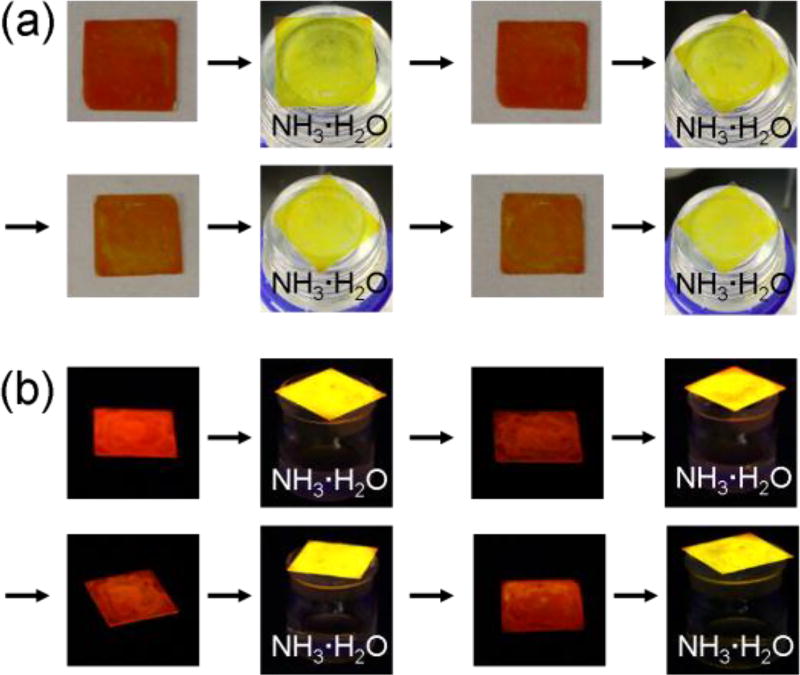
Next, gas detection was performed by using the intriguing optical behaviours of the assembly in the solid state. When the thin film of 1 was exposed to ammonia gas, an obvious colour change from red to yellow was observed either under a UV lamp or under the sun (Figure 5). This process of colour change was very sensitive, just in a few seconds. Fluorescence spectra of the films were recorded to investigate the process. Upon exposure to ammonia, the emission maximum of 1 showed a great blue shift (75 nm) (Figure S13a). After removing from ammonia, the original emission of the film recovered. The recovered thin film was still valid for ammonia detection again and could be used circularly. Certainly, with the increased cycles for ammonia detection, both the responsive time to ammonia and the recovery time of the film from yellow to red increased accordingly. And at last, the film would lose its ability as an indicator. Thin film of 2 had similar performance when exposed to or removed from ammonia. However, the film of free ligand 3 has no such functional feature (Figure S15).
Figure 5.
Photographs of films of 1 when exposed to or removed from ammonia circularly at room temperature. (a) under the sun; (b) under a UV lamp at 365 nm.
In conclusion, two multicomponent rectangular metallacycles were constructed by means of coordination-driven self-assembly. By introducing the AIE-active ligand, DPA 3, into the systems, the resulting SCCs, 1 and 2, exhibited both AIE and solvatochromism. Interestingly, they can emit near-infrared fluorescence in the solid state. Furthermore, upon exposure to ammonia, thin films of 1 and 2 functioned as the gas indicator by changing their colours from red to yellow and vice versa upon removal of it, making these two films good tools for biochemical analysis. Exploration and research of biological applications of these materials are now ongoing in our lab.
Supplementary Material
Acknowledgments
F.H. thanks the NSFC/China (21620102006), the State Key Laboratory of Chemical Engineering, and the Fundamental Research Funds for the Central Universities for financial support. P.J.S. thanks the NIH (RO1-CA215157) for financial support.
