Abstract
The existence of small-molecule signals that influence development in Caenorhabditis elegans has been known for several decades, but only in recent years have the chemical structures of several of these signals been established. The identification of these signals has enabled connections to be made between these small molecules and fundamental signaling pathways in C. elegans that influence not only development, but also in metabolism, fertility, and lifespan. Spurred by these important discoveries and aided by recent advances in comparative metabolomics and NMR spectroscopy, the field of nematode chemistry has the potential to expand dramatically in the coming years. This review will focus on small-molecule pheromones and hormones that influence developmental events in the nematode life cycle (ascarosides, dafachronic acids, and nemamides), will cover more recent work regarding the biosynthesis of these signals, and will explore how the discovery of these signals is transforming our understanding of nematode development and physiology.
Introduction
C. elegans is an ideal model system for studying the role of pheromonal, hormonal, and nutritional cues in controlling developmental decisions. The transparency of C. elegans has allowed for its (invariant) cell lineage to be completely mapped from the single fertilized egg to the fully developed hermaphrodite adult (with 959 somatic cells). Furthermore, the fact that C. elegans is primarily hermaphroditic (but still forms the occasional male) has facilitated genetic screens that have implicated genes in specific developmental events. However, our current state of knowledge regarding the role of small-molecule cues in C. elegans and other nematodes is relatively rudimentary. For example, C. elegans has over 1,200 G protein-coupled receptors (GPCRs) for the detection of external small-molecule cues, but only a small subset of these receptors have been characterized.1,2 C. elegans also has 284 nuclear hormone receptors (NHRs), many of which likely respond to internal small-molecule cues, but the ligand for only one of these receptors has been characterized.3,4
C. elegans can alter or arrest its development at several distinct points in response to environmental signals. One of the best studied developmental events is formation of the stress-resistant dauer larval stage. The dauer has attracted intense scientific interest in part because the neuroendocrine pathways that control dauer formation include the insulin / insulin-like growth factor-1 (IGF-1) and TGFβ pathways, which lie at the nexus of development, metabolism, stress-resistance, and lifespan in C. elegans. Favorable conditions allow C. elegans to develop from an egg, through four larval stages (L1-L4), to the reproductive adult, but high population densities, low amounts of food (bacteria), and high temperatures promote the formation of the dauer, an alternative L3 larval stage. C. elegans senses its population density using a secreted pheromone, the dauer pheromone, which consists of several derivatives of the 3,6-dideoxy-L-sugar ascarylose (“ascarosides”) (Fig. 1a,b).5–9 In addition to the dauer, C. elegans can undergo other types of arrest, including starvation-induced larval arrest. If C. elegans is faced with complete starvation at any larval stage, it will arrest at that larval stage and can recover and resume development once it encounters food.10,11 Most work on this type of arrest has been done for L1 arrest, which is achieved by hatching C. elegans eggs in the absence of food. An adult reproductive diapause has also been reported in which starvation leads to apoptotic death of the germline, followed by germline regeneration once conditions improve.12,13
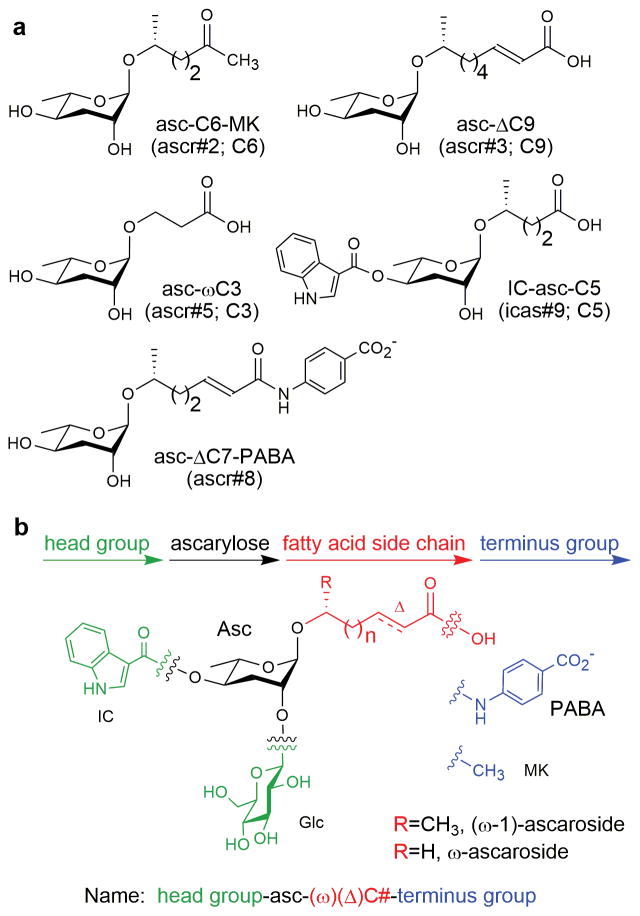
Figure 1. The dauer pheromone ascarosides and the modular structure of the ascaroside family of pheromones.
a, Chemical structures of the primary components of the dauer pheromone.6–9 In addition to the structure-based name (see b for nomenclature), additional names are given including a name based on an earlier nomenclature (indicating only the number of carbons in the side chain) and a name based primarily on the order of discovery (ascr#, icas#). b, The simplest ascarosides in C. elegans have an ascarylose sugar attached to a saturated fatty acid side chain of a particular length (C#) at its penultimate (ω-1) carbon. Deviations from that simple structure are indicated in a structure-based nomenclature: head group-asc-(ω)(Δ)C#-terminus group. ω indicates attachment at the terminal (ω), instead of the penultimate (ω-1) carbon. Δ indicates a double bond at the α-β position. Head groups include indole-3-carbonyl (IC), 4-hydroxybenzoyl (HB), 2-(E)-methyl-2-butenoyl (MB), and octopamine succinyl (OS) at the 4′-position and glucosyl (Glc) at the 2′-position. Terminus groups include a methyl ketone (MK), instead of a carboxylic acid, para-aminobenzoic acid (PABA), and ethanolamine (EA). Figure is adapted.61
Dauers have specialized features, including a thickened cuticle and a closed buccal cavity (mouth), that help to protect the worm from dessication, chemical agents, and pathogens.14 As the dauer stage does not feed, it must rely on fat stores in the form of triacylglycerols to supply its energy needs.15 These fats are converted to glucose via the glyoxylate cycle, and this glucose is used in glycolysis and fermentative metabolism, while aerobic respiration is suppressed.16,17 The glucose is also diverted to the biosynthesis of trehalose, which provides osmotic protection against dessication.16,18,19 C. elegans can survive as a dauer for up to several months, and only begins to die once its fat stores become depleted.15 The stress resistance of the dauer is due in part to altered gene expression in which genes involved in starvation, heat, and oxidative stress are up-regulated.20–22 C. elegans survives in the wild on rotting plant material,23 and the dauer stage serves as a stress-resistant dispersal stage, enabling the worm to seek out a new microbially rich food source, often by engaging in nictation behavior (waving while standing on its tail) and hitching a ride on an invertebrate carrier, such as a fruit fly or snail.
The insulin/IGF-1 and TGFβ pathways that control dauer formation were pieced together through genetic screens to identify mutant worms that either constitutively formed dauers under favorable conditions (Daf-c mutants) or failed to form dauers under unfavorable conditions (Daf-d mutants) (Fig. 2).3,24,25 Under favorable conditions (high food / low dauer pheromone), chemosensory neurons secrete insulin and TGFβ ligands, and downstream signaling ultimately leads to the production of dafachronic acids, which are hormones that promote reproductive growth and suppress dauer formation (Fig. 2). Unfavorable conditions (low food /high dauer pheromone), on the other hand, suppress insulin and TGFβ signaling, thereby inhibiting dafachronic acid production and promoting dauer formation (Fig. 2). In addition to dauer formation, the dafachronic acid pathway plays a central role in controlling fertility and lifespan.22,26–28
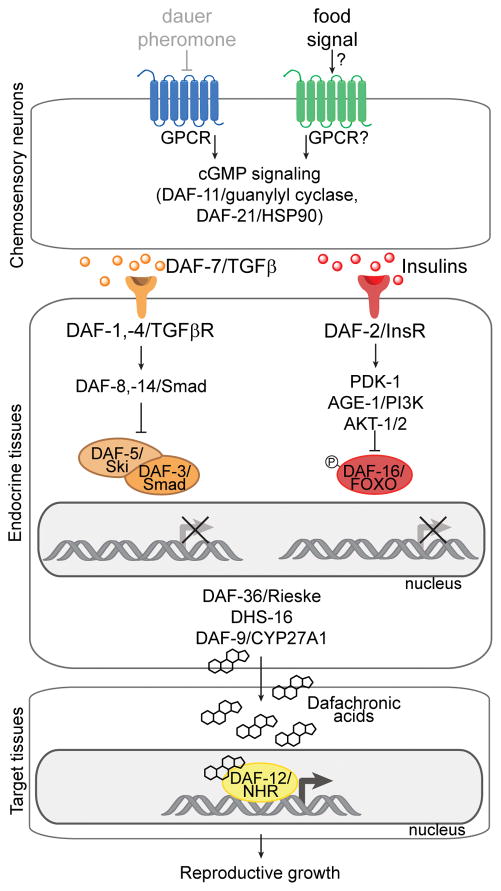
Figure 2. Role of insulin/IGF-1 and TGFβ pathways in controlling reproductive growth versus dauer development.
