Abstract
Objectives:
Minimally invasive epiduroscopy has recently been reported as an effective treatment procedure for chronic and intractable low back pain. However, no study has determined safe anesthetics for monitored anesthesia care during epiduroscopy. We aimed to compare and evaluate conventional monitored anesthesia care drugs with dexmedetomidine.
Methods:
A retrospective study including all patients who underwent epiduroscopy at the JR Tokyo General Hospital from April 2011 to March 2016 was designed. The epiduroscopy procedures were performed under anesthesia with dexmedetomidine plus fentanyl (dexmedetomidine group) or droperidol plus fentanyl (neuroleptanalgesia group). Patients who received analgesics other than fentanyl, another analgesic combined with fentanyl, any sedative other than dexmedetomidine or droperidol, or who had incomplete data were excluded. We compared (1) the type and dose of medication during the epiduroscopy and (2) the incidence of postoperative nausea and vomiting.
Results:
We identified 45 patients (31 and 14 in the dexmedetomidine and neuroleptanalgesia groups, respectively) with a mean age of 69.0 years. The two groups had comparable characteristics, such as age, sex, body mass index, the American Society of Anesthesiologists Physical Status, analgesics used in the clinic, comorbidities, history of smoking, and the duration of anesthesia. The dexmedetomidine group received a significantly lower fentanyl dose during surgery (126 ± 14 vs 193 ± 21 µg, mean ± standard deviation, p = 0.014) and exhibited a significantly lower incidence of postoperative nausea and vomiting (1 vs 3, p = 0.047) than the neuroleptanalgesia group.
Conclusion:
This study involved elderly patients, and the use of dexmedetomidine in monitored anesthesia care during epiduroscopy procedures in these patients may reduce the required fentanyl dose during surgery and the incidence of postoperative nausea and vomiting. This strategy may help prevent respiratory depression and aspiration.
Keywords: Spinal canal endoscopy, low back pain, neuroleptanalgesia, droperidol, respiratory depression
Introduction
The lifetime prevalence of low back pain, which is reported to exceed 70% in developed countries, peaks between the ages of 35 and 55 years.1 Although medications, nerve block, and heat therapy are available for the management of low back pain,2 a recent study found that two-thirds of patients develop chronic low back pain.3 Inflammation and adhesions in the epidural space have been recently considered a cause of chronic, intractable low back pain.4,5 Inflammation of the epidural space causes production of inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and leads to epidural space adhesion,6–9 which can cause chronic pain.10
Minimally invasive epiduroscopy may be an effective treatment for such conditions;11 therefore, the number of epiduroscopy procedures performed is currently increasing worldwide.12,13 This procedure consists of observation, washing, irrigation, lysis of the adhesion in the epidural space under direct visual observation, and direct drug administration to the affected part of the spinal canal.12,14 However, anesthesia is required to alleviate pain during the lysis of the adhesions in the epidural space.15 Thus, monitored anesthesia care (MAC) should be used to provide anesthesia during epiduroscopy.16–18
Sedation levels range from conscious sedation to general anesthesia, and MAC is an intermediate state. MAC involves the anesthesiological assessment and management of physiological changes, such as fluctuations in blood pressure and respiratory depression, and medical issues that can arise in patients because of invasive medical care by an anesthesiologist.19 Epiduroscopy is often performed on the elderly, and, therefore, it is necessary to consider the possibility of respiratory depression and aspiration of postoperative nausea and vomiting (PONV) induced by the anesthetic. PONV is one of the most common perioperative complications, with a reported frequency of 20%–30%.20 However, no studies have examined the anesthetics used in MAC during an epiduroscopy and the anesthetic that would provide the maximum benefit is unclear.
