Summary
Background
Squamous cell carcinoma of the anal canal (SCCA) is a rare malignancy associated with infection by human papillomavirus (HPV). No consensus treatment approach exists for the treatment of metastatic disease. Because intratumoral HPV oncoproteins upregulate immune checkpoint proteins such as PD-1 to evade immune-mediated cytotoxicity, we did a trial of the anti-PD-1 antibody nivolumab for patients with metastatic SCCA.
Methods
We did this single-arm, multicentre, phase 2 trial at ten academic centres in the USA. We enrolled patients with treatment-refractory metastatic SCCA, who were given nivolumab every 2 weeks (3 mg/kg). The primary endpoint was response according to Response Evaluation Criteria in Solid Tumors, version 1.1, in the intention-to-treat population. At the time of data cutoff, the study was ongoing, with patients continuing to receive treatment. The study is registered with ClinicalTrials.gov, number NCT02314169.
Findings
We screened 39 patients, of whom 37 were enrolled and received at least one dose of nivolumab. Among the 37 patients, nine (24% [95% CI 15–33]) had responses. There were two complete responses and seven partial responses. Grade 3 adverse events were anaemia (n=2), fatigue (n=1), rash (n=1), and hypothyroidism (n=1). No serious adverse events were reported.
Interpretation
To our knowledge, this is the first completed phase 2 trial of immunotherapy for SCCA. Nivolumab is well tolerated and effective as a monotherapy for patients with metastatic SCCA. Immune checkpoint blockade appears to be a promising approach for patients with this orphan disease.
Funding
National Cancer Institute/Cancer Therapy Evaluation Program, the HPV and Anal Cancer Foundation, the E B Anal Cancer Fund, The University of Texas MD Anderson Moon Shots Program, and an anonymous philanthropic donor.
Introduction
Squamous cell carcinoma of the anal canal (SCCA) is rare, with roughly 27 000 new cases per year worldwide.1 Although most patients with localised SCCA are cured by chemoradiation,2,3 25% of patients develop distant metastases.4,5 There is no consensus for the treatment of refractory metastatic disease. To our knowledge, no phase 2 study using immunotherapy has thus far been completed for metastatic SCCA.
More than 90% of cases of SCCA are linked to prior infection with human papillomavirus (HPV).6–9 Preventive vaccinations against HPV are underused,10 with fewer than half of adolescent males and females receiving HPV vaccinations.11 Still, the incidence of SCCA is increasing annually worldwide,12 a trend expected to continue over the coming decades.
HPV viral proteins E6 and E7 contribute to the oncogenic transformation of anal squamous epithelium into invasive cancer.13–15 Within tumour cells, HPV oncoproteins are immunogenic and can trigger an anti-tumour host immune response by recruitment of tumour-infiltrating lymphocytes.16,17 Tumour cells express PD-L1 and, on binding its inhibitory receptor PD-1 on the surface of T cells, downregulate T-cell activation and thwart the local anti-tumour immune response.18,19 Nivolumab is a humanised monoclonal antibody against PD-1 that disrupts this interaction, enabling T-cell cytotoxicity. It has activity as a monotherapy in advanced solid cancers, such as head and neck cancer, melanoma, non-small-cell lung cancer, and renal cell carcinoma.20–24 We did a multicentre, phase 2 study of nivolumab for patients with previously treated metastatic SCCA.
Methods
Study design and participants
NCI9673 was a multicentre, single-arm, phase 2 trial of nivolumab done through the National Cancer Institute’s Experimental Therapeutics Clinical Trials Network (ETCTN) at ten academic centres in the USA. We included patients aged at least 18 years with histologically confirmed SCCA, a life expectancy of at least 6 months, and an Eastern Cooperative Oncology Group performance status of 0 or 1. We excluded patients with adenocarcinoma of the anal canal. Participants had to have measurable disease according to the standard Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1,25 and at least one previous systemic therapy for surgically unresectable or metastatic disease. However, patients developing new metastatic disease within 6 months of completion of chemoradiation for management of limited-stage disease were allowed to participate. A minimum period of 28 days was required between any previous chemotherapy for metastatic disease and initiation of nivolumab. At least 3 months must have elapsed between any surgery or radiotherapy for oligometastatic disease and administration of treatment during the study. Patients who had previously received immunotherapeutic drugs were ineligible.
Patients had to have adequate bone marrow, renal, and hepatic function, including an absolute neutrophil count greater than 1500 cells per μL; haemoglobin concentration of at least 90 g/L; platelet count greater than 100 000 per μL; total bilirubin no more than 1.5 times the institutional upper limit of normal (ULN), with the exception of patients with Gilbert’s syndrome, who were allowed a total bilirubin no more than 30 g/L; alanine aminotransferase and aspartate aminotransferase concentrations of no more than 2.5 times the ULN; and a serum creatinine concentration of no more than 1.5 times the ULN, or at least 50 mL/min according to the Cockcroft-Gault formula to assess renal function in patients whose creatinine clearance exceeded the 1.5 times the ULN limit. HIV-positive patients were allowed to participate if their CD4-positive cell count exceeded 300 cells per μL, their HIV viral load was undetectable, and they were compliant with antiretroviral treatment. Patients with hepatitis B virus or hepatitis C virus were eligible if their liver function tests were less than the treating institution’s ULN.
