Abstract
The majority of our knowledge of avian energetics is based on studies of birds from temperate and high latitudes. Using the largest existing sample of wild-caught Old World tropical species, we showed that birds from Southern Vietnam had lower basal metabolic rate (BMR) than temperate species. The strongest dissimilarity between tropical and temperate species was the low scaling exponent in the allometric relation between BMR and body mass in tropical birds (the regression slope was 0.573). The passerine migrants to temperate and high latitudes had higher BMR than tropical sedentary passerines. Body mass alone accounted for 93% of the variation in BMR (body mass ranged from 5 to 252 g). Contrary to some other studies, we did not find evidence besides the above mentioned that phylogeny, taxonomy, behavior, or ecology have a significant influence on BMR variation among tropical birds.
Keywords: allometry, BMR, body mass, energy metabolism, tropical birds
The notable differences in numerous life-history and other traits between tropical and temperate birds led to the notion that tropical birds have a “slow pace of life”: they live longer, have fewer offspring, and invest more resources in self-maintenance, whereas temperate birds have high rates of mortality and invest more resources in reproduction (Williams et al. 2010; Jimenez et al. 2014a). The diversification of life histories in animals is limited by physiological mechanisms (Ricklefs and Wikelski 2002). Physiological differences between birds of high and low latitudes remain insufficiently known, although some recent studies have made great progress in this respect (Tieleman et al. 2005, 2006; Wiersma et al. 2007a, 2007b; Williams et al. 2010; Wiersma et al. 2012; Jimenez et al. 2013, 2014b; Jimenez and Williams 2014).
The most commonly examined comparative measure of the metabolic rate of endotherms is basal metabolic rate (BMR), which is the minimum metabolic rate of an adult normothermic animal in postabsorptive state and in nonreproductive phase, at rest and at temperatures within the thermoneutral zone (TNZ; McNab 1997). BMR is the lowest cost of body maintenance and, therefore, an important characteristic of physiological heterogeneity of communities and separate populations of endotherms. To date, there is much evidence supporting the dependence of the variation in avian BMR on many factors, such as season, temperature, habitat, phylogeny, behavior, etc. (McNab 2012). However, the importance of each factor with respect to the others often is not clear. For example, it is hard to separate the effects of temperature and pace of life (Londoño et al. 2015; Bech et al. 2016) or temperature and migratory tendency (Jetz et al. 2008).
Several physiological characteristics, which may relate to BMR variation, are different in tropical and temperate species. In particular, tropical birds have a smaller organ size, lower flight muscle, and feather mass (Williams et al. 2010; Wiersma et al. 2012), smaller muscle fiber size, and increased costs of maintaining muscle mass (Jimenez and Williams 2014), lower daily metabolized energy in nestlings (Bryant and Hails 1983), lower summit metabolism and field metabolic rate (Wagner et al. 2013), lower peak metabolic rate (Wiersma et al. 2007a, 2007b), and cellular metabolic rate (Jimenez et al. 2014b). At least some of these features may be considered as the reasons for BMR reduction in tropical birds. Although avian BMR was studied in many works over the last century (Downs and Brown 2012), the number of studies on free-living birds in tropics is much smaller compared to temperate zone. There are only 3 studies on BMR of tropical birds containing adequate sample sizes of individuals and/or species. Two of them were done in the Neotropical region: the study by Wiersma et al. (2007b), based on 69 species from a lowland rainforest in Panama, and the very recent work by Londoño et al. (2015), based on 253 species from forests in Peru. The only comprehensive study of energetics in tropical birds of the Old World is the recent monograph by McNab (2013), based on 45 free-living New Guinea species and 32 species obtained from captivity.
Some studies on avian energetics have found a reduced rate of metabolism in tropical birds (Weathers 1977; Hails 1983; Klaassen 1995; Wikelski et al. 2003; Tieleman et al. 2006; McNab 2009), but some have found it to be similar to the metabolic rate of birds from higher latitudes or that the difference depends on species and their ecology (Scholander et al. 1950; MacMillen 1974; Vleck and Vleck 1979; Weathers 1979,1997; Pettit et al. 1985; Bennett and Harvey 1987; McNab 2013). Nevertheless, all phylogenetically controlled multispecies studies have confirmed low metabolic rate in tropical birds (Wiersma et al. 2007a, 2007b; Londoño et al. 2015; Bech et al. 2016). The substantial studies by Wiersma et al. (2007a, 2007b) demonstrated lower basal and peak metabolic rates in Neotropical birds, which perfectly fit the concept of slow “intensity of life” in tropical species. Londoño et al. (2015) found that BMR of temperate breeders is on average 16.4% higher than that of Neotropical species. At the same time, McNab (2013) has showed that lowland tropical passerines from his and Wiersma et al. (2007b) datasets, on average, had a higher BMR than expected from a general avian-scaling curve. However, unlike studies on Neotropical birds, McNab (2013) intentionally did not correct his analyses for phylogeny. In our study, we wanted to investigate whether the Old World birds follow the trend observed in the New World.
The higher BMR of passerines was one of the main taxonomic differences observed in the energetics of endotherms (e.g., Lasiewski and Dawson 1967; Aschoff and Pohl 1970; Kendeigh et al. 1977; Bennett and Harvey 1987; Gavrilov 1997; Jetz et al. 2008; McNab 2009, 2015a; Bech et al. 2016). However, with the exceptions of Wiersma et al. (2007b), McNab (2013), Londoño et al. (2015), and Bech et al. (2016), all comparisons between passerines and non-passerines were done using datasets substantially amassed on metabolic rates of temperate species. On the other hand, a comprehensive phylogenetically controlled analysis by Jetz et al. (2008) revealed that BMR in migrants is much higher than in nonmigrant birds. These researchers partially explained high BMR in long-distance migrants with lower temperatures on their breeding grounds, but the BMR data for migrants on their tropical wintering grounds are too scarce to test their hypothesis. In our study, we partly fill this gap. We predict that long-distance migration requires higher metabolic capacity (e.g., based on higher mass of metabolically active tissues), which should be reflected in higher BMR of tropical migrants compared to residents. Since most of the temperate passerines are migratory species, we think that one of the main reasons of increased BMR in the temperate passerines is their migratory style of life. Therefore, we hypothesized that the energetic asymmetry between passerines and non-passerines should be less pronounced in tropical residents than in breeding birds of higher latitudes.
Together with migratory tendency and taxonomy, some ecological factors may have a great impact on animal energetics (McNab 1988, 2009, 2015b). Among these factors we tested those that were shown to have influence on BMR in tropical birds: diet (arthropods, seeds, nectar, etc.) and some characteristics of behavior, which are important in terms of risk of overheating (habitat, foraging in the sun or shade, etc.).
In this study, we use the largest original dataset on BMR of Old World wild-caught tropical birds to reveal features of tropical birds. Specifically, we wish to: (1) estimate the influence of phylogeny, ecology, and migratory tendency on their BMRs; and (2) compare BMR of tropical and temperate birds.
Materials and Methods
Capturing birds
We caught birds using mist nets in Cat Tien National park (Vườn quốc gia Cát Tiên), Southern Vietnam (11°25′N, 107°25′E; elevation ∼120–140 m) in a deciduous tropical forest with strongly degraded vegetation (Vandekerkhove et al. 1993; Blanc et al. 2000). Birds were captured during 7–26 May 2011, 10–18 April 2012, and 15 March–11 May 2013. We weighed birds just after the capture. Juvenile birds and females with a strongly pronounced brood patch were released immediately after weighing and did not participate in BMR measurements. We housed birds in soft mesh cages and provided water and food ad libitum. Birds were kept in cages for 10 h at maximum (mean is 5.5 h). Some birds (such as broadbills, pittas, doves, kingfishers, woodpeckers, and drongos) refused to eat and were force-fed every 2 h with zophobas, mealworms, crickets, mango fruits, and dry feed Padovan (Valman s.r.l., Italy). The last feeding was done before 5 PM. We weighed all captured birds every 1–2 h, depending on the size of species. If a bird’s weight had dropped noticeably (∼10% of body-weight loss), we immediately released that individual.
We obtained a total of 368 BMR measurements (equal to the number of individuals) during 79 nights. The number of individuals per species ranged from 1 to 47 (average of 5.6). Our BMR database includes 66 species from 29 families belonging to 9 orders (Supplementary Table S1). Latin and common names of species were taken from IOC World Bird Names v. 7.1 (http://www.worldbirdnames.org/).