The pathways are depicted under favorable conditions in which the concentration of dauer pheromone is low (grayed out) and the concentration of an uncharacterized food signal is high. Under these conditions, the food signal is thought to promote GPCR signaling in chemosensory neurons, leading to DAF-11/guanylyl cyclase activity and increased levels of cGMP, resulting in secretion of TGFβ and insulin ligands. DAF-7/TGFβ binds to DAF-1,-4/TGFβR, which regulates Smad/co-Smad transcriptional complexes. Insulins bind to DAF-2/InsR, leading to phosphorylation by AKT-1/2 of DAF-16/FOXO, which is retained in the cytosol. TGFβ and insulin signaling promotes the biosynthesis of the dafachronic acids, which bind to DAF-12/NHR in target tissues that functions transcriptionally to promote reproductive growth. Under unfavorable conditions in which dauer pheromone is high and food signal is low, chemosensory neurons do not secrete insulin and TGFβ ligands. DAF-16/FOXO becomes dephosphorylated, translocates into the nucleus, and acts as a transcription factor, while DAF-3/Co-Smad and DAF-5/Sno/Ski are no longer inhibited by DAF-8/-14 and thus can act as a transcription factor. Consequently, the biosynthesis of the dafachonic acids is inhibited, unliganded DAF-12 binds to its inhibitor DIN-1 and promotes a transcriptional program that triggers dauer formation.
The first part of this review will describe the discovery of the dauer pheromone ascarosides in C. elegans and other nematode species and how this discovery has enabled the identification of the ascaroside receptors that lie upstream of the insulin/IGF-1 and TGFβ pathways. It will also describe how C. elegans controls the biosynthesis of specific ascarosides in order to modulate its chemical message under different conditions. The second part of this review will focus on the discovery, biological role, and biosynthesis of the dafachronic acids in C. elegans and in other nematode species. The last part of this review will focus on the discovery of a polyketide-nonribosomal peptide in C. elegans that plays an important role in L1 recovery. Overall, this review will describe how the discovery of small-molecule cues in C. elegans is providing the missing link that is enabling many connections to be made between important signaling pathways and developmental processes.
Role of the dauer pheromone in C. elegans
The dauer pheromone was first described in the early 1980s as several small molecules of unknown structure that could be extracted from conditioned culture medium and used to induce dauer formation and to block dauer recovery.29,30 It was not until the mid-2000s that the dauer pheromone was shown to consist of several structurally related ascarosides (Fig. 1a).5–9 Most of these dauer pheromone ascarosides were identified through activity-guided fractionation of crude pheromone extract and then structural characterization of the purified, active compounds using NMR spectroscopy and mass spectrometry.5–8 The ascarosides, which have a modular structure, have fatty acid-derived side chains and can have various modifications on the ascarylose sugar (“head groups”) or at the end of the fatty acid side chain (“terminus groups”) (Fig. 1b). Several naming schemes exist for the ascarosides, based either on the order of their discovery or on elements of their chemical structure, such as the number of carbons in their side chain (see Fig. 1b for structure-based nomenclature).31 The ascarosides can be divided into two main classes, the (ω-1)-ascarosides, in which the fatty acid side chain is attached at its penultimate (or ω-1) carbon to the ascarylose sugar, and the ω-ascarosides, in which the fatty acid side chain is attached at its terminal (or ω) carbon to the ascarylose sugar. Interestingly, only one of the dauer pheromone components is an ω-ascaroside, asc-ωC3 (ascr#5; C3), and this ascaroside works synergistically with the other dauer pheromone components to induce dauer (Fig. 1a).7 The dauer pheromone ascarosides must accumulate to low- to mid-nanomolar concentrations before inducing dauer,6–8 and a high population density of worms is required to produce these high concentrations of pheromone.
The dauer pheromone ascarosides are only a small subset of a much larger number of ascarosides produced by C. elegans. Overlapping subsets of ascarosides influence dauer formation and/or a variety of behaviors, including hermaphrodite aggregation,32,33 male attraction to hermaphrodites,34 hermaphrodite attraction to males,35 avoidance32,36,37, and foraging suppression.38,39 Many of the ascarosides that induce dauer also induce avoidance in adults, which may provide a survival advantage as C. elegans should want to avoid the environmental conditions that induce dauer. Most ascarosides that influence behaviors, however, tend to work at much lower concentrations (femtomolar to picomolar) than those that influence dauer, which is consistent with these ascarosides being involved in communication between two individuals or small groups of worms.
The discovery of the dauer pheromone ascarosides has enabled the identification of several of their target receptors. It is important to note that in the past, these target receptors were for the most part not picked up in forward genetic screens, such as those screening for Daf mutants, possibly because the phenotypes of these receptors are only apparent in the presence of their ligands. Thus, the identification of the ascaroside pheromones has opened the door in a dramatic fashion to further discoveries regarding chemosensory control of development and behavior in C. elegans. Several different approaches have been taken in order to identify the receptor targets of the dauer pheromone ascarosides. The fact that the dauer pheromone down-regulates the expression of certain chemosensory genes was exploited to screen for mutations that blocked this down-regulation and led to the identification of SRBC-64 and SRBC-66 as targets of the dauer pheromone components, asc-C6-MK (ascr#2; C6) and asc-ΔC9 (ascr#3; C9).40 Efforts to identify the receptors of asc-ωC3 (ascr#5; C3) utilized strains that had been cultured at high densities for multiple years and had developed mutations such that they no longer formed dauers in response to dauer pheromone.41 After a resistant strain was crossed with wild-type worms to generate recombinant inbred lines, quantitative trait locus mapping was used to pinpoint the genomic region responsible for asc-ωC3 (ascr#5; C3) sensitivity, leading to the identification of its receptors SRG-36 and SRG-37. Additional receptors for the ascarosides were identified using a biochemical approach in which a component of the TGFβ pathway, the SMAD DAF-8, was immunoprecipitated and copurified with the DAF-37 and DAF-38 GPCRs.42 Whereas the daf-37 mutant was defective in responding to asc-C6-MK (ascr#2; C6), the daf-38 mutant was defective in responding to multiple ascarosides. Presumably, many of the GPCRs targeted by the ascarosides remain to be identified, given the number of ascarosides without identified targets and given that the ascarosides often have multiple developmental and behavioral activities and thus likely have multiple targets. Interestingly, the receptors that have been identified thus far come from several different families of GPCRs, including the serpentine receptor class BC family (SRBC-64 and -66), class G family (SRG-36 and -37), and class W family (DAF-37), and a GPCR with homology to the human gonatodropin-releasing hormone receptor (DAF-38). Thus, it is unlikely that some structural similarity between these GPCR targets can be used to identify additional ascaroside receptors in C. elegans.
Role of the dauer pheromone in other nematode species
Pristionchus pacificus
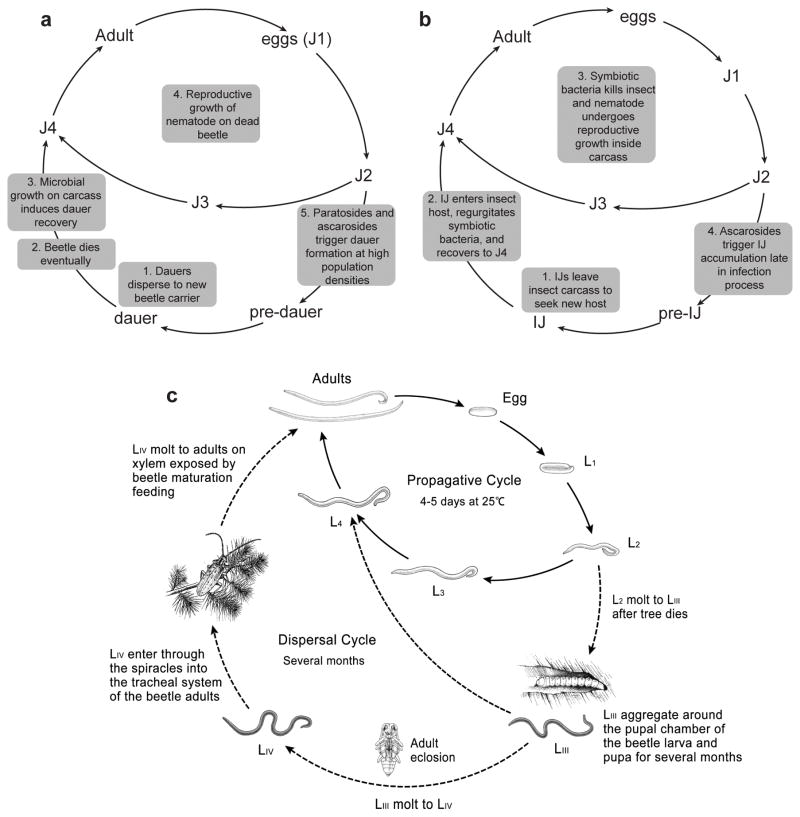
Ascarosides are, in fact, widely produced by many nematode species, including some parasitic species.43–48 Of the nematode species examined to date, P. pacificus produces the most structurally diverse set of pheromones that includes ascarosides, dimeric ascarosides, as well as paratosides (derivatives of the the 3,6-dideoxy-L-sugar paratose).45,46 Furthermore, these ascarosides and paratosides can have elaborate head and terminus groups derived from modular assembly of building blocks from lipid, amino acid, and nucleoside metabolic pathways.45,46 P. pacificus, which engages in a necromenic association with scarab beetles, survives as a dauer on the beetle until the beetle dies and then recovers and feeds on the microbes that proliferate on the beetle carcass (Fig. 3a).49 Once microbial food begins to run out, specific ascarosides and paratosides constitute a dauer pheromone that induces dauer formation. Furthermore, exposure of early larval stages to starvation and specific ascarosides and paratosides induces the development of teeth-like denticles in the adult that enable. P. pacificus to add fungi and other nematodes to its diet.45,46,49,50 It has been speculated that the diversity of pheromones produced by P. pacificus is due to heavy competition of different P. pacificus strains for resources in a very narrow ecological niche (the beetle). Indeed, strains that produce the most of a given pheromone do not necessarily correspond to the strains that respond most strongly to that pheromone, suggesting that certain strains may produce pheromones to induce other strains to enter dauer in order to out-compete them.46,50 Perhaps reflecting the importance of its association with scarab beetles, P. pacificus engages in an extreme form of nictation, in which it secretes an extremely long-chain polyunsaturated wax ester (“nematoil”) that helps hundreds of worms to stick together and form waving towers of nematodes (“dauer towers”) to further increase the likelihood of finding a beetle carrier.51
Figure 3. Life cycles of P. pacificus (a), H. bacteriophora (b), and pinewood nematode (c).
