Abstract
Objectives
The Patient-Reported Outcomes Measurement Information System (PROMIS) is a universally applicable set of instruments, including item banks, short forms and computer adaptive tests (CATs), measuring patient-reported health across different patient populations. PROMIS CATs are highly efficient and the use in practice is considered feasible with little administration time, offering standardized and routine patient monitoring. Before an item bank can be used as CAT, the psychometric properties of the item bank have to be examined. Therefore, the objective was to assess the psychometric properties of the Dutch-Flemish PROMIS Physical Function item bank (DF-PROMIS-PF) in Dutch patients receiving physical therapy.
Design
Cross-sectional study.
Setting and participants
805 patients >18 years, who received any kind of physical therapy in primary care in the past year, completed the full DF-PROMIS-PF (121 items).
Methods
Unidimensionality was examined by Confirmatory Factor Analysis and local dependence and monotonicity were evaluated. A Graded Response Model was fitted. Construct validity was examined with correlations between DF-PROMIS-PF T-scores and scores on two legacy instruments (SF-36 Health Survey Physical Functioning scale [SF36-PF10] and the Health Assessment Questionnaire Disability-Index [HAQ-DI]). Reliability (standard errors of theta) was assessed.
Results
The results for unidimensionality were mixed (scaled CFI = 0.924, TLI = 0.923, RMSEA = 0.045, 1th factor explained 61.5% of variance). Some local dependence was found (8.2% of item pairs). The item bank showed a broad coverage of the physical function construct (threshold-parameters range: -4.28–2.33) and good construct validity (correlation with SF36-PF10 = 0.84 and HAQ-DI = -0.85). Furthermore, the DF-PROMIS-PF showed greater reliability over a broader score-range than the SF36-PF10 and HAQ-DI.
Conclusions
The psychometric properties of the DF-PROMIS-PF item bank are sufficient. The DF-PROMIS-PF can now be used as short forms or CAT to measure the level of physical function of physiotherapy patients.
Introduction
Patient Reported Outcome Measures (PROMs) have become standard instruments to measure patients’ perceived health, and are used to assist in patient-physician shared-decision making and to monitor patients’ health over time. However, its application in daily clinical practice is not without problems. Many traditional PROMs are too long for use in daily clinical practice, and sometimes contain irrelevant and poorly formulated questions [1,2]. The measurement error is often too large to make decisions based on the individual PROM scores and to evaluate changes in individual patients. Furthermore, the scores of traditional PROMs are often difficult to interpret and cannot be compared between the many different existing PROMs [3].
The Patient-Reported Outcomes Measurement Information System (PROMIS®) has the potential to overcome some of the shortcomings of existing PROMs [4]. PROMIS is an innovative universally, that is a generic or non-disease specific, applicable set of instruments designed to measure patient-reported health across different populations, including patient populations, in a more efficient way [5,6]. PROMIS instruments are developed using Item Response Theory (IRT) and consist of item banks. These item banks (a set of items [questions] measuring one common construct) can be applied as short forms (fixed length subsets of items out of the item bank) or highly efficient computer adaptive tests (CAT). A CAT is a computer-administered measure in which successive items are selected by a computer algorithm based on responses to previous items. Patients only have to respond to a small number (3–7) of highly informative and relevant items. As a consequence, PROMIS tools are, if applied as short form or CAT, relatively short and the administration time is much less compared to traditional PROMs. Moreover, the CAT application results in estimates with a low measurement error [7]. PROMIS tools were carefully developed in close collaboration with patients and experts, ensuring its content validity. All PROMIS scores are standardized and expressed as T-scores with a population mean of 50 and a Standard Deviation (SD) of 10, enabling easy interpretation. In short, PROMIS instruments are easy to interpret, are less burdensome, have less measurement error, and have better content validity than traditional PROMs [7–9].
The PROMIS Physical Function (PROMIS-PF) item bank is one of the PROMIS instruments which is highly relevant for physical therapists and their patients [10]. Physical function refers to the ability to perform activities of daily living and instrumental activities of daily living [10]. Limitations in physical function are a major concern for elderly and patients with musculoskeletal diseases [11,12]. Physical therapy treatment often focuses on improving physical function. Physical function is therefore frequently a core outcome of treatment. The PROMIS-PF item bank has shown to have less measurement error, stronger content validity and other desirable psychometric properties, compared to traditional physical function PROMs such as the SF-36 Health Survey Physical Functioning scale (SF36-PF10) and the Health Assessment Questionnaire-Disability Index (HAQ-DI) [5,8,9,13,14]. The PROMIS-PF item bank was translated into Dutch-Flemish and showed good psychometric properties in Dutch patients with chronic pain and rheumatoid arthritis [15–18]. However, the PROMIS-PF item bank has not yet been validated in patients receiving physical therapy.
In line with the international PROMIS goals to re-do the calibration of item banks and evaluate its psychometric properties in multiple validation studies and in patients with multiple conditions before an item bank can be used as CAT, the aim of current study was to examine the psychometric properties of the V1.2 Dutch-Flemish PROMIS-PF item bank (DF-PROMIS-PF) in Dutch patients receiving physical therapy in primary care. This is the first study re-doing the calibration of the PROMIS-PF in patients receiving physical therapy. The ultimate aim is to obtain a user-friendly, efficient, precise and valid instrument to measure physical function in patients receiving physical therapy in daily clinical practice and research.
