Abstract
In this paper, we report an improved magnesium hydroxide, Mg(OH)2, coprecipitation method for the determination of 16 trace elements (Al, V, Cr, Mn, Mn, Fe, Co, Ni, Cu, Zn, Ga, As, Se, Sb, Sn and Pb) and 18 rare earth elements (REEs), including Sc, Y, U and Th in seawater and estuarine water samples. The procedure involves coprecipitation of the trace elements and REEs on Mg(OH)2 upon addition of a small volume of triethylamine (TEA) followed by analysis of the dissolved pellet solutions by inductively coupled plasma mass spectrometry (ICP-MS). Three-step sequential coprecipitation was carried out on 10 mL aliquots of seawater to eliminate the matrix ions and to preconcentrate the analytes of interest into a 1 mL final volume. Spike recoveries varied from 85% (Th) to 105% (Y). Calcium (Ca), sodium (Na) and potassium (K) matrices were virtually eliminated from the analysis solutions. Collision reaction interface (CRI) technology utilizing H2 and He gases was employed to determine its effectiveness in removing the spectral interferences originating from the residual Mg matrix, TEA and plasma gases. H2 was more effective than He in reducing spectral interferences from TEA and plasma gases. Limits of detection (LODs) ranged from 0.01 ng L−1 (Ho) to 72 ng L−1 (Al). The method was validated by using certified seawater (CASS-4) and estuarine water (SLEW-3) reference materials. Precision for five (n = 5) replicate measurements were between 1.2% (Pr) and 18% (Lu). Fe, Pb, Sn, and Zn impurities in TEA were significant in comparison to the levels in CASS-4 and SLEW-3, while relatively high background signals impacted determinations of low levels of Sc and Th. The effects of these hurdles on precision and accuracy were alleviated by measuring these elements in spiked CASS-4 and SLEW-3.
Keywords: Trace elements, Rare earth elements, seawater, triethylamine, Mg(OH)2 coprecipitation, ICP-MS
Introduction
Elemental impurities, including rare earth elements (REEs) in seawater originate from a variety of natural and anthropogenic processes. Accurately determining the concentrations of these elements in seawater has become of increasing interest because they play important roles in oceanic production as well as provide important information about geochemical cycling of metals, anthropogenic inputs and coastal pollution [1, 2]. For instance, cadmium, cobalt, copper, iron, manganese, nickel, and zinc are micronutrients for phytoplankton and other marine microorganisms [1–3]. Lead is often utilized as an indicator of anthropogenic inputs and coastal pollution [4] while aluminum and scandium are tracers of oceanographic processes [5,6]. REEs have also long been used as tracers of oceanic mixing and surface water complexation [7–10]. In recent years, they are released to aquatic environments as a result of increasing use in industrial and medical applications as catalyst and magnetic resonance imaging (MRI) contrast agents [7–11]. Today, REEs are also realized as indicators of coastal pollution due to increasing discharges to coastal and estuarine waters [7–11].
Despite instrumental advancements, accurate determination of trace elements in seawater remains a challenge. This is mostly because of very low elemental concentrations ranging from parts per trillion (ppt) to low parts per billion (ppb) in a highly saline water (ca. 3.5% salt). Among various instrumental approaches, inductively coupled plasma mass spectrometry (ICP-MS) is a powerful technique offering excellent sensitivity, multielement detection capability and the ability to perform isotopic ratio analysis. ICP-MS has also been the most relied technique of the international trace metal measurement research program (GEOTRACES) which was initiated for fast and accurate determination a suite of trace metals (e.g., Al, Cd, Cu, Fe, Mn, Pb and Zn) in the oceanic waters to improve understanding of the processes involved in oceanic trace element cycles and their effects on the changing environmental conditions [12,13]. Nevertheless, direct determinations from seawater by ICP-MS are often hampered by spectral and non-spectral interferences. In addition to sample introduction challenges and reduced sensitivity due to heavy salt matrix, salt-based and Ar-based polyatomic interferences interfere with isotopes in the mid-mass (m/z) range [14, 15]. Seawater matrix could also cause spectral interferences on REEs, such as 135Ba16O+ and 137Ba16O+ on 151Eu+ and 153Eu+, and 138BaH+ on 139La+, and 138Ba16OH+ on 155Gd+. Though several groups reported direct determination of trace elements from seawater by high resolution magnetic sector instruments (HR-ICP-MS) [16, 17], separation procedures are preferred, even with HR-ICP-MS determinations, to eliminate the salt matrix prior to analysis to avoid problems from exposure of the instrument to salt matrices [18, 19].
Coprecipitation methods have been attractive means for eliminating the salt matrix and preconcentrating the trace elements in seawater [20–32]. Coprecipitation as metal hydroxides has also added advantage for ICP-MS since acid dissolution yields metals ions in slightly acidic solution [20–32]. Various coprecipitation methods have been reported utilizing solutions of lanthanum, La(III) [20], gallium, Ga(III) [21,22], yttrium, Y(III) [23] and iron, Fe(III) [24–26] and Mg(II) [27–32] for scavenging the trace metal ions in seawater onto insoluble La(OH)3, Ga(OH)3, Y(OH)3, Fe(OH)3, Mg(OH)2 respectively. Among these approaches, Mg(OH)2 coprecipitation has been popular for ultra-trace analysis as it uses Mg(II) available in seawater. The first published report of Mg(OH)2 precipitation was based on the precipitation of Ga(OH)3 in the presence of Mg(II) using sodium hydroxide (NaOH) at pH 9 resulting in coprecipitation of Al, Co, Cr, Fe, La, Mn, Ni, Ti, V, Zn, Y and Pb for determination by atomic emission spectroscopy (ICP-AES) [22]. Since then, Mg(OH)2 coprecipitation has been studied for ICP-MS determination with various modifications without adding any additional coprecipitation agent. Elements, including Fe, Cr, Mn and Pb [27–32] were quantitatively coprecipitated from seawater.
Ammonium hydroxide (NH4OH) has been the preferred reagent in recent studies concerning Mg(OH)2 coprecipitation for ICP-MS applications as it is commercially available as high-purity reagent. Secondly, it is a weak base and thus precipitation could be achieved with little to no adjustment of solution pH. The major limitation of NH4OH-induced Mg(OH)2 coprecipitation, however, is that it only scavenges metals ions (e.g., Fe, Cr, Mn and Pb, and REEs) whose hydroxides are insoluble in water. Published literature indicates that attempts have also been made to coprecipitate Cd, Co, Cu and Zn, but the results are somewhat sporadic [28,31,32]. While Co and Zn were scavenged partially in some studies [31,32], Cd and Cu could not be precipitated with Mg(OH)2 [28]. This is due to the fact that formation of hydroxides of these elements is very sensitive to pH of the medium. In slight excess of NH4OH, these elements form soluble ammonia complexes (e.g., Cu(NH3)42+ and Cd(NH3)42+) and tend to stay in solution. In their conclusion statement, Ardini et al. [31] indicated the need for coprecipitation of Cu, Cd, Co and proposed several approaches of coprecipitation. Yet, no significant improvement has occurred over the last decade. Within this context, Mg(OH)2 coprecipitation deserves implementation of new tactics to overcome its known limitations to be a large-scale multielement coprecipitation method in seawater analysis.
In this paper, we described a Mg(OH)2 coprecipitation using triethylamine (TEA), an aprotic base, for scavenging the trace metals and REEs from seawater. Simultaneous coprecipitations were performed with NH4OH and TEA to verify the relative strengths and deficiencies of the procedures with pre-cleaned real seawater samples. The coprecipitation with TEA afforded quantitative scavenging of a large suite of trace elements including those that form ammonia complexes (e.g., Cd, Cu, Co, Ni, and Zn). Experimental conditions were optimized for removal of seawater matrix via sequential coprecipitations. The CRI was optimized using H2 and He gases. The performances of the cell gases were examined for eliminating the spectral interferences associated with residual TEA and Mg matrix, and plasma argon. To the best of our knowledge, this is first report demonstrating the analytical merits of TEA as a unique reagent for improving the scope of Mg(OH)2 coprecipitation in seawater analysis by ICP-MS.
2. Experimental
2.1. Reagents and materials
Ultra-pure deionized water (18.2 MΩ cm resistivity) obtained by dual system cleaning was used throughout. Tap water was first passed through MaxCap® reverse-osmosis deionization (RO/DI) unit (SpectraPure Inc., Tempe, AZ). The pre-cleaned water was gravity-fed into a 4-stage Barnstead™ E-Pure deionization system. A multielement working standard solution containing 10 mg L−1 of Al, As, Ba, Cd, Ca, Co, Cr, Cu, Fe, Ga, K, Mg, Mn, Mo, Na, Ni, Pb, Sb, Se, Sr, Tl, V and Zn in 5% HNO3 (Trace metal grade, Fisher Scientific) was prepared from single element stock solutions containing the appropriate elements at 1000 mg L−1 (High Purity Standards, Fisher Scientific). A 1.0 mg L−1 rare earth elements (REEs) working standard solution was prepared in 5% HNO3 from a 100 mg L−1 REEs standard solution (Ce, Dy, Er, Eu, Gd, Ho, La, Lu, Nd, Pr, Sc, Sm, Tb, Th, Tm, U, Y, Yb) (BDH Chemicals, VWR). A 10 mg L−1 multielement working solution of Au, Ir, Os, Pd, Pt, Ru, Sn and Te was made in 2% HCl and 2% HNO3 from 1000 mg L−1 single element solutions of each element. Triethylamine (Trace metal grade, 99.8%, Lot# A0374495) was purchased from Acros Organics (Fair Lawn, NJ). Trace metal grade ammonium hydroxide (NH4OH, Lot# 7113051) was obtained from Fisher Scientific. All dilute HCl, HNO3 and wash solutions used in this study are prepared on a volume by volume basis (v/v) unless noted otherwise.
