Abstract
Tautomeric and anionic Watson-Crick-like mismatches play important roles in replication and translation errors through mechanisms that are not fully understood. Using NMR relaxation dispersion, we resolved a sequence-dependent kinetic network connecting G•T/U wobbles with three distinct Watson-Crick mismatches consisting of two rapidly exchanging tautomeric species (Genol•T/U⇌G•Tenol/Uenol; population <0.4%) and one anionic species (G•T−/U−; population ≈0.001% at neutral pH). Inserting the sequence-dependent tautomerization/ionization step into a minimal kinetic mechanism for correct incorporation during replication following initial nucleotide binding leads to accurate predictions of dG•dT misincorporation probability across different polymerases, pH conditions, and for a chemically modified nucleotide, and provides mechanisms for sequence-dependent misincorporation. Our results indicate that the energetic penalty for tautomerization/ionization accounts for ≈10−2−10−3–fold discrimination against misincorporation, which proceeds primarily via tautomeric dGenol•dT and dG•dTenol with contributions from anionic dG•dT− dominating at pH ≥8.4 or for some mutagenic nucleotides.
In their paper describing the structure of the DNA double helix1, Watson and Crick proposed that if nucleotide bases adopted their energetically unfavorable tautomeric forms, mismatches (Fig. 1a) could pair up in a Watson-Crick-like (WC-like) geometry (Fig. 1b) and potentially give rise to spontaneous mutations. Decades later, it is well established that the replicative and translational machineries form a tight grip around the WC geometry to discriminate against mismatches2–5. There is also evidence that both tautomeric6–13 (Fig. 1b) and anionic7–9,14,15 (Fig. 1c) WC-like mismatches can evade such fidelity checkpoints and give rise to replication6,7 and translation errors16. Despite their centrality to the fidelity of information transfer in the central dogma of molecular biology, and growing evidence showing the involvement of spontaneous mutations in generating cancer causing mutations17, the very existence of these species and their contributions to replication and translation errors remain to be definitively established.
Figure 1. Tilting rapid tautomeric equilibria in excited state WC-like mismatches.
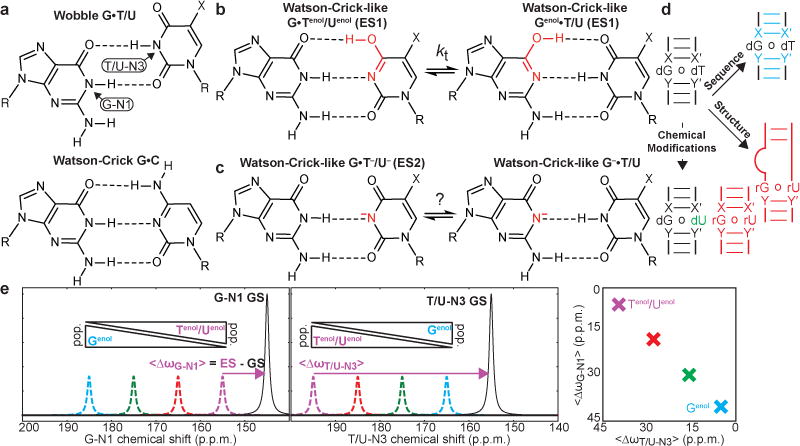
Chemical structures of ground state wobble (a) and excited state tautomeric (b) and anionic (c) WC-like G•T/U (X = H or CH3 for uridine and thymidine, respectively) mismatches. d, Tilting rapid tautomeric equilibrium using sequence (cyan), structure (red), and chemical modifications (green). X-X′ and Y-Y′ denote WC base pairs adjacent to the G•T/U mismatches. e, Perturbations that differentially tilt the tautomeric equilibrium are expected to give rise to anti-correlated linear changes in the <∆ωG-N1> and <∆ωT/U-N3> values (color-coded).
Tautomeric and anionic mismatches come in a variety of chemical forms (Extended Data Fig. 1). For example, WC-like G•T/U mismatches can form when either the guanine (Genol•T/U and G−•T/U) or thymidine/uridine (G•Tenol/Uenol and G•T−/U−) base assumes a rare enolic (Fig. 1b) or anionic (Fig. 1c) form. While it remains unclear which WC-like mismatch contributes to replication and translation errors, factors (e.g. changes in pH7,8,14,18 and chemical modifications19) that stabilize different forms have been shown to increase misincorporation probabilities14,20. Misincorporation probabilities can also vary significantly with sequence context through mechanisms that remain poorly understood21,22. Resolving these different WC-like mismatches and their chemical dynamics is key for elucidating their potential roles in replication, transcription, and translation errors. However, this presents a formidable challenge to current biophysical methods because these mismatches differ by the placement of a single proton and π-bond (Fig. 1b,c and Extended Data Fig. 1). Protons are generally invisible to X-ray crystallography and cryo-EM12, and consequently it has not been possible to unambiguously resolve the identity of WC-like mismatches captured within active sites of polymerases6,7,15,23 and the ribosome decoding site9,24. Moreover, WC-like mismatches are predicted to exist in rapid tautomeric (Genol•T/U⇌G•Tenol/Uenol)25,26 equilibria (Fig. 1b,c and Extended Data Fig. 1) making them exceptionally difficult to capture experimentally.
Techniques based on NMR relaxation dispersion (RD)27–29 make it possible to characterize low-abundance short-lived conformational states, or ‘excited states’ (ESs), in biomolecules30. Using NMR RD, we recently provided evidence that wobble G•T/U mismatches exist in dynamic equilibrium with tautomeric (ES1) and anionic (ES2) WC-like mismatches within DNA and RNA duplexes8,31. The guanine N1 (G-N1) and thymidine/uridine N3 (T/U-N3) chemical shifts measured for tautomeric ES1 were consistent with Genol•T/U, but were partially skewed toward G•Tenol/Uenol. This was interpreted as evidence for a rapid (on the chemical shift timescale) equilibrium between a major Genol•T/U and minor G•Tenol/Uenol species8. The anionic ES2 was only detectable at high pH (≥7.8) and was heavily skewed in favor of G•T−/U− with no evidence for G−•T/U. The roles of these various WC-like mismatches in replication and translation errors remains unknown. Here, by combining NMR RD and measurements of misincorporation rates, we resolved a kinetic network connecting two distinct tautomeric and one anionic WC-like mismatches, and established their relative contributions to dG•dTTP misincorporation.
Tilting the tautomeric equilibrium
If ES1 does indeed represent two tautomeric species in rapid equilibrium (Fig. 1b), it should be feasible to tilt the equilibrium (Kt = pGenol/pTenol/Uenol) by changing the local sequence or structural context around the mismatch, or by using base modifications (Fig. 1d). This in turn should lead to very specific changes in the ES1 G-N1 and T/U-N3 chemical shifts, which are population weighted averages over the two species (Fig. 1e; left). Tilting the equilibrium in favor of Genol•T/U should induce a downfield shift in the ES1 G-N1, because it increases the population of deprotonated Genol, and an upfield shift in ES1 T/U-N3, because it decreases the population of deprotonated Tenol/Uenol, and vice versa (Fig. 1e; left). ∆ωT/U-N3 versus ∆ωG-N1 is predicted to be linear (Fig. 1e; right) with negative slope and intercept determined by the fundamental chemical shifts of the tautomeric species (Equation 1).