Measurements of BMR
BMR of birds was estimated during the night after capture by flow-through respirometry (see Supplementary Materials for details about respirometry equipment, calibration, leak testing, etc.). At 6 PM, after sunset, we placed up to 7 (average of 4.8) birds inside cylindrical polypropylene chambers (1.3–2.7 L). We put metabolic chambers in boxes made from sound-proofing and heat-insulating foam plastic. We used 20 L chambers for the largest species (Amaurornis phoenicurus, Arborophila chloropus, and Centropus sinensis). The rates of gas exchange were measured until 6:00 AM. We did not use thermostats because the ambient temperature in the laboratory during measurements was very stable (recorded using type T thermocouple probe [Sable Systems International, USA]). The temperature inside the chambers was within 28–30°C (recorded every 20 min with iButton thermologgers), which should be within the TNZ of most tropical birds (McNab 2013).
We used 8 independent membrane pumps (AC-500, Resun, China) to push the outdoor air through columns containing self-indicating granulated fine-pored silica gel to remove water vapor and then into metabolic chambers with birds. The flow rate was set at 250–1,200 mL/min (depending on the size of measured species). We used a custom built valve system, which alternately routed the air stream from each chamber with birds and an empty reference chamber (for baselining) to the FoxBox-C Respirometry System (Sable Systems International, USA), which included build-in air filters, mass flowmeter, flow controller, membrane pump, O2 and CO2 analyzers. After the air left the valve system, it was routed through a small column (V = 45–70 mL) with Drierite® 10–20 mesh absorbent (W.A. Hammond Drierite Co. Ltd, USA) and then through mass flowmeter into O2/CO2 gas analyzers. Birds were measured alternately in cycles. The time of measurement for each bird within a cycle and the length of each cycle depended on the number of birds within a night session and were at average of 25 and 139 min, correspondingly. Baselining was performed 1–3 times during each cycle, depending on the number of measured birds. It was done in such a way that measurements of each bird or at least each second bird adjoined with baselining. Around 6:00 AM, birds were removed from the chambers, weighed with a precision of 0.1 g, and released.
To estimate BMR we selected the lowest stable part of the curve (average of 11 min) over the entire night. We rejected data from individuals that did not become quiescent. The volume of consumed oxygen was calculated from fractional concentrations of O2 and CO2 according to the principle of Haldane transformation (Luft et al. 1973; Wagner et al. 1973; Wilmore and Costill 1973):
where VE is the flow of dry air out of the animal chamber in volume per time unit, FI is volume fractional concentration of respective gas in dry inlet air, FE is volume fractional concentration of respective gas in dry outlet air. We calculated the respiratory quotient (VCO2 produced/VO2 consumed) and used it to convert the volume of oxygen consumption (VO2) to the values of energy expenditure (kJ/day) using the equation 5 from Lusk (1924). Nevertheless, the mean BMR was less than 0.01% different from the value calculated through commonly used coefficient 19.8 J/mL O2 (Gessaman and Nagy 1988).
Categorization of ecological and behavioral factors
Following McNab (2009, 2013, 2015b), we used analysis of covariance (ANCOVA) to determine which factors had an impact on BMR. We used the following taxonomic, ecological, and behavioral rankings: passerine/non-passerine and oscine/suboscine dichotomies, migratory behavior (migrant/resident), habitat type (exposed, forest or intermediate), foraging substrate, feeding in the shade or sun, food habits (diet). We categorized species to ecological groups according to personal observations of our colleagues, I.V. Palko and M.V. Kalyakin (Supplementary Table S1), who conduct long-term observations on ecology and behavior of tropical birds in the study site.
Exposed (open) habitat type did not always equal to feeding in the sun, as some species from exposed habitats feed in the shade of thick grass or shrub (e.g., Acrocephalus sp., Locustella lanceolata, Phragmaticola aedon, Timalia pileata). Migratory species only included migrants to temperate and high latitudes (54 species were residents and 12 were migrants). All tropical breeders were categorized as residents (sedentary birds), including Pitta moluccensis, which performs winter migration to the Malay Archipelago.
Statistical analysis
All scaling exponents in allometric equations in our study were based on ordinary least squares (OLS) regressions, unless specifically mentioned. The body mass and BMR data were log10-transformed before analysis to account for allometric scale. To test for difference in intercepts of log(BMR) ∼ log(M) regressions in different groups of birds (categorical predictor, e.g., residents/migrants), we used ANCOVA with log(BMR) as dependent variable and log(M) as covariate. We tested the differences between observed and predicted values of BMR using a t-test (predicted values were calculated using different allometric equations from Table 1). The differences between observed slopes and theoretical slopes of 2/3 and 3/4 were tested with Welch’s t-test. To test for difference in slopes of 2 regression lines, we tested the model with the interaction term of log(M) and the grouping factor versus the model without interaction using function “ANOVA.” All regression residuals were normally distributed, which was checked using Shapiro–Wilk test. All analyses were conducted in R (R Core Team 2016). The significance level was set as α = 0.05. Standard errors of intercepts and slopes are shown in equations in brackets.
Table 1.
The average ratio of observed whole-organism BMR of tropical resident birds of different taxonomic groups from Vietnam to predicted BMR, estimated using allometric relationships between BMR and body mass from literature on corresponding groups
| Literature source | a | b | Avian group | Ratio (%) |
|---|---|---|---|---|
| This study | 195.75 | 0.573 | Tropical (including migrants) | 98.8 |
| This study | 200.18 | 0.581 | Tropical (including migrants) (PC) | 99.4 |
| This study | 267.49 | 0.622 | Tropical migrants | 84.9** |
| This study | 202.80 | 0.589 | Tropical residents | 100.0 |
| Brody and Proctor (1932) | 372.63 | 0.640 | Temperate | 65.6** |
| King and Farner (1961) | 335.36 | 0.659 | Temperate | 78.0** |
| King and Farner (1961) | 311.08 | 0.744 | Large temperate (M > 125 g) | 80.9* |
| Lasiewski and Dawson (1967) | 361.74 | 0.668 | Temperate | 74.7** |
| Lasiewski and Dawson (1967) | 540.10 | 0.724 | Temperate passerines | 64.6** |
| Lasiewski and Dawson (1967) | 327.83 | 0.723 | Temperate non-passerines | 90.8* |
| Zar (1969) | 324.06 | 0.739 | Temperate | 107.7 |
| Zar (1969) | 473.11 | 0.632 | Temperate passerines | 51.2** |
| Zar (1969) | 319.03 | 0.743 | Temperate non-passerines | 98.8 |
| Aschoff and Pohl (1970) | 480.64 | 0.726 | Temperate passerines | 73.2** |
| Aschoff and Pohl (1970) | 307.73 | 0.734 | Temperate non-passerines | 99.8 |
| Bennett and Harvey (1987) | 240.93 | 0.670 | All | 112.9** |
| Daan et al. (1990) | 361.32a | 0.677 | All | 77.2** |
| Reynolds and Lee (1996) | 343.17 | 0.670 | All | 79.3** |
| Reynolds and Lee (1996) | 339.25 | 0.635 | All (PC) | 70.8** |
| Gavrilov (1997) | 435.08 | 0.700 | Passerines in summer | 72.9** |
| Gavrilov (1997) | 349.65 | 0.710 | Non-passerines in summer | 82.0** |
| Tieleman and Williams (2000) | 308.32 | 0.638 | All | 78.8** |
| Tieleman and Williams (2000) | 279.90 | 0.677 | All (PC) | 99.7 |
| Frappell et al. (2001) | 471.39a | 0.680 | Basically temperate | 59.8** |
| Frappell et al. (2001) | 445.36a | 0.680 | Basically temperate (PC) | 63.3** |
| Rezende et al. (2002) | 329.64a | 0.635 | All | 72.9** |
| Rezende et al. (2002) | 399.98a | 0.721 | All (PC) | 81.7** |
| McKechnie and Wolf (2004) | 303.75 | 0.669 | All | 89.3** |
| McKechnie and Wolf (2004) | 243.51 | 0.677 | All (PC) | 114.6** |
| Speakman (2005) | 350.41 | 0.671 | All | 77.9** |
| McKechnie et al. (2006) | 315.1 | 0.744 | All wild-caught birds (PC) | 114.6* |
| White et al. (2006) | 243.35 | 0.640 | All | 100.5 |
| Wiersma et al. (2007b) | 307.97 | 0.644 | Tropical passerines | 82.4** |
| Wiersma et al. (2007b) | 262.73 | 0.644 | Tropical non-passerines | 90.7* |
| McNab (2009) | 314.47 | 0.652 | All | 81.2** |
| McNab (2009) | 429.69 | 0.713 | Passerines | 77.7** |
| McNab (2009) | 317.40 | 0.724 | Non-passerines | 94.1 |
| McNab (2009) | 451.02 | 0.708 | Temperate passerines | 71.6** |
| McNab (2013) | 234.97 | 0.581 | Tropical (including non-residents) | 84.7** |
| McNab (2013) | 245.39 | 0.634 | Tropical residents | 97.6 |
| McNab (2013) | 293.61 | 0.686 | Tropical passerines | 102.1 |
| McNab (2013) | 167.36 | 0.686 | Tropical non-passerines | 160.1** |
| Londoño et al. (2015) | 220.73a | 0.551 | Tropical residents | 82.3** |
| Londoño et al. (2015) | 193.95a | 0.543 | Tropical residents (PC) | 91.2** |
| Londoño et al. (2015) | 300.48a | 0.644 | Tropical resident passerines | 86.0** |
| Londoño et al. (2015) | 283.25a | 0.644 | Tropical resident non-passerines | 84.1** |
| Londoño et al. (2015) | 298.51a | 0.627 | Tropical resident passerines (PC) | 81.0** |
| Londoño et al. (2015) | 277.73a | 0.701 | Tropical resident non-passerines (PC) | 100.7 |
Notes: a is the allometric coefficient and b is the scaling exponent from equation BMR = aMb, where BMR is basal metabolic rate in kJ/day and M is body mass in kg. PC means “phylogenetically corrected.” Temperate birds here include also species from high latitudes.
