Abstract
A prominent example of seasonal phenotypic flexibility is the winter increase in thermogenic capacity (=summit metabolism, ) in small birds, which is often accompanied by increases in pectoralis muscle mass and lipid catabolic capacity. Temperature or photoperiod may be drivers of the winter phenotype, but their relative impacts on muscle remodeling or lipid transport pathways are little known. We examined photoperiod and temperature effects on pectoralis muscle expression of myostatin, a muscle growth inhibitor, and its tolloid-like protein activators (TLL-1 and TLL-2), and sarcolemmal and intracellular lipid transporters in dark-eyed juncos Junco hyemalis. We acclimated winter juncos to four temperature (3 °C or 24 °C) and photoperiod [short-day (SD) = 8L:16D; long-day (LD) = 16L:8D] treatments. We found that myostatin, TLL-1, TLL-2, and lipid transporter mRNA expression and myostatin protein expression did not differ among treatments, but treatments interacted to influence lipid transporter protein expression. Fatty acid translocase (FAT/CD36) levels were higher for cold SD than for other treatments. Membrane-bound fatty acid binding protein (FABPpm) levels, however, were higher for the cold LD treatment than for cold SD and warm LD treatments. Cytosolic fatty acid binding protein (FABPc) levels were higher on LD than on SD at 3 °C, but higher on SD than on LD at 24 °C. Cold temperature groups showed upregulation of these lipid transporters, which could contribute to elevated Msum compared to warm groups on the same photoperiod. However, interactions of temperature or photoperiod effects on muscle remodeling and lipid transport pathways suggest that these effects are context-dependent.
Keywords: birds, FABPpm, FABPc, FAT/CD36, myostatin, pectoralis, phenotypic flexibility, photoperiod, temperature
Seasonal phenotypes in small birds are one well-known example of reversible phenotypic flexibility and allow birds to better match their phenotypes to seasonal climates (Swanson 2010; Swanson and Vézina 2015). Small birds experience high thermoregulatory demands in cold climates, and due to their high surface area-to-volume ratios, they are expected to undergo substantial variation in metabolic phenotypes between seasons. The winter phenotype in small birds is distinguished by improved cold tolerance and elevated basal (BMR; minimum maintenance metabolism) and summit (Msum; maximum cold-induced metabolism) metabolic rates relative to summer (Dubois et al. 2016). Such increments of metabolic rates, especially Msum, are positively correlated with cold tolerance (Swanson, 2001; Swanson and Liknes 2006). As a result, Msum is commonly interpreted as an indicator of a bird’s ability to endure cold environments.
Adjustments in Msum throughout the annual cycle in birds may be mediated through variation in skeletal muscle (Marsh 1981; Evans et al. 1992; Liknes and Swanson 2011b) and heart (Piersma 1998; Battley et al. 2001; Swanson et al. 2014b) masses and/or cellular metabolic intensities (Liknes and Swanson 2011a; Zheng et al. 2014) coupled with enhanced substrate transport (Liknes et al. 2014a). Previous studies indicated that flight muscle hypertrophy contributed to increases in organismal metabolic rates (Marsh 1981, 1984; Evans and Rose 1988; Dietz et al. 1999; Petit and Vézina 2014), especially for winter phenotypes in birds (Swanson et al. 2009, 2013; Liknes and Swanson 2011b; Petit et al. 2014). Because seasonal increases in pectoralis muscle and heart masses consistently contribute to the winter phenotype in small birds, the mechanisms regulating seasonal muscle remodeling are an important research target for understanding the flexible metabolic phenotypes in birds. One candidate for the flexible regulation of skeletal muscle mass is myostatin, which belongs to the TGF-β family of growth factors and is an autocrine/paracrine inhibitor of muscle growth in birds and mammals (Lee 2004). Myostatin acts to inhibit skeletal muscle growth and reduces muscle mass in birds and mammals, thereby regulating muscle remodeling in adults (Lee 2004; Rodgers and Garikipati 2008). Several studies have examined variation in expression of myostatin in birds during migration and winter acclimatization accompanied by variation in muscle mass (Swanson et al. 2009, 2014a; King et al. 2015). These studies offer variable support for a role for myostatin in regulating flexible muscle masses throughout the annual cycle in birds, with some finding reduced expression of either mRNA or protein for myostatin in pectoralis muscle (Swanson et al. 2009), or no changes in other species for migratory or wintering phenotypes (Swanson et al. 2014a; King et al. 2015). However, white-throated sparrows Zonotrichia albicollis under migratory photoperiod exhibited the opposite effect, with elevated mRNA expression of myostatin compared to the winter photoperiod, but European starlings Sturnus vulgaris did not show any changes in myostatin mRNA expression with exercise training (Price et al. 2011). In contrast, exercise- and cold-trained house sparrows Passer domesticus showed significantly reduced myostatin protein levels associated with increased pectoralis mass compared to their controls (Zhang et al. 2015b).
Myostatin is secreted as an inactive form and requires cleavage by metalloproteinases, including the tolloid-like proteins TLL-1 and TLL-2, to form the active C-terminal dimer myostatin (Huet et al. 2001). Thus, upstream of myostatin receptors, regulation of muscle mass through myostatin can involve both synthesis of myostatin and activation of the protein via cleavage (Lee 2004, 2008). Myostatin and TLL-1 gene expression were both downregulated in winter house sparrows relative to summer sparrows (Swanson et al. 2009). Moreover, mRNA expression for either or both TLL-1 and TLL-2 were downregulated in winter relative to summer for American goldfinches (Spinus tristis) and black-capped chickadees (Poecile atricapillus, Swanson et al. 2014a). In contrast, Price et al. (2011) found that TLL-1 mRNA expression did not differ from controls for exercise-trained European starlings. Moreover, exercise- and cold-trained house sparrows showed only non-significant variation in TLL mRNA expression in trained groups compared to their controls (Zhang et al. 2015b). Thus, although some evidence suggests that modulation of myostatin and myostatin processing capacity may regulate phenotypic flexibility of metabolic capacities in birds in response to changing energy demands, this modulation does not appear to be universal for birds under all such conditions.