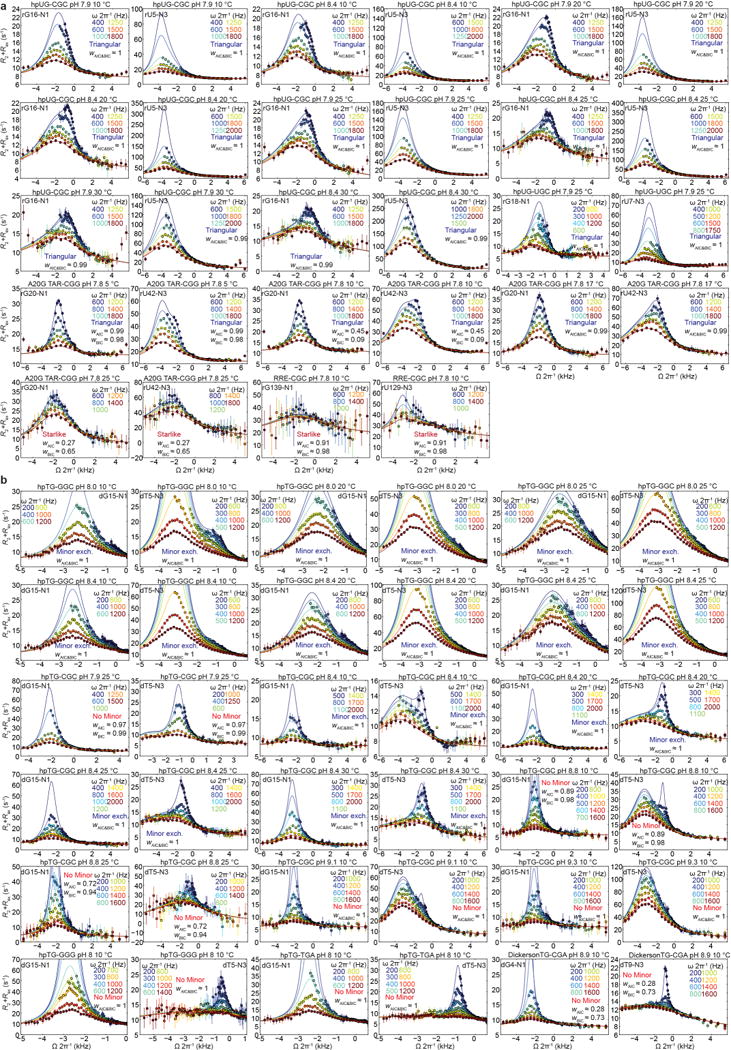
We measured 15N RD for five dG•dT mismatches within distinct sequence contexts and for thirteen rG•rU mismatches in nine structurally unique non-coding RNAs (Fig. 2a and Extended Data Fig. 2a). Experiments were carried out at near-neutral pH (6.4–6.9) so as to lower the anionic ES2 below detection8 (Extended Data Fig. 2b). The RD experiments measure spin-relaxation rates in the rotating frame (R1ρ) during a relaxation period in which a radiofrequency field is applied with variable offset (Ω 2π−1, in Hz) and power (ω 2π−1, in Hz) to suppress the chemical exchange contribution (Rex) to the transverse spin relaxation rate (R2) arising due to chemical exchange between the energetically more stable ground state (GS) and ES27,28.
Figure 2. Resolving rapidly interconverting tautomers.
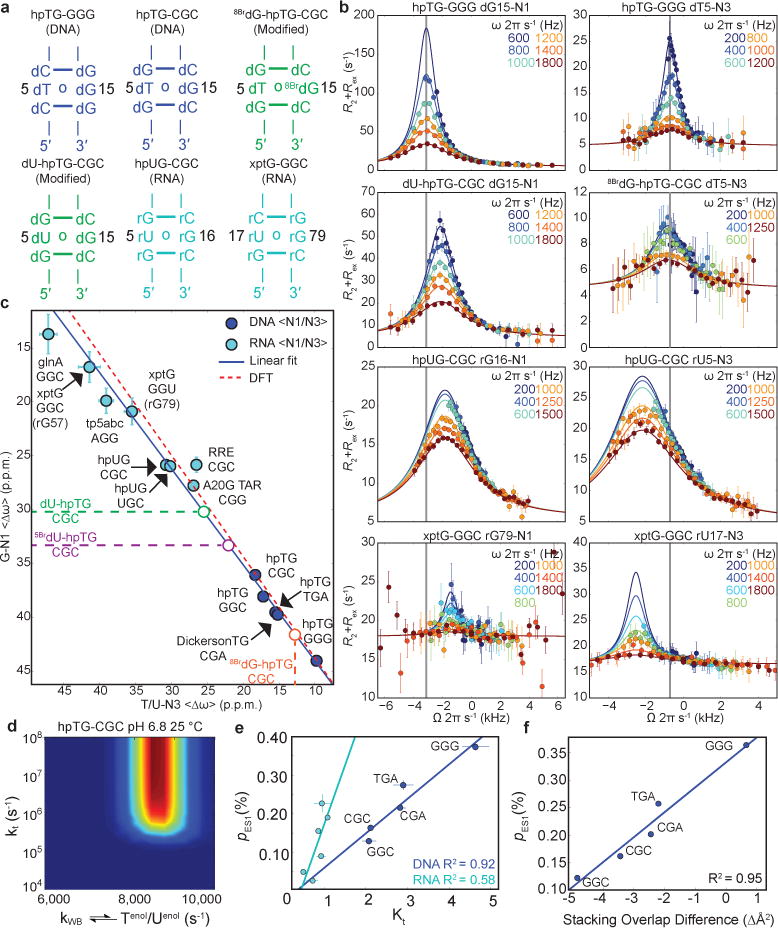
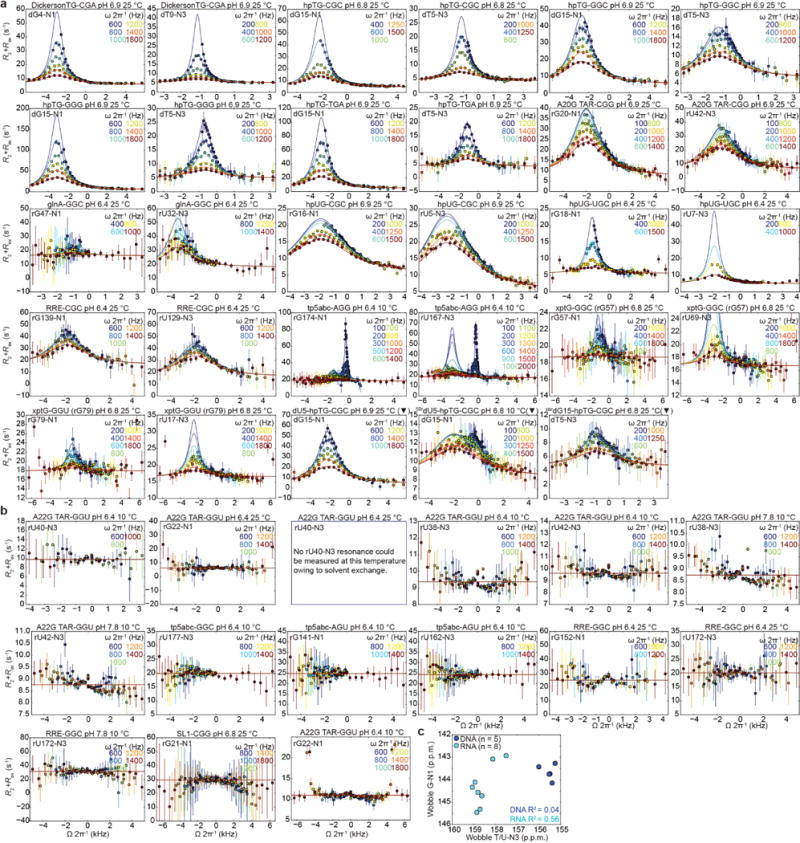
a, Representative hairpin (“hp”) DNA, RNA, and chemically modified constructs. Name denotes mismatch and sequence context (5′-3′). b, Representative G-N1 and T/U-N3 RD profiles (pH 6.8-6.9 and 25 °C). Best fits to the B-M equations are shown. c, <∆ωT/U-N3> versus <∆ωG-N1> ES1 chemical shift differences measured by NMR RD. Blue line indicates fit to the ∆ω’s using the fundamental tautomer chemical shifts as variables (Methods). Red line indicates DFT-predictions. d, Lower bounds for the rate of tautomeric Genol•T/U⇌G•Tenol/Uenol exchange. Contour plots showing scaled weights for combinations of kTenol/Uenol versus kt; red indicates better fit. e, A plot of Kt versus tautomeric ES1 population for DNA (blue, n=5) and RNA (cyan, n=6) constructs determined at pH 6.9 and 25 °C. f, Change in stacking overlap (∆Å2=Å2(WC)–Å2(WB)) between wobble and Watson-Crick-like mismatches versus ES1 population (pH 6.9 and 25 °C) for five DNA sequence contexts (Methods). Error bars in b, c, e reflect experimental uncertainty (one s.d., Methods).
We observed G-N1 and T/U-N3 RD consistent with WC-like ES1 exchange for all five dG•dT and eight rG•rU mismatches located within helical environments (Fig. 2b and Extended Data Fig. 3a), thus establishing their robust occurrence in DNA and RNA. No RD was observed (Extended Data Fig. 3b) for rG•rU mismatches adjacent to apical loops, three-way junctions, or bulges (Extended Data Fig. 2a). This could be due to the lower abundance of WC-like mismatches when outside the grip of the helical environment, though we cannot rule out that the exchange is orders of magnitude faster and beyond detection.
