Abstract
Background:
Microfracture is a single-stage arthroscopic procedure used to treat small- and medium-sized cartilage defects, the clinical results of which have been mixed to date.
Purpose:
To retrospectively evaluate prospectively collected patient-reported outcomes (PROs) after microfracture as well as to determine patient-related and defect-related factors associated with clinical outcomes and which factors predict the need for additional surgery.
Study Design:
Case-control study; Level of evidence, 3.
Methods:
All patients between the ages of 10 and 70 years who underwent microfracture by the senior author for a focal chondral defect of the knee between January 1, 2005, and March 1, 2010, were eligible for study enrollment. Patients were excluded if they underwent concomitant procedures that violated the subchondral bone. Functional outcomes were determined using preoperative and final follow-up PROs, including the Lysholm, International Knee Documentation Committee (IKDC), Knee injury and Osteoarthritis Outcome Score (KOOS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Short Form–12 (SF-12), and overall satisfaction scores. Patient-related factors (sex, age, body mass index [BMI]) and defect-related factors (lesion size, location, concomitant procedures, prior procedures) were analyzed for correlations with outcome scores. All patient-related and defect-related factors were also analyzed as predictors for subsequent surgery.
Results:
Overall, 101 patients (102 knees; 55 male, 46 female; mean age, 35.87 ± 12.52 years; mean BMI, 26.3 ± 5.5 kg/m2; mean defect size, 2.635 ± 1.805 cm2) were included. Lesion location included 44.90% at the medial femoral condyle, 21.43% at the trochlea, 11.22% at the lateral femoral condyle, 10.20% at multiple sites, 8.16% at the patella, and 4.08% at the tibial plateau. Microfracture was performed alone in 72 of 102 (71%) knees. At a mean follow-up of 5.66 ± 2.54 years (range, 2-11 years), clinically meaningful and statistically significant improvements were seen in all PROs (P < .05) except the SF-12 mental component score. Patients who had an isolated tibial plateau defect or multiple defects demonstrated reduced improvements in the symptom rate (P = .0237). Patients with a BMI >30 kg/m2 had lower postoperative scores on the KOOS activities of daily living subscale (P = .0261) and poorer WOMAC function and WOMAC pain scores (P = .029 and .0307, respectively). Patient BMI, age, sex, defect location, concomitant procedures, and operative side were not significant predictors for additional surgery. Larger defect size (>3.6 cm2) and prior knee surgery were independent risk factors for additional knee surgery after microfracture.
Conclusion:
After microfracture, all PROs demonstrated clinically and statistically significant improvements at 5.7 years. Functionally, male patients benefited more from microfracture than female patients. Microfracture of tibial lesions and multisite microfracture provided less benefit than microfracture of isolated femoral defects. Larger lesion size (>3.6 cm2) and prior knee surgery predicted the need for additional knee surgery after microfracture.
Keywords: microfracture, articular cartilage, cartilage preservation, focal chondral injury
Cartilage defects of the knee often cause significant pain and disability to the injured patient.1 It is well established that these defects have a limited ability to independently heal because of the intrinsic properties of articular cartilage, specifically a relative avascularity and hypocellular composition.5 Therefore, surgical management has become the mainstay for symptomatic defects that interfere with knee function. Estimates are that greater than 300,000 cartilage procedures are performed annually in the United States alone.1,15,28
Cartilage procedures can largely be classified into 3 categories: (1) palliation (debridement or chondroplasty), (2) repair (microfracture or drilling), and (3) restoration (autologous chondrocyte implantation [ACI], osteochondral autograft transfer, and osteochondral allograft transplantation).14,21,23 The microfracture technique exposes the subchondral bone marrow and creates a blood clot in the chondral defect, ultimately recruiting mesenchymal stem cells that heal the defect with a fibrocartilaginous scar.27 Given the technical simplicity, relatively short surgical times, low cost, and lack of need for additional equipment, microfracture has become a popular first-line treatment for chondral defects.15,22
The treating surgeon must consider patient-related (sex, age, body mass index [BMI], activity level) and defect-related (lesion size, location, concomitant procedures, prior procedures) factors when selecting the appropriate cartilage procedure.3 Microfracture has historically demonstrated good to excellent results in active patients with small (<2-4 cm2) defects at short-term follow-up.6,9,18,26 Midterm and long-term studies have demonstrated mixed results, with the vast majority of studies to date involving the treatment of isolated femoral condyle defects without concomitant meniscal, ligament, or osseous procedures.7,13,24,29
The purpose of the current study was 2-fold: (1) to review prospectively collected functional outcomes of a mixed group of patients who underwent microfracture with a minimum 2-year follow-up, and (2) to determine which patient-related and defect-related factors are associated with improved outcomes and which factors predict the need for additional surgery. The primary hypothesis was that microfracture, regardless of whether it was performed for isolated or multisite defects or concomitantly with other procedures, would lead to improved functional and clinical outcomes at final follow-up. The secondary hypothesis was that patient-related and defect-related factors would dictate final follow-up outcomes as well as the need for additional knee surgery.