In addition to flight muscle hypertrophy, winter and migratory enhancement of lipid transport (Liknes et al. 2014; Zhang et al. 2015d) and catabolism (Marsh and Dawson 1989; Liknes and Swanson 2011a; Corder et al. 2016) also occurs in birds. Prolonged shivering thermogenesis in birds is almost exclusively fueled by exogenous lipids from adipose tissues (Swanson 2010). As a result, capacities for non-esterified fatty acid (NEFA) uptake into the myocyte and their subsequent transport to mitochondrial membranes are potential regulatory steps for lipid catabolic capacity in support of shivering (Swanson 2010; Zhang et al. 2015a). Once circulating NEFAs reach the myocyte, plasma membrane-bound fatty acid binding protein (FABPpm) and fatty acid translocase (FAT/CD36) cooperate to transport NEFAs across the endothelium, the interstitial space, and the sarcolemma to enter muscle cells (Kiens 2006; Glatz et al. 2010). The effects of winter acclimatization or migration on skeletal muscle FAT/CD36 and FABPpm levels are incompletely resolved. Some studies showed elevated FAT/CD36 and FABPpm levels during migration compared to non-migrants (McFarlan et al. 2009; Zhang et al. 2015c), but others indicated stable FAT/CD36 and FABPpm during migration or wintering (Zhang et al. 2015c, 2015d). Moreover, results from exercise and cold training studies suggest that responses of FAT/CD36 and/or FABPpm levels to increasing energy demands also vary among species (Price et al. 2010; Zhang et al. 2015a). Intramyocyte fatty acid transport in skeletal muscles is mediated by cytosolic fatty acid binding protein (FABPc) (Kiens 2006; Guglielmo 2010). FABPc also acts as an intramyocyte fatty acid receptor, acting to regulate and potentially limit fatty acid uptake (Glatz et al. 2010). Most studies of migration (Guglielmo et al. 2002; McFarlan et al. 2009), cold acclimation (Stager et al. 2015), or winter acclimatization (Liknes et al. 2014; Zhang et al. 2015d) show that increases in pectoralis FABPc levels are consistent correlates of enhanced flight and shivering performance in birds.
The regulation of winter phenotypes in small resident birds in temperate-zone climates could potentially be driven by environmental cues such as photoperiod (Carey and Dawson 1999) and temperature (Swanson and Olmstead 1999). To examine mechanistic bases for photoperiod and temperature-induced variation in organismal metabolic capacities, we studied dark-eyed juncos Junco hyemalis exposed to different photoperiod [long-day (LD) and short-day (SD)] and temperature (cold and warm) treatments. Dark-eyed juncos are a common winter resident in South Dakota and resident populations in western North America show seasonal variation in pectoralis mass and Msum (Swanson 1990, 1991). Moreover, a previous study using the same individual birds indicated that cold exposure, but not short photoperiod, elevated Msum compared to warm exposed birds (Swanson et al. 2014c). This study also demonstrated that even though pectoralis muscle mass did not vary significantly among acclimation treatments, cellular metabolic intensity in the pectoralis was generally higher for LD and cold groups (Swanson et al. 2014c). In addition, Stager et al. (2015) conducted a study of genome-wide transcriptional profiles on pectoralis muscle samples from the same individual birds and found that mRNA expression for muscle hypertrophy, angiogenesis and lipid transport and oxidation pathways were generally upregulated by cold exposure. Several studies, however, have documented that mRNA and protein expression in pectoralis muscle during migration and winter acclimatization in small birds are sometimes not correlated for myostatin or lipid transport pathways (e.g., Swanson et al. 2014a, 2017; King et al. 2015; Zhang et al. 2015d). Consequently, the present study builds upon these previous studies by evaluating, in pectoralis muscle (the main thermogenic organ in birds), both gene and protein expression for myostatin, gene expression for myostatin activators TLL-1 and TLL-2, and gene and protein expression for the lipid transporters, FABPpm, FABPc (protein only) and FAT/CD36 for these same individual juncos, as well as by examining correlations among these factors and with Msum and pectoralis muscle mass. We hypothesize that cold exposure will reduce expression of the myostatin system and increase lipid transport capacities, but that photoperiod will have limited impacts on these systems. These analyses will identify the relative variation in pectoralis gene and protein expression for the myostatin and lipid transport systems in response to temperature and photoperiod in small birds and how such variation might relate to seasonal metabolic flexibility.
Materials and Methods
Bird collection and acclimation treatments
All procedures reported herein were approved by the University of South Dakota Institutional Animal Care and Use Committee (Protocol 79-01-11-14C). Details of bird collection and acclimation treatments are provided in Swanson et al. (2014b). Briefly, dark-eyed juncos were captured near Vermillion, South Dakota (approximately 42° 47' N, 97° W) during mid-December under appropriate state (11-7, 12-2) and federal (MB758442) scientific collecting permits. After capture, birds were individually housed in 59 × 45 × 36 cm stainless-steel cages with controlled temperature (± 2 °C) and photoperiod with ad libitum mixed seed, protein supplement (mixture of homogenized dog food and hard-boiled egg with seed) and vitamin-enriched (Wild Harvest Multi-Drops vitamin supplement for all birds, United Pet Group, Inc., Cincinnati, OH, USA) water. Birds were acclimated to captive conditions, at room temperature (23 °C) and natural photoperiod (9 h:15 h, light:dark), for at least two weeks. We randomly assigned birds into four temperature–photoperiod treatments: 24 °C, 8 h:16 h light:dark (warm SD); 24 °C, 16 h:8 h light:dark (warm LD); 3 °C, 8 h:16 h light:dark (cold SD); and 3 °C, 16 h:8 h light:dark (cold LD). We included 12 birds in each treatment and acclimation treatments lasted for 6 weeks.
After the 6-week acclimation treatments, we euthanized birds by cervical dislocation and quickly excised the pectoralis muscles on ice. We weighed the left pectoralis muscle to the nearest 0.1 mg and divided it into two sub-samples, one of which was placed in RNAlater (Ambion, Grand Island, NY, USA) for real-time quantitative reverse transcription PCR (qRT–PCR) and one flash-frozen in liquid nitrogen for Western blot analysis. Both sub-samples were stored frozen at −80 °C until later assays.
Quantitative real-time RT–PCR
We measured pectoralis mRNA expression for myostatin, TLL-1, TLL-2, FABPpm, and FAT/CD36 by qRT–PCR, as described in Swanson et al. (2014a) and Zhang et al. (2015a, 2015b, 2015c, 2015d), using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a housekeeping gene. For these assays, we first extracted total RNA with β-mercaptoethanol and the RNeasy Fibrous Tissue Mini kit (QIAGEN, Valencia, CA, USA). We then quantified extracted RNA with an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) and used 50 ng of purified RNA for qRT–PCR reactions with a TaqMan RNA-to-CT 1-Step Kit (Applied Biosystems, Carlsbad, CA, USA) and a Step-One-Plus Real-Time PCR System (Applied Biosystems). For all genes, we used the custom qRT–PCR probe and primer sets (Applied Biosystems) containing the sequences listed in Swanson et al (2014a, see their Table 1), which were derived from partial cDNA sequences from house sparrows (Swanson et al. 2009; GenBank Accession Numbers KP337454–KP337456). We conducted the qRT–PCR at 48 °C for 15 min, then 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s and 60 °C for 1 min. We optimized protocols for all six genes to verify efficiencies for these probe and primer sets for dark-eyed juncos. The FABPc probe and primer sets failed to amplify mRNA using the StepOnePlus Real-Time PCR System, so we were unable to quantify gene expression for FABPc. Slopes and efficiencies for each gene were: GAPDH (−3.35, 98.8%), Myostatin (−3.5, 93.1%), TLL-1 (−3.42, 96.1%), TLL-2 (−3.49, 93.4%), FABPpm (−3.45, 94.9%), and FAT/CD36 (−3.36, 98.4%). We quantified changes in mRNA expression using the 2−ΔΔCT method (Livak and Schmittgen, 2001). We used the mean value for all acclimation treatments for each gene as the reference sample and set the value for this reference sample equal to 1. We then normalized mRNA expression to this reference sample to determine relative amounts of mRNA expression (relative to GAPDH expression) for all other samples for the same tissue and species (e.g., Zhang et al. 2015a, 2015b, 2015c, 2015d).
