Abstract
Background
Burkholderia pseudomallei is an environmental Gram-negative bacillus and the cause of melioidosis. B. thailandensis, some strains of which express a B. pseudomallei-like capsular polysaccharide (BTCV), is also commonly found in the environment in Southeast Asia but is considered non-pathogenic. The aim of the study was to determine the distribution of B. thailandensis and its capsular variant in Thailand and investigate whether its presence is associated with a serological response to B. pseudomallei.
Methodology/principal findings
We evaluated the presence of B. pseudomallei and B. thailandensis in 61 rice fields in Northeast (n = 21), East (n = 19) and Central (n = 21) Thailand. We found BTCV in rice fields in East and Central but not Northeast Thailand. Fourteen fields were culture positive for B. pseudomallei alone, 8 for B. thailandensis alone, 11 for both B. pseudomallei and B. thailandensis, 6 for both B. thailandensis and BTCV, and 5 for B. pseudomallei, B. thailandensis and BTCV. Serological testing using the indirect hemagglutination assay (IHA) of 96 farmers who worked in the study fields demonstrated that farmers who worked in B. pseudomallei-positive fields had higher IHA titers than those who worked in B. pseudomallei-negative fields (median 1:40 [range: <1:10–1:640] vs. <1:10 [range: <1:10–1:320], p = 0.002). In a multivariable ordered logistic regression model, IHA titers were significantly associated with the presence of B. pseudomallei (aOR = 3.7; 95% CI 1.8–7.8, p = 0.001) but were not associated with presence of B. thailandensis (p = 0.32) or BTCV (p = 0.32). One sequence type (696) was identified for the 27 BTCV isolates tested.
Conclusions/significance
This is the first report of BTCV in Thailand. The presence of B. pseudomallei and B. thailandensis in the same field was not uncommon. Our findings suggest that IHA positivity of healthy rice farmers in Thailand is associated with the presence of B. pseudomallei in rice fields rather than B. thailandensis or BTCV.
Author summary
Burkholderia thailandensis is a non-pathogenic soil-dwelling bacterium and is genetically closely related to Burkholderia pseudomallei, the cause of melioidosis. In mouse models, inoculation of a variant of B. thailandensis which express a B. pseudomallei-like capsular polysaccharide (BTCV) induces antibodies and partial protection against melioidosis. Here, we evaluated the presence of B. pseudomallei, B. thailandensis, and BTCV in 61 rice fields in Northeast, East and Central Thailand, determined whether they co-existed, and if their presence was associated with a serological response in farmers. We report the presence of BTCV in Thailand for the first time and describe the distribution of the three organisms. Co-localization between these organisms in the same rice fields was not uncommon. Our findings suggested that serological positivity based on the indirect hemagglutination assay (a test commonly used to detect antibodies to B. pseudomallei) in healthy rice farmers in Thailand was associated with exposure to B. pseudomallei, but not exposure to B. thailandensis or BTCV.
Introduction
Burkholderia pseudomallei is a soil-dwelling Gram-negative bacterium and the cause of melioidosis, a frequently fatal infectious disease of humans and animals. Humans acquire the disease following skin inoculation, inhalation or ingestion of the bacterium from the environment. The disease is highly endemic in Southeast Asia and Northern Australia [1], and is increasingly being reported in South Asia, Africa and Central and South America [2, 3]. A recent modeling study estimated that there are about 165,000 human melioidosis cases per year worldwide, of whom 89,000 (54%) die [4]. The current diagnostic standard for melioidosis is microbiological culture [5]. However, melioidosis is difficult to diagnose due to its diverse clinical manifestations, the inadequacy of conventional bacterial identification methods, and a lack of microbiology laboratories in tropical developing countries [5]. An indirect haemagglutination assay (IHA) is the most frequently used serological test for melioidosis, but may be misleading when used for the diagnosis of melioidosis in disease-endemic regions [5]. This is because the background seropositivity (IHA titers ≥1:160) ranges from 4% to 32% in healthy individuals living in areas where melioidosis is endemic [6–8]. Therefore, IHA is recommended as a serological standard to assess exposure to B. pseudomallei [5].
Burkholderia thailandensis was first recognized by Wuthiekanun et al. in 1996 [9]. The organism is genetically closely related to B. pseudomallei, can be isolated from environmental soil and water, and is non-pathogenic [9–11]. The colony morphology of B. thailandensis and B. pseudomallei are very similar, but B. thailandensis can assimilate L-arabinose [12, 13]. In addition, B. thailandensis has polysaccharide-related genes that are distinct from B. pseudomallei (74.8% and 72.8% nucleotide and protein similarity, respectively) and usually lacks the virulence-associated capsular polysaccharide (also referred to as CPS or CPS-I) of B. pseudomallei [14–17]. The geographical distribution of B. thailandensis is uncertain but the organism has rarely been isolated from fields that are culture positive for B. pseudomallei [18, 19]. It was recently shown that B. pseudomallei can inhibit the growth and motility of B. thailandensis in the laboratory [20]. However, previous environmental studies did not systematically evaluate the presence of both organisms, so the presence of B. thailandensis and co-localization of both organisms may have been underestimated [18, 19]. In an experimental mouse model, lipopolysaccharide extracted from B. thailandensis induced measurable IgG and IgM, and provided partial protection against melioidosis [21]. The association between exposure to environmental B. thailandensis and IHA seropositivity in humans is still largely unknown.
A variant of B. thailandensis originally isolated from soil in Cambodia (E555) that contained genes encoding a B. pseudomallei-like capsular polysaccharide cluster (BTCV) was described in 2010 [17]. This organism exhibited several B. pseudomallei-like phenotypes including colony wrinkling, resistance to human complement binding, and intracellular macrophage survival. However, in mice E555 was avirulent [17], induced higher levels of IgG and gave better protection against melioidosis than non-capsulated B. thailandensis [22]. The capsular polysaccharide (CPS) biosynthesis gene cluster of E555 and that of B. pseudomallei are highly similar (94.4% and 96% nucleotide and protein similarity, respectively) [17], and nuclear magnetic resonance spectroscopy has shown that the structures of CPS produced by E555 and that of B. pseudomallei are identical [23]. Previously, BTCV has been isolated from human blood in the USA in 2003 (strain CDC3015869; ST101, USA [24]) and from environmental samples in Cambodia in 2010 (strain E555; ST696), Gabon in 2013 (strain D50; ST1126 [25]) and Laos in 2015 (strain ST_10; ST696 [26]). BTCV has not been reported in Thailand and its distribution is unknown.
