Abstract
Skeletal muscle cells are highly abundant and metabolically active and are known to ‘communicate’ their energy demands to other organs through active secretion. Muscle-derived secretory proteins include a variety of cytokines and peptides collectively referred to as “myokines” that exert auto-, para- or endocrine effects. Analyses of the skeletal muscle secretome revealed that numerous myokines are secreted in response to contraction or strength training, and that these factors not only regulate energy demand but also contribute to the broad beneficial effects of exercise on cardiovascular, metabolic, and mental health. Herein we review recent studies on the myokines that regulate muscle function and those that mediate cross talk between skeletal muscle and other organs including adipose tissue, liver, pancreas, the cardiovascular system, brain, bones, and skin.
Keywords: muscle, secretion, cell communication, paracrine, endocrine, myokine
Introduction
Detailed proteomic analysis of the human skeletal muscle secretome combined with strict computational filtering revealed that a complex mixture of over 300 myokines were actively secreted from these cells [1–3]. The vast majority of these myokines are known circulating factors that are not unique to skeletal muscle, though a few are skeletal muscle selective. As myokines with autocrine functions are discussed in more detail in the Demonbreun and McNally review in this issue, we will focus herein on paracrine myokines that influence muscle development and homeostasis by regulating angio/vasculogenesis, innervations, adipogenesis, and bone formation as well as on endocrine myokines that have diverse systemic effects. We will also address critical unanswered questions regarding the molecular underpinnings of skeletal muscle secretory vesicle trafficking. As numerous myokines have been linked to improved clinical outcomes in a range of disorders from diabetes to cancer[4–6], future strategies to control the release of these biologically active peptides from skeletal muscle could provide robust and novel therapeutic opportunities.
Muscle Angiogenesis
As in other solid tissues, angiogenesis is fundamental to skeletal muscle growth and differentiation and vascular endothelial growth factor-A (VEGFA) is arguably the most well-characterized paracrine factor secreted from skeletal muscle[7]. Genetically engineered mice with skeletal muscle-specific deletion of VEGFA have significantly reduced interstitial VEGFA levels accompanied by vascular rarefaction, reduced exercise capacity, and insulin resistance [8,9]. Coupled with evidence that these mice exhibit normal circulating levels of VEGFA, these findings indicate that skeletal muscle cells provide a critical source of intramuscular VEGFA that is necessary locally (not systemically) for muscle growth and metabolic homeostasis [8,9]. Other exercise-induced myokines that act in a paracrine fashion to regulate muscle angiogenesis include the cytokine interleukin- (IL)-8 and angiopoietin 1[6].
Muscle Innervation
Skeletal muscle cells also secrete several neurotrophic factors including those that impart coordinated control of motor neuron innervation, neuromuscular junction (NMJ) formation and muscle terminal maintenance. Such factors include, but are not limited to brain-derived neurotrophic factor (BDNF), fibroblast growth factor binding protein-1 (FGFBP1), ciliary neurotrophic factor receptor-A (CNTFR-A), and low-density lipoprotein receptor-4 (LRP4) [4–6]. While skeletal muscle cells are not a major contributor to circulating BDNF, they do release low levels of this myokine which may play a paracrine role in regulating survival, growth and maintenance of intramuscular neurons [6]. Skeletal muscle cells also secrete FGFBP1- a factor that is concentrated at neuromuscular junctions and is essential for NMJ maintenance [10]. However, to date studies confirming a requirement for skeletal muscle-dependent elaboration of these factors is lacking. Both CNTFR-A and LRP-4 are key signal-peptide containing plasma membrane receptors that are critical for maintenance of the NMJ. CNTFR-A is a cytokine receptor that is secreted from both neurons and skeletal muscle cells, but tissue-specific deletion studies revealed that these tissues play a non-redundant role in NMJ function [11,12]. CNTFR is released in a soluble functional form and attached to the extracellular surface of PMs by a glycosylphosphatidylinositol linkage where it functions as a co-receptor along with leukemia inhibitory factor receptor (LIFR) and gp130[13]. Ligands for this receptor complex include CNTF, cytokine like factor-1, and cardiotrophin-like cytokine factor 1 although studies suggest that the latter two are likely the ligands responsible for muscle terminal maintenance. LRP4 is a single-pass transmembrane receptor that is targeted to the plasma membrane of skeletal muscle cells via secretory vesicle trafficking and interacts with nerve-derived agrin. Recent studies showed that conditional deletion of LRP4 from adult mouse skeletal muscle resulted in degeneration of both pre- and post-synaptic nerve terminals [14]. Indeed, within one month following LRP4 depletion, these mice exhibited phenotypes that resembled myasthenic syndromes including weight loss, muscle weakness, scoliosis, and premature death [14]. Future studies are needed to determine the extent to which defects in muscle-nerve connections that are observed during aging and are exacerbated in neuromuscular diseases are due to impaired secretion of these or other myokines.