Figure in c is adapted.54
Entomopathogenic nematodes
Over a third of nematode species are parasitic, infecting either insects, plants, or animals (including humans). Entomopathogenic nematodes, which infect insect hosts, serve as a model system for studying parasitism in nematodes and have been shown to use ascarosides to control their development.44 Most parasitic nematodes have a dauer-like infective stage, called the infective juvenile (IJ) or infective third larval stage (iL3) that is important for survival outside of their host. For example, the entomopathogenic nematode Heterorhabditis bacteriophora survives in the environment as an IJ as it seeks out an insect host (Fig. 3b). Once inside its host, it regurgitates a symbiotic bacteria that kills the host, and it recovers and undergoes reproductive growth until the population density reaches a certain threshold. IJs accumulate late in the infection process in response to a secreted ascaroside pheromone with an ethanolamide (EA) moiety (asc-C11-EA) and ultimately break out of the insect carcass in order to seek a new insect host.44 Interestingly, while the H. bacteriophora IJ pheromone blocks recovery from the IJ stage, C. elegans dauer pheromone does not, indicating that the pheromones in nematodes are largely species-specific.44
Pinewood nematode
Pinewood nematode is a plant-parasitic nematode that relies on a Monochamus beetle vector to transport it between pine tree hosts.52 Not only does pinewood nematode produce ascarosides, but also its vector beetle produces them, and these ascarosides play an important role in mediating interactions between the two partners.47 The Monochamus beetle, in fact, is the first example of ascaroside production in an organism other than a nematode. The pinewood nematode enters its pine tree host as its beetle vector engages in maturation feeding on the tree during the summer (Fig. 3c). The nematode then enters the reproductive phase of its life cycle, in which it passes from the egg through four larval stages (L1-L4) to the reproductive adult. During this phase, the nematode increases its population density, feeds, and weakens the pine tree. In autumn, the beetle lays eggs on the weakened pine tree, and the resulting beetle larvae make chambers within the tree. During the cold winter months, the beetle larvae produce ascarosides that help it to postpone its molt to the pupal stage. The deteriorating conditions in the tree trigger the formation of the dispersal third-stage (LIII) nematode larvae, which aggregate around the beetle chambers.53 The nematode LIII larvae produce ascarosides (specifically, asc-C5 (ascr#9) and asc-ΔC6) that promote beetle pupation. As the beetle enters the adult stage, they produce fatty acid ethyl esters that promote development of dispersal fourth-stage (LIV) nematode larvae.54 Ascarosides produced by the adult beetle (specifically, asc-C9 (ascr#10)) are attractive to the nematode LIV larvae, potentially inducing them to enter the tracheal system of the beetle for subsequent transport to a new pine tree host.
Biosynthesis of the dauer pheromone in C. elegans
C. elegans biosynthesizes the ascaroside pheromones by producing ascarosides with very long side chains and then shortening these side chains through peroxisomal β-oxidation cycles, which shorten the side chains by two carbons per cycle. This model is based on several lines of evidence. First, very long-chain ascarosides have been identified in C. elegans.55 In fact, the first ascarosides identified in any organism were very long-chain ascarosides (29–33 carbons) identified over 50 years ago in the parasitic worm Ascaris (a large parasitic worm that infects humans and livestock).56,57 These very long-chain ascarosides are found in the inner lipid layer of the Ascaris egg shell and are thought to contribute to the extreme resistance of the eggs to chemicals and salts.58 Second, mutant worms defective in peroxisomal β-oxidation accumulate medium and long chain-length ascarosides, suggesting that these ascarosides are biosynthetic precursors to the short-chain ascaroside pheromones.33,59,60 Lastly, peroxisomal β-oxidation enzymes have been shown to process directly in vitro ascarosides, rather than fatty acids.61
The biosynthetic origin of the long-chain ascarosides in C. elegans is not known, although it has been speculated that long-chain fatty acids are hydroxylated at the (ω-1)- or ω-position by an unknown cytochrome P450 and that this hydroxylated fatty acid is then coupled to an activated form of the ascarylose sugar, NDP-ascarylose. The biosynthetic origin of this putative NDP-ascarylose is also not known. Biosynthesis of CDP-ascarylose has been studied extensively in bacteria. However, C. elegans does not have close homologs of all of the genes in the bacterial pathway.62 Genetic screens to identify genes that are important for the integrity of the C. elegans egg shell have identify some candidate genes potentially involved in sugar modification, but it remains to be seen whether any of these candidate genes are involved in ascarylose biosynthesis.63,64
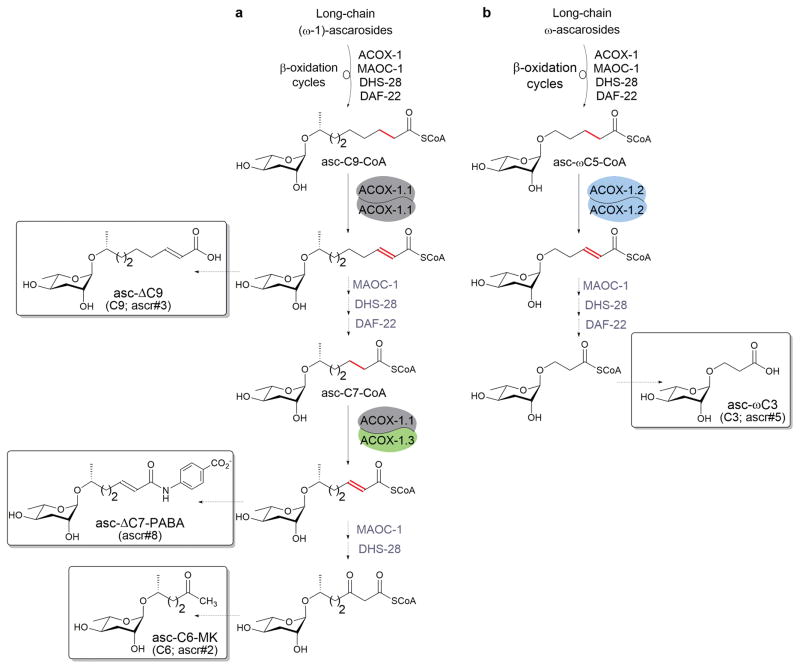
Ascaroside side-chain shortening occurs through two parallel β-oxidation pathways, one shortening (ω-1)-ascarosides and one shortening ω-ascarosides (Fig. 4a,b).61 Each β-oxidation cycle requires four enzymes: an acyl-CoA oxidase, an enoyl-CoA hydratase (MAOC-1), a (3R)-hydroxyacyl-CoA dehydrogenase (DHS-28), and a 3-ketoacyl-CoA thiolase (DAF-22).33,59,61,65,66 The acyl-CoA oxidases (ACOXs) that participate in ascaroside biosynthesis form both protein homo- and heterodimers, which have specific side chain-length preferences and help to control the mixture of ascarosides produced.61 Recently, the names of the ACOX enzymes have been changed to reflect their similarities to either human ACOX-1 or ACOX-3, and this review incorporates this new nomenclature (summarized in Fig. 4 legend). An ACOX-1.1 homodimer can process both fatty acyl-CoA substrates and ascaroside-CoA substrates, including an (ω-1)-ascaroside with a 9-carbon side chain (Fig. 4a). An ACOX-1.1/ACOX-1.3 heterodimer is specifically active towards an (ω-1)-ascaroside with a 7-carbon side chain (Fig. 4a). An ACOX-1.2 homodimer is specifically active towards an ω-ascaroside with a 5-carbon side chain (Fig. 4b).
Figure 4. Role of the acyl-CoA oxidases, ACOX-1.1, -1.2, and -1.3, in the biosynthesis of the ascarosides.
Two parallel β-oxidation pathways shorten the side chains of the (ω-1)-ascarosides (a) and the ω-ascarosides (b). Each cycle shortens the side chains of the ascarosides by two carbons. The boxed structures are four of the five dauer pheromone components shown in Figure 1a. Figure is adapted.61 Note that in order to reflect their similarity to mammalian ACOX-1, C. elegans ACOX-1, -2, -3, -4, -5, and F59F4.1 have been officially renamed ACOX-1.1, -1.2, -1.3, -1.4, -1.5, and -1.6, respectively. To reflect its similarity to mammalian ACOX-3, C. elegans ACOX-6 has been renamed ACOX-3. There are no C. elegans homologs of mammalian ACOX-2.