We recently reported the presence of B. pseudomallei in 61 rice fields in the Northeast, East and Central Thailand, and its association with soil physicochemical properties [27]. Here, we report the presence of B. thailandensis and co-localization between B. pseudomallei and B. thailandensis in the same rice fields, and provide the first report of the BTCV in Thailand. In addition, we explored whether exposure to B. thailandensis and BTCV is associated with background seropositivity to B. pseudomallei by evaluating IHA levels in healthy adults who worked in the sampled rice fields.
Materials and methods
Study area
East, Central and Northeast Thailand consist of 7, 21 and 20 provinces, cover 34,381, 93,005 and 168,854 km2, and had estimated populations in 2013 of 3.9, 18.7 and 23.3 million, respectively [28]. Northeast Thailand is a plateau surrounded by mountain ranges, and most of the arable land consists of tropical sandy soil. East Thailand is characterized by short mountain ranges alternating with alluvial plains. Central Thailand is a large plain consisting of clay soil. Rice farming is the predominant form of agriculture in all three regions. In Thailand, for administrative purposes each province is sub-divided into districts, sub-districts, communes and villages. The majority of the population in all three regions live in rural settings and most adults are engaged in agriculture, particularly rice farming. In 2013, areas used for agriculture were 57%, 48% and 60% in East, Central and Northeast Thailand, respectively [29].
Study design
We conducted a cross-sectional environmental survey as described previously [27]. All B. pseudomallei, B. thailandensis and BTCV reported in this work were from the same environmental survey [27]. In brief, we collected soil from randomly-selected rice fields in the East, Central and Northeast regions during the dry season (from April–June) in 2013, 2014 and 2015, respectively. We sampled rice fields that had been used for rice farming in the 12 months prior to the sampling date. We collected the blood from farmers who were exposed to the sampled rice fields in the 12 months prior to the blood collection date.
Ethics statement
Written, informed consent was obtained from land owners and farmers prior to soil sampling and blood collection, respectively. The study protocol was approved by the Ethics Committee of the Faculty of Tropical Medicine, Mahidol University (MUTM 2013-021-01) and the Oxford Tropical Research Ethics Committee, University of Oxford (OXTREC 1013–13).
Soil sampling and soil properties
The method used for soil sampling was described previously [27, 30]. In brief, each rice field was divided into a 10x10 grid system to generate 100 sampling points per field. At each sampling point, around 30 grams of soil was removed from the base of a 30-cm hole. All soil samples were processed within 48 hours of collection for the identification of B. pseudomallei and B. thailandensis, and for soil physicochemical properties. One kilogram of soil from each sampling field was made by aggregating 100 soil samples (10 g per each sampling point) and evaluated for soil physicochemical properties as described previously [27].
Identification of B. pseudomallei and B. thailandensis
Ten grams of soil from each sampling point was mixed with 10 ml of enrichment broth consisting of threonine-basal salt solution plus colistin 50mg/L (TBSS-C50 broth) and incubated at 40°C in air for 48 hours. Ten microliters of surface liquid was then streaked onto Ashdown agar containing gentamicin 8mg/L and crystal violet 5mg/L using a calibrated loop, incubated at 40°C in air, and examined every day for 4 days for bacterial colonies suggestive of B. pseudomallei or B. thailandensis [31, 32]. B. pseudomallei can have seven colony morphotypes on Ashdown agar [32], and cannot readily be distinguished from the colonies of B. thailandensis [9, 12, 13]. For each soil specimen, a total of up to five presumptive colonies of B. pseudomallei or B. thailandensis were picked and evaluated by a latex agglutination test that is highly specific for B. pseudomallei CPS [16, 33, 34], and the L-arabinose assimilation test. B. pseudomallei was defined based on a positive latex agglutination test and negative L-arabinose assimilation. B. thailandensis was defined based on a negative latex agglutination test and positive L-arabinose assimilation [9, 16, 17]. Colonies which had positive results for both the latex agglutination and L-arabinose assimilation tests were defined as BTCV, as previously described [17]. The monoclonal antibody used in the latex agglutination test has been shown to be positive for B. pseudomallei and BTCV (strains E555 and CDC3015869), and negative for B. thailandensis [35, 36].
Genotyping of BTCV
Because BTCV was found in rice fields in East and Central Thailand, we randomly selected three isolates of BTCV from each culture positive field and genotyped these using multilocus sequence typing (MLST) [37]. The alleles at each of the seven loci were assigned by comparing the sequences to those on the B. pseudomallei MLST website (https://pubmlst.org/bpseudomallei/).
Information and the sequence type of BTCV reported in this work has been deposited in the global MLST database (https://pubmlst.org/bpseudomallei/).
Serological response
Blood samples were collected from rice farmers who had worked in the sampled rice fields in the 12 months prior to the sampling date. The detection of antibodies against B. pseudomallei was performed using the IHA, as described previously [38, 39]. The antigen used in the IHA was derived from a pool of two clinical B. pseudomallei isolates, 199a and 207a, obtained from melioidosis patients in Ubon Ratchathani, Thailand. The negative control was pooled sera from three patients with no detectable IHA titers. The positive control was pooled sera from three patients with known positive IHA. In this study, for binary comparisons, an IHA titer of <1:80 was defined as negative based on the previous report that healthy US donor could have IHA titers up to 1:40 [40].