As predicted based on variably tilting the Genol•T/U⇌G•Tenol/Uenol equilibrium (Fig. 1e; right), the fitted <∆ωG-N1> and <∆ωT/U-N3> values obtained from two-state analysis (GS⇌ES1) of the RD profiles (Fig. 2b; Extended Data Figure 3a; and Supplementary Table 1) fell along a line with negative slope (Fig. 2c). As a negative control, no correlation was observed between the corresponding GS G-N1 and T/U-N3 chemical shifts (Extended Data Fig. 3c). We confirmed these linear trends using chemical modifications that tilt the tautomeric equilibrium toward enolic dT (dG•dU and dG•5BrdU) or enolic dG (8BrdG•dT) (Fig. 2c; Extended Data Fig. 3a; and Supplementary Discussion 1).
Sequence-dependent Genol•T/U⇌G•Tenol/Uenol
A linear fit to the <∆ωT/U-N3> versus <∆ωG-N1> values, assuming physically reasonable ranges, yielded fundamental chemical shifts for the tautomeric species that are in excellent agreement with values predicted by DFT calculations (Fig. 2c and Supplementary Tables 2,3)8. The tautomeric equilibria (Supplementary Table 2) obtained from this analysis and from re-fitting the RD data using a 3-state model with linear topology25 (wobble⇌G•Tenol/Uenol⇌Genol•T/U) are slightly tilted in favor of dGenol•dT in DNA (Kt = 2.1-4.6) whereas the populations of rGenol•rU and rG•rUenol are more comparable in RNA (Kt = 0.5-1.1). These differences may be attributed to the electron-donating methyl group in dT which destabilizes dTenol relative to rUenol(32). The RD data also allowed us to estimate a lower bound for the fast tautomeric exchange rate >≈500,000-1,000,000 s−1 (Fig. 2d and Extended Data Fig. 4)33 and a G•Tenol/Uenol→Genol•T/U transition state barrier <9-10 kcal mol−1 (pre-exponential factor = kBT h−1(34) and κ = 1) that is in good agreement with values (≈11.5 kcal mol−1) reported using computational methods25. These results establish the existence of G•Tenol/Uenol and Genol•T/U in an ultra-fast equilibrium, each of which can potentially contribute to replication and translation errors.
Interestingly, the exchange parameters vary significantly with sequence context (Supplementary Table 1). The ES1 population (pES1 = pGenol + pTenol/Uenol) varies 3-fold in DNA and 8-fold in RNA while the forward (kGS →ES1) and reverse (kES1→GS) rate constants vary by 4- and 5-fold, respectively, for DNA, and by 38- and 6-fold, respectively, for RNA (Supplementary Table 1). A linear correlation is observed between pES1 and Kt (Fig. 2e and Supplementary Table 2), indicating that the Genol•T/U population dominates these variations with sequence and structural context. In DNA, these variations can potentially be explained by sequence-specific changes in stacking with the immediate neighbors that accompany the transition from the wobble to Watson-Crick geometry (Fig. 2f). For example, GGG, has the highest pES1 and is predicted to gain stacking overlap whereas CGG has the lowest pES1 and is predicted to lose stacking overlap. Similar sequence-dependent effects have been reported in lesion repair by methyltransferases35. Interestingly, dG dominates the changes in stacking, potentially explaining the stronger sequence dependence of the Genol•T/U population compared to G•Tenol/Uenol.
Sequence-dependent anion equilibria
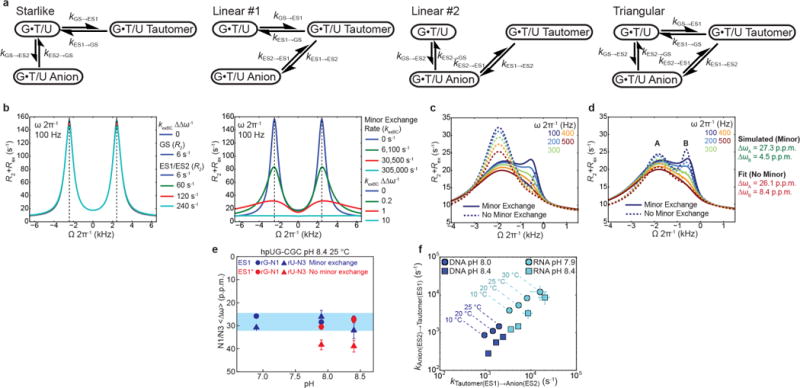
Next, we examined whether anionic WC-like G•T−/U− (Fig. 1c) also form robustly in DNA and RNA and whether anionic G−•T/U remains undetectable under these different environments. We measured RD at high pH (≥7.8) for G•T/U mismatches in a subset of our RNA (Fig. 3a and Extended Data Fig. 5a) and DNA constructs (Fig. 3b and Extended Data Fig. 5b). In all cases, we observed RD consistent with wobble⇌anion exchange, thus establishing the robustness of this process across different sequence contexts (Fig. 3a,b and Extended Data Fig. 5). 3-state fitting of the RD data assuming a starlike topology (Extended Data Fig. 6a) yielded large ∆ωT/U-N3(ES2) ≈55 p.p.m. and much smaller ∆ωG-N1(ES2) ≈5 p.p.m., consistent with a dominant G•T−/U− species and with no evidence for G−•T/U. Again, we observe strong sequence-specific variations in ES2 population (pES2) and the kGS→ES2 and kES2→GS rates robustly across different temperatures and pH conditions (Supplementary Tables 4,5).
Figure 3. 3-State exchange with triangular topology and minor exchange between tautomeric and anionic WC-like excited states.
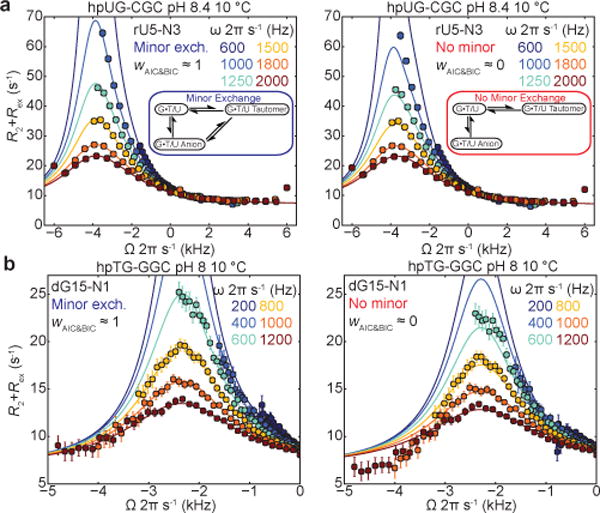
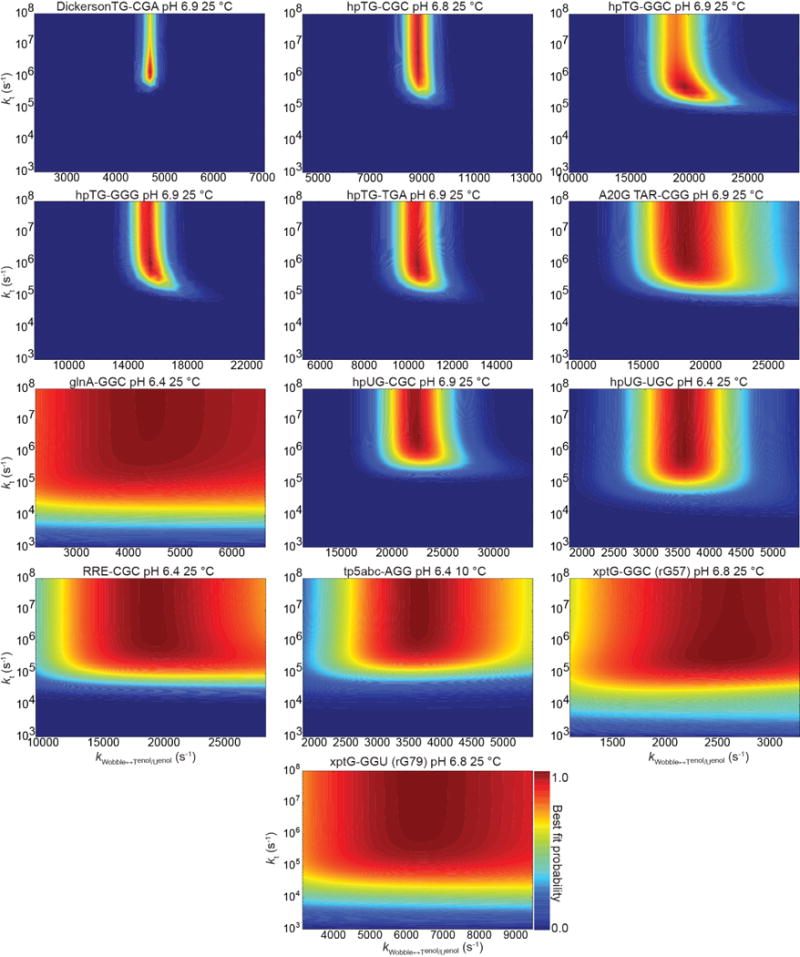
Comparison of 3-state B-M fit with triangular (left) and starlike (right) topology to the RD profiles measured in (a) hpUG-CGC RNA and (b) hpTG-GGC DNA. Statistical AIC (wAIC) and BIC (wBIC) weights comparing starlike and triangular topologies are shown. Error bars reflect experimental uncertainty (one s.d., Methods).