aMarks recalculations based on equation 1 L of O2 = 20.083 kJ of energy (Schmidt-Nielsen 1997).
P < 0.05;
P < 0.001.
Phylogenetic analysis
The phylogenetic relationships between studied species were extracted from the BirdTree.org database (http://www.birdtree.org) using the study by Hackett et al. (2008) as the backbone for phylogenetic reconstruction. A total of 1,000 trees were downloaded from BirdTree.org, which is enough to obtain robust phylogenies (Rubolini et al. 2015). The resultant MCC consensus tree (Supplementary Figure S1) was obtained by Treeannotator of BEAST 1.8.2 (Drummond et al. 2012).
Phylogenetic signal in traits was estimated with Pagel’s λ (Pagel 1999; Freckleton et al. 2002) using function “phylosig” from package “phytools” (Revell 2012). We used phylogenetic generalized least squares model (PGLS) to take the phylogenetic signal into account in allometric analysis (Grafen 1989; Freckleton et al. 2002). We did not find any phylogenetic signal in the residual variation of the regression of log(BMR) on log(M) (i.e., mass-independent BMR). Consequently, the OLS method was more suitable for fitting the regression models than PGLS (Revell 2010). We provided several phylogenetic regressions to show that regression coefficients from PGLS were very close to those that were obtained by OLS. We fit PGLS via maximum likelihood (ML) approach using the function “gls” from package “nlme” (Pinheiro et al. 2014). We specified 4 different ways in which the tree structure is expected to affect the covariance in trait values across species (Brownian motion [BM], Grafen’s ρ, Pagel’s λ, and Ornstein–Uhlenbeck [OU] models of evolution) using package “ape” (Paradis et al. 2004).
We estimated the effect of within-species sampling (intraspecific variation) on the strength of phylogenetic signal using function “phylosig” from package “phytools” (Revell 2012). We also used function “pgls.Ives” from the same package (BM is assumed) to incorporate sampling error in the estimation of species means by fitting the phylogenetic reduced major axis (RMA) regression of Ives et al. (2007).
Results
The relation between BMR and body mass
We found the mass exponent in the allometric relation between BMR and body mass in tropical birds to be very small (Figure 1). The OLS regression between log(BMR) and log(M) was log(BMR) = 0.573[0.029] + 0.573[0.019]*log(M) (R2adj = 0.930). The slope value (b = 0.57) differed from the theoretical slopes b = 0.75 (Kleiber’s law) and b = 0.67 (Rubner’s rule) (P < 0.001). If we included in the analysis only species with more than 3 individuals [n ≥ 3 is requirement from McKechnie and Wolf (2004)], the equation was log(BMR) = 0.598[0.040] + 0.546[0.030]*log(M) (R2adj = 0.928). The slope of the regression in the reduced sample was still significantly lower than 0.67 (P < 0.001).
Figure 1.
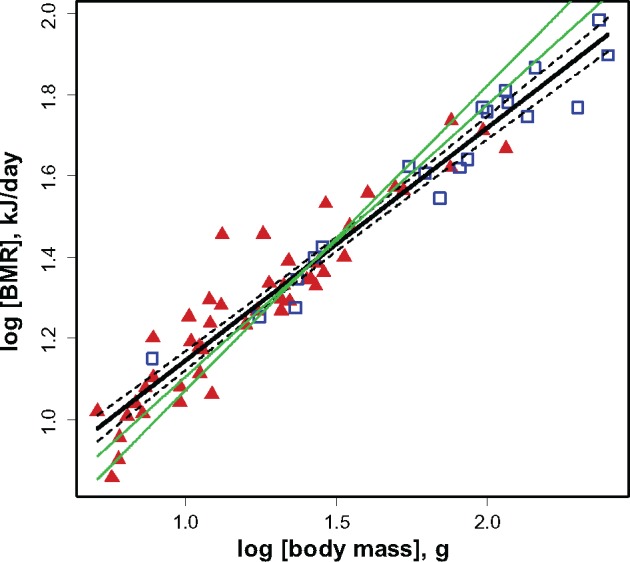
The relationship between BMR and body mass in tropical birds of Southern Vietnam (black thick solid line). Red solid triangles indicate passerines; blue open squares indicate non-passerines. Green thin solid lines indicate theoretical slopes b = 0.75 (Kleiber’s law) and b = 0.67 (Rubner’s rule). Black thin dashed lines indicate 95% confidence interval of the regression.
Phylogenetic analysis
The coefficients of log(BMR) ∼ log(M) regression did not change significantly after taking phylogeny into account. We did not find a phylogenetic signal in mass-independent BMR of tropical birds from our sample.
The Pagel’s λ for log(M), log(BMR), and mass-independent BMR were 0.989 (P < 0.001), 0.943 (P < 0.001), and 0.00007 (P = 1.00), respectively (P values indicate the significance of the difference from zero). The Pagel’s λ for log(M) and log(BMR) were not significantly different from unity (P > 0.3), for example, we cannot confidently distinguish our estimated phylogenetic signal in those traits from BM.
Comparison of different evolutionary models using the general sample of migrant and resident species revealed that the best fit was the model with Pagel’s covariance structure (AIC = −172.32). It was followed by the OU model (AIC = −155.59) and covariance under BM (AIC = −137.20). The phylogenetic regression from the model, which finds and fits the ML value for Pagel’s λ, was log(BMR) = 0.557[0.047] + 0.581[0.025]*log(M) (R2 = 0.956). The best-fit value of λ was determined to be very close to zero by ML and not different from zero (P = 1). The phylogenetic regression from the OU model was log(BMR) = 0.573[0.030] + 0.573[0.020]*log(M), α (strength of the evolutionary constraint) was equal to 1.00. Assuming the BM model of evolution (Pagel’s λ fixed to unity), the phylogenetic regression was log(BMR) = 0.529[0.089] + 0.589[0.034]*log(M).
We repeated analysis with all branches of the tree set to unity. The differences in phylogenetic signal were negligible from the above results. The best-fit model (with Pagel’s covariance structure) gave the phylogenetic regression log(BMR) = 0.541[0.045] + 0.588[0.024]*log(M) (R2 = 0.952). Incorporating the standard errors for each species into the model led to considerable reduction of sample size, as 23 of 66 species were presented only by a single individual; however, the estimates of Pagel’s λ did not change substantially for all traits. The equation of the model, which took standard errors into account using Ives et al. (2007) regression method, was log(BMR) = 0.580 + 0.564*log(M).
The influence of taxonomy on BMR of tropical birds
We did not find differences in BMR between tropical passerine and non-passerine birds: log(BMR) ∼ log(M) regression lines of passerines and non-passerines did not differ in slopes (P > 0.5), nor in intercepts (P > 0.7). The corresponding OLS regressions were log(BMR) = 0.555[0.043] + 0.588[0.033]*log(M) and log(BMR) = 0.596[0.060] + 0.559[0.032]*log(M). When we excluded migratory species (all of them were passerines) from the analysis, absence of significant differences between passerines and non-passerines remained, and the equation for passerines changed to log(BMR) = 0.513[0.040] + 0.609[0.029]*log(M).
