Abstract
Objective:
Using compound W (a 3,3′-diiodothyronine sulfate [T2S] immuno-crossreactive material)-specific polyclonal antibodies and homogeneous time-resolved fluorescence immunoassay assay techniques (AlphaLISA) to establish an indirect competitive compound W (ICW) quantitative detection method.
Method:
Photosensitive particles (donor beads) coated with compound W or T2S and rabbit anti-W antibody were incubated with biotinylated goat anti-rabbit antibody. This constitutes a detection system with streptavidin-coated acceptor particle. We have optimized the test conditions and evaluated the detection performance.
Results:
The sensitivity of the method was 5 pg/mL, and the detection range was 5 to 10 000 pg/mL. The intra-assay coefficient of variation averages <10% with stable reproducibility.
Conclusions:
The ICW-AlphaLISA shows good stability and high sensitivity and can measure a wide range of compound W levels in extracts of maternal serum samples. This may have clinical application to screen congenital hypothyroidism in utero.
Keywords: Homogeneous time-resolved fluorescence immunoassay, pregnancy, thyroid hormone analogs, compound W
Introduction
Prior studies identified that a 3,3′-diiodothyronine sulfate (T2S)-crossreactive material, compound W, is not authentic T2S, and does not cochromatograph with synthetic T2S on high-performance liquid chromatography (HPLC) in fetal and maternal serum samples.1,2 Maternal levels increase with the progression of pregnancy and rapidly peaked before parturition.1 Serial measurements of serum W in pregnant women have been found to be useful as a noninvasive technique for the diagnosis of congenital hypothyroidism in pregnancies associated with fetal goiter.2-5
Congenital hypothyroidism is caused by a variety of causes that affect the pituitary-thyroid axis during prenatal period. Congenital hypothyroidism may result in serious abnormalities in the fetal central nervous system (CNS).6,7 It may also associate with other developmental and physical abnormalities. Optimal thyroid hormone (TH) level is essential for normal development of the CNS.2,6 Thus, early diagnosis and treatment are essential to ensure the normal CNS development and prevent the sequelae of congenital hypothyroidism; cretinism is the most serious form. Currently, screening for congenital hypothyroidism is initiated at 2 to 3 days after birth by measuring thyrotropin levels in the neonatal heel blood and starting therapy in the postnatal period. This neonatal screening strategy may be late for securing a normal brain development that starts at first trimester of pregnancy.8,9 A fetal functional marker in maternal serum or urine would provide a convenient method for screening congenital hypothyroidism in utero, rather than postnatally.
The only other method for the measurement of compound W involves the use of radioimmunoassay (RIA) which was developed over 2 decades ago by Wu et al.1,2 Radioimmunoassay, in general, is not convenient to most clinical laboratories due to the involvement of using a radioisotope (I-125). In this study, we have applied a highly sensitive and rapid homogeneous time-resolved fluorescence immunoassay to establish an indirect competitive compound W quantitative detection method AlphaLisa (ICW-AlphaLisa) to measure the levels of compound W in maternal serum during pregnancy.
Materials and Methods
Reagents and serum samples
The T2S-specific polyclonal rabbit antiserum was developed in the Thyroid Laboratory at Long Beach VA Medical Center.1 Bovine serum albumin (BSA) was purchased from Sigma Company. The affinity column–purified biotinylated goat anti-rabbit IgG was obtained from the Luoyang Sino-American Biotechnology Company. The light-emitting particles (acceptor beads), the photosensitive particles (donor beads), opaque white microplate, and the AlphaLISA LiCA detector (AlphaLisa Signal Reader) are provided by Yang Haibo Biological Technology Co., Ltd. Serum samples from near-term pregnant women were the banked samples left over for other routine prenatal testing in the Jiang Yuan Hospital with the approval of Human Use Committee.
