Abstract
The development of peripheral lymphoid tissues from the mesoderm is the result of a complex convergence combining lymphohematopoietic differentiation with the local specification of nonhematopoietic mesenchymal components. Although the various transcriptional regulators with fate-determining effects in diversifying the mobile leukocyte subsets have been thoroughly studied and identified, the tissue-specific determinants promoting the regional differentiation of resident mesenchyme are less understood. Of these factors, various members of the NK-class Nkx paralogues have emerged as key regulators for the organogenesis of spleen and mucosal lymphoid tissues, and recent data have also indicated their involvement in various pathological events, including gut inflammation and hematopoietic malignancies. Here, we summarize available data on the roles of Nkx2-3 in lymphoid tissue development and discuss its possible value as a developmental marker and disease-associated pathogenic trait.
Keywords: Nkx2-3, lymphoid organs, lymphoma, inflammation
Introduction: Roles of Nkx Family Members in Cellular Differentiation and Organogenesis—A General Overview
The development of complex organisms such as the mammalian body requires a highly regulated coordination of gene expression. Homeobox genes containing helix-turn-helix DNA-binding motif first described in Drosophila are master regulators of developmental processes such as mesodermal patterning. The largest group of homeobox genes comprises the HOX (including the extended HOX) class of transcription factors, which may be phylogenetically related to the NK class of homeobox genes that constitutes the second largest group of homeobox-encoding genes. In Drosophila, there are 2 classes of NK genes, the NK-1 class containing the NK-1 family and the NK-2 class with the highly related NK-2, NK-3, and NK-4 families. Two NK-2 class genes, namely, tinman and bagpipe, are responsible for dorsal heart tube formation, mesodermal differentiation, and development of gut musculature, respectively.1 Vertebrate homologues of ancient NK-class homeobox containing genes were identified on their conserved tyrosine residues.2,3
All NK-2 members are responsible for the development of a certain organ or tissue, and most of them share an overlapping expression pattern. They are able to activate signaling pathways responsible for cell differentiation, migration, and maturation, thus their activity is essential to the formation and maintenance of a normally structured and functioning organism.4
Regulation through Nkx transcription factors encoded by NK-2 genes is a well-balanced system, where every small change can lead to severe alterations. This complexity is well illustrated by the multiple roles of the NK-2 class members. Nkx2.1 and Nkx2.8 are responsible for lung epithelial development and surfactant production, whereas Nkx2.1 is also crucial in the development of thyroid and pituitary glands.5 Alterations in the Nkx2.1 gene can cause neurological defects, congenital hypothyroidism, and lung malformations in humans.6 In addition, Nkx2.1 also acts as a proto-oncogene in lung tumors,7 and it is overexpressed in lung adenocarcinoma and small cell lung cancer.8 Due to its tissue specificity, it can be used for the identification of metastatic cells of lung epithelial origin.9 Furthermore, Nkx2.1 and Nkx2.8 also have a prognostic value, as their upregulation in human lung squamous carcinoma cells resulted in resistance to cisplatin, a chemotherapeutic agent.10,11
Nkx2.2 and Nkx2.9 share an overlapping expression pattern in neural progenitors. They are necessary for the development of spinal cord V3 interneurons and hindbrain visceral motor neurons, as the spinal cords of mice lacking these factors have reduced number of V3 neurons and expanded motor neurons.12
Another important factor is Nkx2.5, an early myogenic marker detected in mouse embryos from embryonic day 7.5 (E7.5), which is responsible for the development of the atrioventricular node and the myocardium. Nkx2.5 is responsible for cardiac looping and the expression of several other transcription factors essential for heart development, angiogenesis, and hematopoiesis, thus its mutation causes an arrest in heart development and embryonic lethality in mice.13,14 In humans, mutations of Nkx2.5, such as a single-nucleotide deletion that was correlated with congenital heart disease, can cause atrial septal defect and sudden cardiac death.15 Furthermore, Nkx2.6 has a similar role in heart development; however, its role is less important because Nkx2.6 null mice are viable due to the compensatory effect of Nkx2.5. This compensation is probably related to the ectopic expression of Nkx2.5 in the lateral pharynx where normally only Nkx2.6 is expressed.16
Besides the nervous system, the vascular system and airways, combinatorial expression of Nkx factors can also be observed in abdominal visceral organs, especially in the stomach (Nkx2.5 and Nkx2.6) and spleen (Nkx2.5 and Nkx2.3).