Table 1.
Correlations among mass-independent summit metabolic rate residuals (from regressions of metabolic rate versus Mb
|
Pearson correlation coefficient | ||||||
|---|---|---|---|---|---|---|
| Msum residual | PEC residual | Myostatin mRNA level | TLL-1 mRNA level | TLL-2 mRNA level | Myostatin protein level | |
| Msum residual | −0.028 | −0.201 | 0297 | 0.2 | 0.277 | |
| PEC residual | −0.063 | 0.095 | 0.06 | −0.187 | ||
| mRNA expression | ||||||
| Myostain | 0.119 | 0.127 | 0.044 | |||
| TLL-1 | 0.738** | 0.375* | ||||
| TLL-2 | 0.251 | |||||
Swanson et al. (2014b), pectoralis muscle mass (PEC), myostatin mRNA level, tolloid-like protein 1 (TLL-1) mRNA level, tolloid-like protein 2 (TLL-2) mRNA level, protein levels of myostatin in pectoralis muscles of dark-eyed junco. *P < 0.05; **P < 0.01.
Western blots
We measured pectoralis protein levels for myostatin, FAT/CD36, FABPc, and FABPpm using Western blots with glyceraldehyde phosphate dehydrogenase (GAPDH) as a housekeeping protein (Zhang et al. 2015a,2015b, 2015c, 2015d). We were unable to acquire functional antibodies for TLL-1 and TLL-2, so we did not conduct Western blots for TLL-1 and TLL-2. For Western blot assays, we removed pectoralis samples from −80 °C storage and homogenized small samples by sonication on ice with a Cole-Parmer (Chicago, IL, USA) 4710 Series Ultrasonic homogenizer for three 10 s bursts, with 30 s in between bursts to disrupt membranes and separate membrane proteins from phospholipids. The homogenizing buffer contained 50 mM Tris, pH 7; 100 mM NaCl; 2% SDS. We then separated soluble and phospholipid fractions by centrifugation, and collected the soluble fraction for analysis. We determined protein concentrations on the soluble fraction using a modified DC Lowry improved protein assay and we used 10 μg of protein for analysis via sodium dodecyl sulfate–polyacrylamide gel electrophoresis. We ran all samples on NuPAGE® Novex® 4–12% Bis-Tris protein gels with the same random sample included on every gel to serve as a standard for detecting gel-to-gel variation. After transferring proteins onto membranes, we probed blots with antibodies against myostatin (goat polyclonal; R&D Systems, Minneapolis, MN, USA; 1:100 dilution), FAT/CD36 (rabbit polyclonal; Novus Biologicals, Littleton, CO, USA; 1:1,000 dilution), FABPpm (rabbit polyclonal, from Christopher G. Guglielmo; 1:10,000 dilution), FABPc (rabbit polyclonal, from Christopher G. Guglielmo; 1:8,000 dilution) (McFarlan et al. 2009), and GAPDH (chicken polyclonal; Millipore, Temecula, CA, USA; 1:8,000 dilution). We washed membranes with TBS-T and incubated them with horseradish peroxidase-conjugated secondary antibodies: anti-rabbit (1:1,000; Santa Cruz Biotechnology, Dallas, TX, USA) for FAT/CD36, FABPpm, and FABPc, anti-chicken (1:1,500; Abcam, Cambridge, MA, USA) for GAPDH, and anti-Goat (1:1,000 dilution; Santa Cruz Biotechnology) for myostatin. For analysis, we visualized blots with the ECL Plus Western Blotting Detection System (GE Healthcare, Buckinghamshire, UK) and captured chemiluminescent images with a VersaDoc 3000 Molecular Imager (Bio-Rad, Hercules, CA, USA). We analyzed images with Quantity One software (Bio-Rad Laboratories), normalizing each protein level by dividing by GAPDH protein levels for the same tissue sample (Zhang et al. 2015b). We used these normalized protein levels for subsequent statistical comparisons.
Statistical analyses
We present data as means ± SE, unless otherwise noted. We compared mean values of mRNA and protein expression among different treatments with two-way ANOVA. If parametric assumptions of normal distribution (Kolmogorov–Smirnov test) or homogenous variances (Levene’s test) were violated, we log10-transformed data prior to comparisons. If significant differences were detected by two-way ANOVA, we used Tukey tests to identify which means differed significantly. We further calculated Pearson correlation coefficients to examine relationships among separate components for each pathway. We obtained values for Msum, body mass (Mb), pectoralis mass, and pectoralis activities of carnitine palmitoyl transferase (CPT, an indicator of fatty acid transport across the mitochondrial membrane), citrate synthase (CS, a key regulatory enzyme of the Krebs cycle), and β-hydroxyacyl Co-A dehydrogenase (HOAD, a key enzyme regulating fat oxidation capacity) for the same individual birds from Swanson et al. (2014c). We tested for correlations for Msum, body mass (Mb), and pectoralis mass with myostatin mRNA and protein expression, and with mRNA expression for the TLLs to examine the muscle remodeling pathway. We tested for correlations for Msum and enzyme activities with fatty acid transporter mRNA and protein expression to examine lipid transport and metabolism pathways. To remove the effects of Mb from analyses of relationships for pectoralis mass and Msum, we calculated residuals from allometric regressions with Mb, and then used least squares linear regression of residuals to test for correlations with these variables. All statistical analyses were conducted with SigmaStat Version 3.5 (Systat, Point Richmond, CA, USA). We accepted statistical significance for all tests at P < 0.05.