In a previous study8, the emergence of anionic ES2 at high pH was accompanied by unexpected changes in the ES1 tautomeric chemical shifts. Similar deviations are observed here for both RNA and DNA (Supplementary Table 4). We postulated that ‘minor’ exchange36 between ES1 and ES2 could ‘mix’ their chemical shifts and give rise to such deviations (Extended Data Fig. 6a,b and Supplementary Table 4). Indeed, all five RD profiles with peculiar ES1 chemical shifts showed a statistically significant improvement when fitting data to a 3-state model with minor exchange in a triangular rather than starlike topology (Fig. 3a,b; Extended Data Fig. 6c,d; and Supplementary Tables 4–6). The resulting ES1 15N rG-N1 and rU-N3 chemical shifts vary less significantly with pH (Extended Data Fig. 6e and Supplementary Table 4) and the rate constants (kES1→ES2 and kES2→ES1) exhibit the expected temperature dependence (Extended Data Fig. 6f), neither of which is expected if the data were being spuriously over fitted. Additionally, limited or poor quality RD data can make it difficult to resolve different topologies37 (Supplementary Table 6).
Tautomerization/ionization during misincorporation
dG•dT misincorporation is the most frequent base substitution error committed by high fidelity DNA polymerases with misincorporation frequency Fpol= (kpol/Kd)incorrect/(kpol/Kd)correct ≈10−4−10−5 for most studied polymerases38,39, i.e. an error is committed with frequency of 1 in 104−105 nucleotide incorporations, in which kpol is the maximum rate of nucleotide incorporation and Kd is the apparent nucleotide equilibrium dissociation constant. Differences in nucleotide binding affinities (Kd−1(incorrect)/Kd−1(correct)) only account for a factor of ≈10−1−10−2 in discrimination40, whereas differences in the polymerization rates (kpolincorrect/kpolcorrect) account for ≈10−3.
The mechanisms that lower the values of kpolincorrect relative to kpolcorrect remain poorly understood. Many decades ago, Topal and Fresco postulated that the frequency of tautomerization may be an important determinant of misincorporation probability11. Interestingly, the population of the tautomeric species ≈10−3 is comparable to the kpolincorrect/kpolcorrect values. In addition, the rate at which the wobble dG•dT forms either the WC-like tautomeric (kGS→ES1 = 0.3-10 s−1; Supplementary Tables 1,5) or anionic (kGS→ES2 = 1.1-124 s−1; Supplementary Tables 1,5) mismatches (Fig. 4a) is comparable to kpolincorrect (0.16-1.16 s−1) measured for incorrect dG•dTTP or dGTP•dT misincorporation22,39 whereas it is up to ≈1000-fold slower than kpolcorrect (25-275 s−1) measured for correct dG•dCTP or dGTP•dC22,39. If formation of WC-like dG•dT mismatches (Fig. 4a) is required for misincorporation following initial dNTP binding in a wobble conformation, it could provide a mechanism for lowering kpolincorrect relative to kpolcorrect. Indeed, prior studies have shown that DNA polymerases cannot undergo the necessary conformational changes needed for catalysis when dG•dT is in a wobble conformation7 and all available structures of catalytically active polymerases with bound mismatches within the active site feature WC-like dG•dT or dA•dC geometries6,7. Similarly, WC-like rG•rU mismatches have been shown to form in the first and second codon positions of catalytically active ribosomes9, in which wobbles are typically rejected5, potentially helping to explain translational error hotspots41.
Figure 4. Kinetic mechanism for dG•dT misincorporation.
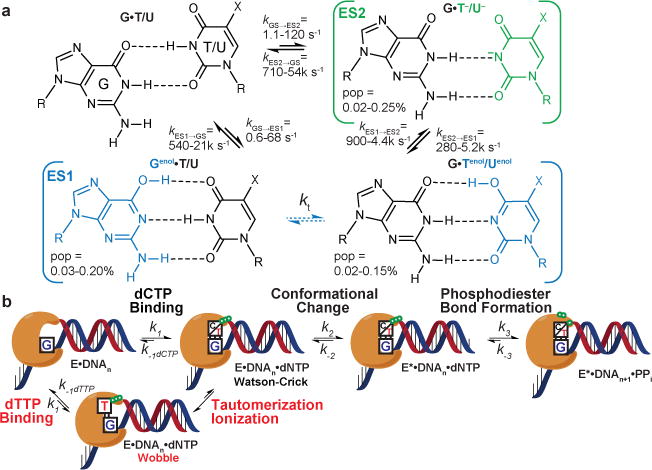
a, Triangular exchange between wobble G•T/U mispair, rapidly interconverting WC-like tautomers (Genol•T/U⇌G•Tenol/Uenol), and anionic WC-like G•T−/U−. Exchange between anionic G•T−/U− and a low-abundance short-lived anionic G−•T/U or other non-WC species that fall outside RD detection cannot be ruled out. WC-like G•T/U populations and ranges at pH 6.4-8.9 and 10-25 °C (Supplementary Tables 1, 5). b, Minimal kinetic mechanism for polymerization42. Incorporation of an incorrect dNTP includes an additional tautomerization/ionization step allowing for the formation of a Watson-Crick-like dG•dT mismatch. Discriminatory steps are in red.
To examine this possibility, we built a kinetic model for dG•dTTP misincorporation by inserting a tautomerization/ionization step (Fig. 4a) following initial nucleotide binding in a wobble conformation and prior to the pre-chemistry conformational change in the existing minimal kinetic model for correct incorporation42 (Fig. 4b). All other steps, including the pre-chemistry conformational change and phosphodiester bond formation, are assumed to have identical kinetic parameters as measured for correct nucleotide incorporation39,43–46 (Supplemental Table 7). The model assumes that misincorporation directly from the wobble conformation is negligible and that the tautomerization and ionization rates measured in duplex DNA by NMR approximate the rates in the polymerase active site. We tested models (Extended Data Fig. 7) in which either the tautomeric (MES1), anionic (MES2), or both (MES1+ES2) species can be misincorporated as well as models that excluded the triangular network all together (MKd).
Strikingly, the most general MES1+ES2 model robustly predicts the measured Fpol values for three polymerases (T7, polymerase ε, and polymerase β) that have varying rate limiting steps and microscopic rate constants (Fig. 5a; Extended Data Fig. 8; and Supplementary Table 7). Similar results are obtained with MES1 under these neutral conditions in which the ES2 population is insignificant (<10−6 at pH 6.9) (Fig. 5a and Extended Data Fig. 8). In contrast, MES2 consistently underestimates Fpol by one to two orders of magnitude whereas MKd overestimates Fpol by one to two orders of magnitude (Fig. 5a)47. Variants of the MKd model in which only pre-formed tautomeric dNTP with populations of 10−4−10−5 bind in a productive WC-like geometry overestimates kpol and Kd by several orders of magnitude (data not shown). These data indicate that formation of tautomeric WC-like dGenol•dT and dG•dTenol with population of ≈0.1% can account for the ≈102−103–fold lower value of kpolincorrect relative to kpolcorrect and that at neutral pH, >99% of misincorporation proceeds via the tautomeric species, which form predominantly via direct exchange from the wobble (Fig. 5b).
Figure 5. Measured versus predicted misincorporation probability and rates.