Although the various NK-2 homologues have different sequences reflecting different functions, they display overlapping homeodomain structure and DNA-sequence specificities as defined by its first helical region and carboxy-terminal region and often overlapping expression patterns. In contrast to most of the NK class members which bind DNA with TAAT core sequences,17,18 homeoproteins of the NK-2 class bind DNA with CAAG core sequences.19,20 The specific overlapping or distinct expression and DNA-binding specificities of these Nkx factors create a special transcriptional setting, which is essential in development, differentiation, and adult tissue patterning in an organ-specific manner, collectively referred to as “Nkx code.”1
Roles of Nkx Family Members in the Formation of Lymphoid Tissues and Intestinal Inflammation
Nkx family members also play crucial roles in lymphoid organ development. The spleen, the largest peripheral lymphoid organ, develops following signals originating from the splanchnic mesodermal plate (SMP) during embryonic development,21 a transient structure expressing Nkx3-2 (also known as Bapx1). The Nkx3-2–producing SMP has an essential role in the formation of left-right asymmetry21 and the development of spleen from E10-10.5. The absence of this factor causes asplenia and disturbed pyloric sphincter formation due to impairments of the visceral mesoderm. As SMP formation is permitted in the absence of Nkx3-2, it is likely that this factor is not necessary for the appearance of SMP but is required for its maturation to provide further factors promoting spleen development.
Another Nkx member, Nkx2-5, is also expressed in the developing spleen and its absence also leads to asplenia.22 Both Nkx3-2 and Nkx2-5 can serve as early markers of splenic development. Nkx2-5 is expressed in specified splenic mesenchymal cells which will form the splenic anlage.23 Among these mesenchymal cells, we can find lymphoid tissue organizer cells that serve as stromal precursors of secondary lymphoid organs.24 According to Castagnaro et al, all splenic stromal cells (fibroblastic reticular cells, marginal reticular cells, follicular dendritic cells [FDCs], and mural cells) derived from Nkx2-5+Islet1+IL-7+ mesenchymal precursors. Interestingly, this derivation is spleen specific, as Nkx2-5 is not involved in the stromal development of mesenteric lymph nodes and Peyer’s patches.25
In contrast to Nkx2-5 and Nkx3-2 which take part only in spleen development among peripheral lymphoid organs, Nkx2-3 is involved in the formation of other lymphoid tissues as well. Nkx2-3 is expressed in the spleen, midgut, hindgut, and pharyngeal endoderm.26 Nkx2-3 is crucial for the formation of visceral mesoderm which gives rise to several cell types, such as vascular and intestinal smooth muscle cells, endothelial cells involved in leukocyte traffic, and stromal cells of secondary lymphoid organs.23 The main effect of Nkx2-3 is the expression of the mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in endothelial cells. MAdCAM-1 has a crucial role in lymphocyte homing to mucosal tissues by binding integrin α4β7 and L-selectin leukocyte homing receptors.27
Nkx2-3 deficiency in mice results in atrophic disorganized spleen, fewer and smaller Peyer’s patches with altered endothelial addressin expression, enlarged and disorganized colonic crypts, abnormal villus formation, and altered lymphocyte homing.28 In the spleen, the possible target cells affected by Nkx2-3 deficiency are the red pulp (RP) sinus endothelial cells, as in mice lacking Nkx2-3, the venous sinus network identifiable by IBL-9/2 rat mAb29 is completely missing. The absence of Nkx2-3 also disrupts the architecture of marginal sinus, including the organization of marginal zone (MZ) macrophage subpopulations and MAdCAM-1–positive marginal sinus-lining endothelial cells, similar to the deficiency of lymphotoxin β receptor (LTβR); however, the RP vasculature is unperturbed in the absence of LTβR.30 In human spleen, the presence of RP venous sinuses lined by CD31+/CD34−/vWF+ endothelial cells that express Nkx2-3 supports the involvement of Nkx2-3 in the regional specification of endothelium.31 Furthermore, the absence of Nkx2-3 in mice causes ectopic differentiation of splenic vessels into lymph node like high endothelial venules (HEVs) displaying PNAd homing addressin in an LTβR-dependent process.32 Similarly, the constitutive activation of the noncanonical nuclear factor κB pathway mediating LTβR signaling in p100−/−/p52knock-in mice also resulted in ectopic PNAd-positive splenic