Oscine passerines did not differ from suboscines in both regression coefficients (P > 0.3 for the slopes, P > 0.5 for the intercepts), although suboscines were represented by only 4 species with similar body weights (52.7–115.3 g).
The influence of ecology and behavior on BMR
Our results suggest that residential birds have a lower BMR than long-distance migrants on their wintering grounds in tropics. The influence of all other ecological and behavioral factors was not significant (habitat, feeding in the shade or sun, diet, foraging substrate). To simplify the analysis, the categories of different factors were variously combined, but the adjusted R2 increased only by 0.011 at maximum. There was also no correlation between mean capture day and BMR (Spearman’s ρ: P > 0.8).
There was no difference between resident (n = 54) and migratory (n = 12) species in slopes (P = 0.75) of log(BMR) ∼ log (M) regressions, but migratory birds had a higher intercept (P = 0.014) (Figure 2). The OLS regressions for resident and migratory birds were log(BMR) = 0.539[0.028] + 0.589[0.018]*log(M) and log(BMR) = 0.563[0.160] + 0.622[0.145]*log(M), correspondingly. The difference in intercepts between migrants and residents only tended to be significant after removal of the barn swallow from the analysis (P = 0.077).
Figure 2.
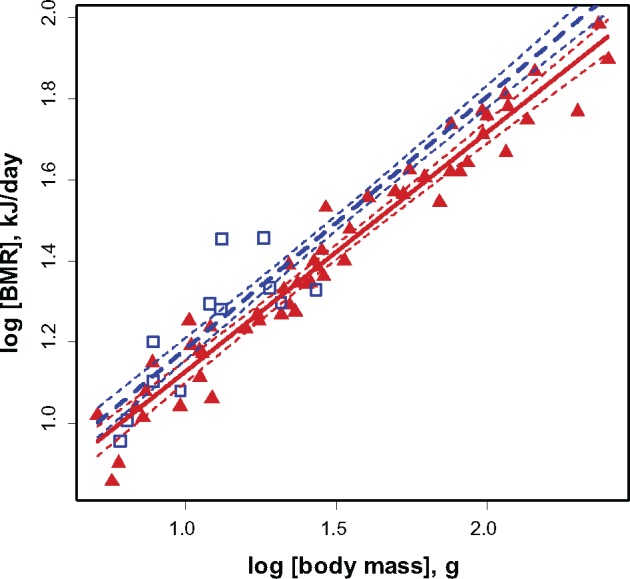
The relationship between BMR and body mass in resident (red thick solid line, red solid triangles) and migratory (blue thick dashed line, blue open squares) tropical birds of Southern Vietnam. Thin dashed lines indicate 95% confidence intervals of the corresponding regressions.
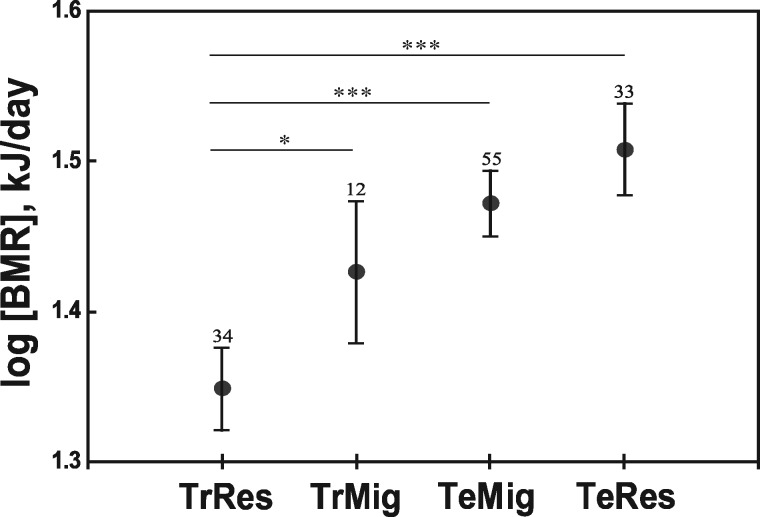
We also compared our raw BMR data with those from McNab’s (2009) comprehensive database (533 species) using ANCOVA. We found that tropical residents in our study had a lower BMR than both residents and migrants from middle/high latitudes on their breeding grounds (P < 0.001). That was true also for passerines (P < 0.001) and non-passerines (P < 0.01) separately. The migrants in our study did not differ in BMR from both migrants and residents on their breeding grounds in middle/high latitudes (P > 0.24). Among passerines, the BMR of long-distance migrants on their wintering grounds was intermediate between tropical and temperate residents (Figure 3).
Figure 3.
Mean BMRs of resident and migratory passerines in tropics and middle/high latitudes. TrRes and TrMig denote tropical residents and migrants, correspondingly (original data); TeMig and TeRes denote migrants and residents on their breeding grounds at middle/high latitudes [data from McNab (2009)]. Current effect of the ANCOVA model was F3, 129 = 23.55, P < 0.001 [log(body mass) was used as a covariate]. Vertical bars denote 95% confidence intervals (sample sizes are shown above them). *P < 0.05; ***P < 0.001.
BMR of tropical versus temperate birds
We implemented some commonly used allometric equations to calculate the predicted BMR of our birds using their mean body weight (Table 1). The considerable part of those equations (especially the old ones) were based on species from temperate areas, which allowed us to compare BMR of tropical birds with estimated BMR of temperate birds of the same body mass. On average, BMR of resident birds from Vietnam was 23% lower than the BMR of temperate species. Using raw data from McNab’s (2009) database, we found that resident birds from Vietnam had a 17.5% lower BMR than residents from middle/high latitudes (P < 0.001). Similarly, when comparing with raw data from other extensive studies on tropical birds (Wiersma et al. 2007b; McNab 2013; Londoño et al. 2015), we found that BMR of our tropical residents was lower than that in the cited works (P < 0.001).
Discussion
The relation between BMR and body mass
We found that the slope of relation between BMR and body mass in tropical birds is very gentle (b = 0.573) relative to temperate-zone birds (b > 0.63 in all studies, see Table 1). The slope of the log(BMR) ∼ log(body mass) regression did not change significantly in different ecological and taxonomic groups of birds, when sample size was reduced to exclude species presented only by few individuals, or after the inclusion of phylogeny into the model. The slopes of the BMR regressions of tropical birds in other studies were similarly gentle, at least within the range of body weights up to 1–2 kg: b = 0.527 [calculated from Vleck and Vleck (1979)], b = 0.542 [calculated from Hails (1983)], b = 0.508 [calculated from Tieleman et al. (2005)], b = 0.581 (McNab 2013), and b = 0.551 (Londoño et al. 2015). Wiersma et al. (2007b) reported a steeper slope, b = 0.644, for birds breeding in tropics. The low scaling exponent relating BMR to body mass in tropical birds could be of a heritable nature or could be an effect of phenotypic plasticity in response to different environmental conditions. For instance, McKechnie et al. (2006) found substantial differences in scaling coefficients for BMR in captive-raised and wild-caught birds, which most likely reflect phenotypic adjustments, and not genotypic divergence.
The allometric coefficient (a) in our study was also lower than reported in all allometric equations for temperate species (Table 1). Together with the very low scaling exponent (b), this indicates that both small and large tropical birds had lower BMR than temperate birds, but the difference in BMR between large temperate and tropical species is more pronounced than in small species. Tropical birds of Southern Vietnam spend a considerable portion of their lives at ambient temperatures that exceed 30°C in the shade, which is close to their body temperature. Moreover, during the lengthy rainy season they endure a very high temperature together with a very high humidity. Such conditions hamper heat dissipation both through nonevaporative and evaporative thermoconductance, especially in large animals due to their unfavorable surface/volume ratio (Schmidt-Nielsen 1997). This physiological constraint is readily apparent in some energetic models, which assume positive and proportional relationship between BMR and maximal aerobic capacity (Bennett and Ruben 1979) or maximal rate of daily work output (Gavrilov 1997). In the body of a large active animal, even a small portion of endogenous heat in addition to BMR may lead to overheating. From this point of view, the gentle allometric slope may reflect the adaptive decrease of BMR in large birds, which are more vulnerable to hot conditions than small birds. That is one of the speculative explanations of the low scaling exponent in tropical birds, although we did not have data to test it.