2.2. Cleaning of stock seawater
Method development studies were carried out with coastal seawater samples collected off the coast of Pensacola, FL. Collected seawater (2 L) was placed into acid-cleaned polypropylene bottles and acidified to 0.1% HNO3 at the sampling site. In the laboratory, the water samples were filtered through 0.45-µm membrane filters and stored in 0.1% HNO3. Prior to use, samples of the seawater were cleaned with TEA coprecipitations. For cleaning, 50 mL aliquots (n = 20) were taken from the filtered seawater and treated with 0.5 mL of trace metal grade TEA. The resulting colloidal solutions were centrifuged at 10,000 rpm by a centrifuge (Thermo Scientific Sorvall ST 16) for 30 min. The supernatant solutions were transferred into acid-cleaned Teflon tubes (Savillex) and heated at 110 °C for 1 h on a digestion block (DigiPrep, SCP Science, Champlain, NY) to evaporate residual TEA from the seawater samples. All individual water samples were then combined in a 1-L volumetric flask. A volume of 100 mL of 10% HNO3 was added and filled to 1 L with deionized water. The cleaned stock seawater was placed into acid-cleaned polypropylene bottle for use. Sub-samples were analyzed by ICP-OES (Perkin Elmer Optima 8000) and ICP-MS to verify Ca and Mg concentrations in the cleaned seawater. The loss in Ca after the cleaning via coprecipitation was negligible. On the other hand, a significant amount of Mg coprecipitated as Mg(OH)2. The seawater Mg is critical component of Mg(OH)2 coprecipitation. To simulate the coprecipitation and matrix removal scenarios in real seawater samples, Mg levels were established to 1200 mg L−1 by adding appropriate volume of 10 mg mL−1 Mg (from 99.995% Mg(NO3)2• 6H2O, Acros). Aliquots of the cleaned stock seawater (2 mL, n = 5) were precipitated with 0.1 mL TEA. Dissolved pellet solutions were analyzed by ICP-MS to verify the removal of trace metals. The residual elemental impurities were not significant to affect the method development studies using spiked seawater solutions.
2.3. Instrumentation
Elemental determinations were carried out using a Varian 820MS inductively coupled plasma mass spectrometer (Varian, Australia). The instrument was equipped with collision reaction interface (CRI) technology utilizing H2 and He gases, a peltier-cooled double-pass glass spray chamber, a teflon Ari-mist nebulizer (SCP Science, Champlain NY), quartz torch, CRI-type Pt sampler and skimmer cones and all-digital detector (DDEM, Model AF250, ETP Australia) providing nine decades of linear dynamic range. Samples were introduced manually. The instrument was optimized daily with 5 µg L−1 138Ba, 25Mg, 115In, 140Ce, 208Pb solution for optimal sensitivity, oxides (156CeO+/140Ce+ < 3%) and doubly charged ions (138Ba2+/138Ba+ < 2%). Data collection was achieved using ICP-MS Expert software package (version 2.2 b126). Hydrogen was used in determinations with CRI mode. CRI H2 was introduced to the plasma through the tip of the skimmer cone. The flow rate of the H2 was optimized with a series of experiments. The operating parameters of the instrument are summarized in Table 1. An internal standard (IS) solution containing 5 µg L−1 germanium (Ge), rhodium (Rh), rhenium (Re) was used to correct for possible instrumental drift and matrix-related signal fluctuations. The IS solution was mixed on-line with the sample solution.
Table 1.
Operating parameters for Varian 820-MS ICP-MS instrument
| RF Power | 1.4 kW |
| Plasma flow | 17 L min−1 |
| Auxiliary flow | 1.7 L min−1 |
| Nebulizer flow | 1.05 L min−1 |
| Sheath flow | 0.15 L min−1 |
| Sampling depth | 6.5 mm |
| Pump rate | 6 rpm; 0.2 mL min−1 |
| Stabilization time | 20 s |
| Spray chamber temperature | 4 °C |
| Scan mode | Peak hopping |
| Dwell time | 20 ms |
| Points/peak | 1 |
| Scans/peak | 5 |
| Scans/replicate | 3 |
|
| |
| CRI mode settings | |
|
| |
| CRI gas/port | H2/Skimmer cone |
| CRI gas flow rate | 65 mL min−1 |
| Isotopes measured with CRI | 27Al, 45Sc, 52Cr, 56Fe, 89Y, 139La, 140Ce |
2.4. Method development
2.4.1. Comparison of NH4OH- and TEA-assisted Mg(OH)2 coprecipitation
One of the limitations of NH4OH-assisted Mg(OH)2 coprecipitation is that several metal ions, including Cd, Co, Cu, Ni, Zn that are of interest in GEOTRACES program cannot be scavenged quantitatively in ammoniacal seawater solutions. Previous attempts show partial scavenging requires the use of matrix-matched calibration [28, 31]. In the beginning of the studies, we carried out a series of Mg(OH)2 coprecipitations with TEA and NH4OH with pre-cleaned seawater. Studies were performed in 2-mL polypropylene micro-centrifuge tubes (Fisher Scientific) that were soaked in 5% HNO3 overnight. A volume of 1 mL seawater was placed into 2-mL centrifuge tubes (n=5), and spiked with 20 µL of 1.0 µg mL−1 trace elements and 0.1 µg mL−1 REEs solutions. Precipitation of Mg(OH)2 was made with 100 µL TEA or 350–400 µL NH4OH. A larger volume of ammonia was required for initiating Mg(OH)2 colloids. Once the appropriate precipitation solution was added, each sample was brought to a final volume of 2 mL with deionized water. The solutions were inverted several times and allowed 10 min for precipitation of trace elements onto the Mg(OH)2 colloids. Each solution was then centrifuged at 10,000 rpm for 15 min using Eppendorf 5415D centrifuge. The supernatant liquid was discarded. The pellets were washed gently with water to remove the artifacts of matrix salts (e.g., Na, Ca, K, Cl etc.) then dissolved in 1 mL of 5% HNO3. Aliquots of 1 mL unspiked seawater (n = 5) were also coprecipitated with 100 µL TEA with 350–400 µL NH4OH using the same procedure for blank corrections (e.g., control). All solutions were analyzed by ICP-MS for spike recoveries. The spike concentrations were 20 µg L−1 for trace elements and 2.0 µg L−1 for REEs in 1 mL analysis solution.
2.4.2. Optimization of coprecipitation conditions with TEA
Experiments were carried out to optimize the coprecipitation conditions from 10 mL seawater into 1 mL to achieve 10-fold preconcentration. The objectives were to (1) quantitatively precipitate a large suite of elements within minimal amount of Mg(OH)2 (2) effectively get rid of other matrix ions (e.g., Na, Ca, K) from solution. In this context, seawater samples were coprecipitated stepwise first to reduce the volume from 10 mL down to 2 mL, then to reduce it from 2 mL to 1 mL. Details of each coprecipitation are described below.
First coprecipitation
10 mL seawater (n = 5) was first spiked with 40 µL of 1.0 µg mL−1 of trace elements and 0.1 µg mL−1 REEs solutions and precipitated with 50, 100 or 150 µL of TEA to determine the optimum TEA volume for coprecipitation of analytes of interest in a 10 mL volume. After sitting for 15 min, colloidal solutions were centrifuged at 6,000 rpm on a VWR Clinical 200 centrifuge for 20 min. The supernatant solution was poured off and the remaining pellet was dissolved in 1 mL of 5% HNO3 and completed to 2 mL with deionized water for ICP-MS analysis. Expected elemental concentrations were 20 µg L−1 for trace elements and 2 µg L−1 for REEs.
Second coprecipitation
This experiment was carried out to optimize TEA volume for Mg(OH)2 coprecipitation when reducing the volume from 2 to 1 mL. As described in first coprecipitation, another set of 10 mL seawater (n = 5) spiked with trace elements was coprecipitated with 100 µL TEA which was determined to be optimum volume from first coprecipitation experiment. After centrifugation, the remaining pellet was dissolved in sufficient volume of 5% HNO3 and completed to 2 mL with water. The volume of 5% HNO3 at this stage was determined to be around 0.6 and 0.7 mL with several trials. This volume was sufficient to dissolve the pellets ensuring that the resulting solution was neutral to slightly acidic prior to second coprecipitation. The contents in 2-mL solutions were coprecipitated again with 15, 25 or 35 µL TEA to determine the optimum TEA volume that provided quantitative elemental recoveries along with effective removal of the salt matrix. After adding TEA, colloidal solutions were allowed to sit 10 min before they were centrifuged at 10,000 rpm for 15 min using Eppendorf 5415D centrifuge. The pellet was washed gently and dissolved in 1 mL with 5% HNO3 for ICP-MS analysis. The expected elemental concentrations were 40 µg L−1 for trace elements and 4 µg L−1 for REEs.
Third coprecipitation
A 10 mL volume seawater (n = 4) was treated as described in the preceding section, but the second coprecipitation was performed with 20 µL TEA which was determined to be optimum TEA volume. The pellets from this second coprecipitation were dissolved with 0.3 to 35 mL of 5% HNO3 and completed to 2 mL with water. Contents were coprecipitated for a third time with 20 µL TEA. After centrifugation, the supernatant was removed and the remaining pellet was dissolved in 1 mL of 5% HNO3. The prepared solutions were analyzed by ICP-MS.