Methods
Study participants
For this study Dutch patients (18 years or older) receiving physical therapy in primary care in the past year were invited. Patients were eligible if they provided informed consent.
Procedures
The study was approved by the local institutional review board of the VU University Medical Center. Physical therapy practices across the Netherlands were approached to recruit patients for the study through the personal network of the authors, and advertisement in a Dutch physical therapy journal. Thereafter, the patients were invited by their physical therapist, by e-mail or flyer, to complete an online questionnaire.
Measures
The questionnaire included items addressing demographic and clinical characteristics. The questionnaire also included all 121 items of the V1.2 DF-PROMIS-PF. The items cover a wide range of activities, from self-care (activities of daily living) to more complex activities that require a combination of skills. The item bank includes items about functioning of the axial regions (neck and back), the upper and lower extremities, and ability to carry out instrumental activities of daily living (i.e. housework, shopping) [10]. There is no time frame set for the items, but current status is inferred. There are three different 5-point Likert response scales: 1) Unable to do/With much difficulty/With some difficulty/With a little difficulty/Without any difficulty, 2) Cannot do/Quite a lot/Somewhat/Very little/Not at all; and, 3) Cannot do because of health/A lot of difficulty/Some difficulty/A little bit of difficulty/No difficulty at all. Higher scores indicate better function.
In addition, two generic legacy instruments were administered: the SF36-PF10 and the HAQ-DI [19,20]. The Dutch version 2 of the SF36-PF10 was used, which consists of 10 items measuring perceived limitations in a variety of physical activities. Scores of the SF36-PF10 are summed and linearly transformed to range between 0 and 100, with higher scores indicating better physical function [20]. The SF36-PF10 has demonstrated good reliability and validity in Canadian patients with musculoskeletal disorders undergoing physical therapy [21], and in Dutch patients with rheumatoid arthritis [22]. The Dutch version of the HAQ-DI was used, which contains 20 items measuring physical disabilities over the past week in eight categories of daily living. The category scores were averaged to produce a total score ranging from 0 to 3, with higher scores indicating more disability [19]. Disability scores were calculated according the alternative scoring rule indicating that its scores depends on the amount of self-reported difficulty while performing activities, and not on the use of aids or help. The HAQ-DI has demonstrated good reliability and validity within Dutch patients with rheumatoid arthritis and osteoarthritis [23,24].
Statistical analysis
Demographic and clinical characteristics were described by descriptive statistics. Psychometric analyses were conducted in accordance with the PROMIS analysis plan and were similar to the re-doing of the calibration of the DF-PROMIS-PF in Dutch patients with chronic pain [15,25]. In order to apply an instrument as CAT and to obtain valid scores for CATs and short forms it is important that the items fit an IRT model, because the CAT algorithm and scoring system are based on the parameters of the underlying IRT model. Before applying IRT models, it is important to evaluate the core assumptions of the IRT model: (1) unidimensionality, indicating that the items assess one and only one construct, in this study physical functioning, (2) local independence, indicating that the items are only related to the construct being measured and not to other factors, and (3) monotonicity, indicating that the probability of a affirmative response to the item increases with increasing levels of the underlying construct.
To check unidimensionality, a Confirmatory Factor Analysis (CFA) was fitted using the R-package Lavaan (version 0.5–16) [26,27]. All items were hypothesized to load on a single factor. Model fit was evaluated based on the Comparative Fit Index (CFI), Tucker Lewis Index (TLI) and Root Means Square Error of Approximation (RMSEA). Both scaled and unscaled fit indices were calculated. Since the CFI, TLI and RMSEA are Chi-square based the scaled parameters are considered more exact [28,29]. Model fit criteria include CFI >0.95, TLI >0.95 and RMSEA <0.06 [30]. As an additional approach for assessing unidimensionality, we conducted a Principal Component Analysis (PCA). Sufficient evidence for unidimensionality was considered if the first factor accounts for at least 20% of the variability and when the ratio of the variance explained by the first to the second factor is greater than four [25].
Local independence was evaluated by considering the residual correlation matrix [31]. Residual correlations greater than 0.2 were considered as indicators of possible local dependence [25]. Item pairs which were highly correlated (>0.80) were inspected in more detail with regard to item content and distribution of item response categories. In addition, we inspected model modification indices. Modification indices show improvement in Chi-square if residual correlations of an item pair were left free in the model and thus present a useful tool for identifying problematic items.
Monotonicity was evaluated by estimating a nonparametric Mokken scale with the R-package Mokken [25,32–35]. Fit of model was evaluated by calculating the scalability coefficient H. Monotonicity criteria are met if (1) the scalability coefficients for all item pairs are positive, (2) the scalability coefficients for the items in relation to the scale at issue are at least 0.30, and (3) the scalability coefficient H for the scale is at least 0.30. Higher values for H indicate a better scale. A rule of thumb is that a scale is considered to be strong when H is ≥0.50 [32–35].