Methods
Patient Enrollment
After institutional review board approval, all microfracture procedures performed by the senior investigator (B.J.C.) between January 1, 2005, and March 1, 2010, were prospectively collected for retrospective review. Inclusion criteria included patients between the ages of 10 to 70 years at the time of surgery, single-site and multisite microfracture, and microfracture with concomitant procedures. Concomitant procedures included loose body removal, meniscectomy, patellar tendon excision, medial patellofemoral ligament reconstruction, hardware removal, synovial release, ACI biopsy without subsequent ACI, and cruciate ligament reconstruction. Exclusion criteria were concomitant procedures that included biologic augmentation, meniscus allograft transplantation, and clinical malalignment that would otherwise require a realignment procedure.
Patient information collected from charts included age at the time of surgery, patient height and weight, lesion size, lesion location, previous surgeries, and subsequent surgeries. The patient-reported outcomes (PROs) analyzed in this study were the Lysholm knee scale; International Knee Documentation Committee (IKDC); Knee injury and Osteoarthritis Outcome Score (KOOS) pain, symptoms, activities of daily living (ADL), sport, and quality of life subscores; Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain, stiffness, and function subscores; overall satisfaction; symptom rate; and Short Form–12 (SF-12) physical and mental component scores. Clinically meaningful differences in these outcome scores were determined based on the minimal clinically important difference (MCID) for the IKDC subjective score (11.5 in the setting of a knee injury), the minimal detectable change (MDC) for KOOSscores (range, 5-12 in the setting of a knee injury), and the MDC for the Lysholm score (8.9 in the setting of anterior cruciate ligament reconstruction).2,4,8 PROs were only collected and reported for the most recent follow-up. For patients who required a second procedure (eg, total knee arthroplasty), we reported PROs that were collected before the second procedure.
Surgical Technique
Microfracture was performed according to previously described techniques.26 In short, the surgical technique involved debridement of the chondral defect, establishment of stable shoulders around the defect, removal of the calcified cartilage layer, and penetration of the subchondral bone with a microfracture awl. The microfracture holes were evenly spaced 3 to 4 mm apart to preserve the structure and function of the subchondral bone plate. Marrow product egress (blood and fat droplets) was confirmed before the conclusion of each surgical procedure.
Rehabilitation
Postoperatively, the patients with tibiofemoral lesions were restricted to toe-touch weightbearing on the operative limb for the first 2 weeks, followed by gradual progression to full weightbearing. Patients with patellofemoral lesions only were allowed to bear weight as tolerated immediately in a hinged knee brace locked in full extension. Patients were encouraged to use a continuous passive motion machine for 6 to 8 hours per day for 6 weeks. This protocol promoted adhesion and maturation of the fibrocartilage clot to the subchondral bone and has demonstrated clinical efficacy.24 Closed kinetic chain exercises were started at 2 weeks, and open kinetic chain exercises were started at 8 weeks. Impact exercises resumed at 4 months, plyometric training at 5 months, and sport-specific drills (cutting, pivoting, twisting) at 6 months. The postoperative rehabilitation course was tailored to each individual patient based on symptoms, demand level, associated procedures, and the tempo of recovery.