2.4.3 Optimization of CRI conditions for reducing spectral interferences
The collision reaction interface (CRI) of the ICP-MS instrument allows the spectral interferences to be removed using H2 and He that are directly injected into the plasma at tip of the skimmer or sampler cone as the ion beam from the plasma passes through the orifice of the cones. The CRI conditions were optimized with simulated matrix solutions. A reagent blank containing 20 µL TEA in 10 mL of 2% HNO3 solution was used to elucidate the extent of interferences from residual TEA. A second matrix solution was made similarly in 2% HNO3 with 800 µg mL−1 Mg and 20 µL TEA along with 10 µg L−1 trace elements and 1 µg L−1 REEs. These test solutions were analyzed simultaneously with 2% HNO3 blank and 10 µg L−1 standard solution for 52Cr, 56Fe, 65Cu and 66Zn in CRI mode using H2 and He gases. The optimum CRI conditions were determined based on the removal of interferences on 52Cr and 56Fe isotopes.
2.5. Method validation and procedure
The Mg(OH)2 coprecipitation protocol described here is based on the coprecipitation of analytes of interest in 10 mL seawater (n = 5). Three sequential coprecipitations were performed to increase the removal of Mg in analysis solution. First, 0.1 mL TEA was added into 10 mL seawater in 0.1% HNO3 (pH 1.8 – 2.0) in a 15-mL conical test tube (Falcon™). The solution was inverted several times for mixing and then allowed to sit for 15 min for precipitation. The contents were then centrifuged at 6,000 rpm for 15 min on a centrifuge (VWR Clinical 200). The supernatant was discarded and the pellet was dissolved in 0.5 to 0.6 mL of 5% HNO3. If dissolution was incomplete, acid was added dropwise until a clear solution was obtained. This was to ensure the solution was either neutral or slightly acidic before second precipitation. After dissolution, the solutions were transferred to 2-mL acid-cleaned micro-centrifuge tubes and brought to 2 mL volume with deionized water. For second coprecipitation, 20 µL TEA was added to initiate the precipitation of Mg(OH)2. At this stage, precipitation usually occurred slowly with visible turbidity within 30 s to a minute. If the solution did not become turbid, TEA was added dropwise until solutions developed visible cloudiness. Turbid solutions were allowed to sit 15 min for complete precipitation; the contents were centrifuged at 10,000 rpm for 10 min on an Eppendorf Model 5415D centrifuge. After pouring off the supernatant, the pellet was dissolved in 0.3 mL of 5% HNO3 and brought to a 2 mL volume with water. Final coprecipitation (third one) was made with by adding 20 µL TEA. The contents were centrifuged and the resulting pellet was gently washed and then dissolved in 1 mL 5% HNO3. The final acidity of the solutions was estimated to be 2 to 3% HNO3.
For method validation, Nearshore seawater (CASS-4) and Estuarine water (SLEW-3) certified reference materials from National Research Council of Canada (NRCC) were treated with the same procedure as described above. Five replicate coprecipitations (n = 5) were implemented in that 10 mL sub-samples of CASS-4 and SLEW-3 were placed into 15-mL tubes and coprecipitated individually. Calibration was performed with multielement external standards over a concentration range of 0.02 to 100 µg L−1 for trace elements and 0.002 to 10 µg L−1 for REEs. The calibration standards were analyzed without any procedural preconcentration treatment or matrix matching.
3. Results and discussion
3.1. Comparison of Mg(OH)2 coprecipitation with TEA and NH4OH
To date, Mg(OH)2 coprecipitation of trace elements from seawater has been implemented with NH4OH or NaOH [20–32]. The latter is a strong base and hence requires very strict control of the solution pH to ensure reproducible precipitation of elements onto Mg(OH)2. In contrast, NH4OH is a weak base (pKb = 4.75) and generates a pH of 10 to 11 in its alkaline solutions, where strong Mg(OH)2 precipitation scavenges Fe, Mn, Cr, and Pb. Other metal ions, including Cd, Co, Cu, Ni, and Zn, despite various modifications of the Mg(OH)2 approach, could not be quantitatively scavenged from the seawater matrix due to formation of ammonia complexes [28,31,32]. Triethylamine (TEA) is an alkylamine (weak base, pKb = 3.25) and readily produces solutions with a pH between 10 and 11, but does not form any complexes with metals ions, because the coordinating abilities of alkylamines decrease in the order of NH3 > RNH2 > R2NH > R3N (R = -CH3, -C2H5 etc.) [33].
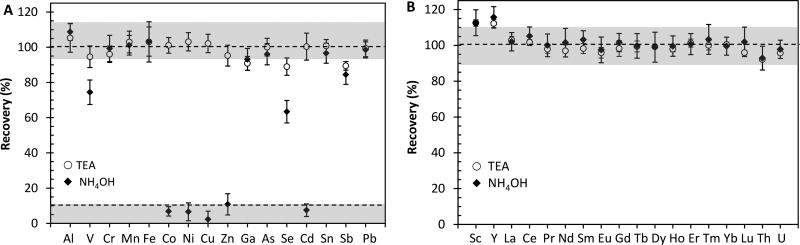
Prior to optimization of the procedural conditions, we examined the precipitation of trace elements via TEA- and NH4OH-induced Mg(OH)2 coprecipitation using the cleaned seawater as described in Section 2.4.1. The elemental recoveries are shown in Fig. 1A and 1B for trace elements and REEs, respectively. Cd, Co, Cu, Ni and Zn virtually remained in solution when NH4OH was added. Only a small fraction, 3% (Cu) to 11% (Zn), of these elements was precipitated from seawater. In contrast, Cd, Co, Cu, Ni and Zn were quantitatively scavenged in seawater when TEA was added for Mg(OH)2 precipitation. Recoveries varied from 95% (Zn) to 103% (Ni). V, Se and Sb also showed partial scavenging from seawater with NH4OH; the recoveries were 74%, 63% and 83%, respectively. Precipitation with TEA was more effective for these elements, improving the recoveries to 95% for V, 80% for Se and 89% for Sb. Other elements, including Al, Cr, Mn, Fe, Ga, As, Sn and Pb were coprecipitated from seawater effectively with both TEA and NH4OH. Recoveries for these elements ranged from 91% (Ga) to 105% (Al) with TEA, and from 93% (Ga) and 109% (Al) with NH4OH. The precipitation of REEs occurred quantitatively and almost to the same extent with TEA and NH4OH (Fig. 1B) However, it should be noted that recoveries for 45Sc and 89Y were high (113–116%). Analysis solutions contained significantly high matrix ions after first precipitation (see Section 3.2 and Table 2) and thus these high recoveries could be from the overlaps of 10B35Cl+ and 44CaH+ on 45Sc, and 88SrH+ and 44Ca10B35Cl+ on 89Y due to incomplete removal of NaCl and other matrix species.
Fig. 1.
Comparison of Mg(OH)2 coprecipitation of trace elements and REEs in seawater with TEA and NH4OH. Values are mean ± standard deviation for five replicates (n = 5).
Table 2.
Effect of TEA volume on the spike recoveries of trace elements after the first Mg(OH)2 coprecipitation of 10 mL seawater into 2 mL volume. Values are for mean ± standard deviation of five replicate measurements (n = 5)
| Element/ Isotope |
Recovery (%) | Element/ Isotope |
Recovery (%) | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 50 µL TEA |
100 µL TEA |
150 µL TEA | 50 µL TEA |
100 µL TEA |
150 µL TEA | ||
|
|
|
||||||
| 27Al | 96 ± 6 | 102± 5 | 102 ± 7 | 45Sc | 106 ± 4 | 109 ± 3 | 111 ± 6 |
| 51V | 87 ± 4 | 93 ± 3 | 91 ± 5 | 89Y | 101 ± 3 | 106 ± 5 | 112 ± 4 |
| 52Cr | 95 ± 6 | 98 ± 4 | 101 ± 4 | 139La | 100 ±5 | 101 ± 5 | 102 ± 6 |
| 55Mn | 96 ± 3 | 101 ± 4 | 98 ± 6 | 140Ce | 102 ± 3 | 102 ± 4 | 102 ± 4 |
| 56Fe | 97± 7 | 101 ± 4 | 102 ± 5 | 141Pr | 98± 5 | 99 ± 6 | 99 ± 5 |
| 59Co | 83 ± 4 | 94 ± 2 | 98 ± 2 | 146Nd | 97 ± 3 | 96 ± 5 | 96 ± 3 |
| 60Ni | 94 ± 5 | 97 ± 5 | 101 ± 5 | 149Sm | 96 ± 3 | 98± 2 | 100 ± 5 |
| 63Cu | 92 ± 6 | 94 ± 5 | 97 ± 6 | 153Eu | 98 ± 4 | 98 ± 6 | 98± 4 |
| 68Zn | 78 ± 5 | 92 ± 6 | 93 ± 7 | 157Gd | 93 ± 5 | 94 ± 4 | 99 ± 2 |
| 69Ga | 95 ± 3 | 99 ± 3 | 99 ± 4 | 159Tb | 94 ± 6 | 95 ± 5 | 97 ± 6 |
| 75As | 94 ± 5 | 96 ± 5 | 100 ± 3 | 163Dy | 96 ± 5 | 98 ± 3 | 97 ± 3 |
| 82Se | 82 + 3 | 93 ± 4 | 94 ± 5 | 165Ho | 97 ± 2 | 100 ± 5 | 98 ± 4 |
| 112Cd | 84 ± 4 | 95 ± 3 | 98 ± 4 | 167Er | 92± 3 | 96 ± 2 | 97 ± 3 |
| 120Sn | 93 ± 5 | 97 ± 3 | 98 ± 3 | 169Tm | 93 ± 5 | 98 ± 5 | 100 ± 4 |
| 121Sb | 82 ± 4 | 93 ± 6 | 95 ± 3 | 172Yb | 93 ± 4 | 100 ± 2 | 98 ± 3 |
| 208Pb | 84 ± 5 | 96± 4 | 94 ± 5 | 175Lu | 97 ± 6 | 101 ± 3 | 100 ± 4 |
| 232Th | 82 ± 4 | 92 ± 5 | 98 ± 4 | ||||
| 238U | 83 ± 3 | 95± 4 | 98 ± 6 | ||||
3.2. Optimization of TEA volume for initial Mg(OH)2 coprecipitation
A sequential two-step or three-step Mg(OH)2 coprecipitation was performed for scavenging the trace elements from 10 mL seawater to 1 mL final volume with optimal removal of the seawater matrix. The first coprecipitation was performed with 50, 100 or 150 µL volumes of TEA (n = 5) to determine the optimum TEA volume for scavenging all of the metal ions of interest into a matrix containing only Mg(OH)2. Colloidal solutions formed rapidly for each volume of TEA used; however, coprecipitation was more effective with an increasing volume of TEA. The results are summarized in Table 2 for the analytes of interest. Elemental recoveries for certain trace elements were low for 50 µL TEA that included Co (83%), V (87%), Se (82%), Zn (78%), Cd (84%), Sb (82%) and Pb (84%). For other trace elements, recoveries were better than 90%; 92% (Cu) and 97% (Fe). Recoveries for the weakly scavenged trace elements improved with 100 µL TEA and were between 92% (Zn) and 96% (Pb). The values did not change significantly with 150 µL TEA and the recoveries ranged from 91% (V) to 102% (Fe).