Phylogenetic analysis of BMR
Contrary to some other studies on energetics of tropical birds (Wiersma et al. 2007b; Londoño et al. 2015), we did not find a significant phylogenetic signal in mass-independent BMR. We demonstrated that this trait has evolved independently of phylogeny, for example, close relatives are not more similar than distant relatives. The best-fit phylogenetic regression of BMR on body mass was the model with Pagel’s covariance structure, but this result should be treated with caution. Due to the low values of α and λ, there was not enough power to adequately distinguish between BM and other evolution models. The different models of evolution demonstrated very close values of regression coefficients in our study, which is in agreement with special articles on this topic (Jhwueng 2013). Although our number of species (n = 66) is sufficient for phylogenetic analysis (Boettiger et al. 2012), the taxonomical and mass range was too limited to reliably extend this conclusion for all Southeast Asian tropical birds.
The lack of phylogenetic signal in BMR of our sample of birds may reflect their strong adaptation to constant environment in tropics: the body mass undertook the majority of BMR variation and leaved too little for the other traits, including phylogeny (see below). Another reason why interspecific differences in mass-independent BMR were not predicted by phylogeny may be related to methodological problems. One of them is possible inaccuracies in the phylogenetic tree, particularly in branch lengths. However, the effect of incorporating branch length information is negligible for most of phylogenetic tests, including our main test, Pagel’s λ (Freckleton et al. 2002; Münkemüller et al. 2012). The phylogenetic regressions are also robust to errors in tree topology and branch lengths (Stone 2011). The more plausible methodic cause of the lack of phylogenetic signal in BMR could be related to an insufficient sample size of different taxa. Our non-passerines were represented substantially by only Coraciiformes and Piciformes (Supplementary Table S1). Another possible bias could be an insufficient intraspecific sample size, which is known to have a great impact on phylogenetic analysis (Harmon and Losos 2005; Garamszegi and Møller 2010; Münkemüller et al. 2012). Of 66 species in our BMR database, 23 were represented only by 1 individual, 12 by 2 individuals, and 4 by 3 individuals.
The influence of taxonomy on BMR of tropical birds
One of the reasons why passerine birds are so widespread and numerous may be related to their high BMR in comparison with other taxa of endotherms (Gavrilov 1994, 1999a, 1999b, 2011, 2014). Some studies attributed this energetic asymmetry to the phylogenetic relationships between species (Reynolds and Lee 1996; Garland and Ives 2000; Rezende et al. 2002; McKechnie and Wolf 2004; Wiersma et al. 2007b), although all of them were based on outdated and inaccurate phylogeny from Sibley and Ahlquist (1990). In our study, tropical resident passerines did not show higher BMR than non-passerines. This result contradicts previous comparisons of BMR in these 2 groups of tropical birds (Wiersma et al. 2007b; McNab 2013; Londoño et al. 2015), but is in agreement with Gavrilov’s (2011, 2014) hypothesis. According to his conjecture, passerine birds had to reduce flight speed for settlement in forest habitats. Passerines of the temperate zone could not reduce speed by an increase in head resistance as tropical birds do, because it is not energetically compatible with long-distance migrations. They used other means to reduce flight speed, namely adopting a new style of flight, which consists of the active work of wings in down-stroke only. Such flight requires more energy, and migrating passerines obtained it by increasing their metabolic capacity, which was reflected in their BMR. Besides passerines there are 3 orders of birds with a similarly high BMR: Anseriformes, Procellariiformes, and Charadriiformes (McNab 2015a). All these groups are also characterized by high mobility and seasonal long-distance migration. It is likely that natural selection does not act directly on BMR, but on correlated energetic traits. Among those, the most ecologically important traits are daily energy expenditure, maximal aerobic metabolism, potential productive energy, and maximum rate of a daily locomotor activity (Bennett and Ruben 1979; Gavrilov 1997; Nilsson 2002; White and Seymour 2004). Interspecific studies in birds generally support the aerobic capacity model [see references in Swanson et al. (2012)], although the positive relationship between BMR and work output rate was not found in some studies (Ricklefs et al. 1996; Wiersma 2003; Welcker et al. 2015).
Wiersma et al. (2007b) have found that tropical passerines had higher BMR than tropical non-passerines, but this difference was not significant in phylogenetic models. McNab (2013) had found that passerines in his sample of tropical birds had a mean BMR that is 75% greater than non-passerines of the same mass. He did not use phylogenetic methods in this comparison and pointed at several important aspects against the use of phylogenetic correction before factor analysis. Using OLS models, Londoño et al. (2015) have found that passerine BMR averaged 12% higher than that of non-passerines. Moreover, the PGLS analysis showed a difference in these groups in slopes as well.
We did not find any differences in BMR in Old World suboscines comparing to oscine passerines. BMRs of all 4 suboscine species fell close to the general regression line. If tropical passerines had higher BMR than non-passerines, one could expect that more primitive Eurylaimides would have a lower BMR compared to oscines. Using a mixed sample of temperate and tropical birds, Swanson and Bozinovic (2011) found that oscines have higher summit metabolic rates (maximum rate of thermogenesis) than New World suboscines. This result favors the hypothesis, which explains competitive superiority of oscines by their higher metabolic capacities (Swanson and Bozinovic 2011).
The influence of ecology and behavior on BMR
We did not find any ecological or behavioral factors to have an impact on BMR, with the exception of migratory tendency. Our data suggest that migratory passerines from temperate and high latitudes on their wintering grounds in tropics have a higher BMR than tropical residents. This is in agreement with the results of a study comparing migrants and residents based on a global database (Jetz et al. 2008). Moreover, relatively high BMR was observed in several species of migratory shorebirds (Kersten and Piersma 1987; Lindström 1997; Lindström and Klaassen 2003). Sedentary New Zealand ducks have a lower BMR than migratory species from the same genera (McNab 2003b). Intraspecific studies on captive-raised common stonechats Saxicola torquata also showed that BMR was lower in individuals from a sedentary tropical population than in individuals from a migratory temperate population (Klaassen 1995; Wikelski et al. 2003).
In addition, wintering passerine migrants from our study did not differ in BMR from passerine migrants on their breeding grounds in temperate and high latitudes. Our results on BMR of migrants in tropics support the aforementioned Gavrilov’s (2011, 2014) hypothesis about migratory tendency as an important cause of high BMR in passerines. Jetz et al. (2008) found no significant difference in summer BMR between migrants and nonmigrants after accounting for temperature, and concluded that higher BMR of migrants is determined in part by temperature effects through phenotypic flexibility. But, since we measured BMR of migrants and residents at the same time and place, where representatives of both groups had been living for several months, we conclude that higher BMR of migrants could reflect the elevated maintenance costs of metabolic machinery for long-distance migration. On the other hand, BMR of passerine migrants on their tropical wintering grounds in Vietnam was lower than that of passerine residents from temperate and high latitudes. This result suggests that migratory tendency is not the only driver of increased metabolic power. Following the notion of Jetz et al. (2008), we consider low ambient temperatures at high latitudes as another obvious factor of BMR elevation (see next section).
In contrast to our study, some researchers found BMR to be lower in tropical birds that forage in the sun, than in those that forage in the shade (Weathers 1979, 1997; Hails 1983). Additionally, we did not find any relation between BMR and characteristics of habitat, diet, and foraging substrates. Of the variation in BMR of 13 species of birds of paradise, 99% can be accounted for by interspecific variation in body mass, food habits, and altitudinal distribution (McNab 2003a, 2005). The frugivorous birds of paradise had the lowest BMR compared to omnivores and insectivores (McNab 2005). In contrast to birds of paradise, the BMR of New Guinean herbivores was 23% higher than in those with an animal diet (McNab 2013). At the same time, there was no effect of food habits on BMR of honeyeaters (McNab 2016). The habitat type did not affect BMR of tropical birds from New Guinea, whereas foraging substrate did: the highest BMR was found in species that forage on trees compared to species that use aerial/ground feeding substrates (McNab 2013).
Body mass on its own accounted for 93.0% of the variation of avian BMR in our study (94.2% if we excluded the barn swallow from the analysis, which was represented by a single individual with exceptionally high BMR). The inclusion of any other factors did not improve the model. In a similar analysis, McNab (2013) found that body mass accounted for 86.6% of the variation in BMR of tropical birds. Londoño et al. (2015) reported R2 ∼80% in a similar model and R2 ∼74% in phylogenetic analysis. McNab (2013) found at least 9 significant factors of BMR variation, but with the exception of migratory tendency, these factors increased R2 by 2.8% at most. Bringing passerine/nonpasserine dichotomy into analysis, McNab (2013) increased R2 to 94.7%. Wiersma et al. (2007b) reported R2 ∼93% for the same model.