Adipogenesis and Metabolism: Regulation by Paracrine and Endocrine Signaling
Myokines that influence adipogenesis include IL-6, myostatin, myonectin, and irisin and these factors likely exert both local (paracrine) and long-range (endocrine) effects[15]. There are three major adipose depots in the body, the visceral adipose tissue (VAT; which includes all cellular adipose depots in the peritoneal cavity); subcutaneous adipose tissue (SAT); and intramuscular adipose tissue (iMAT) which includes adipocytes located between muscles and perimysial adipocytes located within a single muscle fiber. iMAT depots have been reported to vary between 28 and 82% of VAT mass in animals and patients. The importance of iMAT is underscored by findings indicating positive correlations between iMAT levels and insulin resistance while no such relationship was found between SAT and insulin sensitivity. Significant correlations were also found between iMAT and cardiovascular risk that were independent of other fat depots. Adipose tissue is comprised of two types of fat, white (WAT) and brown (BAT). While WAT stores excess energy, it is largely detrimental as this tissue is metabolically unfavorable and secretes numerous pro-inflammatory cytokines. BAT on the other hand is largely beneficial, because this tissue consumes energy and clears triglycerides. It is well-known that physical activity limits WAT and promotes BAT production and studies indicate that these metabolic benefits are mediated, at least in part, by myokines[16,17]. Due to their close proximity to muscle cells, iMATs are likely to be an important target tissue for paracrine myokines that influence fat browning such as musclin, a myokine released from muscles during exercise that activates PPARα-dependent browning of WAT[16,17].
Other myokines act in an endocrine fashion to influence metabolism by triggering regulatory pathways in VAT or other target organs including the liver and pancreas. One of the best-studied metabolic myokines is IL6, and studies have shown that physical activity induces muscle IL6 production and leads to elevated circulating IL6 levels[18]. Target tissues for circulating IL6 include the liver, pancreas, and adipose tissue. Acute IL6 signaling (akin to levels induced by exercise) promotes glucose production in the liver, favors lipolysis in adopose tissue[18] and promotes pancreatic beta-cell viability and insulin secretion[19,20]. Furthermore, intramuscular IL6 promotes beneficial glucose uptake and fat oxidation via PI3K and AMPK signaling pathways, respectively. However, this cytokine is also released by adipose tissue and chronic levels of IL6 in response to high-fat diets can contribute to obesity through a mechanism that involves macrophage recruitment into adipose tissue[21]. Interestingly, very recent studies revealed that voluntary running suppresses tumor growth through the regulation of NK-cell mobilization and trafficking, processes that are mediated by IL6 and epinephrine. In this manner, this study links exercise-dependent IL6 secretion with cancer and immunity [22]. Future studies using genetically engineered animals with tissue-restricted IL6 deletion will be necessary to determine the extent to which skeletal muscle and/or adipose tissues contribute to these IL6-dependent processes. IL15, a related myokine, also regulates metabolic homeostasis. Notably, transgenic mice that ectopically express IL15 under the control of a skeletal muscle-specific promoter exhibit significantly elevated circulating IL15 accompanied by reduced body fat and increased bone mineral content (see below for further discussion)[23].
Irisin is another exercise-induced myokine that influences adipogenesis and metabolism[24]. Irisin is secreted as a 112 amino acid polypeptide hormone after proteolytic cleavage of its cellular form, fibronectin-type III domain-containing-5 (FNDC5)[24]. Irisin improves obesity by stimulating WAT browning and activation of thermogenesis to promote energy expenditure [24–26] and it has thus been proposed as a therapeutic target for obesity and type 2 diabetes [27]. Interestingly, activation of the Rho-kinase-1 (ROCK1) pathway in skeletal muscle limits irisin production and transgenic mice engineered to express active ROCK1 in skeletal muscle exhibit reduced circulating irisin levels, reduced adipocyte browning, obesity and insulin resistance. The finding that systemic irisin administration reversed these outcomes confirmed the importance of this myokine in muscle-adipose tissue communication and homeostasis[28].
Myonectin (CTRP15) is a member of the C1q/TNF-related protein (CTRP) family that is secreted by skeletal muscles in response to exercise and nutrients[29]. Diet-induced obesity leads to a reduction in myonectin mRNA and serum levels, while voluntary exercise increases mRNA and circulating levels. Myonectin administration into mice reduces circulating free fatty acid levels but does not change adipose tissue lipolysis[29]. Moreover, myonectin induces fatty acid uptake in cultured adipocytes and hepatocytes [29]. Collectively, these studies indicate that myonectin links the skeletal muscle energy state to lipid homeostasis through its effects on both liver and adipose tissue [29].
