Abstract
Background
Endogenous regenerative capacity, assessed as circulating progenitor cell (PC) numbers, is an independent predictor of adverse outcomes in patients with cardiovascular disease. However, their predictive role in heart failure (HF) remains controversial. We assessed the relationship between the number of circulating PCs and the etiology and severity of HF and their impact on incident HF events.
Methods and Results
We recruited 2049 adults of which 651 had heart failure diagnosis. PCs were enumerated by flow cytometry as CD45med+ blood mononuclear cells expressing CD34, CD133, VEGFR2 and CXCR4 epitopes. PC subsets were lower in number in HF and after adjustment for clinical characteristics in multivariable analyses, a low CD34+ and CD34+/CXCR+ cell count remained independently associated with a diagnosis of HF (P<0.01). PCs levels were not significantly different in reduced [HFrEF] vs preserved [HFpEF] ejection fraction patients. In 514 subjects with HF, there were 98 (19.1%) all cause deaths during a 2.2 ± 1.5 year follow-up. In a Cox regression model adjusting for clinical variables, hematopoietic-enriched PCs (CD34+, CD34+/CD133+ and CD34+/CXCR4+) were independent predictors of all-cause death (Hazard ratio [HR] 2.0, 1.6, 1.6-fold higher mortality, respectively, P<0.03) among HF patients. Endothelial-enriched PCs (CD34+/VEGF+) were independent predictors of mortality in patients with HFpEF only (HR=5.0, P=0.001).
Conclusions
PC levels are lower in patients with HF and lower PC counts are strongly and independently predictive of mortality. Strategies to increase PCs and exogenous stem cell therapies designed to improve regenerative capacity in HF, especially in HFpEF need to be further explored.
Keywords: HF, progenitor cells, CD34+, CD133+, VEGFR2+, CXCR4+, HFpEF, HFrEF, non-ischemic cardiomyopathy, ischemic cardiomyopathy, cardiovascular outcomes
Progenitor cells (PCs) are mononuclear cells that are mostly derived from the bone marrow and can be identified in peripheral blood. CD34-expressing mononuclear cells have the potential to differentiate into diverse phenotypes including hematopoietic, endothelial, and non-hematopoietic (mesenchymal, lacking CD45 expression) lineages and participate in vascular and myocardial regeneration1, 2. CD133 is a 5-transmembrane antigen on primitive stem cells that is lost during maturation and dual expression of these markers (CD34+/CD133+) identifies a PC-enriched subpopulation3, 4. Co-expression of vascular endothelial growth factor receptor-2 (VEGFR2) appears to identify a rarer subpopulation of PCs further enriched for endothelial progenitors5, 6. Lastly, co-expression of Chemokine (C-X-C Motif) Receptor 4 (CXCR4), which promotes homing of PCs to stromal-derived factor-rich hypoxic environments, may further characterize CD34+ PCs with capacity for tissue repair7. The role of PCs in myocardial regeneration and vascular repair and remodeling is recognized to be largely through angiogenic and paracrine mechanisms1, 8.
Low circulating PC levels are associated with endothelial dysfunction, accelerated atherosclerosis, and adverse outcomes in patients with coronary artery disease and peripheral vascular disease9, 10. However, the role of PCs in HF remains controversial. Whether PCs levels are altered in HF compared to appropriate control populations, whether the etiology of HF differentially affects circulating PCs, and whether the alteration of PCs in HF have an impact on clinical outcomes remains unclear.
In this study, we investigated the frequency of circulating PCs enriched for hematopoietic and endothelial PCs in subjects with different types and severity of HF in comparison to non-HF controls, and studied the impact of circulating PC numbers on long term HF outcomes. Our hypothesis was that circulating PC counts will be significantly lower in patients with HF, correlate with its severity, and predict long term outcomes in HF.
METHODS
Study design
We enrolled patients from the Emory Cardiovascular Biobank, a prospective registry of adult patients undergoing cardiac catheterization for suspected or known coronary artery disease at three Emory Healthcare hospitals in Atlanta, GA. We excluded patients with (1) history of cardiac transplantation (2) acute myocardial infarction (3) evidence of significant infection as these would alter the levels of PC counts and (4) patients with severe valvular disease. Demographic characteristics, medical history, medication use, and behavioral habits were documented as previously described9. To validate the association between HF and PC counts, we analyzed this relationship in an independent cohort of 582 subjects (137 with HF) from the Mental Stress Ischemia Prognosis Study (MIPS), a prospective study that recruited patients with stable CAD between June 2011 and August 2014 at Emory University affiliated hospitals as previously described11. Both studies were approved by the Institutional Review Board at Emory University (Atlanta, GA). All subjects provided written informed consent.
Defining heart failure phenotypes
HF was defined by the presence of physician diagnosis of HF, or ICD-9 discharge diagnosis of HF. HF was categorized into HF with reduced ejection fraction (HFrEF) with ejection fraction (EF)≤40% and HF with preserved ejection fraction (HFpEF) with EF>40%. Ischemic cardiomyopathy was defined as presence of history of myocardial infarction, coronary revascularization, or obstructive coronary artery disease (>50% stenosis). Echocardiograms of patients with HF performed within 3 months of enrollment were reviewed for: ejection fraction, diastolic dysfunction, left atrial size, left posterior wall thickness, and right ventricular systolic pressure.
Progenitor cells assays
PC assays were performed using flow cytometry as previously described9, 10 as CD45med cells co-expressing CD34+, CD133+, VEGFR2+, or CXCR4+. Further details are described in the appendix.
Follow-up and Outcomes
We conducted follow-up as previously described9 to identify incident adverse cardiovascular outcomes including all-cause death, death from cardiovascular causes, and hospitalization for HF. Follow-up data was available for 495 HF patients (96.3%) with mean follow-up time of 2.17 ± 1.47 years.