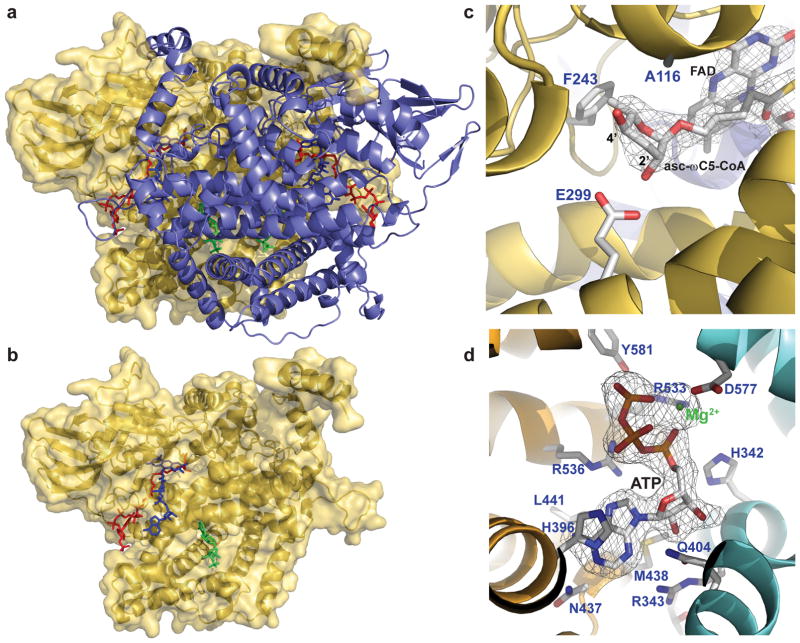
The crystal structure of an ACOX-1.1 homodimer bound to an FAD cofactor, as well as an ACOX-1.2 homodimer bound to an FAD cofactor and its specific substrate (asc-ωC5-CoA; see Fig. 4b for structure) have been solved, revealing important differences between the two active sites and providing a molecular basis for their substrate specificities (Fig. 5a,b).67 ACOX-1.1, which has a broader substrate range and can process longer substrates, has a larger active site that opens to two channels on the outer surface of the protein. ACOX-1.2, on the other hand, which specifically processes the short-chain ascaroside asc-ωC5-CoA, has a smaller active site that is largely closed to the outer surface of the protein (Fig. 5c). Furthermore, the ACOX-1.2 active site has specific amino acid residues responsible for recognizing the ascarylose ring of its specific substrate (Fig. 5c). In fact, mutating ACOX-1.1 at these residues to the corresponding residues found in ACOX-1.2 enables it to process asc-ωC5-CoA.67
Figure 5. Structure of the C. elegans acyl-CoA oxidases ACOX-1.1 and ACOX-1.2.
a,b The ACOX-1.2 homodimer, showing one subunit in yellow and one in purple (a) or only showing one in yellow (b), bound to an FAD cofactor (blue), an asc-ωC5-CoA substrate (red, structure shown in Fig. 4b), and ATP (green). c, Close up of the ACOX-1.2 active site, showing the importance of specific amino acid residues in binding the ascarylose ring of the asc-ωC5-CoA substrate. d, ATP-binding site in ACOX-1.1 located at the dimer interface (between the orange and light blue subunits). Figure is adapted.67
Interestingly, the ACOX-1.1 and the ACOX-1.2 crystal structures revealed that the enzymes bind ATP at the dimer interface (Fig. 5a,b,d).67 An ATP binding site is found in most C. elegans ACOX enzymes, but does not appear to be conserved in C. elegans ACOX-3 (previously named ACOX-667) or in the ACOX enzymes of more distantly related nematodes, plants, or mammals.67 Comparison of the ACOX-1.1 crystal structure with bound FAD and ATP to an apo-ACOX-1.1 structure with no bound FAD or ATP suggests that ATP binding may lead to a conformational change that stabilizes FAD binding. Mutational studies in which residues in the ATP binding site or FAD binding site were mutated provide further evidence that ATP and FAD binding are correlated.67 ACOX-1.1 mutants that could not bind ATP showed reduced enzymatic activity, presumably because they did not bind FAD as well. Although it remains to be shown whether the ACOX enzymes bind ATP in vivo, one possibility is that ATP levels in the worm may influence the biosynthesis of the ascarosides.
Production of the ascarosides in C. elegans has been shown to be influenced by a variety of factors, including environmental conditions, such as food availability and temperature33,61,68, larval stage69, and sex35. Increased food availability tends to increase the production of most ascarosides,61,68 and a dietary restricted mutant with reduced pharynx pumping produces less ascarosides.68 Transcriptional regulation of the ACOX enzymes appears to affect the relative production of certain ascarosides. For example, bacterial food downregulates the expression of acox-1.3 in L4 larvae and suppresses the production of short-chain (ω-1)-ascarosides that lie downstream of ACOX-1.3, such as the dauer pheromone asc-C6-MK (ascr#2; C6) (Fig. 4a).61 Non-dauer inducing conditions (high food / low population density) relative to dauer-inducing conditions (low food / high population density) upregulate in early stage larvae the expression of acox-1.2 and induce the production of the very short-chain ω-ascaroside asc-ωC3 (ascr#5; C3), more so than other ascarosides (Fig. 4b).61 Heat stress has been shown to induce the production of several ascarosides61,66,68, and the transcription factor HSF-1 may contribute to this induction by up-regulating the expression of several β-oxidation genes.68
Relatively little is known about the biosynthesis of the various head groups and terminus groups and how these groups become attached to the ascarosides. It has been shown that the indole-3-carbonyl (IC) group present in one of the dauer pheromones, IC-asc-C5 (icas#9; C5) and several aggregation pheromones is derived from tryptophan, although its biosynthetic pathway is unknown.32,33 It is thought that attachment of the IC group to the ascarosides occurs relatively late in the biosynthesis of ascarosides since daf-22 worms (which cannot biosynthesize the ascarosides), when exogenously provided an ascaroside with a 9-carbon side chain, can produce the corresponding IC-modified ascaroside.33 Future work will need to address how the β-oxidation process and head/terminus group attachment are coordinated.
Role of the dafachronic acids in C. elegans and other nematode species
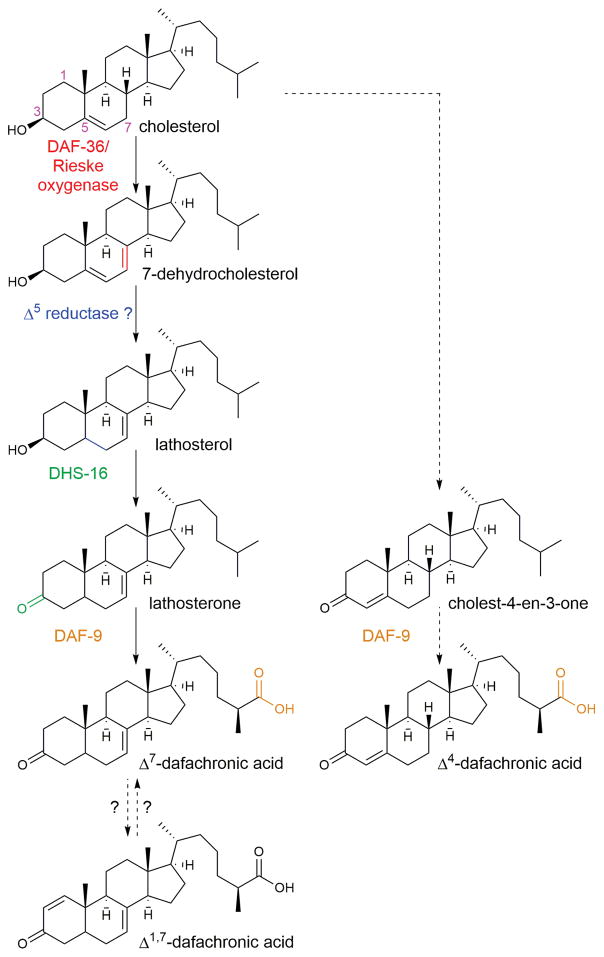
The ultimate output of the insulin/IGF-1 and TGFβ pathways is the biosynthesis of the dafachronic acids, the ligands for the NHR DAF-12. DAF-12 controls the decision of whether or not to enter dauer and also regulates adult lifespan in response to signals from the gonad.70,71 Insulin and TGFβ signaling promotes the biosynthesis of the dafachronic acids from cholesterol. Although C. elegans is typically cultured in the lab on a bacterial lawn, these cultures must be supplemented with cholesterol to promote growth and fertility, since nematodes are unable to synthesize sterols. Cholesterol deprivation leads to the occasional formation of dauer-like larvae and the development of a Mig phenotype, in which the gonad fails to migrate properly.28 DAF-9 is a cytochrome P450 necessary for dafachronic acid biosynthesis,4,72 and daf-9 mutants have phenotypes that are very similar to those induced by cholesterol deprivation.72 The first dafachronic acids to be discovered, Δ4-dafachronic acid and Δ7-dafachronic acid, were identified by incubating candidate substrates for DAF-9 with the enzyme and analyzing whether any products were produced that could stimulate the transcriptional activity of DAF-12.4 Partial purification of endogenous activators of DAF-12 transcriptional activity also gave LC-MS peaks that were consistent with the two dafachronic acids, although Δ7-dafachronic acid appeared to be more abundant than Δ4-dafachronic acid.4 More recent work to partially purify and spectroscopically characterize endogenous activators of DAF-12 transcriptional activity has confirmed Δ7-dafachronic acid, but could not confirm Δ4-dafachronic acid, as an endogenous DAF-12 ligand.73 This work also identified a novel DAF-12 ligand, Δ1,7-dafachronic acid, and showed that this ligand has similar potency as Δ7-dafachronic acid.
