Abstract
Clusterin, a widely expressed, tissue-specific glycoprotein, is a diagnostic marker of several tumor types, including anaplastic large cell lymphoma, follicular dendritic cell sarcoma, and tenosynovial giant cell tumor. A recent study has suggested it is highly expressed by well-differentiated neuroendocrine tumors (NET) arising at most anatomic sites, with the exception of jejunoileal tumors, and that it is similarly not expressed by poorly differentiated neuroendocrine carcinomas (NEC). We sought to validate this result in a large cohort of NETs and NECs. Clusterin immunohistochemistry was performed on tissue microarrays of 255 NETs [45 lung, 4 stomach, 8 duodenum, 75 pancreas (62 primary, 13 metastatic), 107 jejunoileum (69 primary, 38 metastatic), 16 appendix] and 88 NECs (43 visceral, 45 Merkel cell). Extent (%) and intensity (0, 1+, 2+, 3+) of staining were assessed and an H-score (extent x intensity) calculated. An average H-score >5 was considered positive. Clusterin expression was noted in 82.4% of 148 non-jejunoileal NETs (average H-score 183) and only 8.4% of 107 jejunoileal NETs (average H-score 31), as well as 19.3% of NECs (average H-score 36). Clusterin is frequently, strongly expressed by NETs of diverse anatomic sites, with the exception of jejunoileal tumors, in which it is only rarely, weakly expressed. It is occasionally, weakly expressed by NECs. Most metastatic NETs of occult origin arise in the pancreas or the jejunoileum. For cases in which an initial site of origin immunopanel (e.g., islet 1, PAX6, CDX2) is ambiguous, addition of clusterin may be diagnostically useful, with absence of expression suggesting a jejunoileal origin.
Keywords: Clusterin, immunohistochemistry, neuroendocrine, carcinoma of unknown primary, differential diagnosis
Introduction
The reported incidence of well-differentiated neuroendocrine tumors (NETs) has increased 5-fold over the last 30 years, and given relatively indolent disease biology, their population prevalence is greater than that of gastric, pancreatic, or esophageal cancer.(1) NETs arise at diverse anatomic sites, predominantly in the lung, tubal gut, and pancreas. Ten to twenty percent of tumors present as metastases of occult origin, and determining the site of origin is prognostically and therapeutically relevant.(2–4) Most NETs presenting as metastasis of unknown primary arise from the ileum or pancreas.(5–8) Immunohistochemistry is useful for assigning NET site of origin.(4) At the University of Iowa our primary panel includes islet 1, PAX6 (both for pancreas), and CDX2 (midgut). In our experience, up to 10% of tumors of pancreatic or jejunoileal origin are negative with our primary panel, and additional markers in this setting are of interest.(9)
Clusterin is a widely expressed, tissue-specific glycoprotein (Table 1) with numerous roles in health and disease. Named for its ability to aggregate Sertoli cells and erythrocytes, clusterin was independently discovered over a dozen times, reflecting its diverse biologic functions, which are generally cytoprotective (Table 2).(10–25) Fundamentally, it is a molecular chaperone, able to bind diverse molecules via hydrophobic interactions.(26, 27)
Table 1.
Distribution of Clusterin Expression
| Organ | Cell Type |
|---|---|
| Esophagus | Squamous epithelium (excepting basal cells) |
| Stomach | Foveolar epithelium (proximal stomach) Chief cells Antral glands |
| Duodenum | Brunner glands |
| Liver | Hepatocytes Bile duct epithelium |
| Pancreatobiliary tree | Pancreatic ductal epithelium Islets of Langerhans* Extrahepatic biliary epithelium Gallbladder epithelium |
| Testis | Sertoli cells |
| Epididymis | Epididymal epithelium |
| Ovary | Granulosa cells |
| Uterus | Endometrial epithelium Endocervical epithelium Ectocervical epithelium |
| Vagina | Squamous epithelium |
| Kidney | Distal convoluted tubules Transitional epithelium of pelvicalyceal system |
| Ureter | Transitional epithelium |
| Bladder | Transitional epithelium |
| Adrenal cortex | Zona glomerulosa |
| Larynx/trachea | Submucosal glands |
| Heart | Myocytes of left and right atria |
| Brain | Choroid plexus Ependyma Subpopulation of neurons and glia |
| Eye | Ciliary body epithelium |
| Joint space | Synovial lining cells |
Based on in situ hybridization data from: Aronow BJ, Lund SD, Brown TL, Harmony JA, Witte DP. Apolipoprotein J expression at fluid-tissue interfaces: potential role in barrier cytoprotection. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(2):725–9.
See Discussion
Table 2.