We excluded patients with active autoimmune disease or history of autoimmune disease that might recur with the potential to affect vital organ function or require immunosuppressive treatment including chronic prolonged systemic corticosteroids (defined as corticosteroid use for ≥1 month). Patients with any other concomitant condition requiring systemic corticosteroids were ineligible. Patients with treated brain metastases were allowed to participate provided that their lesions were stable and asymptomatic for at least 3 months. Patients with symptomatic congestive heart failure, unstable angina pectoris, or dysrhythmias were not allowed to participate. Pregnant women were not eligible, and both men and women of childbearing potential who were treated with nivolumab were required to use contraception if sexually active. Approval for the study was granted through the central institutional research board for all participating institutions. All patients provided written informed consent before study entry. This trial was done according to the Declaration of Helsinki.
Procedures
Nivolumab was administered intravenously every 2 weeks at a dose of 3 mg/kg. Toxic effects were assessed at baseline and every 2 weeks before each dose of nivolumab. Adverse events were measured according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. No dose reductions were allowed. However, patients who had grade 2 or more adverse events requiring corticosteroids for drug-related toxic effects had their treatment paused until the degree of severity had improved to at least grade 1. Treatment was otherwise continued until objective disease progression, development of intercurrent illness preventing further drug administration, unacceptable adverse events, dose delays of more than 6 weeks or more than two dose delays for the same adverse event, or withdrawal of consent. Treatment beyond progression was permitted during the initial 12-week treatment period provided that no more than four new lesions (the sum of which could not exceed 40% of the baseline sum, including new lesions) developed and that the patient remained clinically stable. Responses to treatment were assessed by the site investigator by RECIST 1.1 criteria using CT or MRI scans obtained at baseline and every 6 weeks thereafter. No central review to assess radiographic response was done. An electrocardiogram, thyroid profile, and pregnancy test (for women of childbearing age) were obtained at baseline. Liver, renal, and blood counts (white blood cell count, haemoglobin, and platelet count) were assessed at baseline and before every dose of nivolumab.
Optional tumour biopsies were collected at baseline and after two doses of nivolumab. To test for HPV, available pretreatment tumour tissue was tested for expression of p16 by immunohistochemistry and for HPV subtypes 16, 18, 31, 33, and 51 as previously described.7 Formalin-fixed, paraffin-embedded tumour specimens were stained with primary antibodies against CD8 (ThermoFisher Scientific, Waltham, MA, USA), Granzyme B (Leica Microsystems, Wetzlar, Germany), PD-L1 (E1L3N clone, Cell Signaling, Danvers, MA, USA), or PD-1 (Abcam, Epitomics, Cambridge, UK). All immunohistochemistry slides were scanned and digitised (Scanscope XT, Aperio Technologies, Wetzlar, Germany), and quantitative analyses of protein expression were done with ImageScope software (Aperio). Two pathologists independently reviewed immunohistochemistry data. Fresh tumour tissue was collected in a single cell suspension (GentleMacs Octo dissociator, Miltenyi, CA, USA). Staining for flow cytometry was carried out for the following markers: CD8-Alexa Fluor 700 (ThermoFisher Scientific), PD-1-BV650 (BD Biosciences, San Jose, CA, USA), CD3-PerCP-Cy5.5 (BioLegend, San Diego, CA, USA), TIM-3-BV605 (BioLegend), CD4-Alexa Fluor 532 (ThermoFisher Scientific), LAG-3-PE (eBioscience, San Diego, CA, USA), live/dead fixable yellow stain (ThermoFisher Scientific), PD-L1-BV786 (BD Biosciences), and CD45-Alexa Fluor 532 (eBioscience). Samples were run on a FACS Fortessa (BD Biosciences) and analysed using FlowJo software (Tree Star, OR, USA). These immune monitoring studies were done by the Immunotherapy Platform at MD Anderson Cancer Center.
All patients had the opportunity to participate in an optional translational component of the study in which serial blood samples were collected before treatment and before the second, fourth, and sixth doses of nivolumab. Genomic alterations (mutations and amplifications) detected from circulating cell-free DNA extracted from pretreatment plasma samples were analysed by next-generation sequencing of a targeted 70-gene panel using a CLIA-approved Illumina Hi-Seq 2500 platform (Guardant Health; Redwood City, CA, USA).26
Outcomes
The primary endpoint was response, defined as the percentage of patients achieving a partial or complete radiographic response. Secondary endpoints were progression-free survival (time from start of treatment to progression or death, whichever occurred first), overall survival (time from start of treatment to death of any cause), and the development of grade 3 or 4 toxic effects. Exploratory objectives included characterisation of immune biomarkers with paired tissue biopsies and genomic profiling from plasma samples and correlation with radiographic response. Duration of response and depth of response were assessed in a post-hoc analysis. Depth of response was defined as the percentage change from baseline, using the prespecified target lesions (chosen by the primary treating physician), in the sum of measurable diameters of assessed tumours. Duration of response was the time from identified tumour response according to RECIST criteria until the onset of radiographic disease progression.