Dexmedetomidine (DEX) has been used in cases where evaluation or treatment needs to be performed using approaches such as gastrointestinal endoscopy21 or cardiac catheterization,22 because of its sedative effects and general safety owing to its extremely low potential for respiratory depression. DEX is a strong and highly selective α2 adrenergic agent that has various pharmacologic actions.23 It is believed that the sedative effect occurs through the central α2A receptor of the locus coeruleus nucleus, and the blockage of pain occurs through the central α2A receptor of the spinal cord posterior horn.23 The combination of droperidol plus fentanyl (neuroleptanalgesia (NLA)), which provides balanced anesthesia, has been used for a long time.24 Droperidol is a strong neuroplegic with an α receptor blocking effect. It produces kinetic reflex restraint, psychic apathy, and a nerve interception state with the stabilization of the autonomic nervous system.25,26 Fentanyl is a synthetic opioid used worldwide.27 It is a full agonist with a very high selectivity for the μ-opioid receptor. The analgesic effect of fentanyl is considered to be approximately 200 times greater than that of morphine.28,29 Side effects of fentanyl include somnolence, respiratory depression, nausea, vomiting, and itch. However, the extent of nausea, vomiting, and itch has been reported to be less than that experienced as a side effect of morphine.30–35
Thus, the aim of this study was to establish a strategy for reducing the PONV associated with epiduroscopy procedures using DEX plus fentanyl or NLA, which are often used in MAC during epiduroscopy procedures in Japan. Therefore, we compared the two regimens in two different groups of patients (DEX and NLA groups) and investigated the differences in the type and dose of medication used during surgery and incidence of PONV.
Methods
Subjects and setting
All the patients who underwent an epiduroscopy at the JR Tokyo General Hospital from April 2011 to March 2016 were included in this study. Informed patient consent was obtained after epiduroscopy for collecting surgical and clinical data retrospectively, and this study was approved by the Research Ethics Committee of JR Tokyo General Hospital (approval no. H28-01). During the study period, there were no changes in the surgical facilities at JR Tokyo General Hospital, and the number of operating rooms (10), the shifts for nurses working in the operating room, the frequency of epiduroscopy procedures (2/month), the devices used, and the number of surgeons performing the procedure (2) did not change.
Exclusion criteria
This study compared the effect of DEX and droperidol, and, therefore, excluded patients who received an analgesic other than fentanyl or combined with fentanyl and those who received any sedative other than DEX or droperidol. Patients who had incomplete data, did not undergo the procedure in its entirety, or received a dose other than that listed earlier were also excluded.
Study design
This retrospective study used the clinical, anesthesia, and nursing records of patients. The anesthetics used in MAC during the epiduroscopy procedures were DEX plus fentanyl or droperidol plus fentanyl, and, based on these two regimens, the patients were classified into DEX and NLA groups, respectively. In both groups, prior to the start of surgery, the patients were placed in the prone position and their sacral hiatuses were sterilized with 10% povidone-iodine and then draped. The MAC was then initiated, an approximately 1-cm incision was created after induction of local anesthesia with mepivacaine 3 mL, the incision was dilated, and the epiduroscope was inserted.
The DEX group received an initial loading dose of DEX (6.0 μg kg−1 h−1, 10 min), as directed, and 50–150 μg of fentanyl was administered prior to the local anesthetic. After the surgery had commenced, the DEX group was administered a maintenance dose of DEX (0.2–0.7 μg kg−1 h−1) and a fentanyl 25–50 μg/dose was intermittently administered during the MAC until the conclusion of the surgery. The NLA group was administered fentanyl (50–200 μg) and droperidol (2.5–5.0 mg) before the local anesthetic was administered. After the surgery commenced, fentanyl (25–50 μg/dose) and droperidol (1.25 mg/dose) were intermittently administered during the MAC in accordance with the patient’s condition. At the discretion of the anesthesiologists in charge, both groups were administered the necessary doses of oxygen, vasopressors (ephedrine or phenylephrine), antihypertensives/vasodilators (nicardipine or diltiazem), or antiarrhythmic drugs (atropine, landiolol, or lidocaine).
Data analysis and outcome parameters
The following patient characteristics were recorded: age, sex, body mass index (BMI), the American Society of Anesthesiologists Physical Status (ASA-PS), regular use of analgesics, comorbidities, history of smoking, diagnosis, operating time, duration of anesthesia, the time from the conclusion of surgery until discharge, total fluid volume infused, oxygen dose, the type and dose of the anesthetic used, the type of medication used other than anesthetics, and the incidence of PONV.
Statistical analysis
The statistical analysis was designed to compare the DEX and NLA groups. Continuous parameters were expressed as means ± standard deviations (SDs) and independent t-tests were conducted to compare the groups. Categorical variables were expressed as frequencies and percentages, and chi-square tests were conducted to compare the groups. All the analyses were performed using STATA 13.1 software (Stata Corporation, College Station, TX, USA). The threshold for significance was set at p < 0.05.