Sample size calculation
To determine the optimal sample size, we performed a pilot study of soil sampling in four rice fields in Chachoengsao province, East Thailand. Three and four rice fields were culture positive for B. pseudomallei (75%; 3 of 4) and B. thailandensis (100%; 4 of 4), respectively. We calculated that 60 rice fields (3 rice fields per province) and 60 rice farmers were needed to determine environmental factors associated with presence of B. pseudomallei and B. thailandensis, and evaluate the association between IHA titers and presence of both organisms, respectively, with a power of 80% at an alpha error of 5%.
Statistical analysis
We evaluated (1) positivity of B. thailandensis and BTCV in the rice fields, (2) co-localization and correlation between B. pseudomallei, B. thailandensis and BTCV in the rice fields, (3) soil properties associated with presence of B. thailandensis and BTCV, and (4) association between IHA levels and presence of those organisms in the rice fields. Fisher’s exact test and Mann-Whitney test were performed to compare binary and ordinal variables, respectively. McNemar’s test was used to compare the presence of two organisms. We assessed co-localization between organisms in the rice fields by using Kappa value. The Kappa value was used to describe the agreement of presence and absence of the organism in rice fields, beyond that caused by chance, as follows: 0.00–0.20, slight; 0.21–0.40, fair; 0.41–0.60, moderate; 0.61–0.80, substantial; 0.81–1.00, high [41]. A Spearman rank correlation coefficient (Spearman’s rho) was used to assess correlations among the total number of sampling points culture-positive for B. pseudomallei, B. thailandensis and BTCV in the rice fields. Spearman’s rho close to 0 indicates no correlation, while Spearman’s rho close to 1 (or -1) indicates a strong positive (or negative) correlation between the organisms [42]. We used ordered logistic regression to determine the associations between IHA level and presence of the three organisms. As we sampled more than one rice farmer per field, the analysis was stratified by rice field. Multivariable ordered logistic regression models were developed using a purposeful selection method [43]. In brief, a univariable ordered logistic regression model was used to preliminarily evaluate the crude association between presence of B. pseudomallei, B. thailandensis and BTCV and IHA titers. We decided a priori to evaluate their independent associations in a multivariable ordered logistic regression model, and presence of B. pseudomallei, B. thailandensis and BTCV were all included in the final multivariable model. Sensitivity analysis was conducted by grouping B. thailandensis and BTCV as B. thailandensis. All statistical tests were performed using STATA version 14.0 (StataCorp LP, College Station, Texas). The final database with the data dictionary are publicly available online (https://doi.org/10.6084/m9.figshare.4928993).
Results
Distribution of B. thailandensis and BTCV
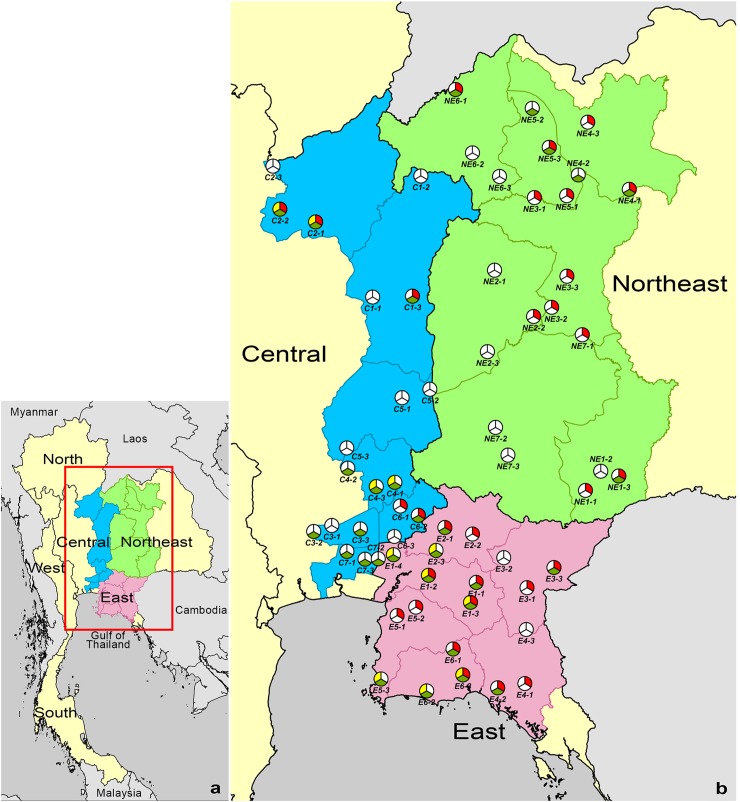
Of 6,100 soil samples collected from 61 rice fields, 826 (14%) were culture positive for B. thailandensis. The percentages of rice fields that were culture-positive for B. thailandensis were 29% (6 of 21 rice fields), 63% (12 of 19 rice fields) and 57% (12 of 21 rice fields) in Northeast, East and Central Thailand, respectively (Fig 1). There was borderline evidence that culture-positivity for B. thailandensis was higher in the East than the Northeast (63% vs. 29%, p = 0.055), while there was no significant difference between East and Central (63% vs. 57%, p = 0.76) or Northeast and Central regions (29% vs. 57%, p = 0.12).
Fig 1. Map of the presence of B. pseudomallei, B. thailandensis and B. thailandensis expressing B. pseudomallei-like capsular polysaccharide (BTCV) in 61 rice fields in Northeast (n = 21), East (n = 19) and Central (n = 21) Thailand.
(a) Map of Thailand. (b) Location of the 61 rice fields evaluated. Red, green and yellow pies represent rice fields that were culture positive and negative for B. pseudomallei, B. thailandensis, and BTCV, respectively. Province codes represent Burirum (NE1), Chaiyaphum (NE2), Khon Kaen (NE3), Udon Thani (NE4), Nong Bua Lam Phu (NE5), Loei (NE6) and Nakhon Ratchasima (NE7) in the Northeast, Chachoengsao (E1), Prachinburi (E2), Sa Kaeo (E3), Chanthaburi (E4), Chonburi (E5) and Rayong (E6) in the East, Phetchabun (C1), Phitsanulok (C2), Pathum Thani (C3), Saraburi (C4), Lopburi (C5), Nakhon Nayok (C6) and Bangkok (C7) in Central Thailand. ArcGis Version 10.2 (ESRI, Redlands, CA, USA) was used to map the sampled rice fields. The location of sampled rice fields was recorded by using the EpiCollect application (www.epicollect.net, Imperial College, London).