Our inability to find significant ecological and behavioral factors of BMR variation may reflect the homogeneity of our sample of species and individuals: all birds were captured in roughly the same season within a very confined territory without much difference in biotopes and altitude. McNab (2013) had a much more diverse sample: birds from altitude up to 3,000 m, species with torpor, birds from small islands, flightless species, etc.
BMR of tropical versus temperate birds
The BMR of tropical birds in our study was on average 23% lower than predicted by most allometric equations for temperate species or 17.5% lower than BMR of temperate birds from extensive McNab’s (2009) database. The common explanation of reduced BMR in tropical birds is the conformity of their life-history traits together with a slow pace of life (Wiersma et al. 2007b). However, besides life history, there is another possible explanation for the latitudinal trend in basal and field metabolic rates—temperature on breeding grounds (Anderson and Jetz 2005; Jetz et al. 2008; Bech et al. 2016). The heritable nature of BMR was shown in free-living birds (Bushuev et al. 2011, 2012). If there is an impact of ambient temperature on energetics, the lower BMR of tropical birds is not necessarily evidence for heritable differences in BMR. It could simply reflect the high phenotypic plasticity of the energetics of birds (McKechnie 2008; Swanson 2010; Kerimov et al. 2014).
The low BMR of tropical species could result from the absence of costly long-distance migrations and, in particular, the low demands for thermogenesis in warm stable climate. Wiersma et al. (2007b) have found that tropical migrants breeding in temperate habitats had a lower BMR than temperate residents. Jetz et al. (2008) proposed that higher BMR of migrants is partly the result of the negative effects of temperature on BMR. White et al. (2007) have concluded that low BMR of birds from hot arid environments results mainly from extreme temperatures. They found that BMR was negatively associated with ambient temperature and annual temperature range, but was not correlated with low annual net primary productivities. Bech et al. (2016) have showed that “slow pace of life” in Australian old-endemic passerine birds was not accompanied by low BMR. On the other hand, Londoño et al. (2015) have found no difference in BMR across a 2.6-km altitude gradient in tropical Peru. According to Jetz et al. (2008), a 20°C decrease in temperature was associated with a 50% increase in BMR. Taking into account that the high-altitude site was on average 12°C colder than the lowland site, the result of Londoño et al. (2015) argues against the “temperature” explanation of low BMR in tropical birds and provides support in favor of the “slow life history” hypothesis. On the other hand, low BMR in tropical birds, as well as similarity of avian BMR across altitudes in Peru (Londoño et al. 2015), may be explained not by average ambient temperatures, but by seasonal stability in tropics (Bech et al. 2016). In summary, the relative roles of temperature and life histories in latitudinal variation of BMR remain unclear.
In conclusion, using our sample of 54 sedentary and 12 migratory avian species from Southern Vietnam, we showed that tropical birds have 17.5–23% lower BMR than temperate birds. Also, the most pronounced difference between tropical and temperate species was the low scaling exponent in the allometric relation between BMR and body mass in tropical birds. This indicates that the difference in BMR between large temperate and tropical species is more pronounced than in small species. Furthermore, we found evidence that tropical migrants to temperate and high latitudes on their wintering grounds might have higher BMR than tropical resident species. Apart from this observation, we did not find any evidence that phylogeny, taxonomy, behavior, and ecology have a significant influence on BMR variation among birds of Southern Vietnam. Body mass alone accounted for ∼93% of the variation in BMR in our study, which is higher than in other studies on tropical birds despite the narrower range of body mass in our sample of species. One of the possible reasons for the poor correlations we observed between ecological factors and BMR, contrary to other studies on tropical birds, could be related to the uniformity of our study area with respect to climate conditions and biotopes. The revealed absence of differences in BMR between tropical passerines and non-passerines as well as the increased BMR in migratory species are consistent with the predictions of Gavrilov’s (2011, 2014) hypothesis.
Supplementary Material
Acknowledgments
We are grateful to the directorate of main and south divisions of the Joint Russian-Vietnamese Tropical and Technological Center for providing rooms and facilities (A.N. Kuznetsov, V.L. Trunov, Nguyễn Thị Nga, Nguyễn Văn Khuê). We are much obliged to V.V. Rozhnov for acquisition of respirometry equipment and to Nguyễn Văn Diện, the director of Cát Tiên National Park, for permission to work there. We thank A.E. Anichkin, Nguyễn Văn Thịnh, Vũ Mạnh, and Nguyễn Bảo Ngọc for arranging our experiments; our colleagues I.V. Palko and S.S. Gogoleva for help in the field; I.V. Palko and M.V. Kalyakin for expert categorization of ecological factors for our avian species; E.N. Rakhimberdiev for help in statistics; E.A. Ershova for corrections in English; A.B. Savinetsky for providing the original software used to control the multi-channel system; and L.P. Korzun for the help on all preliminary stages of our research.
Ethical note
The design of our study was approved by the ethics committee of Lomonosov Moscow State University [resolution of the committee no 26(6)]. We have exerted every effort to carry out our work in compliance with present international ethical standards. All our experiments were intravital and did not require prolonged treatment and handling of birds. None of the species from our study were included in “Threatened” category of the IUCN Red List of Threatened Species.
Funding
Field work and initial data analysis was supported by the Tropical Center (project Ekolan E-1.2) and partially by the Russian Foundation for Basic Research (RFBR Grant nos 12-04-01440, 15-04-07407, and 15-04-08407). The maintenance of the database and final data analysis was financially supported by the Russian Science Foundation (RSF Grant no. 14-50-00029).
References
- Anderson KJ, Jetz W, 2005. The broad-scale ecology of energy expenditure of endotherms. Ecol Lett 8:310–318. [Google Scholar]
- Aschoff J, Pohl HJ, 1970. Der Ruheumsatz von Vögeln als Funktion der Tageszeit und der Körpergröße. J Ornith 111:38–47. [Google Scholar]
- Bech C, Chappell MA, Astheimer LB, Londoño GA, Buttemer WA, 2016. A ‘slow pace of life’ in Australian old-endemic passerine birds is not accompanied by low basal metabolic rates. J Comp Physiol B 186:503–512. [DOI] [PubMed] [Google Scholar]
- Bennett AF, Ruben JA, 1979. Endothermy and activity in vertebrates. Science 206:649–654. [DOI] [PubMed] [Google Scholar]
- Bennett PM, Harvey PH, 1987. Active and resting metabolism in birds: allometry, phylogeny and ecology. J Zool 213:327–344. [Google Scholar]
- Blanc L, Maury-Lechon G, Pascal J-P, 2000. Structure, floristic composition and natural regeneration in the forests of Cat Tien National Park, Vietnam: an analysis of the successional trends. J Biogeogr 27:141–157. [Google Scholar]
- Boettiger C, Coop G, Ralph P, 2012. Is your phylogeny informative? Measuring the power of comparative methods. Evolution 66:2240–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody S, Proctor RC, 1932. Growth and development, with special reference to domestic animals. XXIII. Relation between basal metabolism and mature body weight in different species of mammals and birds. Univ Miss Agr Exp Sta Res Bull 166:89–101. [Google Scholar]
- Bryant DM, Hails CJ, 1983. Energetics and growth patterns of three tropical bird species. Auk 100:425–439. [Google Scholar]
- Bushuev AV, Husby A, Sternberg H, Grinkov VG, 2012. Quantitative genetics of basal metabolic rate and body mass in free-living pied flycatchers Ficedula hypoleuca. J Zool 288:245–251. [Google Scholar]
- Bushuev AV, Kerimov AB, Ivankina EV, 2011. Estimation of heritability and repeatability of resting metabolic rate in birds by the example of free-living Pied Flycatchers Ficedula hypoleuca (Aves: Passeriformes). Biol Bull Rev 1:26–46. [PubMed] [Google Scholar]
- Daan S, Masman D, Groenewold A, 1990. Avian basal metabolic rates: their association with body composition and energy expenditure in nature. Am J Physiol 259:R333–R340. [DOI] [PubMed] [Google Scholar]
- Downs CT, Brown M, 2012. Is respirometry a standardized technique? A review of measurement of avian resting metabolic rates. J Therm Biol 37:531–536. [Google Scholar]
- Drummond AJ, Suchard MA, Xie D, Rambaut A, 2012. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol 29:1969–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frappell PB, Hinds DS, Boggs DF, 2001. Scaling of respiratory variables and the breathing pattern in birds: an allometric and phylogenetic approach. Physiol Biochem Zool 74:75–89. [DOI] [PubMed] [Google Scholar]
