Abstract
Stem cells have the ability to self-renew and differentiate into specialized cell types, and, in the human body, they reside in specialized microenvironments called “stem cell niches.” Although several niches have been described and studied in vivo, their functional replication in vitro is still incomplete. The in vitro culture of pluripotent stem cells may represent one of the most advanced examples in the effort to create an artificial or synthetic stem cell niche. A focus has been placed on the development of human stem cell microenvironments due to their significant clinical implications, in addition to the potential differences between animal and human cells. In this concise review we describe the advances in human pluripotent stem cell culture, and explore the idea that the knowledge gained from this model could be replicated to create synthetic niches for other human stem cell populations, which have proven difficult to maintain in vitro.
Keywords: pluripotent stem cell, hematopoietic stem cell, cancer stem cell, stem cell niche, engineered surfaces, polymers
Introduction
Culturing cells outside of the human body is inherently difficult. Biologists have dedicated decades of research to learning what conditions are most conducive to culturing cells in vitro. This included adaptation of substrates to allow cells to adhere and proliferate for extended periods of time. When polystyrene petri dishes were first implemented for cell culture, they were unable to sustain cell growth due to insufficient cell spreading on the surface [1, 2]. Surface modification techniques, such as chemical treatments with sulfuric acid [1] or glow discharge [2], were utilized to allow enhanced cell adhesion through the negative surface charge of the polystyrene [3]. These surface modification technologies enabled fundamental studies of how cells interact with their environment, and specifically how the substrate affects cell behavior [4]. Surface-modified plastics were among the first man-made microenvironments produced for the sole purpose of culturing cells, and they paved the way to the tissue-culture plastic which is still the gold standard in laboratories around the world today.
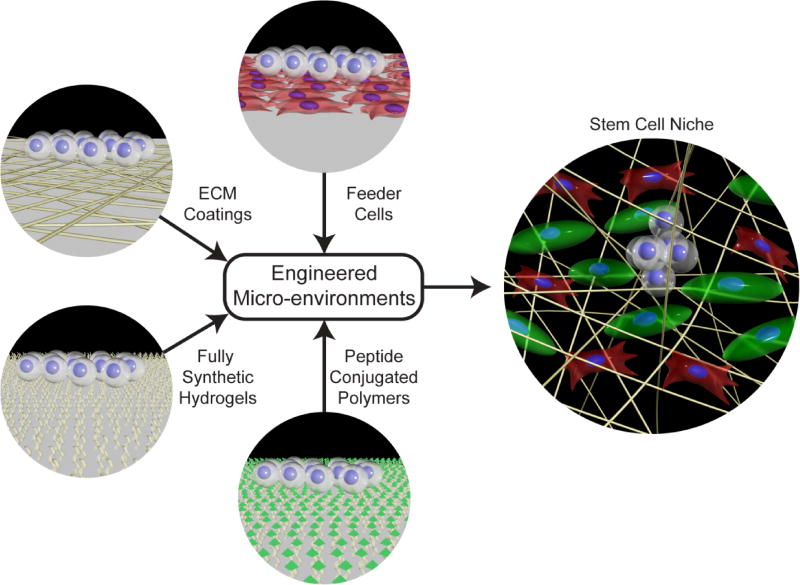
The discovery of stem cells brought about a new challenge in cell culture substrate design. Instead of simply growing cells, specific cell properties such as self-renewal and pluripotency or multipotency, need to be maintained or controlled. Maintaining these unique stem cell properties during expansion is crucial to create sufficient populations of undifferentiated cells that can then be terminally differentiated [5]. While standard tissue culture plastic can be suitable for culturing both primary cells and cell lines, they are not well-suited for maintaining stemness for prolonged periods of time. Recent trends in development of stem cell substrates have focused on recapitulating the stem cell niche ex vivo in a tissue culture environment. This has been accomplished using strategies such as feeder cells, purified extracellular matrix proteins (ECM), peptide conjugated surfaces or hydrogels, and specialized synthetic polymers, to create a milieu that is conducive to stem cell expansion and maintenance of stem cell properties outside the body (Figure 1). The development of surfaces capable of preserving the pluripotency of human embryonic stem cells (hESCs) and human induced pluripotent stem cells (iPSCs) is a major advancement towards defined stem cell microenvironments, and may serve as a blueprint for other stem cells with high levels of phenotypic plasticity, such as cancer stem cells or hematopoietic stem cells. This perspective outlines current knowledge in the composition of the stem cell niche, and how the niche can be recapitulated in vitro using engineered microenvironments. This is highlighted by examining current trends in the expansion of pluripotent stem cells, and relating this progress to the expansion of other stem cells that are difficult to culture. While this review focuses on the development of culture substrates for stem cells, it should be noted that the soluble factors comprising the culture medium also play a significant role in the maintenance of the stem cell phenotype. These aspects are outside of the scope of the perspective, and we refer the interested reader to other reviews that cover this topic in detail [6].
Fig. 1.
Engineered stem cell microenvironments draw inspiration from the in vivo stem cell niche. In an effort to recapitulate functional elements of the stem cell niche, culture substrates have been developed using stromal cells, extracellular matrix proteins, or peptide conjugated polymers. Fully synthetic hydrogels help maintain stem cell pluripotency and self-renewal by supporting matrix proteins from the medium or secreted by cells. It has also been demonstrated that topology or stiffness are important considerations when creating stem cell microenvironments. In the illustration stem cells are white, different types of stromal cells are red and green, and ECM proteins are yellow fibrils.
The Stem Cell Niche
Stem cells have the specific function of producing and replenishing specialized cells during the life of eukaryotic organisms. During early mammalian development, the fertilized egg divides into blastomeres with stem cell properties that give rise to the first two cell lineages: the throphoectoderm cells from the outer blastomeres of the embryo which will form the placenta, and the inner blastomeres will become the inner cell mass (ICM), a population of cells with pluripotent properties [7]. The ICM eventually differentiates into specialized cell types of the three germ layers, namely the ectoderm, mesoderm, and endoderm [7]. In vitro, this pluripotent stage can be maintained over longer periods of time by isolating and expanding the cells comprising the ICM, which are called embryonic stem cells. Throughout life, fetal and adult life stem cells, called somatic stem cells (SSC), are developed and maintained in specialized microenvironments termed stem cell niches. These niches serve as protective environments that provide supportive conditions to maintain stem cell self-renewal and differentiation properties.
The niche is comprised of soluble signaling from cytokines and growth factors, direct interactions with other cells, and the extracellular matrix (ECM). In combination with one another, these components activate signaling pathways involved in the maintenance of the undifferentiated and quiescent states of stem cells. While some signaling pathways and niche elements are commonly expressed among stem cells, there is not a defined pathway present in all of them [8–10]. However, literature suggests that a common characteristic between stem cells and their niches can be found in the expression of a specific ECM protein, laminin, and its receptor in the stem cells, integrin α6. The laminin and integrin α6 interaction has been documented as a critical niche component in corneal [11], colonic [12], epithelial [13], hepatic [14], spermatogonial [15], neuronal [16], glioblastoma [17], and embryonic stem cells [18]. Interestingly, laminin is one the first ECM proteins deposited during embryo development, and it is specifically expressed in the blastocyst stage [19], when the ICM is formed and, as explained above, a transient state of pluripotency exists. Human embryonic stem cells, the in vitro counterpart of the pluripotent ICM cells, express integrin α6 [18] and specific isoforms of laminin are able to support their self-renewal and proliferation [20]. Furthermore, although other ECM proteins such as vitronectin [21] and fibronectin [22] can support self-renewal of hESCs, it has recently been shown that hESCs cultured on ECM-coated surfaces remodeled their microenvironment by depositing their own laminin [23]. Due to the similarities among pluripotent stem cells and several somatic stem cells and their corresponding niches, we propose that the knowledge of the in vitro culture of human pluripotent stem cells could be exploited to bioengineer stem cell niches for somatic stem cells.