For the rice fields that were culture-positive for B. thailandensis, the median numbers of positive sampling points were 6.5 (range 1 to 27), 26.5 (range 1 to 100) and 10.5 (range 1 to 85) in Northeast, East and Central Thailand, respectively (S1 Table). There was a trend towards the median number of positive sampling points for B. thailandensis being lower in the Northeast than the East (p = 0.08), while there was no significant difference between Central versus Northeast or East Thailand (p = 0.40 and 0.52, respectively).
BTCV was isolated from 11 of 61 (18%) rice fields in the East (7 fields) and Central (4 fields) regions but it was not isolated from rice fields in Northeast Thailand (Fig 1). Overall, the proportion of fields positive for the BTCV was lower than that for B. thailandensis (18% vs. 49%, p<0.001). The percentage of rice fields that were culture positive for BTCV in the East and Central regions was not significantly different (37% [7/19] vs. 19% [4/21], p = 0.29). The median numbers of positive sampling points in the East and Central regions were also not significantly different (3 [range 1 to 24] vs. 4 [range 1 to 8], p = 0.45).
Co-localization and correlation between presence of B. pseudomallei, B. thailandensis and BTCV in the same rice fields
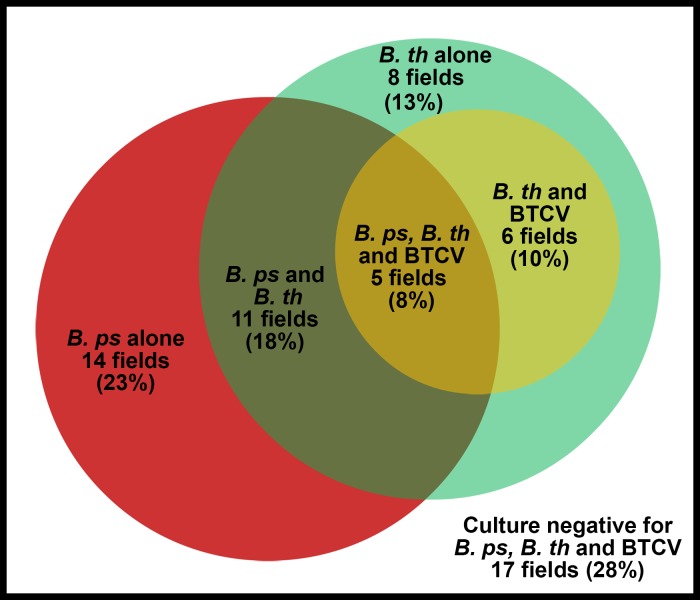
We previously reported the isolation of B. pseudomallei from 30 of 61 rice fields included in this study [27]. Fig 2 shows the number of rice fields from which B. pseudomallei, B. thailandensis and BTCV were isolated. Of 61 rice fields, 14 (23%) were positive for B. pseudomallei alone, 8 (13%) positive for B. thailandensis alone, 11 (18%) positive for both B. pseudomallei and B. thailandensis, 6 (10%) positive for B. thailandensis and BTCV, and 5 (8%) positive for B. pseudomallei, B. thailandensis and BTCV. Co-localization of B. pseudomallei and B. thailandensis in the same rice field was not more frequent than expected by chance (Kappa value 0.08, p = 0.26). The numbers of sampling points per rice field that were culture positive for B. pseudomallei and for B. thailandensis were not correlated (Spearman’s rho -0.02, 95%CI -0.27 to 0.23, p = 0.89). A sensitivity analysis was conducted by considering BTCV as B. thailandensis, which gave a comparable result (Spearman’s rho -0.02, 95%CI -0.27 to 0.23, p = 0.87). All eleven fields culture positive for BTCV were also culture positive for B. thailandensis (Fig 2). There was a fair agreement between presence of B. thailandensis and BTCV (Kappa value 0.37, p<0.001), and a strong correlation between the total number of sampling points culture positive for the two organisms (Spearman’s rho 0.68, 95% CI 0.51 to 0.79, p<0.001).
Fig 2. Overlap between presence of B. pseudomallei (B. ps; red), B. thailandensis (B. th; green) and B. thailandensis expressing B. pseudomallei-like capsular polysaccharide (BTCV; yellow) in 61 sampled rice fields.
Co-localization and correlation between presence of B. pseudomallei, B. thailandensis and BTCV in the same sampling points
Of 6,100 soil samples collected, 975 (16%) were positive for B. pseudomallei alone, 706 (12%) positive for B. thailandensis alone, 24 (0.4%) positive for BTCV alone, 69 (1%) positive for both B. pseudomallei and B. thailandensis, 1 (0.02%) positive for B. pseudomallei and BTCV, 50 (0.8%) positive for B. thailandensis and BTCV, and 1 (0.02%) positive for B. pseudomallei, B. thailandensis and BTCV. There was a slight agreement between presence of B. thailandensis and BTCV in the same soil sample (Kappa value 0.09, p<0.001). Co-localization of B. pseudomallei and B. thailandensis in the same soil sample was also not greater than that expected by chance (p>0.99).
Soil physicochemical properties associated with presence of B. thailandensis and BTCV
Associations between soil physicochemical properties and the presence of B. thailandensis were not observed (S2 Table). Presence of BTCV was negatively associated with cation exchange capacity, which represents the total nutrient fixing capacity of soil (p = 0.05), and associated with the level of total nitrogen (p = 0.04; S3 Table). The associations were also observed in the multivariable model (S4 Table).