Settings in which Clusterin was Independently Discovered
| Study | Name Assigned | Comment |
|---|---|---|
| Fischer-Colbrie et al (1982) | Glycoprotein III | Membrane-bound and soluble glycoprotein of chromaffin granules |
| Blaschuk et al (1983) | Clusterin | Isolated from ram rete testis fluid based on ability to aggregate Sertoli cells and erythrocytes; secreted by Sertoli cells |
| Collard and Griswold (1987) | Sulfated glycoprotein 2 (SGP-2) | Primary Sertoli cell secretory protein; binds to acrosome and distal tail of spermatozoa |
| Léger et al (1987) | Testosterone-repressed prostate message (TRPM-2) | Most upregulated ventral prostate protein upon castration (i.e., androgen withdrawal) |
| Michel et al (1989) | T64 | Most abundant mRNA in Rous sarcoma virus-transformed quail neuroretinal cells |
| Kirszbaum et al (1989) | Serum protein 40 kD,40kD (SP-40,40) | Member of the SC5b-9 complement complex (i.e., soluble terminal complement complex) |
| Jenne and Tschopp (1989) | Complement cytolysis inhibitor (CLI) | Inhibitor of SC5b-9 complex |
| de Silva et al (1990) | Apolipoprotein J (apoJ) | High density lipoprotein (HDL)-associated glycoprotein |
| May et al (1990) | Alzheimer’s disease hippocampus clone 9 (p-ADHC-9) | Increased mRNA expression in Alzheimer’s disease hippocampus |
| Hartmann et al (1991) | 80-kD glycoprotein (gp 80) | Glycoprotein secreted from the apical surface of Madin-Darby canine kidney (MDCK) cell line |
| James et al (1991) | NA1/NA2 | High density lipoprotein (HDL)-associated glycoprotein |
| Danik et al (1991) | pTB16 | Increased mRNA expression in high-grade astrocytoma |
| Jones et al (1992) | K611 | Increased mRNA expression in retinitis pigmentosa retinas |
| Diemer et al (1992) | 38-kD protein (pc38K) | Increased protein expression in differentiating (i.e., nodular) smooth muscle cells in culture |
Modified from Table in: Rosenberg ME, Silkensen J. Clusterin: physiologic and pathophysiologic considerations. Int J Biochem Cell Biol. 1995;27(7):633–45.
Clusterin came to prominence in diagnostic pathology in 2000, when gene expression profiling of 31 hematolymphoid neoplasm cell lines identified expression limited to anaplastic large cell lymphoma (ALCL).(28) It was subsequently independently validated as an ALCL marker.(29–31) It has also found use as a marker of follicular dendritic cell sarcoma,(32, 33) tenosynovial giant cell tumor,(34) large cell transformation in mycosis fungoides,(35) and, most recently, hepatocellular carcinoma.(36)
We read with interest a recent report by Mourra and colleagues describing clusterin expression in most NETs from diverse anatomic sites, except the jejunoileum in which expression was rare; the protein was also not expressed by poorly differentiated neuroendocrine carcinomas (NECs). (37) This was an extension of earlier work from the group, which had shown that clusterin was frequently expressed by pancreatic NETs but not by solid pseudopapillary neoplasm.(38) We sought to validate these results in a large cohort of NETs and NECs, in which case absence of clusterin expression in a metastatic NET of occult origin would suggest a jejunoileal origin.
Materials and methods
Neoplasms were identified from the surgical pathology archives of the University of Iowa Hospitals and Clinics. Original glass slides were reviewed, the diagnosis was confirmed, and a “best tumor block” was identified. Tissue microarrays (TMAs) were constructed using the Manual Tissue Arrayer MTA-1 (Beecher Instruments; Sun Prairie, WI), with the following 343 tumors arrayed as triplicate 1 mm cores: 255 NETs (45 lung, 4 stomach, 8 duodenum, 62 pancreas primary, 13 pancreas metastatic, 69 jejunoileum primary, 38 jejunoileum metastatic, 16 appendix) and 88 NECs (43 visceral, 45 Merkel cell).
Clusterin immunohistochemistry was performed manually on 4-μm-thick tissue sections after deparaffinization, rehydration, and pressure cooker heat-induced epitope retrieval in Target Retrieval Solution (pH 6.1; Dako; Carpinteria, CA) using a mouse monoclonal antibody directed against the α-chain (clone 41D; 1:20,000 dilution; EMD Millipore; Temecula, CA) and the polymer-based Dako EnVision+ detection system. Palatine tonsil served as the positive (follicular dendritic cells, squamous epithelium, and endothelium) and negative (lymphocytes) external control.
Clusterin expression was evaluated for extent (0–100%) and intensity (0–3+) in each TMA core and an overall H-score (mean extent*intensity) was calculated for each neoplasm. An average H-score >5 was considered positive. This research was conducted with University of Iowa Institutional Review Board approval.
Results
Clusterin expression was noted in 82.4% of 148 non-jejunoileal NETs (average positive H-score 183) (Figure 1a–b) and only 8.4% of 107 jejunoileal NETs (average positive H-score 31) (Figure 1c–f), as well as 19.3% of NECs (average positive H-score 36) (see Figure 2a–d). We also observed strong expression in non-neoplastic islets, which predominated at the periphery (Figure 3a). There was moderately strong staining in the Paneth cells of ileal mucosa, but enteroendocrine cells appeared negative (Figure 3b). Detailed expression data are presented in Tables 3 and 4.
Figure 1.