The T2S in 63% ethanol in concentration of 1, 5, 10, 50, 100, 500, 1000, and 10 000 pg/mL was served as standard curve. Maternal serum samples were extracted by adding 2 volumes of 95% ethanol (final concentration, 63%) and incubated at −20°C overnight. Then, mixtures were centrifuged at 4°C and the supernatants were used for measurement.4
Preparation of T2S antigen–coated microparticles
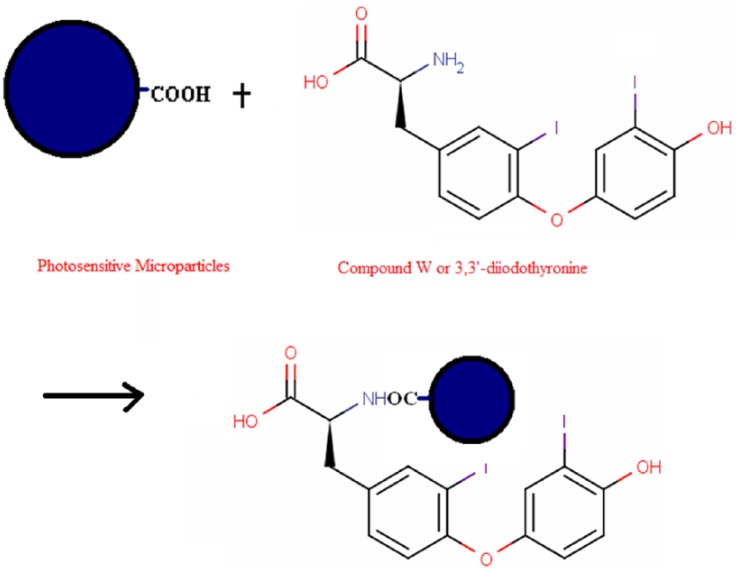
The compound W or T2S antigen–coated microparticles were prepared by adding 1 mg of photosensitive microparticles prepared in an Eppendorf tube together with 12.5 μL 1% Tween-20, 0.05 mg T2S, 10 μL sodium borohydride cyanide (25 mg/mL), with 2-(N-morpholino) ethanesulfonic acid buffer (0.1 M, pH 6.0). The volume was made up to 200 μL at 37°C and then incubated for 48 hours in dark. Subsequently, 10 μL of carboxyl methoxyamine hydrochloride solution (0.3 M, pH 5.0) was added to block the unbound sites. The reaction was continued at 37°C in dark for 1 hour. Afterward, the tubes were centrifuged in an Eppendorf Centrifuge (5415D) at 6000 r/min for 20 minutes. The preparation of T2S or compound W–photosensitive microparticle is shown in Figure 1.
Figure 1.
The conjugation reaction of photosensitive donor beads and T2S (or compound W). The carboxyl group over the surface of donor beads and the amino group on T2S were linked covalently. T2S indicates 3,3′-diiodothyronine sulfate.
The preparation of biotinylated goat anti-rabbit IgG antibody
The preparation of biotinylated antibody
The goat anti-rabbit IgG antibody was placed in borate buffer (0.05 M, pH 8.6) and the concentration of antibody was adjusted to 1 mg/mL with the same buffer solution.10,11 In the centrifuge tube, to every milliliter of antibody, add 80 μg of biotin preparation and incubate at room temperature for 3 hours. After incubation, samples were added to a 5-mL Sephadex G-25 column (equilibrated with 1× phosphate-buffered saline [PBS] buffer, pH 7.5 containing 0.1% BSA) and then washed with PBS buffer. The eluate was collected in 1-mL fractions and the A280 absorbance was measured. Eluted peaks were collected and stored in 0.2% sodium azide at 4°C.
The procedure of detection
Holes on a white opaque microtiter plate were filled with standard or sample, T2S-coated donor beads, rabbit antibodies and biotinylated goat anti-rabbit IgG. The volume of each reagent was 20 μL. The plates were then placed inside the apparatus and incubated for 20 minutes at 37°C. Subsequently, 175 μL of streptavidin-coated acceptor beads (100 μg/mL) were added. The reaction mixtures were incubated in the dark for another 10 minutes and the plates were then read on the AIphaLISA Signal Reader (LiCA detector). The concentrations of compound W in the samples were calculated from the standard curve. The reaction is shown in Figure 2.