Genetic diversity of BTCV
A total of 27 isolates of BTCV from 76 culture positive sampling points for BTCV in 11 rice fields in East and Central Thailand were randomly selected for MLST (S5 Table). All 27 isolates belonged to sequence type (ST) 696, which was identical to the ST of BTCV strain E555 reported from soil in Cambodia [17]. We had previously reported a single B. pseudomallei isolate (strain A-330-05-1-04) from drinking water in Ubon Ratchathani, northeast Thailand, as ST696 [44]. The isolate was re-evaluated. The isolate was found to be positive for both latex agglutination and L-arabinose assimilation and was thus re-classified as BTCV.
IHA titers and their association with presence of B. pseudomallei, B. thailandensis and BTCV
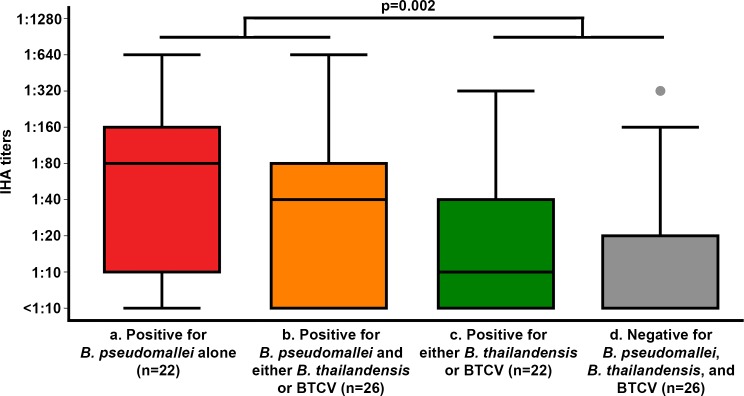
Of 96 rice farmers included in the analysis, 29, 35 and 32 were from Northeast, East and Central Thailand, respectively. The median number of farmers per rice field was 1 (range 1 to 5). Sixty-two farmers (65%) were male and median age was 51 years (range 23–75 years). Six farmers (6%) had a known diagnosis of diabetes. Overall, 27 (28%) farmers had a positive IHA (IHA titers ≥1:80). Forty eight farmers who worked in rice fields culture-positive for B. pseudomallei had higher IHA titers than the 48 farmers who worked in rice fields culture-negative for the organism (median 1:40 [range: <1:10–1:640] vs. <1:10 [range: <1:10–1:320], p = 0.002) (Fig 3). Proportion of farmers who had positive IHA (IHA ≥1:80) was also significantly higher in rice field culture-positive for B. pseudomallei than the farmers who work in rice field culture-negative for B. pseudomallei (44% vs. 13%, p = 0.001).
Fig 3. IHA titers associated with the presence of B. pseudomallei, B. thailandensis and B. thailandensis expressing B. pseudomallei-like capsular polysaccharide (BTCV) in the rice fields, respectively.
Box-and-whisker plots indicate median, interquartile range and distribution of IHA titers. Dots indicate outliers (data located outside 1.5 times of interquartile range). (a) IHA titers of farmers whose rice fields were culture positive for B. pseudomallei alone (22 farmers). (b) IHA titers of farmers whose rice fields were culture positive for B. pseudomallei and either B. thailandensis or BTCV (26 farmers), (c) IHA titers of farmers whose rice fields culture positive for either B. thailandensis or BTCV (22 farmers), and (d) IHA titers of farmers whose rice fields culture negative for B. pseudomallei, B. thailandensis and BTCV (26 farmers).
In the univariable ordered logistic regression model, IHA titers were associated with the presence of B. pseudomallei (OR = 3.39; 95% CI 1.66–6.90, p = 0.001) but not with presence of B. thailandensis (OR = 0.92; 95% CI 0.44–1.91, p = 0.82) or BTCV (OR = 1.04; 95% CI 0.60–1.80, p = 0.89). A multivariable ordered logistic regression model was used to evaluate independent association between IHA titers of rice farmers and presence of each organism. IHA titers were independently associated with the presence of B. pseudomallei (aOR = 3.72; 95% CI 1.76–7.84, p = 0.001) but not associated with the presence of B. thailandensis (p = 0.32) or BTCV (p = 0.32, Table 1).
Table 1. Factors associated with indirect hemagglutination assay (IHA) results in 96 healthy rice farmers.
| Organisms cultured from rice fields | IHA results | Odds Ratios (95% confidence interval)* | ||
|---|---|---|---|---|
| IHA positive ** (n = 27 famers) | IHA negative ** (n = 69 farmers) | Univariable analysis | Multivariable analysis | |
| B. pseudomallei | 21/27 (78%) | 27/69 (39%) | 3.39 (1.66–6.90), p = 0.001 | 3.72 (1.76–7.84), p = 0.001 |
| B. thailandensis | 12/27 (44%) | 36/69 (52%) | 0.92 (0.44–1.91), p = 0.82 | 0.63 (0.25–1.57), p = 0.32 |
| BTCV | 4/27 (15%) | 17/69 (25%) | 1.04 (0.60–1.80), p = 0.89 | 1.62 (0.63–4.17), p = 0.32 |
* Estimated by ordered logistic regression models stratified by sampled rice field.
** IHA titers ≥ 1:80 is defined as positive; IHA titers <1:80 is defined as negative.
Discussion
Here, we present data on the spatial distribution of B. pseudomallei, B. thailandensis and BTCV in Northeast, East and Central Thailand. This is the first study to report the isolation of BTCV from soil in Thailand, although we did find that a Thai water isolate previously identified as B. pseudomallei was actually an example of BTCV [44]. B. thailandensis was commonly isolated in all three regions, while BTCV was less common but associated with B. thailandensis. Co-localization of B. thailandensis and B. pseudomallei was not uncommon. Our findings also suggest that IHA positivity of healthy rice farmers was associated with exposure to B. pseudomallei rather than to B. thailandensis or BTCV. This supports the recommendation that IHA could be used to measure exposure to environmental B. pseudomallei [5], even in areas containing other closely related Burkholderia species.