Results
Patient selection
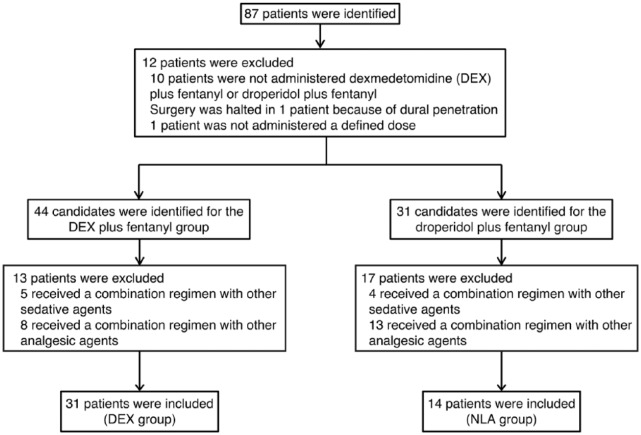
During the period of this study, 87 patients underwent epiduroscopy procedures, and 12 were excluded; 10 were not administered DEX plus fentanyl or droperidol plus fentanyl, 1 had the surgery halted because of dural penetration, and 1 was not administered a defined dose. Finally, 44 and 31 candidates were identified and included in the DEX and NLA groups, respectively. In the DEX group, 13 additional patients were excluded; 5 who received DEX plus fentanyl combined with other sedative agents and 8 who received DEX plus fentanyl combined with other analgesic agents.
In the NLA group, 17 patients were excluded; 4 who received droperidol plus fentanyl combined with other sedative agents and 13 who received droperidol plus fentanyl combined with other analgesic agents. Thus, 31 and 14 patients were analyzed in the DEX and NLA groups, respectively (Figure 1).
Figure 1.
Flowchart of patient selection.
Participants
A comparison of the patient characteristics and diagnoses in the DEX and NLA groups is shown in Table 1. Age, sex, BMI, ASA-PS, comorbidities (i.e. hypertension, diabetes mellitus, dyslipidemia, malignancy, cardiovascular disease, asthma, urological disease, thyroid disease, brain disease, peripheral nerve disease, dementia, and depression), analgesic agents used in the clinic (i.e. NSAIDs, narcotics, pregabalin, adjuvant remedy, and others), and smoking history were not significantly different between the two groups. Significant differences were not found between the DEX and NLA groups in the proportion of patients with a specific diagnosis (i.e. spinal stenosis, herniated disk, spondylolisthesis, or other condition). Pre-medication and PONV prophylaxis were not administered to all patients. The Apfel PONV factors of female patient ratio and non-smoker ratio were not significantly different between the groups. The PONV observation period was from the day of epiduroscopy until the day of discharge in both groups.
Table 1.
Comparison of patient characteristics, American Society of Anesthesiologists (ASA) physical status classification system, comorbidities, and analgesic agents between the DEX and NLA groups.
| Variables | DEX group | NLA group | p-value |
|---|---|---|---|
| Number enrolled | 31 | 14 | |
| Age (years), mean ± SD | 68.5 ± 1.83 | 70.1 ± 2.76 | 0.631 |
| Male/Female (%) | 12/19 (38.7/61.3) | 8/6 (57.1/42.9) | 0.249 |
| BMI (kg/m2, mean ± SD) | 23.4 ± 0.47 | 22.7 ± 0.70 | 0.426 |
| Tobacco | 0.428 | ||
| No (never smoked) | 16 | 9 | |
| Yes (former/current smoker) | 15 | 5 | |