Statistical Analysis
Demographic data were summarized using descriptive statistics, including means, SDs, ranges, and frequencies as appropriate. Demographic data, lesion size, age, and BMI were analyzed in a continuous fashion for any associations with functional outcome scores. For continuous data available preoperatively and postoperatively, changes in values were assessed using analysis of variance with post hoc Tukey correction for multiple comparisons. For categorical data available preoperatively and postoperatively, changes in frequency were assessed using the Fisher exact test. A multivariate logistic regression analysis was performed to identify independent factors associated with a reoperation. A receiver operating characteristic analysis was performed to identify the optimal cutoff lesion size to predict subsequent surgery. Statistical significance was defined as a P value of <.05 for all tests. All statistical analyses were performed using SPSS Statistics version 21 (IBM Corp).
Results
Patient Demographics
After inclusion and exclusion criteria were applied, a total of 102 knees (101 patients) with a mean follow-up of 5.66 ± 2.54 years (range, 2-11 years) were included (Table 1). Of the 101 participants, there were 55 male and 46 female patients, with a mean age of 35.9 ± 12.5 years (range, 12.0-65.4 years) at the time of surgery. The mean BMI was 26.3 ± 5.5 kg/m2 (range, 17.8-47.0 kg/m2), and the mean lesion size was 2.63 ± 1.81 cm2 (range, 0.36-9.00 cm2) (Table 1). The lesion location included 11.22% at the lateral femoral condyle, 44.90% at the medial femoral condyle, 8.16% at the patella, 21.43% at the trochlea, 4.08% at the tibial plateau, and 10.20% at multiple sites. In 72 of 102 knees, microfracture was performed in isolation. In the remaining 30 knees, ≥1 concomitant procedures were performed in addition to microfracture (Table 2).
TABLE 1.
Patient-Related Factors of the Microfracture Cohort
| Sex, n (%) | |
| Male | 55 (54.5) |
| Female | 46 (45.5) |
| Age at surgery, mean ± SD (range), y | 35.87 ± 12.52 (12.00-65.37) |
| Body mass index, mean ± SD (range), kg/m2 | 26.26 ± 5.51 (17.75-46.98) |
| Defect size, mean ± SD (range), cm2 | 2.64 ± 1.80 (0.36-9.00) |
| Prior surgery, n (%) | 46 (45.5) |
TABLE 2.
Concomitant Procedures at the Time of Microfracture
| Concomitant Procedure | n (%) |
|---|---|
| Autologous chondrocyte implantation biopsy | 18 (21.1) |
| Loose body removal | 15 (17.6) |
| Medial meniscectomy | 15 (17.6) |
| Lateral meniscectomy | 10 (11.8) |
| Lateral release | 8 (9.4) |
| Synovectomy | 4 (4.7) |
| Anterior cruciate ligament reconstruction | 4 (4.7) |
| Medial meniscal repair | 3 (3.5) |
| Lateral meniscal repair | 2 (2.4) |
| Medial patellofemoral ligament reconstruction | 2 (2.4) |
| Suprapatellar pouch release | 2 (2.4) |
| Patellar tendon excision | 1 (1.2) |
| Hardware removal | 1 (1.2) |
| Total | 85 (100.0) |
Preoperative Considerations
The analysis of preoperative and demographic information found that patients with a higher BMI were more likely to have undergone prior knee surgery (P = .045). Additionally, there was a positive relationship between increased age and the presence of multiple chondral lesions (P = .003). Preoperatively, both increased age and increased BMI showed a negative correlation with preoperative PRO scores. Increased age was associated with lower baseline Lysholm scores (P = .0173), higher baseline KOOS pain scores (P = .0229), lower baseline KOOS ADL scores (P = .0244), poorer baseline WOMAC pain scores (P = .0205), poorer baseline WOMAC stiffness scores (P = .0051), and poorer baseline WOMAC function scores (P = .0244). Additionally, a higher preoperative BMI was associated with lower preoperative Lysholm scores (P = .0091), lower KOOS ADL scores (P = .0170), poorer WOMAC function scores (P = .0170), and lower SF-12 physical scores (P = .0386). There was no difference in preoperative PROs by sex.