Among the REEs, Th and U showed slightly weak scavenging with 50 µL TEA; 82% and 83%, respectively, while all other REEs were quantitatively precipitated yielding recoveries between 92% (Er) and 102% (Ce) (see Table 2). 45Sc and 89Y recoveries were relatively high again for all TEA volumes ranging between 106% and 112%. Values for Th and U improved to 92% and 95%, respectively, with 100 µL TEA and to 98% with 150 µL TEA. A volume of 100 µL TEA appeared to be optimum for successful scavenging of all trace elements, however, matrix concentration was substantial in the 2-mL analysis solutions after a single step coprecipitation. Mg levels showed a strong correlation with TEA volume and were 1167, 2033 and 2837 µg mL−1 for 50, 100 and 150 µL TEA. Sodium levels were also substantial that ranged from 2320 to 2540 µg mL−1, while those for Ca and K ranged from 35 to 46 and 51 to 73 µg mL−1, respectively.
3.2.1. Matrix removal and elemental recoveries with two-step Mg(OH)2 coprecipitation
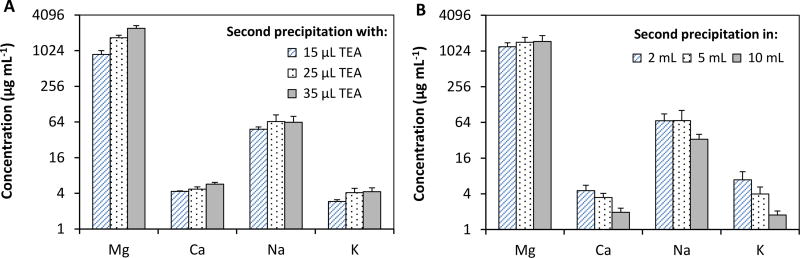
A volume of 100 µL TEA was determined to be optimum for the first coprecipitation of 10 mL seawater into 2 mL volume. In the second precipitation to further remove the matrix ions, the pellet from the first Mg(OH)2 precipitation was dissolved with about 0.6 mL of 5% HNO3 as described in Section 2.4.2. Mg(OH)2 and trace metals were coprecipitated with 15, 25 or 35 µL TEA. The pellets were recovered after centrifugation and dissolved to a final volume of 1 mL with 5% HNO3. The results for matrix ion levels are shown in Fig. 2A. Magnesium levels decreased significantly to 912, 1691, 2447 µg mL−1 for 15, 25 and 35 µL TEA, respectively. These values corresponded to about 4-, 2.3- and 1.6-fold decrease in Mg levels from those measured after the first precipitation with 100 µL TEA (e.g., 2033 µg mL−1 Mg). Sodium concentration did decrease to about 48–65 µg mL−1 from 2320 – 2540 µg mL−1. The levels of Ca and K were very low ranging around 4.3–5.7 µg mL−1 (Ca) and 2.9–4.3 µg mL−1 (K). Although 15 µL TEA afforded lowest Mg levels, pipetting of 20 µL and higher volumes of volatile TEA was more precise ensuring more controlled and consistent coprecipitation; therefore, 20 µL TEA was selected to be optimum.
Fig. 2.
The effect of TEA volume and solution volume on the removal of matrix ion. Bars show the concentration of matrix elements remained in solution after second Mg(OH)2 coprecipitation. Results are mean ± standard deviation for five replicates (n=5).
Additional experiments were performed to minimize the precipitation of Mg(II) in that second coprecipitation was performed in 2, 5 and 10 mL volumes. Namely, the dissolved pellet solutions from the first coprecipitation were brought to 2, 5 or 10 mL with deionized water prior to second coprecipitation. Then, 20 µL TEA was added to the solutions precipitation of Mg(OH)2. No significant improvement occurred in reducing Mg(II) matrix (Fig. 2B); instead precipitation occurred very slowly, especially in 10 mL volumes due to low Mg concentration available for coprecipitation. Other cations, Na, Ca and K, were removed to some extent in 10 mL solutions, but were virtually remained similar for 2 and 5 mL solutions, indicating the coprecipitation in larger volumes was not effective for matrix removal.
The elemental recoveries obtained from second Mg(OH)2 coprecipitation are summarized in Table 3 for 2, 5 and 10 mL volumes. Besides inefficient removal of Mg matrix, recoveries in 10 mL solutions tended to be lower for Cd, Ni, Se, Zn, and Pb, Th and U between 85% (Pb and Zn) and 88% (Cd and U), suggesting weak scavenging due to low Mg levels. For 2 mL solutions possessing higher Mg levels (ca. 2000 µg mL−1), recoveries were better varying between 92% (Zn, Ni, and Sb) and 103% (Al) for trace elements and 91% (Th) to 105% (Y) for REEs. These results indicated that 2 mL volume was more suitable for successive coprecipitations.
Table 3.
Elemental recoveries when second Mg(OH)2 coprecipitation was performed in 2, 5 or 10 mL volumes into 1 mL final volume. Values are for mean ± standard deviation of five replicate measurements (n = 5)
| Element/ Isotope |
Recovery (%) | Element/ Isotope |
Recovery (%) | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 2 mL | 5 mL | 10 mL | 2 mL | 5 mL | 10 mL | ||
|
|
|
||||||
| 27Al | 103 ± 6 | 96± 7 | 93 ± 6 | 45Sc | 98 ± 3 | 96 ± 5 | 94± 8 |
| 51V | 93 ± 8 | 91 ± 5 | 91 ± 6 | 89Y | 105 ± 4 | 97 ± 5 | 90 ± 5 |
| 52Cr | 95 ± 8 | 94 ± 8 | 90 ± 6 | 139La | 98 ± 6 | 97 ± 7 | 97 ± 6 |
| 55Mn | 94 ± 4 | 102 ± 6 | 91 ± 5 | 140Ce | 104 ± 2 | 100 ± 5 | 94 ± 5 |
| 56Fe | 95 ± 8 | 100 ± 4 | 97 ± 8 | 141Pr | 99 ± 3 | 91 ± 3 | 93 ± 7 |
| 59Co | 100± 3 | 93 ± 7 | 96 ± 1 | 146Nd | 99 ± 3 | 90 ± 4 | 101± 4 |
| 60Ni | 92 ± 4 | 92 ± 4 | 88 ± 2 | 149Sm | 102 ± 5 | 89± 4 | 94 ± 2 |
| 63Cu | 99 ± 7 | 93± 6 | 90± 5 | 153Eu | 98 ± 4 | 93 ± 6 | 98± 3 |
| 68Zn | 92 ± 7 | 93 ± 4 | 85 ± 5 | 157Gd | 97 ± 65 | 96 ± 3 | 94 ± 3 |
| 69Ga | 103 ± 5 | 89 ± 7 | 91 ± 3 | 159Tb | 93 ± 4 | 93 ± 3 | 94 ± 2 |
| 75As | 93 ± 3 | 95 ± 7 | 97 ± 2 | 163Dy | 95 ± 5 | 94 ± 3 | 90 ± 2 |
| 82Se | 101 + 6 | 97 ± 5 | 87 ± 4 | 165Ho | 99 ± 3 | 94 ± 2 | 89 ± 3 |
| 112Cd | 97 ± 4 | 100 ± 5 | 89 ± 3 | 167Er | 102± 3 | 89 ± 3 | 89 ± 5 |
| 120Sn | 93 ± 4 | 92 ± 6 | 94± 4 | 169Tm | 103 ± 4 | 92 ±5 | 97 ± 3 |
| 121Sb | 92 ± 3 | 97 ± 7 | 92 ± 6 | 172Yb | 101 ± 5 | 94 ± 6 | 92 ± 3 |
| 208Pb | 93± 5 | 92 ± 3 | 85 ± 3 | 175Lu | 99 ± 4 | 92 ± 2 | 92 ± 3 |
| 232Th | 91 ± 6 | 93 ± 4 | 87 ± 4 | ||||
| 238U | 95 ± 5 | 88± 5 | 88 ± 2 | ||||
3.2.2. Recoveries and matrix removal with three-step Mg(OH)2 coprecipitation
The decrease in concentration of Mg and other matrix ions through first and second precipitation indicated that successive precipitations were more effective for reducing the salt matrix. Based on this, a three-step coprecipitation was implemented on a different set of 10 mL seawater samples (n = 4). Briefly, the dissolved pellet solution from second precipitation was brought to 2 mL with water. The solution was coprecipitated for a third time using 20 µL TEA. The pellet from this precipitation was dissolved in 1 mL with 5% HNO3. The matrix composition of the analysis solutions measured from first to third precipitation are summarized in Table 4. Mg concentration decreased from about 2196 µg mL−1 was to 737 µg mL−1 in the analysis solutions. In addition, the analysis solutions were almost entirely depleted in Ca, Na and K after the third precipitation, which was advantageous to avoid spectral interferences associated with polyatomic ions of Ca, Na and K, such as 44Ca16O+ on 60Ni, 23Na40Ar+ on 63Cu, and 39K40Ar+ on 69Ga. The elemental recoveries (Table 5) after third precipitation did not change significantly in comparison to those from second coprecipitation (see Table 3). The recoveries for trace elements were between 88% (Sb) and 101% (Sn), and those for REEs ranged from 85% (Th) to 105% (Y) indicating successful scavenging of the elements even after repetitive coprecipitations.