Our finding of co-localization of B. pseudomallei and B. thailandensis is consistent with a previous environmental study in Khon Kaen, northeast Thailand [11]. B. thailandensis has been reported from many melioidosis-endemic countries; including Thailand, Laos, Vietnam [37], Cambodia (https://pubmlst.org/bpseudomallei/), Australia [45], Papua New Guinea [46], Kenya [37] and Gabon [25]. This is probably highly influenced by the locations of melioidosis research groups. B. thailandensis has also been reported in two non melioidosis-endemic countries; France and the United States [24, 37]. B. thailandensis strain 82172 (ST73) was isolated from the intestine of a foal in France in 1982, while B. thailandensis strain CDC2721121 (ST73) was isolated from the pleural wound of a 76 year old male from Louisiana, USA, in 1997 [24, 37]. As strain CDC2721121 was isolated from a wound sample its pathogenicity in humans cannot be assumed and this strain was avirulent in a mouse model [22].
Although BTCV was common in both Central and East Thailand, its prevalence is lower than that of wild-type B. thailandensis. Our analysis suggested that BTCV was associated with soil with low cation exchange capacity and high levels of total nitrogen. Although Sim et al. raised the possibility that acquisition of the B. pseudomallei-like CPS in E555 might improve its environmental fitness [17], this is not supported by our findings. It is also possible that the environment we studied is not representative of the environmental niche which induced B. thailandensis to acquire the B. pseudomallei-like gene cluster.
The finding that all BTCV isolates obtained from different geographical areas in Thailand, Laos and Cambodia were ST696 suggests that these may have arisen from a single ancestor. BTCV isolates in USA (ST101) [24] and Gabon (ST1126) [25] are single- and triple-locus variants of ST696, respectively (S5 Table). Previous phylogenetic analysis suggested that ST101 and ST696 are closely related and possibly share the same ancestor [17]. Studies using whole genome sequencing of BTCV and B. thailandensis from different regions are required to further understand the genetic diversity and evolution of this organism.
Our results suggest that exposure to environmental B. thailandensis and BTCV makes a limited contribution to IHA seropositivity in farmers. In animal models, antibodies can be detected after intraperitoneal inoculation of B. thailandensis and BTCV [22]. Intraperitoneal inoculation can lead to rapid dissemination of B. thailandensis or BTCV by bypassing natural host defenses [22], and induces a serological response. Nonetheless, human exposure to B. thailandensis and BTCV in the natural environment rarely if ever leads to infection, unlike exposure to B. pseudomallei. This is also supported by the finding that intra-nasal inoculation of high dose B. thailandensis or BTCV (106 colony forming unit [CFU]) did not cause death in BALB/c mice with rapid bacterial clearance and no visible abscess formation in sacrificed mice, whilst the LD50 of B. pseudomallei was less than 300 CFU in the same experiment [17].
Our study has several limitations. Soil sampling was performed during the dry season over a period of three years. We chose to sample during the dry season to control the variation in presence of the three organisms, human exposure and soil physicochemical properties associated with seasonal changes. It is possible that the presence of B. thailandensis and BTCV could vary according to the season. Farmers may work in multiple rice fields, and be exposed to B. pseudomallei in untested fields. Our study may have also detected more positive samples for B. thailandensis and BTCV if more than five colonies had been tested from each sampling point or using other identification methods such as PCR.
In summary, our large cross-sectional environmental survey has defined the distribution of B. thailandensis and BTCV in Thailand. This is the first report of BTCV in Thailand, which appears to be less common than wild-type B. thailandensis. Our findings also suggest that exposure to B. thailandensis or BTCV in the environment makes a limited contribution to IHA positivity amongst healthy farmers.
Supporting information
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
We thank the farmers, village heads and soil sampling volunteers who participated in the study. We thank Dr Sabine Dittrich for the additional information regarding BTCV in Laos. We thank Weerawat Wongasa, Sittikorn Rongsumlee, Taveewat Boonyakamolset, Prapass Wannapinij, Boonkoed Siriphong and Piyamas Buakaew for their laboratory and administrative support.
Data Availability
All 3 files are available from the Figshare database(https://figshare.com/s/9ad409f0d16f3845076a).
Funding Statement
The study was funded by the National Institute of Allergy and Infectious Diseases (Y1-AI-4906-09). DL is supported by an Intermediate Fellowship awarded by the Wellcome Trust (101103/Z/13/Z) and DABD by Wellcome Trust (106698/Z/14/Z). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Wiersinga WJ, Currie BJ, Peacock SJ. Melioidosis. N Engl J Med. 2012;367(11):1035–44. doi: 10.1056/NEJMra1204699 . [DOI] [PubMed] [Google Scholar]
- 2.Redondo MC, Gomez M, Landaeta ME, Rios H, Khalil R, Guevara RN, et al. Melioidosis presenting as sepsis syndrome: a case report. Int J Infect Dis. 2011;15(3):e217–8. doi: 10.1016/j.ijid.2010.11.009 . [DOI] [PubMed] [Google Scholar]
- 3.Currie BJ, Dance DA, Cheng AC. The global distribution of Burkholderia pseudomallei and melioidosis: an update. Trans R Soc Trop Med Hyg. 2008;102 Suppl 1:S1–4. doi: 10.1016/S0035-9203(08)70002-6 . [DOI] [PubMed] [Google Scholar]
- 4.Limmathurotsakul D, Golding N, Dance DAB, Messina JP, Pigott DM, Moyes CL, et al. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nature Microbiol. 2016;1:15008 doi: 10.1038/nmicrobiol.2015.8 [DOI] [PubMed] [Google Scholar]