Figure 2.
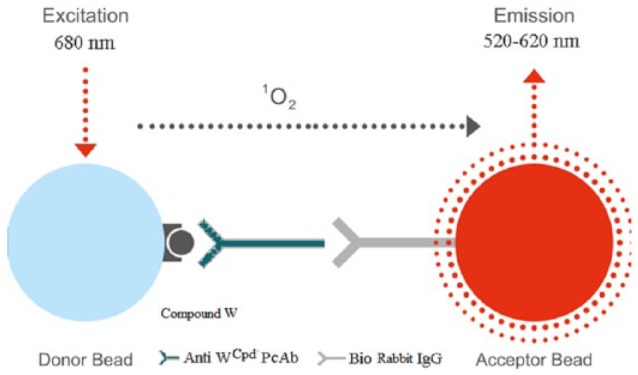
The detection of compound W in the AlphaLISA: the amplified signal is generated when the T2S-conjugated donor beads are brought near the streptavidin-coated acceptor beads through the immune interaction between the biotinylated goat anti-rabbit and rabbit anti-T2S antibodies. Singlet state oxygen molecules produced by the excited donor beads activate the acceptor beads in proximity to emit luminescence (Anti-W PcAb or Anti-WCpd PcAb, Anti-W Compound [anti-T2S] polyclonal antibody; Bio Rabbit IgG, Biotinylated goat anti-rabbit IgG).
Optimization of the reaction condition
To optimize the indirect competitive method measuring compound W, we have determined the optimal dilutions of W antigen–photosensitive particulate matter, anti-W polyclonal antibody, and the biotinylated goat anti-rabbit antibody dilution to achieve the best reaction condition.
The optimal dilution of compound W antigen–coated donor beads and the W polyclonal antibody working concentration were determined by applying their serial dilutions in reaction microplates. Each dilution was measured by LiCA detector, and results were analyzed statistically. The optimal dilution was chosen when the IC50 value of the antiserum-binding capability was obtained.
After the establishment of the optimal working dilution of W-coated particulate matter and polyclonal antibody, the optimal dilution of biotinylated goat anti-rabbit IgG was determined by testing a serial dilution of 1:100, 1:200, 1:300, 1:400, 1:500, and 1:600. Each dilution was measured by the emitting signal and IC50 was determined. Using the aforementioned detecting procedure, the biotinylated goat anti-rabbit IgG dilution with high emission counts (at zero concentration of antigen) and low nonspecific binding (NSB) is selected as a working concentration.
After the establishment of all working concentrations, the optimal reaction time was determined by incubating (at 37°C) for 10, 15, 20, and 30 minutes, to give the light-emitting particles a sufficient reaction time to form. Other steps following the aforementioned procedure of detection, trends were obtained under different competition time at zero concentration of the antigen. Thus, the suitable tracer reaction time was chosen.
The standard curve was plotted by the Log-Logit process (Origin Software) of count values (CPS [counts per second]) obtained from homogeneous time-resolved detector for each dose point correspondingly.
T2S radioimmunoassay
The 3,3′-T2S and [125I]T2S were prepared by the method of Eelkman-Rooda and co-workers.12,13 The T2S was further purified and quantitatively recovered by reversed-phase HPLC with a preparative column, as described previously.1 The RIA employed an anti-L-T2S antibody (W0213) obtained from rabbits immunized with L-T2S BSA conjugate.1 In a final dilution of 1:15 000, anti-T2S antibody W0213 bound 33% to 45% of a tracer amount (~5 pg or 6.8 fmol) of [125I]T2S in 0.075 mol/L barbital buffer (pH 8.6; containing 0.1% sodium azide and 0.125% normal rabbit serum) and 19% ethanol.