HEVs; however, these endothelial cells coexpressed both MAdCAM-1 and PNAd,33 whereas the HEVs in adult Nkx2-3–deficient mice only produced PNAd addressin.32 Interestingly, Nkx2-3 may also affect the vascular commitment of spleen at a very early stage, as its absence also induced the expression of sac-like structures within the spleen lined by endothelial cells displaying LYVE-1 hyaluronan receptor, characteristic for lymphatic endothelia.34 In summary, although some features of the absence of Nkx2-3 are reminiscent of the consequences of LTβR inactivation, the “gradient” of splenic tissue alterations manifests in an opposite directionality—in Nkx2-3−/− mice, the most severe splenic defect affects the RP, with somewhat lesser defects of white pulp and follicles (including preserved FDCs), whereas in Ltbr−/− mice, the RP appears intact, but the white pulp and follicles (lacking FDCs) are severely perturbed. The MZ is affected in both conditions. Furthermore, the formation of Peyer’s patches in Nkx2-3–deficient mice is partially blocked,35 and in the mucosal HEVs, the MAdCAM-1 addressin is also replaced by PNAd.36
Microvascular endothelial cells are important mediators of intestinal homeostasis and inflammation. Activation of this endothelial layer by bacterial and other agents results in the upregulation of adhesion molecules and chemokines necessary for the vascular attachment and migration of leukocytes. Altered expression of endothelial markers such as Nkx2-3 and its potential target MAdCAM-1 can cause a perturbation in leukocyte traffic and inflammatory response. Consequently, the development of inflammatory bowel diseases (IBD) such as Crohn disease (CD) and ulcerative colitis (UC) can be linked to these endothelial factors.37,38 In the past few years, several genome-wide association studies have demonstrated an association between altered expression of Nkx2-3 and IBDs. According to Xiao et al, single-nucleotide polymorphisms of Nkx2-3 (namely, rs10883365 and rs1190140) are associated with CD and UC. Polymorphism rs10883365 may contribute significantly to the emergence of both CD and UC, whereas occurrence of the T allele of rs1190140 can increase the risk of CD.39
Increased expression of Nkx2-3 at both RNA and protein level was also demonstrated in intestinal samples of patients with CD.38 Connor et al40 reported that elevated Nkx2-3 level may lead to the upregulation of MAdCAM-1 at inflammatory sites. Tumor necrosis factor α can further augment adhesion molecule expression in the intestines and it may crossregulate the genes affected by Nkx2-3.38
In other studies, 125 Nkx2-3–regulated genes were identified in an Nkx2-3 knockdown B-cell line using genome-wide gene expression microarray analysis. This cell line was originated from a patient with CD and showed downregulation of 33 and upregulation of 92 genes following Nkx2-3 knockdown.41 In this study, a comprehensive list of inflammation-associated genes and their subpathways that are regulated by Nkx2-3 was also provided. According to these findings, Nkx2-3 knockdown caused downregulation of CXCR7 and upregulation of CXCL1, CCL22, and CXCL10 chemokines, hence affecting the chemotactic activities in immune responses.41 Decreased Nkx2-3 levels also influenced the MEF2/KLF2 pathway, which has an important role in the maintenance of microvascular endothelial balance and induction of inflammation.42 However, KLF2 also regulates the vasoactive peptide endothelin-1 (END-1), which may have role in the development of IBD. END-1 expression negatively correlates with Nkx2-3 as it was downregulated in the intestinal tissue of patients with UC and CD.38
Recently, a positive feedback loop in the expression of AOC3 (amine oxidase, copper containing 3, a marker expressed on cell surface, thus enabling sorting of viable cells) and Nkx2-3 was observed both in human myofibroblast cell lines and primary cultures as colorectal epithelial stem cell niche components. Intriguingly, Nkx2-3 expression not only distinguished intestinal myofibroblasts from skin fibroblasts but it also turned out to be a determinant in the preservation of myofibroblast identity, presumably involved in regulating colonic stem cell niche.43 This was further proven using small interfering RNA–mediated knockdown of Nkx2-3 in myofibroblast lines, inducing cells to acquire a fibroblast-like genetic profile. The exact connection between the Nkx2.3+ myofibroblasts and the recently discovered CD34+ gp38+ nonmyofibroblastic mesenchymal cells that create a niche for intestinal epithelial stem cells44 remains to be elucidated.