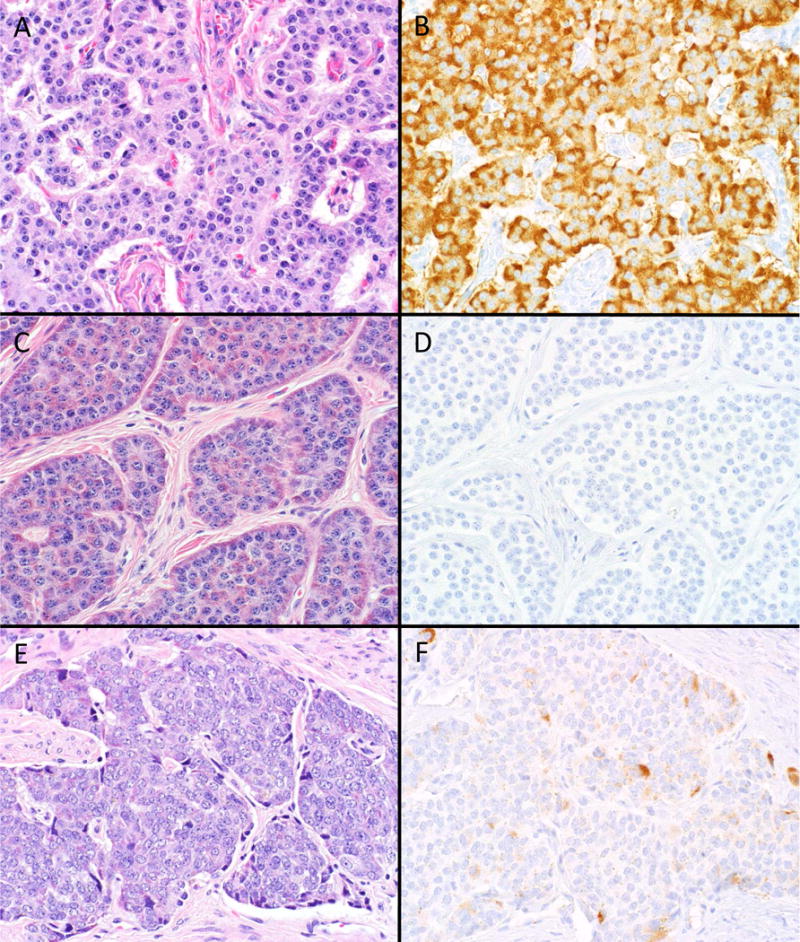
A–F, Clusterin Staining in Well-Differentiated Neuroendocrine Tumors (NET). The vast majority of non-jejunoileal NETs (pancreatic primary depicted in A) demonstrate strong clusterin expression (B); most jejunoileal tumors (C) are entirely negative (D), while occasional jejunoileal tumors (E) demonstrate weak, patchy staining (F) (original magnification of each image 400×).
Figure 2.
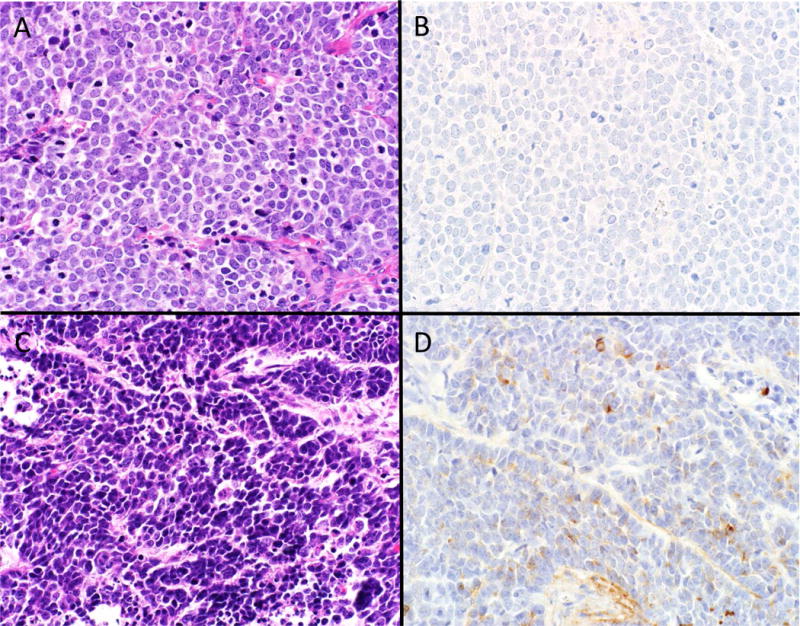
A–D, Clusterin Staining in Poorly Differentiated Neuroendocrine Carcinomas (NEC). A majority of NECs (Merkel cell carcinoma depicted in A) do not express clusterin (B), while up to 20% (visceral small cell carcinoma depicted in B) show weak, patchy staining (D) (original magnification of each image 400×).
Figure 3.
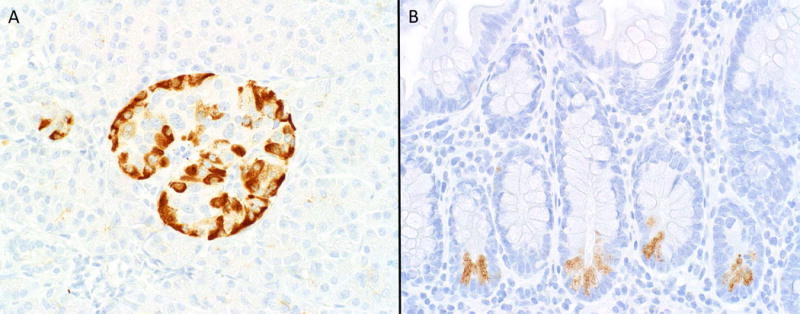
A–B, Clusterin Staining in Cognate Normal Tissues. Clusterin is strongly expressed by non-neoplastic islets, with expression predominantly at the periphery, where glucagon-expressing alpha cells reside (A); In non-neoplastic ileal mucosa moderately strong staining is noted in Paneth cells (readily identified by their location in crypt bases and luminal orientation of cytoplasmic granules), while no staining is noted in enteroendocrine cells (which also reside in crypt bases but with abluminal orientation of cytoplasmic granules) (B) (original magnification of both images 400×).
Table 3.