PROs After Microfracture
After microfracture, most patients demonstrated improvements in all measured outcomes between the preoperative state and final follow-up (Figure 1). All measured outcomes demonstrated statistically significant improvements aside from the SF-12 mental score (P = .076), which improved, although not significantly (Figure 1). In addition to statistically significant improvements, mean PRO scores demonstrated clinically meaningful improvements for all outcomes in which an MCID or MDC had been elucidated (Figure 2). Compared with female patients, male patients had a 14.249-point greater improvement in the KOOS symptoms score (95% CI, 4.2954-24.2026; P = .0056) and a 13.625-point greater improvement in the KOOS sport score (95% CI, 0.8102-26.4398; P = .0374). Patients who had an isolated tibial plateau defect or multiple defects demonstrated reduced improvements in the symptom rate (P < .05 each). In addition to the statistically significant improvements in the magnitude of change for male over female patients, a postoperative analysis by sex demonstrated that male patients generally had statistically higher postoperative PRO scores. This included male patients having higher postoperative IKDC scores (P = .0099); improved KOOS pain, symptoms, and sport scores (P = .0199, .0011, and .0089, respectively); and lower WOMAC pain and stiffness scores (P = .0173 and .0068, respectively).
Figure 1.
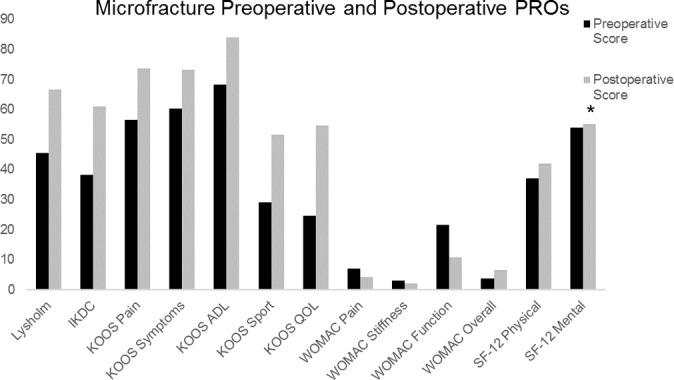
Mean preoperative and postoperative patient-reported outcome scores. *All changes were significant aside from the SF-12 mental score. ADL, activities of daily living; IKDC, International Knee Documentation Committee; KOOS, Knee injury and Osteoarthritis Outcome Score; PRO, patient-reported outcome; QOL, quality of life; SF-12, Short Form–12; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Figure 2.
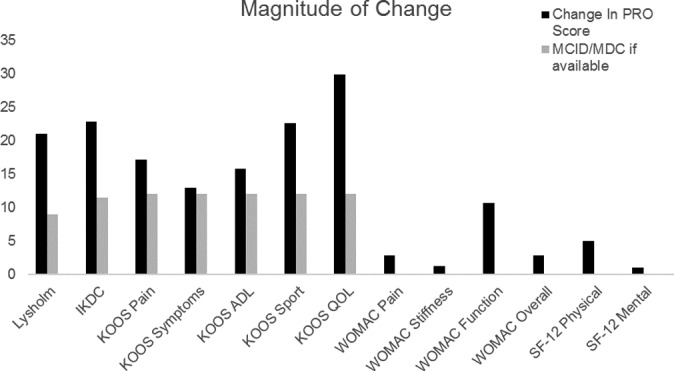
Magnitude of change for all patient-reported outcomes. Reference minimal clinically important difference or minimal detectable change are shown where these values were elucidated. ADL, activities of daily living; IKDC, International Knee Documentation Committee; KOOS, Knee injury and Osteoarthritis Outcome Score; MCID, minimal clinically important difference; MDC, minimal detectable change; PRO, patient-reported outcome; QOL, quality of life; SF-12, Short Form–12; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Final Follow-up Considerations
Increased BMI, older age, and increased lesion size all exhibited negative correlations with postoperative PROs. Concomitant procedure status did not correlate with final follow-up PROs. A higher BMI significantly correlated with multiple postoperative PROs including poorer KOOS pain scores (P = .0328), lower KOOS ADL scores (P = .0115), poorer WOMAC pain scores (P = .0121), higher WOMAC stiffness scores (P = .0061), and poorer WOMAC function scores (P = .0102). When BMI was analyzed in a noncontinuous fashion, those with a BMI >30 kg/m2 had lower postoperative KOOS ADL scores (P = .0261) and poorer WOMAC function and pain scores (P = .029 and .0307, respectively). Having undergone previous surgery before microfracture correlated with significantly lower KOOS quality of life scores (P = .0495). Older age correlated with a significantly lower symptom rate (P = .0321). Larger lesion size significantly correlated with lower SF-12 physical component scores (P = .0186).