Statistical analysis
Subject characteristics were reported as descriptive statistics with means, medians, standard deviations and ranges. Differences between groups were assessed using the t-test for continuous variables, and chi-square or Fischer exact tests for categorical variables where appropriate. Spearman rank correlation coefficients were used for examining associations between PCs and echocardiographic parameters as well as BNP levels. Two-sided P-value < 0.05 were considered statistically significant. For non-normally distributed variables such as PCs counts, Mann-Whitney U test was used to compare groups in unadjusted analyses. For multivariable analyses, PCs counts were examined as continuous variables after log-transformation (log 2) to achieve normality. PCs counts were examined also as a dichotomous variable stratified by previously identified cutoffs using receiver operating characteristic analyses and Youden index (sensitivity + specificity − 1) as previously described9. The following cutoffs were used: 737 cells/milliliter for CD34+ cells, 504 cells/ milliliter for CD34+/CD133+ cells, 533 cells/ milliliter for CD34+/CXCR4+ cells and median for CD34+/VEGF+ cells. Characteristics incorporated in multivariable analyses included variables that correlated in bivariate analysis with the outcome studied (age, gender, body mass index, smoking history, presence of CAD, ejection fraction, estimated glomerular filtration rate, beta blocker use, and angiotensin pathway antagonist use). We also used stepwise backwards regression to reach a final set of variables for each outcome, with P<0.1 as the threshold to retain a variable and obtained similar results. The association between PCs count and all-cause death, cardiovascular death, HF hospitalizations adjusting for the aforementioned variables was examined in Cox regression models. Analyses were performed using IBM SPSS Statistics Version 22, (Armonk, NY, USA).
RESULTS
Out of 1467 patients enrolled in this study, 514 had a diagnosis of HF, Table 1. Patients with HF were more likely to be older, black, with a higher burden of cardiovascular risk factors. Of the total HF cohort, 330 patients (64.2%) had HFrEF and 286 (86.7%) had ischemic cardiomyopathy. Patients with HFrEF were more likely to be male, black, with a history of previous myocardial infarction compared to those with HFpEF Table 2.
Table 1.
Patients Characteristics among patients with and without heart failure
| Variable | Total (n=1467) | No heart failure (n=953) | Heart failure (n=514) | P-value* |
|---|---|---|---|---|
| Age, years mean (SD) | 65 (13) | 65 (13) | 66 (14) | 0.01 |
| Male, n (%) | 887 (60.5) | 578 (60.7) | 309 (60.1) | 0.84 |
| Black, n (%) | 328 (22.4) | 184 (19.3) | 144 (28) | 0.0001 |
| Body Mass Index kg/m2,mean (SD) | 29 (6) | 30 (6) | 29 (7) | 0.28 |
| Smoking, n (%) | 974 (66.4) | 640 (67.2) | 334 (65) | 0.40 |
| Diabetes, n (%) | 597 (41.5) | 368 (39.4) | 229 (45.4) | 0.03 |
| Hypertension, n (%) | 1309 (89.8) | 840 (88.2) | 469 (92.9) | 0.01 |
| Hypercholesterolemia, n (%) | 1070 (73.9) | 695 (73.3) | 375 (75) | 0.49 |
| Low density lipoprotein, mg/dL mean (SD) | 91 (38) | 93 (39) | 87 (35) | 0.002 |
| Estimated GFR mL/min/1.73 m2, mean (SD) | 69 (26) | 72 (25) | 63 (28) | 0.0001 |
| Ejection fraction%, mean (SD) | 53 (13) | 58 (6) | 41 (16) | <0.01 |
| Coronary artery disease, n (%) | 1292 (88.1) | 841 (88.2) | 451 (87.7) | 0.77 |
| Unstable Angina, n (%) | 339 (23.1) | 218 (22.9) | 121 (23.5) | 0.77 |
| Revascularization history, n (%) | 742 (50.6) | 480 (50.4) | 262 (51) | 0.82 |
| History of myocardial infarction, n (%) | 278 (20.2) | 139 (15.3) | 139 (29.5) | <0.0001 |
| ACE/ARB use, n (%) | 745 (50.8) | 457 (48.0) | 288 (56.0) | 0.003 |
| Aspirin use, n (%) | 1130 (77.0) | 727 (76.3) | 403 (78.4) | 0.36 |
| Plavix use, n (%) | 551 (37.6) | 362 (38.0) | 189 (36.8) | 0.65 |
| Statin use, n (%) | 1003 (68.4) | 647 (67.9) | 356 (69.3) | 0.59 |
| Beta Blocker use, n (%) | 1058 (72.1) | 648 (68.0) | 410 (79.8) | <0.0001 |
| CD34+ cell/mL median (IQR) | 1665 (1048–2514) | 1687 (1096–2562) | 1593 (979–2389) | 0.03 |
| CD34+/CD133+ cell/mL median (IQR) | 763 (456–1207) | 786 (472–1226) | 732 (433–1183) | 0.12 |
| CD34+/CXCR4+ cell/mL median (IQR) | 801 (487–1360) | 852 (515–1395) | 754 (439–1247) | 0.003 |
| CD34+/VEGF2R+ cell/mL median (IQR) | 41 (12–138) | 47 (13–148) | 34 (11–110) | 0.02 |
P values comparing patients with and without heart failure
Table 2.