When the assumptions of unidimensionality, local independence, and monotonicity were met, the fit of the Graded Item Response model (GRM) was examined, indicating if the IRT model fits to the response data. A logistic GRM was used to estimate item slopes, thresholds, and individual theta scores, using IRT PRO [36]. The item slopes represent the discriminative ability of the items. The item thresholds represent the item difficulties, and locate the items along the measured trait. The theta represents the individual’s physical function score. For standardization and international comparison, the theta scores were transformed into T-scores, where a T-score 50 represents the average score of the general US population, with a SD of 10.
Differential Item Functioning (DIF) analyses evaluate if persons from different groups (for example male vs. female), with similar levels of physical function, respond similar to the items which implies validity of comparisons between the groups at issue [25,37,38]. DIF was evaluated by ordinal logistic regression models in the R package Lordif (version 0.3–3), in which a McFadden’s pseudo R2 change of 2% was used as the critical value to flag for possible DIF [25,39–41]. DIF was evaluated for age (median split) and gender (male vs. female). When items were flagged for DIF, the impact of DIF was examined by plotting item characteristic curves (ICCs) and test characteristic curves (TCCs).
Construct validity indicates whether the item bank really measures the intended construct (physical function). Therefore, construct validity of the DF-PROMIS-PF was evaluated by calculating Spearman correlations between the T-scores of the DF-PROMIS-PF and scores on the two legacy instruments (SF36-PF10 and HAQ-DI). If an instrument measures the intended construct its scores should be highly correlated to scores of other PROMs measuring the same construct. We hypothesized that the DF-PROMIS-PF would have strong correlations with both legacy instruments (r>0.60), but the strongest correlation (r>0.70) with the SF36-PF10, because both DF-PROMIS-PF and SF36-PF10 were both developed for use in a general population whereas the HAQ-DI in the first instance was developed for patients with rheumatoid arthritis.
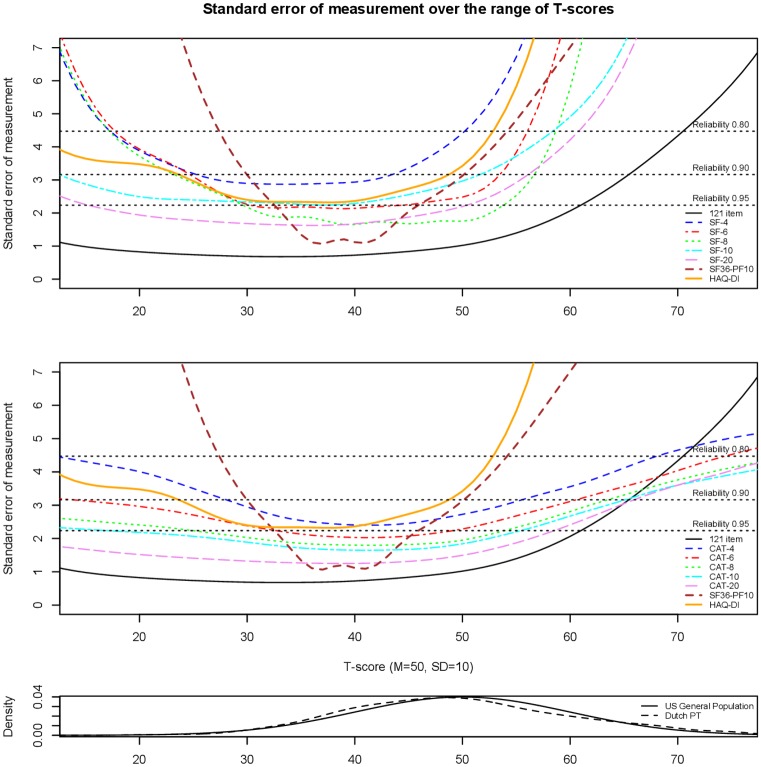
Reliability indicates whether a measure is precise in estimating the level of the construct, in other words, precise in estimating the physical function T-scores. Reliability within IRT is conceptualized as “information”, in which the fact that measurement precision can differ across levels of the measured trait (θ = Theta) is taken into account [42,43]. Increased information is related to smaller standard errors (SEs) and, therefore, greater measurement precision [42,43]. Plots were overlaid showing SEs, as a parameter of reliability, across the score range of the full DF-PROMIS-PF, the 4-, 6-, 8-, 10-, and 20-item DF-PROMIS-PF Short Forms, and 4-, 6-, 8-, 10-, and 20-item simulated fixed-length CATs. The simulated fixed-length CATs were conducted with use of the R-package catR (version 3.13) [44,45]. Furthermore, the plot included also SEs from the SF36-PF10 and the HAQ-DI, to compare their reliability to the reliability of the DF-PROMIS-PF measures. The distribution of T-scores of the Dutch physiotherapy patients sample was plotted under the reliability plot to show the relation between the reliability of the item bank and the distribution of scores in the sample.
Results
Study participants
A total of 805 patients completed the questionnaire. Their demographic and clinical characteristics are summarized in Table 1. The statistical analyses used for checking model assumptions were performed on 753 respondents who had complete DF-PROMIS-PF data because the R packages used cannot handle missing items. However, because IRTPRO can accommodate incomplete data, all 805 patients were used for estimation the IRT model parameters and T-scores were calculated for all 805 patients.