The T2S RIA procedure was a modification of the RIA described previously.1 Serum samples (0.2-1.0 mL) were extracted with 2 volumes of 95% ethanol (final concentration, 63%) before assay. Preliminary experiments showed that the extraction efficiency of T2S in serum exceeded 96%. Final T2S concentrations were not corrected for recovery. The lower limit of detection of the assay was 3.3 fmol (2 pg) or 33.1 pmol/L in a 300-μL ethanol extract of serum. Intra-assay variations were 1.9% to 9.1% and interassay variations were 6.0% to 19.5%, depending on the measured concentrations.14
Results
The establishment of the optimal concentration of compound W–conjugated donor beads and anti-W polyclonal antibody
The concentrations of the W antigen and the conjugate of compound W antigen-donor beads exerted critical influence on the sensitivity of the standard curve. Therefore, the selection of an appropriate dilution ratio was the key to the success of the assay. Further studies explored the optimal working concentration of photosensitive material and antiparticles—W PcAb. The results are shown in Table 1. Based on fluorescence count from Table 1, W–photosensitive particles dilution 1:100 and compound W PcAb dilution of 1:2000 were selected.
Table 1.
The selection of the working concentration of W-donor bead and anti-W polyclonal antibody (counts are means of triplicates).
| Conjugated compound W–photosensitive microparticles | Anti-W polyclonal antibody |
|||
|---|---|---|---|---|
| 1:500 | 1:1000 | 1:2000 | 1:10 000 | |
| 1:10 | 168 232 | 99 801 | 59 291 | 44 479 |
| 1:100 | 80 573 | 42 568 | 25 180 | 21 516 |
| NSB | 10 | 9 | 11 | 11 |
Abbreviation: NSB, nonspecific binding.
The establishment of the dilutions of biotinylated goat anti-rabbit IgG
The selection of appropriate biotinylated goat anti-rabbit IgG dilution is not only to ensure a sufficient amount of antibody bound but also to avoid causing an increase in NSB. In the ICW-AlphaLISA, the antibody (the biotinylated goat anti-rabbit IgG) was diluted into different concentrations. The result is shown in Table 2.
Table 2.
The selection of biotinylated goat anti-rabbit IgG antibody dilution (counts are means of triplicates).
| Biotinylated goat anti-rabbit IgG antibody dilution | 1:100 | 1:200 | 1:300 | 1:400 | 1:500 | 1:600 |
|---|---|---|---|---|---|---|
| Zero concentration net luminescent signal | 36 172 | 30 179 | 26 517 | 10 532 | 5432 | 2371 |
| NSB | 85 | 52 | 21 | 11 | 6 | 5 |
Abbreviations: IgG, immunoglobulin G; NSB, nonspecific binding.
Table 2 shows that when the dilution of biotinylated goat anti-rabbit IgG increases, the NSB and zero concentration (of sample) fluorescence count began to decline significantly after 1:300 dilution. Thus, 1:300 dilution of biotinylated goat anti-rabbit IgG was selected as the optimal dilution.
The determination of the reaction time
The reaction temperature was set at 37°C, giving an adequate response time (biotin-avidin binding reaction), measuring the fluorescence count at different time points of the competition (W-coated beads and antibody conjugation). The fluorescence count (zero concentration of sample) showed no significant difference after 15 minutes of reaction. The experiments with different times of reaction (the biotin-avidin binding) showed that the reaction had virtually completed between 15 and 30 minutes. Therefore, the reaction time of 15 minutes was chosen.
The calibration of the compound W (T2S)-AlphaLISA standard curve
The standard curve for compound W-AlphaLISA was obtained under the following optimal detection conditions: W-donor particles, 1:100 dilution; W PcAb, 1:2000 dilution; biotinylated goat anti-rabbit IgG, 1:300 dilution; incubation temperature 37°C; and 15 minutes of each incubation. The standard curve of the ICW-AlphaLISA method was generated by Origin Software is shown in Figure 3.
Figure 3.