Feeder Cells
The isolation and successful culture of hESCs opened an entirely new outlook on the future of cell and tissue culture. However, this early milestone came with its own set of challenges. While hESCs can adhere to normal tissue-culture plastic, the unique attribute of maintaining self-renewal is lost over time under those conditions. Thus, mitotically inactivated feeder cell layers, a technique derived from earlier work which successfully maintained the pluripotency of mouse embryonic stem cells (mESCs) and mouse embryonal carcinoma cells (mECCs) [24–26], were used to support the culture of hESCs. A study using non-proliferative human oviductal epithelial cells as a feeder layer and human leukemia inhibitory factor (HLIF) to culture the inner cell mass (ICM) of a human blastocyst proved to be the first isolation and culture of human ICM cells, although the cells differentiated towards a fibroblast-like phenotype after two passages [27]. The first hESC lines that could be sustained indefinitely in vitro were cultured on feeder layers composed of mouse embryonic fibroblasts (MEFs) in medium supplemented with FBS [5, 7, 28]. An alternative source of human pluripotent stem cells is provided by human fibroblasts reprogrammed into pluripotent stem cells, termed induced pluripotent stem cells (hiPSCs) [29]. The establishment of hESC and hiPSC lineages provided the platform necessary to investigate i) what microenvironment is necessary to sustain these cells in a pluripotent state, ii) how to eliminate components within the microenvironment which are undefined or derived from xenogeneic sources, and iii) how the physical properties of the microenvironment affect the differentiation and maintenance of these cells.
Similarly, feeder cells and co-cultures of stem cells with somatic cells are also used in the expansion and maintenance of human hematopoietic stem cells (HSCs). HSCs are a relatively rare cell type located primarily within the bone marrow, peripheral blood, or umbilical cord blood and are capable of differentiating into all mature blood cells of the human body. HSCs play a critical role in bone marrow transplants used in clinical treatments for hematological disorders such as leukemia, lymphoma, and sickle cell anemia [30]. Ex vivo expansion and maintenance of these rare cells could therefore provide an attractive platform for improving the treatment of several debilitating diseases. Engineering a culture substrate capable of expanding long-term repopulating hematopoietic stem cells began by examining the niche in which they reside. This led to co-culture of isolated human HSCs with stromal cells associated with the bone marrow microenvironment, and cells which secrete a specific set of cytokines and growth factors which have been attributed to maintaining HSCs [31–35]. Human feeder cells, such as osteoblasts [36] and mesenchymal stem cells [37–39], as well as porcine endothelial cells [40], mouse fetal liver [41], and mouse stromal cells [33] are some examples of the types of feeder cells associated with the expansion of human HSCs in vitro. While the interactions between these supporting cells and HSCs have not been fully characterized, the ability of feeder cells to promote HSC expansion in vitro has shown some degree of success (Table 1). The direct clinical relevance of HSCs to the treatment of blood disorders has led to the extensive use of human feeder cells to provide a microenvironment free from xenogeneic contamination, as opposed to MEFs used in early hESC microenvironments. This has contributed to successful human trials which have demonstrated that ex vivo expansion of HSCs can provide an effective means of improving engraftment efficiency over standard cord blood transplants [42]. While in vitro expansion over short periods of time have demonstrated successes, long term maintenance of HSCs has yet to be achieved, as it is possible for hESCs. Despite the successes provided by feeder cells to the culture of both hESCs and HSCs, the unknown interactions between the cells in co- culture systems contributed to a desire for feeder-free cell culture systems with fully-defined media components. Such a culture platform would be used to directly study how the composition of the microenvironment affects stemness.
Table 1.
Representative list of stem cell culture substrates
| Culture Substrate | Cell Type | Highlighted Results | Reference |
|---|---|---|---|
|
| |||
| Xenogenic Feeder Cells | |||
|
| |||
| MEF feeders | ICM of blastocyst (derived lines H1, H7, H9, H13, H14) | Continuously cultured and remained undifferentiated for over 8 months | [7] |
| MEF feeders | iPSC | Remained undifferentiated and proliferated for at least 4 months | [29] |
| MEF feeders | hESC (derived HES-1, HES-2) | Sustained culture for up to 64 passages | [5] |
| Mouse fetal liver feeders (AFT024) | CD34+ UC-HSPC | More efficient engraftment in mice than human BM stromal cell monolayers | [41] |
| PMVEC | CD34+ UC-HSPC | 470 fold increase in CD34+ CD38− population compared to stroma-free culture | [40] |
|
| |||
| Human Feeder Cells | |||
|
| |||
| Human Oviductal epithelial feeders | ICM of blastocyst | All cells differentiate after second sub-culture | [27] |
| Human AFT epithelial feeders | hESC (HES-3, HES-4) | Self-renewal maintained for at least 20 passages | [43] |
|
| |||
| Culture Substrate | Cell Type | Highlighted Results | Reference |
|
| |||
| Human FM feeders | hESC (HES-3, HES-4) | Self-renewal maintained for at least 20 passages | [43] |
| Human FS feeders | hESC (HES-3, HES-4) | Self-renewal maintained for at least 20 passages | [43] |
| Human foreskin fibroblast feeders | hESC (derived lines HS181, HS207) | Self-renewal maintained for over 40 weeks | [44] |
| hMSC feeders | CD34+ UC-HSPC | ~2–7 fold Increase LTC-IC population | [83] |
| Human osteoblast feeders | CD34+ BM-HSPC | 8 fold increase in CD34+ population | [36] |
| BM stromal feeders | CD34+ BM-HSPC | Supported HSPC culture for 5 weeks and maintained LTC-IC populations | [84] |
| Human prostate CAF | PC3 | Enriched CD44hi/CD24lo CSC population | [85] |
| Primary colonic human myofibroblasts | Primary human colon carcinoma | CSC differentiation prevented in 2D culture | [86] |
| BM-hMSC | SUM159, SUM149, MCF-7 | Three-fold increase in cancer stem cell population to 14% of total population | [87] |
|
| |||
| ECM Coatings/Gels | |||
|
| |||
| Matrigel | hESC (H1, H7, H9, H14) | Sustained culture for up to 21 passages in defined media | [45] |
| Combination of laminin, collagen IV, fibronectin, vitronectin | hESC (H1, H9; derived WA15, WA16) | Sustained culture for up to 7 months in defined media | [45] |
| Matrigel | hESC (H1, H7, H9, H14) | Undifferentiated cells maintained for over 6 months with conditioned media | [18] |
| Laminin | hESC (H1, H7, H9, H14) | Undifferentiated cells maintained for at least 7 passages with conditioned media | [18] |
| Collagen IV | hESC (H1, H7, H9) | Some undifferentiated colonies present after 6passages with conditioned media | [18] |
|
| |||
| Culture Substrate | Cell Type | Highlighted Results | Reference |
|
| |||
| Fibronectin | hESC (H1, H7, H9) | Some undifferentiated colonies present after 6 passages with conditioned media | [18] |
| Vitronectin | hESC (HUES1, HES2, HESC-NL3) | Sustained culture for up to 12 weeks in defined media | [21] |
| Laminin | hESC (HUES1, HES2, HESC-NL3) | hESC growth not supported in defined media | [21] |
| Fibronectin | hESC (HUES1, HES2, HESC-NL3) | hESC growth not supported in defined media | [21] |
| Collagen IV | hESC (HUES1, HES2, HESC-NL3) | hESC growth not supported in defined media | [21] |
| Fibronectin | hESC (I3, I6, H9) | Sustained undifferentiated hESCs for up to 38 passages with growth factor addition | [46] |
| Chitosan/Alginate scaffold | hESC (BG01V) | Maintained for 21 days in culture in defined media | [88] |
| Gelatin | hESC (I3, I6, H9) | 70% more differentiation after 6 days compared to fibronectin coating | [46] |
| Laminin-511 | hESC (HS420, HS207, HS401), iPSC (BJ#12, LDS1.4) | Self-renewal maintained for over 4 months | [47] |