Table 1. Demographic and clinical characteristics of the study population.
| Physical therapy patients (n = 805) | |
|---|---|
| Age mean (SD) range | 53 (14) 18–88 |
| Gender n (%) | |
| Male | 331 (41) |
| Female | 474 (59) |
| Country of birth n (%) | |
| Netherlands | 761 (95) |
| Other | 44 (5) |
| Educational level n (%) | |
| Less than High School degree | 21 (3) |
| High School degree | 82 (10) |
| Some college | 301 (37) |
| College degree | 37 (5) |
| Advanced degree | 364 (45) |
| Body region of treatment n (%) | |
| Head | 14 (2) |
| Breast/abdomen | 25 (3) |
| Neck/upper back | 152 (19) |
| Shoulders/upper arm | 113 (14) |
| Elbow/forearm/hand | 23 (3) |
| Low back | 157 (20) |
| Pelvis/hip/upper leg | 76 (9) |
| Knee | 86 (11) |
| Lower leg/ankle/foot | 52 (6) |
| More than 1 region | 107 (13) |
| Disorder type for treatment n (%) | |
| Disorder of muscles, bones or joints without surgery | 391 (49) |
| Recovery after surgery | 100 (12) |
| Condition resulting from an accident without surgery | 70 (9) |
| Cardiac, vascular or lymphatic disorder | 25 (3) |
| Pulmonary affection | 20 (2) |
| Other internal disorder | 4 (1) |
| Neurological disorder | 15 (2) |
| Gynaecological disorder | 7 (1) |
| Disorder with no known cause | 11 (1) |
| Rheumatic disease | 17 (2) |
| Osteoarthritis | 45 (6) |
| Other | 100 (12) |
| Duration of pain n (%) | |
| 0–3 months | 126 (16) |
| 3–6 months | 116 (14) |
| 6–12 months | 166 (21) |
| 1–2 years | 146 (18) |
| 2–5 years | 85 (10) |
| >5 years | 166 (21) |
| T-score of the PROMIS Physical Function item bank mean (SD) range | 48.2 (9.4) 21.4–73.5 |
| Legacy instruments mean (SD) | |
| SF36-PF10 (n = 710) | 75.2 (26) |
| HAQ-DI (n = 739) | 0.4 (0.5) |
SF36-PF10 = Short Form Health Survey Physical Functioning (range 0–100, higher scores indicate better physical function); HAQ-DI = Health Assessment Questionnaire-Disability Index (range 0–3, higher scores indicate less physical functioning).
Model assumptions
The results for unidimensionality were mixed: The CFA analyses showed unscaled fit indices of CFI = 0.982, TLI = 0.982 and RMSEA = 0.091, and scaled indices of CFI = 0.924, TLI = 0.923 and RMSEA = 0.045. The scaled CFI and TLI did not met the criterion of >0.95, while the scaled RMSEA did met the criterion of <0.06. Furthermore, the first factor accounted for 61.5% of the variance and the ratio of the variance explained by the first to the second factor was 7.6, well above the criterion of 4. Altogether showing sufficient unidimensionality.
Some violations of local independence were found: The residual correlation matrix showed that 592 out of 7260 item pairs (8.2%) were flagged for local dependence (S1 Appendix). It was found that upper extremity related items addressing easy activities (e.g. tooth brushing) were often highly residual correlated with items related to running. Further inspection of these item pairs showed that only few patients answered ‘unable to do’ or ‘with much difficulty’ to these easy items, while the distribution of responses to the running items were more balanced. This might explain the residual correlation. As a further approach to evaluate local independence, modification indices were examined to see if freeing the residual correlations would improve the model. It was found that the item pairs with the highest modification indices show strong overlap in item content (S1 Appendix). Many items related to running, lifting, and items with very similar wording were residually related, which may indicate a second factor in the data. Therefore, we examined if items related to running or lifting could form a factor by themselves. A CFA on 11 items related to walking or running resulted in scaled CFI = 0.98, TLI = 0.98, and RMSEA = 0.16. A CFA on 12 items related to lifting resulted in scaled CFI = 0.99, TLI = 0.98, and RMSEA = 0.11. The RMSEA values were considered too high to consider a second factor.
No violations of monotonicity were found. The scalability coefficients for all item pairs were positive, only one item had a scalability coefficient <0.30 and the scalability coefficient H for the full scale was 0.57, suggesting strong scalability.
IRT model parameters
The GRM item slope parameters ranged from 1.36 to 4.29 (mean 2.74) and the item threshold parameters ranged from -4.28 to 2.33, indicating good coverage of the physical function construct. The mean T-score of the DF-PROMIS-PF for the Dutch sample was 48.2 (SD = 9.4), with a range from 21.4 to 73.5, indicating a slightly lower average level of physical function in Dutch patients currently receiving physical therapy treatment or had completed physical therapy treatment in the past year compared to persons from the US general population, with large variation among patients.
Differential Item Functioning
Only two out of 121 Dutch-Flemish PROMIS Physical Function items were flagged for DIF for age and fourteen for DIF for gender (S2 Appendix). However, investigation of ICCs and TCCs showed that their impact on the T-scores was negligible.
Construct validity
The DF-PROMIS-PF correlated strongly with both the SF36-PF10 (r = 0.84)) and the HAQ-DI (r = -0.85), as expected. The correlation with the SF-PF10 was, however, not higher than the correlation with the HAQ-DI.