Multivariate Analysis
In the multivariate logistic regression model examining the need for additional surgery, larger defect size (P = .0117) and previous knee surgery (P = .0187) were the only independent predictive factors for subsequent surgery after microfracture (Figure 3). Patient BMI, age, sex, defect location, concomitant procedures, and operative side were not significant predictors for additional surgery. The receiver operating characteristic analysis revealed that defects >3.6 cm2 treated with microfracture were predictive of requiring additional surgical intervention in the future (area under the curve, 0.587; specificity, 0.8; sensitivity, 0.4146). There were 43 knees (42.1%) that required additional surgery at a mean 2.63 years (range, 0.23-10.18 years) after the index microfracture procedure. The most common subsequent procedure was arthroscopic debridement (27.9%), followed by total knee arthroplasty (11.6%).
Figure 3.
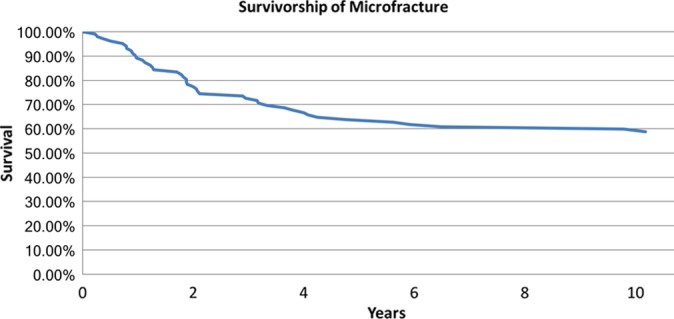
Survivorship of the operative knee as determined by the need for additional procedures related to the articular cartilage after microfracture.
Discussion
Microfracture has become a common treatment option for relatively small, symptomatic cartilage defects in the knee. Our hypotheses were confirmed, as patients who underwent microfracture showed clinically meaningful and statistically significant improvements in PROs after a mean of 5.7 years, regardless of whether it was performed at a single site, multiple sites, or with concomitant procedures. Overall, male patients demonstrated better final postoperative PROs as well as a greater magnitude of improvement when compared with their female counterparts. Older age, higher BMI, larger defect size, and multiple defect locations were the patient-specific and defect-specific predictors of diminished postoperative outcomes. Larger lesion size (>3.6 cm2) and prior surgery predicted the need for additional surgery after microfracture.
Microfracture is one of the most commonly employed surgical techniques for the treatment of cartilage defects in the knee.15 Multiple level 3 and 4 studies have reported statistically significant improvements in clinical outcomes and PROs after microfracture.12,16,19,24,26 In a retrospective review of 48 patients undergoing microfracture for isolated chondral defects without concomitant procedures, Krych et al12 demonstrated significant improvements in multiple PROs. The change in IKDC (22.7 vs 22.8, respectively) and KOOS ADL (20.3 vs 15.8, respectively) scores was similar to that in our study at 5 years. Steadman et al24 retrospectively reviewed their results of microfracture in a cohort of isolated defects without concomitant procedures and found significant improvements in Lysholm and Tegner scores at 11 years after surgery. Our study showed somewhat more moderate improvements in the Lysholm score (increased by 21.0) compared with the study by Steadman et al24 (increased by 30.1). Pain and stiffness as measured by the WOMAC also decreased by a similar amount, even at 11 years of follow-up, showing a potentially durable result. Miller et al16 retrospectively evaluated 350 patients undergoing microfracture at an average of 4 years and found that PROs improved over the first 2 years and plateaued thereafter. Steadman et al24 also found that there were no differences in outcomes based on sex; however, age younger than 45 years correlated with improved outcomes.16
However, there are relatively few high-level prospective studies currently available.7,13,29 Lim et al13 prospectively compared microfracture, ACI, and osteochondral autograft transfer at a minimum of 3 years’ follow-up. They found no superiority of one technique over the others in PROs or objective findings, including postoperative magnetic resonance imaging and second-look arthroscopic surgery.13 Knutsen et al10 recently reported long-term results (14-15 years) in a prospective randomized controlled trial of ACI versus microfracture and demonstrated no difference in survivorship, clinical outcomes, or advancement of radiographically identified osteoarthritis. However, at final follow-up, approximately 50% of the patients who had not experienced a failure had early radiographic signs of osteoarthritis, and they reported a failure rate of 42.5% and 32.5% for the ACI and microfracture groups, respectively. In a systematic review of 5-year outcomes, Kraeutler et al11 confirmed the equivalence of microfracture and first-generation ACI in PROs and reported similar failure rates of 18.5% and 17.1%, respectively. Ulstein et al29 compared the results of 11 patients undergoing microfracture with 14 patients undergoing osteochondral autograft transfer at an average of 10 years and found no significant differences. Last, Gudas et al7 compared microfracture with osteochondral autograft transfer in a randomized clinical trial of isolated femoral defects and found the 2 techniques to be comparable at an average of 10 years’ follow-up, aside from a diminished sporting level in the microfracture group.