| Laminin-521 + E-Cadherin | hESC (H1, HS401) | Efficient derivation and self-renewal of hESCs for over 20 passages with high cloning efficacy | [48] |
| A variety of ECMs and sera coatings | hESC (HS237, HS293, HS360, HS401, Regea 06/105, HS237) | All substrates tested were significantly inferrior to Matrigel in maintaining hESC cultures | [50] |
| Vitronectin coated TCPS | hESC (H9, H14), IPSC (iPS(IMR-90)-3, iPS(IMR-90)-4, iPS(foreskin)-2) | Maintained for 9 passages | [89] |
| Vitronectin coated UVPS | iPSC | Sustained culture for up to 2 months | [49] |
| Puramatrix™ Synthetic ECM + hMSC feeders | CD34+ BM-HSPC | Increased LTC-IC population | [90] |
|
| |||
| Culture Substrate | Cell Type | Highlighted Results | Reference |
|
| |||
| Fibrin gel + hMSC feeders | CD34+ UC-HSPC | More efficient CD34+ population enhancement than collagen I or polymer scaffolds | [91] |
| Fibronectin conjugated PET film | CD34+ UC-HSPC | 19 fold increase in CD34+ cells | [92] |
| Fibronectin adsorbed PET fibers | CD34+ UC-HSPC | 2 fold increase in CD34+ cells | [92] |
| Fibronectin conjugated PET fibers | CD34+ UC-HSPC | 100 fold increase in CD34+ cells, with 45 foldincrease in LTC-IC population | [92] |
| Collagen conjugated PET fibers | CD34+ UC-HSPC | 73 fold increase in CD34+ cells with 4 fold increase in LTC-IC population | [92] |
| Fibrin Gel | A2780, HepG2, Primary Patient | Formed spheroids indicating enrichment of CSC | [93] |
| Laminin coated TCPS | Primary Brain Tumors (derived G144, G166, G179) | Supported cell lines consisting mostly of glioma neural stem cells | [61] |
| Collagen Scaffold | MCF-7 | Increasedangiogenic GF and MMP expression and enhanced CD44+/CD24− population | [94] |
| Fibronectin coated PDMS | SUM159, MDA-MB-468 | No enrichment of CSCs, not affected by stiffness | [95] |
| BSA coated PDMS | SUM159, MDA-MB-468 | More than doubled CSC population, not affected by stiffness | [95] |
| Collagen coated PDMS | SUM159, MDA-MB-468 | No enrichment of CSCs, not affected by stiffness | [95] |
| Laminin-511 coating | CD44+/CD24− Src-transformed MCF10A, SUM 1315 | Increased adhesion and enhanced TAZ gene expression | [62] |
| FBS coated PCL fibers | MCF-7, T47D, SK-BR-3 | Three-fold increase in proportion of ALDH+ cells, increased mammosphere formation | [96] |
|
| |||
| Culture Substrate | Cell Type | Highlighted Results | Reference |
|
| |||
| Peptide Modified Surfaces | |||
|
| |||
| BSP-PAS | hESC (H1, H7) | Undifferentiated cells maintained for over 16 passages in defined media | [68] |
| VN-PAS | hESC (H1, H7) | Undifferentiated cells maintained for over 16passages in defined media | [68] |
| sFN-PAS | hESC (H1, H7) | No cell adhesion observed | [68] |
| lFN-PAS | hESC (H1, H7) | No cell adhesion observed | [68] |
| LM-PAS | hESC (H1, H7) | No cell adhesion observed | [68] |
| Peptide SAM microarray | hESC (H1, H9) | Cell adhesion observed with integrin binding peptides, but these peptides did not effectively maintain hESCs. Heparin-binding peptide sequences bound cells and allowed for self-renewal. | [69] |
| GKKQRFRHRNRKG SAM | hESC (H1, H7, H9, H14), iPSC (IMR-90, DF19-9 7T) | Heparin binding peptide SAM sustained undifferentiated cells for 2–3 months | [69] |
| Amine functional TCPS modified with CRGD | hESC (H9, H14) | Continuously cultured and remained undifferentiated for at least 10 passages. Superior cell adhesion compared to linear RGDS sequence. | [66] |
| VN-pDA-PS | hESC (H9), iPSC | Sustained culture for over 3 months in defined media | [97] |
| FN patterned PEGDA | CD34+ UC-HSPC | Enhanced adhesion, no effect on stemness | [98] |
| OPN patterned PEGDA | CD34+ UC-HSPC | Enhanced adhesion, no effect on stemness | [98] |
| RGD patterned PEGDA | CD34+ UC-HSPC | Enhanced adhesion, no effect on stemness | [98] |
| RGDSK-PEG-Acrylate hydrogel + hMSC feeders | CD34+ UC-HSPC | Increased expansion, CD34+ expression, and early progenitor cell population | [71] |
| RGD conjugated PEGDA | 4T1, MCF-7 | Decreased CD44hi/CD24lo sub-population of breast cancer cells | [70] |
|
| |||
| Culture Substrate | Cell Type | Highlighted Results | Reference |
|
| |||
| FHBP conjugated PEGDA | 4T1, MCF-7 | Enriched CD44hi/CD24lo sub-population of breast cancer cells | [70] |
| CD44BP conjugated PEGDA | 4T1, MCF-7 | Decreased CD44hi/CD24lo sub-population of breast cancer cells | [70] |
|
| |||
| Fully Synthetic Polymer Hydrogels | |||
|
| |||
| PMEDSAH | hESC (BG01, H9) | Pluripotency maintained for over 20 passages, up to 10 passages in defined media | [74] |
| APMAAm | hESC (H1s, H9-hOct4-pGZs) | Sustained culture for over 20 passages in defined media | [78] |
| PMVE-alt-MA | hESC (HUES1, HUES9), iPSC | Increased endogenous ECM production, maintained pluripotency for five passages | [79] |
The strategies outlined in the table have been used to create microenvironments, which expand stem cell populations while preserving their phenotype. Comparing the strategies of culturing different types of stem cells reveals common elements, indicating advances in culturing one type of stem cell may have implications in other stem cell fields as well. However, it should be noted that the use of different growth factors, medias, or culture techniques may contribute to some of the observed results. However, the purpose of this table is to highlight the different substrates, and the similarity between different stem cell types, used in culturing of a variety of stem cells.
Abbreviations: MEF=mouse embryonic fibroblast, ICM=inner cell mass, hESC=human embryonic stem cell, iPSC=induced pluripotent stem cell, UC=umbilical cord, HSPC= hematopoietic stem progenitor cell, PMVEC=porcine microvascular endothelial cell, FS=fetal skin, FM=fetal muscle, hMSC=human mesenchymal stem cell, BM=bone marrow, LTC-IC=long term culture initiating cells, CAF=cancer associated fibroblast, CSC=cancer stem cell, TCPS=tissue culture polystyrene, PET=polyethylene terephthalate, GF=growth factor, MMP=matrix metalloprotease, ECM=extracellular matrix, PDMS=polydimethylsiloxane, UVPS=ultraviolet-ozone treated polystyrene, BSA=bovine serum albumin, FBS=fetal bovine serum, PCL=polycaprolactone, BSP=bone sialoprotein derived peptide, VN=vitronectin derived peptide, sFN=short fibronectin binding peptide, lFN=long fibronectin binding peptide, LM=laminin derived peptide, PAS=peptide-acrylate surface, OPN=osteopontin, FN=fibronectin, SAM=self-assembled monolayer, pDA=polydopamine, PS=polystyrene, PEG=polyethylene glycol, FHBP=fibronectinheparan binding peptide, CD44BP=CD44 binding peptide, PEGDA=polyethylene glycol diacrylate, PMVE-alt-MA= poly(methyl vinyl ether-alt-maleic anhydride), APMAAm=aminopropylmethacrylamide hydrogel, PMEDSAH=poly[2-(methacryloyloxy)ethyl dimethyl-(3-sulfopropyl)ammonium hydroxide]
Feeder Free Culture Using Purified Extracellular Matrix Proteins
While the expansion of stem cells on human [43, 44] and xenogenic feeder layers has shown success in maintaining pluripotent stem cells for prolonged culture times and multiplying adult human HSCs for therapeutic use, these systems lack control over which signals stem cells are exposed to. Deconstruction of the stem cell niche has led to ECM-based microenvironments that provide an adhesive substrate that interacts directly with cells through integrin signaling, which is suspected to contribute to stem cell maintenance. However, the biological activity of the ECM is more complex than a simple adhesive substrate, as it provides a reservoir for growth factors which can alter their release or presentation, contains cryptic signaling domains, modulates the mechanical stiffness of a substrate, and can be remodeled by cells based on environmental cues. A major advance in the development of more defined microenvironments using ECM coatings came with the first ‘feeder-free’ hESC culture system [18]. This study demonstrated that feeder layers could be eliminated through use of various matrix proteins, either a product of Engelbreth-Holm-Swarm mouse sarcoma cells (Matrigel) or purified matrix proteins laminin, collagen IV, or fibronectin coated on tissue-culture plastic in conditioned medium supplemented by human basic fibroblast growth factor (hbFGF).