Results
Myostatin and TLLs
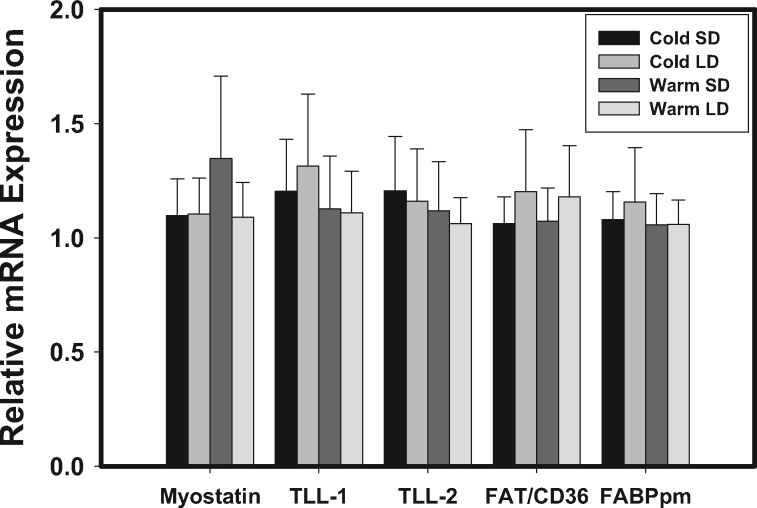
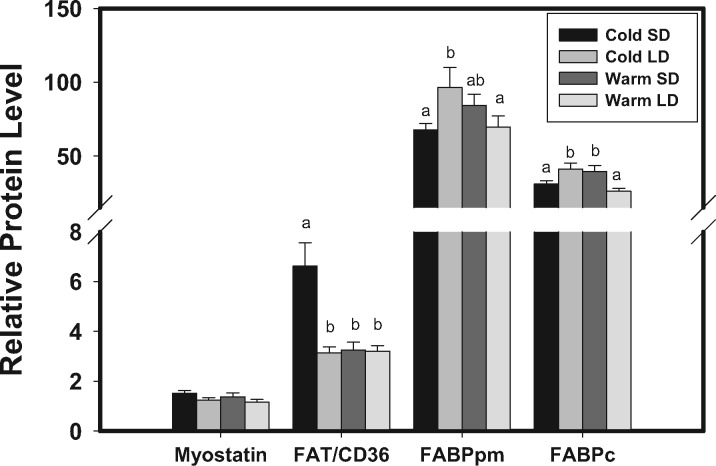
Both GAPDH mRNA expression and protein levels did not differ significantly among groups, so GAPDH should serve and effective housekeeping role in this study. No significant differences were detected in pectoralis mRNA expression for myostatin or the TLLs among acclimation treatments (Figure 1). We detected a single band for the myostatin antibody in our Western blots, which correspond to the 52 kDa unprocessed latent form of myostatin. As for mRNA expression, protein expression of myostatin did not vary significantly among acclimation groups (Figure 2).
Figure 1.
Temperature and photoperiod effects on relative mRNA expression levels from qRT-PCR for myostatin, tolloid-like protein 1 (TLL-1), tolloid-like protein 2 (TLL-2), plasma membrane-bound fatty acid binding protein (FABPpm), and fatty acyl translocase (FAT/CD36) in pectoralis muscles of dark-eyed junco (Junco hyemalis). Error bars represent SE. Sample sizes for the different treatment groups were: cold short day (Cold SD), n = 11; cold long day (Cold LD), n = 10; warm short day (warm SD), n = 10; warm long day (warm LD), n = 10.
Figure 2.
Temperature and photoperiod effects on relative protein levels from western blot for myostatin, tolloid-like protein 1 (TLL-1), tolloid-like protein 2 (TLL-2), plasma membrane-bound fatty acid binding protein (FABPpm), and fatty acyl translocase (FAT/CD36) in pectoralis muscles of dark-eyed junco (Junco hyemalis). Error bars represent SE. Sample sizes for the different treatment groups were: cold short day (Cold SD), n = 11; cold long day (Cold LD), n = 10; warm short day (warm SD), n = 10; warm long day (warm LD), n = 10. Different letters denote significant differences between treatment groups.
Trans-sarcolemmal and intramyocyte lipid transport
No significant differences among acclimation treatments were detected for pectoralis mRNA expression of FAT/CD36 or FABPpm (Figure 1). Pectoralis protein expression, however, showed more variation, with significant differences among acclimation treatments for all three fat transporters (Figure 2; FAT/CD36: F1,40 = 11.734, P = 0.002; FABPpm: F1,40 = 6.053, P = 0.019; FABPc: F1,40 = 13.741, P < 0.001). FAT/CD36 protein expression was highest in the cold SD treatment and was significantly higher than for the other acclimation treatments. FABPpm protein expression was highest in the cold LD acclimation treatment and was significantly higher than cold SD and warm LD treatments. FABPc protein levels were significantly higher in the cold LD and warm SD than in the cold SD and warm LD groups.
Correlations
Neither pectoralis muscle mass residuals, myostatin mRNA or protein expression, fatty acid transporter mRNA or protein expression, nor cellular catabolic enzyme activities were significantly correlated with Msum residuals (Tables 1 and 2). In the muscle remodeling pathway, mRNA expression of TLL-1 and TLL-2 were significantly positively correlated. In addition, myostatin protein expression was significantly positively correlated with TLL-1 mRNA expression (Table 1). In the fatty acid transport and catabolism pathway, mRNA expression of FAT/CD36 and FABPpm were strongly positively correlated. Protein expression of FABPc was also positively correlated with both mRNA and protein expression of FABPpm. In addition, FABPpm mRNA expression was significantly positively correlated with both CS and HOAD activities. Finally, CS, CPT, and HOAD enzyme activities were significantly positively correlated with each other (Table 2).
Table 2.
Correlations among mass-independent metabolic rate residuals (from regressions of metabolic rate versus Mb
|
Pearson correlation coefficient | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Msum residual | FAT/CD36 mRNA level | FABPpm mRNA level | FAT/CD36 Protein level | FABPpm Protein level | FABPc Protein level | CPT activity | CS activity | HOAD activity | |
| Msum Residuals | 0.307 | 0.179 | 0.167 | −0.092 | 0.127 | 0.278 | 0.144 | 0.185 | |
| mRNA expression | |||||||||
| FAT/CD36 | 0.783** | 0.054 | 0.051 | 0.255 | 0.187 | 0.245 | 0.279 | ||
| FABPpm | 0.087 | 0.099 | 0.315* | 0.116 | 0.338* | 0.364* | |||
| Protein level | |||||||||
| FAT/CD36 | −0.13 | −0.026 | 0.108 | −0.02 | −0.172 | ||||
| FABPpm | 0.763** | −0.103 | 0.253 | 0.196 | |||||
| FABPc | −0.116 | 0.218 | 0.256 | ||||||
| Enzyme activities | |||||||||
| CPT | 0.345* | 0.353* | |||||||
| CS | 0.557** | ||||||||
Swanson et al. (2014b), activities of carnitine palmitoyl transferase (CPT), citrate synthase (CS), β-hydroxyacyl CoA-dehydrogenase (HOAD), protein levels of cytosolic fatty acid binding protein (FABPc), and protein levels and mRNA expression of fatty acyl translocase (FAT/CD36) and plasma membrane-bound fatty acid binding protein (FABPpm) in pectoralis muscles of dark-eyed junco. *P < 0.05; **P < 0.01.
Discussion
Results from the present study suggest that regulation of lipid transporter protein levels in pectoralis muscle is complex, with variation in either temperatures or photoperiod potentially resulting in changes to lipid transport capacities. For example, high protein expression for all three lipid transporters occurred in cold temperatures, but high expression in the cold was dependent on photoperiod, with some transporter protein levels reaching maximum levels under cold and short days (FAT/CD36) and others under cold and long days (FABPpm and FABPc). Both of these responses could be interpreted as occurring to match to the higher energetic demands associated with cold and/or migratory status, the latter promoted by exposure of winter-collected birds to long days. Previous studies of these same juncos detected upregulation in genes and enzyme activities involved in fatty acid metabolism (Swanson et al. 2014b, Stager et al. 2015), but, importantly, this study extended these previous findings to protein expression in the pectoralis. Swanson et al. (2014c) previously documented for these same individual juncos that cold temperatures increased Msum, but this was not associated with increased pectoralis muscle mass. In addition, CS and HOAD activities in these same juncos generally increased on long days, but activities were highest on the cold LD treatment and lowest on the warm SD treatment, potentially due to stimulation of the migratory disposition by long days (Swanson et al. 2014c). Stager et al. (2015) built upon these findings with analyses of genome-wide patterns of mRNA expression, documenting cold-induced increases in transcripts for muscle growth (although not myostatin), angiogenesis, and lipid transport and catabolic pathways.