The biosynthesis of Δ7-dafachronic acid is the most fully delineated branch of the dafachronic acid biosynthetic pathway (Fig. 6). Cholesterol is desaturated to 7-dehydrocholesterol by the DAF-36/Rieske oxygenase.74 An unidentified Δ5-reductase is then thought to convert 7-dehydrocholesterol to lathosterol, which is subsequently oxidized at the 3 position to lathosterone by DHS-16, a short-chain dehydrogenase.75 Finally, the lathosterone side chain is oxidized to a carboxylic acid by DAF-9, presumably with the help of the cytochrome P450 oxidoreductase EMB-8.72,75 Meanwhile, the biosynthesis of Δ4-dafachronic acid is much less well understood. HSD-1, an ortholog of human of 3β-hydroxysteroid dehydrogenase/Δ5-Δ 4 isomerases, has been implicated in the hypothetical step that converts cholesterol to 4-cholesten-3-one76, however, other possible roles for this enzyme have also been proposed.73,75
Figure 6. Biosynthetic pathway of the dafachronic acids.
The biosynthesis of Δ4-dafachronic acid from cholesterol is unknown, and it is not clear whether this dafachronic acid is produced at physiologically relevant concentrations in the worm. The biosynthesis of Δ1,7-dafachronic acid has not been studied, and it is unknown whether it is derived from or converted to Δ7-dafachonic acid (or neither). Dotted lines indicate proposed reactions and pathways.
The insulin/IGF-1 and TGFβ pathways regulate the dafachronic acid biosynthetic pathway by promoting DAF-9 expression under replete conditions and inhibiting it under dauer-inducing conditions.77,78 Dafachronic acid biosynthesis is also influenced by the methyltransferase STRM-1, which is thought to methylate upstream intermediates in the biosynthetic pathway (such as lathosterol) at the C-4 position and thus, to syphon off intermediates for dafachronic acid biosynthesis.79,80 Interestingly, the metabolic changes that occur with dauer formation lead to decreased NADPH levels and may also represent a mechanism to inhibit dafachonic acid biosynthesis, given that DAF-9 is NADPH dependent.18 Specifically, the increased trehalose biosynthesis that comes with dauer formation diverts glucose-6-phosphate from the pentose phosphate pathway, a major contributor to cellular NADPH levels, thereby reducing cellular NADPH levels and indirectly inhibiting dafachronic acid biosynthesis to promote dauer formation.18
An inhibitor of DAF-9, dafadine, has been identified by screening for compounds that induce a Daf-c phenotype in worms.81 DAF-9 was considered a candidate target for dafadine, given that (1) dafadine gives rise to both Daf-c and Mig phenotypes, (2) epistasis analysis with Daf-d mutants suggested dafadine functions just upstream or at the level of DAF-12, and (3) dafadine contains an azole moiety that could bind the heme iron at the DAF-9 catalytic center. Dafadine was confirmed to inhibit biochemically DAF-9, as well as the human ortholog of DAF-9, CYP27A1, which is involved in bile acid biosynthesis.81
Interestingly, the enzymes involved in dafachronic acid biosynthesis are found in several different tissues. DAF-36 is expressed primarily in the intestine,74 DHS-16 in the pharynx, head neurons, and hypodermis,75 DAF-9 in the XXX neuroendocrine cells, hypodermis, and spermatheca,28,72,77,78,82 and HSD-1 in the XXX cells76. The distribution of the biosynthetic enzymes across multiple tissues suggests that either different dafachronic acids are produced in different tissues and perhaps play different roles or that distribution of the biosynthetic genes enables multi-tissue regulation of the biosynthetic pathway. A photocleavable, masked derivative of Δ7-dafachronic acid has been synthesized that enables light-mediated release of the hormone into the worm.83 This derivative could potentially be employed to study tissue-specific functions or biosynthetic steps of the dafachronic acids.
The role of DAF-12 in development appears to be largely conserved through nematode evolution.84–87 DAF-12 homologs been shown to control the formation of the dauer-like iL3 stage in certain parasitic species. In a cell-based assay, the dafachronic acids stimulated the transcriptional activity of DAF-12 homologs from the human threadworm (Strongyloides stercoralis) and several species of hookworm (Ancylostoma spp. and Necator americanus).85 The crystal structures of the S. stercoralis DAF-12 homolog85 and an Ancylostoma DAF-12 homolog86 in complex with dafachronic acids have been solved, revealing a conserved ligand-binding domain for the dafachronic acids.85,86 Analogous to dauer recovery in C. elegans, the iL3 larvae of parasitic nematodes will recover and resume feeding once they enter their host or if they are exposed to serum. The dafachronic acids can induce feeding in S. stercoralis and Ancylostoma spp. iL3 larvae in the absence of serum treatment.85 Although many parasitic nematode species must proceed through the iL3 stage and infect their host with each generation, S. stercoralis can complete one generation outside its host before forming iL3 larvae in the next generation. Importantly, the dafachronic acids block iL3 formation in this next generation and, in fact, force the nematodes to develop into free-living fourth stage larvae that ultimately die.85,87 Additionally, the dafachronic acids have been shown to block the formation of infective larvae in Strongyloides papillosus, a parasite of ruminants that can undergo multiple generations outside its host.84 The ability of the dafachronic acids to force iL3 recovery leading to nematode death suggests that these compounds could be developed as new therapeutics to treat parasitic nematode infections.
Role of the nemamides in C. elegans and other nematode species
In addition to dauer, C. elegans can arrest its life cycle at several other stages.10,11 Starvation-induced larval arrest is typically studied at the L1 stage, since C. elegans eggs hatched in the absence of food directly enter this arrested L1 stage. L1 arrest is not technically a diapause as is dauer because arrested L1 larvae are morphologically the same as non-arrested L1 larvae.10 The insulin/IGF-1,88–90 AMP-activated kinase (AMPK),91 and Target of Rapamycin (TOR)92 pathways have been implicated in starvation-induced L1 arrest maintenance, recovery, and survival.10 In comparison to dauer development, however, L1 arrest is poorly understood. For example, it is unclear how nutrient availability is sensed, how different signaling pathways respond to this nutrient availability, the sites of action of these signaling pathways, and how they are integrated.10
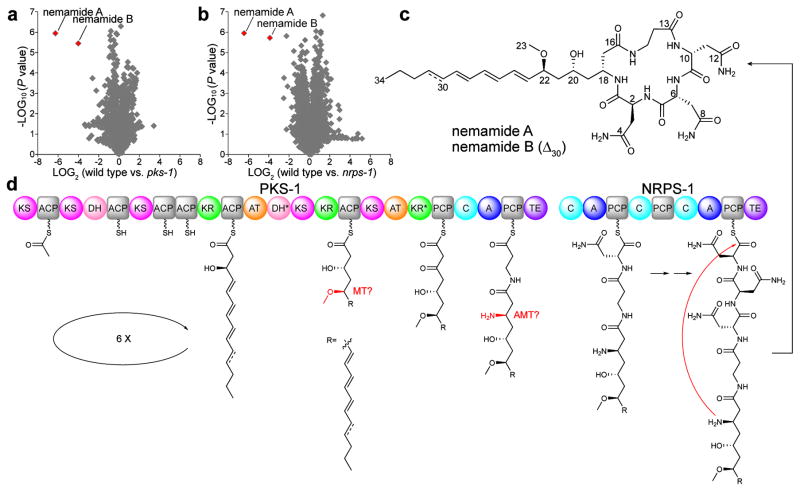
Two hybrid polyketide-nonribososomal peptides, nemamides A and B, have been discovered that promote survival during and recovery from L1 arrest (Fig. 7a–c).93 The nemamides are produced by a hybrid polyketide-nonribosomal peptide synthetase, PKS-1, and a nonribosomal peptide synthetase, NRPS-1, that are expressed in the canal-associated neurons, two essential neurons that extend the length of the worm and are associated with the excretory canal.93 The nemamides were identified by performing comparative metabolomics between wild-type, pks-1 mutant, and nrps-1 mutant worms to discover specific masses associated with the genes (Fig. 7a,b). The compounds were then purified from the worms and characterized using NMR spectroscopy (Fig. 7c).93 Although polyketides and nonribosomal peptides are produced by many species of bacteria and fungi, they are extremely rare in animals.93–95 The nemamides, in fact, represent the first polyketides or nonribosomal peptides identified that are biosynthesized in an assembly-line manner in animals (Fig. 7d).
Figure 7.