While the feeder-free systems were able to successfully eliminate the complexity and inconsistency associated with feeder cells, the reliance on MEF conditioned medium remained a source of xenogeneic contamination and unknown composition. Nevertheless, the concept of coating tissue-culture plastic with matrix proteins and utilizing conditioned medium both continue to be a popular technique for stem cell culture, including both pluripotent stem cells and adult stem cells. Development and validation of the first defined hESC media, TeSR1, on a substrate coated with purified human matrix proteins marked a significant progression towards the ultimate goal of obtaining a fully defined microenvironment that preserves the pluripotency of hESCs and hiPSCs [45]. Initial work with TeSR1 demonstrated that hESCs could be grown on a matrix coating consisting of collagen IV, fibronectin, laminin, and vitronectin, an ECM combination that was determined through a screening process. Further studies have demonstrated the ability to maintain pluripotent stem cells on substrates coated with purified fibronectin [46], laminin [47, 48], or vitronectin [21, 49]. Although these ECM-coated surfaces can maintain hESC and iPSC stem cells, not all surfaces coated with these purified ECM components are successful in maintaining the pluripotent stem cell phenotype [21, 50]. This could be explained in part by the conformational changes of ECM proteins on the coated substrates, which have been demonstrated to depend significantly on the underlying substrate and been shown to impact integrin binding interactions [51–55]. Therefore, proteins adsorbed onto different surfaces could express differences in its ability to support pluripotent stem cell culture [49]. Specifically, in the case of pluripotent stem cells, different conformations of Matrigel coated on glass, polystyrene, or tissue culture surfaces have been shown to drastically alter ESC proliferation and differentiation [56]. Similarly, conformational change in osteopontin (Osp) by thrombin-cleavage (tc) results in an inhibitory effect on proliferation and differentiation of human HSCs (CD34+CD38− cells) compared to native Osp coated surfaces, which induce apoptosis in these cells [57]. Interestingly, the effect of tc-Osp is lost in committed hematopoietic progenitor cells (CD34+CD38+).
The reason for the success of these substrates is not clearly understood. However, speculation over integrin binding with matrix molecules [21, 58], and several cell-signaling pathways are suggested to play a role in stem cells retaining their pluripotency. Nevertheless, limited success of culturing pluripotent stem cells on collagen substrates [59], which contain integrin binding domains complementary to receptors found on the surfaces of hESCs, leads to questions as to what specific interactions are necessary to preserve the cell’s ability to self-renew. One interaction to note that is found in hESCs and hiPSCs is integrin α6β1, which binds to different subtypes of the laminin family of ECM proteins [17], and recently has been associated with maintaining stemness of pluripotent stem cells and other stem cells. Furthermore, it has recently been shown that integrin α6 can be used as a marker to identify long-term repopulating human hematopoietic stem cells from other multipotent progenitors within a cord blood sample [60]. Integrin α6 has also been implicated as an important regulator of glioblastoma cancer stem cell self-renewal, proliferation, and tumor formation capacity [17]. This has led to recent successes of laminin coated culture substrates in maintaining the cancer stem cell (CSC) phenotype [61]. Primary brain tumor cells cultured on laminin coated tissue culture flasks led to the isolation of multiple glioma neural stem cell lines, a significant achievement in the field of cancer stem cells. Laminin has also been identified as an important ingredient in the expansion of CSCs from other tissue types as well, including breast cancer where laminin 511 has been linked to enhanced CSC adhesion and growth [62]. With the demonstrated similarities between the transcriptional factors and signaling pathways associated with hESCs and cancer stem cells [63], it is not surprising that similar culture methods for maintaining these stem cells has been achieved. These results indicate ECM coatings have some ability to mimic the stem cell niche, without the integration of stromal cells.
The importance of the elimination of the feeder cells, in terms of contributing to basic understanding of stem cell pluripotency, cannot be overstated simply due to the reduction of variables within the culture system. Despite the successful elimination of the unknowns contributed by the feeder cells, conditioned media, and xenogenic materials through the use of matrix coated substrates, the inherent complexity of these systems continues to mask the mechanism of how these systems work. This has led to the development of newer surfaces through peptide modified hydrogel substrates which can be utilized to examine specific details and interactions between cells and their microenvironment.
Peptide-Conjugated Polymer Substrates
It has been demonstrated that proteins bind to cells through specific oligopeptides, often consisting of only a few amino acid units. This concept was first demonstrated with the discovery that the peptide sequence RGD is responsible for cell attachment to the extracellular matrix protein fibronectin [64]. Since then additional peptide sequences have been discovered within other ECM proteins, such as laminin and collagen, and include: KQAGDV, YGYGDALR, FYFDLR, KRLDGS and LDV [55]. These binding motifs have been found to associate with different integrins on the cell surface, making these peptide sequences a potentially powerful tool to study how integrin binding affects the fundamental stem cell characteristics of self-renewal and differentiation. However, studies using peptide sequences for binding have found that the conformation can drastically alter how the peptides interact with their corresponding integrin [65]. These types of conformational changes that alter cell binding to peptides may also explain some of the discrepancies seen in the protein coatings on the substrates. This concept has been demonstrated in pluripotent stem cell culture through the attachment of a cyclic-RGD peptide to an amine modified tissue culture plate [66]. Coupling of an NHS-PEG- maleimide linker to the surface allowed for the immobilization of cyclic-RGD through a Michael addition reaction. As result, it was shown that the cyclic-RGD surfaces were capable of expanding pluripotent stem cells, while the non-cyclic form of the RGD was not.
Attaching adhesion peptide sequences to other types of substrates has also been explored. Modification of bioinert substrates, such as polyethylene glycol (PEG), with adhesion peptides has been proposed as a method for modifying culture surfaces to create tunable tissue engineering substrates [67]. Attachment of adhesion peptides to bulk hydrogels, or to self-assembled monolayers provided a new method for creating bio-inspired synthetic substrates for pluripotent stem cell expansion. The first example of these types of substrates were acrylate gels modified with peptide sequences from either fibronectin, vitronectin, bone sialoprotein (BSP), or laminin, and were studied in parallel to determine which surface was superior in pluripotent stem cell maintenance [68]. Interestingly, each of the peptide sequences studied contained an RGD sequence motif, however, only the BSP and vitronectin modified acrylate gels were capable of sustaining the stem cell self-renewal, providing further evidence for the importance of the adhering peptide conformation. This study led to the first commercialized biosynthetic substrate, Synthemax™, which promotes the sustained self-renewal and proliferation of human pluripotent stem cells. Other peptide conjugated substrates have also been reported, including those which do not contain the well documented RGD sequence [69]. A notable example is the heparin- binding peptide GKKQRFRHRNRKG, which when bound to the surface using self-assembled monolayers (SAMs), was able to maintain the pluripotency of multiple hES and iPS cell lines for 1–3 months. This peptide is thought to interact with the glycosaminoglycans on the surface of the cells, facilitating adhesion to the substrate and aiding in the maintenance of the stem cell phenotype. The peptide conjugated onto surface was found to have superior performance compared to surfaces with integrin binding RGD sequences by instead interacting with the glycosaminoglycans on the cell surface. Performance of these surfaces could be further enhanced through co-presentation of RGD containing peptides and the glycosaminoglycan binding regime, suggesting that both adhesion and integrin interactions contribute to the success of this type of surface modification. Therefore, while integrin interactions play an important role in pluripotent stem cell maintenance, there may be other signaling pathways involved. Peptide conjugated polymers in defined conditions allow for studying these alternative interactions, demonstrating the power of this technique.