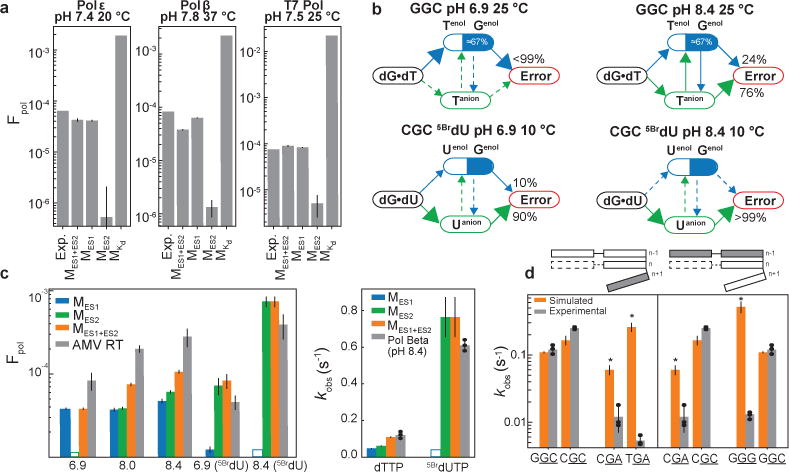
a, Fpol measured experimentally for dTTP•dG misincorporation for human DNA polymerase ε, rat DNA polymerase β, and T7 DNA polymerase with values simulated using MES1, MES2, MES1+ES2 and MKd (error bars represent one s.d., Methods). b, Flux pathways for dT•dG(GGC) and 5BrdU•dG(CGC). c, Left. Measured and simulated Fpol values for dTTP/5BrdUTP in AMV RT14. Right, measured and simulated kobs values for dTTP/5BrdUTP misincorporation for human DNA polymerase β. Error is s.d. of n=3 biological replicates for kinetic assays, or previously published error for AMV RT14. Error for kinetic simulations is described in Methods. d, Measured and simulated kobs for dTTP misincorporation for human DNA polymerase β in different sequence contexts. (*) indicates that ES2 exchange rates were extrapolated (Methods). Error is s.d. of n=3 biological replicates for kinetic assays. Error for kinetic simulations is described in Methods.
Impact of pH, modifications, and sequence
We also examined whether the MES1+ES2 model can reproduce the dependence of misincorporation probability on pH, base modification, and sequence. MES1+ES2 accurately predicts the ≈3-fold increase in misincorporation probability observed with increasing pH (Fig. 5c; left). This can be attributed to an increase in the population of dG•dT−, which accounts for >70% of the net misincorporation at pH 8.4 (Fig. 5b,c). In contrast, MES1 fails to predict this increase in misincorporation probability (Fig. 5c, left; MKd not shown due to absence of pH-dependent Kd values). Under high pH, the tautomeric and anionic species have comparable populations, and there is significant flux (>20%) toward both tautomeric and anionic species through the indirect minor exchange pathway (Fig. 5b). In this manner, the tautomeric and anionic contributions to misincorporation are coupled.
MES1+ES2 and NMR RD measurements also accurately predict Fpol and kobs for 5-bromo-2′-deoxyuridine triphosphate (5BrdUTP) (Fig. 5c). This includes a steeper ≈6-fold increase in FpoldG•5BrdUTP measured for AMV RT when increasing the pH from 7.0 to 8.4 (Fig. 5c; left). This can be attributed to the lower pKa of dG•5BrdU− (pKa ≈9)14 relative to dG•dT− (pKa ≈11.8)8. We further verified the robustness of these predictions by measuring kobsdG•5BrdUTP and kobsdTTP for human DNA polymerase β at high pH (8.4). The model accurately predicts the ≈4-fold enhancement in kobsdG•5BrdUTP relative to kobsdTTP (Fig. 5c; right). Again, MES1 fails to predict these variations (Fig. 5c). Indeed, at both neutral and high pH, 5BrdUTP is predicted to be predominantly misincorporated via the more populated dG•dT− (Fig. 5b,c). These data indicate that misincorporation due to dG•dT− can dominate at pH≥8.4 or for chemically modified nucleotides at neutral pH.
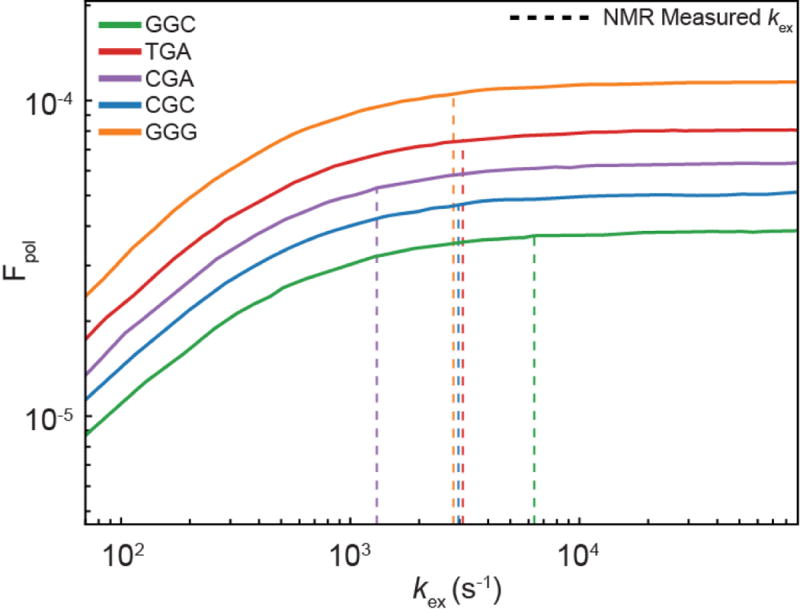
Importantly, due to the sequence-dependence of tautomerization and ionization, MES1+ES2 also predicts ≈8-fold sequence-specific variations in Fpol at pH 8.4 (Fig. 5d). Comparable (5-fold) sequence-specific variations have been reported previously21. We tested these predictions using human DNA polymerase β at pH 8.4 for nine different sequence contexts (Supplementary Table 8). While kobsdG•dCTP varied weakly (<1.2-fold) with sequence, kobsdG•dTTP varied ≈45-fold (Extended Data Fig. 9), with larger changes observed when varying the base pair at the n-1 position, which stacks with dG•dTTP in the polymerase active site (Fig. 5d). While the MES1+ES2 predictions slightly underestimate the sequence-specific variations in kobsdG•dTTP, this is not too surprising considering that other microscopic steps could also vary with sequence. The predictions do recapitulate the lower kobsdG•dTTP for CGA and comparable values for GGC and CGC (Fig. 5d). Interestingly, the two major outliers (TGA and GGG) arise primarily due to a large ES2 population. It is likely that the polymerase environment, including absence of base pairs at the n+1 position (Fig. 5d), can influence the sequence-specific dependence of tautomerization/ionization and consequently misincorporation.
Our data indicate that formation of WC-like anionic and tautomeric mismatches help determine the frequency of dG•dT misincorporation and its dependence on pH, chemical modifications, and possibly sequence. Our analysis indicates that Fpol is determined primarily by the ES1 population and that significant reductions in kex = kGS→ES1 + kES1→GS, outside the range detected here, would be required to significantly reduce Fpol (Extended Data Fig. 10). While it is likely that differences in the polymerase active site environment will tune tautomerization/ionization dynamics, the robustness of the predictions across different polymerases, pH conditions, and modified nucleotides suggests that it will not cause significant perturbations relative to the broad kinetic range examined here. Indeed, very small differences in tautomerization/ionization dynamics are observed for DNA and RNA, which have different helical structures and stabilities. It is possible that tautomerization/ionization is dominated by the energetics of hydrogen-bonding and proton transfer and that the natural grip for the WC geometry in the double helix is similar to that achieved by the polymerase in the context of an isolated dNTP paired to the template. Other mechanisms may be applicable for purine-purine mismatches where alterations in the active site have been proposed rather than adoption of a WC-like base pair43,48. The approach presented here can be applied to examine the roles of other tautomeric and anionic mismatches in replication, transcription, translation, and mismatch repair49.
Extended Data
Extended Data Figure 1. Watson-Crick-like mismatches.
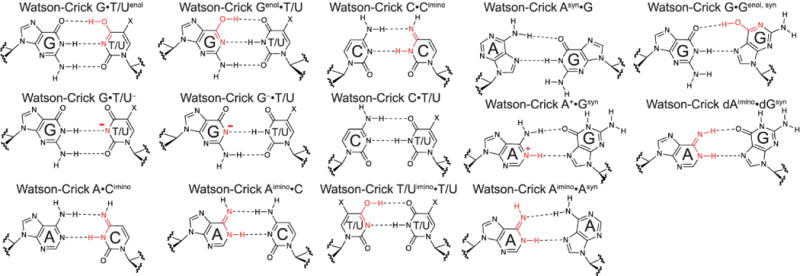
a, Watson-Crick-like mismatches stabilized by tautomeric and ionic base forms. Tautomeric purine•pyrimindine and purine•purine mismatches were first proposed by Topal and Fresco11. For G•T/U mismatches, X = H or CH3 for uridine and thymidine, respectively.