Patients Characteristics among different HF subtypes
| Variable | HFpEF (n=184, 35.8%) |
HFrEF (n=330, 64.2%) |
P-value* | NICM (n=44, 13.3%) |
ICM (n=286, 86.7%) |
P-value† |
|---|---|---|---|---|---|---|
| Age, years mean (SD) | 67.3 (14.6) | 65.9 (13.2) | 0.20 | 61.3 (15.4) | 66.6 (12.7) | 0.01 |
| Male, n (%) | 91 (49.5) | 218 (66.1) | <0.001 | 22 (50) | 196 (68.5) | 0.02 |
| Black, n (%) | 40 (21.7) | 104 (31.5) | 0.02 | 20 (45.5) | 84 (29.4) | 0.03 |
| Body Mass Index kg/m2, mean (SD) | 28.9 (6.4) | 29.2 (6.6) | 0.73 | 30.4 (6.9) | 29.1 (6.6) | 0.20 |
| Smoking, n (%) | 115 (62.5) | 219 (66.4) | 0.37 | 28 (63.6) | 191 (66.8) | 0.68 |
| Diabetes, n (%) | 86 (47.8) | 143 (44.1) | 0.43 | 14 (33.3) | 129 (45.7) | 0.13 |
| Hypertension, n (%) | 173 (94) | 296 (92.2) | 0.44 | 39 (97.5) | 257 (91.5) | 0.18 |
| Hypercholesterolemia, n (%) | 137 (74.5) | 238 (75.3) | 0.83 | 20 (50) | 218 (79) | <0.001 |
| Low density lipoprotein, mg/dL mean (SD) | 84 (34) | 89 (35) | 0.11 | 90 (40) | 88 (34) | 0.01 |
| Estimated GFR mL/min/1.73 m2, mean (SD) | 60.7 (28.3) | 64.9 (27.4) | 0.08 | 67.5 (29.1) | 64.5 (27.2) | 0.004 |
| Ejection fraction%, mean (SD) | 56 (7) | 29 (9) | <0.001 | 28 (11) | 29 (8) | 0.01 |
| Revascularization history, n (%) | 91 (49.5) | 171 (51.8) | 0.60 | 0 (0) | 171 (59.8) | <0.001 |
| History of myocardial infarction, n (%) | 41 (23.7) | 98 (32.9) | 0.03 | 0 (0) | 98 (38.3) | <0.001 |
| ACE/ARB use, n (%) | 95 (51.6) | 193 (58.5) | 0.13 | 17 (38.6) | 176 (61.5) | <0.001 |
| Aspirin use, n (%) | 141 (76.6) | 262 (79.4) | 0.46 | 27 (61.4) | 235 (82.2) | <0.001 |
| Clopidogrel use, n (%) | 60 (32.6) | 129 (39.1) | 0.14 | 4 (9.1) | 125 (43.7) | <0.001 |
| Statin use, n (%) | 125 (67.9) | 231 (70) | 0.62 | 22 (50) | 209 (73.1) | <0.001 |
| Beta Blocker use, n (%) | 151 (82.1) | 259 (78.5) | 0.33 | 26 (59.1) | 233 (81.5) | <0.001 |
| CD34+ cell/mL median (IQR) | 1550 (944–2234) | 1612 (1011–2470) | 0.22 | 1404 (812–2369) | 1659 (1047–2479) | 0.08 |
| CD34+/CD133+ cell/mL median (IQR) | 737 (398–1083) | 730 (467–1223) | 0.40 | 636 (347–1133) | 740 (471–1230) | 0.13 |
| CD34+/CXCR4+ cell/mL median (IQR) | 739 (440–1183) | 772 (439–1320) | 0.51 | 587 (317–1124) | 794 (478–1343) | 0.04 |
| CD34+/VEGF2R+ cell/mL median (IQR) | 31 (11–91) | 38 (11–123) | 0.54 | 57 (16–138) | 33 (9–117) | 0.16 |
P Value comparing patients with HFpEF vs. HFrEF
P Value comparing patients with NICM vs. ICM
Relationship between progenitor cells and demographic and clinical features
Lower hematopoietic PC-enriched cell populations (CD34+, CD34+/CD133+, CD34+/CXCR4+) were associated with advanced age, female gender, low BMI, and impaired renal function. Lower endothelial PC-enriched CD34+/VEGFR2+ PCs were associated with age, smoking and diabetes mellitus. Supplementary table 1
Progenitor cells and HF
In univariate analyses, all PC subsets were significantly lower in patients with HF except for the CD34+/CD133+ PCs Table 3. After adjustment for clinical characteristics in multivariable analyses, only the CD34+/CXCR+ cell counts remained independently associated with a diagnosis of HF. Thus, a 50% decrease in CD34+/CXCR4+ cell counts was associated with a 20% (P=0.008) increased odds of having HF, Table 3.
Table 3.
Logistic regression models for heart failure prediction*
| Emory Biobank (n=1467) | MIPS study (n=582) | BioBank and MIPS (n=2049) | ||||
|---|---|---|---|---|---|---|
| Variables† | OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value |
| CD34+ | 1.13 (0.98–1.3) | 0.08 | 1.34 (1.07–1.68) | 0.01 | 1.16 (1.04–1.28) | 0.006 |
| CD34+/CD133+ | 1.06 (0.96–1.2) | 0.17 | 1.24 (1.01–1.53) | 0.04 | 1.08 (0.99–1.19) | 0.08 |
| CD34+/CXCR4+ | 1.17 (1.05–1.3) | 0.008 | 1.33 (1.1–1.61) | 0.003 | 1.2 (1.09–1.31) | <0.001 |
| CD34+/VEGF2R+ | 1.01 (0.99–1.05) | 0.31 | 0.98 (0.94–1.03) | 0.5 | 1.01 (0.98–1.04) | 0.44 |
Adjusted for age, gender, race, BMI, history of MI, estimated glomerular filtration rate, diabetes mellitus, hypertension, hypercholesterolemia, and smoking
per 50% reduction in PC level
PC levels were not significantly different by the etiology of HF (HFrEF vs HFpEF). The CD34+/CXCR4+ PC counts were significantly lower in patients with non-ischemic compared to those with ischemic cardiomyopathy (median 587 (317–1124) and 794 (478–1343) cell/mL, P=0.04, respectively), but the reduction in other cell types did not reach statistical significance Table 2.
Validation analysis
The associations between PC subsets and HF were validated in an independent cohort of 582 subjects with stable CAD enrolled in the MIPS study (Supplemental table 2) in which there were significant correlations between lower PC counts and HF (Supplemental Table 3). These associations persisted after adjusting for clinical characteristics and risk factors (table 3). In the pooled cohort of 2049 subjects recruited for both studies, all hematopoietic-enriched PC subsets were associated with HF (table 3). The association between PC and HF persisted even after adjusting for white blood cells counts which didn’t correlate with HF.