| ASA physical status | 0.514 | ||
| Class 1 | 3 | 3 | |
| Class 2 | 24 | 10 | |
| Class 3 | 4 | 1 | |
| Diagnosis (%) | |||
| Spinal stenosis | 29 (93.6) | 11 (78.6) | 0.139 |
| Disk herniation | 5 (16.1) | 3 (21.4) | 0.667 |
| Spondylolisthesis | 2 (14.3) | 2 (6.5) | 0.393 |
| Other | 6 (19.4) | 2 (14.3) | 0.681 |
| Comorbidities (%) | |||
| Hypertension | 18 (58.1) | 8 (57.1) | 0.954 |
| Diabetes mellitus | 2 (6.5) | 1 (7.1) | 0.931 |
| Dyslipidemia | 14 (45.2) | 5 (35.7) | 0.552 |
| Malignancy | 4 (12.9) | 3 (21.4) | 0.465 |
| Cardiovascular disease | 3 (9.7) | 1 (7.1) | 0.782 |
| Asthma | 4 (12.9) | 1 (7.1) | 0.569 |
| Urological disease | 4 (12.9) | 2 (14.3) | 0.900 |
| Thyroid disease | 1 (3.2) | 1 (7.1) | 0.555 |
| Brain disease | 5 (16.1) | 1 (7.1) | 0.411 |
| Peripheral nerve disease | 2 (6.5) | 1 (7.1) | 0.931 |
| Dementia | 1 (3.2) | 1 (7.1) | 0.555 |
| Depression | 2 (6.5) | 0 | 0.331 |
| Analgesic agents used in clinic (%) | |||
| NSAIDs | 12 (38.7) | 7 (50.0) | 0.478 |
| Narcotics | 5 (16.1) | 2 (14.3) | 0.875 |
| Pregabalin | 14 (45.2) | 5 (35.7) | 0.553 |
| Adjuvant remedy | 22 (71.0) | 12 (85.7) | 0.287 |
| Other | 24 (77.4) | 13 (92.3) | 0.210 |
ASA: The American Society of Anesthesiologists; DEX group: dexmedetomidine plus fentanyl; NLA group: droperidol plus fentanyl (neuroleptanalgesia); BMI: body mass index; NSAIDs: non-steroidal anti-inflammatory drugs; SD: standard deviation.
Main results
A comparison of the incidence of PONV and the dose of fentanyl during surgery between the DEX and NLA groups is shown in Figure 2. The presence or absence of PONV was determined based on the history of receiving an antiemetic (metoclopramide or domperidone), which was noted in significantly fewer patients in the DEX group than in the NLA group (1 vs 3, p = 0.047). A significantly lower dose of the anesthetic fentanyl was used during surgery in the DEX group than in the NLA group (126 ± 14 vs 193 ± 21 µg, respectively; mean ± SD, p = 0.014). Significant intergroup differences were not observed in the use of other intraoperative drugs during surgery (Table 2).
Figure 2.
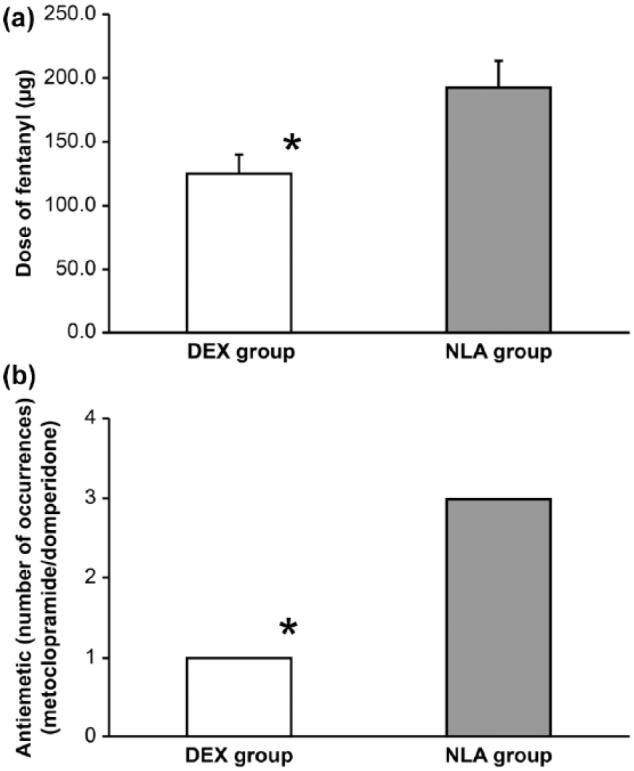
(a) Comparison of the doses of fentanyl and (b) incidence of postoperative nausea and vomiting (PONV) between the dexmedetomidine plus fentanyl (DEX group) and neuroleptanalgesia (droperidol plus fentanyl (NLA group)) groups. Doses of fentanyl are expressed as mean ± standard deviations (SD). PONV was determined based on history of receiving an antiemetic (metoclopramide or domperidone).
*p < 0.05 vs corresponding NLA group.
Table 2.