- Freckleton RP, Harvey PH, Pagel M, 2002. Phylogenetic analysis and comparative data: a test and review of evidence. Am Nat 160:712–726. [DOI] [PubMed] [Google Scholar]
- Garamszegi LZ, Møller AP, 2010. Effects of sample size and intraspecific variation in phylogenetic comparative studies: a meta-analytic review. Biol Rev Camb Philos Soc 85:797–805. [DOI] [PubMed] [Google Scholar]
- Garland T Jr, Ives AR, 2000. Using the past to predict the present: confidence intervals for regression equations in phylogenetic comparative methods. Am Nat 155:346–364. [DOI] [PubMed] [Google Scholar]
- Gavrilov VM, 1994. Migrations and evolution of metabolism in birds. Ring 16:22–37. [Google Scholar]
- Gavrilov VM, 1997. Energetics and avian behavior In: Turpaev TM, editor. Physiology and General Biology Reviews. Vol. 11 Amsterdam: Harwood Academic Publishers, 1–225. [Google Scholar]
- Gavrilov VM, 1999a. Ecological phenomena of Passeriformes as a derivative of their energetics. Acta Ornithol 34:165–172. [Google Scholar]
- Gavrilov VM, 1999b. Energy responses of passerine and non–passerine birds to their thermal environment: differences and ecological effects. Avian Ecol Behav 3:1–21. [Google Scholar]
- Gavrilov VM, 2011. Energy expenditures for flight, aerodynamic quality, and colonization of forest habitats by birds. Biol Bull 38:779–788. [Google Scholar]
- Gavrilov VM, 2014. Ecological and scaling analysis of the energy expenditure of rest, activity, flight, and evaporative water loss in Passeriformes and non-Passeriformes in relation to seasonal migrations and to the occupation of boreal stations in high and moderate latitudes. Q Rev Biol 89:107–150. [DOI] [PubMed] [Google Scholar]
- Gessaman JA, Nagy KA, 1988. Energy metabolism: errors in gas-exchange conversion factors. Physiol Zool 61:507–513. [Google Scholar]
- Grafen A, 1989. The phylogenetic regression. Philos Trans R Soc B 326:119–157. [DOI] [PubMed] [Google Scholar]
- Hackett SJ, Kimball RT, Reddy S, Bowie RCK, Braun EL. et al. , 2008. A phylogenomic study of birds reveals their evolutionary history. Science 320:1763–1768. [DOI] [PubMed] [Google Scholar]
- Hails CJ, 1983. The metabolic rate of tropical birds. Condor 85:61–65. [Google Scholar]
- Harmon LJ, Losos JB, 2005. The effect of intraspecific sample size on type I and type II error rates in comparative studies. Evolution 59:2705–2710. [PubMed] [Google Scholar]
- Ives AR, Midford PE, Garland T Jr, 2007. Within-species variation and measurement error in phylogenetic comparative methods. Syst Biol 56:252–270. [DOI] [PubMed] [Google Scholar]
- Jetz W, Freckleton RP, McKechnie AE, 2008. Environment, migratory tendency, phylogeny and basal metabolic rate in birds. PLoS ONE 3:e3261.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhwueng D-C, 2013. Assessing the goodness of fit of phylogenetic comparative methods: a meta-analysis and simulation study. PLoS ONE 8:e67001.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez AG, Cooper-Mullin C, Calhoon EA, Williams JB, 2014a. Physiological underpinnings associated with differences in pace of life and metabolic rate in north temperate and neotropical birds. J Comp Physiol B 184:545–561. [DOI] [PubMed] [Google Scholar]
- Jimenez AG, Harper JM, Queenborough SA, Williams JB, 2013. Linkages between the life-history evolution of tropical and temperate birds and the resistance of cultured skin fibroblasts to oxidative and non–oxidative chemical injury. J Exp Biol 216:1373–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez AG, Van Brocklyn J, Wortman M, Williams JB, 2014b. Cellular metabolic rate is influenced by life-history traits in tropical and temperate birds. PLoS ONE 9:e87349.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez AG, Williams JB, 2014. Differences in muscle fiber size and associated energetic costs in phylogenetically paired tropical and temperate birds. Physiol Biochem Zool 87:752–761. [DOI] [PubMed] [Google Scholar]
- Kendeigh SC, Dolnik VR, Gavrilov VM, 1977. Avian energetics In: Pinowski J, Kendeigh SC, editors. Granivorous Birds in Ecosystem. Cambridge: Cambridge University Press, 127–204. [Google Scholar]
- Kerimov AB, Grinkov VG, Ivankina EV, Ilyina TA, Bushuev AV, 2014. The influence of spring temperature on the intensity of advertising behavior and basal metabolic rate in bright and pale pied flycatcher Ficedula hypoleuca males. Zool Zhurn 93:1288–1302. In Russian with English summary. [Google Scholar]
- Kersten M, Piersma T, 1987. High levels of energy expenditure in shorebirds: metabolic adaptations to an energetically expensive way of life. Ardea 75:175–187. [Google Scholar]
- King JR, Farner DS, 1961. Energy metabolism, thermoregulation and body temperature In: Marshall AJ, editor. Biology and Comparative Physiology of Birds. New York: Academic Press, 215–288. [Google Scholar]
- Klaassen M, 1995. Molt and basal metabolic costs in males of 2 subspecies of stonechats - the European Saxicola torquata rubicula and the east-African Saxicola torquata axillaris. Oecologia 104:424–432. [DOI] [PubMed] [Google Scholar]
- Lasiewski RC, Dawson WR, 1967. A re-examination of the relation between standard metabolic rate and body weight in birds. Condor 69:13–23. [Google Scholar]
- Lindström Ǻ, 1997. Basal metabolic rates of migrating waders in the Eurasian Arctic. J Avian Biol 28:87–92. [Google Scholar]
- Lindström Ǻ, Klaassen M, 2003. High basal metabolic rates of shorebirds while in the Arctic: a circumpolar view. Condor 105:420–427. [Google Scholar]
- Londoño GA, Chappell MA, Castañeda MR, Jankowski JE, Robinson SK, 2015. Basal metabolism in tropical birds: latitude, altitude, and the “pace of life”. Funct Ecol 29:338–346. [Google Scholar]
- Luft UC, Myhre LG, Loeppky JA, 1973. Validity of Haldane calculation for estimating respiratory gas exchange. J Appl Physiol 34:864–865. [DOI] [PubMed] [Google Scholar]
- Lusk G, 1924. Animal calorimetry: twenty-fourth paper. Analysis of the oxidation of mixtures of carbohydrate and fat. J Biol Chem 59:41–42. [Google Scholar]
- MacMillen RE, 1974. Bioenergetics of Hawaiian honeycreepers: the Amakihi Loxops virens and the Anianiau L. parva. Condor 76:62–69. [Google Scholar]
- McKechnie AE, 2008. Phenotypic flexibility in basal metabolic rate and the changing view of avian physiological diversity: a review. J Comp Physiol B 178:235–247. [DOI] [PubMed] [Google Scholar]
- McKechnie AE, Freckleton RP, Jetz W, 2006. Phenotypic plasticity in the scaling of avian basal metabolic rate. Proc R Soc B 273:931–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKechnie AE, Wolf BO, 2004. The allometry of avian basal metabolic rate: good predictions need good data. Physiol Biochem Zool 77:502–521. [DOI] [PubMed] [Google Scholar]
- McNab BK, 1988. Food habits and the basal rate of metabolism in birds. Oecologia 77:343–349. [DOI] [PubMed] [Google Scholar]
- McNab BK, 1997. On the utility of uniformity in the definition of basal rate of metabolism. Physiol Zool 70:718–720. [DOI] [PubMed] [Google Scholar]
- McNab BK, 2003a. Ecology shapes bird bioenergetics. Nature 426:620–621. [DOI] [PubMed] [Google Scholar]
- McNab BK, 2003b. The energetics of New Zealand's ducks. Comp Biochem Physiol A 135:229–247. [DOI] [PubMed] [Google Scholar]
- McNab BK, 2005. Food habits and the evolution of energetics in birds of paradise (Paradisaeidae). J Comp Physiol B 175:117–132. [DOI] [PubMed] [Google Scholar]
- McNab BK, 2009. Ecological factors affect the level and scaling of avian BMR. Comp Biochem Physiol A 152:22–45. [DOI] [PubMed] [Google Scholar]
- McNab BK, 2012. Extreme Measures: The Ecological Energetics of Birds and Mammals. Chicago (IL: ): University of Chicago Press. [Google Scholar]
- McNab BK, 2013. The ecological energetics of birds in New Guinea. Bull Florida Mus Nat Hist 52:95–159. [Google Scholar]
- McNab BK, 2015a. Avian energetics: the passerine/non-passerine dichotomy. Comp Biochem Physiol A 191:152–155. [DOI] [PubMed] [Google Scholar]
- McNab BK, 2015b. Behavioral and ecological factors account for variation in the mass-independent energy expenditures of endotherms. J Comp Physiol B 185:1–13. [DOI] [PubMed] [Google Scholar]
- McNab BK, 2016. Analysis of factors that influence energy expenditure in honeyeaters (Meliphagidae). New Zeal J Zool 43:179–190. [Google Scholar]
- Münkemüller T, Lavergne S, Bzeznik B, Dray S, Jombart T. et al. , 2012. How to measure and test phylogenetic signal. Methods Ecol Evol 3:743–756. [Google Scholar]
- Nilsson JA, 2002. Metabolic consequences of hard work. Proc R Soc B 269:1735–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagel M, 1999. Inferring the historical patterns of biological evolution. Nature 401:877–884. [DOI] [PubMed] [Google Scholar]
- Paradis E, Claude J, Strimmer K, 2004. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20:289–290. [DOI] [PubMed] [Google Scholar]
- Pettit TN, Ellis HI, Whittow GC, 1985. Basal metabolic rate in tropical seabirds. Auk 102:172–174. [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team, 2014. nlme: linear and nonlinear mixed effects models. R package version 3.1-118. [cited 2017 March 26]. Available from: https://CRAN.R-project.org/package=nlme.