We found little evidence in this study for upregulation of gene or protein expression of the myostatin system in pectoralis muscle in response to cold or short days. The absence of such an upregulation is consistent with the lack of an increase in pectoralis muscle mass in response to cold or short days (Swanson et al. 2014c) as well as the absence of a transcriptomic signature for the myostatin pathway (Stager et al. 2015) for these same juncos. Surprisingly, muscle remodeling mechanisms leading to hypertrophy, at least as mediated through the myostatin pathway, appear less important to temperature-induced variation in organismal metabolic capacities for dark-eyed juncos than is typical for the winter phenotype in birds (Swanson 2010). Collectively, these results suggests that enhanced lipid transport and catabolism capacities, rather than muscle hypertrophy, are one of the primary drivers of enhanced thermogenic capacity during cold acclimation for dark-eyed juncos.
Myostatin and TLLs
Myostatin, TLL-1 and TLL-2 mRNA expression and myostatin protein levels did not differ among acclimation treatments in this study. The qRT-PCR and Western blot analyses in the present study validate the absence of variation in myostatin expression documented via transcriptomics in these same individual birds (Stager et al. 2015). Because pectoralis muscle mass also did not vary significantly among treatment groups for these birds (Swanson et al. 2014c), our results do not preclude a general role for the myostatin system in mediating seasonal muscle remodeling in birds. Even though increases in pectoralis muscle mass are a common component of phenotypic flexibility associated with winter or cold acclimation in many small birds (Vézina et al. 2006, 2007; Swanson 2010; Liknes and Swanson 2011b), a few previous studies have also documented an absence of pectoralis muscle mass changes for wintering (Swanson et al. 2014a) or migratory (King et al. 2015) phenotypes.
Trans-sarcolemmal lipid transport
Neither temperature nor photoperiod significantly altered pectoralis FAT/CD36 and FABPpm mRNA expression in this study. Winter or migratory status in most bird species also fail to promote changes in mRNA expression for these two sarcolemmal lipid transporters (Zhang et al. 2015c, 2015d) and Stager et al. (2015) found no significant variation in mRNA expression of pectoralis FAT/CD36 among temperature and photoperiod treatments for these same juncos. Moreover, cold and exercise training in house sparrows also did not change mRNA expression for FAT/CD36 or FABPpm (Zhang et al. 2015a). In contrast, pectoralis muscle mRNA expression of FAT/CD36 was upregulated for captive white-throated sparrows photostimulated to migratory condition (Zajac et al. 2011) and yellow-rumped warblers Setophaga coronata showed higher FAT/CD36 and FABPpm mRNA expression during spring migration than during fall migration (Zhang et al. 2015c). The present study detected a strong positive correlation in pectoralis mRNA, but not protein, expression between FAT/CD36 and FABPpm, suggesting co-expression of these genes in response to acclimation treatments. Regarding the difference in mRNA and protein expression with acclimation, some studies in mammals have also failed to detect correlations between mRNA or protein expression and the rate of fatty acid transport into myocytes (Luiken et al. 2003; Chabowski et al. 2006). In contrast, some recent studies document that FAT/CD36 and FABPpm are ubiquitously expressed (Nickerson et al. 2009), suggesting that the rate of trans-sarcolemmal fatty acid uptake depends on a cooperative role for the two fatty acid transporters (Chabowski et al. 2007). The positive correlation between pectoralis mRNA expression for FAT/CD36 and FABPpm in this study provided support for a cooperative role between these two trans-sarcolemmal transporters in birds.
In contrast to results for mRNA expression in this study, temperature and photoperiod treatments in the present study both induced changes in pectoralis protein expression for both FAT/CD36 and FABPpm, with cold exposure effects being modified by photoperiod treatments. Pectoralis protein expression for these two transporters had different responses to photoperiod, with FAT/CD36 increasing on cold SD treatments and FABPpm increasing on cold LD treatments. Because protein levels of these sarcolemmal lipid transporters are more directly indicative of fatty acid transport capacities than mRNA expression, post-transcriptional processing of FABPpm and FAT/CD36 may alter protein levels (Bonen et al. 1999). As a consequence, fatty acid transporter protein expression might be expected to show more variation than mRNA expression (Glatz et al. 2010) with varying energy demands, as detected in the present study. Previous studies of wintering American goldfinches and cold-trained house sparrows both showed elevated pectoralis FAT/CD36 protein expression with incresing energy demands (Zhang et al. 2015a, 2015d). In addition, black-capped chickadees increased pectoralis FABPpm protein expression during winter compared to summer (Zhang et al. 2015d). Migratory birds may also increase pectoralis FABPpm protein levels during migration compared to non-migratory periods (McFarlan et al. 2009). Moreover, pectoralis FAT/CD36 protein expression increased during migration relative to summer for warbling vireos Vireo gilvus and yellow warblers Setophaga petechia and increased during spring relative to fall migration for yellow-rumped warblers (Zhang et al. 2015c). Pectoralis FABPpm protein levels also showed a similar seasonal pattern of variation for yellow and yellow-rumped warblers, but not for warbling vireos (Zhang et al. 2015c). Collectively, these studies suggest that pectoralis levels of the two sarcolemmal lipid transporters often increase in concert with increasing energy demands, but this is not always the case.
Reasons for the inconsistency between patterns of FAT/CD36 and FABPpm protein expression in the present study are unclear. Some studies have, however, documented different responses between these two fat transporters in skeletal muscles after contraction or insulin-treatment in mammals (Chabowski et al. 2004). These two proteins often act in conjunction at the plasma membrane, but translocation of FAT/CD36 and FABPpm to other locations in the myocyte under conditions of altered energy demand may differ (Chabowski et al. 2004; Han et al. 2007). Such differences in translocation could lead to different patterns of protein expression for the two sarcolemmal transporters, as documented in the present study.
Intramyocyte lipid transport
Variation in FABPc protein expression among acclimation treatments in this study was difficult to interpret, as levels increased on cold LD compared to cold SD and on warm SD compared to warm LD. Pectoralis intramyocyte lipid transport is consistently an important target of upregulation for migratory (Guglielmo et al. 2002; McFarlan et al. 2009) and winter (Liknes et al. 2014; Zhang et al. 2015d) phenotypes in birds. In addition, photo-stimulated migratory white-throated sparrows (Zajac et al. 2011) and cold- and exercise-trained house sparrows (Zhang et al. 2015a) also showed increases in FABPc expression. These data suggest that intracellular lipid transport is an important target of adjustment underlying elevation of metabolic capacities with increasing energy demands. The increase in FABPc on LD under cold treatment in this study supports the migratory disposition hypothesis, as exposure to a LD photoperiod for winter-collected birds could induce the spring migratory phenotype. On the other hand, within the warm treatment, SD birds had significantly higher FABPc levels than LD birds, possibly to maintain thermogenic function on a winter photoperiod. Complicating this interpretation further, Stager et al. (2015) documented cold-induced increases in pectoralis FABPc mRNA expression for these same individual juncos. We were unable to amplify FABPc mRNA in the present study, so we were unable to validate this finding.