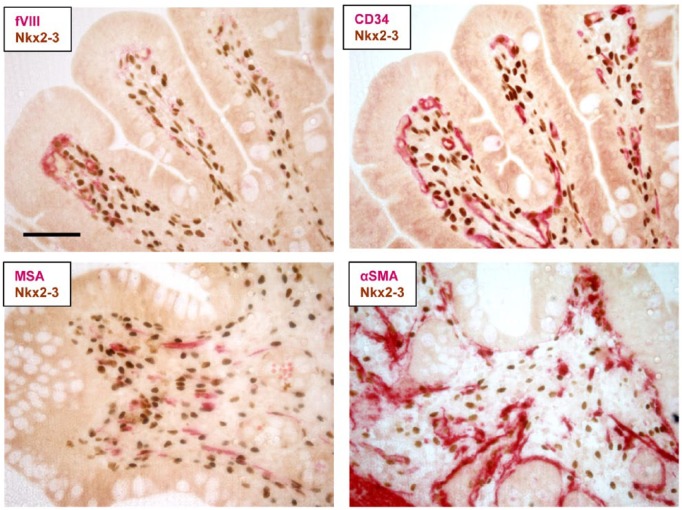
The lineage-specific expression of Nkx2-3 has not been formally defined yet either in humans or in mice. Using a recently developed and validated immunohistochemistry-grade anti-human Nkx2-3 mAb31,45 may in the future enable a conclusive assessment to be achieved, even though its expression is probably shared by several mesenchymal cells in a regionally restricted manner of the colonic subepithelial connective tissue (Figure 1).
Figure 1.
Immunohistochemical localization of Nkx2-3 protein expression in human colon in formaldehyde-fixed paraffin-embedded (FFPE) biopsy. Using reference labeling (in red color) for endothelial markers (factor VIII [upper left] or CD34 [upper right]) or myofibroblast-smooth muscle markers (muscle-specific actin, MSA [lower left] or alpha-smooth muscle actin, αSMA [lower right]) in dual immunohistochemistry reveals both shared expression and partial overlap with Nkx2-3–positive cells (brown nuclear staining). Scale bar, 100 µm.
Involvement of Nkx2-3 in Hematopoietic Malignancies
In addition to exerting important functions in the vascular specification of spleen and Peyer’s patches, recently, Nkx2-3 has also been implicated in lymphocyte differentiation. Although lack of Nkx2-3 blocks the development of splenic MZ B cells probably through an altered microenvironment,35 a recent study showed that the B–cell-restricted overexpression of Nkx2-3 due to the translocation of NKX2-3 gene to the Ig heavy chain (IgH) causes splenic MZ lymphoma. This juxtaposition of 14g32.33 (IgH) and 10q24.2 (NKX2-3) was identified by break point cloning analysis of B-cell lymphoma samples.45 In a transgenic (Tg) mouse model using a similar translocation, an oligoclonal expansion occurs first as a result of increased B-cell receptor signaling and enhanced adhesiveness through several adhesion molecules, including LFA-1 integrin, ICAM-1, and MAdCAM-1 adhesion molecules. This condition later evolves into a nongerminal center-type diffuse large B-cell lymphoma (non-GC DLBCL) similar to human mucosa-associated lymphoid tissue and MZ lymphomas. Interestingly, despite the presence of the translocation, the lymphoma usually occurred at a slow pace, beyond 1 year of age in Tg mice. In a large cohort of B-cell malignancies, increased Nkx2-3 expression was detected in 3% of the samples, with higher frequency (6%-7%) in splenic MZ lymphomas and MALT lymphomas, corresponding to the regional expression of Nkx2-3; however, in other forms of B-cell lymphomas without such regional confinement (DLBCL, follicular lymphoma, mantle cell lymphoma, chronic lymphocytic leukemia, or multiple myeloma), upregulated Nkx2-3 was found in less than 1% of the cases.45 It remains to be seen whether the expression of Nkx2-3 may reflect differences either in the clinical course (including regional involvement) or in therapeutic responses within homogeneous groups of various B-cell malignancies.