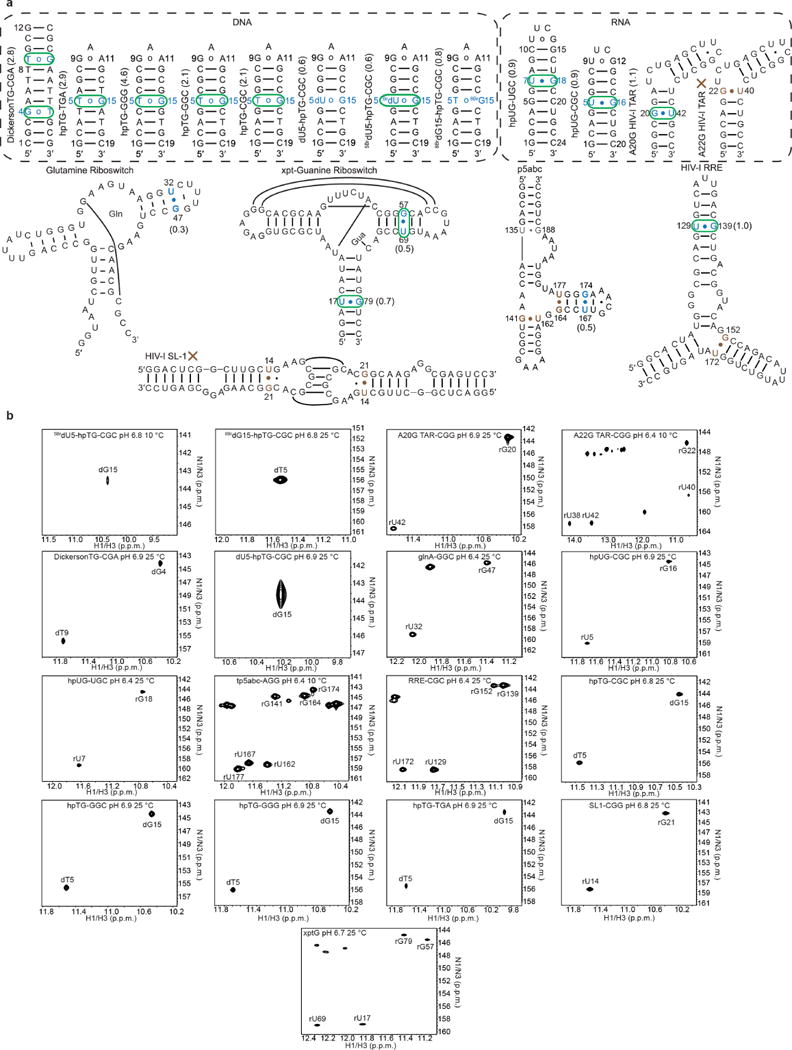
Extended Data Figure 2. DNA and RNA constructs used in this study.
a, Secondary structures of the various DNA and RNA constructs used in this study. G•T/U mismatches that show signs of chemical exchange directed toward tautomeric and/or anionic WC-like mismatches are highlighted in blue and green, respectively. G•T/U mismatches that show no evidence for WC-like RD are highlighted in brown. The value of Kt measured at near-neutral pH is shown next to each mismatch. The DickersonTG-CGA, hpTG-CGC and hpUG-CGC sequences contexts were studied in a prior publication8. b, 2D [15N, 1H] HSQC spectra of DNA and RNA constructs used in this study showing the imino resonances of G-N1/H1 and T/U-N3/H3 targeted for RD measurements. Spectra shown for xptG was collected at pH 6.7 and 25 °C in potassium acetate buffer described elsewhere8.
Extended Data Figure 3. RD profiles measured in DNA and RNA at near-neutral pH.
a, 15N G-N1 and T/U-N3 RD measured for G•T/U mismatches at pH 6.4-6.9 and 10-25 °C showing wobble⇌tautomer exchange. Note that in addition to wobble⇌tautomer exchange, tp5abc undergoes an independent slower exchange process involving a change in secondary structure that is described in detail elsewhere53. The trend lines represent B-M 2-/3-state fits. Constructs containing a chemically modified base are indicated with (▼).b, Absence of 15N RD for rG•rU mismatches near bulges, apical loops, or three-way junctions. Error bars reflect experimental uncertainty (one s.d., see Methods). c, No correlation is observed between ground state (GS) wobble G-N1 and T/U-N3 chemical shifts for DNA (n = 5) or RNA (n = 8). Error bars in a, b reflect experimental uncertainty (one s.d., see Methods).
Extended Data Figure 4. Establishing lower limits for rates of base pair tautomeric exchange.
Agreement between measured and predicted R1ρ values (scaled weight, Eq. 2) when varying the wobble⇌G•Tenol/Uenol ( ) and G•Tenol/Uenol ⇌Genol•T/U (kt) rate of exchange. See Methods for additional details.
Extended Data Figure 5. RD profiles measured in DNA and RNA at high pH.
B-M 3-state fits of a, RNA and b, DNA 15N RD data for starlike and triangular topologies (indicated within the plots). The relative statistical Akaike’s Information Criterion (wAIC) and Bayesian Information Criterion (wBIC) weights50,60 for each fit were used to select the model (representative starlike versus triangular, comparisons with linear models shown in Supplementary Table 6). Error bars reflect experimental uncertainty (one s.d., see Methods).
Extended Data Figure 6. Discerning minor exchange between WC-like tautomeric and anionic G•T/U mismatches.
a, Topologies used to model chemical exchange. Individual rate constants are shown for each leg of the different topologies. b, Left: B-M simulations showing that when R2(GS) ≠ R2(ES1) and R2(GS) ≠ R2(ES2) no apparent peak asymmetry is observed. Right: B-M simulations showing that minor exchange between two ESs in a triangular topology induces asymmetry in the RD profiles and opposite changes in the apparent chemical shift for the two ESs. c, B-M simulations (solid lines) showing the fitted exchange parameters for hpUG-CGC at pH 8.4 and 10 °C (Supplementary Table 5) when including minor exchange in a triangular topology. For comparison, simulations using the same parameters without minor exchange (k3 = 0 and k−3 = 0) are also shown (dashed lines). d, Dashed lines denote 3-state B-M best fit to starlike topology (kES1→ES2 = 0 and kES2→ES1 = 0) to data simulated with triangular topology with minor exchange (solid lines). Shown to the right is the over/under estimation of the true (green) versus fitted (red) ES chemical shifts when fitting RD profiles with triangular topology with minor exchange to a starlike model that has no minor exchange. e, The ES1 <∆ωrG-N1> and <∆ωrU-N3> values as a function of pH derived from the 3-state B-M fit with triangular and starlike topology. f, Forward (kES1→ES2) and reverse (kES2→ES1) minor exchange rate constants for hpTG-GGC (pH 8 and 8.4) and hpUG-CGC (pH 7.9 and 8.4) as a function of temperature. Error bars in e, f reflect experimental uncertainty (one s.d., Methods).
Extended Data Figure 7. Kinetic mechanisms used to model misincorporation.
Rate constants for each step are listed in Supplementary Table 7.
Extended Data Figure 8. Benchmarking kinetic simulations of misincorporation.
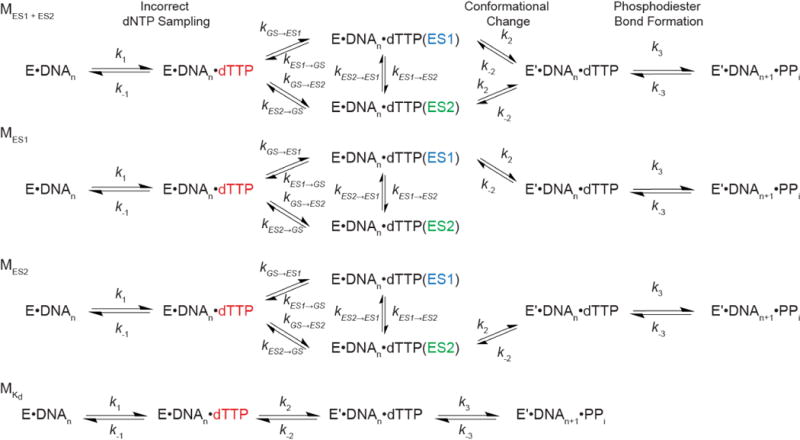
a, Comparison of kpol and Kd values for correct incorporation measured experimentally for human DNA polymerase ε with values computed based on pre-steady state simulations using the microscopic rate constants provided in ref. 39. Error bars reflect fitting uncertainty as previously published39. b, Robustness of calculated kpol values for human DNA polymerase ε when varying rate constants (forward rate constant: blue; and reverse rate constant: orange) for steps other than tautomerization/ionization by 2-fold (n = 200 independent simulations in which rate constants were randomly varied within 2-fold). As expected, the only rate constant with a substantial effect on the reported kpol values was the rate limiting k2 conformational change step (middle).