Progenitor cells and severity of HF
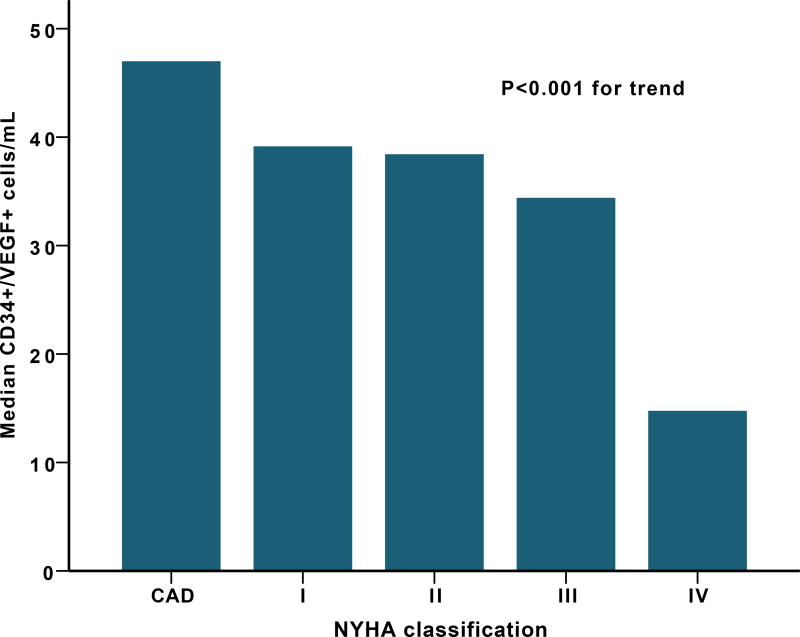
Worse NYHA functional class was associated with low CD34+/VEGF2R+ cell counts (median of 39 (8–133) vs. 14 (6–40) cell/mL P=0.003 for Stage I vs IV), Figure 1. Other PC subsets (CD34+, CD34+/CD133+, and CD34+/CXCR4+) were not different among different NYHA classification groups.
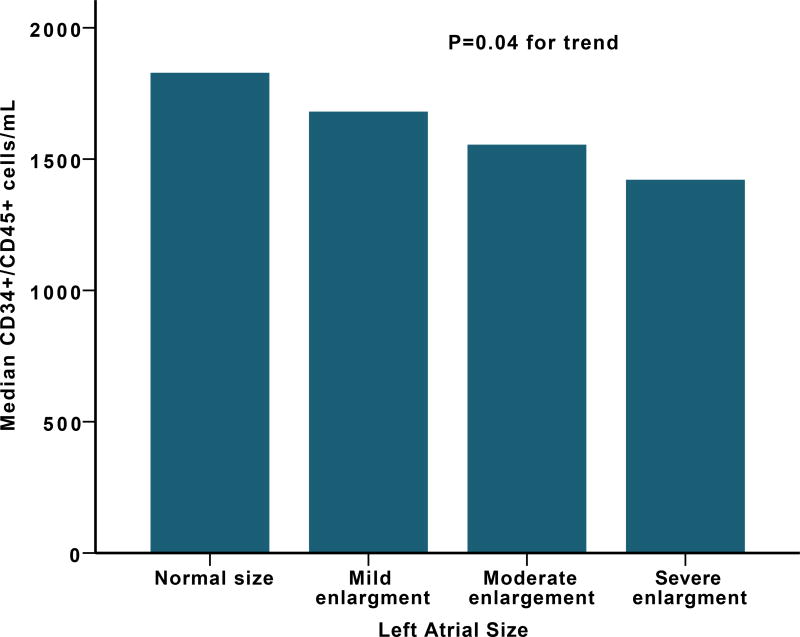
Figure 1.
(A) Median CD34+/VEGF+ cells levels among different NYHA classification (B) Median CD34+ cells levels and left atrial size.
In a subset of patients (n=359) with available echocardiogram, those with diastolic dysfunction had lower levels of CD34+/VEGF+ PCs with a median of 25 (9–77) compared to those without diastolic dysfunction with median of 41 (14–157) cell/mL P=0.003. Left atrial size correlated negatively with CD34+ (rho=−0.15, P=0.005), CD34+/CD133+ (rho=−0.15, P=0.006) and CD34+/CXCR4+ (rho=−0.13, P=0.02) Supplemental Table 4, Figure 1, while CD34+/VEGF+ cells correlated negatively with left ventricular posterior wall thickness (rho=−0.16, P=0.04). Right ventricular systolic pressures correlated inversely with CD34+ (rho=−0.15, P=0.004), CD34+/CD133+ (rho=−0.15, P=0.002), and CD34+/CXCR4+ (rho=−0.11, P=0.03) cells but not CD34+/VEGF+ (rho=−0.06, P=0.28). There was no significant association between PC counts and ejection fraction. In a subset of 131 patients with available levels, B-type natriuretic peptide (BNP) levels correlated negatively with CD34+ (rho=−0.18, p=0.04), CD34+/CD133+ (rho=−0.19, P=0.03), CD34+/CXCR4+ (rho=−0.16, P=0.06), and CD34+/VEGF+ (rho=0.17, P=0.046) populations.
Progenitor cell counts and Outcomes in HF
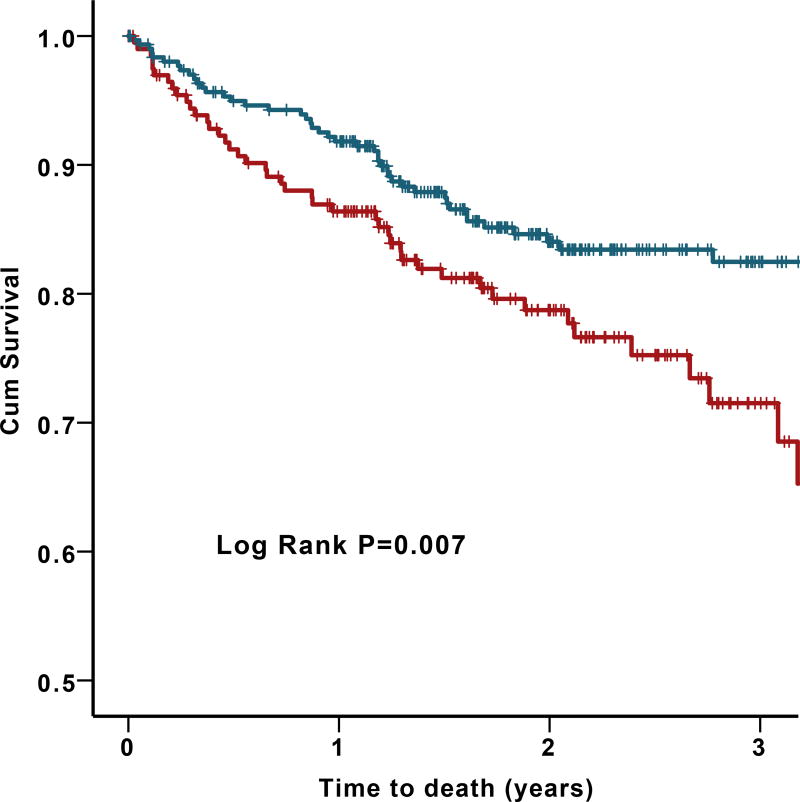
Among 514 patients with HF, there were 98 (19.1%) deaths from all causes, 76 (14.8%) deaths from cardiovascular causes, and 93 (18.1%) hospitalizations for HF. In univariate analyses, subjects with low CD34+, CD34+/CD133+, and CD34+/CXCR4+ counts had higher rates of all-cause death and cardiovascular death (Figure 2). This remained significant after adjusting for clinical variables in Cox regression models. Moreover, patients with PC counts below the previously reported cut-off values9 for CD34+, CD34+/CD133+, and CD34+/CXCR4+ cell counts had a 1.8-, 1.6-, and 1.8-fold increased mortality compared to those with higher circulating PC numbers, respectively Table 4. We found no significant correlation between PC counts and time to first HF hospitalizations.