Regarding various precursor B-cell alterations induced by Nkx2-3 deviations, the absence of Nkx2-3 in mice caused no marked alteration in the bone marrow B-cell lineage composition.35 However, in a type of B-cell acute lymphoblastic leukemia (B-ALL) characterized by ETV6/RUNX1 positivity, Nkx2-3 may also be involved. This fusion is created through a t(12;21)(p13;q22) translocation of ETS variant 6 and Run-related transcription factor-1 (RUNX1). A recent study investigated ETV6/RUNX1-positive B-cell precursor-ALL (BCP-ALL)–associated long-coding (lnc) messenger RNA (mRNA) expression patterns, and they identified 16 lnc mRNAs associated with ETV6/RUNX1 in BCP-ALL samples. They also described binding of a histone modification H3K27ac—a regulatory enhancer element—with the ETV6/RUNX1-specific lnc mRNAs such as lnc-NKX2-3-1 in REH cells. Short hairpin RNA silencing of the fusion protein has also confirmed that lnc-NKX2-3-1 and other 3 lnc mRNAs are regulated by ETV6/RUNX1. Although most of these lnc mRNAs have an effect on the translational level, lnc-NKX2-3 and lnc-RTN4R-1 rather act transcriptionally and cause significant alterations of gene expression.46–48
In addition to BCP malignancies, Nkx2-3 among other members of NK-like (NKL) homeobox proteins has recently also been involved in T-cell ALL (T-ALL). In cases with blocked T-cell receptor α-chain rearrangement, the maturation arrest in a subset of T-ALL was associated with ectopic expression of Nkx2-3 or other members of NKL factors, causing rearrangement inhibition through the repression of TcRα enhancer (Eα).49
The potential involvement of other Nkx members in T-ALL was previously established, which also demonstrated a connection between TAL1, miR-17-92, and leukemic transformation. Nkx3-1 may, in a tissue-specific manner, act as tumor suppressor in prostate epithelium50 but its promoter is a target for TAL1-mediated activation and consequent transformation in T-cell precursors. As downstream target, miR-17-92 is also involved in cell cycle regulation through E2F1 and Notch1-induced T-ALL, although the exact relationship between these participants is not fully determined.51 In addition, human pediatric T-ALL has also been associated with Nkx2-5 translocated to either BCL11B or TcRδ,52,53 indicating that although in normal T-cell maturation these NK genes play no demonstrable role, they possess transformation potential on ectopic expression.
Conclusions and Perspectives
According to available data, Nkx2-3 and its paralogues exert essential functions in normal developmental patterning and differentiation of several peripheral lymphoid organs and may play role(s) in hematopoietic/lymphoid malignancies. Although the normal physiological tissue maturation proceeds in a spatially and temporally defined order under the influence of Nkx family members, the involvement of ectopic Nkx2-3 expression in malignant transformation appears to be a random event, coupled with diverse cellular signaling alterations. The most widely established consequence of the deregulated expression of Nkx2-3 is closely linked to IBD, and as this condition represents a possible precursor for colonic cancer, monitoring the tissue expression of Nkx2-3 protein may entail further diagnostic importance (Table 1).
Table 1.
Effects of Nkx mutations on lymphoid organ formation and hematopoietic malignancies.
| Nkx family member | Spleen | Peyer’s patches | Association with hematopoietic malignancies |
|---|---|---|---|
| Nkx2.1 (TFF-1) | — | — | Rearrangement in T-cell acute lymphoblastic leukemia54 |
| Nkx2.2 | — | — | Rearrangement in T-cell acute lymphoblastic leukemia54 |
| Nkx2.3 | Smaller, atrophic red pulp, disorganized stroma, lymph node like vasculature, defect in B-cell maturation28 | Fewer, smaller, with altered vascular MAdCAM-1/PNAd switch, abnormal lymphocyte homing28 | Ectopic expression maturation arrest in T-ALL49
Involvement in ETV6/RUNX1-positive B-ALL46 IgH-related translocation in MZBL45 |
| Nkx2.5 | Asplenia55 | No contribution25 | Translocation to BCL11B in T-ALL52 |
| Nkx3.1 (BAPX2) | — | — | Induces proliferation in TAL1-positive human T-ALL50
Ectopic expression in T-ALL cell lines53 |
| Nkx3.2 (BAPX1) | Asplenia/hyposplenia22
Perturbation of LR asymmetry21 |
— | Ectopic expression in T-ALL cell lines53 |
Abbreviations: B-ALL, B-cell acute lymphoblastic leukemia; IgH, Ig heavy chain; LR, left-right; MZBL, marginal zone B-cell lymphoma; T-ALL, T-cell acute lymphoblastic leukemia.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Z.K. is supported by the ÚNKP-17-4-I New National Excellence Program of the Ministry of Human Capacities and the postdoctoral research grant of the Faculty of Medicine, University of Pécs. This work was supported by OTKA K108429, GINOP-232-15-2016-00050, and EFOP-361-16-2016-00004 research funds.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: The review’s concept was conceived and the manuscript was written by PB, DV and ZK; immunohistochemistry for Nkx2-3 was contributed by GR and BK; human tissue biopsy samples were provided by ÁV.