Footnotes
ASSOCIATED CONTENT
Experimental details and additional data. These materials are available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
References
- 1.(a) Gamelin DR, Güdel HU. Acc. Chem. Rsc. 2000;33:235–242. doi: 10.1021/ar990102y. [DOI] [PubMed] [Google Scholar]; (b) Wong KMC, Yam VWW. Acc. Chem. Rsc. 2011;33:424–434. doi: 10.1021/ar100130j. [DOI] [PubMed] [Google Scholar]; (c) Cui Y, Yue Y, Qian G, Chen B. Chem. Rev. 2012;112:1126–1162. doi: 10.1021/cr200101d. [DOI] [PubMed] [Google Scholar]; (d) Wang F, Liu X. Acc. Chem. Rsc. 2014;47:1378–1385. doi: 10.1021/ar5000067. [DOI] [PubMed] [Google Scholar]; (e) Zhou J, Liu Q, Feng W, Sun Y, Li F. Chem. Rev. 2015;115:395–465. doi: 10.1021/cr400478f. [DOI] [PubMed] [Google Scholar]; (f) Beppu T, Tomiguchi K, Masuhara A, Pu YJ, Katagiri H. Angew. Chem. Int. Ed. 2015;54:7332–7335. doi: 10.1002/anie.201502365. [DOI] [PubMed] [Google Scholar]; (g) Henthorn HA, Pluth MD. J. Am. Chem. Soc. 2015;137:15330–15336. doi: 10.1021/jacs.5b10675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Jathoul AP, Grounds H, Anderson JC, Pule MA. Angew. Chem. Int. Ed. 2014;53:13059–13063. doi: 10.1002/anie.201405955. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hyun H, Wada H, Bao K, Gravier J, Yadav Y, Laramie Ml, Henary M, Frangioni JV, Choi HS. Angew. Chem. Int. Ed. 2014;53:10668–10672. doi: 10.1002/anie.201404930. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Liu HY, Wu PJ, Kuo SY, Chen CP, Chang EH, Wu CY, Chan YH. J. Am. Chem. Soc. 2015;137:10420–10429. doi: 10.1021/jacs.5b06710. [DOI] [PubMed] [Google Scholar]; (d) Ren L, Liu F, Shen X, Zhang C, Yi Y, Zhu X. J. Am. Chem. Soc. 2015;137:11294–11302. doi: 10.1021/jacs.5b03899. [DOI] [PubMed] [Google Scholar]; (e) Palner M, Pu K, Shao S, Rao J. Angew. Chem. Int. Ed. 2015;54:11477–11480. doi: 10.1002/anie.201502736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Serin JM, Brousmiche DW, Fréchet JMJ. J. Am. Chem. Soc. 2002;124:11848–11849. doi: 10.1021/ja027564i. [DOI] [PubMed] [Google Scholar]; (b) Ye Y, Li WP, Anderson CJ, Kao J, Nikiforovich GV, Achilefu S. J. Am. Chem. Soc. 2003;125:7766–7767. doi: 10.1021/ja034186o. [DOI] [PubMed] [Google Scholar]; (c) Xing B, Khanamiryan A, Rao J. J. Am. Chem. Soc. 2005;127:4158–4159. doi: 10.1021/ja042829+. [DOI] [PubMed] [Google Scholar]; (d) Umezawa K, Nakamura Y, Makino H, Citterio D, Suzuki K. J. Am. Chem. Soc. 2008;130:1550–1551. doi: 10.1021/ja077756j. [DOI] [PubMed] [Google Scholar]; (e) Palma A, Alvarez LA, Scholz D, Frimannsson DO, Grossi M, Quinn SJ, O’Shea DF. J. Am. Chem. Soc. 2011;133:19618–19621. doi: 10.1021/ja208086e. [DOI] [PubMed] [Google Scholar]; (f) Zeng L, Jiao C, Huang X, Huang K-W, Chin W-S, Wu J. Org. Lett. 2011;13:6026–6029. doi: 10.1021/ol202493c. [DOI] [PubMed] [Google Scholar]; (g) Poirel A, Nicola AD, Ziessel R. Org. Lett. 2012;14:5696–5699. doi: 10.1021/ol302710z. [DOI] [PubMed] [Google Scholar]; (h) Yuan L, Lin W, Zheng K, He L, Huang W. Chem. Soc. Rev. 2013;42:622–661. doi: 10.1039/c2cs35313j. [DOI] [PubMed] [Google