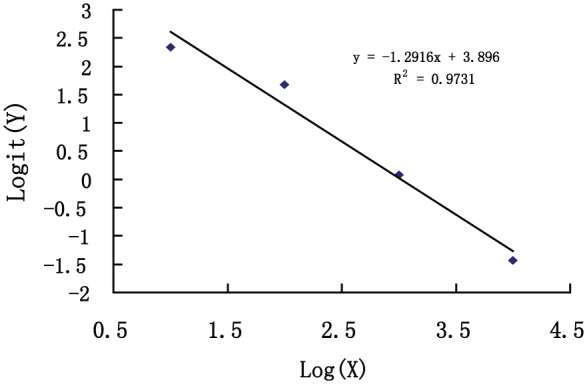
Compound W homogeneous time-resolved fluorescence immunoassay standard curve (X, concentration of standards [pg/mL]; Y, signal counts [counts per second, CPS]).
The evaluation of methodology
We investigated the sensitivity, precision, soundness, and stability for the ICW-AlphaLISA method. A comparison of methodology was obtained by samples assayed by both RIA method and AlphaLISA. The results to compare the 2 methods of detection were plotted with correlation coefficient.
Sensitivity and linearity range
The mean and standard deviation were determined by the evaluation of 10 experiments. The limit of detection was determined from the mean ± 2.0 x SD. The sensitivity (mean ± 2 x SD) was calculated from the mean fluorescence of the zero concentration ICW-AlphaLISA shows a sensitivity of 5 pg/mL and the linear range was 5 to 10 000 pg/mL.
Precision
The intra-assay variation was determined by the preparation of 3 different concentrations of W reference standard, as shown in Table 3. The intra-assay coefficient of variation was less than 10%, and the results meet the basic requirements immunoassay detection.
Table 3.
Indirect competitive compound W-AlphaLISA intra-assay and interassay variability of assessment.
| Standard conc., pg/mL | Intra-assay (n = 12) |
Interassay (n = 12) |
||
|---|---|---|---|---|
| X ± 2SD, pg/mL | CV, % | X ± 2SD, pg/mL | CV, % | |
| 20 | 18.8 ± 1.5 | 8.0 | 19.1 ± 1.8 | 9.4 |
| 200 | 198.6 ± 8.6 | 4.3 | 190.9 ± 15.2 | 8.0 |
| 2000 | 1942.2 ± 72.3 | 3.7 | 1961.8 ± 163.4 | 8.3 |
Soundness
The results were obtained by the measurement of a serial dilutions, from 1:2 to 1:256, of a sample with high value of compound W identified by RIA are shown in Table 4. In Table 4, based on data processing of correlation equations, we obtained dilution and the measured value is Y = 1.0842X − 7.3025, R2 = 0.9994. This illustrates that the dilution of a blood sample with high compound W concentration does not affect the measurement. It shows that ICW-AlphaLISA detection system for detecting serum compound W levels is not interfered by substances co-existing in the sample.
Table 4.
Soundness results (data are the means of triplicates).
| Dilutions | W predicted values, pg/mL | W measured values, pg/mL |
|---|---|---|
| 1:1 | 3895 | |
| 1:2 | 1947.5 | 2098.3 |
| 1:8 | 486.9 | 551.4 |
| 1:32 | 121.7 | 94.3 |
| 1:64 | 60.9 | 53.2 |
| 1:128 | 30.4 | 38.5 |
| 1:256 | 15.2 | 7.2 |
Stability
Three repeated assays at different times determined the interassay variation of the ICW-AlphaLISA. The assays showed the variation being less than 10%, indicating that the dose-response curve had a relatively small in variation. Similarly, repeated assay revealed that an exposure of the assay reagents at 37°C for 7 days had an average rate of decline of 11.3%. Thus, it indicated that ICW-AlphaLISA kit met the shelf life requirement.
Comparison between RIA and ICW-AlphaLISA
Figure 4 shows linear regression analysis on the result of measurement of 32 samples of serum extracts by both RIA and W-AlphaLISA. The correlation coefficient was 0.87, indicating a statistically significant correlation between the 2 methods.