Table 4.
Effect of sequential precipitations on removal of matrix ions from seawater with TEA-assisted Mg(OH)2 coprecipitation. Values (µg mL−1) are mean ± standard deviation for four replicates (n = 4)
| Element | Coprecipitation number | ||
|---|---|---|---|
|
| |||
| 1st | 2nd | 3rd | |
|
|
|||
| Na | 2650 ± 225 | 74 ± 16 | 4.5 ± 1.0 |
| K | 71 ± 12 | 3.8 ± 0.8 | 0.7 ± 0.1 |
| Ca | 48 ± 8 | 5.8 ±1.2 | 1.2 ± 0.05 |
| Mg | 2196 ± 154 | 935 ± 118 | 737 ± 127 |
Table 5.
Elemental recoveries for trace elements and REEs after third Mg(OH)2 coprecipitation of 10 mL seawater to 1 mL volume. Results are mean ± standard deviation for four replicates (n = 4)
| Element/Isotope | Recovery (%) | Element/Isotope | Recovery (%) |
|---|---|---|---|
| 27Al | 97 ± 7 | 45Sc | 104 ± 7 |
| 51V | 96 ± 4 | 89Y | 105 ± 6 |
| 52Cr | 96 ± 4 | 139La | 101 ± 7 |
| 55Mn | 97 ± 5 | 140Ce | 101 ± 8 |
| 56Fe | 98 ± 7 | 141Pr | 101 ± 6 |
| 59Co | 93± 4 | 146Nd | 97 ± 7 |
| 60Ni | 92 ± 6 | 149Sm | 98 ± 7 |
| 63Cu | 99 ± 7 | 153Eu | 96 ± 7 |
| 68Zn | 92 ± 8 | 157Gd | 100 ± 6 |
| 69Ga | 93 ± 4 | 159Tb | 96 ± 5 |
| 75As | 92 ± 5 | 163Dy | 94 ± 6 |
| 82Se | 96 + 5 | 165Ho | 96 ± 5 |
| 112Cd | 92 ± 5 | 167Er | 94± 7 |
| 120Sn | 101 ± 5 | 169Tm | 94 ± 6 |
| 121Sb | 88 ± 4 | 172Yb | 101 ± 5 |
| 208Pb | 94± 4 | 175Lu | 93 ± 6 |
| 232Th | 85 ± 7 | ||
| 238U | 90 ± 7 |
3.3. Optimization of CRI conditions for reducing spectral interferences on 56Fe and 52Cr
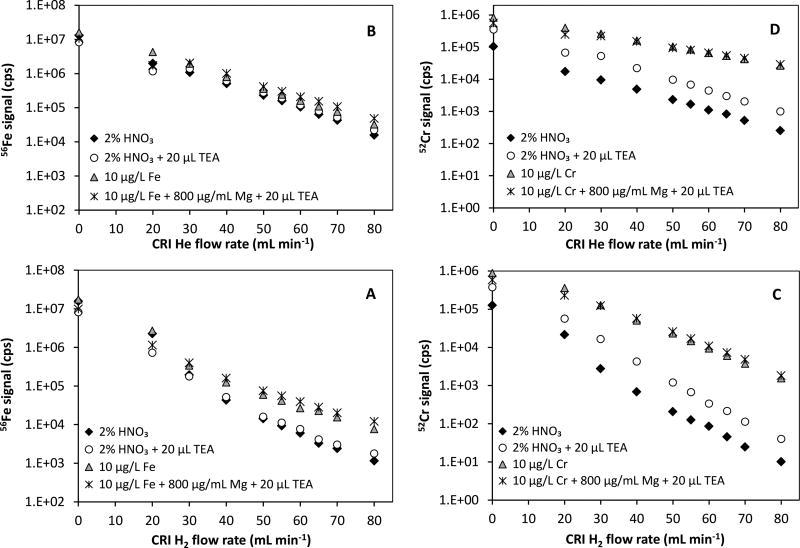
Fig. 3 shows the performances of H2 and He CRI gases on the removal of 40Ar12C+ and 40Ar16O+ interferences on 52Cr and 56Fe isotopes, respectively. A logarithmic decay was observed in the background levels with increasing CRI gas flow rate for both H2 and He. However, H2 CRI was more effective than He in terms of reducing the background signals. H2 is a reactive gas that reduces polyatomic ion interferences by converting them into species at different mass-to-charge ratio (m/z). It should be noted that H2 was also more effective in reducing the background signals from ArH+ and ArOH+ on 45Sc and 57Fe, respectively. These indicate that 40Ar12C+ and 40Ar16O+ species were mainly converted to Ar+, CH+ and OH+ in H2 CRI mode. A flow rate of 65 mL min−1 for H2 was found to be optimal for which the background and analytical signals are summarized in Table 6 along with relevant signal-to-noise (S/N) ratios. For 2% HNO3 blank, 56Fe background signals (e.g., 40Ar16O+) dropped from 1.6×107 to about 2,630 cps with H2 and to 62,262 cps with He (see Fig. 3A and B), indicating that H2 CRI reduced background signal for 56Fe by 24-fold. The S/N ratios were calculated by dividing 56Fe signals from 10 µg L−1 Fe in 2% HNO3 or in Mg matrix (e.g., 800 µg mL−1 Mg + 20 µL TEA) to that from 2% HNO3 blank or 2% HNO3 + 20 µL TEA blank, respectively. With H2 CRI, S/N ratio for 56Fe were 8.7 and 8.8 in the presence and absence Mg matrix, respectively. These values were sufficiently high to discriminate Fe signal from the 40Ar16O+ background for accurate determination of low levels of Fe in seawater (see Fig. 3A). For He CRI, the S/N ratios were 1.8 with and without Mg matrix that were not high enough for differentiating the analytical signal from the background. This effect is also depicted in Fig. 3B where the background signal constantly overlapped on the signal from 10 µg L−1 Fe solution indicating that He was not suitable for 56Fe measurement.
Fig. 3.
The performance of H2 and He CRI modes in alleviating the background signal on 56Fe and 52Cr and differentiating analytical signals for 10 µg L−1 Fe and Cr in the absence and presence of Mg matrix.
Table 6.
Ions signals (cps) and signal-to noise (S/N) ratio for 56Fe and 52Cr isotopes obtained with H2 and He CRI gases for blank (2% HNO3), reagent blank (2% HNO3 + 20 µL TEA), and 10 µg L−1 Fe and Cr in the absence and presence of Mg matrix. Signal-to-noise (S/N) ratio is based on the ratio of average ion signals from 10 µg L−1 Fe or Cr to that from 2% HNO3 in the absence of matrix. In the presence of Mg matrix, S/N is the ratio of ion signals from 10 µg L−1 Fe and Cr in 800 µg mL−1 Mg + 20 µL TEA to that of reagent blank (2% HNO3 + 20 µL TEA)
| Solution/medium | 56Fe | 52Cr | |||
|---|---|---|---|---|---|
|
| |||||
| H2 | He | H2 | He | ||
|
|
|||||
| 2% HNO3 | 2,630 | 62,262 | 45 | 823 | |
| 2% HNO3 + 20 µL TEA | 2,942 | 79,573 | 215 | 2,997 | |
| 10 µg L−1 Fe and Cr | 22,575 (S/N = 8.7) | 112786 (S/N = 1.8) | 6,004 (S/N = 133) | 53,461 (S/N = 65) | |
| 10 µg L−1 Fe and Cr in 800 µg mL−1 Mg + 20 µL TEA | 25,245 (S/N = 8.6) | 142,121 (S/N = 1.8) | 7,226 (S/N = 29) | 54,804 (S/N = 18) | |
Hydrogen as CRI gas also reduced the 40Ar12C+ background significantly on 52Cr in both 2% HNO3 and 2% HNO3 + 20 µL TEA in comparison to relatively high backgrounds with He CRI (Fig. 3C and D). The S/N ratio for 10 µg L−1 Cr in 2% HNO3 (e.g., 133) was about 2-fold higher than the S/N ratio with He (e.g., 65). Although S/N values dropped to 29 for H2 and 18 for He in Mg matrix, H2 CRI was still more effective in reducing the interferences on 52Cr in Mg matrix in comparison to He CRI.