The reoperation rate reported in the present study (42.1%) is within the range of those reported by other studies with PROs, which ranged from 2.8% to 54.0%. Although the current study lacks a comparison with an alternate treatment modality, the size of the cohort and the addition of multisite microfracture and concomitant procedures set the present study apart from both the retrospective and prospective studies to date. Despite the addition of multisite microfracture and concomitant knee procedures, the current study demonstrated significant improvements in PROs at midterm follow-up for all patients. The present study presents a mixed patient population that reflects the typical patient population seen in “real-world” clinical practice, which is not always addressed in a randomized controlled trial.
There is growing clinical evidence to suggest midterm and long-term success for microfracture in the knee.7,13,16,24,29 In each of these studies, isolated femoral condyle defects were treated with microfracture, and concomitant procedures were excluded. The present study is the first to examine the midterm results of microfracture in a mixed patient cohort including multisite microfracture and concomitant procedures. We found that microfracture with concomitant procedures did not influence final outcomes; however, multisite microfracture was a predictor of poorer outcomes. One explanation for the comparable results with or without concomitant procedures may be the strict observation of standard-of-care postoperative guidelines in all of our patients undergoing microfracture.24 All patients were instructed, when relevant, to be toe-touch weightbearing and were given a continuous passive motion machine to promote motion without compression in the early postoperative period. Additionally, the finding of poorer outcomes in the multisite microfracture cohort is to be expected, as multiple chondral injuries are indicative of a more advanced disease process.
Prior studies have examined the patient-related factors that affect final outcomes after microfracture.16,25 Miller et al16 reported that patients younger than 45 years had significantly improved outcomes after microfracture as compared with those older than 45 years. Moreover, recent pooling of the data in the form of systematic reviews and meta-analyses has demonstrated patient age and BMI to be significant predictors of postoperative improvement after microfracture.6,9,18,19 These findings are corroborated in the current study, as we found that younger age and lower BMI correlated with improved postoperative results. To our knowledge, this is the first study to demonstrate that male patients may benefit more than female patients from microfracture. This trend had been noted on a limited basis for the Lysholm score by Miller et al16,17 but was ultimately not statistically significant at short-term follow-up. It may be that male patients report worse PROs at baseline compared with female patients and thus have greater improvements postoperatively.
Defect-related factors have also been previously demonstrated to correlate with outcomes after microfracture.7,17,20,26 Mithoefer et al20 demonstrated significantly higher rates of return to sport for athletes with defects <2 cm2 as compared with athletes with larger lesions. Harris et al9 found that a defect >4 cm2 was the only factor predictive of improved outcomes with ACI over microfracture. Additionally, our data suggest that the presence of a single chondral lesion, having a lower BMI, or being male also independently predict greater functional improvement after microfracture. Again, the current study corroborates previous findings that defect size influences clinical outcomes. A novel finding of the current study is that larger defects (>3.6 cm2) and prior surgery were both independent predictors for future surgery after microfracture.
Although this study reports the outcomes on a large number of patients from a single surgeon, there are several limitations. We evaluated the longitudinal results of microfracture in a mixed patient cohort without a control group for comparison. While the presence of multisite microfracture and concomitant procedures in the present study adds a level of complexity to the analysis, it also provides heterogeneity to the study cohort and a potential confounding effect on the interpretation of the results. Last, while PROs are critical for understanding the effects of a given treatment on the patient experience, the addition of an objective measure of microfracture success, such as radiographic or magnetic resonance imaging follow-up, would help to strengthen the conclusions of the study.