Discovery and biosynthesis of the nemamides. (a,b) Comparison of average peak areas for metabolite features in wild-type worms versus pks-1 mutant worms (a) and in wild-type worms versus nrps-1 mutant worms (b), with nemamide A and B highlighted in red. (c) Chemical structures of nemamide A and B. (d) Proposed biosynthetic assembly line for the nemamides. Domain abbreviations: acyl transferase (AT), acyl carrier protein (ACP), ketosynthase (KS), ketoreductase (KR), dehydratase (DH), methyltransferase (MT), aminotransferase (AMT), adenylation (A), peptidyl carrier protein (PCP), condensation (C), and thioesterase (TE). Domains labeled with an asterisk are predicted to be inactive based on the nemamide structures. Figure is adapted.93
The pks-1 and nrps-1 mutant worms are slow to recover from L1 arrest and also display reduced survival during prolonged L1 arrest, suggesting that the nemamides may be involved in regulating the worm’s response to nutrient cues. Analysis of the expression of the 40 C. elegans insulins in arrested and recovered L1s showed that the mutants fail to up-regulate certain insulins during recovery in response to food.93 Thus, the nemamides may regulate insulin signaling. However, epistasis experiments suggest that pks-1 and nrps-1 function in a pathway that is at least partially independent of the insulin/IGF-1 pathway. Although C. elegans is known to secrete an uncharacterized, density-dependent pheromone that promotes L1 arrest survival, the nemamides do not appear to be a component of this pheromone.93 Future work is needed to determine the exact mechanism of action of the nemamides. Interestingly, homologs of pks-1 and nrps-1 are found in most nematode species, suggesting that the nemamides play a conserved role across nematode evolution.93,94
Future directions
Although C. elegans has been extensively studied in terms of its genes and proteins, relatively little is known about its small molecules and biosynthetic pathways. Future small-molecule discovery in nematodes will be facilitated by more recently developed techniques in mass spectrometry-based and 2D NMR-based metabolomics. The initial dauer pheromone ascarosides and sex pheromone ascarosides were identified through activity-guided fractionation.5–8 Although this approach is the best one for establishing causality (i.e., that a given small molecule is the most potent one in a crude extract that gives rise to a particular activity), it is painstaking and slow. An LC-MS/MS technique has been developed for rapidly profiling the ascarosides (and related compounds) in a crude pheromone extract.33 This technique has been used to analyze the ascarosides in many nematode species, including P. pacificus and pinewood nematode, as well as to analyze the ascarosides in biosynthetic mutants in C. elegans.33,45,47,61 In a complementary technique, partial fractionation of crude extracts, followed by 2D NMR spectroscopy of the fractions, has been used to facilitate the identification of novel ascarosides and dafachronic acids.9,45,73 For example, this technique was used to compare conditioned culture extracts from wild-type C. elegans and a biosynthetic mutant (daf-22) in order to identify one of the five dauer pheromone components.9 Newer techniques in comparative mass spectrometry-based metabolomics are also facilitating the identification of natural products with completely novel structural frameworks, such as the nemamides.93 Unfortunately, many of the pheromones and hormones in nematodes are produced in extremely low quantities. Thus, since full structural characterization of a structurally novel natural product by NMR spectroscopy requires 50–100 μg of compound (and preferably much more), tens of liters of worms must often be cultivated to obtain sufficient compound. There are no good solutions to this issue that continues to impede progress in the field as a whole.
For the ascarosides, dafachronic acids, and nemamides, many questions remain. The specific biological roles of many of the ascarosides and dafachronic acids are unknown. Identification of the receptor targets of the ascarosides will help to define their individual roles, and use of quantitative trait locus mapping appears to be an extremely promising method for receptor discovery. Studies of the biosynthesis of the ascarosides could potentially uncover how C. elegans regulates the production of different ascarosides under different conditions and could perhaps shed light on the function of specific ascarosides. Of particular interest is how C. elegans integrates the β-oxidation process, which shortens the ascaroside side chains, with the addition of various head groups and terminus groups. The coordination of the biosynthesis of the dafachronic acids across multiple tissues is also poorly understood and could potentially reveal the individual contribution of different dafachronic acids to developmental arrest and lifespan regulation. For the nemamides, their receptor target has not been identified, and it is unclear how they promote L1 survival and whether they have additional important functions.
Previous work suggests the existence of many as-yet-unidentified chemical signals in C. elegans. For example, C. elegans L1 larvae have been shown to secrete a (non-ascaroside) pheromone that promotes L1 survival.96 As another example, C. elegans males secrete a pheromone that reduces the survival of hermaphrodites.97 Male-specific ascarosides contribute to this reduced survival, but additional signals may contribute as well.97,98 An uncharacterized male signal accelerates larval development and promotes reproductive maturation of hermaphrodites, while male-specific ascarosides act as a separate signal, delaying the loss of germline precursor cells in adult hermaphrodites.98 Furthermore, identification of the pheromones and hormones in C. elegans is only a starting point. Given that there are thousands of nematode species and the chemistries of only a few of them have been examined to any degree, much is left to be discovered in other species as well.
Acknowledgments
This work was supported by grants from the NIH (GM118775), NSF (Career Award, 1555050), Ellison Medical Foundation, Alfred P. Sloan Foundation, and the Research Corporation for Science Advancement.
Footnotes
Competing interests. The author declares no competing financial interests.
References
- 1.Bargmann CI. Chemosensation in C. elegans. WormBook. 2006 doi: 10.1895/wormbook.1.123.1. www.wormbook.org. [DOI] [PMC free article] [PubMed]
- 2.Robertson HM, Thomas JH. The putative chemoreceptor families of C. elegans. WormBook. 2006:1–12. doi: 10.1895/wormbook.1.66.1. [DOI] [PMC free article] [PubMed]
- 3.Antebi A. Nuclear receptor signal transduction in C. elegans. WormBook. 2015:1–49. doi: 10.1895/wormbook.1.64.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Motola DL, et al. Identification of ligands for DAF-12 that govern dauer formation and reproduction in C. elegans. Cell. 2006;124:1209–1223. doi: 10.1016/j.cell.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 5.Jeong PY, et al. Chemical structure and biological activity of the Caenorhabditis elegans dauer-inducing pheromone. Nature. 2005;433:541–545. doi: 10.1038/nature03201. [DOI] [PubMed] [Google Scholar]
- 6.Butcher RA, Fujita M, Schroeder FC, Clardy J. Small-molecule pheromones that control dauer development in Caenorhabditis elegans. Nat Chem Biol. 2007;3:420–422. doi: 10.1038/nchembio.2007.3. [DOI] [PubMed] [Google Scholar]
- 7.Butcher RA, Ragains JR, Kim E, Clardy J. A potent dauer pheromone component in C. elegans that acts synergistically with other components. Proc Natl Acad Sci U S A. 2008;105:14288–14292. doi: 10.1073/pnas.0806676105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butcher RA, Ragains JR, Clardy J. An indole-containing dauer pheromone component with unusual dauer inhibitory activity at higher concentrations. Org Lett. 2009;11:3100–3103. doi: 10.1021/ol901011c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pungaliya C, et al. A shortcut to identifying small molecule signals that regulate behavior and development in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2009;106:7708–7713. doi: 10.1073/pnas.0811918106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baugh LR. To grow or not to grow: nutritional control of development during Caenorhabditis elegans L1 arrest. Genetics. 2013;194:539–555. doi: 10.1534/genetics.113.150847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schindler AJ, Baugh LR, Sherwood DR. Identification of late larval stage developmental checkpoints in Caenorhabditis elegans regulated by insulin/IGF and steroid hormone signaling pathways. PLoS Genet. 2014;10:e1004426. doi: 10.1371/journal.pgen.1004426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Angelo G, Van Gilst MR. Starvation protects germline stem cells and extends reproductive longevity in C. elegans. Science. 2009;326:954–958. doi: 10.1126/science.1178343. [DOI] [PubMed] [Google Scholar]
- 13.Seidel HS, Kimble J. The oogenic germline starvation response in C. elegans. PLoS One. 2011;6:e28074. doi: 10.1371/journal.pone.0028074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cassada RC, Russell RL. The dauerlarva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Dev Biol. 1975;46:326–342. doi: 10.1016/0012-1606(75)90109-8. [DOI] [PubMed] [Google Scholar]
- 15.Narbonne P, Roy R. Caenorhabditis elegans dauers need LKB1/AMPK to ration lipid reserves and ensure long-term survival. Nature. 2009;457:210–214. doi: 10.1038/nature07536. [DOI] [PubMed] [Google Scholar]
- 16.Erkut C, Gade VR, Laxman S, Kurzchalia TV. The glyoxylate shunt is essential for desiccation tolerance in C. elegans and budding yeast. Elife. 2016:5. doi: 10.7554/eLife.13614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wadsworth WG, Riddle DL. Developmental regulation of energy metabolism in Caenorhabditis elegans. Dev Biol. 1989;132:167–173. doi: 10.1016/0012-1606(89)90214-5. [DOI] [PubMed] [Google Scholar]