Clusterin Expression in Well-Differentiated Neuroendocrine Tumors
| Anatomic Site | n | % Positive | Average H-score (if positive) |
|---|---|---|---|
| Lung | 45 | 73.3 | 141 |
| Stomach | 4 | 50 | 165 |
| Duodenum | 8 | 62.5 | 224 |
| Pancreas Primary | 62 | 88.7 | 215 |
| Pancreas Metastasis | 13 | 100 | 215 |
| Jejunoileum Primary | 69 | 5.8 | 11 |
| Jejunoileum Metastasis | 38 | 13.2 | 50 |
| Appendix | 16 | 93.8 | 138 |
Table 4.
Clusterin Expression in Poorly Differentiated Neuroendocrine Carcinomas
| Tumor Type | n | % Positive | Average H-score (if positive) |
|---|---|---|---|
| Small Cell Carcinoma | 43 | 21 | 51 |
| Merkel Cell Carcinoma | 45 | 18 | 22 |
Discussion
We detected clusterin expression in the vast majority (82.4%) of non-jejunoileal NETs, with expression typically strong (average positive H-score 183). Notably this included 89% of 62 pancreatic primaries and 100% of 13 pancreatic metastases (average positive H-score 215 in both of these settings). This contrasted with infrequent, weak expression in jejunoileal tumors (6% of 69 primaries and 13% of 38 metastases with average positive H-scores of 11 and 50, respectively). We also found weak clusterin expression in 20% of NECs, with similar frequencies in visceral and cutaneous primaries.
Our results are nearly identical to those of Mourra and colleagues, who found clusterin expression in 85% of 80 non-jejunoileal and 8% of 51 jejunoileal NETs.(37, 38) In one of these studies they also found no expression in 13 WHO G3 tumors.(37) In the only other study of clusterin expression in neuroendocrine epithelial neoplasms, which was limited to the pancreas, Henderson-Jackson et al reported staining in 92% of 59 neoplasms, with strong staining in 61%.(39) Six of the neoplasms in this study were G3. Specific clusterin results were not presented for the G1–G2 vs G3 neoplasms, and, based on our results, it is likely that the overall positive and strong positive rates in their NETs are greater than those reported for the entire cohort.
Among the myriad contexts in which it was independently discovered, clusterin (initially known as glycoprotein III in this setting) was found to be associated with the membrane and soluble contents of adrenal chromaffin granules.(12, 40, 41) Clusterin was subsequently identified as a component of the large dense core secretory vesicles of thyroid parafollicular cells, anterior and posterior pituitary, and parathyroid; in these endocrine tissues, expression is limited to secretory vesicles.(41–43) Clusterin expression has also been described in the developing rat and porcine endocrine pancreas, with initially widespread distribution in fetal pancreas becoming ultimately restricted to a subset of glucagon-expressing cells in the adult.(44–46) We similarly detected clusterin expression in non-neoplastic islets, strongest at the islet periphery, where glucagon-producing alpha cells reside. Of note, though Aronow and colleagues, in their detailed anatomic description of clusterin expression described strong expression in pancreatic acinar cells and no expression in pancreatic islets, review of the relevant image from this manuscript demonstrates moderately strong expression in islets and not in acinar parenchyma.(47) In colonic mucosa, Andersen and colleagues found clusterin expression limited to a subset of the total enteroendocrine cell population; in this same report, a colonic NET also showed strong expression.(48)
Tumors recapitulate normal cell types. NETs variously recapitulate the neuroendocrine cell types native to the anatomic site at which they arise (e.g., enterochromaffin-like cells in the body of the stomach, alpha or beta cells in the pancreas, L cells in the rectum). Jejunoileal NETs recapitulate serotonin-producing, enterochromaffin (EC) cells. We hypothesize that most NETs express clusterin because clusterin is expressed by these tumors’ cognate normal cell types. Given clusterin’s function as a molecular chaperone, one would speculate a role in the secretion of peptide hormones/biogenic amines from chromaffin granules/dense core secretory vesicles, possibly involving the loading or recycling of these organelles.
As opposed to expression in non-neoplastic islets, we failed to demonstrate significant clusterin expression in the neuroendocrine cells of non-neoplastic ileum. Among neuroendocrine cells, EC cells are unique in the small amount of hormone that is released with each exocytosis event (i.e., 70x less than is released by chromaffin cells), which is more akin to that seen at synapses.(49) EC cell hormone-release kinetics are attributed to a smaller fusion pore size and may be due to a preference for “kiss-and-run” rather than full vesicle fusion.(50) The unique biology of the EC cell should be reflected in its structure, and lack of clusterin expression appears to be at least a marker of this phenotype.
Clusterin has been shown to function as a tumor suppressor and an oncoprotein.(51–53) Clusterin is known to inhibit NF-κB signaling, resist the epithelial-to-mesenchymal transition, and is typically down-regulated in cancer relative to normal tissue. On the other hand, clusterin is known to be upregulated in cancers resistant to chemo-, hormone, or radiotherapy, probably related to the protein’s anti-apoptotic function. Custirsen (OGX-111) is an antisense oligonucleotide to clusterin that has made it to phase III clinical trials (in combination with conventional chemotherapy) in metastatic castration-resistant prostate cancer and metastatic non-small cell lung cancer.(53–56) Given frequent, strong expression in non-jejunoileal NETs, a clinical trial in this setting (e.g., in advanced tumors progressing on somatostatin analogue therapy) could be considered.