Given the inconsistent results in the present study, it is, perhaps, important to note that FABPc serves not only as an intramyocyte fatty acid transporter, but also as a fatty acid receptor and may be co-regulated with fatty acid binding proteins on the cell membrane (Luiken et al. 2003). The membrane-bound and cytosolic forms of FABP in pectoralis were positively correlated in the present study. FABPc may also be more important to overall lipid transport capacity than membrane-associated lipid transporters because very little fatty acid exists as free or unbound molecules inside muscle cells (Kiens 2006), whereas at least some fatty acid transport across membranes occurs by simple diffusion (Hamilton et al. 2002). Intramyocyte lipid transport capacity in birds may be especially important to organismal metabolic capacities because birds rely almost exclusively on exogenous lipids to fuel prolonged muscular activity (Jenni-Eiermann et al. 2002; Guglielmo 2010). In contrast, mammalian aerobic muscular activity is mainly fueled by carbohydrates and lipid droplets inside the myocyte (Guglielmo 2010). Despite the importance of FABPc to overall lipid transport in birds during elevated energy demands, protein expression was not consistently correlated with elevated energy demands for juncos in this study, suggesting complex interactions between temperature and photoperiod in the regulation of lipid transport in these birds.
Taken together, cold exposure, at least under some photoperiod conditions, increased all three fat transporters, suggesting that changes in this pathway could contribute to elevated Msum under cold exposure in these same individual birds (Swanson et al. 2014c). However, photoperiod did not consistently alter either muscle mass, the myostatin system, or fatty acid transport pathways. This result is, perhaps, not surprising given that the transcriptomic study of Stager et al. (2015) on these same birds demonstrated increased concerted gene expression of the citric acid cycle and oxidative phosphorylation pathways on SD, but increased concerted gene expression in fatty acid metabolism pathways on LD. Because migratory phenotypes can be stimulated by either or both photoperiod or exercise (Price et al. 2011), it is possible that fuel transport and catabolism capacities did not change in the same direction due to photoperiod alone. Exercise might also be important for inducing changes in metabolic pathways and physiological responses for the migratory phenotype. Indeed, exercise training for house sparrows resulted in increases in pectoralis muscle mass, trans-sarcolemmal and intramyocyte lipid transport capacities and cellular metabolic intensities on constant photoperiods (Zhang et al. 2015a).
Correlations
In this study, neither pectoralis muscle mass, fat transport capacity, the myostatin system, nor regulatory enzymes for cellular metabolic intensity were significantly correlated with Msum. Positive correlations of pectoralis muscle mass or cellular metabolic intensity with Msum have been observed in previous avian studies (Petit and Vézina 2014; Swanson et al. 2013, 2014b). We observed significant positive correlations between protein expression of the active form of myostatin and mRNA expression of TLL-1, but not TLL-2, which suggests that TLL-1 serves as the major activator for myostatin in juncos. Prominent changes in mRNA expression of TLL-1 but not TLL-2 have also been observed for wintering house sparrows (Swanson et al. 2009), but this was not true for other bird species, as pectoralis TLL-2 expression in winter black-capped chickadees exceeded that for summer but TLL-1 expression did not, and similar seasonal trends for both TLLs occurred for American goldfinches (Swanson et al. 2014a). In addition, neither the migratory condition (King et al. 2015) nor exercise-training (Price et al. 2011) resulted in alterations of pectoralis mRNA expression for the TLLs relative to non-migratory or non-exercised conditions.
For fatty acid transporters, we observed a positive correlation of pectoralis FAT/CD36 and FABPpm mRNA expression, emphasizing the importance of cooperation between these two sarcolemmal lipid transporters. Moreover, FABPc protein expression was positively correlated with both FABPpm mRNA and protein expression, highlighting the potential role for FABPc as a receptor for NEFAs from membrane-bound FABPpm. Similar correlations between these FABPs have also been observed for exercise- and acute cold-trained house sparrows (Zhang et al. 2015a). Together with the positive correlations of pectoralis CS with CPT and HOAD, the data suggest parallel variation and integration of the various steps in fatty acid transport and catabolism pathways in dark-eyed juncos in this study. Such relationships are consistent with the concept of symmorphosis and suggest that correlated variation is important for acclimatization to temperature and photoperiod in birds (Suarez, 1998, Swanson, 2010). Similar correlations among these pathways were also observed in exercise- and cold-trained house sparrows (Zhang et al. 2015a) and during migration in white-throated sparrows (McFarlan et al. 2009), but were not observed during migration or winter acclimatization for other birds (Zhang et al. 2015c, 2015d). As a result, evidence for the concept of symmorphosis of metabolic pathways under conditions of increased energy demands is still inconclusive, but is seemingly more consistent for experimental manipulation under standardized laboratory conditions than under natural conditions requiring elevated energy demands.
In conclusion, along with the data of Swanson et al. (2014c) and Stager et al. (2015), these data suggest that regulation of flight muscle mass, the myostatin system, and lipid transport capacities are common methods for regulating metabolic capacities of birds in general. Effects of cold acclimation or winter acclimatization, migratory status, or photoperiod on these pathways, however, might be species- or context-specific, so firm generalizations about regulation of these pathways under different conditions promoting increased energy demands are currently difficult to delineate.
Acknowledgments
We thank Sol Redlin, Kathie Rasmussen, and Bob Garner for access to their property or helps on collection of juncos. We thank Ming Liu, Kyle Kirby, Stephanie Owens, and Will Culver III for technical assistance in the laboratory and field. We also thank Jianqiu Zou and Yi-Fan Li for their advice on Western blots. Two anonymous reviewers provided helpful comments on a previous version of this article and we thank them for their efforts.
Funding
This research was funded by NSF IOS-1021218 to D.L.S.