Reliability
Fig 1 shows plots of the SEs across the range of the full DF-PROMIS-PF, short forms, simulated CATs, and the SF36-PF10 and HAQ-DI. The reliability of the total item bank was greater than 0.95 for the range of the scale where most of the study sample was located (between T-scores 35–60), indicating very good reliability. The plots demonstrate that CATs show greater reliability than the short forms. Furthermore, the DF-PROMIS-PF instruments show greater reliability than the SF36-PF10 and HAQ-DI across most of the trait.
Fig 1. The two upper plots show the standard errors of the total Dutch-Flemish PROMIS Physical Function item bank (121 item), the 4-, 6-, 8-, 10-, and 20-item short forms (SFs) and CATs, and the SF36-PF10 and HAQ-DI, respectively.
The horizontal axis represents the different physical function abilities with T = 50 representing the mean of the US general population with a standard deviation of 10. The vertical axis represents the standard error (reliability), with reference reliabilities of 0.80, 0.90 and 0.95. The lower the curve, the greater the reliability. The lower plot shows the distribution of the Dutch physiotherapy patients (Dutch PT) sample and the US general population sample along the T-score scale.
Discussion
This is the first validation study of PROMIS in a physical therapy population. The results of the current study add to the evidence on the psychometric properties of the DF-PROMIS-PF. The results supported sufficient unidimensionality, showed some local dependence, good monotonicity, good IRT model fit, good coverage of the construct of physical function and negligible DIF for age and gender. Furthermore, good construct validity and high reliability across the physical function construct was found. Although further improvement of the item bank may be possible, we consider the psychometric properties of the DF-PROMIS-PF sufficient to measure the level of physical function of physiotherapy patients if being applied as short forms or CAT.
The average age (53yr) and percentages of females (60%) in our sample matches with the average physical therapy patient population in the Netherlands in 2015, but the percentage of patients with complaints longer than 6 months was much higher in our sample (70% compared to 25% in the Netherlands) [46]. Due to the selection procedure of the study, we do not know how many patients were still under treatment. The mean T-score (48.2) was only slightly lower (0.18SD) than the average US general population, which could indicate that many patients had completed their treatment.
Our results regarding the psychometric properties of the DF-PROMIS-PF were similar to those of previous studies of the PROMIS-PF. Previous studies in different patient populations and in people from the general population also found small problems with the IRT assumptions unidimensionality and local dependence (S3 Appendix) [10,15,17,47,48]. On the contrary, Paz et al. found good results regarding the IRT assumptions in Spanish speaking persons (S3 Appendix) [49]. Regarding local dependence, 8% local dependence was found in the current study, whereas this was 6% in Dutch chronic pain patients and 10% in Spanish speaking persons [15,48]. The current study showed that sparsity in the item response categories (few patients who answered ‘unable to do’ or ‘with much difficulty’ to the easy items) was a major cause of misfit and local dependence. Sparsity in item response categories was also found by Rose et al. and Hung et al. [10,47]. This indicates that some of the ‘easy’ items such as ‘are you able to brush your teeth’ may be less relevant for these populations. Furthermore, in the current study there were items that showed high overlap in item content, mostly regarding running and lifting. However, analyses showed that there was no running or lifting factor present besides the physical function factor.
In the current study items with DIF with respect to age and gender were found, however their impact on the physical function scores was negligible. Previous studies on the PROMIS-PF also showed no or minimal impact of DIF for age and gender [10,15,17]. Overall, we conclude that the DF-PROMIS-PF can be used across physical therapy patients that differ in age or gender.
The current study supports the construct validity of the DF-PROMIS-PF, by showing strong correlations between the DF-PROMIS-PF and the traditionally used SF36-PF10 (r = 0.84) and HAQ-DI (r = -0.85). This was also found by Oude Voshaar et al., who found similar strong correlations between the DF-PROMIS-PF and both the SF36-PF10 (r = 0.84) and the HAQ-DI (r = -0.76) [16].
The current study as well as several previous studies showed that the PROMIS-PF measures have good reliability; the reliability of the total item bank as well as the short forms and CATs were greater than 0.80 or even 0.90 or 0.95 for the range of the scale where the study samples were located [10,15,16]. These studies also showed that CATs outperform short forms [10,15,16]. Furthermore, the current and previous studies showed that the PROMIS-PF measures have better precision (smaller measurement error) over a broader score-range compared to the SF36-PF10 and the HAQ-DI [8,9,14]. Moreover, PROMIS CATs are more efficient and more feasible for use in daily clinical practice than the SF36-PF10 and HAQ-DI. The administration time for the physician, which is now a big problem within physical therapy, is much less with the PROMIS CATs compared to other PROMs. A recent study in patients undergoing meniscal surgery showed that the majority (89%) of the patients completed the PROMIS-PF CAT after answering 4 items [50]. Moreover, PROMIS T-scores are easy to interpret and can easily be used for patient-physician communication and goalsetting, for monitoring patients and for monitoring the progress of the treatment on physical function level.
Were the current study focused on examining the psychometric properties of the whole item bank and showed sufficient properties of the DF-PROMIS-PF to be used as CAT, future research is recommended on known-groups validity, test-retest reliability and responsiveness of the CAT, because the CAT would most likely be used in pre- and post-intervention measurements. A recent study in orthopedic reconstructions patients showed that the PROMIS-PF CAT outperformed the legacy instruments Knee injury and Osteoarthritis Outcome Score Joint Replacement (KOOS-JR) and Hip disability and Osteoarthritis Outcome Score Joint Replacement (HOOS-JR) with respect to responsiveness [51].