Current trends in stem cell culture have expanded the use of biofunctional hydrogels into 3D culture matrices; with the goal to mimic functional aspects of the extracellular matrix. A 3D polyethylene glycol diacrylate hydrogel conjugated with the heparin-binding peptide WQPPRARI, similar to the SAM surface that was able to expand pluripotent stem cells, was utilized for the proliferation of CD44+/CD24− breast cancer stem cells in vitro [70]. Other peptides, such as an integrin binding peptide or CD44 binding peptide, conjugated to the PEGDA hydrogel led to decreased CD44 expression, indicating a reduction in the CSC population. Similar peptide conjugation strategies that utilize integrin-binding peptides, such as RGD, have also been implemented in 3D hydrogels for culturing HSCs [71]. However, these studies often demonstrate little efficacy in expanding stem cell populations. Current research in tuning the hydrogel mechanics without altering the ligand density [72], as well as creating enzymatically degradable hydrogels, is leading the way towards fabricating synthetic ECM mimics which can study specific interactions of cells and their environment [73]. Peptide-conjugated hydrogels therefore provide the ability to probe whether specific cellular interactions are required or sufficient to promote stem cell self-renewal and expansion, making them a unique tool to study specific interactions in the stem cell microenvironment.
Fully Synthetic Polymer Substrates
While bio-inspired culture platforms provided a means for examining how specific interactions affect stem cell culture, the surfaces are costly and have limited shelf-life. Therefore, the development of a fully synthetic surface for pluripotent stem cell culture could result in affordable culture surfaces for scaled up cell expansion of stem cells in an environment never exposed to undefined biological components[6, 74]. This could lead to widespread use and implementation resulting in consistent and comparable results, unlike the ECM coated surfaces that have shown conflicting reports due to batch-to- batch inconsistency. The first fully synthetic surface for pluripotent stem cell culture, which contains no biological moieties was a zwitterionic hydrogel, with negatively charged sulfate groups and positively charged ammonium groups, called PMEDSAH, short for poly[2-(methacryloyloxy)ethyl dimethyl-(3-sulfopropyl)ammonium hydroxide] [74]. This hydrogel is attached to culture surfaces using surface initiated atom transfer radical polymerization (ATRP), and has been shown to maintain multiple human iPSC [75] and hESC lines[74], for extended number of passages in both chemically-defined medium or medium conditioned by human feeder cells. A recent report of iPS cell lines derived on PMEDSAH, and subsequent maintenance of their pluripotency for over nine months of continuous culture, demonstrates that this platform is a cost effective and consistent alternative to recombinant protein coatings for the derivation and long-term culture of hiPSCs [76]. The mechanism for how these surfaces work is not yet fully understood. However, despite the non-fouling nature of the PMEDSAH surfaces, a possible protein interaction on the surface of the zwitterionic hydrogel has been proposed in which the sulfate groups of PMEDSAH act as a mimic of heparan sulfate [59]. The mechanism proposes that the surface would sequester bFGF from the growth media, effectively concentrating it on the surface and protecting it from degradation, thereby enhancing the performance of the surface in maintaining stem cell pluripotency [59]. The role of PMEDSAH in supporting pluripotent stem cells is refined further in recent work investigating how PMEDSAH properties affect pluripotent stem cell growth and maintenance [77]. The conformational state of the PMEDSAH was demonstrated to play an important role in the growth of pluripotent stem cells. At intermediate hydrogel thicknesses, the hESCs studied show a significantly higher growth rate of undifferentiated colonies when compared to thin or thick coatings of PMEDSAH. It is thought to be due to a combination of different properties including hydrophilicity, surface charge, and the number of inter-chain interactions of the hydrogel brush.
Since the development of PMEDSAH there have been other synthetic hydrogel systems developed for culturing pluripotent stem cells. A poly(aminopropylmethacrylamide) surface has been used to maintain cell pluripotency in the defined medium, mTESR-1 [78]. Analysis of its surface dynamics suggests that adsorption of bovine serum albumin (BSA) from the culture media plays a role in the attachment of hESCs on the culture surface. Advancement of high throughput microarray screening platforms has led to the development of additional synthetic hydrogel substrates capable of supporting pluripotent stem cell self-renewal and pluripotency [79]. Screening of polymer arrays for cell adhesion, proliferation, and differentiation potential capabilities determined that 16 polymers could sustain short-term maintenance of pluripotent stem cells. However, only the poly(methyl vinyl ether-alt-maleic anhydride) polymer was capable of supporting pluripotent stem cell growth over five passages [79].
Despite the successes of synthetic culture surfaces in maintaining pluripotent stem cells, reports of culturing other difficult to culture stem cells, such as cancer stem cells or hematopoietic stem cells, on these types of surfaces are scarce in the current literature. A recent study documenting the importance of laminin and integrin interactions demonstrated that the ability of these surfaces is at least partially due to their ability to support cell secreted laminin [23, 76]. With laminin and integrin α6 being crucial components in different types of stem cells, fully synthetic hydrogel surfaces could potentially provide a culture platform for expanding and maintaining stem cells beyond just pluripotent stem cells.
Future Perspectives
The ability to preserve the phenotype of stem cells in vitro has been a major challenge over the last few decades. This is true for pluripotent stem cells including embryonic stem cells and more recently, induced pluripotent stem cells, as well as some difficult to culture adult stem cells, such as cancer stem cells and hematopoietic stem cells. Expansion and preservation of these rare stem cells is critical for tissue engineering or regeneration, in vitro drug testing, and treatment of many diseases. While each of these stem cells reside in unique microenvironments in the body, their niche components each consist of extracellular matrix, stromal cells, and soluble signals. This has led to similar in vitro culture strategies for the maintenance of each of these stem cell types. Interestingly, techniques, which have been successful in maintaining pluripotent stem cells, have also been found useful in culturing other types of stem cells. These platforms typically utilize specific elements of the niche to recapitulate specific functions or signals thought to be important in maintaining stem cells. This includes implementation of feeder cells, laminin coated substrates, and peptide conjugated hydrogels to engineer microenvironments suitable to human stem cell preservation.
The development of pluripotent stem cell culture platforms has provided great insight into the necessary interactions to preserve the stem cell phenotype, and has been instrumental in developing techniques useful in culturing other types of stem cells. It has been revealed that there are many similarities between the preservation of pluripotent stem cells and other adult stem cells. One such commonality includes signaling through the laminin binding α6 integrin, which is expressed by stem cells ranging from hematopoietic stem cells, cancer stem cells, and pluripotent stem cells [17, 18, 60–62]. This expression has led to many successful culture techniques using laminin-coated surfaces. However, it should be emphasized that the success of these coating technologies relies on maintaining the conformational state of the protein, which has been demonstrated to affect pluripotent stem cells cultured on Matrigel coated surfaces [56]. Therefore, exploring how ECM conformation affects stem cell maintenance may reveal new strategies for their expansion and preservation. Another consideration to be taken into account is the effect of substrate topography and mechanical properties on stemness, with recent reports demonstrating that both roughness and stiffness affect the self-renewal capabilities of hESCs [80–82]. With this in mind, the creation of a 3D extracellular matrix microenvironments engineered into natural conformational states could provide a unique culture environment that could be used to study stem cell interactions with specific matrix components.