In summary, physical activity influences the communication between skeletal muscle and adipose tissue, pancreas, and liver. Two of the most important consequences of this cross talk are: (a) generation of a less pro-inflammatory environment and thus the reduction of sarcopenia and visceral fat accumulation, and (b) modulation of insulin sensitivity and glucose metabolism. Therefore, the endocrine functions of skeletal muscle in response to physical activity play a major role in restricting obesity, insulin resistance, and related disorders including type 2 diabetes mellitus[30].
Skeletal muscle to bone formation, repair, and maintenance
The cross talk between muscle and bone is very well established as exercise stimulates bone formation and, inversely, muscle loss causes bone loss. Studies have shown that bi-directional biomechanical, paracrine and endocrine signals control muscle and bone growth[31,32]. Insulin like growth factor-1 (IGF1), fibroblast growth factor-2 (FGF2), IL15, matrix metalloproteinase-2 (MMP2) are released from muscles in response to contraction and positively contribute to bone formation and maintenance[32]. On the other hand, muscle injury induces release of myostatin that in turn interferes with bone repair and healing[32]. Indeed, myostatin-deficient mice exhibit increased in the bone mineral content and density[32]. Other myokines involved in muscle-to-bone communication are irisin and the CNTF. Conditioned media from myoblast cultures enhances osteoblast differentiation in vitro and this effect is dependent on irisin[33]. Injection of recombinant irisin in mice increases cortical bone mass and strength through the suppression of sclerostin, which is an inhibitor of Wnt signaling[33]. CNTF in contrast inhibits osteoblast differentiation[11] and bone formation in a sex-specific manner[34]. The extent to which muscle to bone communication is defective in relevant in diseases such as osteoporosis, sarcopenia, and aging is yet to be explored.
Myokines and regulation of the cardiovascular system
In general, there is an inverse correlation between muscle mass and cardiovascular disease risk and recent studies have confirmed that sarcopenia (skeletal muscle wasting) is a risk factor for cardiovascular disease[35,36]. This concept has led to the thesis that some myokines might confer cardio- and/or vascular protection. Certainly, the cardiovascular system can indirectly benefit from the aforementioned metabolic effects of numerous myokines. Additionally, cells within the cardiovascular system are also direct targets for certain myokines. For example, folliostatin like 1 (FSTL-1) a glycoprotein secreted by skeletal muscle, cardiomyocytes, and epicardial cells has received a tremendous amount of attention of late because local administration of this myokine (via an epicardial patch) leads to remarkable cardioprotection from ischemia-induced myocardial infarction in mouse and swine models[37]. This effect has been attributed to an increase number of dividing cardiomyocytes and increased angiogenesis in the peri-infarct region. FSTL1 is an exercise-induced myokine and in patients as little as 1hr of aerobic exercise can lead to a 22% increase in circulating FSTL-1 levels[38,39]. Moreover, in animal models, skeletal muscle FSTL1 expression is significantly correlated with its circulating levels and with heart function. However, the extent to which skeletal muscle produced FSTL1 contributes to the beneficial effects of regular exercise performed shortly after myocardial infarction[40] has yet to be determined. Nonetheless it is clear from transgenic mouse models that muscle-derived FSTL1 can attenuate neointimal formation in response to arterial injury by suppressing SMC proliferation and can improve revascularization following hind-limb ischemia by promoting angiogenesis[41,42]. Thus, skeletal muscle-dependent release of FSTL1 could have multiple therapeutic effects on the cardiovascular system. Other myokines reported to have salutary effects on the cardiovascular system include fibroblast growth factor 21 (FGF21), musclin, and apelin which have been linked to cardioprotection, protection from atherosclerosis and control of blood pressure respectively[15,43–46]. However, again, future studies are required to determine if skeletal muscle is a major contributor to the circulating levels of these factors.
Skeletal muscle to brain
Exercise and myokine signaling has also been linked to brain neurogenesis and cognitive functions. Endurance exercise increases the levels of circulating irisin which induces the expression of FNDC5 in the hippocampus that in turn promotes the expression of BDNF and thus neurogenesis[47]. Exercise also leads to elevated plasma levels of the myokine cathepsin-B (CTSB), which promotes BDNF expression in the hippocampus, promotes neurogenesis and increases spatial memory abilities [48]. While correlations were found between patient CTSB levels, fitness, and hippocampus-dependent memory functions, the extent to which this myokine is causative for the beneficial effects of physical activity on cognitive abilities remains to be determined.