Statistical analysis
For this Simon’s optimal, two-stage phase 2 study, we established a null hypothesis p≤0.05 and an alternative hypothesis p≥0.20, where p represents the percentage of patients with a partial or complete radiographic response to nivolumab. Using an α of 0.10 and β of 0.10, 12 patients were treated in the first stage. The study was designed so that after at least one radiographic response, 25 additional patients were included. We calculated 95% CIs around proportions using an exact binomial calculation. All analyses were done on the intention-to-treat population. We estimated median progression-free survival, overall survival, and duration of response by Kaplan-Meier analyses. We calculated 95% CIs around median survival outcomes using the Greenwood formula.27 We calculated the percentage of patients with durable responses as the number of patients with a documented radiographic response lasting past the first detection of partial or complete response, relative to the total number of patients who achieved a radiographic response. Adverse events were recorded and tabulated according to type and grade. To assess associations between response and various clinicopathological factors, we calculated odds ratios, corrected for multiple comparisons, in a post-hoc analysis according to methods previously described.28 Using pretreatment specimens for both the immunohistochemistry and flow cytometry analyses, we compared mean biomarker expression values using a Mann–Whitney test between the patients with response and without response according to the time of first restaging. Patients were restaged for response by diagnostic imaging by CT or MRI every 6 weeks.
We analysed data using GraphPad Prism for Windows, version 6.00 (La Jolla, California, USA).
This trial is registered with ClinicalTrials.gov (NCT02314169).
Role of the funding source
The study was designed by the investigators. The National Cancer Institute/Cancer Therapy Evaluation Program oversaw all data collection. None of the funders, other than the National Cancer Institute/Cancer Therapy Evaluation Program, had any role in the data analysis or interpretation of the results. None of the funders had a role in writing the Article. VKM, LX, LV, JB, CO, AM, KB, HS, JA, PSh, and CE had access to raw data. The corresponding author had full access to all of the data and the final responsibility for the decision to submit for publication.
Results
We screened 39 patients, all with surgically unresectable or metastatic disease, between May 14, 2015, and Nov 11, 2015 (appendix p 4). Two patients were ineligible and 37 patients were enrolled, received at least one dose of nivolumab, and were included in the analyses. All patients had previously been treated for advanced disease (median lines of previous therapy 2 [IQR 1–2.5]). 32 (86%) of 37 patients had received a platinum-based treatment for metastatic disease. 31 patients (84%) had previously received radiation to the primary tumour. Table 1 shows baseline demographic data. Two patients were HIV-positive. No patients had a history of hepatitis B virus or hepatitis C virus infection.
Table 1.
Baseline demographics
| n=37 | |
|---|---|
| Median age (years) | 56 (51–64) |
|
| |
| Race | |
| White | 33 (90%) |
| Black | 2 (5%) |
| Asian | 2 (5%) |
|
| |
| Sex | |
| Male | 10 (27%) |
| Female | 27 (73%) |
|
| |
| ECOG performance status | |
| 0 | 10 (27%) |
| 1 | 27 (73%) |
|
| |
| HIV positive | 2 (5%) |
|
| |
| Median number of prior lines of therapy | 2 (1–7) |
|
| |
| Distribution of unresectable disease | |
| Local recurrence | 15 (41%) |
| Distant metastasis | 37 (100%) |
|
| |
| Sites of distant metastases | |
| Lung | 19 (51%) |
| Liver | 14 (38%) |
| Lymph node | 10 (27%) |
| Soft tissue | 5 (14%) |
Data are n (%) or median (IQR). ECOG=Eastern Cooperative Oncology Group.
The data cutoff date was May 16, 2016. The median follow-up time for all patients was 10.1 months (95% CI 9.4–12.2). Patients received a median of six doses of nivolumab (IQR 3–10). Three patients were ineligible for assessment of radiographic response (had only one dose before withdrawing from the study), and 34 patients underwent at least one restaging scan for assessment of response to nivolumab. All 37 patients were included in all analyses.
In the first stage of the two-stage design, four of the first 12 patients had partial responses, which allowed the recruitment of 25 additional patients for the second stage. Nine (24% [95% CI 15–33]) of 37 patients achieved a response (seven partial responses and two complete responses; figure 1A). One of the two HIV-positive patients had a partial radiographic response. Durable responses were recorded in seven (78%) of nine patients with a median duration of response of 5.8 months (IQR 3.9–8.1) among responders (figure 1B). As of the date of data cutoff, six (67%) of the nine patients who responded remained on study, with the longest ongoing duration of response of 10.4 months. 17 (47% [95% CI 30–63]) of 37 patients had stable disease and 27 (72% [95% CI 53–84]) had disease control. Median reduction in target lesions for the responders from baseline (depth of response) was 70% (IQR 57–90).
Figure 1. Tumour response in 34 assessable patients.