- 18.Penkov S, et al. Integration of carbohydrate metabolism and redox state controls dauer larva formation in Caenorhabditis elegans. Nat Commun. 2015;6:8060. doi: 10.1038/ncomms9060. [DOI] [PubMed] [Google Scholar]
- 19.Erkut C, et al. Trehalose renders the dauer larva of Caenorhabditis elegans resistant to extreme desiccation. Curr Biol. 2011;21:1331–1336. doi: 10.1016/j.cub.2011.06.064. [DOI] [PubMed] [Google Scholar]
- 20.Murphy CT, et al. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424:277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- 21.McElwee JJ, Schuster E, Blanc E, Thomas JH, Gems D. Shared transcriptional signature in Caenorhabditis elegans Dauer larvae and long-lived daf-2 mutants implicates detoxification system in longevity assurance. J Biol Chem. 2004;279:44533–44543. doi: 10.1074/jbc.M406207200. [DOI] [PubMed] [Google Scholar]
- 22.Shaw WM, Luo S, Landis J, Ashraf J, Murphy CT. The C. elegans TGFb dauer pathway regulates longevity via insulin signaling. Curr Biol. 2007;17:1635–1645. doi: 10.1016/j.cub.2007.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Felix MA, Duveau F. Population dynamics and habitat sharing of natural populations of Caenorhabditis elegans and C. briggsae. BMC Biol. 2012;10:59. doi: 10.1186/1741-7007-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fielenbach N, Antebi A. C. elegans dauer formation and the molecular basis of plasticity. Genes Dev. 2008;22:2149–2165. doi: 10.1101/gad.1701508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu P, Dauer J. WormBook. 2007 doi: 10.1895/wormbook.1.144.1. http://www.wormbook.org. [DOI] [PMC free article] [PubMed]
- 26.Hsin H, Kenyon C. Signals from the reproductive system regulate the lifespan of C. elegans. Nature. 1999;399:362–366. doi: 10.1038/20694. [DOI] [PubMed] [Google Scholar]
- 27.Gerisch B, et al. A bile acid-like steroid modulates Caenorhabditis elegans lifespan through nuclear receptor signaling. Proc Natl Acad Sci U S A. 2007;104:5014–5019. doi: 10.1073/pnas.0700847104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerisch B, Weitzel C, Kober-Eisermann C, Rottiers V, Antebi A. A hormonal signaling pathway influencing C. elegans metabolism, reproductive development, and life span. Dev Cell. 2001;1:841–851. doi: 10.1016/s1534-5807(01)00085-5. [DOI] [PubMed] [Google Scholar]
- 29.Golden JW, Riddle DL. A pheromone influences larval development in the nematode Caenorhabditis elegans. Science. 1982;218:578–580. doi: 10.1126/science.6896933. [DOI] [PubMed] [Google Scholar]
- 30.Golden JW, Riddle DL. A Caenorhabditis elegans dauer-inducing pheromone and an antagonistic component of the food supply. J Chem Ecol. 1984;10:1265–1280. doi: 10.1007/BF00988553. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X, Noguez JH, Zhou Y, Butcher RA. Analysis of ascarosides from Caenorhabditis elegans using mass spectrometry and NMR spectroscopy. Methods Mol Biol. 2013;1068:71–92. doi: 10.1007/978-1-62703-619-1_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Srinivasan J, et al. A modular library of small molecule signals regulates social behaviors in Caenorhabditis elegans. PLoS Biol. 2012;10:e1001237. doi: 10.1371/journal.pbio.1001237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.von Reuss SH, et al. Comparative metabolomics reveals biogenesis of ascarosides, a modular library of small-molecule signals in C. elegans. J Am Chem Soc. 2012;134:1817–1824. doi: 10.1021/ja210202y. This paper developed an LC-MS/MS technique to analyze rapidly the ascarosides present in a crude extract that has proven extremely useful for the characterization of ascarosides in a variety of nematode species and in mutants in the ascaroside biosynthetic pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Srinivasan J, et al. A blend of small molecules regulates both mating and development in Caenorhabditis elegans. Nature. 2008;454:1115–1118. doi: 10.1038/nature07168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Izrayelit Y, et al. Targeted metabolomics reveals a male pheromone and sex-specific ascaroside biosynthesis in Caenorhabditis elegans. ACS Chem Biol. 2012;7:1321–1325. doi: 10.1021/cb300169c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Macosko EZ, et al. A hub-and-spoke circuit drives pheromone attraction and social behaviour in C. elegans. Nature. 2009;458:1171–1175. doi: 10.1038/nature07886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Artyukhin AB, et al. Succinylated octopamine ascarosides and a new pathway of biogenic amine metabolism in Caenorhabditis elegans. J Biol Chem. 2013;288:18778–18783. doi: 10.1074/jbc.C113.477000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greene JS, et al. Balancing selection shapes density-dependent foraging behaviour. Nature. 2016;539:254–258. doi: 10.1038/nature19848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greene JS, Dobosiewicz M, Butcher RA, McGrath PT, Bargmann CI. Regulatory changes in two chemoreceptor genes contribute to a Caenorhabditis elegans QTL for foraging behavior. Elife. 2016;5:e21454. doi: 10.7554/eLife.21454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim K, et al. Two chemoreceptors mediate developmental effects of dauer pheromone in C. elegans. Science. 2009;326:994–998. doi: 10.1126/science.1176331. This paper identified the first GPCR targets of the ascarosides. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGrath PT, Xu Y, Ailion M, Garrison JL, Butcher RA, Bargmann CI. Parallel evolution of domesticated Caenorhabditis species targets pheromone receptor genes. Nature. 2011;477:321–325. doi: 10.1038/nature10378. This paper used pheromone sensitive and insensitive C. elegans strains and quantitative trait locus mapping to identify a new family of GPCR targets of the ascarosides. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park D, et al. Interaction of structure-specific and promiscuous G-protein-coupled receptors mediates small-molecule signaling in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2012;109:9917–9922. doi: 10.1073/pnas.1202216109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choe A, et al. Ascaroside signaling is widely conserved among nematodes. Curr Biol. 2012;22:772–780. doi: 10.1016/j.cub.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Noguez JH, et al. A novel ascaroside controls the parasitic life cycle of the entomopathogenic nematode Heterorhabditis bacteriophora. ACS Chem Biol. 2012;7:961–966. doi: 10.1021/cb300056q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bose N, et al. Complex small-molecule architectures regulate phenotypic plasticity in a nematode. Angew Chem Int Ed Engl. 2012;51:12438–12443. doi: 10.1002/anie.201206797. This paper demonstrated that the nematode P. pacificus produces far more complex ascaroside architectures than seen in other nematode species that include many different types of primary metabolism-derived building blocks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yim JJ, Bose N, Meyer JM, Sommer RJ, Schroeder FC. Nematode signaling molecules derived from multimodular assembly of primary metabolic building blocks. Org Lett. 2015;17:1648–1651. doi: 10.1021/acs.orglett.5b00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao L, Zhang X, Chinta S, Lu M, Wei Y, Zhou J, Zhang W, Kong X, Liu Y, Zou Z, Butcher RA, Sun J. Ascarosides coordinate the dispersal of a plant-parasitic nematode with the metamorphosis of its vector beetle. Nature Communications. 2016;7:e12341. doi: 10.1038/ncomms12341. This paper was the first to detect ascarosides in an organism other than a nematode, specifically the vector beetle of pinewood nematode. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choe A, et al. Sex-specific mating pheromones in the nematode Panagrellus redivivus. Proc Natl Acad Sci U S A. 2012;109:20949–20954. doi: 10.1073/pnas.1218302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bento G, Ogawa A, Sommer RJ. Co-option of the hormone-signalling module dafachronic acid-DAF-12 in nematode evolution. Nature. 2010;466:494–497. doi: 10.1038/nature09164. [DOI] [PubMed] [Google Scholar]
- 50.Bose N, et al. Natural variation in dauer pheromone production and sensing supports intraspecific competition in nematodes. Curr Biol. 2014;24:1536–1541. doi: 10.1016/j.cub.2014.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Penkov S, et al. A wax ester promotes collective host finding in the nematode Pristionchus pacificus. Nat Chem Biol. 2014;10:281–285. doi: 10.1038/nchembio.1460. [DOI] [PubMed] [Google Scholar]
- 52.Zhao L, Mota M, Vieira P, Butcher RA, Sun J. Interspecific communication between pinewood nematode, its insect vector, and associated microbes. Trends Parasitol. 2014;30:299–308. doi: 10.1016/j.pt.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 53.Zhao LL, Wei W, Kang L, Sun JH. Chemotaxis of the pinewood nematode, Bursaphelenchus xylophilus, to volatiles associated with host pine, Pinus massoniana, and its vector Monochamus alternatus. J Chem Ecol. 2007;33:1207–1216. doi: 10.1007/s10886-007-9289-y. [DOI] [PubMed] [Google Scholar]
- 54.Zhao L, et al. Chemical signals synchronize the life cycles of a plant-parasitic nematode and its vector beetle. Curr Biol. 2013;23:2038–2043. doi: 10.1016/j.cub.2013.08.041. [DOI] [PubMed] [Google Scholar]
- 55.Zagoriy V, Matyash V, Kurzchalia T. Long-chain O-ascarosyl-alkanediols are constitutive components of Caenorhabditis elegans but do not induce dauer larva formation. Chem Biodivers. 2010;7:2016–2022. doi: 10.1002/cbdv.201000012. [DOI] [PubMed] [Google Scholar]
- 56.Jezyk PF, Fairbairn D. Ascarosides and ascaroside esters in Ascaris lumbricoides (Nematoda) Comp Biochem Physiol. 1967;23:691–705. doi: 10.1016/0010-406x(67)90334-9. [DOI] [PubMed] [Google Scholar]
- 57.Bartley JP, Bennett EA, Darben PA. Structure of the ascarosides from Ascaris suum. J Nat Prod. 1996;59:921–926. doi: 10.1021/np960236+. [DOI] [PubMed] [Google Scholar]
- 58.Fairbairn D. The biochemistry of Ascaris. Exp Parasitol. 1957;6:491–554. doi: 10.1016/0014-4894(57)90037-1. [DOI] [PubMed] [Google Scholar]