Skeletal muscle to skin
Very recent studies indicate that skin also responds to myokines. Crane and colleagues demonstrated that exercise attenuated skin changes associated with aging in both humans and mice [49]. Physical activity induces IL15 release from skeletal muscles through the AMPK pathway[49] and IL15 modulates mitochondrial function in skin. Interestingly, IL15 injections into mice mimic anti-aging effects of exercise both in muscle and skin. Overall, this pioneering study describes a mechanism that explains, at the molecular level, how physical activity attenuates skin aging through IL15-dependent processes[49].
Molecular mechanisms of skeletal muscle secretory vesicle trafficking
As noted above, skeletal muscle cells secrete numerous bioactive polypeptides that influence both muscle and whole body homeostasis and the clinical benefits of these factors makes them promising targets for therapeutic strategies to treat a myriad of diseases. However, despite their clinical relevance, almost nothing is known about the mechanisms that regulate the release of these factors. Indeed, there is a paucity of information regarding the final steps in recruitment and exocytosis of specific secretory vesicles; events that must be tightly and temporally controlled for beneficial outcomes. Although skeletal muscle cells express several canonical secretory vesicle transport proteins including specific VAMPs (2–5,7, and 8) and associated t-SNAREs [50], the mechanisms that target VAMP-containing vesicles to particular regions in the plasma membrane to control myokine secretion are largely unknown. In many cell types, the initial stage of vesicle translocation from the Golgi to the periphery involves movement along growing microtubules and/or filamentous or branched actin bundles that radiate towards the plasma membrane[51]. However, most of the actin in skeletal muscle cells is organized into distinct striated fibers and the microtubules are organized in a stable orthogonal lattice comprised of MT bundles [52] suggesting that vesicle transport, docking, and release are likely coordinated in a unique fashion in these cells.
Surprisingly little is known about the control of vesicle transport in skeletal muscle cells. In vitro studies in cultured myoblasts have shown that the Glut4 glucose receptor is trafficked to the plasma membrane in VAMP2-labeled vesicles and Glut4 translocation requires a dynamic cycle of actin filament remodeling which can be induced by insulin[53]. While it is not presently clear which VAMP containing vesicles carry VEGFA, TEM of human muscle revealed that VEGFA-containing vesicles accumulate in the sub-sarcolemmal regions and between the contractile elements in resting myocytes and that these vesicles are more prominent in the sub-sarcolemmal regions following exercise [54]. Collectively, these findings indicate the possibility that peripheral (non-striated) actin fibers act as a barrier that limits secretory vesicle access to plasma membrane-associated t-SNARES. However, several critical questions remain including 1) how is information relayed from extracellular stimuli to mobilize specific myokine-containing vesicles and what are the molecular components that drive the plasma membrane recruitment/docking and release of these vesicles? 2) what is the contribution of secretory vesicle recruitment to the mechanical membrane damage/repair response and to what extent does membrane repair influence myokine release? 3) Do changes that occur during pathological muscle degeneration or aging alter the composition and quantity of the secretory output? Acquisition of such knowledge will be helpful in the rational design of strategies to control the release of bioactive molecules in a temporal and spatial manner and should improve clinical outcomes of therapies developed to target a range of disorders from type 2 diabetes and associate sequelae to cancer, depression and dementia, atherosclerosis, and/or other cardiovascular disorders[30].
Conclusion
Secretion of soluble peptides by muscle has a major influence on skeletal muscle growth and whole body homeostasis. Understanding the molecular underpinnings that control myokine secretion will be important to harness the beneficial effects of exercise to improve human health.
Figure 1. Skeletal muscle as a paracrine and endocrine organ.
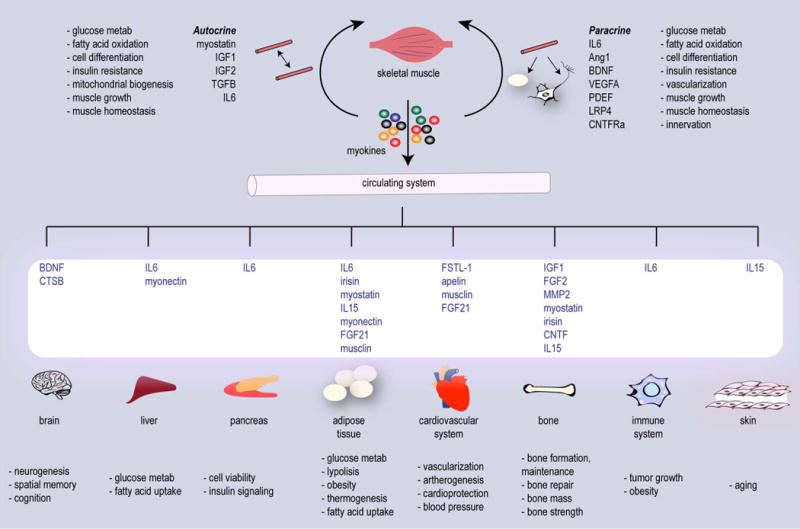
Skeletal muscle is one of the largest tissues in the body and myofibers are one of the most metabolically active tissues. Skeletal muscles ‘communicate’ their energy demands to other organs through active release of factors. Muscle-derived secretory proteins include a variety of cytokines and peptides collectively termed “myokines” that exert auto-, para- or endocrine effects.