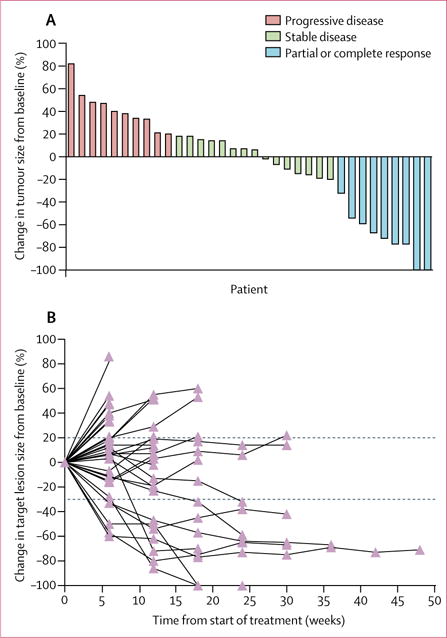
(A) Waterfall plot. (B) Duration of response. The dotted line represents RECIST 1.1 markers of disease progression. Three patients who received only one dose of nivolumab were not assessed.
At the time of data cutoff, 24 (65%) of the 37 patients who received treatment had disease progression while taking nivolumab. There were no treatment-related deaths; 16 (43%) of 37 patients died because of progression of disease. Median progression-free survival was 4.1 months (95% CI 3.0–7.9; figure 2A). 6-month progression-free survival was 38% (95% CI 24–60). Median overall survival was 11·5 months (95% CI 7.1–not estimable; figure 2A), with an estimated 1-year overall survival of 48% (32–74).
Figure 2.
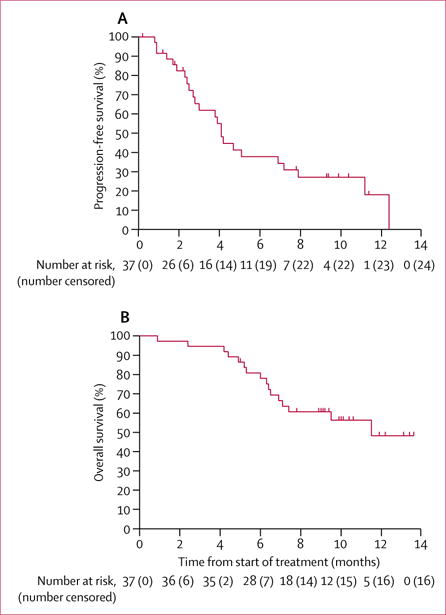
Progression-free survival (A) and overall survival (B)
Of the 37 patients treated with nivolumab, the most common adverse events were anaemia (26 [70%]), fatigue (25 [68%]), and rash (11 [30%]; table 2). Five patients had grade 3 adverse events: anaemia (two patients), fatigue (one patient), rash (one patient), and hypothyroidism (one patient). One patient developed treatment-related grade 2 pneumonitis requiring steroid therapy and a temporary treatment break. Only one other patient required immunosuppressive drugs for treatment of nivolumab-related autoimmune hypothyroidism, which resolved after a short course of corticosteroids. No grade 3 or 4 adverse events occurred in the HIV-positive patients. No patients discontinued the study because of drug-related toxic effects.
Table 2.
All adverse events
| Grade 1 | Grade 2 | Grade 3 | |
|---|---|---|---|
| Anaemia | 13 (35%) | 11 (30%) | 2 (5%) |
| Fatigue | 17 (46%) | 7 (19%) | 1 (3%) |
| Rash | 8 (22%) | 2 (5%) | 1 (3%) |
| Constipation | 8 (22%) | 2 (5%) | 0 |
| Anorexia | 5 (14%) | 4 (11%) | 0 |
| Diarrhoea | 8 (22%) | 0 | 0 |
| Weight loss | 5 (14%) | 1 (3%) | 0 |
| Arthralgia | 3 (8%) | 3 (8%) | 0 |
| Hyperglycaemia | 3 (8%) | 1 (3%) | 0 |
| Hypothyroidism | 1 (3%) | 1 (3%) | 1 (3%) |
| Lymphoedema | 1 (3%) | 1 (3%) | 0 |
| Nausea | 2 (5%) | 0 | 0 |
| Pneumonitis | 0 | 1 (3%) | 0 |
Data are n (%). n=37.
30 patients had pretreatment plasma samples available for analysis of cell-free DNA. We detected 58 alterations, 47 mutations, and 11 gene amplifications, accounting for 1.6 mutations per patient (appendix p 5). The most common mutations were in TP53 (n=8, 27%) and PIK3CA (n=7, 23%). We noted no significant association with radiographic response for mutations either in TP53 (OR 0.42 [95% CI 0.07–2.60], p=0.36) or in PIK3CA (10.00 [0.87–110.00], p=0.064). All other genes were mutated in fewer than three of the 30 analysed patients.