The psychometric properties of the DF-PROMIS-PF item bank are sufficient for use as short forms or CAT to measure the level of physical function in Dutch physical therapy practice. Using the highly efficient DF-PROMIS-PF CAT in clinical practice is considered feasible with little administration time, and has the potential for standardized and routine patient monitoring across a wide range of patients receiving physical therapy.
Supporting information
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
The Dutch-Flemish PROMIS group is an initiative that aims to translate and implement PROMIS item banks and CATS in the Netherlands and Flanders (www.dutchflemishpromis.nl). The Dutch-Flemish translation of the PROMIS item banks was supported by a grant from the Dutch Arthritis Association (BP 10-1-261). Funding for current study was provided by a grant from the Royal Dutch Society for Physical Therapy. We would like to thank Lisa Edelaar for her administrative support and Oguzhan Ogreden for his support with the reliability analyses.
Data Availability
All relevant data are available via Figshare; https://doi.org/10.6084/m9.figshare.5711971.
Funding Statement
The Dutch-Flemish translation of the PROMIS item banks was supported by a grant from the Dutch Arthritis Association (BP 10-1-261). Funding for current study was provided by a grant from the Royal Dutch Society for Physical Therapy. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Boyce MB, Browne JP, Greenhalgh J. The experiences of professionals with using information from patient-reported outcome measures to improve the quality of healthcare: a systematic review of qualitative research. BMJ Qual Saf. 2014;23(6):508–18. doi: 10.1136/bmjqs-2013-002524 [DOI] [PubMed] [Google Scholar]
- 2.Jette DU, Halbert J, Iverson C, Miceli E, Shah P. Use of standardized outcome measures in physical therapist practice: perceptions and applications. Phys Ther. 2009;89(2):125–35. doi: 10.2522/ptj.20080234 [DOI] [PubMed] [Google Scholar]
- 3.Porter I, Goncalves-Bradley D, Ricci-Cabello I, Gibbons C, Gangannagaripalli J, Fitzpatrick R, et al. Framework and guidance for implementing patient-reported outcomes in clinical practice: evidence, challenges and opportunities. J Comp Eff Res. 2016;5(5):507–19. doi: 10.2217/cer-2015-0014 [DOI] [PubMed] [Google Scholar]
- 4.Witter J. The Promise of Patient-Reported Outcomes Measurement Information System-Turning Theory into Reality: A Uniform Approach to Patient-Reported Outcomes Across Rheumatic Diseases. rheum dis clin north am. 2016;42(2):377–94. doi: 10.1016/j.rdc.2016.01.007 [DOI] [PubMed] [Google Scholar]
- 5.Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol [Internet]. 2010. November [cited 2014 Jul 12];63(11):1179–94. Available from: http://www.sciencedirect.com/science/article/pii/S0895435610001733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cella D, Gershon R, Lai J, Choi S. The future of outcomes measurement: item banking, tailored short-forms, and computerized adaptive assessment. Qual Life Res. 2007;16(1):133–41. [DOI] [PubMed] [Google Scholar]
- 7.Fries JF, Bruce B, Bjorner J, Rose M. More relevant, precise, and efficient items for assessment of physical function and disability: moving beyond the classic instruments. Ann Rheum Dis [Internet]. 2006. November [cited 2014 Jul 19];65 Suppl 3:iii16–21. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1798376&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fries J, Rose M, Krishnan E. The PROMIS of better outcome assessment: responsiveness, floor and ceiling effects, and Internet administration. J Rheumatol [Internet]. 2011. August [cited 2014 Jul 11];38(8):1759–64. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3827974&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fries JF, Krishnan E, Rose M, Lingala B, Bruce B. Improved responsiveness and reduced sample size requirements of PROMIS physical function scales with item response theory. Arthritis Res Ther [Internet]. 2011. January [cited 2014 Jul 12];13(5):R147 Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3308075&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rose M, Bjorner JB, Gandek B, Bruce B, Fries JF, Ware JE. The PROMIS Physical Function item bank was calibrated to a standardized metric and shown to improve measurement efficiency. J Clin Epidemiol [Internet]. 2014. May [cited 2014 Jul 11];67(5):516–26. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24698295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dworkin RH, Turk DC, Farrar JT, Haythornthwaite J a, Jensen MP, Katz NP, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain [Internet]. 2005. January [cited 2014 Oct 11];113(1–2):3–19. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15621359 [DOI] [PubMed] [Google Scholar]
- 12.Paterson DH, Warburton DE. Physical activity and functional limitations in older adults: a systematic review related to Canada’s Physical Activity Guidelines. Int J Behav Nutr Phys Act [Internet]. 2010;7:38 Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2882898&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruce B, Fries J, Lingala B, Hussain YN, Krishnan E. Development and assessment of floor and ceiling items for the PROMIS physical function item bank. Arthritis Res Ther [Internet]. 2013. January [cited 2014 Jul 19];15(5):R144 Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3978724&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fries JF, Witter J, Rose M, Cella D, Khanna D, Morgan-DeWitt E. Item response theory, computerized adaptive testing, and PROMIS: assessment of physical function. J Rheumatol [Internet]. 2014. January [cited 2014 Jul 19];41(1):153–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24241485 [DOI] [PubMed] [Google Scholar]