Highlights.
-
◦
Skeletal muscle secretes myokines that exert auto- para- and endocrine effects
-
◦
Paracrine factors modulate muscle angiogenesis, adipogenesis, and innervation
-
◦
Myokine secretion is regulated by exercise
-
◦
Exercise benefits cardiovascular, metabolic and mental health via endocrine factors
-
◦
The molecular mechanisms regulating myokine secretion remain to be fully elucidated
Acknowledgments
This work was supported by NIH/National Heart, Lung, and Blood Institute grant HL-081844 and an American Heart Association grant 0355776U to J.M. Taylor. We thank the following funding support to J. Giudice: start up funds, a Junior Faculty Development Award, and the Nutrition Obesity Research Center (NORC) Pilot and Feasibility grant (P30DK056350) from The University of North Carolina at Chapel Hill.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
There are no conflicts of interest
References
- 1.Hartwig S, Raschke S, Knebel B, Scheler M, Irmler M, Passlack W, Muller S, Hanisch FG, Franz T, Li X, et al. Secretome profiling of primary human skeletal muscle cells. Biochim Biophys Acta. 2014;1844:1011–1017. doi: 10.1016/j.bbapap.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Le Bihan MC, Bigot A, Jensen SS, Dennis JL, Rogowska-Wrzesinska A, Laine J, Gache V, Furling D, Jensen ON, Voit T, et al. In-depth analysis of the secretome identifies three major independent secretory pathways in differentiating human myoblasts. J Proteomics. 2012;77:344–356. doi: 10.1016/j.jprot.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Raschke S, Eckardt K, Bjorklund Holven K, Jensen J, Eckel J. Identification and validation of novel contraction-regulated myokines released from primary human skeletal muscle cells. PLoS One. 2013;8:e62008. doi: 10.1371/journal.pone.0062008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iizuka K, Machida T, Hirafuji M. Skeletal muscle is an endocrine organ. J Pharmacol Sci. 2014;125:125–131. doi: 10.1254/jphs.14r02cp. [DOI] [PubMed] [Google Scholar]
- 5.Walsh K. Adipokines, myokines and cardiovascular disease. Circ J. 2009;73:13–18. doi: 10.1253/circj.cj-08-0961. [DOI] [PubMed] [Google Scholar]
- 6.Pedersen BK. Muscles and their myokines. The Journal of Experimental Biology. 2011;214:337–346. doi: 10.1242/jeb.048074. [DOI] [PubMed] [Google Scholar]
- 7.Hoier B, Hellsten Y. Exercise-induced capillary growth in human skeletal muscle and the dynamics of VEGF. Microcirculation. 2014;21:301–314. doi: 10.1111/micc.12117. [DOI] [PubMed] [Google Scholar]
- 8.Bonner JS, Lantier L, Hasenour CM, James FD, Bracy DP, Wasserman DH. Muscle-specific vascular endothelial growth factor deletion induces muscle capillary rarefaction creating muscle insulin resistance. Diabetes. 2013;62:572–580. doi: 10.2337/db12-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olfert IM, Howlett RA, Tang K, Dalton ND, Gu Y, Peterson KL, Wagner PD, Breen EC. Muscle-specific VEGF deficiency greatly reduces exercise endurance in mice. J Physiol. 2009;587:1755–1767. doi: 10.1113/jphysiol.2008.164384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taetzsch T, Tenga MJ, Valdez G. Muscle fibers secrete FGFBP1 to slow degeneration of neuromuscular synapses during aging and progression of ALS. Journal of Neuroscience. 2016 doi: 10.1523/JNEUROSCI.2992-16.2016. EPub online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson RW, White JD, Walker EC, Martin TJ, Sims NA. Myokines (muscle-derived cytokines and chemokines) including ciliary neurotrophic factor (CNTF) inhibit osteoblast differentiation. Bone. 2014;64:47–56. doi: 10.1016/j.bone.2014.03.053. [DOI] [PubMed] [Google Scholar]
- 12.Lee N, Spearry RP, Leahy KM, Robitz R, Trinh DS, Mason CO, Zurbrugg RJ, Batt MK, Paul RJ, Maclennan AJ. Muscle ciliary neurotrophic factor receptor alpha promotes axonal regeneration and functional recovery following peripheral nerve lesion. J Comp Neurol. 2013;521:2947–2965. doi: 10.1002/cne.23324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunt LC, White J. The Role of Leukemia Inhibitory Factor Receptor Signaling in Skeletal Muscle Growth, Injury and Disease. Adv Exp Med Biol. 2016;900:45–59. doi: 10.1007/978-3-319-27511-6_3. [DOI] [PubMed] [Google Scholar]