13 patients (four responders, nine non-responders) treated at MD Anderson Cancer Center consented to tumour biopsies and had pretreatment tumour samples collected for assessment by immunohistochemistry. We also had HPV test results for 15 pre-existing archival tissue tumour samples from these patients. HPV was detected in all 15 of these tumour samples. By immunohistochemistry, responders had higher baseline percentages of T cells expressing CD8 (figure 3A) and granzyme B (figure 3B), than did non-responders. Immune cells in the tumour microenvironment of responders had higher concentrations of PD-1 relative to the non-responders (figure 3C). Similarly, PD-L1 was expressed on more than 40% of tumour cells analysed from the patients who responded to nivolumab, higher than that in the non-responders (figure 3D).
Figure 3. Immune profiling comparing expression of various biomarkers in pretreatment biopsies between responders and non-responders.
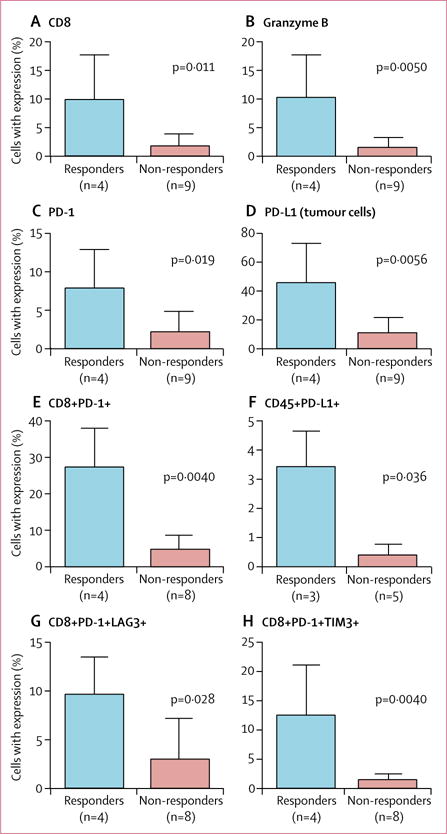
Assessed by immunohistochemistry (A–D) and flow cytometry (E–H). The bars are means (SDs).
Nine patients (four responders, five non-responders) had adequate fresh tissue from the pretreatment biopsy to analyse by flow cytometry. Flow cytometry analysis of fresh tumours specimens showed that PD-1 expression on CD8+ T cells was higher for responders than for non-responders at baseline (figure 3E), as were LAG-3 and TIM-3 (appendix p 2). CD45+ leucocytes had higher baseline expression of PD-L1 in responding patients than in non-responding patients (figure 3F, appendix p 1). Among the CD8+ T cells, responders had higher dual expression of PD-1 and LAG-3 and of PD-1 and TIM-3 compared with non-responders (figure 3G, 3H). The prevalence of these CD8+ T-cell subsets in the biopsy samples was lower, but not significantly, after two doses of nivolumab compared with baseline for patients who had a response (appendix p 3). In patients who did not have a response, the prevalence of these cells increased, although not significantly (appendix p 3).
In a post-hoc analysis, we recorded no effect on response according to sex (odds ratio [OR] 0.26 [95% CI 0.03–2.50], p=0.25), previous exposure to platinum chemotherapy for management of systemic disease (1.30 [0.13–14.00], p=0.81), or previous radiotherapy treatment (0.24 [0.04–1.50], p=0.13). After correction for multiple comparisons, we recorded no association between treatment and sites of distant metastases, including the liver (1.10 [0.25–5.20], p=0.88), lung (0.96 [0.24–3.80], p=0.96), lymph node (8.30 [1.30–53.00], p=0.03), or soft tissue (0.18 [0.01–3.50], p=0.26). We recorded no cases of pseudoprogression,29 in which tumours decrease in size following an upfront enlargement.
Discussion
Nivolumab resulted in objective responses in 24% of patients with metastatic SCCA. All patients had received previous treatment for incurable, metastatic SCCA. Although the median progression-free survival was 4.1 months, the longest duration of treatment has been almost 1 year and is continuing as of the data cutoff date. Nivolumab was well tolerated, with side-effect profiles similar to what has been reported with nivolumab for other solid tumours.20
Historically, doublet chemotherapy with cisplatin and fluorouracil has been the most common treatment for patients with metastatic SCCA, based on small, retrospective case series of fewer than 20 patients.30,31 Given the paucity of treatment options available for this population, novel effective therapies are greatly needed. Our findings are encouraging with regard to improving outcomes for patients with refractory metastatic SCCA, especially since, to our knowledge, this trial is the first prospective phase 2 study of immunotherapy for patients with previously treated metastatic anal cancer.
Median overall survival was 11.5 months. Although the usual overall survival for this population is unclear, one case series32 of patients with metastatic SCCA treated with only palliative chemotherapy reported a median overall survival of 17 months from diagnosis of metastatic disease. Because the patients in our study were heavily pretreated, our results suggest that immune checkpoint blockade agents might extend overall survival beyond currently available therapies, especially if provided early in the disease treatment course, although future trials are needed for confirmation.