Scholar]; (i) Li Y, Sun Y, Li J, Su Q, Yuan W, Dai Y, Han C, Wang Q, Feng W, Li F. J. Am. Chem. Soc. 2015;137:6407–6416. doi: 10.1021/jacs.5b04097. [DOI] [PubMed] [Google Scholar]; (j) Shao A, Xie Y, Zhu S, Guo Z, Zhu S, Guo J, Shi P, James TD, Tian H, Zhu W-H. Angew. Chem. Int. Ed. 2015;54:7275–7280. doi: 10.1002/anie.201501478. [DOI] [PubMed] [Google Scholar]; (k) Shi B, Jie K, Zhou Y, Zhou J, Xia D, Huang F. J. Am. Chem. Soc. 2016;138:80–83. doi: 10.1021/jacs.5b11676. [DOI] [PubMed] [Google Scholar]; (l) Hu Y, Wang Z, Zhang X, Yang X, Ge C, Fu L, Gao X. Org. Lett. 2017;19:468–471. doi: 10.1021/acs.orglett.6b03614. [DOI] [PubMed] [Google Scholar]; (m) Chen N, Zhang W, Chen S, Wu Q, Yu C, Wei Y, Xu Y, Hao E, Jiao L. Org. Lett. 2017;19:2026–2029. doi: 10.1021/acs.orglett.7b00601. [DOI] [PubMed] [Google Scholar]
- 4.(a) Xie N-H, Li C, Liu J-X, Gong W-L, Tang BZ, Li G, Zhu M-Q. Chem. Commun. 2016;52:5808–5811. doi: 10.1039/c6cc01187j. [DOI] [PubMed] [Google Scholar]; (b) Chang Z-F, Jing L-M, Chen B, Zhang M, Cai X, Liu J-J, Ye Y-C, Lou X, Zhao Z, Liu B, Wang J-L, Tang BZ. Chem. Sci. 2016;7:4527–4536. doi: 10.1039/c5sc04920b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kang M, Gu X, Kwok RTK, Leung CWT, Lam JWY, Li F, Tang BZ. Chem. Commun. 2016;52:5957–5960. doi: 10.1039/c6cc01797e. [DOI] [PubMed] [Google Scholar]
- 5.(a) Das N, Mukherjee PS, Arif AM, Stang PJ. J. Am. Chem. Soc. 2003;125:13950–13951. doi: 10.1021/ja037788g. [DOI] [PubMed] [Google Scholar]; (b) Mukherjee PS, Das N, Kryschenko YK, Arif AM, Stang PJ. J. Am. Chem. Soc. 2004;126:2464–2473. doi: 10.1021/ja039235b. [DOI] [PubMed] [Google Scholar]; (c) Bar AK, Gole B, Ghosh S, Mukherjee PS. Dalton Trans. 2009:6701–6704. doi: 10.1039/b911622m. [DOI] [PubMed] [Google Scholar]; (d) Klosterman JK, Yamauchi Y, Fujita M. Chem. Soc. Rev. 2009;38:1714–1725. doi: 10.1039/b901261n. [DOI] [PubMed] [Google Scholar]; (e) Inokuma Y, Kawano M, Fujita M. Nat. Chem. 2011;3:349–358. doi: 10.1038/nchem.1031. [DOI] [PubMed] [Google Scholar]; (f) Chen S, Chen L-J, Yang H-B, Tian H, Zhu W. J. Am. Chem. Soc. 2012;134:13596–13599. doi: 10.1021/ja306748k. [DOI] [PubMed] [Google Scholar]; (g) Yan X, Jiang B, Cook TR, Zhang Y, Li J, Yu Y, Huang F, Yang H-B, Stang PJ. J. Am. Chem. Soc. 2013;135:16813–16816. doi: 10.1021/ja4092193. [DOI] [PubMed] [Google Scholar]; (h) Cook TR, Zheng Y-R, Stang PJ. Chem. Rev. 2013;113:734–777. doi: 10.1021/cr3002824. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Yan X, Li S, Pollock JB, Cook TR, Chen J, Zhang Y, Ji X, Yu Y, Huang F, Stang PJ. Proc. Natl. Acad. Sci. U.S.A. 2013;110:15585–15590. doi: 10.1073/pnas.1307472110. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Chen L-J, Zhao G-Z, Jiang B, Sun B, Wang M, Xu L, He J, Abliz Z, Tan H, Li X, Yang H-B. J. Am. Chem. Soc. 2014;136:5993–6001. doi: 10.1021/ja500152a. [DOI] [PubMed] [Google Scholar]; (k) Wei P, Li J, Yan X, Zhou Q. Org. Lett. 2014;16:126–129. doi: 10.1021/ol403111e. [DOI] [PubMed] [Google Scholar]; (l) Wei P, Cook TR, Yan X, Huang F, Stang PJ. J. Am. Chem. Soc. 2014;136:15497–15500. doi: 10.1021/ja5093503. [DOI] [PubMed] [Google Scholar]; (m) Zhou Z, Yan X, Cook TR, Saha ML, Stang PJ. J. Am. Chem. Soc. 2016;138:806–809. doi: 10.1021/jacs.5b12986. [DOI] [PubMed] [Google Scholar]; (n) Zhang Z, Wang H, Wang X, Li Y, Song B, Bolarinwa O, Reese RA, Zhang T, Wang X-Q, Cai J, Xu B, Wang M, Liu C, Yang H-B, Li X. J. Am. Chem. Soc. 2017;139:8174–8185. doi: 10.1021/jacs.7b01326. [DOI] [PubMed] [Google Scholar]; (o) Li X-Z, Zhou L-P, Yan L-L, Yuan D-Q, Lin C-S, Sun Q-F. J. Am. Chem. Soc. 2017;139:8237–8244. doi: 10.1021/jacs.7b02764. [DOI] [PubMed] [Google Scholar]
- 6.(a) Yan X, Cook TR, Wang P, Huang F, Stang PJ. Nat. Chem. 2014;7:342–348. doi: 10.1038/nchem.2201. [DOI] [PubMed] [Google Scholar]; (b) Yan X, Wang H, Hauke CE, Cook TR, Wang M, Saha ML, Zhou Z, Zhang M, Li X, Huang F, Stang PJ. J. Am. Chem. Soc. 2015;137:115276–15286. doi: 10.1021/jacs.5b10130. [DOI] [PubMed] [Google Scholar]; (c) Yan X, Wang M, Cook TR, Zhang M, Saha ML, Zhou Z, Li X, Huang F, Stang PJ. J. Am. Chem. Soc. 2016;138:4580–4588. doi: 10.1021/jacs.6b00846. [DOI] [PubMed] [Google Scholar]; (d) Zhang M, Li S, Yan X, Zhou Z, Saha ML, Wang Y-C, Stang PJ. Proc. Natl. Acad. Sci. U.S.A. 2016;113:11100–11105. doi: 10.1073/pnas.1612898113. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Zhang M, Saha ML, Wang M, Zhou Z, Song B, Lu C, Yan X, Li X, Huang F, Yin S, Stang PJ. J. Am. Chem. Soc. 2017;139:5067–5074. doi: 10.1021/jacs.6b12536. [DOI] [PubMed] [Google Scholar]
- 7.(a) Lu H, Xu B, Dong Y, Chen F, Li Y, Li Z, He J, Li H, Tian W. Langmuir. 2010;26:6838–6844. doi: 10.1021/la904727t. [DOI] [PubMed] [Google Scholar]; (b) Wang F, Wen J, Huang L, Huang J, Quyang J. Chem. Commun. 2012;48:7395–7397. doi: 10.1039/c2cc33172a. [DOI] [PubMed] [Google Scholar]; (c) Lu H, Su F, Mei Q, Tian Y, Tian W, Johnson RH, Meldrum DR. J. Mater. Chem. 2012;22:9890–9900. doi: 10.1039/C2JM30258F. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Zhang X, Zhang X, Wang S, Liu M, Zhang Y, Tao L, Wei Y. ACS Appl. Mater. Interfaces. 2013;5:1943–1947. doi: 10.1021/am302512u. [DOI] [PubMed] [Google Scholar]; (e) Sun B, Yang X, Ma L, Niu C, Wang F, Na N, Wen J, Ouyang J. Langmuir. 2013;29:1956–1963. doi: 10.1021/la3048278. [DOI] [PubMed] [Google Scholar]
- 8.Chowdhury A, Howlader P, Mukherjee PS. Chem. Eur. J. 2016;22:7468–7478. doi: 10.1002/chem.201600698. [DOI] [PubMed] [Google Scholar]
- 9.(a) Chien W-L, Yang C-M, Chen T-L, Li S-T, Hong J-L. RSC Adv. 2013;3:6930–6938. [Google Scholar]; (b) Dong Y, Zhang J, Tan X, Wang L, Chen J, Li B, Ye L, Xu B, Zou B, Tian W. J. Mater. Chem. C. 2013;1:7554–7559. [Google Scholar]
- 10.(a) Zheng Y-R, Zhao Z, Wang M, Ghosh K, Pollock JB, Cook TR, Stang PJ. J. Am. Chem. Soc. 2010;132:16873–16882. doi: 10.1021/ja106251f. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Li S, Huang J, Cook TR, Pollock JB, Kim H, Chi K-W, Stang PJ. J. Am. Chem. Soc. 2013;135:2084–2087. doi: 10.1021/ja3118812. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.