It should be noted that the residual Mg levels after the third coprecipitation negatively affected the determinations with 65Cu and 66Zn isotopes due to 25Mg40Ar+ and 26Mg40Ar+ overlaps, respectively. Unlike H2 CRI, He was more effective in attenuating the interferences from 25Mg40Ar+ and 26Mg40Ar+ in Mg matrix. Theoretical 63Cu/65Cu (2.24) and 66Zn/68Zn (1.48) ratios were attained in 800 µg mL−1 Mg matrix at a He CRI flow rate of 65 mL min−1. However, He CRI was not used to avoid switching between different modes of operations. All CRI determinations were made in H2 mode as it was more effective for 56Fe and 52Cr determination. 65Cu and 66Zn isotopes were included in measurements, but were excluded from calculations and reporting.
3.4. Limits of detection and method blanks
The limits of detection (LOD) and blanks for TEA-assisted Mg(OH)2 coprecipitation are summarized in Table 7 and Table 8 for trace elements and REEs, respectively. Reagent blanks (n = 10) were prepared with 140 µL trace metal grade TEA which were evaporated in pre-cleaned 2-mL tubes at 100 °C on a block digestion unit and brought to a 1 mL volume with 5% HNO3. Blank solutions were analyzed against aqueous calibration standards ranging from 0.010 to 2 µg L−1 for trace elements and 0.002 to 0.5 µg L−1 for REEs. The LODs were calculated from the calibration curves as the concentration equivalent to three times the standard deviation of blank signal (3σ). A multiplier of 10 was used for preconcentration from 10 mL seawater into a 1 mL final volume for estimating the method detection limit. Besides the relative sensitivity of a particular element or isotope in argon plasma, LODs are influenced from blank contamination and extent of background signals. In general, blank contamination from the reagents were very low for most trace elements, except for Al, Fe, Sn, Zn, and Pb. LODs ranged from 0.0003 µg L−1 (Cd) to 0.024 µg L−1 (Cu) that were sufficiently low to achieve accurate determination of the elements in seawater. Al, Fe and Zn impurities in TEA were higher than 0.1 µg L−1 resulting in relatively high LODs. The blank values from TEA for Al, Fe, and Zn were as high as 7%, 28% and 30% of the total Al, Fe and Zn concentration in seawater (CASS-4), respectively. A 7% blank contribution may not impact accuracy on Al as it is one of the most abundant elements in seawater (3–10 µg L−1 levels), but could confound the accuracy on Fe and Zn determinations. In addition, Pb and Sn impurities in TEA were significant; 0.062 µg L−1 for Pb and 0.032 µg L−1 for Sn. The blank signals for Sn were similar to that measured in CASS-4 and SLEW-3, while that for Pb were about 4-fold higher than Pb levels in CASS-4 and SLEW-3. Accurate blank correction and coprecipitation with spiked samples could alleviate the difficulties from blank contamination. Both approaches were used for Fe, Pb, Sn and Zn determination in CASS-4 and SLEW-3 (see Section 3.5).
Table 7.
Procedural blanks, limits of detection (LOD) and the results for trace elements measured in CASS-4 and SLEW-3 with optimized Mg(OH)2 coprecipitation procedure. Values are for mean ± standard deviation of five replicate measurements (n = 5)
| Element/ Isotope |
Blank (µg L−1) |
LOD (µg L−1) |
CASS-4 (µg L−1) |
SLEW-3 (µg L−1) |
||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Measured | Certified | %RSD | Measured | Certified | %RSD | |||
|
|
||||||||
| 27Al | 0.22 | 0.072 | 3.11 ± 0.25 | NR | 8.0 | 2.28 ± 0.20 | NR | 8.8 |
| 51V | 0.014 | 0.006 | 1.08 ± 0.05 | 1.18 ± 0.16 | 4.6 | 2.59 ± 0.20 | 2.57 ± 0.31 | 7.7 |
| 52Cr | 0.025 | 0.011 | 0.162 ± 0.025 | 0.144 ± 0.029 | 15 | 0.198 ± 0.031 | 0.183 ± 0.019 | 16 |
| 55Mn | 0.015 | 0.004 | 2.59 ± 0.14 | 2.78 ± 0.19 | 5.4 | 1.47 ± 0.12 | 1.61 ± 0.22 | 8.2 |
| 56Fe | 0.204 | 0.033 | 0.732± 0.071 | 0.713 ± 0.058 | 9.6 | 0.644 ±0.026 | 0.568 ± 0.059 | 4.1 |
| 56Fea | 0.718± 0.056 | 7.8 | 0.582 ±0.03 | 5.1 | ||||
| 59Co | 0.003 | 0.0006 | 0.026 ± 0.003 | 0.026 ± 0.003 | 11 | 0.039 ± 0.006 | 0.042 ± 0.010 | 15 |
| 60Ni | 0.065 | 0.016 | 0.306 ± 0.034 | 0.314 ± 0.030 | 11 | 1.12 ± 0.082 | 1.23 ± 0.07 | 7.3 |
| 63Cu | 0.070 | 0.024 | 0.642 ± 0.045 | 0.592 ± 0.055 | 7.0 | 1.35 ± 0.15 | 1.55 ± 0.12 | 11 |
| 68Zn | 0.125 | 0.045 | 0.420 ± 0.12 | 0.381 ± 0.057 | 28 | 0.256 ± 0.035 | 0.210 ± 0.037 | 13 |
| 68Zna | 0.405 ± 0.06 | 15 | 0.234 ± 0.02 | 8.5 | ||||
| 69Ga | 0.004 | 0.0003 | BDL | NR | BDL | NR | ||
| 75As | 0.006 | 0.004 | 0.987 ± 0.14 | 1.11 ± 0.16 | 14 | 1.22 ± 0.08 | 1.36 ± 0.09 | 6.5 |
| 82Se | 0.014 | 0.008 | 0.053 + 0.004 | NR | 7.5 | 0.044 ± 0.008 | NR | 18 |
| 112Cd | 0.002 | 0.0003 | 0.029 ± 0.004 | 0.026 ± 0.003 | 14 | 0.043 ± 0.004 | 0.048 ± 0.004 | 9.3 |
| 120Sn | 0.032 | 0.006 | 0.032 ± 0.008 | NR | 25 | 0.043 ± 0.008 | NR | 18 |
| 120Sna | 0.036 ± 0.004 | 11 | 0.048 ± 0.005 | 10 | ||||
| 121Sb | 0.017 | 0.005 | 0.126 ± 0.012 | NR | 9.5 | 0.148 ± 0.015 | NR | 10 |
| 208Pb | 0.062 | 0.008 | 0.015 ± 0.010 | 0.0098 ± 0.0036 | 66 | 0.013 ± 0.008 | 0.0090 ± 0.0014 | 61 |
| 208Pba | 0.013 ± 0.0028 | 22 | 0.012 ± 0.003 | 25 | ||||
Results obtained for CASS-4 and SLEW-3 spiked with 0.05 µg L−1 Pb, Sn, and 0.5 µg L−1 Fe, Zn.
NR: not reported.
BDL: below detection limit.
Table 8.