Conclusion
We found frequent, strong clusterin expression in non-jejunoileal and only rare, weak expression in jejunoileal NETs. Clusterin is known to be expressed by chromaffin granules and dense core secretory vesicles of neuroendocrine cells, and we speculate that lack of expression in jejunoileal tumors may reflect the unique character of jejunoileal EC cells. We frequently encounter metastatic NETs of occult origin and perform a primary panel of immunostains including islet 1, PAX6, and CDX2 to assist in assigning site of origin. Given our results here, we have added clusterin to the second tier of our site of origin algorithm, applied when the initial panel is negative (Figure 4). Clusterin provides the additional advantage of multiple potential diagnostic uses (e.g., in ALCL, follicular dendritic sarcoma, and tenosynovial giant cell tumor). Finally, given frequent, strong expression, clusterin represents a logical therapeutic target in advanced NETs, in which custirsen would be expected to act as a chemo- and/or radiosensitizer.
Figure 4.
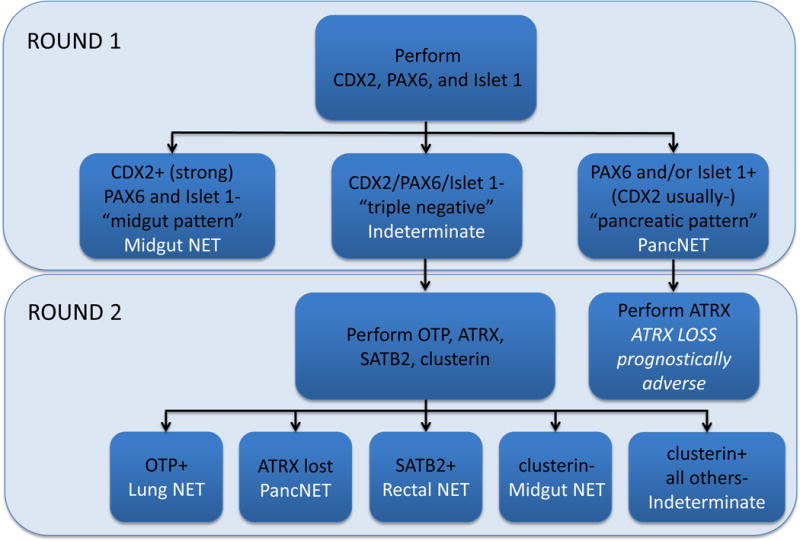
Iowa Well-Differentiated Neuroendocrine Tumor Site of Origin Immunohistochemical Classifier. Clusterin immunohistochemistry is potentially useful in tumors considered “indeterminate” for site of origin on initial islet 1/PAX6/CDX2 staining. In this context, lack of clusterin staining would support a jejunoileal origin.
Acknowledgments
This work was supported by NIH grant P50 CA174521-01A1 (TMO, JRH, AMB).
Footnotes
Presented in part at the 104th Annual Meeting of the United States and Canadian Academy of Pathology (USCAP), Boston, MA; March 2015.
Nothing To Disclose – The authors have indicated that they have no conflicts of interest that relate to the content of this manuscript.
Author Contributions:
AMB designed the study; JLH’s laboratory performed the immunohistochemical staining; AMB, TWC, KMS, and JEM performed the research; AMB analyzed the data; AMB wrote the paper; TMO and JRH contributed patients; all authors edited the paper.
References
- 1.Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008;26(18):3063–72. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 2.Pavel M, Baudin E, Couvelard A, Krenning E, Oberg K, Steinmuller T, et al. ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology. 2012;95(2):157–76. doi: 10.1159/000335597. [DOI] [PubMed] [Google Scholar]
- 3.Kunz PL, Reidy-Lagunes D, Anthony LB, Bertino EM, Brendtro K, Chan JA, et al. Consensus guidelines for the management and treatment of neuroendocrine tumors. Pancreas. 2013;42(4):557–77. doi: 10.1097/MPA.0b013e31828e34a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellizzi AM. Assigning site of origin in metastatic neuroendocrine neoplasms: a clinically significant application of diagnostic immunohistochemistry. Advances in anatomic pathology. 2013;20(5):285–314. doi: 10.1097/PAP.0b013e3182a2dc67. [DOI] [PubMed] [Google Scholar]
- 5.Kirshbom PM, Kherani AR, Onaitis MW, Feldman JM, Tyler DS. Carcinoids of unknown origin: comparative analysis with foregut, midgut, and hindgut carcinoids. Surgery. 1998;124(6):1063–70. doi: 10.1067/msy.1998.93105. [DOI] [PubMed] [Google Scholar]
- 6.Wang SC, Parekh JR, Zuraek MB, Venook AP, Bergsland EK, Warren RS, et al. Identification of unknown primary tumors in patients with neuroendocrine liver metastases. Archives of surgery. 2010;145(3):276–80. doi: 10.1001/archsurg.2010.10. [DOI] [PubMed] [Google Scholar]
- 7.Savelli G, Lucignani G, Seregni E, Marchiano A, Serafini G, Aliberti G, et al. Feasibility of somatostatin receptor scintigraphy in the detection of occult primary gastro-entero-pancreatic (GEP) neuroendocrine tumours. Nuclear medicine communications. 2004;25(5):445–9. doi: 10.1097/00006231-200405000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Prasad V, Ambrosini V, Hommann M, Hoersch D, Fanti S, Baum RP. Detection of unknown primary neuroendocrine tumours (CUP-NET) using (68)Ga-DOTA-NOC receptor PET/CT. European journal of nuclear medicine and molecular imaging. 2010;37(1):67–77. doi: 10.1007/s00259-009-1205-y. [DOI] [PubMed] [Google Scholar]
- 9.Maxwell JE, Sherman SK, Stashek KM, O’Dorisio TM, Bellizzi AM, Howe JR. A practical method to determine the site of unknown primary in metastatic neuroendocrine tumors. Surgery. 2014;156(6):1359–65. doi: 10.1016/j.surg.2014.08.008. discussion 65–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blaschuk O, Burdzy K, Fritz IB. Purification and characterization of a cell-aggregating factor (clusterin), the major glycoprotein in ram rete testis fluid. J Biol Chem. 1983;258(12):7714–20. [PubMed] [Google Scholar]