- R Core Team, 2016. A language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing [cited 2017 March 26]. Available from: https://www.R-project.org/.
- Revell LJ, 2010. Phylogenetic signal and linear regression on species data. Methods Ecol Evol 1:319–329. [Google Scholar]
- Revell LJ, 2012. phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol Evol 3:217–223. [Google Scholar]
- Reynolds PS, Lee IIIRM, 1996. Phylogenetic analysis of avian energetics: passerines and nonpasserines do not differ. Am Nat 147:735–759. [Google Scholar]
- Rezende EL, Swanson DL, Novoa FF, Bozinovic F, 2002. Passerines versus nonpasserines: so far, no statistical differences in the scaling of avian energetics. J Exp Biol 205:101–107. [DOI] [PubMed] [Google Scholar]
- Ricklefs RE, Konarzewski M, Daan S, 1996. The relationship between basal metabolic rate and daily energy expenditure in birds and mammals. Am Nat 147:1047–1071. [Google Scholar]
- Ricklefs RE, Wikelski M, 2002. The physiology/life-history nexus. Trends Ecol Evol 17:462–468. [Google Scholar]
- Rubolini D, Liker A, Garamszegi LZ, Møller AP, Saino N, 2015. Using the BirdTree.org website to obtain robust phylogenies for avian comparative studies: a primer. Curr Zool 61:959–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Nielsen K, 1997. Animal Physiology: Adaptation and Environment. New York: Cambridge University Press. [Google Scholar]
- Scholander PF, Walters V, Hock R, Irving L, 1950. Adaptation to cold in arctic and tropical mammals and birds in relation to body temperature, insulation, and basal metabolic rate. Biol Bull 99:259–271. [DOI] [PubMed] [Google Scholar]
- Sibley CG, Ahlquist JE, 1990. Phylogeny and Classification of Birds: A Study in Molecular Evolution. New Haven (CT: ): Yale University Press. [Google Scholar]
- Speakman JR, 2005. Body size, energy metabolism and lifespan. J Exp Biol 208:1717–1730. [DOI] [PubMed] [Google Scholar]
- Stone EA, 2011. Why the phylogenetic regression appears robust to tree misspecification. Syst Biol 60:245–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson DL, 2010. Seasonal metabolic variation in birds: functional and mechanistic correlates. Curr Ornithol 17:75–129. [Google Scholar]
- Swanson DL, Bozinovic F, 2011. Metabolic capacity and the evolution of biogeographic patterns in oscine and suboscine passerine birds. Physiol Biochem Zool 84:185–194. [DOI] [PubMed] [Google Scholar]
- Swanson DL, Thomas NE, Liknes ET, Cooper SJ, 2012. Intraspecific correlations of basal and maximal metabolic rates in birds and the aerobic capacity model for the evolution of endothermy. PLoS ONE 7:e34271.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieleman BI, Dijkstra TH, Lasky JR, Mauck RA, Visser GH. et al. , 2006. Physiological and behavioural correlates of life-history variation: a comparison between tropical and temperate zone house wrens. Funct Ecol 20:491–499. [Google Scholar]
- Tieleman BI, Williams JB, 2000. The adjustment of avian metabolic rates and water fluxes to desert environments. Physiol Biochem Zool 73:461–479. [DOI] [PubMed] [Google Scholar]
- Tieleman BI, Williams JB, Ricklefs RE, Klasing KC, 2005. Constitutive innate immunity is a component of the pace-of-life syndrome in tropical birds. Proc R Soc B 272:1715–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandekerkhove K, De Wulf R, Chin NN, 1993. Dendrological composition and forest structure in Nam Bai Cat Tien National Park, Vietnam. Silva Gandavensis 58:41–83. [Google Scholar]
- Vleck CM, Vleck D, 1979. Metabolic rate in five tropical bird species. Condor 81:89–91. [Google Scholar]
- Wagner DN, Mineo PM, Sgueo C, Wikelski M, Schaeffer PJ, 2013. Does low daily energy expenditure drive low metabolic capacity in the tropical robin, Turdus grayi? J Comp Physiol B 183:833–841. [DOI] [PubMed] [Google Scholar]
- Wagner JA, Horvath SM, Dahms TE, Reed S, 1973. Validation of open-circuit method for the determination of oxygen consumption. J Appl Physiol 34:859–863. [DOI] [PubMed] [Google Scholar]
- Weathers WW, 1977. Temperature regulation in the dusky munia Lonchura fuscans (Cassin) (Estrildidae). Aust J Zool 25:193–199. [Google Scholar]
- Weathers WW, 1979. Climatic adaptation in avian standard metabolic rate. Oecologia 42:81–89. [DOI] [PubMed] [Google Scholar]
- Weathers WW, 1997. Energetics and thermoregulation by small passerines of the humid, lowland tropics. Auk 114:341–353. [Google Scholar]
- Welcker J, Speakman JR, Elliott KH, Hatch SA, Kitaysky AS, 2015. Resting and daily energy expenditures during reproduction are adjusted in opposite directions in free–living birds. Funct Ecol 29:250–258. [Google Scholar]
- White CR, Blackburn TM, Martin GR, Butler PJ, 2007. Basal metabolic rate of birds is associated with habitat temperature and precipitation, not primary productivity. Proc R Soc B 274:287–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CR, Phillips NF, Seymour RS, 2006. The scaling and temperature dependence of vertebrate metabolism. Biol Lett 2:125–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CR, Seymour RS, 2004. Does basal metabolic rate contain a useful signal? Mammalian BMR allometry and correlations with a selection of physiological, ecological, and life-history variables. Physiol Biochem Zool 77:929–941. [DOI] [PubMed] [Google Scholar]
- Wiersma P, 2003. Working for a living: physiological and behavioural trade-offs in birds facing hard work [PhD dissertation]. Zoological Laboratory, Groningen, The Netherlands: University of Groningen.
- Wiersma P, Chappell MA, Williams JB, 2007a. Cold- and exercise-induced peak metabolic rates in tropical birds. Proc Natl Acad Sci USA 104:20866–20871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersma P, Muñoz-Garcia A, Walker A, Williams JB, 2007b. Tropical birds have a slow pace of life. Proc Natl Acad Sci USA 104:9340–9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersma P, Nowak B, Williams JB, 2012. Small organ size contributes to the slow pace of life in tropical birds. J Exp Biol 215:1662–1669. [DOI] [PubMed] [Google Scholar]
- Wikelski M, Spinney L, Schelsky W, Scheuerlein A, Gwinner E, 2003. Slow pace of life in tropical sedentary birds: a common-garden experiment on four stonechat populations from different latitudes. Proc R Soc B 270:2383–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JB, Miller RA, Harper JM, Wiersma P, 2010. Functional linkages for the pace of life, life-history, and environment in birds. Integr Comp Biol 50:855–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmore JH, Costill DL, 1973. Adequacy of the Haldane transformation in the computation of exercise VO2 in man. J Appl Physiol 35:85–89. [DOI] [PubMed] [Google Scholar]
- Zar JH, 1969. The use of the allometric model for avian standard metabolism-body weight relationships. Comp Biochem Physiol 29:227–234. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.