To our knowledge, this is the first completed study of an immune checkpoint inhibitor that included HIV-positive patients, who are at high risk of developing SCCA. Provided that HIV-positive patients maintain adequate CD4+ T-cell counts under careful clinical observation with an infectious diseases specialist, we recommend that HIV-positive patients be considered for participation in future clinical trials with immune checkpoint inhibitors so that the safety and activity of these drugs can be studied further in a larger series.
In our cell-free DNA analysis, there was no association with mutation status for any gene and response to anti-PD-1 treatment. Consistent with our findings of only 1.6 mutations per tumour according to a 70-gene next-generation sequencing test, a mean 3.5 mutations per tumour were detected in a separate cohort of patients with SCCA using a larger 255-gene tissue-based next-generation sequencing test.33 No comprehensive whole exome analysis has been done to characterise fully the mutational burden of SCCA. Although limited by a fixed number of analysed genes, our data, together with the series using the 255-gene panel, suggest that squamous cell carcinomas of the anal canal do not feature a high prevalence of somatic mutations across the genome. Therefore, a high mutation burden might not drive the immunogenicity associated with responses to immune checkpoint blockade.34 Rather, given that HPV was detected in all tested specimens in our study and the high prevalence of HPV in SCCA, we postulate that the viral interaction of the host tumour cells and surrounding microenvironment could be responsible for the immune responses to nivolumab.
Analysis of pretreatment biopsies revealed an association between treatment responses and the presence of an inflammatory milieu within the tumour before administration of nivolumab. Tumours from responders had more activated effector T cells at baseline than did non-responders. Analyses using both immunohistochemistry and flow cytometry showed higher PD-1 and PD-L1 expression in baseline tissue samples among responders than non-responders. Among the PD-1-positive CD8+ T cells identified by flow cytometry, higher pretreatment coexpression of other coinhibitory immune markers like LAG-3 and TIM-3 was associated with response to nivolumab. Although our tissue analysis was limited by a small number of specimens, these findings are consistent with data from other solid tumours such as melanoma.35
Our study had several limitations. First, the dose of nivolumab we used differs from the 2016 recommendation of a fixed 240 mg every 2 weeks.36 Although we support fixed dosing as a consideration for future trials for this population, our data show that nivolumab has clinical activity in patients with metastatic SCCA. Also, because of the small number of tissue samples from patients, we could not do a comprehensive characterisation comparing the patients who did or did not have radiographic responses to nivolumab. For example, we were unable to construct a quantitative score to identify responders and nonresponders on the basis of the frequency of CD8+ T cells and expression of various biomarkers. Nonetheless, our findings should encourage further analysis of a possible association between biomarker expression and response to immunotherapeutic drugs in more patients with SCCA. In a forthcoming trial of nivolumab plus ipilimumab for patients with metastatic SCCA, paired biopsies will be analysed to assess for an association between response to treatment and expression of p16 and p53 by immunohistochemistry, changes in T-cell receptor profiling, and immune response against viral proteins E6 and E7 as measured by ELISPOT assay.
In summary, nivolumab is well tolerated, and is a promising treatment for patients with refractory metastatic SCCA. Based on these results, we remain optimistic that immune checkpoint blockade drugs represent the next step towards improving clinical outcomes for patients with this disease.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed for reports in English published between Jan 1, 2005, and Feb 1, 2014, using the terms “anal cancer”, “immunotherapy”, and “phase 2”. We identified no clinical trials. When this study was first designed, the recommendation for treatment of unresectable or metastatic squamous cell carcinoma of the anal canal (SCCA) was a chemotherapy doublet with cisplatin and fluorouracil. No consensus exists regarding treatment following progression on front-line therapy for unresectable or metastatic anal cancer. No previous prospective phase 2 or 3 clinical trial has been completed using immunotherapy agents for the treatment of metastatic anal cancer. This trial was developed to characterise the safety and activity for nivolumab in patients with previously treated SCCA.
Added value of this study
Most cases of SCCA are linked to previous infection with the human papillomavirus. We conducted a multicentre, phase 2 study of the anti-PD-1 antibody nivolumab for patients with previously treated metastatic SCCA. Nivolumab was well tolerated and effective with two complete responses and seven partial responses with limited toxic effects.
Implications of all the evidence
Nivolumab is well tolerated and is a promising therapy for patients with refractory metastatic anal cancer. Immune checkpoint blockade drugs are a promising approach towards improving clinical outcomes for patients with metastatic SCCA.
Acknowledgments
This work was funded by the HPV and Anal Cancer Foundation, the EB Anal Cancer Fund, the NIH UM1CA186688 and N01-CM-2011-0003 Awards, generous contributions to The University of Texas MD Anderson Moon Shots Program, and an anonymous donor.
Footnotes
Contributors
VKM, LX, LV, JB, MP, GB, HS, JA, PSh, and CE designed the study. VKM, MES, HN, SI, PSi, KC, BP, DD, EC, JLW, TBS, AM, WCF, AO, RAW, and CE collected data and recruited patients. VKM, LX, LV, JB, WCF, CO, AO, JR, AM, KB, RL, HS, JA, PSh, and CE analysed and interpreted the data. All authors wrote and reviewed the report and gave final approval to submit for publication.