- 11.Fritz IB, Burdzy K, Setchell B, Blaschuk O. Ram rete testis fluid contains a protein (clusterin) which influences cell-cell interactions in vitro. Biol Reprod. 1983;28(5):1173–88. doi: 10.1095/biolreprod28.5.1173. [DOI] [PubMed] [Google Scholar]
- 12.Fischer-Colbrie R, Schachinger M, Zangerle R, Winkler H. Dopamine beta-hydroxylase and other glycoproteins from the soluble content and the membranes of adrenal chromaffin granules: isolation and carbohydrate analysis. J Neurochem. 1982;38(3):725–32. doi: 10.1111/j.1471-4159.1982.tb08691.x. [DOI] [PubMed] [Google Scholar]
- 13.Collard MW, Griswold MD. Biosynthesis and molecular cloning of sulfated glycoprotein 2 secreted by rat Sertoli cells. Biochemistry. 1987;26(12):3297–303. doi: 10.1021/bi00386a008. [DOI] [PubMed] [Google Scholar]
- 14.Leger JG, Montpetit ML, Tenniswood MP. Characterization and cloning of androgen-repressed mRNAs from rat ventral prostate. Biochemical and biophysical research communications. 1987;147(1):196–203. doi: 10.1016/s0006-291x(87)80106-7. [DOI] [PubMed] [Google Scholar]
- 15.Michel D, Gillet G, Volovitch M, Pessac B, Calothy G, Brun G. Expression of a novel gene encoding a 51.5 kD precursor protein is induced by different retroviral oncogenes in quail neuroretinal cells. Oncogene research. 1989;4(2):127–36. [PubMed] [Google Scholar]
- 16.Kirszbaum L, Sharpe JA, Murphy B, d’Apice AJ, Classon B, Hudson P, et al. Molecular cloning and characterization of the novel, human complement-associated protein, SP-40,40: a link between the complement and reproductive systems. The EMBO journal. 1989;8(3):711–8. doi: 10.1002/j.1460-2075.1989.tb03430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jenne DE, Tschopp J. Molecular structure and functional characterization of a human complement cytolysis inhibitor found in blood and seminal plasma: identity to sulfated glycoprotein 2, a constituent of rat testis fluid. Proceedings of the National Academy of Sciences of the United States of America. 1989;86(18):7123–7. doi: 10.1073/pnas.86.18.7123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Silva HV, Harmony JA, Stuart WD, Gil CM, Robbins J. Apolipoprotein J: structure and tissue distribution. Biochemistry. 1990;29(22):5380–9. doi: 10.1021/bi00474a025. [DOI] [PubMed] [Google Scholar]
- 19.May PC, Lampert-Etchells M, Johnson SA, Poirier J, Masters JN, Finch CE. Dynamics of gene expression for a hippocampal glycoprotein elevated in Alzheimer’s disease and in response to experimental lesions in rat. Neuron. 1990;5(6):831–9. doi: 10.1016/0896-6273(90)90342-d. [DOI] [PubMed] [Google Scholar]
- 20.Hartmann K, Rauch J, Urban J, Parczyk K, Diel P, Pilarsky C, et al. Molecular cloning of gp 80, a glycoprotein complex secreted by kidney cells in vitro and in vivo. A link to the reproductive system and to the complement cascade. The Journal of biological chemistry. 1991;266(15):9924–31. [PubMed] [Google Scholar]
- 21.James RW, Hochstrasser AC, Borghini I, Martin B, Pometta D, Hochstrasser D. Characterization of a human high density lipoprotein-associated protein, NA1/NA2. Identity with SP-40,40, an inhibitor of complement-mediated cytolysis. Arteriosclerosis and thrombosis: a journal of vascular biology. 1991;11(3):645–52. doi: 10.1161/01.atv.11.3.645. [DOI] [PubMed] [Google Scholar]
- 22.Danik M, Chabot JG, Mercier C, Benabid AL, Chauvin C, Quirion R, et al. Human gliomas and epileptic foci express high levels of a mRNA related to rat testicular sulfated glycoprotein 2, a purported marker of cell death. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(19):8577–81. doi: 10.1073/pnas.88.19.8577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones SE, Meerabux JM, Yeats DA, Neal MJ. Analysis of differentially expressed genes in retinitis pigmentosa retinas. Altered expression of clusterin mRNA. FEBS letters. 1992;300(3):279–82. doi: 10.1016/0014-5793(92)80863-c. [DOI] [PubMed] [Google Scholar]
- 24.Diemer V, Hoyle M, Baglioni C, Millis AJ. Expression of porcine complement cytolysis inhibitor mRNA in cultured aortic smooth muscle cells. Changes during differentiation in vitro. The Journal of biological chemistry. 1992;267(8):5257–64. [PubMed] [Google Scholar]