Comparison of intraoperative drugs between the DEX and NLA groups.
| DEX group (n = 31) | NLA group (n = 14) | p-value | |
|---|---|---|---|
| Nicardipine | 6 | 5 | 0.237 |
| Diltiazem | 1 | 0 | 0.497 |
| Landiolol | 0 | 2 | 0.031 |
| Lidocaine | 0 | 1 | 0.132 |
| Atropine | 5 | 1 | 0.412 |
| Phenylephrine | 0 | 1 | 0.132 |
| Ephedrine | 1 | 0 | 0.497 |
DEX group: dexmedetomidine plus fentanyl; NLA group: droperidol plus fentanyl (neuroleptanalgesia).
The comparison of the surgery- and anesthesia-related items between the DEX and NLA groups is shown in Table 3. The differences in the operating time, duration of anesthesia, time from the conclusion of surgery until discharge, total fluid volume infused, and oxygen dose were not significant between the two groups.
Table 3.
Comparison of surgery- and anesthesia-related items between the DEX and NLA groups.
| DEX group (n = 31) | NLA group (n = 14) | p-value | |
|---|---|---|---|
| Operation time (minimum, mean ± SD) | 61.5 ± 1.9 | 61.1 ± 2.9 | 0.930 |
| Anesthetic time (minimum, mean ± SD) | 100.5 ± 2.95 | 97.9 ± 4.39 | 0.333 |
| The time from the conclusion of surgery until discharge (minimum, mean ± SD) | 13.5 ± 0.80 | 12.1 ± 1.20 | 0.333 |
| Infusion volume (mL, mean ± SD) | 459.7 ± 20.8 | 483.6 ± 31.0 | 0.526 |
| Oxygen flow rate (L, mean ± SD) | 215.2 ± 28.1 | 274.9 ± 41.9 | 0.243 |
DEX group: dexmedetomidine plus fentanyl; NLA group: droperidol plus fentanyl (neuroleptanalgesia); SD: standard deviation.
Discussion
Summary of findings
The current results indicate that the use of DEX during epiduroscopy procedures results in a lower incidence of PONV, a reduced dose of fentanyl use during surgery, and no change in the duration of anesthesia.
Impact of DEX during epiduroscopy
We found that the use of DEX reduced the intraoperative requirement for fentanyl compared to that with the use of NLA. Previous studies of other anesthetics similarly showed that the intraoperative use of DEX reduced the required dose of fentanyl,36–40 which was attributed to the slight analgesic activity exhibited by DEX.41 The patients who underwent epiduroscopy were elderly, and this demographic typically exhibits reduced clearance and a prolonged elimination half-life of fentanyl, suggesting that they are far more sensitive to the drug than younger individuals are.42 Thus, elderly patients have a higher risk of developing more severe respiratory depression faster during sedation than younger individuals do. The increased susceptibility of elderly patients to respiratory depression during sedation with fentanyl precludes the prediction of its effect site concentration during target-controlled infusion (TCI). In respiratory depression during MAC, maintaining an open airway in elderly patients can prove difficult, and circumstances can hamper those efforts, such as the patient being in a prone position and breathing spontaneously. Thus, reducing the dose of fentanyl during surgery is crucial, and the use of DEX could facilitate the prevention of perioperative respiratory depression.
Reduction of PONV
In this study, we noted no significant differences between the two groups with respect to age, sex, duration of anesthesia, and history of smoking. However, patients who were anesthetized with DEX had a significantly lower incidence of PONV than those who were anesthetized with NLA. Previous meta-analyses of randomized controlled trials of other anesthetics showed similar results, that is, the use of DEX reduced the incidence of PONV.36–39,43 This study indicates that the use of DEX could contribute to reducing fentanyl dosage.
PONV has little immediate effect on the life expectancy of patients, but persistent PONV can cause serious complications, such as dehydration, electrolyte abnormalities, aspiration, and aspiration pneumonia.44 This situation is more serious in the elderly. The elderly often have physiological difficulties in swallowing. In addition, there are often comorbidities and regularly used medications that could influence swallowing function. As such, they often have a higher risk of aspiration than younger individuals.45–47 Postoperatively, aspiration is likely to be caused by the residual action of a sedative or narcotic, and the antiemetics used to treat PONV can also cause aspiration.47 Therefore, initiating steps to treat PONV is vital,48 especially to prevent dehydration, electrolyte abnormalities, aspiration, and aspiration pneumonia. DEX is an exceptional sedative that can reduce the incidence of PONV in MAC when an elderly patient undergoes epiduroscopy.