References
- 1. Stanfel MN, Moses KA, Schwartz RJ, Zimmer WE. Regulation of organ development by the NKX-homeodomain factors: an NKX code. Cell Molec Biol (Noisy-le-grand). 2005. Suppl 51:OL785–99. [PubMed] [Google Scholar]
- 2. Hayashi S, Scott MP. What determines the specificity of action of Drosophila homeodomain proteins? Cell. 1990;63:883–894. [DOI] [PubMed] [Google Scholar]
- 3. Jagla K, Bellard M, Frasch M. A cluster of Drosophila homeobox genes involved in mesoderm differentiation programs. Bioessays. 2001;23:125–133. [DOI] [PubMed] [Google Scholar]
- 4. Hombria JC, Lovegrove B. Beyond homeosis—HOX function in morphogenesis and organogenesis. Differentiation. 2003;71:461–476. [DOI] [PubMed] [Google Scholar]
- 5. Kimura S, Hara Y, Pineau T, et al. The T/ebp null mouse: thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev. 1996;10:60–69. [DOI] [PubMed] [Google Scholar]
- 6. Safi KH, Bernat JA, Keegan CE, Ahmad A, Hershenson MB, Arteta M. Interstitial lung disease of infancy caused by a new NKX2-1 mutation. Clin Case Rep. 2017;5:739–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weir BA, Woo MS, Getz G, et al. Characterizing the cancer genome in lung adenocarcinoma. Nature. 2007;450:893–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang L, Lin M, Ruan WJ, et al. Nkx2-1: a novel tumor biomarker of lung cancer. J Zhejiang Univ Sci B. 2012;13:855–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stenhouse G, Fyfe N, King G, Chapman A, Kerr KM. Thyroid transcription factor 1 in pulmonary adenocarcinoma. J Clin Pathol. 2004;57:383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang P, Han Y, Huang L, Li Q, Ma D. Expression and clinical significance of TTF-1 and p63 in NSCLC. Zhongguo Fei Ai Za Zhi/Chinese J Lung Cancer. 2009;12:995–999. [DOI] [PubMed] [Google Scholar]
- 11. Hsu DS, Acharya CR, Balakumaran BS, et al. Characterizing the developmental pathways TTF-1, NKX2-8, and PAX9 in lung cancer. Proc Nat Acad Sci U S A. 2009;106:5312–5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Briscoe J, Sussel L, Serup P, et al. Homeobox gene Nkx2.2 and specification of neuronal identity by graded sonic hedgehog signalling. Nature. 1999;398:622–627. [DOI] [PubMed] [Google Scholar]
- 13. Lyons I, Parsons LM, Hartley L, et al. Myogenic and morphogenetic defects in the heart tubes of murine embryos lacking the homeo box gene Nkx2-5. Genes Dev. 1995;9:1654–1666. [DOI] [PubMed] [Google Scholar]
- 14. Tanaka M, Chen Z, Bartunkova S, Yamasaki N, Izumo S. The cardiac homeobox gene Csx/Nkx2.5 lies genetically upstream of multiple genes essential for heart development. Development. 1999;126:1269–1280. [DOI] [PubMed] [Google Scholar]
- 15. Ellesoe SG, Johansen MM, Bjerre JV, Hjortdal VE, Brunak S, Larsen LA. Familial atrial septal defect and sudden cardiac death: identification of a novel NKX2-5 mutation and a review of the literature. Congen Heart Dis. 2016;11:283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tanaka M, Yamasaki N, Izumo S. Phenotypic characterization of the murine Nkx2.6 homeobox gene by gene targeting. Molec Cell Biol. 2000;20:2874–2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mennerich D, Hoffmann S, Hadrys T, Arnold HH, Bober E. Two highly related homeodomain proteins, Nkx5-1 and Nkx5-2, display different DNA binding specificities. Biol Chem. 1999;380:1041–1048. [DOI] [PubMed] [Google Scholar]
- 18. Jorgensen MC, Petersen HV, Ericson J, Madsen OD, Serup P. Cloning and DNA-binding properties of the rat pancreatic beta-cell-specific factor Nkx6.1. FEBS Lett. 1999;461:287–294. [DOI] [PubMed] [Google Scholar]
- 19. Guazzi S, Price M, De Felice M, Damante G, Mattei MG, Di Lauro R. Thyroid nuclear factor 1 (TTF-1) contains a homeodomain and displays a novel DNA binding specificity. EMBO J. 1990;9:3631–3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen CY, Schwartz RJ. Identification of novel DNA binding targets and regulatory domains of a murine tinman homeodomain factor, nkx-2.5. J Biol Chem. 1995;270:15628–15633. [DOI] [PubMed] [Google Scholar]