- 25.Rosenberg ME, Silkensen J. Clusterin: physiologic and pathophysiologic considerations. The international journal of biochemistry & cell biology. 1995;27(7):633–45. doi: 10.1016/1357-2725(95)00027-m. [DOI] [PubMed] [Google Scholar]
- 26.Humphreys DT, Carver JA, Easterbrook-Smith SB, Wilson MR. Clusterin has chaperone-like activity similar to that of small heat shock proteins. The Journal of biological chemistry. 1999;274(11):6875–81. doi: 10.1074/jbc.274.11.6875. [DOI] [PubMed] [Google Scholar]
- 27.Poon S, Easterbrook-Smith SB, Rybchyn MS, Carver JA, Wilson MR. Clusterin is an ATP-independent chaperone with very broad substrate specificity that stabilizes stressed proteins in a folding-competent state. Biochemistry. 2000;39(51):15953–60. doi: 10.1021/bi002189x. [DOI] [PubMed] [Google Scholar]
- 28.Wellmann A, Thieblemont C, Pittaluga S, Sakai A, Jaffe ES, Siebert P, et al. Detection of differentially expressed genes in lymphomas using cDNA arrays: identification of clusterin as a new diagnostic marker for anaplastic large-cell lymphomas. Blood. 2000;96(2):398–404. [PubMed] [Google Scholar]
- 29.Lae ME, Ahmed I, Macon WR. Clusterin is widely expressed in systemic anaplastic large cell lymphoma but fails to differentiate primary from secondary cutaneous anaplastic large cell lymphoma. Am J Clin Pathol. 2002;118(5):773–9. doi: 10.1309/N3GJ-N7A3-T98G-RYJL. [DOI] [PubMed] [Google Scholar]
- 30.Saffer H, Wahed A, Rassidakis GZ, Medeiros LJ. Clusterin expression in malignant lymphomas: a survey of 266 cases. Mod Pathol. 2002;15(11):1221–6. doi: 10.1097/01.MP.0000036386.87517.AA. [DOI] [PubMed] [Google Scholar]
- 31.Nascimento AF, Pinkus JL, Pinkus GS. Clusterin, a marker for anaplastic large cell lymphoma immunohistochemical profile in hematopoietic and nonhematopoietic malignant neoplasms. Am J Clin Pathol. 2004;121(5):709–17. doi: 10.1309/GQ2R-LNDW-LB9W-Y6UU. [DOI] [PubMed] [Google Scholar]
- 32.Grogg KL, Lae ME, Kurtin PJ, Macon WR. Clusterin expression distinguishes follicular dendritic cell tumors from other dendritic cell neoplasms: report of a novel follicular dendritic cell marker and clinicopathologic data on 12 additional follicular dendritic cell tumors and 6 additional interdigitating dendritic cell tumors. Am J Surg Pathol. 2004;28(8):988–98. doi: 10.1097/01.pas.0000112536.76973.7f. [DOI] [PubMed] [Google Scholar]
- 33.Grogg KL, Macon WR, Kurtin PJ, Nascimento AG. A survey of clusterin and fascin expression in sarcomas and spindle cell neoplasms: strong clusterin immunostaining is highly specific for follicular dendritic cell tumor. Mod Pathol. 2005;18(2):260–6. doi: 10.1038/modpathol.3800294. [DOI] [PubMed] [Google Scholar]
- 34.Boland JM, Folpe AL, Hornick JL, Grogg KL. Clusterin is expressed in normal synoviocytes and in tenosynovial giant cell tumors of localized and diffuse types: diagnostic and histogenetic implications. Am J Surg Pathol. 2009;33(8):1225–9. doi: 10.1097/PAS.0b013e3181a6d86f. [DOI] [PubMed] [Google Scholar]
- 35.Chandra P, Plaza JA, Zuo Z, Diwan AH, Koeppen H, Duvic M, et al. Clusterin expression correlates with stage and presence of large cells in mycosis fungoides. Am J Clin Pathol. 2009;131(4):511–5. doi: 10.1309/AJCPH43ZDVLSOSNB. [DOI] [PubMed] [Google Scholar]
- 36.Lai JP, Chen ZM, Lok T, Chan OT, Himmelfarb E, Zhai Q, et al. Immunohistochemical stains of proliferating cell nuclear antigen, insulin-like growth factor 2 and clusterin help distinguish malignant from benign liver nodular lesions. J Clin Pathol. 2014;67(6):464–9. doi: 10.1136/jclinpath-2013-201907. [DOI] [PubMed] [Google Scholar]
- 37.Mourra N, Scriva A, Mansiaux Y, Gozlan S, Bennis M, Balaton A. Clusterin expression in gastrointestinal neuroendocrine tumours is highly correlated with location and is helpful in determining the origin of liver metastases. Histopathology. 2014;65(5):642–50. doi: 10.1111/his.12450. [DOI] [PubMed] [Google Scholar]
- 38.Mourra N, Couvelard A, Tiret E, Olschwang S, Flejou JF. Clusterin is highly expressed in pancreatic endocrine tumours but not in solid pseudopapillary tumours. Histopathology. 2007;50(3):331–7. doi: 10.1111/j.1365-2559.2007.02608.x. [DOI] [PubMed] [Google Scholar]
- 39.Henderson-Jackson EB, Nasir A, Chen DT, Nandyala P, Djeu J, Strosberg J, et al. Cytoplasmic Clusterin expression correlates with pancreatic neuroendocrine tumor size and pathological stage. Pancreas. 2013;42(6):967–70. doi: 10.1097/MPA.0b013e318293734b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fischer-Colbrie R, Zangerle R, Frischenschlager I, Weber A, Winkler H. Isolation and immunological characterization of a glycoprotein from adrenal chromaffin granules. J Neurochem. 1984;42(4):1008–16. doi: 10.1111/j.1471-4159.1984.tb12704.x. [DOI] [PubMed] [Google Scholar]