Implementation of synthetic hydrogels, often in conjunction with conjugated peptides, has also shown promise in expanding a variety of stem cell populations. With laminin being naturally secreted by embryonic stem cells and cancer stem cells, a culture surface that can properly support the secreted laminin could possibly be able to support stem cells in vitro. For example, synthetic culture surfaces, such as PMEDSAH, which do not directly interact with cell signaling receptors but provide a conductive environment for cells to create their own niche [23], could result in more accurate models of in vivo stem cell niches. Expanding the use of these fully synthetic hydrogel surfaces to other types of stem cells, beyond pluripotent stem cells, will be an important step towards scalable stem cell technologies required for therapeutic uses or in vitro drug testing. In conjunction with the important progress with fully defined media, the work on synthetic substrates will play a critical role in controlling stem cell differentiation and widespread implementation.
Acknowledgments
J.H.J. acknowledges the support of NIH's Microfluidics in Biomedical Sciences Training Program: NIH NIBIB T32 EB005582. L.V.D acknowledges the support of NIH grant R01DE016530-08. We would also like to thank Bradley Plummer for his useful insight into pluripotent stem cell culture strategies.
Footnotes
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
Jacob H. Jordahl, Luis Villa-Diaz, Paul H. Krebsbach, and Joerg Lahann declare that they have no conflict of interest.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Altering bacteriological plastic petri dishes for tissue culture use. Public health reports. 1966;81:843–4. [PMC free article] [PubMed] [Google Scholar]
- 2.Amstein CF, Hartman PA. Adaptation of plastic surfaces for tissue culture by glow discharge. Journal of clinical microbiology. 1975;2:46–54. doi: 10.1128/jcm.2.1.46-54.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maroudas NG. Adhesion and spreading of cells on charged surfaces. J Theor Biol. 1975;49:417–24. doi: 10.1016/0022-5193(75)90182-4. [DOI] [PubMed] [Google Scholar]
- 4.Maroudas NG. Sulphonated polystyrene as an optimal substratum for the adhesion and spreading of mesenchymal cells in monovalent and divalent saline solutions. Journal of cellular physiology. 1977;90:511–9. doi: 10.1002/jcp.1040900314. [DOI] [PubMed] [Google Scholar]
- 5.Reubinoff BE, Pera MF, Fong CY, et al. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- 6.Villa-Diaz LG, Ross AM, Lahann J, et al. Concise Review: The evolution of human pluripotent stem cell culture: From feeder cells to synthetic coatings. Stem Cells. 2013;31:1–7. doi: 10.1002/stem.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 8.Adams GB, Scadden DT. The hematopoietic stem cell in its place. Nat Immunol. 2006;7:333–7. doi: 10.1038/ni1331. [DOI] [PubMed] [Google Scholar]
- 9.Doetsch F. A niche for adult neural stem cells. Curr Opin Genet Dev. 2003;13:543–50. doi: 10.1016/j.gde.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Blanpain C, Fuchs E. Epidermal stem cells of the skin. Annu Rev Cell Dev Biol. 2006;22:339–73. doi: 10.1146/annurev.cellbio.22.010305.104357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Z, de Paiva CS, Luo L, et al. Characterization of putative stem cell phenotype in human limbal epithelia. Stem Cells. 2004;22:355–66. doi: 10.1634/stemcells.22-3-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujimoto K, Beauchamp RD, Whitehead RH. Identification and isolation of candidate human colonic clonogenic cells based on cell surface integrin expression. Gastroenterology. 2002;123:1941–8. doi: 10.1053/gast.2002.37065. [DOI] [PubMed] [Google Scholar]
- 13.Jones PH, Watt FM. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell. 1993;73:713–24. doi: 10.1016/0092-8674(93)90251-k. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki A, Zheng Y, Kondo R, et al. Flow-cytometric separation and enrichment of hepatic progenitor cells in the developing mouse liver. Hepatology. 2000;32:1230–9. doi: 10.1053/jhep.2000.20349. [DOI] [PubMed] [Google Scholar]
- 15.Shinohara T, Avarbock MR, Brinster RL. beta1- and alpha6-integrin are surface markers on mouse spermatogonial stem cells. Proc Natl Acad Sci U S A. 1999;96:5504–9. doi: 10.1073/pnas.96.10.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lathia JD, Patton B, Eckley DM, et al. Patterns of laminins and integrins in the embryonic ventricular zone of the CNS. J Comp Neurol. 2007;505:630–43. doi: 10.1002/cne.21520. [DOI] [PubMed] [Google Scholar]
- 17.Lathia JD, Gallagher J, Heddleston JM, et al. Integrin alpha 6 regulates glioblastoma stem cells. Cell stem cell. 2010;6:421–32. doi: 10.1016/j.stem.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu CH, Inokuma MS, Denham J, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971–4. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 19.Leivo I, Vaheri A, Timpl R, et al. Appearance and distribution of collagens and laminin in the early mouse embryo. Dev Biol. 1980;76:100–14. doi: 10.1016/0012-1606(80)90365-6. [DOI] [PubMed] [Google Scholar]
- 20.Miyazaki T, Futaki S, Hasegawa K, et al. Recombinant human laminin isoforms can support the undifferentiated growth of human embryonic stem cells. Biochem Biophys Res Commun. 2008;375:27–32. doi: 10.1016/j.bbrc.2008.07.111. [DOI] [PubMed] [Google Scholar]
- 21.Braam SR, Zeinstra L, Litjens S, et al. Recombinant vitronectin is a functionally defined substrate that supports human embryonic stem cell self-renewal via alpha V beta 5 integrin. Stem Cells. 2008;26:2257–65. doi: 10.1634/stemcells.2008-0291. [DOI] [PubMed] [Google Scholar]
- 22.Lu J, Hou R, Booth CJ, et al. Defined culture conditions of human embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103:5688–93. doi: 10.1073/pnas.0601383103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23•.Laperle A, Hsiao C, Lampe M, et al. Alpha-5 laminin synthesized by human pluripotent stem cells promotes self-renewal. Stem Cell Reports. 2015;5:195–206. doi: 10.1016/j.stemcr.2015.06.009. These authors examined how endogenously secreted ECM proteins interact with defined synthetic substrates, and how that affects pluripotent stem cell culture. It was also shown that some activity of endogenous ECM can be replaced with exogenous sources. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin GR, Evans MJ. Differentiation of clonal lines of teratocarcinoma cells - formation of embryoid bodies in vitro. P Natl Acad Sci USA. 1975;72:1441–5. doi: 10.1073/pnas.72.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–6. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 26.Martin GR. Isolation of a pluripotent cell-line from early mouse embryos cultured in medium Conditioned by Teratocarcinoma Stem-Cells. P Natl Acad Sci-Biol. 1981;78:7634–8. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bongso A, Fong CY, Ng SC, et al. Isolation and culture of inner cell mass cells from human blastocysts. Hum Reprod. 1994;9:2110–7. doi: 10.1093/oxfordjournals.humrep.a138401. [DOI] [PubMed] [Google Scholar]
- 28.Amit M, Carpenter MK, Inokuma MS, et al. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Developmental Biology. 2000;227:271–8. doi: 10.1006/dbio.2000.9912. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 30.Copelan EA. Medical progress: Hematopoietic stem-cell transplantation. New Engl J Med. 2006;354:1813–26. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 31.Majumdar MK, Thiede MA, Haynesworth SE, et al. Human marrow-derived mesenchymal stem cells (MSCs) express hematopoietic cytokines and support long-term hematopoiesis when differentiated toward stromal and osteogenic lineages. Journal of hematotherapy & stem cell research. 2000;9:841–8. doi: 10.1089/152581600750062264. [DOI] [PubMed] [Google Scholar]