- 21. Hecksher-Sorensen J, Watson RP, Lettice LA, et al. The splanchnic mesodermal plate directs spleen and pancreatic laterality, and is regulated by bapx1/Nkx3.2. Development. 2004;131:4665–4675. [DOI] [PubMed] [Google Scholar]
- 22. Lettice LA, Purdie LA, Carlson GJ, Kilanowski F, Dorin J, Hill RE. The mouse bagpipe gene controls development of axial skeleton, skull, and spleen. Proc Nat Acad Sci U S A. 1999;96:9695–9700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brendolan A, Rosado MM, Carsetti R, Selleri L, Dear TN. Development and function of the mammalian spleen. Bioessays. 2007;29:166–177. [DOI] [PubMed] [Google Scholar]
- 24. Katakai T, Suto H, Sugai M, et al. Organizer-like reticular stromal cell layer common to adult secondary lymphoid organs. J Immunol. 2008;181:6189–6200. [DOI] [PubMed] [Google Scholar]
- 25. Castagnaro L, Lenti E, Maruzzelli S, et al. Nkx2-5(+)islet1(+) mesenchymal precursors generate distinct spleen stromal cell subsets and participate in restoring stromal network integrity. Immunity. 2013;38:782–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pabst O, Schneider A, Brand T, Arnold HH. The mouse Nkx2-3 homeodomain gene is expressed in gut mesenchyme during pre- and postnatal mouse development. Dev Dynamics. 1997;209:29–35. [DOI] [PubMed] [Google Scholar]
- 27. Mebius RE, Streeter PR, Michie S, Butcher EC, Weissman IL. A developmental switch in lymphocyte homing receptor and endothelial vascular addressin expression regulates lymphocyte homing and permits CD4+ CD3- cells to colonize lymph nodes. Proc Nat Acad Sci U S A. 1996;93:11019–11024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pabst O, Zweigerdt R, Arnold HH. Targeted disruption of the homeobox transcription factor Nkx2-3 in mice results in postnatal lethality and abnormal development of small intestine and spleen. Development. 1999;126:2215–2225. [DOI] [PubMed] [Google Scholar]
- 29. Balazs M, Horvath G, Grama L, Balogh P. Phenotypic identification and development of distinct microvascular compartments in the postnatal mouse spleen. Cell Immunol. 2001;212:126–137. [DOI] [PubMed] [Google Scholar]
- 30. Balogh P, Balazs M, Czompoly T, Weih DS, Arnold HH, Weih F. Distinct roles of lymphotoxin-beta signaling and the homeodomain transcription factor Nkx2.3 in the ontogeny of endothelial compartments in spleen. Cell Tissue Res. 2007;328:473–486. [DOI] [PubMed] [Google Scholar]
- 31. Kellermayer Z, Hayasaka H, Kajtar B, et al. Divergence of vascular specification in visceral lymphoid organs—genetic determinants and differentiation checkpoints. Int Rev Immunol. 2016;35:489–502. [DOI] [PubMed] [Google Scholar]
- 32. Czompoly T, Labadi A, Kellermayer Z, Olasz K, Arnold HH, Balogh P. Transcription factor Nkx2-3 controls the vascular identity and lymphocyte homing in the spleen. J Immunol. 2011;186:6981–6989. [DOI] [PubMed] [Google Scholar]
- 33. Guo F, Weih D, Meier E, Weih F. Constitutive alternative NF-kappaB signaling promotes marginal zone B-cell development but disrupts the marginal sinus and induces HEV-like structures in the spleen. Blood. 2007;110:2381–2389. [DOI] [PubMed] [Google Scholar]
- 34. Kellermayer Z, Labadi A, Czompoly T, Arnold HH, Balogh P. Absence of Nkx2-3 homeodomain transcription factor induces the formation of lYVE-1-positive endothelial cysts without lymphatic commitment in the spleen. J Histochem Cytochem. 2011;59:690–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pabst O, Forster R, Lipp M, Engel H, Arnold HH. NKX2.3 is required for MAdCAM-1 expression and homing of lymphocytes in spleen and mucosa-associated lymphoid tissue. EMBO J. 2000;19:2015–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kellermayer Z, Mihalj M, Labadi A, et al. Absence of Nkx2-3 homeodomain transcription factor reprograms the endothelial addressin preference for lymphocyte homing in Peyer’s patches. J Immunol. 2014;193:5284–5293. [DOI] [PubMed] [Google Scholar]
- 37. Hatoum OA, Binion DG. The vasculature and inflammatory bowel disease: contribution to pathogenesis and clinical pathology. Inflammat Bowel Dis. 2005;11:304–313. [DOI] [PubMed] [Google Scholar]