Figure 2.
Survival curves for all-cause death (A, B) and Cardiovascular death (C) by CD34+, CD34+/CD133+, and CD34+/CXCR4+ cell counts. (Blue lines represent counts above cutoff and red lines represent counts below cutoff). The following cutoffs were used: 737 cells/milliliter for CD34+ cells, 504 cells/ milliliter for CD34+/CD133+ cells, 533 cells/ milliliter for CD34+/CXCR4+ cells.
Table 4.
Association between progenitor cells and outcomes in patients with heart failure (n=514)
| Continuous* Adjusted HR (95% CI) |
P-Value | Cutoff Adjusted R (95% CI) |
P-Value | ||
|---|---|---|---|---|---|
| Death† | CD34+ | 1.35 (1.11–1.66) | 0.003 | 2.0 (1.23–3.26) | 0.005 |
| (n=98, 19.1%) | CD34+/CD133+ | 1.27 (1.06–1.52) | 0.008 | 1.59 (1.04–2.45) | 0.03 |
| CD34+/CXCR4+ | 1.33 (1.11–1.61) | 0.002 | 1.56 (1.01–2.39) | 0.04 | |
| CD34+/VEGF2R+ | 1.01 (0.99–1.04) | 0.36 | 1.56 (0.97–2.53) | 0.06 | |
| CV Death† | CD34+ | 1.38 (1.11–1.72) | 0.004 | 2.25 (1.33–3.82) | 0.002 |
| (n=76, 14.8%) | CD34+/CD133+ | 1.3 (1.07–1.58) | 0.008 | 1.67 (1.03–2.69) | 0.03 |
| CD34+/CXCR4+ | 1.38 (1.13–1.69) | 0.001 | 1.73 (1.07–2.79) | 0.02 | |
| CD34+/VEGF2R+ | 1.01 (0.99–1.05) | 0.32 | 1.52 (0.9–2.59) | 0.12 | |
| HF | CD34+ | 1.06 (0.81–1.38) | 0.66 | 1.15 (0.57–2.33) | 0.7 |
| Hospitalizations† | CD34+/CD133+ | 0.97 (0.77–1.22) | 0.78 | 0.72 (0.4–1.28) | 0.26 |
| (n=93, 18.1%) | CD34+/CXCR4+ | 0.88 (0.66–1.16) | 0.35 | 0.87 (0.51–1.49) | 0.61 |
| CD34+/VEGF2R+ | 1.04 (0.99–1.08) | 0.11 | 1.24 (0.65–2.36) | 0.51 |
50% reduction in individual PC levels
number of events
Adjusted for age, gender, smoking history, body mass index, estimated glomerular filtration rate, ischemic etiology, ejection fraction, beta blocker use, and angiotensin pathway antagonist use.
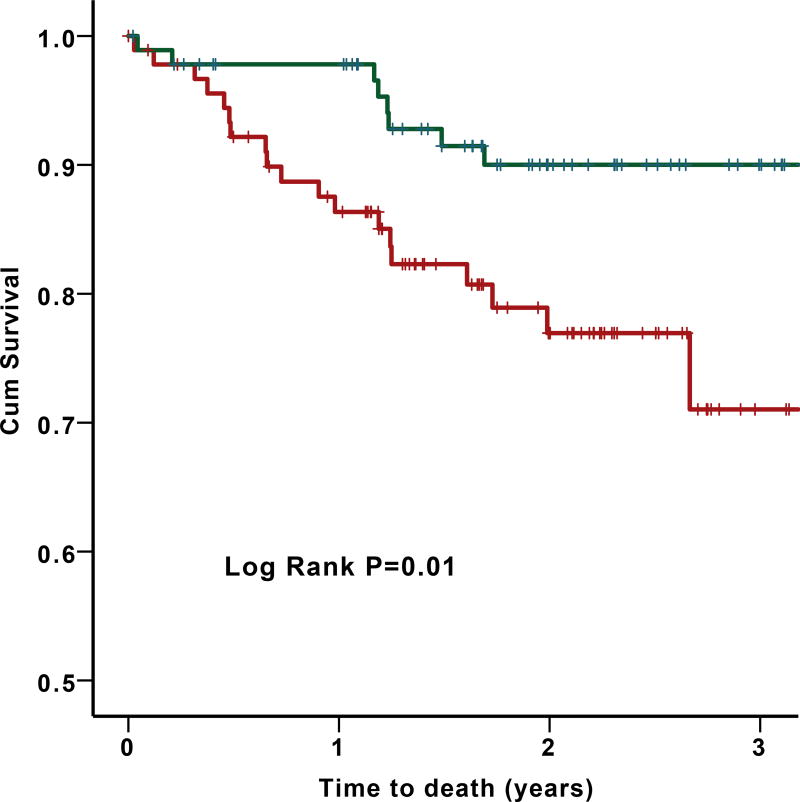
In a subgroup analysis of HFpEF patients (n=184) with 29 deaths (25 from cardiovascular causes), a low CD34+/VEGFR2+ cell count (median of 30 cell/mL as cutoff) was predictive of all-cause death (HR=5.0 95%CI 2.0–12.3 P=0.001) and cardiovascular death (HR=4.0 95%CI 1.6–10.0; P=0.003); Figure 3. Similar results were obtained with cell counts as continuous variables or as tertiles.
Figure 3.
Survival curves for the primary end point of (A) all-cause death and (B) cardiovascular death in HFpEF population by median (30 cell/mL) of CD34+/VEGF+ counts.