Conclusion
After microfracture, nearly all PROs demonstrated clinically and statistically significant improvements at a mean follow-up of 5.7 years, despite a 42% reoperation rate. Functionally, male patients may benefit more from microfracture than female patients. Microfracture of tibial lesions and multisite microfracture may provide less benefit than microfracture of isolated femoral defects. Larger lesion size (>3.6 cm2) and prior knee surgery may predict the need for additional knee surgery after microfracture.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: B.J.C. receives research support from Aesculap/B. Braun, Geistlich, the National Institutes of Health (National Institute of Arthritis and Musculoskeletal and Skin Diseases and Eunice Kennedy Shriver National Institute of Child Health and Human Development), Novartis, Sanofi-Aventis, and Zimmer; has stock/stock options in Aqua Boom, Biomerix, GiteliScope, Ossio, and Regentis; receives other financial or material support from Athletico, JRF Ortho, Operative Techniques in Sports Medicine, Smith & Nephew, and Tornier; receives royalties from DJ Orthopedics, Elsevier, and Saunders/Mosby-Elsevier; and is a paid consultant for Flexion, Smith & Nephew, and Zimmer. A.B.Y. receives research support from Arthrex and NuTech.
Ethical approval for this study was obtained from the Rush University Medical Center (FWA No. 00000482).
References
- 1. Alford JW, Cole BJ. Cartilage restoration, part 1: basic science, historical perspective, patient evaluation, and treatment options. Am J Sports Med. 2005;33(2):295–306. [DOI] [PubMed] [Google Scholar]
- 2. Briggs KK, Lysholm J, Tegner Y, Rodkey WG, Kocher MS, Steadman JR. The reliability, validity, and responsiveness of the Lysholm score and Tegner activity scale for anterior cruciate ligament injuries of the knee: 25 years later. Am J Sports Med. 2009;37(5):890–897. [DOI] [PubMed] [Google Scholar]
- 3. Cole BJ, Pascual-Garrido C, Grumet RC. Surgical management of articular cartilage defects in the knee. Instr Course Lect. 2010;59:181–204. [PubMed] [Google Scholar]
- 4. Collins NJ, Misra D, Felson DT, Crossley KM, Roos EM. Measures of knee function: International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, Knee Injury and Osteoarthritis Outcome Score (KOOS), Knee Injury and Osteoarthritis Outcome Score Physical Function Short Form (KOOS-PS), Knee Outcome Survey Activities of Daily Living Scale (KOS-ADL), Lysholm Knee Scoring Scale, Oxford Knee Score (OKS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Activity Rating Scale (ARS), and Tegner Activity Score (TAS). Arthritis Care Res (Hoboken). 2011;63(suppl 11):S208–S228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gomoll AH, Minas T. The quality of healing: articular cartilage. Wound Repair Regen. 2014;22(suppl 1):30–38. [DOI] [PubMed] [Google Scholar]
- 6. Goyal D, Keyhani S, Lee EH, Hui JH. Evidence-based status of microfracture technique: a systematic review of level I and II studies. Arthroscopy. 2013;29(9):1579–1588. [DOI] [PubMed] [Google Scholar]
- 7. Gudas R, Gudaite A, Pocius A, et al. Ten-year follow-up of a prospective, randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint of athletes. Am J Sports Med. 2012;40(11):2499–2508. [DOI] [PubMed] [Google Scholar]
- 8. Harris JD, Hussey K, Saltzman BM, et al. Cartilage repair with or without meniscal transplantation and osteotomy for lateral compartment chondral defects of the knee: case series with minimum 2-year follow-up. Orthop J Sports Med. 2014;2(10):23259 67114551528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Knutsen G, Drogset JO, Engebretsen L, et al. A randomized multicenter trial comparing autologous chondrocyte implantation with microfracture: long-term follow-up at 14 to 15 years. J Bone Joint Surg Am. 2016;98(16):1332–1339. [DOI] [PubMed] [Google Scholar]
- 11. Kraeutler MJ, Belk JW, Purcell JM, McCarty EC. Microfracture versus autologous chondrocyte implantation for articular cartilage lesions in the knee: a systematic review of 5-year outcomes [published online April 1, 2017]. Am J Sports Med. doi:10.1177/0363546517701912 [DOI] [PubMed] [Google Scholar]