Extended Data Figure 9. kobs values measured for human DNA polymerase β dCTP•dG (left) or dTTP•dG (right) insertion at pH 8.4, 25 °C, and 100 μM dNTP.
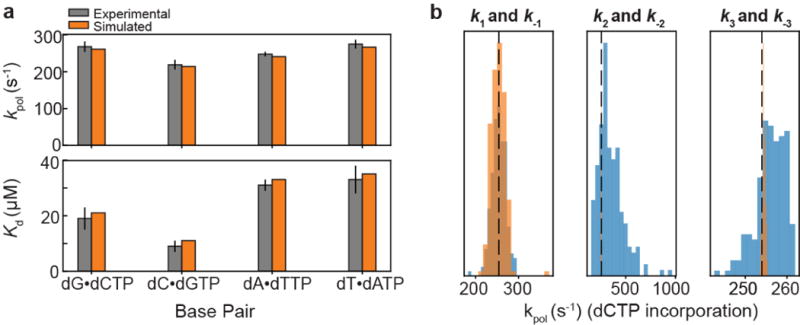
DNA template sequence (5´ to 3´) is read from bottom (n+1 position) to top (n-1 position). Individual replicates (n = 3 independent experiment) are indicated by grey circles. Bar height reflect average of replicates and error bars reflect one s.d.
Extended Data Figure 10. Fpol is primarily governed by ES1 populations.
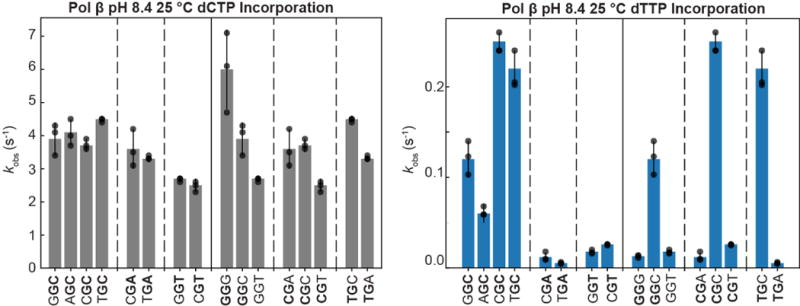
Simulated Fpol values as a function of scaling up or down the kinetic exchange rate for ES1 formation (kex = kGS→ES1 + kES1→GS) without altering the ES1 population. Increasing kex beyond values measured in this study experimentally (green dotted line) minimally affects Fpol. Decreasing the rate of exchange within the range measured experimentally in this study (purple dotted line) also minimally affects the value of Fpol. Much larger decreases in kex are required to significantly reduce the value of Fpol.
Supplementary Material
Acknowledgments
We thank members of the Al-Hashimi laboratory and Prof. T. Oas (Duke University) for stimulating discussions and critical input. We acknowledge technical support and resources from the Duke Magnetic Resonance Spectroscopy Center and the Duke Shared Cluster Resource. This work was supported by grants from the National Institutes of Health (NIH R01GM089846, P01GM0066275, and P50GM103297) and an Agilent Thought Leader Award given to H.M.A., and a grant from the National Science Foundation (MCB-1716168) to Z.S. W.J.Z. was supported by a Pelotonia Graduate Fellowship from The Ohio State University Comprehensive Cancer Center. The authors declare no conflict of interest.
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author Contributions I.J.K and E.S.S. contributed equally to this work. I.J.K., E.S.S., and H.M.A. conceived the research and experimental design and performed data interpretation. I.J.K, E.S.S., W.J.Z., Z.S., and H.M.A. wrote the manuscript. I.J.K. synthesized all DNA constructs as well as the RNA hpUG-CGC, hpUG-CGU, and xptG riboswitch constructs and performed all NMR RD data collection and analyses. E.S.S. constructed and tested the models of misincorporation. W.J.Z. performed all kinetic experiments. A.S. synthesized A20G and A22G HIV-I TAR constructs Y.X. synthesized and assigned p5abc and glnA riboswitch constructs. C-C.C. synthesized and assigned the HIV-I RRE construct. B.S. synthesized and assigned the HIV-I SL1 dimer complex.
The authors declare no competing financial interests. Readers are welcome to comment on the online version of the paper. .
References
- 1.Watson JD, Crick FH. The structure of DNA. Cold Spring Harb Symp Quant Biol. 1953;18:123–131. doi: 10.1101/sqb.1953.018.01.020. [DOI] [PubMed] [Google Scholar]
- 2.Oertell K, et al. Kinetic selection vs. free energy of DNA base pairing in control of polymerase fidelity. Proc Natl Acad Sci USA. 2016;113:E2277–E2285. doi: 10.1073/pnas.1600279113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voorhees RM, Ramakrishnan V. Structural basis of the translational elongation cycle. Annu Rev Biochem. 2013;82:203–236. doi: 10.1146/annurev-biochem-113009-092313. [DOI] [PubMed] [Google Scholar]
- 4.Kool ET. Active site tightness and substrate fit in DNA replication. Annu Rev Biochem. 2002;71:191–219. doi: 10.1146/annurev.biochem.71.110601.135453. [DOI] [PubMed] [Google Scholar]
- 5.Demeshkina N, Jenner L, Westhof E, Yusupov M, Yusupova G. A new understanding of the decoding principle on the ribosome. Nature. 2012;484:256–259. doi: 10.1038/nature10913. [DOI] [PubMed] [Google Scholar]
- 6.Wang W, Hellinga HW, Beese LS. Structural evidence for the rare tautomer hypothesis of spontaneous mutagenesis. Proc Natl Acad Sci USA. 2011;108:17644–17648. doi: 10.1073/pnas.1114496108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bebenek K, Pedersen LC, Kunkel TA. Replication infidelity via a mismatch with Watson-Crick geometry. Proc Natl Acad Sci USA. 2011;108:1862–1867. doi: 10.1073/pnas.1012825108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimsey IJ, Petzold K, Sathyamoorthy B, Stein ZW, Al-Hashimi HM. Visualizing transient Watson-Crick-like mispairs in DNA and RNA duplexes. Nature. 2015;519:315–320. doi: 10.1038/nature14227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rozov A, Demeshkina N, Westhof E, Yusupov M, Yusupova G. Structural insights into the translational infidelity mechanism. Nat Commun. 2015;6:7521. doi: 10.1038/ncomms8251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Topal MD, Fresco JR. Base pairing and fidelity in codon-anticodon interaction. Nature. 1976;263:289–293. doi: 10.1038/263289a0. [DOI] [PubMed] [Google Scholar]
- 11.Topal MD, Fresco JR. Complementary base pairing and the origin of substitution mutations. Nature. 1976;263:285–289. doi: 10.1038/263285a0. [DOI] [PubMed] [Google Scholar]
- 12.Loveland AB, Demo G, Grigorieff N, Korostelev AA. Ensemble cryo-EM elucidates the mechanism of translation fidelity. Nature. 2017;546:113–117. doi: 10.1038/nature22397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh V, Fedeles BI, Essigmann JM. Role of tautomerism in RNA biochemistry. RNA. 2015;21:1–13. doi: 10.1261/rna.048371.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu H, Eritja R, Bloom LB, Goodman MF. Ionization of bromouracil and fluorouracil stimulates base mispairing frequencies with guanine. J Biol Chem. 1993;268:15935–15943. [PubMed] [Google Scholar]
- 15.Koag MC, Nam K, Lee S. The spontaneous replication error and the mismatch discrimination mechanisms of human DNA polymerase β. Nucleic Acids Res. 2014;42:11233–11245. doi: 10.1093/nar/gku789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rozov A, Demeshkina N, Westhof E, Yusupov M, Yusupova G. New Structural Insights into Translational Miscoding. Trends Biochem Sci. 2016;41:798–814. doi: 10.1016/j.tibs.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Tomasetti C, Li L, Vogelstein B. Stem cell divisions, somatic mutations, cancer etiology, and cancer prevention. Science. 2017;355:1330–1334. doi: 10.1126/science.aaf9011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eckert KA, Kunkel TA. Effect of reaction pH on the fidelity and processivity of exonuclease-deficient Klenow polymerase. J Biol Chem. 1993;268:13462–13471. [PubMed] [Google Scholar]
- 19.Warren JJ, Forsberg LJ, Beese LS. The structural basis for the mutagenicity of O6-methyl-guanine lesions. Proc Natl Acad Sci USA. 2006;103:19701–19706. doi: 10.1073/pnas.0609580103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dosanjh MK, Galeros G, Goodman MF, Singer B. Kinetics of extension of O6-methylguanine paired with cytosine or thymine in defined oligonucleotide sequences. Biochemistry. 1991;30:11595–11599. doi: 10.1021/bi00113a015. [DOI] [PubMed] [Google Scholar]