Figure 4.
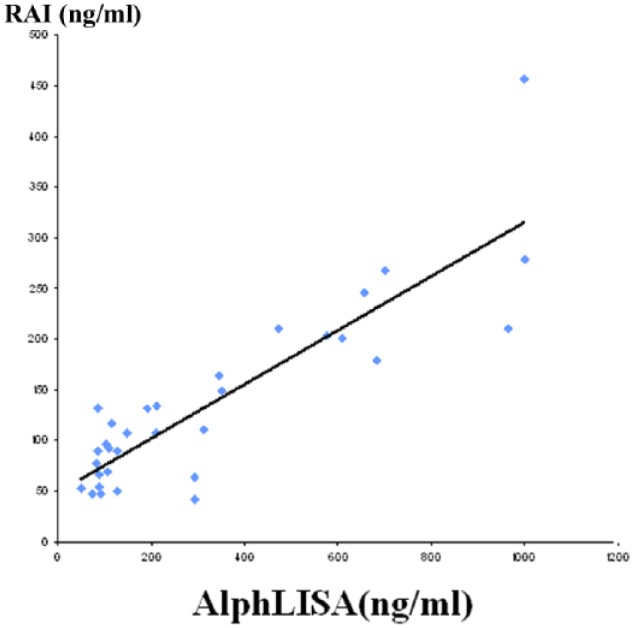
Correlation of the concentrations of W compounds in samples measured by AlphaLISA and radioimmunoassay.
Discussion
AlphaLISA homogeneous chemiluminescence is generated by the excitation of the donor beads and provokes the release of singlet oxygen molecules that results in light emission by triggering a cascade of energy transfer to the acceptor beads. It is a fast and simple operation with high sensitivity and specificity.10,11
The ICW-AlphaLisa involves W (or T2S)-coated donor beads, biotinylated goat anti-rabbit IgG, W-specific polyclonal antibody, and streptavidin-coated acceptor beads. Free W compound in samples (or standards) competes with W-coated donor beads in the conjugation to polyclonal antibody that connects to the biotinylated goat anti-rabbit IgG. An amplified signal is generated when the T2S-conjugated donor beads are brought near the streptavidin-coated acceptor beads through the immune interaction between the biotinylated goat anti-rabbit and rabbit anti-T2S antibodies. Singlet state oxygen molecules produced by the excited donor beads activate the acceptor beads in proximity to emit luminescence that is measured by a detector. The optical signal is inversely proportional to the concentration of W compound in the sample. The concentration is measured against a standard curve plotted with known concentration of 3,3′-T2S.
Prior studies have successfully established RIA for compound W.1,3-5,14-16 The analytical assay by RIA has been used in research. The RIA results have shown that maternal levels increased with the progression of pregnancy and rapidly peaked before parturition.1 Serial measurements of serum W in pregnant women have been found to be useful as a noninvasive technique for the diagnosis of fetal hypothyroidism in pregnancies associated with fetal goiter.3,4 In a prior report,16 we established the “normal” levels, expressed as T2S equivalent, in different gestation periods and showed that the majority of the T2S-crossreactive material is W in maternal serum at term, not T2S. The increased levels of serum W in early pregnancy, prior to the beginning of fetal TH production, are in an agreement with the increase in maternal T4 needs and the apparent maternal to fetal transfer of T4. The presence of TH in the fetus with active sulfation pathway2,16 and the access to the placenta appears to be critical for the production of compound W. We have also shown that there are similar cord and maternal levels at term in samples from the United States as compared with those from Taiwan, Thailand, and Colombia.16
Serum levels of T2S-immunocrossreactive material have been independently measured by Chopra17 with the RIA method. However, this group’s data are somewhat difficult to interpret as HPLC and hot-acid digestion were not used to separate T2S from compound W. Nevertheless, Chopra’s data independently demonstrate that there is an increase in T2S-crossreactive material in pregnant and fetal serum. By the RIA method in the Chinese mainland, Chen et al18 found a mean serum concentration that is similar to our findings. Our nonpregnant female and male T2S-equivalent values are relatively low, with a minimal increase in the hyperthyroid state. These low values are more similar to the levels of other sulfated iodothyronines, ie, T3S, T4S, and rT3S.19-22