DISCUSSION
Novel findings of this study include (1) demonstration that compared to patients without HF, patients with HF have significantly lower circulating levels of hematopoietic enriched PCs; (2) the magnitude of reduction in circulating levels of PC subsets correlates with the severity of HF estimated as NYHA functional class, presence of diastolic dysfunction, left atrial size, pulmonary hypertension, and BNP levels; (3) patients with non-ischemic cardiomyopathy have the lowest levels of CD34+/CXCR4+ PC counts compared to all subsets of HF and non-HF patients; (4) both HFrEF and HFpEF are associated with similar reductions in the levels of circulating PCs compared to non-HF controls; (5) a low hematopoietic-enriched PC count in HF is an independent predictor of higher mortality; and (6) a low endothelial-enriched PC count is predictive of adverse outcomes in the HFpEF, but not in the HFrEF population.
Conflicting relationships between circulating PC numbers and HF have been published12–16. Possible reasons for this inconsistency include the small numbers of subjects studied (the largest study included 128 patients), comparisons with inappropriate controls, variations in phenotyping of PCs with use of functional assays or limited flow cytometry, and the differences in HF phenotypes studied. In contrast, herein we report the results of over 650 patients who were carefully phenotyped for etiology and severity of HF who were compared with over 1400 appropriate controls, and use of standardized and detailed flow cytometric analysis employing an array of specific hematopoietic and endothelial PC markers. Similar to CAD, PCs in ischemic HF are numerically and functionally impaired. However, functional exhaustion in the bone marrow, impaired mobilization or incorporation into damaged tissues, and reduced half-life of PCs may contribute to an unfavorable left ventricular remodeling and adverse outcomes in patients with HF17.
Similarly, conflicting results have been reported regarding the correlation between levels of PCs and severity of HF12, 18, 19. Interestingly, our study shows discordant behavior of PCs subpopulations in HF patients. We observed a progressive decline in endothelial enriched, VEGFR2-expressing PC counts, with more than 3-fold decrease in counts in advanced HF (NYHA IV class) while PC subsets enriched for hematopoietic PCs didn’t show significant changes.
Further, we found correlations between BNP and PCs demonstrating that subjects with higher BNP values had lower levels of both hematopoietic and endothelial enriched PCs. These findings have also been controversial in some previous studies12, 13, 20. Our study also illustrated an association between PC counts and estimated pulmonary artery pressure. Previous studies investigating the role of PCs in patients with pulmonary arterial hypertension have yielded conflicting results21, 22 that may be explained by the differences in the etiologies or stages of pulmonary arterial hypertension. Together, the association with functional class measures, BNP and pulmonary hypertension suggest that subjects with more severe HF are likely to have lower levels of circulating PCs.
In agreement with previous observations, both HFrEF and HFpEF were associated with similar reductions in levels of circulating PCs compared to non-HF controls14, 23. Several studies have reported that patients with non-ischemic cardiomyopathy have higher levels of circulating hematopoietic and endothelial origin PCs24, 25 whereas others have reported lower PC numbers in this group26. In the present study, we demonstrate a significant decrease in the CD34+/CXCR4+ PC subset in patients with non-ischemic cardiomyopathy, suggesting a potential pathophysiologic role of PCs and possibly the CXCR4/stromal derived factor ‘homing’ axis in non-ischemic cardiomyopathy.
There are several potential mechanisms by which PCs may contribute to disease progression and outcomes in patients with HF. Experimental studies have shown that low levels of PCs are associated with impaired endogenous regeneration and repair capacity2. These studies have also demonstrated several ways in which PCs may reduce further deterioration in HF. PCs mitigate adverse left ventricular remodeling after infarction, they prevent apoptosis of cardiomyocytes by secreting insulin growth factor-1, stimulate angiogenesis by either direct incorporation of the cells into the expanding vasculature, or more likely through paracrine secretion of angiogenic growth factors (vascular endothelial growth factor and basic fibroblast growth factor) and exosomes that support the developing microvasculature, help activate cardiac and other resident stem cells, and recruit additional local PCs17, 27. Reduced PCs counts may also be a marker of advanced HF due to several factors such as malnutrition, cardiac cachexia, cardio-renal syndrome, renin-angiotensin system activation, altered endothelial nitric oxide synthase activity, or activation of pro-inflammatory cytokines leading to bone marrow suppression.
Importantly, we found that lower PC counts are independent predictors of increased mortality. Previous studies have shown that reduced PC counts are associated with worse clinical outcomes in patients with coronary and peripheral vascular disease9, 10, but results in HF populations have remained controversial as studies have been mostly in small populations, with predominantly HFrEF and short-term follow-up23, 28, 29. Previous studies have also often employed culture assays to quantify PCs that remain controversial measures of regenerative capacity. In contrast, we have studied more than 500 patients with both HFrEF and HFpEF who have undergone extensive phenotyping of hematopoietic and endothelial PCs, and have detailed long-term follow-up for determination of hard HF outcomes.
An important observation in our study was the association of HFpEF outcomes with the reduction in endothelial progenitors. This relationship may be due to the known association between nitric oxide deficiency and HFpEF. For example, reduced endothelial-enriched PC activity accompanies endothelial dysfunction30. Several studies have demonstrated the association between endothelial dysfunction and the presence and progression of HFpEF31. Experimental studies have shown that endothelial nitric oxide synthase knockout mice have concentric left ventricular hypertrophy and greater diastolic dysfunction after abdominal aortic banding compared to wild-type mice, indicating the role of nitric oxide in left ventricular remodeling and development of HFpEF32. Finally, nitric oxide appears to be pivotal in the mobilization of PCs from the bone marrow33. Together these findings suggest that impaired mobilization of endothelial enriched PCs, likely associated with reduced nitric oxide bioavailability, contributes to the development and progression of HFpEF.
Limitations of our study are that we examined a population undergoing angiography and therefore our conclusions may not be applicable to other HF subtypes including valvular HF. Absence of functional assessments of circulating PCs also limits the conclusions that could be drawn about the proliferative and angiogenic capacity of the lower numbers of PCs, but previous studies have shown correlations between PC numbers and proliferative potential34. Most of the participants in this study had CAD so the results may not be applicable to individuals without CAD. Finally, the observational nature of this analysis does not imply causation, and thus, longer term follow-up and interventional studies directly influencing PC levels are required.