- 32.Flores-Guzman P, Fernandez-Sanchez V, Mayani H. Concise review: ex vivo expansion of cord blood-derived hematopoietic stem and progenitor cells: basic principles, experimental approaches, and impact in regenerative medicine. Stem cells translational medicine. 2013;2:830–8. doi: 10.5966/sctm.2013-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu MJ, Tsuji K, Ueda T, et al. Stimulation of mouse and human primitive hematopoiesis by murine embryonic aorta-gonad-mesonephros-derived stromal cell lines. Blood. 1998;92:2032–40. [PubMed] [Google Scholar]
- 34.Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nature reviews Immunology. 2006;6:93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- 35.Nagasawa T, Omatsu Y, Sugiyama T. Control of hematopoietic stem cells by the bone marrow stromal niche: the role of reticular cells. Trends in immunology. 2011;32:315–20. doi: 10.1016/j.it.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 36.Taichman RS, Emerson SG. Human osteoblasts support hematopoiesis through the production of granulocyte-colony-stimulating factor. J Exp Med. 1994;179:1677–82. doi: 10.1084/jem.179.5.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McNiece I, Harrington J, Turney J, et al. Ex vivo expansion of cord blood mononuclear cells on mesenchymal stem cells. Cytotherapy. 2004;6:311–7. doi: 10.1080/14653240410004871. [DOI] [PubMed] [Google Scholar]
- 38.Kadereit S, Deeds LS, Haynesworth SE, et al. Expansion of LTC-ICs and maintenance of p21 and BCL-2 expression in cord blood CD34(+)/CD38(−) early progenitors cultured over human MSCs as a feeder layer. Stem Cells. 2002;20:573–82. doi: 10.1634/stemcells.20-6-573. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Li CD, Jiang XX, et al. Human placenta-derived mesenchymal progenitor cells support culture expansion of long-term culture-initiating cells from cord blood CD34(+) cells. Exp Hematol. 2004;32:657–64. doi: 10.1016/j.exphem.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 40.Rosler E, Brandt J, Chute J, et al. Cocultivation of umbilical cord blood cells with endothelial cells leads to extensive amplification of competent CD34(+)CD38(−) cells. Exp Hematol. 2000;28:841–52. doi: 10.1016/s0301-472x(00)00177-6. [DOI] [PubMed] [Google Scholar]
- 41.Nolta JA, Thiemann FT, Arakawa-Hoyt J, et al. The AFT024 stromal cell line supports long-term ex vivo maintenance of engrafting multipotent human hematopoietic progenitors. Leukemia. 2002;16:352–61. doi: 10.1038/sj.leu.2402371. [DOI] [PubMed] [Google Scholar]
- 42.de Lima M, McNiece I, Robinson SN, et al. Cord-blood engraftment with ex vivo mesenchymal-cell coculture. New Engl J Med. 2012;367:2305–15. doi: 10.1056/NEJMoa1207285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Richards M, Fong CY, Chan WK, et al. Human feeders support prolonged undifferentiated growth of human inner cell masses and embryonic stem cells. Nat Biotechnol. 2002;20:933–6. doi: 10.1038/nbt726. [DOI] [PubMed] [Google Scholar]
- 44.Hovatta O, Mikkola M, Gertow K, et al. A culture system using human foreskin fibroblasts as feeder cells allows production of human embryonic stem cells. Hum Reprod. 2003;18:1404–9. doi: 10.1093/humrep/deg290. [DOI] [PubMed] [Google Scholar]
- 45.Ludwig TE, Levenstein ME, Jones JM, et al. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol. 2006;24:185–7. doi: 10.1038/nbt1177. [DOI] [PubMed] [Google Scholar]
- 46.Amit M, Shariki C, Margulets V, et al. Feeder layer- and serum-free culture of human embryonic stem cells. Biology of reproduction. 2004;70:837–45. doi: 10.1095/biolreprod.103.021147. [DOI] [PubMed] [Google Scholar]
- 47.Rodin S, Domogatskaya A, Strom S, et al. Long-term self-renewal of human pluripotent stem cells on human recombinant laminin-511. Nat Biotech. 2010;28:611–5. doi: 10.1038/nbt.1620. [DOI] [PubMed] [Google Scholar]
- 48.Rodin S, Antonsson L, Niaudet C, et al. Clonal culturing of human embryonic stem cells on laminin-521/E-cadherin matrix in defined and xeno-free environment. Nature communications. 2014;5:3195. doi: 10.1038/ncomms4195. [DOI] [PubMed] [Google Scholar]
- 49.Saha K, Mei Y, Reisterer CM, et al. Surface-engineered substrates for improved human pluripotent stem cell culture under fully defined conditions. Proc Natl Acad Sci U S A. 2011;108:18714–9. doi: 10.1073/pnas.1114854108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hakala H, Rajala K, Ojala M, et al. Comparison of biomaterials and extracellular matrices as a culture platform for multiple, independently derived human embryonic stem cell lines. Tissue engineering Part A. 2009;15:1775–85. doi: 10.1089/ten.tea.2008.0316. [DOI] [PubMed] [Google Scholar]
- 51.Michael KE, Vernekar VN, Keselowsky BG, et al. Adsorption-induced conformational changes in fibronectin due to interactions with well-defined surface chemistries. Langmuir. 2003;19:8033–40. [Google Scholar]
- 52.Katz BZ, Zamir E, Bershadsky A, et al. Physical state of the extracellular matrix regulates the structure and molecular composition of cell-matrix adhesions. Mol Biol Cell. 2000;11:1047–60. doi: 10.1091/mbc.11.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garcia AJ, Vega MD, Boettiger D. Modulation of cell proliferation and differentiation through substrate-dependent changes in fibronectin conformation. Mol Biol Cell. 1999;10:785–98. doi: 10.1091/mbc.10.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hernandez JCR, Sanchez MS, Soria JM, et al. Substrate chemistry-dependent conformations of single laminin molecules on polymer surfaces are revealed by the phase signal of atomic force microscopy. Biophys J. 2007;93:202–7. doi: 10.1529/biophysj.106.102491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Bi. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 56.Kohen NT, Little LE, Healy KE. Characterization of Matrigel interfaces during defined human embryonic stem cell culture. Biointerphases. 2009;4:69–79. doi: 10.1116/1.3274061. [DOI] [PubMed] [Google Scholar]
- 57.Nilsson SK, Johnston HM, Whitty GA, et al. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood. 2005;106:1232–9. doi: 10.1182/blood-2004-11-4422. [DOI] [PubMed] [Google Scholar]
- 58.Rowland TJ, Miller LM, Blaschke AJ, et al. Roles of integrins in human induced pluripotent stem cell growth on Matrigel and vitronectin. Stem Cells Dev. 2010;19:1231–40. doi: 10.1089/scd.2009.0328. [DOI] [PubMed] [Google Scholar]
- 59.Lambshead JW, Meagher L, O'Brien C, et al. Defining synthetic surfaces for human pluripotent stem cell culture. Cell Regen (Lond) 2013;2:7. doi: 10.1186/2045-9769-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Notta F, Doulatov S, Laurenti E, et al. Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science. 2011;333:218–21. doi: 10.1126/science.1201219. [DOI] [PubMed] [Google Scholar]
- 61.Pollard SM, Yoshikawa K, Clarke ID, et al. Glioma stem cell lines expanded in adherent culture have tumor-specific phenotypes and are suitable for chemical and genetic screens. Cell stem cell. 2009;4:568–80. doi: 10.1016/j.stem.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 62•.Chang C, Goel HL, Gao H, et al. A laminin 511 matrix is regulated by TAZ and functions as the ligand for the alpha6Bbeta1 integrin to sustain breast cancer stem cells. Genes & development. 2015;29:1–6. doi: 10.1101/gad.253682.114. One of the first papers that highlighted the importance of ECM proteins in 2D breast cancer stem cell culture. This study shows that laminin-511 and its interaction with integrin α6 is critical for maintaining cancer stem cells. It also demonstrates that laminin-511 binding activates TAZ, and TAZ activation regulates the expression of laminin-511, indicating laminin-511 is a critical niche component for breast cancer stem cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ben-Porath I, Thomson MW, Carey VJ, et al. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nature genetics. 2008;40:499–507. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pierschbacher MD, Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984;309:30–3. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- 65.Pfaff M, Tangemann K, Muller B, et al. Selective recognition of cyclic RGD peptides of NMR defined conformation by alpha IIb beta 3, alpha V beta 3, and alpha 5 beta 1 integrins. The Journal of biological chemistry. 1994;269:20233–8. [PubMed] [Google Scholar]