The chemical structure of compound W remains to be elucidated and considered to be an important issue in the field of thyroidology.23 The elucidation of the structure depends on the measurement and identification of compound W. However, labeled T2 had to be prepared first in the RIA by using a radioisotope, I-125 to label 3-monoiodothyronine, which was then sulfated to 3,3’-T2S subsequently. The process is cumbersome and requires complex separation and purification. In contrast, the AlphaLISA technology involved mainly a covalent linkage between the carboxyl group over the surface of photosensitive donor beads and the amino group in T2S . Due to the presence of a NH2 group in the T2S molecule, the covalent linkage is very easy to form. This procedure is simple to operate. A 48-hour RIA detection process can be shortened to 30 minutes to complete with higher sensitivity, better stability, wider measurement range, longer reagent shelf life, and no radioactive contamination. Compared with conventional RIA method, correlation between the two is excellent. Thus, W-AlphaLISA can be applied as an alternate method of screening for congenital hypothyroidism as well as to be a helpful tool in the identification of biochemical structure of compound W. Further testing on increased samples is, however, necessary to support its use within a clinical context.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors received financial support from SCIRE (Southern California Institute of Research and Education) for the research.
Declaration Of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: Study conception and design: BH, HY, JB, MZ, WLG, S-YW; Acquisition of data: BH, S-YW; Analysis and interpretation of data: BH, HY, JB, MZ, WLG, S-YW; Drafting of manuscript: BH, HY, JB, MZ, S-YW; Critical revision: WLG, S-YW.
References
- 1. Wu SY, Polk DH, Chen WL, Fisher DA, Huang WS, Yee B. A 3,3′-diiodothyronine sulfate cross-reactive compound in serum from pregnant women. J Clin Endocrinol Metab. 1994;78:1505–1509. [DOI] [PubMed] [Google Scholar]
- 2. Wu SY, Green WL, Huang WS, Hays MT, Chopra IJ. Alternate pathways of thyroid hormone metabolism. Thyroid. 2005;15:945–960. [DOI] [PubMed] [Google Scholar]
- 3. Abuhamad AZ, Fisher DA, Warsof SL, et al. Antenatal diagnosis and treatment of fetal goitrous hypothyroidism: case report and review of the literature. Ultrasound Obst Gynecol. 1995;6:368–371. [DOI] [PubMed] [Google Scholar]
- 4. Cortelazzi D, Morpurgo PS, Zamperini P, Fisher DA, Beck-Peccoz P, Wu SY. Maternal compound W serial measurements for the management of fetal hypothyroidism. Euro J Endocrinol. 1999;141:570–578. [DOI] [PubMed] [Google Scholar]
- 5. Chen DH, Yu J, Bao W, et al. 3,3′-Diiodothyronine sulfate cross-reactive material (Compound W) in human newborns. Pediatr Res. 2012;72:521–524. [DOI] [PubMed] [Google Scholar]
- 6. Morreale de, Escobar G, Obregon MJ, Escobar del Rey F. Role of thyroid hormone during early brain development. Eur J Endocrinol. 2004;151:U25–U37. [DOI] [PubMed] [Google Scholar]
- 7. Leger J, Ecosse E, Roussey M, Lanoe JL, Larroque B; The French Hypothyroidism Study Group. Subtle health impairment socioeducational attainment in young adult patients with congenital hypothyroidism diagnosed by neonatal screening: a longitudinal population-based cohort study. J Clin Endocrinol Metab. 2011;96:1771–1782. [DOI] [PubMed] [Google Scholar]