In conclusion, by examining circulating levels of hematopoietic and endothelial PC subsets, we show that PCs levels are not only predictive of prevalent HF, whether it is HFrEF or HFpEF, but also of outcomes in HF. In this regard, we have provided more definitive data on the unique contribution of PC physiology to the syndrome of HF and its outcomes. These insights are especially important for those with HFpEF where there appears to be a unique role for endothelial-enriched PC populations.
Supplementary Material
Clinical perspective.
What is new?
-
·
Individuals with heart failure (HF) have lower numbers of circulating hematopoietic and endothelial progenitor cells (PC)
-
·
Lower PC counts are associated with greater severity of HF and with higher mortality rates
-
·
Low endothelial-enriched PC counts are strongly associated with adverse outcomes in subjects with HF and preserved ejection fraction
What are the clinical implications?
-
·
Reduced regenerative capacity, estimated as low circulating PC counts, may contribute to development and progression of HF
-
·
Improving endogenous regenerative capacity may be a therapeutic approach for management of HF.
Acknowledgments
Funding Sources: AAQ is supported by 5P01HL101398-02, 1P20HL113451-01, 1R56HL126558-01, 1RF1AG051633-01, R01 NS064162-01, R01 HL89650-01, HL095479-01, 1U10HL110302-01, 1DP3DK094346-01, 2P01HL086773-06A1. AST is supported by the Abraham J. & Phyllis Katz Foundation (Atlanta, GA) and NIH/NIA grant AG051633.
Footnotes
Conflict of Interest Disclosures: None of the authors have conflicts of interest to disclose.
References
- 1.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 2.Kawamoto A, Iwasaki H, Kusano K, Murayama T, Oyamada A, Silver M, Hulbert C, Gavin M, Hanley A, Ma H, Kearney M, Zak V, Asahara T, Losordo DW. CD34-positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation. 2006;114:2163–2169. doi: 10.1161/CIRCULATIONAHA.106.644518. [DOI] [PubMed] [Google Scholar]
- 3.Gehling UM, Ergun S, Schumacher U, Wagener C, Pantel K, Otte M, Schuch G, Schafhausen P, Mende T, Kilic N, Kluge K, Schafer B, Hossfeld DK, Fiedler W. In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood. 2000;95:3106–3112. [PubMed] [Google Scholar]
- 4.Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J, Buck DW. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002–5012. [PubMed] [Google Scholar]
- 5.Fadini GP, Losordo D, Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res. 2012;110:624–637. doi: 10.1161/CIRCRESAHA.111.243386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alaiti MA, Ishikawa M, Costa MA. Bone marrow and circulating stem/progenitor cells for regenerative cardiovascular therapy. Transl Res. 2010;156:112–129. doi: 10.1016/j.trsl.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Seeger FH, Rasper T, Koyanagi M, Fox H, Zeiher AM, Dimmeler S. CXCR4 expression determines functional activity of bone marrow-derived mononuclear cells for therapeutic neovascularization in acute ischemia. Arterioscler Thromb Vasc Biol. 2009;29:1802–1809. doi: 10.1161/ATVBAHA.109.194688. [DOI] [PubMed] [Google Scholar]
- 8.Quyyumi AA. Endothelial function in health and disease: new insights into the genesis of cardiovascular disease. Am J Med. 1998;105:32S–39S. doi: 10.1016/s0002-9343(98)00209-5. [DOI] [PubMed] [Google Scholar]
- 9.Patel RS, Li Q, Ghasemzadeh N, Eapen DJ, Moss LD, Janjua AU, Manocha P, Al Kassem H, Veledar E, Samady H, Taylor WR, Zafari AM, Sperling L, Vaccarino V, Waller EK, Quyyumi AA. Circulating CD34+ progenitor cells and risk of mortality in a population with coronary artery disease. Circ Res. 2015;116:289–297. doi: 10.1161/CIRCRESAHA.116.304187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayek SS, MacNamara J, Tahhan AS, Awad M, Yadalam A, Ko YA, Healy S, Hesaroieh I, Ahmed H, Gray B, Sher SS, Ghasemzadeh N, Patel R, Kim J, Waller EK, Quyyumi AA. Circulating Progenitor Cells Identify Peripheral Arterial Disease in Patients With Coronary Artery Disease. Circ Res. 2016;119:564–571. doi: 10.1161/CIRCRESAHA.116.308802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hammadah M, Al Mheid I, Wilmot K, Ramadan R, Shah AJ, Sun Y, Pearce B, Garcia EV, Kutner M, Bremner JD, Esteves F, Raggi P, Sheps DS, Vaccarino V, Quyyumi AA. The Mental Stress Ischemia Prognosis Study: Objectives, Study Design, and Prevalence of Inducible Ischemia. Psychosom Med. 2017;79:311–317. doi: 10.1097/PSY.0000000000000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valgimigli M, Rigolin GM, Fucili A, Porta MD, Soukhomovskaia O, Malagutti P, Bugli AM, Bragotti LZ, Francolini G, Mauro E, Castoldi G, Ferrari R. CD34+ and endothelial progenitor cells in patients with various degrees of congestive heart failure. Circulation. 2004;110:1209–1212. doi: 10.1161/01.CIR.0000136813.89036.21. [DOI] [PubMed] [Google Scholar]
- 13.Fortini C, Toffoletto B, Fucili A, Puppato E, Olivares A, Beltrami AP, Fiorelli V, Bergamin N, Cesselli D, Morelli C, Francolini G, Ferrari R, Beltrami CA. Circulating stem cell vary with NYHA stage in heart failure patients. J Cell Mol Med. 2011;15:1726–1736. doi: 10.1111/j.1582-4934.2010.01195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiang CH, Huang PH, Leu HB, Hsu CY, Wang KF, Chen JW, Lin SJ. Decreased circulating endothelial progenitor cell levels in patients with heart failure with preserved ejection fraction. Cardiology. 2013;126:191–201. doi: 10.1159/000351973. [DOI] [PubMed] [Google Scholar]
- 15.Shantsila E, Wrigley BJ, Shantsila A, Tapp LD, Gill PS, Lip GY. Monocyte-derived and CD34+/KDR+ endothelial progenitor cells in heart failure. J Thromb Haemost. 2012;10:1252–1261. doi: 10.1111/j.1538-7836.2012.04753.x. [DOI] [PubMed] [Google Scholar]