- 21.Mendelman LV, Boosalis MS, Petruska J, Goodman MF. Nearest neighbor influences on DNA polymerase insertion fidelity. J Biol Chem. 1989;264:14415–14423. [PubMed] [Google Scholar]
- 22.Lee HR, Johnson KA. Fidelity of the human mitochondrial DNA polymerase. J Biol Chem. 2006;281:36236–36240. doi: 10.1074/jbc.M607964200. [DOI] [PubMed] [Google Scholar]
- 23.Xia S, Konigsberg WH. Mispairs with Watson-Crick base-pair geometry observed in ternary complexes of an RB69 DNA polymerase variant. Protein Sci. 2014;23:508–513. doi: 10.1002/pro.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogle JM, Murphy FV, Tarry MJ, Ramakrishnan V. Selection of tRNA by the ribosome requires a transition from an open to a closed form. Cell. 2002;111:721–732. doi: 10.1016/s0092-8674(02)01086-3. [DOI] [PubMed] [Google Scholar]
- 25.Nomura K, et al. DFT calculations on the effect of solvation on the tautomeric reactions for wobble Gua-Thy and canonical Gua-Cyt base-pairs. J Mod Phys. 2013;4:422–431. [Google Scholar]
- 26.Brovarets’ OO, Hovorun DM. The nature of the transition mismatches with Watson-Crick architecture: the G*•T or G•T* DNA base mispair or both? A QM/QTAIM perspective for the biological problem. J Biomol Struct Dyn. 2015;33:925–945. doi: 10.1080/07391102.2014.924879. [DOI] [PubMed] [Google Scholar]
- 27.Trott O, Abergel D, Palmer AG., III An average-magnetization analysis of R1ρ relaxation outside of the fast exchange limit. Mol Phys. 2003;101:753–763. [Google Scholar]
- 28.Korzhnev DM, Orekhov VY, Kay LE. Off-resonance R1ρ NMR studies of exchange dynamics in proteins with low spin-lock fields: an application to a Fyn SH3 domain. J Am Chem Soc. 2005;127:713–721. doi: 10.1021/ja0446855. [DOI] [PubMed] [Google Scholar]
- 29.Hansen AL, Nikolova EN, Casiano-Negroni A, Al-Hashimi HM. Extending the range of microsecond-to-millisecond chemical exchange detected in labeled and unlabeled nucleic acids by selective carbon R1ρ NMR spectroscopy. J Am Chem Soc. 2009;131:3818–3819. doi: 10.1021/ja8091399. [DOI] [PubMed] [Google Scholar]
- 30.Mulder FAA, Mittermaier A, Hon B, Dahlquist FW, Kay LE. Studying excited states of proteins by NMR spectroscopy. Nat Struct Biol. 2001;8:932–935. doi: 10.1038/nsb1101-932. [DOI] [PubMed] [Google Scholar]
- 31.Szymanski ES, Kimsey IJ, Al-Hashimi HM. Direct NMR Evidence that Transient Tautomeric and Anionic States in dG•dT Form Watson-Crick-like Base Pairs. J Am Chem Soc. 2017;139:4326–4329. doi: 10.1021/jacs.7b01156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orozco M, Hernández B, Luque FJ. Tautomerism of 1-methyl derivatives of uracil, thymine, and 5-bromouracil. Is tautomerism the basis for the mutagenicity of 5-bromouridine? J Phys Chem B. 1998;102:5228–5233. [Google Scholar]
- 33.Ban D, et al. Exceeding the limit of dynamics studies on biomolecules using high spin-lock field strengths with a cryogenically cooled probehead. J Magn Reson. 2012;221:1–4. doi: 10.1016/j.jmr.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Vallurupalli P, Chakrabarti N, Pomes R, Kay L. Atomistic picture of conformational exchange in a T4 lysozyme cavity mutant: an experiment-guided molecular dynamics study. Chem Sci. 2016;7:3062–3613. doi: 10.1039/c5sc03886c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delaney JC, Essigmann JM. Effects of Sequence Context of O6-methylguanine Repair and Replication in Vivo. Biochemistry. 2001;40:14968–14975. doi: 10.1021/bi015578f. [DOI] [PubMed] [Google Scholar]
- 36.Trott O, Palmer AG., III Theoretical study of R1p rotating-frame and R2 free-precession relaxation in the presence of n-site chemical exchange. J Magn Reson. 2004;170:104–112. doi: 10.1016/j.jmr.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 37.Sekhar A, et al. Thermal fluctuations of immature SOD1 lead to separate folding and misfolding pathways. Elife. 2015;4:e07296. doi: 10.7554/eLife.07296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kunkel TA, Alexander PS. The base substitution fidelity of eukaryotic DNA polymerases. J Biol Chem. 1986;261:160–166. [PubMed] [Google Scholar]
- 39.Zahurancik WJ, Klein SJ, Suo Z. Significant contribution of the 3′→5′ exonuclease activity to the high fidelity of nucleotide incorporation catalyzed by human DNA polymerase ε. Nucleic Acids Res. 2014;42:13853–13860. doi: 10.1093/nar/gku1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loeb L, Kunkel TA. Fidelity of DNA synthesis. Annu Rev Biochem. 1982;51:429–457. doi: 10.1146/annurev.bi.51.070182.002241. [DOI] [PubMed] [Google Scholar]
- 41.Zhang J, Ieong KW, Johansson M, Ehrenberg M. Accuracy of initial codon selection by aminoacyl-tRNAs on the mRNA-programmed bacterial ribosome. Proc Natl Acad Sci USA. 2015;112:9602–9607. doi: 10.1073/pnas.1506823112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rothwell PJ, Waksman G. Structure and mechanism of DNA polymerases. Adv Protein Chem. 2005;71:401–440. doi: 10.1016/S0065-3233(04)71011-6. [DOI] [PubMed] [Google Scholar]
- 43.Tsai YC, Johnson KA. A new paradigm for DNA polymerase specificity. Biochemistry. 2006;45:9675–9687. doi: 10.1021/bi060993z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patel SS, Wong I, Johnson KA. Pre-steady-state kinetic analysis of processive DNA replication including complete characterization of an exonuclease-deficient mutant. Biochemistry. 1991;30:511–525. doi: 10.1021/bi00216a029. [DOI] [PubMed] [Google Scholar]
- 45.Zahurancik WJ, Klein SJ, Suo Z. Kinetic mechanism of DNA polymerization catalyzed by human DNA polymerase ε. Biochemistry. 2013;52:7041–7049. doi: 10.1021/bi400803v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Balbo PB, Wang EC, Tsai MD. Kinetic mechanism of active site assembly and chemical catalysis of DNA polymerase β. Biochemistry. 2011;50:9865–9875. doi: 10.1021/bi200954r. [DOI] [PubMed] [Google Scholar]
- 47.Maximoff SN, Kamerlin SCL, Florian J. DNA Polymerase lambda Active Site Favors a Mutagenic Mispair between the Enol Form of Deoxyguanosine Triphosphate Substrate and the Keto Form of Thymidine Template: A Free Energy Perturbation Study. J Phys Chem B. 2017;121:7813–7822. doi: 10.1021/acs.jpcb.7b04874. [DOI] [PubMed] [Google Scholar]
- 48.Freudenthal BD, Beard WA, Shock DD, Wilson SH. Observing a DNA polymerase choose right from wrong. Cell. 2013;154:157–168. doi: 10.1016/j.cell.2013.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Freudenthal BD, Beard WA, Cuneo MJ, Dyrkheeva NS, Wilson SH. Capturing snapshots of APE1 processing DNA damage. Nat Struct Mol Biol. 2015;22:924–931. doi: 10.1038/nsmb.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