Declaration of interests
PSi has received fees from Bayer Pharmaceuticals and personal fees from Merrimack Pharma. EC has received grants from National Cancer Institute to her institution, and personal fees for advisory boards from Advaxis, Lilly, Merrimack, Bayer, Taiho, and Amgen. MP has received grants from the National Cancer Institute/Cancer Therapy Evaluation Program. KB has received personal fees from Guardant Health. RL has received personal fees from Guardant Health. JA owns stock in, and has acted as a consultant or on advisory boards for, Jounce, Kite Pharma, Evelo, Constellation, Bristol-Myers Squibb, GlaxoSmithKline, AstraZeneca, Amgen, and Neon. PSh owns stock in, and has acted as a consultant for, Jounce, Kite Pharma, Neon, Bristol-Myers Squibb, GlaskoSmithKline, AstraZeneca, Amgen, Constellation, Evelo. CE has received grants from Daiichi and Keryx, and personal fees from Bayer, Sirtex, Genentech, Roche/Genentech, and Bayer. VKM, MES, HN, SI, KC, BP, DD, JLW, LX, TB-S, LV, JB, AM, WCF, CO, GB, AO, JR, AM, RAW, and HS declare no completing interests.
Contributor Information
Van K Morris, The University of Texas—MD Anderson Cancer Center, Houston, TX, USA.
Mohamed E Salem, Lombardi Comprehensive Cancer Center, Georgetown University, Washington, DC, USA.
Halla Nimeiri, Northwestern University, Chicago, IL, USA.
Syma Iqbal, University of Southern California, Los Angeles, CA, USA.
Preet Singh, Washington University, St Louis, MO, USA.
Kristen Ciombor, The Ohio State University, Columbus, OH, USA.
Blase Polite, University of Chicago, Chicago, IL, USA.
Dustin Deming, University of Wisconsin, Madison, WI, USA.
Emily Chan, Vanderbilt-Ingram Cancer Center, Nashville, TN, USA.
James L Wade, Decatur Memorial Hospital, Decatur, IL, USA.
Lianchun Xiao, The University of Texas—MD Anderson Cancer Center, Houston, TX, USA.
Prof Tanios Bekaii-Saab, The Ohio State University, Columbus, OH, USA.
Luis Vence, The University of Texas—MD Anderson Cancer Center, Houston, TX, USA.
Jorge Blando, The University of Texas—MD Anderson Cancer Center, Houston, TX, USA.
Armeen Mahvash, The University of Texas—MD Anderson Cancer Center, Houston, TX, USA.
Wai Chin Foo, The University of Texas—MD Anderson Cancer Center, Houston, TX, USA.
Chimela Ohaji, The University of Texas—MD Anderson Cancer Center, Houston, TX, USA.
Manolo Pasia, The University of Texas—MD Anderson Cancer Center, Houston, TX, USA.
Gail Bland, The University of Texas—MD Anderson Cancer Center, Houston, TX, USA.
Aki Ohinata, The University of Texas—MD Anderson Cancer Center, Houston, TX, USA.
Jane Rogers, The University of Texas—MD Anderson Cancer Center, Houston, TX, USA.
Amir Mehdizadeh, The University of Texas—MD Anderson Cancer Center, Houston, TX, USA.
Kimberly Banks, Guardant Health, Redwood City, CA, USA.
Richard Lanman, Guardant Health, Redwood City, CA, USA.
Prof. Robert A Wolff, The University of Texas—MD Anderson Cancer Center, Houston, TX, USA.
Howard Streicher, National Cancer Institute, Bethesda, MD, USA.
Prof James Allison, The University of Texas—MD Anderson Cancer Center, Houston, TX, USA.
Prof Padmanee Sharma, The University of Texas—MD Anderson Cancer Center, Houston, TX, USA.
Prof Cathy Eng, The University of Texas—MD Anderson Cancer Center, Houston, TX, USA.