Study limitations
This study had a few limitations that are worth mentioning. First, the sample size was small and was acquired from a single institution. Therefore, larger, multicenter studies will need to be conducted in the future. Second, this was a retrospective study, and the only information available was what was recorded in the existing records. However, factors that could affect the results were identified, and these did not differ between the groups of patients.
Conclusion
This study investigated how the use of DEX in MAC during epiduroscopy may reduce the required dose of fentanyl during surgery and the incidence of PONV in elderly patients. Thus, the avoidance of high doses of fentanyl using DEX may help prevent respiratory depression and aspiration in the elderly.
Acknowledgments
The authors are very grateful to the operational staff who participated in this survey and thank Drs Nagase, Ogawa, Kobayashi, Takeda, and Inoue. T.S. and R.I. conceived the study. R.I. performed the statistical analyses. T.S. collected the data and wrote the first draft of the manuscript. R.I. critically reviewed the manuscript. All the authors contributed to the design, interpretation of the results, and critical revision of the article for intellectually important content.
Footnotes
Availability of data and materials: All datasets, on which the conclusions of the manuscript rely, are presented in the main paper.
Declaration of conflicting interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: This study was approved by the Research Ethics Committee of JR Tokyo General Hospital (approval no. H28-01).
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from all subjects before the study.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Kaplan W, Wirtz VJ, Mantel-Teeuwisse A, et al. Priority medicines for Europe and the World 2013 update. World Health Organization, http://www.who.int/medicines/areas/priority_medicines/MasterDocJune28_FINAL_Web.pdf (accessed 23 December 2017).
- 2. Bredow J, Bloess K, Oppermann J, et al. Conservative treatment of nonspecific, chronic low back pain: evidence of the efficacy—a systematic literature review. Orthopade 2016; 45: 573–578 (in German). [DOI] [PubMed] [Google Scholar]
- 3. Itz CJ, Geurts JW, Van Kleef M, et al. Clinical course of non-specific low back pain: a systematic review of prospective cohort studies set in primary care. Eur J Pain 2013; 17: 5–15. [DOI] [PubMed] [Google Scholar]
- 4. Ruetten S, Meyer O, Godolias G. Epiduroscopic diagnosis and treatment of epidural adhesions in chronic back pain syndrome of patients with previous surgical treatment: first results of 31 interventions. Z Orthop Ihre Grenzgeb 2002; 140: 171–175 (in German). [DOI] [PubMed] [Google Scholar]
- 5. Watanabe H, Takiguchi N, Chiba T, et al. Post-adhesiotomy relationships efficiency of epiduroscopy and X-ray image of peridurography. J Jpn Soc Clin Anesth 2014; 34: 312–318 (in Japanese). [Google Scholar]
- 6. Olmarker K, Larsson K. Tumor necrosis factor-alpha and nucleus-pulposus-induced nerve root injury. Spine 1998; 23: 2538–2544. [DOI] [PubMed] [Google Scholar]
- 7. Igarashi T, Kikuchi S, Shubayev V, et al. 2000 Volvo award winner in basic science studies: exogenous tumor necrosis factor-alpha mimics nucleus pulposus-induced neuropathology. Molecular, histologic, and behavioral comparisons in rats. Spine 2000; 25: 2975–2980. [DOI] [PubMed] [Google Scholar]
- 8. Olmarker K, Rydevik B. Selective inhibition of tumor necrosis factor-alpha prevents nucleus pulposus-induced thrombus formation, intraneural edema, and reduction of nerve conduction velocity: possible implications for future pharmacologic treatment strategies of sciatica. Spine 2001; 26: 863–869. [DOI] [PubMed] [Google Scholar]
- 9. Onda A, Hamba M, Yabuki S, et al. Exogenous tumor necrosis factor-alpha induces abnormal discharges in rat dorsal horn neurons. Spine 2002; 27: 1618–1624. [DOI] [PubMed] [Google Scholar]