- 15.Crins MHP, Terwee CB, Klausch T, Smits N, de Vet HCW, Westhovens R, et al. The Dutch-Flemish PROMIS Physical Function Item Bank Exhibited Strong Psychometric Properties in Patients with Chronic Pain. J Clin Epidemiol. 2017;March 28. [DOI] [PubMed] [Google Scholar]
- 16.Oude Voshaar M a. H, ten Klooster PM, Glas C a. W, Vonkeman HE, Taal E, Krishnan E, et al. Validity and measurement precision of the PROMIS physical function item bank and a content validity–driven 20-item short form in rheumatoid arthritis compared with traditional measures. Rheumatology [Internet]. 2015;kev265. Available from: http://www.rheumatology.oxfordjournals.org/lookup/doi/10.1093/rheumatology/kev265 [DOI] [PubMed] [Google Scholar]
- 17.Oude Voshaar MAH, ten Klooster PM, Glas CAW, Vonkeman HE, Taal E, Krishnan E, et al. Calibration of the PROMIS physical function item bank in Dutch patients with rheumatoid arthritis. PLoS One [Internet]. 2014. January [cited 2014 Jul 19];9(3):e92367 Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3956923&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terwee CB, Roorda LD, de Vet HCW, Dekker J, Westhovens R, van Leeuwen J, et al. Dutch-Flemish translation of 17 item banks from the Patient-Reported Outcomes Measurement Information System (PROMIS). Qual Life Res [Internet]. 2014. August [cited 2014 Jul 12];23(6):1733–41. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24402179 [DOI] [PubMed] [Google Scholar]
- 19.Fries J. The Health Assessment Questionnaire (HAQ) and the Improved HAQ. Standford Univ Sch Med Div Immunol Rheumatoloy. 2009;
- 20.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care [Internet]. 1992. June;30(6):473–83. Available from: http://www.ncbi.nlm.nih.gov/pubmed/1593914 [PubMed] [Google Scholar]
- 21.Beaton DE, Katz JN, Fossel AH, Wright JG, Tarasuk V, Bombardier C. Measuring the whole or the parts? Validity, reliability, and responsiveness of the Disabilities of the Arm, Shoulder and Hand outcome measure in different regions of the upper extremity. J Hand Ther [Internet]. 2001. [cited 2014 Jul 14];14(2):128–46. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11382253 [PubMed] [Google Scholar]
- 22.ten Klooster PM, Vonkeman HE, Taal E, Siemons L, Hendriks L, de Jong AJL, et al. Performance of the Dutch SF-36 version 2 as a measure of health-related quality of life in patients with rheumatoid arthritis. Health Qual Life Outcomes. 2013;11:77 doi: 10.1186/1477-7525-11-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cuperus N, Mahler EA, Vliet Vlieland TP, Hoogeboom TJ, van den Ende CH. Measurement properties of the Health Assessment Questionnaire Disability Index for generalized osteoarthritis. Rheumatology (Oxford). 2015;54(5):821–6. [DOI] [PubMed] [Google Scholar]
- 24.ten Klooster PM, Taal E, van de Laar MA. Rash analysis of the Dutch Health Assessment Questionnaire disability index and the Health Assessment Questionnaire II in patients with rheumatoid arthritis. Arthritis Rheum. 2008;59(12):1721–8. doi: 10.1002/art.24065 [DOI] [PubMed] [Google Scholar]
- 25.Reeve BB, Hays RD, Bjorner JB, Cook KF, Crane PK, Teresi JA, et al. Psychometric Evaluation and Calibration of Health-Related Quality of Life Item Banks: plans for the Patient-Reported Outcomes Measurement Information System (PROMIS). Med Care [Internet]. 2007;45(5):22–31. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17443115 [DOI] [PubMed] [Google Scholar]
- 26.Rosseel Y. Lavaan: An R Package for Structural Equation Modeling. J Stat Softw. 2012;48(2):1–36. [Google Scholar]
- 27.R-Software. www.r-project.org. 2014.
- 28.Satorra A, Bentler PM. A scaled difference chi-square test statistic for moment structure analysis. Psychometrika. 2001;66(4):507–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muthén BO, Du Toit SHC, Spisic D. Robust Inference using Weighted Least Squares and Quadratic Estimating Equations in Latent Variable Modeling with Categorical and Continuous Outcomes [Internet]. Psychometrika. 1997. p. 49 Available from: http://pages.gseis.ucla.edu/faculty/muthen/articles/Article_075.pdf [Google Scholar]
- 30.Bentler PM. Comparative fit indexes in structural models. Psychol Bull. 1990;107(2):238–46. [DOI] [PubMed] [Google Scholar]
- 31.Steinberg L, Thissen D. Uses of item response theory and the testlet concept in the measurement of psychopathology. Psychol Methods. 1996;1:81–97. [Google Scholar]
- 32.Mokken RJ. A Theory and Procedure of Scale Analysis: With Applications in Political Research [Internet]. The Hague: Mouton; 1971. [cited 2014 Jul 12]. http://books.google.com/books?hl=nl&lr=&id=vAumIrkzYj8C&pgis=1 [Google Scholar]
- 33.Sijtsma K, Emons WHM, Bouwmeester S, Nyklícek I, Roorda LD. Nonparametric IRT analysis of Quality-of-Life Scales and its application to the World Health Organization Quality-of-Life Scale (WHOQOL-Bref). Qual life Res [Internet]. 2008. March [cited 2014 Nov 13];17(2):275–90. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2238782&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sijtsma K, Molenaar I. Introduction to nonparametric item response theory. Thousand Oaks: Sage; 2002; [Google Scholar]
- 35.Van der Ark L. Mokken scale analysis in R. J Stat Softw [Internet]. 2007;20(11):1–19. Available from: http://www.jstatsoft.org/v20/i11/paper [Google Scholar]
- 36.Scientific Software International. IRTPRO: user’s guide. Lincolnwood Scientific Software International. 2012.