- 5.Hoffmaster AR, AuCoin D, Baccam P, Baggett HC, Baird R, Bhengsri S, et al. Melioidosis diagnostic workshop, 2013. Emerg Infect Dis. 2015;21(2). doi: 10.3201/eid2102.141045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maude RR, Maude RJ, Ghose A, Amin MR, Islam MB, Ali M, et al. Seroepidemiological surveillance of Burkholderia pseudomallei in Bangladesh. Trans R Soc Trop Med Hyg. 2012;106(9):576–8. doi: 10.1016/j.trstmh.2012.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hii SYF, Kee CC, Ahmad N. Melioidosis: Overview of seropositivity in Malaysia. Trop Biomed. 2016;33(4):4. [PubMed] [Google Scholar]
- 8.Suttisunhakul V, Wuthiekanun V, Brett PJ, Khusmith S, Day NP, Burtnick MN, et al. Development of Rapid Enzyme-Linked Immunosorbent Assays for Detection of Antibodies to Burkholderia pseudomallei. J Clin Microbiol. 2016;54(5):1259–68. doi: 10.1128/JCM.02856-15 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wuthiekanun V, Smith MD, Dance DA, Walsh AL, Pitt TL, White NJ. Biochemical characteristics of clinical and environmental isolates of Burkholderia pseudomallei. J Med Microbiol. 1996;45(6):408–12. doi: 10.1099/00222615-45-6-408 . [DOI] [PubMed] [Google Scholar]
- 10.Trakulsomboon S, Dance DA, Smith MD, White NJ, Pitt TL. Ribotype differences between clinical and environmental isolates of Burkholderia pseudomallei. J Med Microbiol. 1997;46(7):565–70. doi: 10.1099/00222615-46-7-565 . [DOI] [PubMed] [Google Scholar]
- 11.Sermswan RW, Royros P, Khakhum N, Wongratanacheewin S, Tuanyok A. Direct detection of Burkholderia pseudomallei and biological factors in soil. Trans R Soc Trop Med Hyg. 2015;109(7):462–8. doi: 10.1093/trstmh/trv040 . [DOI] [PubMed] [Google Scholar]
- 12.Smith MD, Angus BJ, Wuthiekanun V, White NJ. Arabinose assimilation defines a nonvirulent biotype of Burkholderia pseudomallei. Infect Immun. 1997;65(10):4319–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brett PJ, DeShazer D, Woods DE. Burkholderia thailandensis sp. nov., a Burkholderia pseudomallei-like species. Int J Syst Bacteriol. 1998;48 Pt 1:317–20. doi: 10.1099/00207713-48-1-317 . [DOI] [PubMed] [Google Scholar]
- 14.Reckseidler SL, DeShazer D, Sokol PA, Woods DE. Detection of bacterial virulence genes by subtractive hybridization: identification of capsular polysaccharide of Burkholderia pseudomallei as a major virulence determinant. Infect Immun. 2001;69(1):34–44. doi: 10.1128/IAI.69.1.34-44.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith MD, Wuthiekanun V, Walsh AL, Pitt TL. Latex agglutination test for identification of Pseudomonas pseudomallei. J Clin Pathol. 1993;46(4):374–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wuthiekanun V, Anuntagool N, White NJ, Sirisinha S. Short report: a rapid method for the differentiation of Burkholderia pseudomallei and Burkholderia thailandensis. Am J Trop Med Hyg. 2002;66(6):759–61. . [DOI] [PubMed] [Google Scholar]
- 17.Sim BM, Chantratita N, Ooi WF, Nandi T, Tewhey R, Wuthiekanun V, et al. Genomic acquisition of a capsular polysaccharide virulence cluster by non-pathogenic Burkholderia isolates. Genome Biol. 2010;11(8):R89 doi: 10.1186/gb-2010-11-8-r89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vuddhakul V, Tharavichitkul P, Na-Ngam N, Jitsurong S, Kunthawa B, Noimay P, et al. Epidemiology of Burkholderia pseudomallei in Thailand. Am J Trop Med Hyg. 1999;60(3):458–61. [DOI] [PubMed] [Google Scholar]
- 19.Trakulsomboon S, Vuddhakul V, Tharavichitkul P, Na-Gnam N, Suputtamongkol Y, Thamlikitkul V. Epidemiology of arabinose assimilation in Burkholderia pseudomallei isolated from patients and soil in Thailand. Southeast Asian J Trop Med Public Health. 1999;30(4):756–9. [PubMed] [Google Scholar]
- 20.Ngamdee W, Tandhavanant S, Wikraiphat C, Reamtong O, Wuthiekanun V, Salje J, et al. Competition between Burkholderia pseudomallei and Burkholderia thailandensis. BMC Microbiol. 2015;15(1):56 doi: 10.1186/s12866-015-0395-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ngugi SA, Ventura VV, Qazi O, Harding SV, Kitto GB, Estes DM, et al. Lipopolysaccharide from Burkholderia thailandensis E264 provides protection in a murine model of melioidosis. Vaccine. 2010;28(47):7551–5. doi: 10.1016/j.vaccine.2010.08.058 . [DOI] [PubMed] [Google Scholar]
- 22.Scott AE, Laws TR, D'Elia RV, Stokes MG, Nandi T, Williamson ED, et al. Protection against Experimental Melioidosis following Immunization with Live Burkholderia thailandensis Expressing a manno-Heptose Capsule. Clin Vaccine Immunol. 2013;20(7):1041–7. Epub 2013/05/17. doi: 10.1128/CVI.00113-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bayliss M, Donaldson MI, Nepogodiev SA, Pergolizzi G, Scott AE, Harmer NJ, et al. Structural characterisation of the capsular polysaccharide expressed by Burkholderia thailandensis strain E555:: wbiI (pKnock-KmR) and assessment of the significance of the 2-O-acetyl group in immune protection. Carbohydr Res. 2017;452:17–24. doi: 10.1016/j.carres.2017.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glass MB, Gee JE, Steigerwalt AG, Cavuoti D, Barton T, Hardy RD, et al. Pneumonia and septicemia caused by Burkholderia thailandensis in the United States. J Clin Microbiol. 2006;44(12):4601–4. doi: 10.1128/JCM.01585-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiersinga WJ, Birnie E, Weehuizen TA, Alabi AS, Huson MA, Huis in 't Veld RA, et al. Clinical, environmental, and serologic surveillance studies of melioidosis in Gabon, 2012–2013. Emerg Infect Dis. 2015;21(1):40–7. doi: 10.3201/eid2101.140762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knappik M, Dance DA, Rattanavong S, Pierret A, Ribolzi O, Davong V, et al. Evaluation of Molecular Methods To Improve the Detection of Burkholderia pseudomallei in Soil and Water Samples from Laos. Appl Environ Microbiol. 2015;81(11):3722–7. doi: 10.1128/AEM.04204-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hantrakun V, Rongkard P, Oyuchua M, Amornchai P, Lim C, Wuthiekanun V, et al. Soil Nutrient Depletion Is Associated with the Presence of Burkholderia pseudomallei. Appl Environ Microbiol. 2016;82(24):7086–92. doi: 10.1128/AEM.02538-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thailand Demographic [Internet]. www.bora.dopa.go.th. 2013 [cited 23 October 2015]. Available from: http://stat.bora.dopa.go.th/stat/y_stat56.html.