- 10. Otani K. Pathogenesis of failed back syndrome: including personal opinion of epiduroscopy. J Jpn Soc Pain Clin 2005; 12: 59–68 (in Japanese). [Google Scholar]
- 11. Saberski LR, Kitahata LM. Direct visualization of the lumbosacral epidural space through the sacral hiatus. Anesth Analg 1995; 80: 839–840. [DOI] [PubMed] [Google Scholar]
- 12. Igarashi T, Suzuki H, Murai K, et al. Future of epiduroscopy. J Jpn Soc Clin Anesth 2010; 30: 304–309 (in Japanese). [Google Scholar]
- 13. Igarashi T, Murai K, Shimada N, et al. An update the epiduroscopy/spinal canal endoscopy. J Jpn Soc Clin Anesth 2014; 34: 296–301 (in Japanese). [Google Scholar]
- 14. Taira Y, Higa Y, Kajisa J, et al. Epiduroscopy. Masui 2014; 63: 752–758 (in Japanese). [PubMed] [Google Scholar]
- 15. Takeshima N, Takatani J, Okuda K, et al. An epiduroscopy method using anesthesia, puncture, and adhesiolysis, epidural catheterization. J Jpn Soc Clin Anesth 2010; 30: 291–296 (in Japanese). [Google Scholar]
- 16. Bosscher HA, Heavner JE. Incidence and severity of epidural fibrosis after back surgery: an endoscopic study. Pain Pract 2010; 10: 18–24. [DOI] [PubMed] [Google Scholar]
- 17. Bosscher HA, Heavner JE. Diagnosis of the vertebral level from which low back or leg pain originates. A comparison of clinical evaluation, MRI and epiduroscopy. Pain Pract 2012; 12: 506–512. [DOI] [PubMed] [Google Scholar]
- 18. Bosscher HA, Heavner JE. Lumbosacral epiduroscopy findings predict treatment outcome. Pain Pract 2014; 14: 506–514. [DOI] [PubMed] [Google Scholar]
- 19. Ghisi D, Fanelli A, Tosi M, et al. Monitored anesthesia care. Minerva Anestesiol 2005; 71: 533–538. [PubMed] [Google Scholar]
- 20. Gan TJ, Diemunsch P, Habib AS, et al. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg 2014; 118: 85–113. [DOI] [PubMed] [Google Scholar]
- 21. Takimoto K, Ueda T, Shimamoto F, et al. Sedation with dexmedetomidine hydrochloride during endoscopic submucosal dissection of gastric cancer. Dig Endosc 2011; 23: 176–181. [DOI] [PubMed] [Google Scholar]
- 22. Kunisawa T, Kurosawa A, Hayashi D, et al. Administration of dexmedetomidine alone during diagnostic cardiac catheterization in adults with congenital heart disease: two case reports. J Anesth 2011; 25: 599–602. [DOI] [PubMed] [Google Scholar]
- 23. Kamibayashi T, Maze M. Clinical uses of alpha2-adrenergic agonists. Anesthesiology 2000; 93: 1345–1349. [DOI] [PubMed] [Google Scholar]
- 24. Koga Y. Balanced anesthesia (NLA) in the past. Masui 1995; 44: S144–S146 (in Japanese). [PubMed] [Google Scholar]
- 25. Janssen PA, Niemegeers CJ, Schellekens KH, et al. The pharmacology of dehydrobenzperidol, a new potent and short acting neuroleptic agent chemically related to Haloperidol. Arzneimittelforschung 1963; 13: 205–211. [PubMed] [Google Scholar]
- 26. Yelnosky J, Gardocki JF. A study of some of the pharmacologic actions of fentanyl citrate and droperidol. Toxicol Appl Pharmacol 1964; 6: 63–70. [DOI] [PubMed] [Google Scholar]
- 27. Peng PW, Sandler AN. A review of the use of fentanyl analgesia in the management of acute pain in adults. Anesthesiology 1999; 90: 576–599. [DOI] [PubMed] [Google Scholar]
- 28. Gardocki JF, Yelnosky J. A study of some of the pharmacologic actions of fentanyl citrate. Toxicol Appl Pharmacol 1964; 6: 48–62. [DOI] [PubMed] [Google Scholar]
- 29. Gardocki JE, Yelnosky J, Kuehn WF, et al. A study of the interaction of nalorphine with fentanyl and innovar. Toxicol Appl Pharmacol 1964; 6: 593–601. [DOI] [PubMed] [Google Scholar]
- 30. Fischer RL, Lubenow TR, Liceaga A, et al. Comparison of continuous epidural infusion of fentanyl-bupivacaine and morphine-bupivacaine in management of postoperative pain. Anesth Analg 1988; 67: 559–563. [PubMed] [Google Scholar]