References
- 1.de Martel C, Ferlay J, Franceschi S, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13:607–15. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- 2.Bartelink H, Roelofsen F, Eschwege F, et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol. 1997;15:2040–49. doi: 10.1200/JCO.1997.15.5.2040. [DOI] [PubMed] [Google Scholar]
- 3.Flam M, John M, Pajak TF, et al. Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: results of a phase III randomized intergroup study. J Clin Oncol. 1996;14:2527–39. doi: 10.1200/JCO.1996.14.9.2527. [DOI] [PubMed] [Google Scholar]
- 4.Das P, Bhatia S, Eng C, et al. Predictors and patterns of recurrence after definitive chemoradiation for anal cancer. Int J Radiat Oncol Biol Phys. 2007;68:794–800. doi: 10.1016/j.ijrobp.2006.12.052. [DOI] [PubMed] [Google Scholar]
- 5.Eng C. Anal cancer: current and future methodology. Cancer Invest. 2006;24:535–44. doi: 10.1080/07357900600815208. [DOI] [PubMed] [Google Scholar]
- 6.Frisch M, Glimelius B, van den Brule AJ, et al. Sexually transmitted infection as a cause of anal cancer. N Engl J Med. 1997;337:1350–58. doi: 10.1056/NEJM199711063371904. [DOI] [PubMed] [Google Scholar]
- 7.Morris VK, Rashid A, Rodriguez-Bigas M, et al. Clinicopathologic features associated with human papillomavirus/p16 in patients with metastatic squamous cell carcinoma of the anal canal. Oncologist. 2015;20:1247–52. doi: 10.1634/theoncologist.2015-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daling JR, Madeleine MM, Johnson LG, et al. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer. 2004;101:270–80. doi: 10.1002/cncr.20365. [DOI] [PubMed] [Google Scholar]
- 9.Hoots BE, Palefsky JM, Pimenta JM, Smith JS. Human papillomavirus type distribution in anal cancer and anal intraepithelial lesions. Int J Cancer. 2009;124:2375–83. doi: 10.1002/ijc.24215. [DOI] [PubMed] [Google Scholar]
- 10.Saslow D, Andrews KS, Manassaram-Baptiste D, et al. Human papillomavirus vaccination guideline update: American Cancer Society guideline endorsement. CA Cancer J Clin. 2016;66:375–85. doi: 10.3322/caac.21355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:850–58. doi: 10.15585/mmwr.mm6533a4. [DOI] [PubMed] [Google Scholar]
- 12.Viens LJ, Henley SJ, Watson M, et al. Human papillomavirus-associated cancers—United States, 2008–2012. MMWR Morb Mortal Wkly Rep. 2016;65:661–66. doi: 10.15585/mmwr.mm6526a1. [DOI] [PubMed] [Google Scholar]
- 13.Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–36. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 14.Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76–79. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- 15.Balsitis SJ, Sage J, Duensing S, Munger K, Jacks T, Lambert PF. Recapitulation of the effects of the human papillomavirus type 16 E7 oncogene on mouse epithelium by somatic Rb deletion and detection of pRb-independent effects of E7 in vivo. Mol Cell Biol. 2003;23:9094–103. doi: 10.1128/MCB.23.24.9094-9103.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Welters MJ, de Jong A, van den Eeden SJ, et al. Frequent display of human papillomavirus type 16 E6-specific memory T-helper cells in the healthy population as witness of previous viral encounter. Cancer Res. 2003;63:636–41. [PubMed] [Google Scholar]
- 17.de Jong A, van Poelgeest MI, van der Hulst JM, et al. Human papillomavirus type 16-positive cervical cancer is associated with impaired CD4+ T-cell immunity against early antigens E2 and E6. Cancer Res. 2004;64:5449–55. doi: 10.1158/0008-5472.CAN-04-0831. [DOI] [PubMed] [Google Scholar]
- 18.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–95. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–34. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–35. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–13. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–30. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 24.Ferris RL, Blumenschein GR, Fayette J, et al. Further evaluations of nivolumab (nivo) versus investigator’s choice (IC) chemotherapy for recurrent or metastatic (R/M) squamous cell carcinoma of the head and neck (SCCHN): CheckMate 141. Proc Am Soc Clin Oncol. 2016;34(suppl) abstr 6009. [Google Scholar]
- 25.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 26.Lanman RB, Mortimer SA, Zill OA, et al. Analytical and clinical validation of a digital sequencing panel for quantitative, highly accurate evaluation of cell-free circulating tumor DNA. PLoS One. 2015;10:e0140712. doi: 10.1371/journal.pone.0140712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenwood M. A report of the natural duration of cancer. London: HM Stationery Office; 1926. [Google Scholar]
- 28.Altman DG. Practical statistics for medical research. 1st. London: Chapman and Hall; 1991. [Google Scholar]
- 29.Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–20. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 30.Ajani JA, Carrasco CH, Jackson DE, Wallace S. Combination of cisplatin plus fluoropyrimidine chemotherapy effective against liver metastases from carcinoma of the anal canal. Am J Med. 1989;87:221–24. doi: 10.1016/s0002-9343(89)80702-8. [DOI] [PubMed] [Google Scholar]
- 31.Faivre C, Rougier P, Ducreux M, et al. 5-fluorouracile and cisplatinum combination chemotherapy for metastatic squamous-cell anal cancer. Bull Cancer. 1999;86:861–65. in French. [PubMed] [Google Scholar]
- 32.Eng C, Chang GJ, You YN, et al. The role of systemic chemotherapy and multidisciplinary management in improving the overall survival of patients with metastatic squamous cell carcinoma of the anal canal. Oncotarget. 2014;5:11133–42. doi: 10.18632/oncotarget.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chung JH, Sanford E, Johnson A, et al. Comprehensive genomic profiling of anal squamous cell carcinoma reveals distinct genomically defined classes. Ann Oncol. 2016;27:1336–41. doi: 10.1093/annonc/mdw152. [DOI] [PubMed] [Google Scholar]
- 34.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–20. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–71. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.US Food and Drug Administration. Modification of the dosage regimen for nivolumab. http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm520871.htm (accessed Feb 9, 2017)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