- 29.Lands used for agriculture cencus 2013 [Internet]. 2013 [cited 17 July 2015]. Available from: http://www.oae.go.th/download/use_soilNew/soiNew/landused2556.html.
- 30.Limmathurotsakul D, Dance DA, Wuthiekanun V, Kaestli M, Mayo M, Warner J, et al. Systematic review and consensus guidelines for environmental sampling of Burkholderia pseudomallei. PLoS Negl Trop Dis. 2013;7(3):e2105 doi: 10.1371/journal.pntd.0002105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Limmathurotsakul D, Wuthiekanun V, Amornchai P, Wongsuwan G, Day NP, Peacock SJ. Effectiveness of a simplified method for isolation of Burkholderia pseudomallei from soil. Appl Environ Microbiol. 2012;78(3):876–7. doi: 10.1128/AEM.07039-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chantratita N, Wuthiekanun V, Boonbumrung K, Tiyawisutsri R, Vesaratchavest M, Limmathurotsakul D, et al. Biological relevance of colony morphology and phenotypic switching by Burkholderia pseudomallei. J Bacteriol. 2007;189(3):807–17. doi: 10.1128/JB.01258-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anuntagool N, Panichakul T, Aramsri P, Sirisinha S. Shedding of lipopolysaccharide and 200-kDa surface antigen during the in vitro growth of virulent Ara- and avirulent Ara+ Burkholderia pseudomallei. Acta Trop. 2000;74(2–3):221–8. . [DOI] [PubMed] [Google Scholar]
- 34.Anuntagool N, Naigowit P, Petkanchanapong V, Aramsri P, Panichakul T, Sirisinha S. Monoclonal antibody-based rapid identification of Burkholderia pseudomallei in blood culture fluid from patients with community-acquired septicaemia. J Med Microbiol. 2000;49(12):1075–8. doi: 10.1099/0022-1317-49-12-1075 [DOI] [PubMed] [Google Scholar]
- 35.Duval BD, Elrod MG, Gee JE, Chantratita N, Tandhavanant S, Limmathurotsakul D, et al. Evaluation of a latex agglutination assay for the identification of Burkholderia pseudomallei and Burkholderia mallei. Am J Trop Med Hyg. 2014;90(6):1043–6. doi: 10.4269/ajtmh.14-0025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nualnoi T, Norris MH, Tuanyok A, Brett PJ, Burtnick MN, Keim PS, et al. Development of Immunoassays for Burkholderia pseudomallei Typical and Atypical Lipopolysaccharide Strain Typing. Am J Trop Med Hyg. 2017;96(2):358–67. doi: 10.4269/ajtmh.16-0308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Godoy D, Randle G, Simpson AJ, Aanensen DM, Pitt TL, Kinoshita R, et al. Multilocus sequence typing and evolutionary relationships among the causative agents of melioidosis and glanders, Burkholderia pseudomallei and Burkholderia mallei. J Clin Microbiol. 2003;41(5):2068–79. doi: 10.1128/JCM.41.5.2068-2079.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ileri SZ. The Indirect Haemagglutination Test in the Diagnosis of Melioidosis in Goats. Br Vet J. 1965;121:164–70. [DOI] [PubMed] [Google Scholar]
- 39.Alexander AD, Huxsoll DL, Warner AR Jr., Shepler V, Dorsey A. Serological diagnosis of human melioidosis with indirect hemagglutination and complement fixation tests. Appl Microbiol. 1970;20(5):825–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suttisunhakul V, Chantratita N, Wikraiphat C, Wuthiekanun V, Douglas Z, Day NP, et al. Evaluation of Polysaccharide-Based Latex Agglutination Assays for the Rapid Detection of Antibodies to Burkholderia pseudomallei. Am J Trop Med Hyg. 2015;93(3):542–6. doi: 10.4269/ajtmh.15-0114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Family medicine. 2005;37(5):360–3. . [PubMed] [Google Scholar]
- 42.Mukaka MM. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med J. 2012;24(3):69–71. [PMC free article] [PubMed] [Google Scholar]
- 43.Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:17 doi: 10.1186/1751-0473-3-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Limmathurotsakul D, Wongsuvan G, Aanensen D, Ngamwilai S, Saiprom N, Rongkard P, et al. Melioidosis caused by Burkholderia pseudomallei in drinking water, Thailand, 2012. Emerg Infect Dis. 2014;20(2):265–8. doi: 10.3201/eid2002.121891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levy A, Merritt AJ, Aravena-Roman M, Hodge MM, Inglis TJ. Expanded range of Burkholderia species in Australia. Am J Trop Med Hyg. 2008;78(4):599–604. [PubMed] [Google Scholar]
- 46.Warner JM, Pelowa DB, Gal D, Rai G, Mayo M, Currie BJ, et al. The epidemiology of melioidosis in the Balimo region of Papua New Guinea. Epidemiol Infect. 2008;136(7):965–71. doi: 10.1017/S0950268807009429 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All 3 files are available from the Figshare database(https://figshare.com/s/9ad409f0d16f3845076a).