- 31. Saito Y, Uchida H, Kaneko M, et al. Comparison of continuous epidural infusion of morphine/bupivacaine with fentanyl/bupivacaine for postoperative pain relief. Acta Anaesthesiol Scand 1994; 38: 398–401. [DOI] [PubMed] [Google Scholar]
- 32. Ozalp G, Güner F, Kuru N, et al. Postoperative patient-controlled epidural analgesia with opioid bupivacaine mixtures. Can J Anaesth 1998; 45: 938–942. [DOI] [PubMed] [Google Scholar]
- 33. Berti M, Fanelli G, Casati A, et al. Comparison between epidural infusion of fentanyl/bupivacaine and morphine/bupivacaine after orthopaedic surgery. Can J Anaesth 1998; 45: 545–550. [DOI] [PubMed] [Google Scholar]
- 34. White MJ, Berghausen EJ, Dumont SW, et al. Side effects during continuous epidural infusion of morphine and fentanyl. Can J Anaesth 1992; 39: 576–582. [DOI] [PubMed] [Google Scholar]
- 35. Oda A, Ishiyama T, Suzuki A, et al. Effects of continuous epidural administration of fentanyl and morphine on postcesarean pain. Masui 1996; 45: 1511–1515 (in Japanese). [PubMed] [Google Scholar]
- 36. Jin S, Liang DD, Chen C, et al. Dexmedetomidine prevent postoperative nausea and vomiting on patients during general anesthesia: a PRISMA-compliant meta analysis of randomized controlled trials. Medicine 2017; 96: e5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhong WG, Ge XY, Zhu H, et al. Dexmedetomidine for antiemesis in gynecologic surgery: a meta-analysis of randomized controlled trials. Int J Clin Exp Med 2015; 8: 14566–14576. [PMC free article] [PubMed] [Google Scholar]
- 38. Liang X, Zhou M, Feng JJ, et al. Efficacy of dexmedetomidine on postoperative nausea and vomiting: a meta-analysis of randomized controlled trials. Int J Clin Exp Med 2015; 8: 8450–8471. [PMC free article] [PubMed] [Google Scholar]
- 39. Le Bot A, Michelet D, Hilly J, et al. Efficacy of intraoperative dexmedetomidine compared with placebo for surgery in adults: a meta-analysis of published studies. Minerva Anestesiol 2015; 81: 1105–1117. [PubMed] [Google Scholar]
- 40. Huncke TK, Adelman M, Jacobowitz G, et al. A prospective, randomized, placebo-controlled study evaluating the efficacy of dexmedetomidine for sedation during vascular procedures. Vasc Endovascular Surg 2010; 44: 257–261. [DOI] [PubMed] [Google Scholar]
- 41. Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med 2002; 166: 1338–1344. [DOI] [PubMed] [Google Scholar]
- 42. Bentley JB, Borel JD, Nenad RE, et al. Age and fentanyl pharmacokinetics. Anesth Analg 1982; 61: 968–971. [PubMed] [Google Scholar]
- 43. Wang G, Zhang L, Lou S, et al. Effect of dexmedetomidine in preventing postoperative side effects for laparoscopic surgery: a meta-analysis of randomized controlled trials and trial sequential analysis (PRISMA). Medicine 2016; 95: e2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Apfel CC, Heidrich FM, Jukar-Rao S, et al. Evidence-based analysis of risk factors for postoperative nausea and vomiting. Br J Anaesth 2012; 109: 742–753. [DOI] [PubMed] [Google Scholar]
- 45. Igarashi T, Hirabayashi Y, Seo N, et al. Lysis of adhesions and epidural injection of steroid/local anaesthetic during epiduroscopy potentially alleviate low back and leg pain in elderly patients with lumbar spinal stenosis. Br J Anaesth 2004; 93: 181–187. [DOI] [PubMed] [Google Scholar]
- 46. Wirth R, Dziewas R, Beck AM, et al. Oropharyngeal dysphagia in older persons—from pathophysiology to adequate intervention: a review and summary of an international expert meeting. Clin Interv Aging 2016; 11: 189–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. By the American Geriatrics Society 2015 Beers Criteria Update Expert Panel, Fick DM, Semla TP, et al. American Geriatrics Society 2015 updated beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015; 63: 2227–2246. [DOI] [PubMed] [Google Scholar]
- 48. Scuderi PE, Conlay LA. Postoperative nausea and vomiting and outcome. Int Anesthesiol Clin 2003; 41: 165–174. [DOI] [PubMed] [Google Scholar]