- 16.Pelliccia F, Pasceri V, Cianfrocca C, Vitale C, Pristipino C, Speciale G, Mercuro G, Rosano G. Endothelial progenitor cells in patients with coronary artery disease and left ventricular dysfunction. Coron Artery Dis. 2009;20:303–308. doi: 10.1097/MCA.0b013e328325765e. [DOI] [PubMed] [Google Scholar]
- 17.Kissel CK, Lehmann R, Assmus B, Aicher A, Honold J, Fischer-Rasokat U, Heeschen C, Spyridopoulos I, Dimmeler S, Zeiher AM. Selective functional exhaustion of hematopoietic progenitor cells in the bone marrow of patients with postinfarction heart failure. J Am Coll Cardiol. 2007;49:2341–2349. doi: 10.1016/j.jacc.2007.01.095. [DOI] [PubMed] [Google Scholar]
- 18.Nonaka-Sarukawa M, Yamamoto K, Aoki H, Nishimura Y, Tomizawa H, Ichida M, Eizawa T, Muroi K, Ikeda U, Shimada K. Circulating endothelial progenitor cells in congestive heart failure. Int J Cardiol. 2007;119:344–348. doi: 10.1016/j.ijcard.2006.07.191. [DOI] [PubMed] [Google Scholar]
- 19.Fritzenwanger M, Lorenz F, Jung C, Fabris M, Thude H, Barz D, Figulla HR. Differential number of CD34+, CD133+ and CD34+/CD133+ cells in peripheral blood of patients with congestive heart failure. Eur J Med Res. 2009;14:113–117. doi: 10.1186/2047-783X-14-3-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berezin AE, Kremzer AA. Circulating endothelial progenitor cells as markers for severity of ischemic chronic heart failure. J Card Fail. 2014;20:438–447. doi: 10.1016/j.cardfail.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 21.Yao W, Firth AL, Sacks RS, Ogawa A, Auger WR, Fedullo PF, Madani MM, Lin GY, Sakakibara N, Thistlethwaite PA, Jamieson SW, Rubin LJ, Yuan JX. Identification of putative endothelial progenitor cells (CD34+CD133+Flk-1+) in endarterectomized tissue of patients with chronic thromboembolic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2009;296:L870–8. doi: 10.1152/ajplung.90413.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Junhui Z, Xingxiang W, Guosheng F, Yunpeng S, Furong Z, Junzhu C. Reduced number and activity of circulating endothelial progenitor cells in patients with idiopathic pulmonary arterial hypertension. Respir Med. 2008;102:1073–1079. doi: 10.1016/j.rmed.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 23.Michowitz Y, Goldstein E, Wexler D, Sheps D, Keren G, George J. Circulating endothelial progenitor cells and clinical outcome in patients with congestive heart failure. Heart. 2007;93:1046–1050. doi: 10.1136/hrt.2006.102657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roura S, Planas F, Prat-Vidal C, Leta R, Soler-Botija C, Carreras F, Llach A, Hove-Madsen L, Pons Llado G, Farre J, Cinca J, Bayes-Genis A. Idiopathic dilated cardiomyopathy exhibits defective vascularization and vessel formation. Eur J Heart Fail. 2007;9:995–1002. doi: 10.1016/j.ejheart.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Theiss HD, David R, Engelmann MG, Barth A, Schotten K, Naebauer M, Reichart B, Steinbeck G, Franz WM. Circulation of CD34+ progenitor cell populations in patients with idiopathic dilated and ischaemic cardiomyopathy (DCM and ICM) Eur Heart J. 2007;28:1258–1264. doi: 10.1093/eurheartj/ehm011. [DOI] [PubMed] [Google Scholar]
- 26.Moiseeva OM, Karelkina EV, Moroshkin VS, Seliutin AV, Shliakhto EV. The study of circulating endothelial precursor cells in patients with chronic heart failure. Kardiologiia. 2011;51:36–42. [PubMed] [Google Scholar]
- 27.Andreou I, Tousoulis D, Tentolouris C, Antoniades C, Stefanadis C. Potential role of endothelial progenitor cells in the pathophysiology of heart failure: clinical implications and perspectives. Atherosclerosis. 2006;189:247–254. doi: 10.1016/j.atherosclerosis.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 28.Koller L, Hohensinner P, Sulzgruber P, Blum S, Maurer G, Wojta J, Hulsmann M, Niessner A. Prognostic relevance of circulating endothelial progenitor cells in patients with chronic heart failure. Thromb Haemost. 2016;116:309–316. doi: 10.1160/TH16-01-0051. [DOI] [PubMed] [Google Scholar]
- 29.Alba AC, Lalonde SD, Rao V, Walter SD, Guyatt GH, Ross HJ. Changes in circulating progenitor cells are associated with outcome in heart failure patients: a longitudinal study. Can J Cardiol. 2013;29:1657–1664. doi: 10.1016/j.cjca.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 30.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 31.Akiyama E, Sugiyama S, Matsuzawa Y, Konishi M, Suzuki H, Nozaki T, Ohba K, Matsubara J, Maeda H, Horibata Y, Sakamoto K, Sugamura K, Yamamuro M, Sumida H, Kaikita K, Iwashita S, Matsui K, Kimura K, Umemura S, Ogawa H. Incremental prognostic significance of peripheral endothelial dysfunction in patients with heart failure with normal left ventricular ejection fraction. J Am Coll Cardiol. 2012;60:1778–1786. doi: 10.1016/j.jacc.2012.07.036. [DOI] [PubMed] [Google Scholar]
- 32.Ruetten H, Dimmeler S, Gehring D, Ihling C, Zeiher AM. Concentric left ventricular remodeling in endothelial nitric oxide synthase knockout mice by chronic pressure overload. Cardiovasc Res. 2005;66:444–453. doi: 10.1016/j.cardiores.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 33.Aicher A, Heeschen C, Mildner-Rihm C, Urbich C, Ihling C, Technau-Ihling K, Zeiher AM, Dimmeler S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9:1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- 34.Rustemeyer P, Wittkowski W, Jurk K, Koller A. Optimized flow cytometric analysis of endothelial progenitor cells in peripheral blood. J Immunoassay Immunochem. 2006;27:77–88. doi: 10.1080/15321810500403789. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.