- 59.Butcher RA, et al. Biosynthesis of the Caenorhabditis elegans dauer pheromone. Proc Natl Acad Sci U S A. 2009;106:1875–1879. doi: 10.1073/pnas.0810338106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Izrayelit Y, Robinette SL, Bose N, von Reuss SH, Schroeder FC. 2D NMR-based metabolomics uncovers interactions between conserved biochemical pathways in the model organism Caenorhabditis elegans. ACS Chem Biol. 2013;8:314–319. doi: 10.1021/cb3004644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang X, et al. Acyl-CoA oxidase complexes control the chemical message produced by Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2015;112:3955–3960. doi: 10.1073/pnas.1423951112. This paper directly showed the substrate preference of three ACOX enzymes involved in ascaroside biosynthesis and demonstrated how these enzymes play a key role in determining which ascarosides are produced by C. elegans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johnson DA, Liu H. Mechanisms and pathways from recent deoxysugar biosynthesis research. Curr Opin Chem Biol. 1998;2:642–649. doi: 10.1016/s1367-5931(98)80096-3. [DOI] [PubMed] [Google Scholar]
- 63.Olson SK, Greenan G, Desai A, Müller-Reichert T, Oegema K. Hierarchical assembly of the eggshell and permeability barrier in C. elegans. J Cell Biol. 2012;198:731–748. doi: 10.1083/jcb.201206008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carvalho A, et al. Acute drug treatment in the early C. elegans embryo. PLoS One. 2011;6:e24656. doi: 10.1371/journal.pone.0024656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Joo HJ, et al. Caenorhabditis elegans utilizes dauer pheromone biosynthesis to dispose of toxic peroxisomal fatty acids for cellular homoeostasis. Biochem J. 2009;422:61–71. doi: 10.1042/BJ20090513. [DOI] [PubMed] [Google Scholar]
- 66.Joo HJ, et al. Contribution of the peroxisomal acox gene to the dynamic balance of daumone production in Caenorhabditis elegans. J Biol Chem. 2010;285:29319–29325. doi: 10.1074/jbc.M110.122663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang X, Li K, Jones RA, Bruner SD, Butcher RA. Structural characterization of acyl-CoA oxidases reveals a direct link between pheromone biosynthesis and metabolic state in C. elegans. Proc Natl Acad Sci, USA. 2016;113:10055–10060. doi: 10.1073/pnas.1608262113. This paper obtained the first crystal structures of the ACOX enzymes, providing a structural basis for their substrate specificities and also revealing an ATP binding site that regulates enzymatic activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Joo HJ, et al. HSF-1 is involved in regulation of ascaroside pheromone biosynthesis by heat stress in Caenorhabditis elegans. Biochem J. 2016;473:789–796. doi: 10.1042/BJ20150938. [DOI] [PubMed] [Google Scholar]
- 69.Kaplan F, et al. Ascaroside expression in Caenorhabditis elegans is strongly dependent on diet and developmental stage. PLoS One. 2011;6:e17804. doi: 10.1371/journal.pone.0017804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yamawaki TM, et al. The somatic reproductive tissues of C. elegans promote longevity through steroid hormone signaling. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shen Y, Wollam J, Magner D, Karalay O, Antebi A. A steroid receptor-microRNA switch regulates life span in response to signals from the gonad. Science. 2012;338:1472–1476. doi: 10.1126/science.1228967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jia K, Albert PS, Riddle DL. DAF-9, a cytochrome P450 regulating C. elegans larval development and adult longevity. Development. 2002;129:221–231. doi: 10.1242/dev.129.1.221. [DOI] [PubMed] [Google Scholar]
- 73.Mahanti P, et al. Comparative metabolomics reveals endogenous ligands of DAF-12, a nuclear hormone receptor, regulating C. elegans development and lifespan. Cell Metab. 2014;19:73–83. doi: 10.1016/j.cmet.2013.11.024. This paper provided a revised analysis of the major bioactive dafachronic acids present in C. elegans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rottiers V, et al. Hormonal control of C. elegans dauer formation and life span by a Rieske-like oxygenase. Dev Cell. 2006;10:473–482. doi: 10.1016/j.devcel.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 75.Wollam J, et al. A novel 3-hydroxysteroid dehydrogenase that regulates reproductive development and longevity. PLoS Biol. 2012;10:e1001305. doi: 10.1371/journal.pbio.1001305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Patel DS, Fang LL, Svy DK, Ruvkun G, Li W. Genetic identification of HSD-1, a conserved steroidogenic enzyme that directs larval development in Caenorhabditis elegans. Development. 2008;135:2239–2249. doi: 10.1242/dev.016972. [DOI] [PubMed] [Google Scholar]
- 77.Gerisch B, Antebi A. Hormonal signals produced by DAF-9/cytochrome P450 regulate C. elegans dauer diapause in response to environmental cues. Development. 2004;131:1765–1776. doi: 10.1242/dev.01068. [DOI] [PubMed] [Google Scholar]
- 78.Mak HY, Ruvkun G. Intercellular signaling of reproductive development by the C. elegans DAF-9 cytochrome P450. Development. 2004;131:1777–1786. doi: 10.1242/dev.01069. [DOI] [PubMed] [Google Scholar]
- 79.Matyash V, et al. Sterol-derived hormone(s) controls entry into diapause in Caenorhabditis elegans by consecutive activation of DAF-12 and DAF-16. PLoS Biol. 2004;2:e280. doi: 10.1371/journal.pbio.0020280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hannich JT, et al. Methylation of the sterol nucleus by STRM-1 regulates dauer larva formation in Caenorhabditis elegans. Dev Cell. 2009;16:833–843. doi: 10.1016/j.devcel.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 81.Luciani GM, et al. Dafadine inhibits DAF-9 to promote dauer formation and longevity of Caenorhabditis elegans. Nat Chem Biol. 2011;7:891–893. doi: 10.1038/nchembio.698. [DOI] [PubMed] [Google Scholar]
- 82.Schaedel ON, Gerisch B, Antebi A, Sternberg PW. Hormonal signal amplification mediates environmental conditions during development and controls an irreversible commitment to adulthood. PLoS Biol. 2012;10:e1001306. doi: 10.1371/journal.pbio.1001306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Judkins JC, et al. A photocleavable masked nuclear-receptor ligand enables temporal control of C. elegans development. Angew Chem Int Ed Engl. 2014;53:2110–2113. doi: 10.1002/anie.201307465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ogawa A, Streit A, Antebi A, Sommer RJ. A conserved endocrine mechanism controls the formation of dauer and infective larvae in nematodes. Curr Biol. 2009;19:67–71. doi: 10.1016/j.cub.2008.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang Z, et al. Identification of the nuclear receptor DAF-12 as a therapeutic target in parasitic nematodes. Proc Natl Acad Sci U S A. 2009;106:9138–9143. doi: 10.1073/pnas.0904064106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhi X, et al. Structural conservation of ligand binding reveals a bile acid-like signaling pathway in nematodes. J Biol Chem. 2012;287:4894–4903. doi: 10.1074/jbc.M111.315242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Albarqi MM, et al. Regulation of Life Cycle Checkpoints and Developmental Activation of Infective Larvae in Strongyloides stercoralis by Dafachronic Acid. PLoS Pathog. 2016;12:e1005358. doi: 10.1371/journal.ppat.1005358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Baugh LR, Sternberg PW. DAF-16/FOXO regulates transcription of cki-1/Cip/Kip and repression of lin-4 during C. elegans L1 arrest. Curr Biol. 2006;16:780–785. doi: 10.1016/j.cub.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 89.Chen Y, Baugh LR. Ins-4 and daf-28 function redundantly to regulate C. elegans L1 arrest. Dev Biol. 2014;394:314–326. doi: 10.1016/j.ydbio.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 90.Lee BH, Ashrafi K. A TRPV channel modulates C. elegans neurosecretion, larval starvation survival, and adult lifespan. PLoS Genet. 2008;4:e1000213. doi: 10.1371/journal.pgen.1000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fukuyama M, et al. C. elegans AMPKs promote survival and arrest germline development during nutrient stress. Biol Open. 2012;1:929–936. doi: 10.1242/bio.2012836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fukuyama M, Kontani K, Katada T, Rougvie AE. The C. elegans Hypodermis Couples Progenitor Cell Quiescence to the Dietary State. Curr Biol. 2015;25:1241–1248. doi: 10.1016/j.cub.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 93.Shou Q, et al. A hybrid polyketide-nonribosomal peptide in nematodes that promotes larval survival. Nat Chem Biol. 2016;12:770–772. doi: 10.1038/nchembio.2144. This paper identified the first hybrid polyketide-nonribosomal peptide from an animal and showed that it influences starvation survival in C. elegans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.O’Brien RV, Davis RW, Khosla C, Hillenmeyer ME. Computational identification and analysis of orphan assembly-line polyketide synthases. J Antibiot (Tokyo) 2014;67:89–97. doi: 10.1038/ja.2013.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang H, Fewer DP, Holm L, Rouhiainen L, Sivonen K. Atlas of nonribosomal peptide and polyketide biosynthetic pathways reveals common occurrence of nonmodular enzymes. Proc Natl Acad Sci U S A. 2014;111:9259–9264. doi: 10.1073/pnas.1401734111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Artyukhin AB, Schroeder FC, Avery L. Density dependence in Caenorhabditis larval starvation. Sci Rep. 2013;3:2777. doi: 10.1038/srep02777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Maures TJ, et al. Males shorten the life span of C. elegans hermaphrodites via secreted compounds. Science. 2014;343:541–544. doi: 10.1126/science.1244160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Aprison EZ, Ruvinsky I. Sexually Antagonistic Male Signals Manipulate Germline and Soma of C. elegans Hermaphrodites. Curr Biol. 2016;26:2827–2833. doi: 10.1016/j.cub.2016.08.024. [DOI] [PubMed] [Google Scholar]