- 14.Barik A, Lu Y, Sathyamurthy A, Bowman A, Shen C, Li L, Xiong WC, Mei L. LRP4 is critical for neuromuscular junction maintenance. J Neurosci. 2014;34:13892–13905. doi: 10.1523/JNEUROSCI.1733-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raschke S, Eckel J. Adipo-myokines: two sides of the same coin–mediators of inflammation and mediators of exercise. Mediators Inflamm. 2013;2013:320724. doi: 10.1155/2013/320724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeremic N, Chaturvedi P, Tyagi SC. Browning of White Fat: Novel Insight Into Factors, Mechanisms, and Therapeutics. J Cell Physiol. 2017;232:61–68. doi: 10.1002/jcp.25450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Komolka K, Albrecht E, Wimmers K, Michal JJ, Maak S. Molecular heterogeneities of adipose depots - potential effects on adipose-muscle cross-talk in humans, mice and farm animals. J Genomics. 2014;2:31–44. doi: 10.7150/jgen.5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;88:1379–1406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- 19.Ellingsgaard H, Hauselmann I, Schuler B, Habib AM, Baggio LL, Meier DT, Eppler E, Bouzakri K, Wueest S, Muller YD, et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat Med. 2011;17:1481–1489. doi: 10.1038/nm.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paula FM, Leite NC, Vanzela EC, Kurauti MA, Freitas-Dias R, Carneiro EM, Boschero AC, Zoppi CC. Exercise increases pancreatic beta-cell viability in a model of type 1 diabetes through IL-6 signaling. Faseb J. 2015;29:1805–1816. doi: 10.1096/fj.14-264820. [DOI] [PubMed] [Google Scholar]
- (••)21.Kraakman MJ, Kammoun HL, Allen TL, Deswaerte V, Henstridge DC, Estevez E, Matthews VB, Neill B, White DA, Murphy AJ, et al. Blocking IL-6 trans-signaling prevents high-fat diet-induced adipose tissue macrophage recruitment but does not improve insulin resistance. Cell Metab. 2015;21:403–416. doi: 10.1016/j.cmet.2015.02.006. This study identifies IL6 trans-signaling as a regulator of macrophage recruitment into adipose tissue in response to high-fat diets (obesity models). [DOI] [PubMed] [Google Scholar]
- (••)22.Pedersen L, Idorn M, Olofsson GH, Lauenborg B, Nookaew I, Hansen RH, Johannesen HH, Becker JC, Pedersen KS, Dethlefsen C, et al. Voluntary Running Suppresses Tumor Growth through Epinephrine- and IL-6-Dependent NK Cell Mobilization and Redistribution. Cell Metab. 2016;23:554–562. doi: 10.1016/j.cmet.2016.01.011. In this article authors showed that voluntary running suppresses tumor growth through the regulation of NK-cell mobilization and trafficking, processes that are mediated by IL6 and epinephrine. In this manner, this study links exercise with cancer and immunity. [DOI] [PubMed] [Google Scholar]
- 23.Quinn LS, Anderson BG, Strait-Bodey L, Stroud AM, Argiles JM. Oversecretion of interleukin-15 from skeletal muscle reduces adiposity. Am J Physiol Endocrinol Metab. 2009;296:E191–202. doi: 10.1152/ajpendo.90506.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee P, Linderman JD, Smith S, Brychta RJ, Wang J, Idelson C, Perron RM, Werner CD, Phan GQ, Kammula US, et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 2014;19:302–309. doi: 10.1016/j.cmet.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanchis-Gomar F, Lippi G, Mayero S, Perez-Quilis C, Garcia-Gimenez JL. Irisin: a new potential hormonal target for the treatment of obesity and type 2 diabetes. J Diabetes. 2012;4:196. doi: 10.1111/j.1753-0407.2012.00194.x. [DOI] [PubMed] [Google Scholar]
- 28.Zhou X, Li R, Liu X, Wang L, Hui P, Chan L, Saha PK, Hu Z. ROCK1 reduces mitochondrial content and irisin production in muscle suppressing adipocyte browning and impairing insulin sensitivity. Sci Rep. 2016;6:29669. doi: 10.1038/srep29669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seldin MM, Peterson JM, Byerly MS, Wei Z, Wong GW. Myonectin (CTRP15), a novel myokine that links skeletal muscle to systemic lipid homeostasis. J Biol Chem. 2012;287:11968–11980. doi: 10.1074/jbc.M111.336834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pedersen BK. The diseasome of physical inactivity–and the role of myokines in muscle–fat cross talk. J Physiol. 2009;587:5559–5568. doi: 10.1113/jphysiol.2009.179515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cianferotti L, Brandi ML. Muscle-bone interactions: basic and clinical aspects. Endocrine. 2014;45:165–177. doi: 10.1007/s12020-013-0026-8. [DOI] [PubMed] [Google Scholar]