Procedural blanks, limits of detection (LOD) and the results for REEs measured in CASS-4 and SLEW-3 with optimized Mg(OH)2 coprecipitation procedure. Values are for mean ± standard deviation of five replicate (n=5) measurements
| Element/ Isotope |
Blank (ng L−1) |
LOD (ng L−1) |
CASS-4 (ng L−1) | SLEW-3 (ng L−1) | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Measured | Reported | %RSD | Measured | Reported | %RSD | |||
|
|
||||||||
| 45Sc | 4.8 | 2.5 | 7.2 ± 0.9 | 0.08 + 0.06a | 13 | 9.0 ± 1.2 | 0.55 + 0.09a | 13 |
| 45Sc | 2.7 ± 0.3d | 0.08 + 0.06a | 11 | 2.8 ± 0.2d | 0.55 + 0.09a | 7.1 | ||
| 89Y | 4.2 | 3.0 | 23 ± 2 | 21± 0a | 8.7 | 48 ± 6 | 42 ± 3a | 12 |
| 139La | 3.2 | 1.3 | 13 ± 2 | 9.3 ± 0.2a | 15 | 7.6 ± 0.8 | 7.7 ± 0.4c | 10 |
| 140Ce | 0.8 | 0.09 | 6.5 ± 0.2 | 5.47 ± 0.16b | 3.1 | 8.2 ± 1.0 | 6.6 + 0.3c | 12 |
| 141Pr | 0.8 | 0.09 | 1.3 ± 0.1 | 1.28 ± 0.07b | 7.7 | 1.7 ± 0.02 | 1.64 ± 0.08c | 1.2 |
| 146Nd | 1.2 | 0.32 | 4.7 ± 0.5 | 4.93 ± 0.62b | 11 | 8.4 ± 0.5 | 7.93 ± 0.13c | 6.0 |
| 149Sm | 1.8 | 0.15 | 6.3 ± 0.8 | 5.46 ± 0.22b | 13 | 6.5 ± 0.5 | 7.2 ± 0.3c | 8.5 |
| 153Eu | 0.7 | 0.07 | 0.31 ± 0.04 | 0.28 ± 0.04b | 13 | 0.61 ± 0.04 | 0.48 ± 0.012c | 6.7 |
| 157Gd | 1.2 | 0.07 | 1.3 ± 0.2 | 1.21 ± 0.03b | 15 | 2.6 ± 0.3 | 3.1 ± 0.2c | 11 |
| 159Tb | 0.3 | 0.04 | 0.32 ± 0.02 | 0.26 ± 0.02b | 6.3 | 0.43 ± 0.02 | 0.45 ± 0.013c | 5.0 |
| 163Dy | 0.6 | 0.05 | 1.2 ± 0.10 | 1.41 ± 0.08b | 8.3 | 3.0 ± 0.2 | 3.38 ± 0.09c | 6.7 |
| 165Ho | 0.4 | 0.01 | 0.42 ± 0.02 | 0.46 ± 0.05b | 5.0 | 0.65 ± 0.1 | 0.91 ± 0.02c | 15 |
| 167Er | 0.4 | 0.05 | 1.30 ± 0.15 | 1.22 ± 0.11b | 11 | 2.2 ± 0.2 | 2.70 ± 0.02c | 9.1 |
| 169Tm | 0.4 | 0.04 | 0.45 ± 0.05 | 0.32 ± 0.04b | 11 | 0.65 ± 0.04 | 0.37 ± 0.07c | 6.1 |
| 172Yb | 1.2 | 0.03 | 0.86 ± 0.10 | 1.32 ± 0.11b | 12 | 0.92 ± 0.07 | 1.85 ± 0.002c | 7.6 |
| 175Lu | 1.4 | 0.07 | 0.22 ± 0.04 | 0.21 ± 0.048b | 18 | 0.32 ± 0.05 | 0.291 ± 0.009c | 15 |
| 232Th | 11 | 0.22 | 2.1 ± 0.3 | 0.51 ± 0.02a | 14 | 0.90 ± 0.2 | 0.22 ± 0.01a | 22 |
| 232Th | 1.2 ± 0.09d | 7.5 | 0.65 ± 0.06d | 9.2 | ||||
| 238U | 2.1 | 0.05 | 2.76 ± 0.16e | (3.0) e | 5.8 | 1.62 ± 0.061e | (1.8) e | 3.8 |
Reference values are from Ref.# 46 (Bayon et al., 2011).
Reference values are from Ref. # 33 (Zereen et al., 2013).
Reference values are from Ref.# 54 (Lawrence and Kamber, 2007).
Results obtained for CASS-4 and SLEW-3 spiked with 10 ng L−1 Sc and Th.
Measured and reported values for CASS-4 and SLEW-3 are µg L−1. Values in parentheses are information only.
For REEs, backgrounds signals and contamination from TEA were negligible which produced LODs ranging from 0.01 ng L−1 (Ho) to 1.3 ng L−1 (La), except for 45Sc, 89Y and 232Th. It should be noted that measurement of 45Sc was carried out in CRI mode which provided significant improvement. Still though 45Sc background was relatively high even in 2% HNO3 indicating overlaps from 40ArH+ that could not be overcome completely. In contrast, for 89Y background was low in 2% HNO3 indicating background signals on 89Y were from the impurities in TEA. However, the contamination from the blank was negligible to 89Y levels measured in CASS-4 and SLEW-3. For 232Th, blank levels were also high in 2% HNO3 indicating an unknown source of interference that resulted in relatively high LOD.
3.5. Application to analysis of CASS-3 and SLEW-3
The results for the CASS-4 and SLEW-3 standards are summarized in Table 7 and Table 8 for trace elements and REEs, respectively, along with the certified and reported values (REEs). The precision for five replicate measurements (n = 5) for each CRM is also provided as the relative standard deviation (RSD). The mean values obtained with the coprecipitation method showed good agreement with the certified values for CASS-4 and SLEW-3 within the 95% confidence interval (p < 0.05), except for Pb (p > 0.05). Gallium (Ga) concentrations were below the detection limit (see Table 7). The precision (%RSD) varied between 4.6% (V for CASS-4) and 16% (Cr for SLEW-3). The effect of impurities in TEA was marginal on Fe for which %RSD varied between 4.1 and 9.6%. For Sn, Pb and Zn, precision was poor with %RSDs as high as 25%, 28% and 66%, respectively, due relatively high blank contamination. To overcome this hurdle, 10 mL aliquots (n = 5) of CASS-4 and SLEW-3 were spiked with 0.05 µg L−1 Pb and Sn, and 0.5 µg L−1 Fe and Zn in a separate coprecipitation. Accuracy and precision were improved with the spiked samples. Percent RSD was 7.8% for Fe, 25% for Pb, 11% for Sn and 15% for Zn (see Table 7). Alternatively, the analytical merits of TEA-induced Mg(OH)2 coprecipitation could be also improved for Fe, Sn, Pb and Zn by using distilled TEA. Further, certificate values for Al, Se and Sn are not available for CASS-4 nor for SLEW-3 and hence the values reported here should be considered for reference only.
For the REEs, certificate values for CASS-4 and SLEW-3 are not available; therefore the method accuracy was assessed using the concentrations reported by other groups [26,34,35]. With the exception of Sc and Th, the results agreed within the 95% confidence interval with those obtained by chelating resin solid phase extraction [34,35] and iron hydroxide coprecipitation [26]. Precision varied between 3.1% (Ce) and 18% (Lu) for CASS-4, and 1.2% (Pr) and 22% (Th) for SLEW-3 for five replicate measurements. Values for 139La were about 40% higher than the reference values in CASS-4 [26]. A possible explanation for this discrepancy is 138BaH+ interference though Ba levels after coprecipitation were less than 0.05 µg L−1 in analysis solution. In contrast, 139La results for SLEW-3 samples with similar residual Ba levels were consistent with the reported value [35]. The concentrations of Sc and Th in CASS-4 and SLEW-3 appear to be very low based on the HR-ICP-MS determinations from coprecipitation of 100 mL water samples [26]. In this study, Sc and Th concentrations measured in CASS-4 and SLEW-3 were higher than the reported values [26], which was attributed to high background signals. Similarly, additional determinations were made in CASS-4 and SLEW-3 spiked with 10 ng L−1 Sc and Th. The results are also provided in Table 8. As for the trace elements, precision was improved but the measured concentrations were still higher than the reported values. Eventually, both seawater and estuarine waters contain appreciable levels of boron (B). At very low Sc levels, 10B35Cl+ and 44CaH+ overlaps originating from residual calcium, boron and chloride could be a potential source of interference on 45Sc.
4. Conclusion
In this study, a new approach was developed for improving the performance of traditional Mg(OH)2 coprecipitation method for trace element determination in seawater. Triethlyamine (TEA) was utilized as a unique reagent for coprecipitation of a large suite of trace elements and REEs simultaneously. The procedure allows quantitative scavenging of Co, Cu, Ni, Cd and Zn to be achieved, for which NH4OH-based Mg(OH)2 coprecipitation has been ineffective and problematic. The TEA-induced Mg(OH)2 coprecipitation also afforded quantitative coprecipitation of V, Se and Sb along with U and Th. The procedure is simple and suitable for processing small samples (e.g., 2 or 10 mL) without any need for controlling the pH of solution. Further, TEA is commercially available in trace metal grade and the elemental impurities in TEA are negligible for most trace elements and REEs allowing very low LODs to be achieved for ultra-trace determinations. The impurities in TEA are found to impact the precision and accuracy of determination for Al, Fe, Sn, Pb and Zn. This hurdle could be overcome with several scenarios, such as using large seawater samples (e.g., 50 or 100 mL) and distilled TEA or by isotope dilution analysis.
Acknowledgments
This project is funded in part by grants from the National Institutes of Health (NIH) through Research Centers in Minority Institutions (RCMI) Program (Grant No: G12RR013459) and from the National Science Foundation Research Experience for Undergraduates (NSF-REU) Program at Jackson State University (Grant No: 1461143). The views expressed herein are those of authors and do not necessarily represent the official views of the funding agencies, and any of their sub-agencies. The authors are grateful the Scientific and Technological Council of Turkey (TUBITAK) for post-doctoral fellowship to Dr. Tulay Oymak during the course of this project at Jackson State University.