References
- Battley PF, Dekinga A, Dietz MW, Piersma T, Tang S. et al. , 2001. Basal metabolic rate declines during long-distance migratory flight in great knots. Condor 103:838–845. [Google Scholar]
- Bonen A, Miskovic D, Kiens B, 1999. Fatty acid transporters, FABPpm, FAT, FATP. in human muscle. Can J Appl Physiol 24:515–523. [DOI] [PubMed] [Google Scholar]
- Carey C, Dawson WR, 1999. A search for environmental cues used by birds in survival of cold winters In: Nolan V, Ketterson ED, Thompson CF, editors. Current Ornithology. Boston: Springer, 1–31. [Google Scholar]
- Chabowski A, Chatham JC, Tandon NN, Calles-Escandon J, Glatz JF. et al. , 2006. Fatty acid transport and FAT/CD36 are increased in red but not in white skeletal muscle of ZDF rats. Am J Physiol Endocrinol Metab 291:E675–E682. [DOI] [PubMed] [Google Scholar]
- Chabowski A, Coort SLM, Calles-Escandon J, Tandon NN, Glatz JFC. et al. , 2004. Insulin stimulates fatty acid transport by regulating expression of FAT/CD36 but not FABPpm. Am J Physiol – Endocrinol Metabol 287:E781–E789. [DOI] [PubMed] [Google Scholar]
- Chabowski A, Górski J, Luiken JJFP, Glatz JFC, Bonen A, 2007. Evidence for concerted action of FAT/CD36 and FABPpm to increase fatty acid transport across the plasma membrane. Prostaglandins Leukot Ess 77:345–353. [DOI] [PubMed] [Google Scholar]
- Corder KR, DeMoranville KJ, Russell DE, Huss JM, Schaeffer PJ, 2016. Annual life-stage regulation of lipid metabolism and storage and association with PPARs in a migrant species: the gray catbird Dumetella carolinensis. J Exp Biol 219:3391–3398. [DOI] [PubMed] [Google Scholar]
- Dietz MW, Piersma T, Dekinga A, 1999. Body-building without power training: endogenously regulated pectoral muscle hypertrophy in confined shorebirds. J Exp Biol 202:2831–2837. [DOI] [PubMed] [Google Scholar]
- Dubois K, Hallot F, Vézina F, 2016. Basal and maximal metabolic rates differ in their response to rapid temperature change among avian species. J Comp Physiol B 186:919–935. [DOI] [PubMed] [Google Scholar]
- Evans D, Rose R, 1988. Cardiovascular and respiratory responses to submaximal exercise training in the thoroughbred horse. Pflugers Arch 411:316–321. [DOI] [PubMed] [Google Scholar]
- Evans PR, Davidson NC, Uttley JD, Evans RD, 1992. Premigratory hypertrophy of flight muscles: an ultrastructural Study. Ornis Scand 23:238–243. [Google Scholar]
- Glatz JF, Luiken JJ, Bonen A, 2010. Membrane fatty acid transporters as regulators of lipid metabolism: implications for metabolic disease. Physiol Rev 90:367–417. [DOI] [PubMed] [Google Scholar]
- Guglielmo CG, 2010. Move that fatty acid: fuel selection and transport in migratory birds and bats. Integr Comp Biol 50:336–345. [DOI] [PubMed] [Google Scholar]
- Guglielmo CG, Haunerland NH, Hochachka PW, Williams TD, 2002. Seasonal dynamics of flight muscle fatty acid binding protein and catabolic enzymes in a migratory shorebird. Am J Physiol 282:R1405–R1413. [DOI] [PubMed] [Google Scholar]
- Hamilton JA, Guo W, Kamp F, 2002. Mechanism of cellular uptake of long-chain fatty acids: do we need cellular proteins? Mol Cell Biochem 239:17–23. [PubMed] [Google Scholar]
- Han X-X, Chabowski A, Tandon NN, Calles-Escandon J, Glatz JFC. et al. , 2007. Metabolic challenges reveal impaired fatty acid metabolism and translocation of FAT/CD36 but not FABPpm in obese Zucker rat muscle. Am J Physiol – Endocrinol Metabol 293:E566–E575. [DOI] [PubMed] [Google Scholar]
- Huet C, Li Z-F, Liu H-Z, Black RA, Galliano M-F. et al. , 2001. Skeletal muscle cell hypertrophy induced by inhibitors of metalloproteases; myostatin as a potential mediator. Am J Physiol – Cell Physiol 281:C1624–C1634. [DOI] [PubMed] [Google Scholar]
- Jenni-Eiermann S, Jenni L, Kvist A, Lindstrom A, Piersma T. et al. , 2002. Fuel use and metabolic response to endurance exercise: a wind tunnel study of a long-distance migrant shorebird. J Exp Biol 205:2453–2460. [DOI] [PubMed] [Google Scholar]
- Kiens B, 2006. Skeletal muscle lipid metabolism in exercise and insulin resistance. Physiol Rev 86:205–243. [DOI] [PubMed] [Google Scholar]
- King M, Zhang Y, Carter T, Johnson J, Harmon E. et al. , 2015. Phenotypic flexibility of skeletal muscle and heart masses and expression of myostatin and tolloid-like proteinases in migrating passerine birds. J Comp Physiol 185(3):333–342. [DOI] [PubMed] [Google Scholar]
- Lee S-J, 2004. Regulation of muscle mass by myostatin. Annu Rev Cell Dev Biol 20:61–86. [DOI] [PubMed] [Google Scholar]
- Lee S-J, 2008. Genetic analysis of the role of proteolysis in the activation of latent myostatin. PLoS ONE 3:e1628.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liknes ET, Guglielmo CG, Swanson DL, 2014. Phenotypic flexibility in passerine birds: seasonal variation in fuel storage, mobilization and transport. Comp Biochem Physiol B 174:1–10. [DOI] [PubMed] [Google Scholar]
- Liknes ET, Swanson DL, 2011a. Phenotypic flexibility in passerine birds: seasonal variation of aerobic enzyme activities in skeletal muscle. J Therm Biol 36:430–436. [Google Scholar]
- Liknes ET, Swanson DL, 2011b. Phenotypic flexibility of body composition associated with seasonal acclimatization in passerine birds. J Therm Biol 36:363–370. [Google Scholar]
- Livak KJ, Schmittgen TD, 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T). Method. Methods 25:402–408. [DOI] [PubMed] [Google Scholar]
- Luiken JJ, Koonen DP, Coumans WA, Pelsers MM, Binas B. et al. , 2003. Long-chain fatty acid uptake by skeletal muscle is impaired in homozygous, but not heterozygous, heart-type-FABP null mice. Lipids 38:491–496. [DOI] [PubMed] [Google Scholar]
- Marsh RL, 1981. Catabolic enzyme activities in relation to premigratory fattening and muscle hypertrophy in the gray catbird Dumetella carolinensis. J Comp Physiol B 141:417–423. [Google Scholar]
- Marsh RL, 1984. Adaptations of the gray catbird Dumetella carolinensis to long-distance migration: flight muscle hypertrophy associated with elevated body mass. Physiol Zool 57:105–117. [Google Scholar]
- Marsh R, Dawson W, 1989. Avian adjustments to cold In: Wang LCH, editor. Animal Adaptation to Cold. Berlin, Heidelberg: Springer, 205–253. [Google Scholar]
- McFarlan JT, Bonen A, Guglielmo CG, 2009. Seasonal upregulation of fatty acid transporters in flight muscles of migratory white-throated sparrows Zonotrichia albicollis. J Exp Biol 212:2934–2940. [DOI] [PubMed] [Google Scholar]