- 32.Hamrick MW. The skeletal muscle secretome: an emerging player in muscle-bone crosstalk. BoneKEy Rep. 1 doi: 10.1038/bonekey.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (•)33.Colaianni G, Cuscito C, Mongelli T, Pignataro P, Buccoliero C, Liu P, Lu P, Sartini L, Di Comite M, Mori G, et al. The myokine irisin increases cortical bone mass. Proc Natl Acad Sci U S A. 2015;112:12157–12162. doi: 10.1073/pnas.1516622112. Here, authors revealed an unknown role for the myokine irisin as a mediator of bone-muscle cross talk. Irisin is released by skeletal muscles in response to exercise and profoundly impacts cortical bone mass and strength through the suppression of sclerostin, which is an inhibitor of Wnt signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGregor NE, Poulton IJ, Walker EC, Pompolo S, Quinn JM, Martin TJ, Sims NA. Ciliary neurotrophic factor inhibits bone formation and plays a sex-specific role in bone growth and remodeling. Calcif Tissue Int. 2010;86:261–270. doi: 10.1007/s00223-010-9337-4. [DOI] [PubMed] [Google Scholar]
- 35.Byeon CH, Kang KY, Kang SH, Bae EJ. Sarcopenia is associated with Framingham risk score in the Korean population: Korean National Health and Nutrition Examination Survey (KNHANES) 2010–2011. J Geriatr Cardiol. 2015;12:366–372. doi: 10.11909/j.issn.1671-5411.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou J, Liu B, Liang C, Li Y, Song YH. Cytokine Signaling in Skeletal Muscle Wasting. Trends Endocrinol Metab. 2016;27:335–347. doi: 10.1016/j.tem.2016.03.002. [DOI] [PubMed] [Google Scholar]
- (••)37.Wei K, Serpooshan V, Hurtado C, Diez-Cunado M, Zhao M, Maruyama S, Zhu W, Fajardo G, Noseda M, Nakamura K, et al. Epicardial FSTL1 reconstitution regenerates the adult mammalian heart. Nature. 2015;525:479–485. doi: 10.1038/nature15372. The epicardium is a known source for cardioprotective factors. Herein the authors showed that endogenous FSTL1 declines following myocardial infarction, but replacement of this factor administered via an epicardial patch provided remarkable cardioprotection in this setting in both murine and swine models. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gorgens SW, Raschke S, Holven KB, Jensen J, Eckardt K, Eckel J. Regulation of follistatin-like protein 1 expression and secretion in primary human skeletal muscle cells. Arch Physiol Biochem. 2013;119:75–80. doi: 10.3109/13813455.2013.768270. [DOI] [PubMed] [Google Scholar]
- 39.Norheim F, Raastad T, Thiede B, Rustan AC, Drevon CA, Haugen F. Proteomic identification of secreted proteins from human skeletal muscle cells and expression in response to strength training. Am J Physiol Endocrinol Metab. 2011;301:E1013–1021. doi: 10.1152/ajpendo.00326.2011. [DOI] [PubMed] [Google Scholar]
- 40.Haykowsky M, Scott J, Esch B, Schopflocher D, Myers J, Paterson I, Warburton D, Jones L, Clark AM. A meta-analysis of the effects of exercise training on left ventricular remodeling following myocardial infarction: start early and go longer for greatest exercise benefits on remodeling. Trials. 2011;12:92. doi: 10.1186/1745-6215-12-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miyabe M, Ohashi K, Shibata R, Uemura Y, Ogura Y, Yuasa D, Kambara T, Kataoka Y, Yamamoto T, Matsuo K, et al. Muscle-derived follistatin-like 1 functions to reduce neointimal formation after vascular injury. Cardiovasc Res. 2014;103:111–120. doi: 10.1093/cvr/cvu105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ouchi N, Oshima Y, Ohashi K, Higuchi A, Ikegami C, Izumiya Y, Walsh K. Follistatin-like 1, a secreted muscle protein, promotes endothelial cell function and revascularization in ischemic tissue through a nitric-oxide synthase-dependent mechanism. J