- 41.Palmer DJ, Christie DL. The primary structure of glycoprotein III from bovine adrenal medullary chromaffin granules. Sequence similarity with human serum protein-40,40 and rat Sertoli cell glycoprotein. J Biol Chem. 1990;265(12):6617–23. [PubMed] [Google Scholar]
- 42.Weiler R, Cidon S, Gershon MD, Tamir H, Hogue-Angeletti R, Winkler H. Adrenal chromaffin granules and secretory granules from thyroid parafollicular cells have several common antigens. FEBS letters. 1989;257(2):457–9. doi: 10.1016/0014-5793(89)81595-9. [DOI] [PubMed] [Google Scholar]
- 43.Laslop A, Steiner HJ, Egger C, Wolkersdorfer M, Kapelari S, Hogue-Angeletti R, et al. Glycoprotein III (clusterin, sulfated glycoprotein 2) in endocrine, nervous, and other tissues: immunochemical characterization, subcellular localization, and regulation of biosynthesis. J Neurochem. 1993;61(4):1498–505. doi: 10.1111/j.1471-4159.1993.tb13645.x. [DOI] [PubMed] [Google Scholar]
- 44.Min BH, Jeong SY, Kang SW, Crabo BG, Foster DN, Chun BG, et al. Transient expression of clusterin (sulfated glycoprotein-2) during development of rat pancreas. J Endocrinol. 1998;158(1):43–52. doi: 10.1677/joe.0.1580043. [DOI] [PubMed] [Google Scholar]
- 45.Park IS, Che YZ, Bendayan M, Kang SW, Min BH. Up-regulation of clusterin (sulfated glycoprotein-2) in pancreatic islet cells upon streptozotocin injection to rats. The Journal of endocrinology. 1999;162(1):57–65. doi: 10.1677/joe.0.1620057. [DOI] [PubMed] [Google Scholar]
- 46.Schweiger M, Steffl M, Amselgruber WM. Clusterin expression in porcine endocrine cells during islet development. Horm Metab Res. 2007;39(12):862–6. doi: 10.1055/s-2007-991154. [DOI] [PubMed] [Google Scholar]
- 47.Aronow BJ, Lund SD, Brown TL, Harmony JA, Witte DP. Apolipoprotein J expression at fluid-tissue interfaces: potential role in barrier cytoprotection. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(2):725–9. doi: 10.1073/pnas.90.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andersen CL, Schepeler T, Thorsen K, Birkenkamp-Demtroder K, Mansilla F, Aaltonen LA, et al. Clusterin expression in normal mucosa and colorectal cancer. Mol Cell Proteomics. 2007;6(6):1039–48. doi: 10.1074/mcp.M600261-MCP200. [DOI] [PubMed] [Google Scholar]
- 49.Raghupathi R, Duffield MD, Zelkas L, Meedeniya A, Brookes SJ, Sia TC, et al. Identification of unique release kinetics of serotonin from guinea-pig and human enterochromaffin cells. The Journal of physiology. 2013;591(23):5959–75. doi: 10.1113/jphysiol.2013.259796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raghupathi R, Jessup CF, Lumsden AL, Keating DJ. Fusion Pore Size Limits 5-HT Release From Single Enterochromaffin Cell Vesicles. Journal of cellular physiology. 2016;231(7):1593–600. doi: 10.1002/jcp.25256. [DOI] [PubMed] [Google Scholar]
- 51.Trougakos IP, Gonos ES. Clusterin/apolipoprotein J in human aging and cancer. The international journal of biochemistry & cell biology. 2002;34(11):1430–48. doi: 10.1016/s1357-2725(02)00041-9. [DOI] [PubMed] [Google Scholar]
- 52.Rizzi F, Bettuzzi S. The clusterin paradigm in prostate and breast carcinogenesis. Endocr Relat Cancer. 2010;17(1):R1–17. doi: 10.1677/ERC-09-0140. [DOI] [PubMed] [Google Scholar]
- 53.Koltai T. Clusterin: a key player in cancer chemoresistance and its inhibition. Onco Targets Ther. 2014;7:447–56. doi: 10.2147/OTT.S58622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trougakos IP, So A, Jansen B, Gleave ME, Gonos ES. Silencing expression of the clusterin/apolipoprotein j gene in human cancer cells using small interfering RNA induces spontaneous apoptosis, reduced growth ability, and cell sensitization to genotoxic and oxidative stress. Cancer Res. 2004;64(5):1834–42. doi: 10.1158/0008-5472.can-03-2664. [DOI] [PubMed] [Google Scholar]
- 55.Al-Asaaed S, Winquist E. Custirsen (OGX-011): clusterin inhibitor in metastatic prostate cancer. Curr Oncol Rep. 2013;15(2):113–8. doi: 10.1007/s11912-012-0285-1. [DOI] [PubMed] [Google Scholar]
- 56.Xiu P, Dong XF, Li XP, Li J. Clusterin: Review of research progress and looking ahead to direction in hepatocellular carcinoma. World J Gastroenterol. 2015;21(27):8262–70. doi: 10.3748/wjg.v21.i27.8262. [DOI] [PMC free article] [PubMed] [Google Scholar]