- Nickerson JG, Alkhateeb H, Benton CR, Lally J, Nickerson J. et al. , 2009. Greater transport efficiencies of the membrane fatty acid transporters FAT/CD36 and FATP4 compared with FABPpm and FATP1 and differential effects on fatty acid esterification and oxidation in rat skeletal muscle. J Biol Chem 284:16522–16530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit M, Lewden A, Vezina F, 2014. How does flexibility in body composition relate to seasonal changes in metabolic performance in a small passerine wintering at northern latitude?. Physiol Biochem Zool 87:539–549. [DOI] [PubMed] [Google Scholar]
- Petit M, Vezina F, 2014. Phenotype manipulations confirm the role of pectoral muscles and haematocrit in avian maximal thermogenic capacity. J Exp Biol 217:824–830. [DOI] [PubMed] [Google Scholar]
- Piersma T, 1998. Phenotypic flexibility during migration: optimization of organ size contingent on the risks and rewards of fueling and flight? J Avian Biol: 511–520. [Google Scholar]
- Price ER, McFarlan JT, Guglielmo CG, 2010. Preparing for migration? The effects of photoperiod and exercise on muscle oxidative enzymes, lipid transporters, and phospholipids in white-crowned sparrows. Physiol Biochem Zool 83:252–262. [DOI] [PubMed] [Google Scholar]
- Price ER, Bauchinger U, Zajac DM, Cerasale DJ, McFarlan JT. et al. , 2011. Migration- and exercise-induced changes to flight muscle size in migratory birds and association with IGF1 and myostatin mRNA expression. J Exp Biol 214:2823–2831. [DOI] [PubMed] [Google Scholar]
- Rodgers BD, Garikipati DK, 2008. Clinical, agricultural, and evolutionary biology of myostatin: a comparative review. Endocr Rev 29:513–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stager M, Swanson DL, Cheviron ZA, 2015. Regulatory mechanisms of metabolic flexibility in the dark-eyed junco Junco hyemalis. J Exp Biol 218:767–777. [DOI] [PubMed] [Google Scholar]
- Suarez RK, 1998. Oxygen and the upper limits to animal design and performance. J Exp Biol 201:1065–1072. [DOI] [PubMed] [Google Scholar]
- Swanson DL, 1990. Seasonal variation in cold hardiness and peak rates of cold-induced thermogenesis in the dark-eyed junco Junco hyemalis. The Auk 107(3):561–566. [Google Scholar]
- Swanson DL, 1991. Seasonal adjustments in metabolism and insulation in the dark-eyed junco. Condor 99(2):538–545. [Google Scholar]
- Swanson DL, 2001. Are summit metabolism and thermogenic endurance correlated in winter acclimatized passerine birds?. J Comp Physiol B 171:475–481. [DOI] [PubMed] [Google Scholar]
- Swanson DL, 2010. Seasonal metabolic variation in birds: functional and mechanistic correlates. Curr Ornithol 17:75–129. [Google Scholar]
- Swanson DL, King MO, Culver W III, Zhang Y, 2017. Within-Winter flexibility in muscle masses, myostatin, and cellular aerobic metabolic intensity in passerine birds. Physiol Biochem Zool 90(2): 210–222. [DOI] [PubMed] [Google Scholar]
- Swanson DL, King MO, Harmon E, 2014a. Seasonal variation in pectoralis muscle and heart myostatin and tolloid-like proteinases in small birds: a regulatory role for seasonal phenotypic flexibility? J Comp Physiol B 184:249–258. [DOI] [PubMed] [Google Scholar]
- Swanson DL, Liknes ET, 2006. A comparative analysis of thermogenic capacity and cold tolerance in small birds. J Exp Biol 209:466–474. [DOI] [PubMed] [Google Scholar]
- Swanson DL, Olmstead KL, 1999. Evidence for a proximate influence of winter temperature on metabolism in passerine birds. Physiol Biochem Zool 72:566–575. [DOI] [PubMed] [Google Scholar]
- Swanson DL, Sabirzhanov B, VandeZande A, Clark TG, 2009. Seasonal variation of myostatin gene expression in pectoralis muscle of house sparrows Passer domesticus is consistent with a role in regulating thermogenic capacity and cold tolerance. Physiol Biochem Zool 82:121–128. [DOI] [PubMed] [Google Scholar]
- Swanson DL, Vézina F, 2015. Environmental, ecological and mechanistic drivers of avian seasonal metabolic flexibility in response to cold winters. J Ornithol 156:377–388. [Google Scholar]
- Swanson DL, Zhang Y, King MO, 2013. Individual variation in thermogenic capacity is correlated with flight muscle size but not cellular metabolic capacity in American goldfinches Spinus tristis. Physiol Biochem Zool 86:421–431. [DOI] [PubMed] [Google Scholar]
- Swanson D, Zhang Y, King M, 2014b. Mechanistic drivers of flexibility in summit metabolic rates of small birds. PLoS ONE 9:e101577.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson D, Zhang Y, Liu J-S, Merkord CL, King MO, 2014c. Relative roles of temperature and photoperiod as drivers of metabolic flexibility in dark-eyed juncos. J Exp Biol 217:866–875. [DOI] [PubMed] [Google Scholar]
- Vézina F, Jalvingh KM, Dekinga A, Piersma T, 2006. Acclimation to different thermal conditions in a northerly wintering shorebird is driven by body mass-related changes in organ size. J Exp Biol 209:3141–3154. [DOI] [PubMed] [Google Scholar]
- Vézina F, Jalvingh KM, Dekinga A, Piersma T, 2007. Thermogenic side effects to migratory predisposition in shorebirds. Am J Physiol – Regul, Integr Comp Physiol 292:R1287–R1297. [DOI] [PubMed] [Google Scholar]
- Zajac DM, Cerasale DJ, Landman S, Guglielmo CG, 2011. Behavioral and physiological effects of photoperiod-induced migratory state and leptin on Zonotrichia albicollis: II. Effects on fatty acid metabolism. Gen Comp Endocrinol 174:269–275. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Carter T, Eyster K, Swanson DL, 2015a. Acute cold and exercise training upregulate similar aspects of fatty acid transport and catabolism in house sparrows Passer domesticus. J Exp Biol 218(24):3885–3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Eyster K, Liu J-S, Swanson DL, 2015b. Cross-training in birds: cold and exercise training produce similar changes in maximal metabolic output, muscle masses and myostatin expression in house sparrows Passer domesticus. J Exp Biol 218(14):2190–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, King MO, Harmon E, Eyster K, Swanson DL, 2015c. Migration-induced variation of fatty acid transporters and cellular metabolic intensity in passerine birds. J Comp Physiol B 185:797–810. [DOI] [PubMed] [Google Scholar]
- Zhang Y, King MO, Harmon E, Swanson DL, 2015d. Summer-to-winter phenotypic flexibility of fatty acid transport and catabolism in skeletal muscle and heart of small birds. Physiol Biochem Zool 88:535–549. [DOI] [PubMed] [Google Scholar]
- Zheng WH, Liu JS, Swanson DL, 2014. Seasonal phenotypic flexibility of body mass, organ masses, and tissue oxidative capacity and their relationship to resting metabolic rate in Chinese bulbuls. Physiol Biochem Zool 87:432–444. [DOI] [PubMed] [Google Scholar]