- 38. Yu W, Lin Z, Kelly AA, et al. Association of a Nkx2-3 polymorphism with Crohn’s disease and expression of Nkx2-3 is up-regulated in B cell lines and intestinal tissues with Crohn’s disease. J Crohn Colitis. 2009;3:189–195. [DOI] [PubMed] [Google Scholar]
- 39. Lu X, Tang L, Li K, et al. Contribution of NKX2–3 polymorphisms to inflammatory Bowel diseases: a meta-analysis of 35358 subjects. Sci Rep. 2014;4:3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Connor EM, Eppihimer MJ, Morise Z, Granger DN, Grisham MB. Expression of mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in acute and chronic inflammation. J Leuko Biol. 1999;65:349–355. [DOI] [PubMed] [Google Scholar]
- 41. Yu W, Lin Z, Hegarty JP, et al. Genes regulated by Nkx2–3 in siRNA-mediated knockdown B cells: implication of endothelin-1 in inflammatory bowel disease. Molec Genet Metab. 2010;100:88–95. [DOI] [PubMed] [Google Scholar]
- 42. SenBanerjee S, Lin Z, Atkins GB, et al. KLF2 is a novel transcriptional regulator of endothelial proinflammatory activation. J Exp Med. 2004;199:1305–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hsia LT, Ashley N, Ouaret D, Wang LM, Wilding J, Bodmer WF. Myofibroblasts are distinguished from activated skin fibroblasts by the expression of AOC3 and other associated markers. Proc Nat Acad Sci U S A. 2016;113:E2162–E2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stzepourginski I, Nigro G, Jacob JM, et al. CD34+ mesenchymal cells are a major component of the intestinal stem cells niche at homeostasis and after injury. Proc Nat Acad Sci U S A. 2017;114:E506–E513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Robles EF, Mena-Varas M, Barrio L, et al. Homeobox NKX2-3 promotes marginal-zone lymphomagenesis by activating B-cell receptor signalling and shaping lymphocyte dynamics. Nat Comm. 2016;7:11889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ghazavi F, De Moerloose B, Van Loocke W, et al. Unique long non-coding RNA expression signature in eTV6/RUNX1-driven B-cell precursor acute lymphoblastic leukemia. Oncotarget. 2016;7:73769–73780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kuster L, Grausenburger R, Fuka G, et al. ETV6/RUNX1-positive relapses evolve from an ancestral clone and frequently acquire deletions of genes implicated in glucocorticoid signaling. Blood. 2011;117:2658–2667. [DOI] [PubMed] [Google Scholar]
- 48. Moorman AV, Ensor HM, Richards SM, et al. Prognostic effect of chromosomal abnormalities in childhood B-cell precursor acute lymphoblastic leukaemia: results from the UK medical research council ALL97/99 randomised trial. Lancet Oncol. 2010;11:429–438. [DOI] [PubMed] [Google Scholar]
- 49. Villarese P, Lours C, Trinquand A, et al. TCRα rearrangements identify a subgroup of NKL-deregulated adult t-ALLs associated with favorable outcome. Leukemia. 2017;32:61–71. [DOI] [PubMed] [Google Scholar]
- 50. Shen MM, Abate-Shen C. Roles of the Nkx3.1 homeobox gene in prostate organogenesis and carcinogenesis. Dev Dynamics. 2003;228:767–778. [DOI] [PubMed] [Google Scholar]
- 51. Kusy S, Gerby B, Goardon N, et al. NKX3.1 is a direct TAL1 target gene that mediates proliferation of tAL1-expressing human T cell acute lymphoblastic leukemia. J Exp Med. 2010;207:2141–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nagel S, Kaufmann M, Drexler HG, MacLeod RA. The cardiac homeobox gene NKX2-5 is deregulated by juxtaposition with BCL11B in pediatric T-ALL cell lines via a novel t(5;14)(q35.1;q32.2). Cancer Res. 2003;63:5329–5334. [PubMed] [Google Scholar]
- 53. Nagel S, Pommerenke C, Scherr M, et al. NKL homeobox gene activities in hematopoietic stem cells, T-cell development and T-cell leukemia. PLoS ONE. 2017;12:e0171164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Homminga I, Pieters R, Meijerink JP. NKL homeobox genes in leukemia. Leukemia. 2012;26:572–581. [DOI] [PubMed] [Google Scholar]
- 55. Patterson KD, Drysdale TA, Krieg PA. Embryonic origins of spleen asymmetry. Development. 2000;127:167–175. [DOI] [PubMed] [Google Scholar]