Biol Chem. 2008;283:32802–32811. doi: 10.1074/jbc.M803440200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li YX, Cheng KC, Asakawa A, Kato I, Sato Y, Amitani H, Kawamura N, Cheng JT, Inui A. Role of musclin in the pathogenesis of hypertension in rat. PLoS One. 2013;8:e72004. doi: 10.1371/journal.pone.0072004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu SQ, Roberts D, Kharitonenkov A, Zhang B, Hanson SM, Li YC, Zhang LQ, Wu YH. Endocrine protection of ischemic myocardium by FGF21 from the liver and adipose tissue. Sci Rep. 2013;3:2767. doi: 10.1038/srep02767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Subbotina E, Sierra A, Zhu Z, Gao Z, Koganti SR, Reyes S, Stepniak E, Walsh SA, Acevedo MR, Perez-Terzic CM, et al. Musclin is an activity-stimulated myokine that enhances physical endurance. Proc Natl Acad Sci U S A. 2015;112:16042–16047. doi: 10.1073/pnas.1514250112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhong JC, Zhang ZZ, Wang W, McKinnie SM, Vederas JC, Oudit GY. Targeting the apelin pathway as a novel therapeutic approach for cardiovascular diseases. Biochim Biophys Acta. 2016 doi: 10.1016/j.bbadis.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 47.Wrann CD, White JP, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, Lin JD, Greenberg ME, Spiegelman BM. Exercise induces hippocampal BDNF through a PGC-1alpha/FNDC5 pathway. Cell Metab. 2013;18:649–659. doi: 10.1016/j.cmet.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (•)48.Moon HY, Becke A, Berron D, Becker B, Sah N, Benoni G, Janke E, Lubejko ST, Greig NH, Mattison JA, et al. Running-Induced Systemic Cathepsin B Secretion Is Associated with Memory Function. Cell Metab. 2016;24:332–340. doi: 10.1016/j.cmet.2016.05.025. In this article proteomic approaches identified CTSB as a myokine responsive to physical activity in mice. Authors robustly showed that elevated circulating CTSB promotes BDNF and doublecortin expression in adult hippocampal progenitor cells, which in turn have beneficial effects on cognition. Furthermore, results in humans and monkeys corroborate the role of CTSB in enhancing cognitive abilities. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (••)49.Crane JD, MacNeil LG, Lally JS, Ford RJ, Bujak AL, Brar IK, Kemp BE, Raha S, Steinberg GR, Tarnopolsky MA. Exercise-stimulated interleukin-15 is controlled by AMPK and regulates skin metabolism and aging. Aging Cell. 2015;14:625–634. doi: 10.1111/acel.12341. This is a pioneering study that identifies skin as a novel organ that responds to myokines. The Authors describe a mechanism by which the myokine IL-15 attenuates skin aging via altering skin cell mitochondrial function. Furthermore, this work revealed that when injected into mice IL15 mimics anti-aging effects of exercise both in muscle and skin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tajika Y, Takahashi M, Khairani AF, Ueno H, Murakami T, Yorifuji H. Vesicular transport system in myotubes: ultrastructural study and signposting with vesicle-associated membrane proteins. Histochem Cell Biol. 2014;141:441–454. doi: 10.1007/s00418-013-1164-z. [DOI] [PubMed] [Google Scholar]
- 51.Wu B, Guo W. The Exocyst at a Glance. J Cell Sci. 2015;128:2957–2964. doi: 10.1242/jcs.156398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oddoux S, Zaal KJ, Tate V, Kenea A, Nandkeolyar SA, Reid E, Liu W, Ralston E. Microtubules that form the stationary lattice of muscle fibers are dynamic and nucleated at Golgi elements. J Cell Biol. 2013;203:205–213. doi: 10.1083/jcb.201304063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun Y, Chiu TT, Foley KP, Bilan PJ, Klip A. Myosin Va mediates Rab8A-regulated GLUT4 vesicle exocytosis in insulin-stimulated muscle cells. Mol Biol Cell. 2014;25:1159–1170. doi: 10.1091/mbc.E13-08-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoier B, Prats C, Qvortrup K, Pilegaard H, Bangsbo J, Hellsten Y. Subcellular localization and mechanism of secretion of vascular endothelial growth factor in human skeletal muscle. Faseb J. 2013;27:3496–3504. doi: 10.1096/fj.12-224618. [DOI] [PubMed] [Google Scholar]