- 8. Stiles J, Jernigan TL. The basics of brain development. Neuropsycho Rev. 2010;20:327–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clarman H, Skocic J, Lischinsky JE, Rovet J. Do children with congenital hypothyroidism exhibit abnormal cortical morphology. Pediat Res. 2015;78:286–297. [DOI] [PubMed] [Google Scholar]
- 10. Cauchon E, Liu SM, Percival D, et al. Development of a homogeneous immunoassay for the detection of angiotensin I in plasma using AlphaLISA acceptor beads technology. J Anal Biochem. 2009;388:134–139. [DOI] [PubMed] [Google Scholar]
- 11. Waller H, Chatterji U, Gallay P, Parkinson T, Targett-Adams P. The use of AlphaLISA technology to detect interaction between hepatitis C virusencoded NS5A and cyclophilin A. J Virolog Meth. 2010;165:202–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mol JA, Visser TJ. Synthesis some properties of sulfate esters sulfamates of iodothyronines. Endocrinology. 1985;117:1–7. [DOI] [PubMed] [Google Scholar]
- 13. Eelkman-Rooda SH, Kaptein E, van Loon MA, Visser TJ. Development of a radioimmunoassay for triiodothyronine sulfate. J Immunoassay. 1988;9:125–134. [DOI] [PubMed] [Google Scholar]
- 14. Vanmiddlesworth L, Vanmiddlesworth NR, Egerman RS, et al. Thyroid function and 3,3′-diiodothyronine sulfate cross-reactive substance (compound w) in maternal hyperthyroidism with antithyroid treatment. Endocr Pract. 2011;17:170–176. [DOI] [PubMed] [Google Scholar]
- 15. Wu SY, Fisher DA, Huang WS, et al. Urinary compound W in pregnant women is a potential marker for fetal thyroid function. Am J Obstet Gynecol. 1998;178:886–891. [DOI] [PubMed] [Google Scholar]
- 16. Wu SY, Huang WS, Ho E, Wu ESC, Fisher DA. Compound W, a 3,3′-Diiodothyronine sulfate cross-reactive substance in serum from pregnant women: a potential marker for fetal thyroid function. Pediatr Res. 2007;61:307–312. [DOI] [PubMed] [Google Scholar]
- 17. Chopra IJ. A radioimmunoassay for measurement of 3,3′-diiodothyronine sulfate: studies in thyroidal and nonthyroidal diseases, pregnancy, and fetal/neonatal life. Metabolism. 2004;53:538–543. [DOI] [PubMed] [Google Scholar]
- 18. Chen QS, Fang PH, Zhang BQ, et al. Change of serum Compound W in normal pregnant women. Chinese J Endocrinol Metab. 2003;19:469–470. [Google Scholar]
- 19. Wu SY, Huang WS, Polk DH, et al. The development of a radioimmunoassay for 3,3′,5′-triiodothyronine sulfate (rT3S) in human serum and amniotic fluid. J Clin Endocrinol Metab. 1993;76:1625–1630. [DOI] [PubMed] [Google Scholar]
- 20. Chopra IJ, Wu SY, Chua Teco GN, Santini F. A radioimmunoassay for measurement of 3,5,3′-triiodothyronine sulfate (T3S): Studies in thyroidal and nonthyroidal diseases, pregnancy, and neonatal life. J Clin Endocrinol Metab. 1992;75:189–194. [DOI] [PubMed] [Google Scholar]
- 21. Wu SY, Huang WS, Polk D, et al. Identification of thyroxine-sulfate (T4S) in human serum and amniotic fluid by a novel T4S radioimmunoassay. Thyroid. 1992;2:101–105. [DOI] [PubMed] [Google Scholar]
- 22. Wu SY, Fisher DA. Measurement of 3,3′-diiodothyronine sulfate (T2S) in thyroidal nonthyroidal disease pregnancy fetal/neonatal life. Metabolism. 2004;53:1387. [DOI] [PubMed] [Google Scholar]
- 23. Peeters RP, Visser TJ. Metabolism of thyroid hormone, www.thyroidmanager.org. Updated January 1, 2017.