References
- 1.Sunda WG. Feedback interactions between trace metal nutrients and phytoplankton in the ocean. Front. Microbiol. 2012;3:1–22. doi: 10.3389/fmicb.2012.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Twining BS, Baines SB. The trace metal composition of marine phytoplankton. Ann. Rev. Mar. Sci. 2013;5:191–215. doi: 10.1146/annurev-marine-121211-172322. [DOI] [PubMed] [Google Scholar]
- 3.Gregg WW, Ginoux P, Schopf PS, Casey NW. Phytoplankton and iron: Validation of a global three-dimensional ocean biogeochemical model. Deep. Res. Part II Top. Stud. Oceanogr. 2003;50:3143–3169. doi: 10.1016/j.dsr2.2003.07.013. [DOI] [Google Scholar]
- 4.Boyle EA, Lee J-M, Echegoyen Y, Noble A, Moos S, Carrasco G, Zhao N, Kayser R, Zhang J, Gamo T, Obata H, Norisuye K. Anthropogenic lead emissions in the ocean: The evolving global experiment. Oceanography. 2014;27:69–75. doi:dx.doi.org/10.5670/oceanog.2014.10. [Google Scholar]
- 5.Orians KJ, Bruland KW. Dissolved aluminium in the central North Pacific. Nature. 1985;316:427–429. doi: 10.1038/314731a0. [DOI] [Google Scholar]
- 6.Parker CE, Brown MT, Bruland KW. Scandium in the open ocean: A comparison with other group 3 trivalent metals. Geophys. Res. Lett. 2016;43:2758–2764. doi: 10.1002/2016GL067827. [DOI] [Google Scholar]
- 7.Halicz L, Segal I, Yoffe O. Direct REE determination in fresh waters using ultrasonic nebulization ICP-MS. J. Anal. At. Spectrom. 1999;14:1579–1581. doi: 10.1039/a808387h. [DOI] [Google Scholar]
- 8.Xiang G, Jiang Z, He M, Hu B. Direct determination of trace rare earth elements in ancient porcelain samples with slurry sampling electrothermal vaporization inductively coupled plasma mass spectrometry. Spectrochim. Acta Part B. 2005;60:1342–1348. doi: 10.1016/j.sab.2005.07.005. [DOI] [Google Scholar]
- 9.Arslan Z, Paulson A. Solid phase extraction for analysis of biogenic carbonates by electrothermal vaporization inductively coupled plasma mass spectrometry (ETV-ICP-MS): an investigation of rare earth element signatures in otolith microchemistry. Anal. Chim. Acta. 2003;476:1–13. doi: 10.1016/s0003-2670(02)01368-5. [DOI] [Google Scholar]
- 10.McLing T, Smith W, Smith R. Utilizing rare earth elements as tracers in high TDS reservoir brines in CCS applications. Energy Procedia. 2014;63:3963–3974. doi: 10.1016/j.egypro.2014.11.426. [DOI] [Google Scholar]
- 11.Kramer KJM, Dorten WS, van het Groenewoud H, de Haan E, Kramer GN, Monteiro L, Muntau H, Quevauviller Ph. Collaborative study to improve the quality control of rare earth element determinations in environmental matrices. J. Environ. Monitor. 1999;1:83–89. doi: 10.1039/a807381c. [DOI] [PubMed] [Google Scholar]
- 12.Anderson R, Mawji E, Cutter G, Measures C, Jeandel C. GEOTRACES: Changing the Way We Explore Ocean Chemistry. Oceanography. 2014;27:50–61. doi: 10.5670/oceanog.2014.07. [DOI] [Google Scholar]
- 13.Lagerström ME, Field MP, Séguret M, Fischer L, Hann S, Sherrell RM. Automated on-line flow-injection ICP-MS determination of trace metals (Mn, Fe, Co, Ni, Cu and Zn) in open ocean seawater: Application to the GEOTRACES program. Mar. Chem. 2013;155:71–80. doi: 10.1016/j.marchem.2013.06.001. [DOI] [Google Scholar]
- 14.May TW, Wiedmeyer RH. A table of polyatomic interferences in ICP-MS. At. Spectrosc. 1998;19:150–155. [Google Scholar]
- 15.Pröfrock D, Prange A. Inductively coupled plasma-mass spectrometry (ICP-MS) for quantitative analysis in environmental and life sciences: A review of challenges, solutions, and trends. Appl. Spectrosc. 2012;66:843–868. doi: 10.1366/12-06681. [DOI] [PubMed] [Google Scholar]
- 16.Field MP, Cullen JT, Sherrell RM. Direct determination of 10 trace metals in 50 µL samples of coastal seawater using desolvating micronebulization sector field ICP-MS. J. Anal. At. Spectrom. 1999;14:1425–1431. doi: 10.1039/a901693g. [DOI] [Google Scholar]
- 17.Rodushkin I, Ruth T. Determination of trace metals in estuarine and sea-water reference materials by high resolution inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 1997;12:1181–1185. doi: 10.1039/a702486j. [DOI] [Google Scholar]
- 18.Milne A, Landing W, Bizimis M, Morton P. Determination of Mn, Fe, Co, Ni, Cu, Zn, Cd and Pb in seawater using high resolution magnetic sector inductively coupled mass spectrometry (HR-ICP-MS) Anal. Chim. Acta. 2010;665:200–207. doi: 10.1016/j.aca.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 19.Hatje V, Bruland KW, Flegal AR. Determination of rare earth elements after pre-concentration using NOBIAS-chelate PA-1®resin: Method development and application in the San Francisco Bay plume. Mar. Chem. 2014;160:34–41. doi: 10.1016/j.marchem.2014.01.006. [DOI] [Google Scholar]
- 20.Toyota Y, Okabe S, Kanamori S, Kitano Y. The determination of Mn, Fe, Ni, Cu and Zn in seawater by atomic absorption spectrometry after coprecipitation with lanthanum hydroxide. J. Oceanogr. Soc. Japan. 1983;38:357–361. doi: 10.1007/BF02111032. [DOI] [Google Scholar]
- 21.Sawatari H, Fujimori E, Haraguchi H. Multi-Element determination of trace elements in seawater by gallium coprecipitation and inductively coupled plasma mass spectrometry. Anal. Sci. 1995;11:369–374. [Google Scholar]
- 22.Akagi T, Fuwa K, Haraguchi H. Simultaneous multi-element determination of trace metals in sea water by inductively-coupled plasma atomic emission spectrometry after coprecipitation with gallium. Anal. Chim. Acta. 1985;177:139–151. doi: http://dx.doi.org/10.1016/S0003-2670(00)82946-3. [Google Scholar]
- 23.Kagaya S, Miwa S, Mizuno T, Tohda K. Rapid coprecipitation technique using yttrium hydroxide for the preconcentration and separation of trace elements in saline water prior to their ICP-AES determination. Anal. Sci. 2007;23:1021–4. doi: 10.2116/analsci.23.1021. [DOI] [PubMed] [Google Scholar]
- 24.Zhu Y, Inagaki K, Haraguchi H, Chiba K. On-line elution of iron hydroxide coprecipitate carrier for determination of REEs in natural water by mix-gas ICP-MS. J. Anal. At. Spectrom. 2010;25:364–369. doi: 10.1039/B921214K. [DOI] [Google Scholar]
- 25.Chou CL, Moffatt JD. A simple co-precipitation inductively coupled plasma mass spectrometric method for the determination of uranium in seawater. Fresenius J. Anal. Chem. 2000;368:59–61. doi: 10.1007/s002160000518. [DOI] [PubMed] [Google Scholar]
- 26.Bayon G, Birot D, Bollinger C, Barrat JA. Multi-element determination of trace elements in natural water reference materials by ICP-SFMS after Tm addition and iron co-precipitation. Geostand. Geoanalytical Res. 2011;35:145–153. doi: 10.1111/j.1751-908X.2010.00064.x. [DOI] [Google Scholar]
- 27.Wu J, Boyle EA. Determination of iron in seawater by high-resolution isotope dilution inductively coupled plasma mass spectrometry after Mg(OH)2 coprecipitation. Anal. Chim. Acta. 1998;367:183–191. doi: 10.1016/S0003-2670(98)00145-7. [DOI] [Google Scholar]
- 28.Saito MA, Schneider DL. Examination of precipitation chemistry and improvements in precision using the Mg(OH)2 preconcentration inductively coupled plasma mass spectrometry (ICP-MS) method for high-throughput analysis of open-ocean Fe and Mn in seawater. Anal. Chim. Acta. 2006;565:222–233. doi: 10.1016/j.aca.2006.02.028. [DOI] [Google Scholar]
- 29.Wu J. Determination of picomolar iron in seawater by double Mg(OH)2 precipitation isotope dilution high-resolution ICPMS. Mar. Chem. 2007;103:370–381. doi: 10.1016/j.marchem.2006.10.006. [DOI] [Google Scholar]
- 30.Hsieh H-F, Chen Y-H, Wang C-F. A magnesium hydroxide preconcentration/matrix reduction method for the analysis of rare earth elements in water samples using laser ablation inductively coupled plasma mass spectrometry. Talanta. 2011;85:983–990. doi: 10.1016/j.talanta.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 31.Ardini F, Magi E, Grotti M. Determination of ultra-trace levels of dissolved metals in seawater by reaction cell inductively coupled plasma mass spectrometry after ammonia induced magnesium hydroxide coprecipitation. Anal. Chim. Acta. 2011;706:84–88. doi: 10.1016/j.aca.2011.07.046. [DOI] [PubMed] [Google Scholar]
- 32.Freslon N, Bayon G, Birot D, Bollinger C, Barrat JA. Determination of rare earth elements and other trace elements (Y, Mn, Co, Cr) in seawater using Tm addition and Mg(OH)2 co-precipitation. Talanta. 2011;85:582–587. doi: 10.1016/j.talanta.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 33.Hatfield WE, Yoke JT. Complexes of the Ethylamines with the Halides of Calcium, Cobalt(II), and Zinc. Inorg. Chem. 1962;1:463–470. doi: 10.1021/ic50003a004. [DOI] [Google Scholar]
- 34.Zereen F, Yilmaz V, Arslan Z. Multielement solid phase preconcentration using a chelating resin of styrene divinylbenzene copolymer and application for the analysis of seawater and fish otoliths by inductively coupled plasma mass spectrometry (ICP-MS) Anal. Lett. 2014;47:58–76. doi: 10.1080/00032719.2013.831428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lawrence MG, Kamber BS. Rare earth element concentrations in the natural water reference materials (NRCC) NASS-5, CASS-4 and SLEW-3. Geostand. Geoanalytical Res. 2007;31:95–103. doi: 10.1111/j.1751-908X.2007.00850.x. [DOI] [Google Scholar]