- 12. Krych AJ, Harnly HW, Rodeo SA, Williams RJ., 3rd Activity levels are higher after osteochondral autograft transfer mosaicplasty than after microfracture for articular cartilage defects of the knee: a retrospective comparative study. J Bone Joint Surg Am. 2012;94(11):971–978. [DOI] [PubMed] [Google Scholar]
- 13. Lim HC, Bae JH, Song SH, Park YE, Kim SJ. Current treatments of isolated articular cartilage lesions of the knee achieve similar outcomes. Clin Orthop Relat Res. 2012;470(8):2261–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mall NA, Harris JD, Cole BJ. Clinical evaluation and preoperative planning of articular cartilage lesions of the knee. J Am Acad Orthop Surg. 2015;23(10):633–640. [DOI] [PubMed] [Google Scholar]
- 15. McCormick F, Harris JD, Abrams GD, et al. Trends in the surgical treatment of articular cartilage lesions in the United States: an analysis of a large private-payer database over a period of 8 years. Arthroscopy. 2014;30(2):222–226. [DOI] [PubMed] [Google Scholar]
- 16. Miller BS, Briggs KK, Downie B, Steadman JR. Clinical outcomes following the microfracture procedure for chondral defects of the knee: a longitudinal data analysis. Cartilage. 2010;1(2):108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Miller BS, Steadman JR, Briggs KK, Rodrigo JJ, Rodkey WG. Patient satisfaction and outcome after microfracture of the degenerative knee. J Knee Surg. 2004;17(1):13–17. [DOI] [PubMed] [Google Scholar]
- 18. Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37(10):2053–2063. [DOI] [PubMed] [Google Scholar]
- 19. Mithoefer K, Williams RJ, 3rd, Warren RF, et al. The microfracture technique for the treatment of articular cartilage lesions in the knee: a prospective cohort study. J Bone Joint Surg Am. 2005;87(9):1911–1920. [DOI] [PubMed] [Google Scholar]
- 20. Mithoefer K, Williams RJ, 3rd, Warren RF, Wickiewicz TL, Marx RG. High-impact athletics after knee articular cartilage repair: a prospective evaluation of the microfracture technique. Am J Sports Med. 2006;34(9):1413–1418. [DOI] [PubMed] [Google Scholar]
- 21. Moran CJ, Pascual-Garrido C, Chubinskaya S, et al. Restoration of articular cartilage. J Bone Joint Surg Am. 2014;96(4):336–344. [DOI] [PubMed] [Google Scholar]
- 22. Mundi R, Bedi A, Chow L, et al. Cartilage restoration of the knee: a systematic review and meta-analysis of level 1 studies. Am J Sports Med. 2016;44(7):1888–1895. [DOI] [PubMed] [Google Scholar]
- 23. Richter DL, Schenck RC, Jr, Wascher DC, Treme G. Knee articular cartilage repair and restoration techniques: a review of the literature. Sports Health. 2016;8(2):153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19(5):477–484. [DOI] [PubMed] [Google Scholar]
- 25. Steadman JR, Miller BS, Karas SG, Schlegel TF, Briggs KK, Hawkins RJ. The microfracture technique in the treatment of full-thickness chondral lesions of the knee in National Football League players. J Knee Surg. 2003;16(2):83–86. [PubMed] [Google Scholar]
- 26. Steadman JR, Rodkey WG, Briggs KK. Microfracture to treat full-thickness chondral defects: surgical technique, rehabilitation, and outcomes. J Knee Surg. 2002;15(3):170–176. [PubMed] [Google Scholar]
- 27. Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001;(suppl 391):S362–S369. [DOI] [PubMed] [Google Scholar]
- 28. Tetteh ES, Bajaj S, Ghodadra NS. Basic science and surgical treatment options for articular cartilage injuries of the knee. J Orthop Sports Phys Ther. 2012;42(3):243–253. [DOI] [PubMed] [Google Scholar]
- 29. Ulstein S, Aroen A, Rotterud JH, Loken S, Engebretsen L, Heir S. Microfracture technique versus osteochondral autologous transplantation mosaicplasty in patients with articular chondral lesions of the knee: a prospective randomized trial with long-term follow-up. Knee Surg Sports Traumatol Arthrosc. 2014;22(6):1207–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]