- 37.Embretson SE, Reise SP. Item Response Theory for Psychologists [Internet]. Mahwah, NJ: Lawrence Erlbaum Associates; 2000. [cited 2014 Jul 12]. http://books.google.es/books/about/Item_Response_Theory_for_Psychologists.html?hl=es&id=rYU7rsi53gQC&pgis=1 [Google Scholar]
- 38.Holland P, Wainer H. Differential Item Functioning [Internet]. Hillsdale, NJ: Lawrence Erlbaum Associates; 1993. [cited 2014 Jul 12]. http://books.google.com/books?hl=nl&lr=&id=6YAXJfswvfYC&pgis=1 [Google Scholar]
- 39.Crane PK, Gibbons LE, Jolley L, van Belle G. Differential item functioning analysis with ordinal logistic regression techniques. DIFdetect and difwithpar. Med Care [Internet]. 2006. November [cited 2014 Jul 12];44(11 Suppl 3):S115–23. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17060818 [DOI] [PubMed] [Google Scholar]
- 40.Choi SW, Gibbons LE, Crane PK. Logistic Ordinal Regression Differential Item Functioning using IRT, version 0.3–3 [Internet]. 2016. https://cran.r-project.org/web/packages/lordif/lordif.pdf
- 41.Choi SW, Gibbons LE, Crane PK. Lordif: An R Package for Detecting Differential Item Functioning Using Iterative Hybrid Ordinal Logistic Regression/Item Response Theory and Monte Carlo Simulations. J Stat Softw [Internet]. 2011. March 1 [cited 2014 Jul 12];39(8):1–30. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3093114&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amtmann D, Cook KF, Jensen MP, Chen W-H, Choi S, Revicki D, et al. Development of a PROMIS item bank to measure pain interference. Pain [Internet]. 2010. July [cited 2014 Jul 11];150(1):173–82. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2916053&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Revicki DA, Chen W-H, Harnam N, Cook KF, Amtmann D, Callahan LF, et al. Development and psychometric analysis of the PROMIS pain behavior item bank. Pain [Internet]. 2009. November [cited 2014 Jul 12];146(1–2):158–69. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2775487&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Magis D, Raiche G. Random Generation of Response Patterns under Computerized Adaptive Testing with the R Package catR. J Stat Softw. 2012;48(8):1–31. [Google Scholar]
- 45.Magis D, Barrada JR. Computerized Adaptive Testing with R : Recent Updates of the Package catR. J Stat Softw [Internet]. 2017;76(Code Snippet 1):1–19. Available from: http://www.jstatsoft.org/v76/c01/ [Google Scholar]
- 46.Barten D, Koppes L. Zorg door de fysiotherapeut: Jaarcijfers 2015 en trendcijfers 2011–2015. Nivel. 2016.
- 47.Hung M, Clegg DO, Greene T, Saltzman CL. Evaluation of the PROMIS physical function item bank in orthopaedic patients. J Orthop Res [Internet]. 2011. June [cited 2014 Jul 19];29(6):947–53. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21437962 [DOI] [PubMed] [Google Scholar]
- 48.Paz SH, Spritzer KL, Morales LS, Hays RD. Evaluation of the Patient-Reported Outcomes Information System (PROMIS(®)) Spanish-language physical functioning items. Qual Life Res [Internet]. 2013. September [cited 2014 Jul 11];22(7):1819–30. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23124505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paz SH, Spritzer KL, Morales LS, Hays RD. Age-related Differential Item Functioning for the Patient-Reported Outcomes Information System (PROMIS®) Physical Functioning Items. Prim Heal care open access [Internet]. 2013. March 29 [cited 2014 Jul 19];3(131). Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3774524&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hancock K, Glass N, Antony C, Hettrich C, Albright J, Amendola A, et al. Performance of PROMIS for Healthy Patients Undergoing Meniscal Surgery. J Bone Jt Surg Am. 2017;99(11):954–8. [DOI] [PubMed] [Google Scholar]
- 51.Hung M, Saltzman C, Greene T, Voss M, Bounsanga J, Gu Y, et al. Evaluating instrument responsiveness in joint function: The HOOS JR, the KOOS JR, and the PROMIS PF CAT. J Orthop Res. 2017; [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are available via Figshare; https://doi.org/10.6084/m9.figshare.5711971.