- 66.Kolhar P, Kotamraju VR, Hikita ST, et al. Synthetic surfaces for human embryonic stem cell culture. J Biotechnol. 2010;146:143–6. doi: 10.1016/j.jbiotec.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 67.Hern DL, Hubbell JA. Incorporation of adhesion peptides into nonadhesive hydrogels useful for tissue resurfacing. J Biomed Mater Res. 1998;39:266–76. doi: 10.1002/(sici)1097-4636(199802)39:2<266::aid-jbm14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 68.Melkoumian Z, Weber JL, Weber DM, et al. Synthetic peptide-acrylate surfaces for long-term self-renewal and cardiomyocyte differentiation of human embryonic stem cells. Nat Biotechnol. 2010;28 doi: 10.1038/nbt.1629. 606-U95. [DOI] [PubMed] [Google Scholar]
- 69.Klim JR, Li LY, Wrighton PJ, et al. A defined glycosaminoglycan-binding substratum for human pluripotent stem cells. Nat Methods. 2010;7 doi: 10.1038/nmeth.1532. 989-U72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang X, Sarvestani SK, Moeinzadeh S, et al. Effect of CD44 binding peptide conjugated to an engineered inert matrix on maintenance of breast cancer stem cells and tumorsphere formation. PloS one. 2013;8:e59147. doi: 10.1371/journal.pone.0059147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Raic A, Rodling L, Kalbacher H, et al. Biomimetic macroporous PEG hydrogels as 3D scaffolds for the multiplication of human hematopoietic stem and progenitor cells. Biomaterials. 2014;35:929–40. doi: 10.1016/j.biomaterials.2013.10.038. [DOI] [PubMed] [Google Scholar]
- 72.Schweller RM, West JL. Encoding hydrogel mechanics via network cross-linking structure. ACS biomaterials science & engineering. 2015;1:335–44. doi: 10.1021/acsbiomaterials.5b00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tibbitt MW, Anseth KS. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng. 2009;103:655–63. doi: 10.1002/bit.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Villa-Diaz LG, Nandivada H, Ding J, et al. Synthetic polymer coatings for long-term growth of human embryonic stem cells. Nat Biotechnol. 2010;28:581–3. doi: 10.1038/nbt.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Villa-Diaz LG, Brown SE, Liu Y, et al. Derivation of mesenchymal stem cells from human induced pluripotent stem cells cultured on synthetic substrates. Stem Cells. 2012;30:1174–81. doi: 10.1002/stem.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76•.Villa-Diaz LG, Kim JK, Lahann I, et al. Derivation and Long-Term Culture of Transgene-Free Human Induced Pluripotent Stem Cells on Synthetic Substrates. Stem cells translational medicine. 2014;3:1410–1417. doi: 10.5966/sctm.2014-0087. This work demonstrated for the first time that human iPSCs can be derived and cultured for long periods of time on synthetic polymer coatings in a xeno-free medium, nearing clinically relevant conditions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77•.Qian X, Villa-Diaz LG, Kumar R, et al. Enhancement of the propagation of human embryonic stem cells by modifications in the gel architecture of PMEDSAH polymer coatings. Biomaterials. 2014;35:9581–9590. doi: 10.1016/j.biomaterials.2014.08.015. This work showed how modifcations in the gel architecture of the polymers can impact the biology of stem cells, in this case by promoting large scale expansion of hESCs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Irwin EE, Gupta R, Dashti DC, et al. Engineered polymer-media interfaces for the long-term self-renewal of human embryonic stem cells. Biomaterials. 2011;32:6912–9. doi: 10.1016/j.biomaterials.2011.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brafman DA, Chang CW, Fernandez A, et al. Long-term human pluripotent stem cell self-renewal on synthetic polymer surfaces. Biomaterials. 2010;31:9135–44. doi: 10.1016/j.biomaterials.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen W, Villa-Diaz LG, Sun Y, et al. Nanotopography influences adhesion, spreading, and self-renewal of human embryonic stem cells. ACS Nano. 2012;6:4094–103. doi: 10.1021/nn3004923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sun Y, Villa-Diaz LG, Lam RH, et al. Mechanics regulates fate decisions of human embryonic stem cells. PloS one. 2012;7:e37178. doi: 10.1371/journal.pone.0037178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sun Y, Yong KM, Villa-Diaz LG, et al. Hippo/YAP-mediated rigidity-dependent motor neuron differentiation of human pluripotent stem cells. Nature materials. 2014;13:599–604. doi: 10.1038/nmat3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang JF, Wang LJ, Wu YF, et al. Mesenchymal stem/progenitor cells in human umbilical cord blood as support for ex vivo expansion of CD34(+) hematopoietic stem cells and for chondrogenic differentiation. Haematologica. 2004;89:837–44. [PubMed] [Google Scholar]
- 84.Teixidó J, Hemler ME, Greenberger JS, et al. Role of beta 1 and beta 2 integrins in the adhesion of human CD34hi stem cells to bone marrow stroma. Journal of Clinical Investigation. 1992;90:358–67. doi: 10.1172/JCI115870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Giannoni E, Bianchini F, Masieri L, et al. Reciprocal activation of prostate cancer cells and cancer-associated fibroblasts stimulates epithelial-mesenchymal transition and cancer stemness. Cancer research. 2010;70:6945–56. doi: 10.1158/0008-5472.CAN-10-0785. [DOI] [PubMed] [Google Scholar]
- 86.Vermeulen L, De Sousa E, Melo F, van der Heijden M, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12:468–76. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- 87.Liu S, Ginestier C, Ou SJ, et al. Breast cancer stem cells are regulated by mesenchymal stem cells through cytokine networks. Cancer research. 2011;71:614–24. doi: 10.1158/0008-5472.CAN-10-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li Z, Leung M, Hopper R, et al. Feeder-free self-renewal of human embryonic stem cells in 3D porous natural polymer scaffolds. Biomaterials. 2010;31:404–12. doi: 10.1016/j.biomaterials.2009.09.070. [DOI] [PubMed] [Google Scholar]
- 89.Rowland TJ, Miller LM, Blaschke AJ, et al. Roles of integrins in human induced pluripotent stem cell growth on Matrigel and vitronectin. Stem cells and development. 2010;19:1231–40. doi: 10.1089/scd.2009.0328. [DOI] [PubMed] [Google Scholar]
- 90.Sharma MB, Limaye LS, Kale VP. Mimicking the functional hematopoietic stem cell niche in vitro: recapitulation of marrow physiology by hydrogel-based three-dimensional cultures of mesenchymal stromal cells. Haematologica. 2012;97:651–60. doi: 10.3324/haematol.2011.050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ferreira MS, Jahnen-Dechent W, Labude N, et al. Cord blood-hematopoietic stem cell expansion in 3D fibrin scaffolds with stromal support. Biomaterials. 2012;33:6987–97. doi: 10.1016/j.biomaterials.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 92.Feng Q, Chai C, Jiang XS, et al. Expansion of engrafting human hematopoietic stem/progenitor cells in three-dimensional scaffolds with surface-immobilized fibronectin. Journal of biomedical materials research Part A. 2006;78:781–91. doi: 10.1002/jbm.a.30829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu J, Tan Y, Zhang H, et al. Soft fibrin gels promote selection and growth of tumorigenic cells. Nature materials. 2012;11:734–41. doi: 10.1038/nmat3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen L, Xiao Z, Meng Y, et al. The enhancement of cancer stem cell properties of MCF-7 cells in 3D collagen scaffolds for modeling of cancer and anti-cancer drugs. Biomaterials. 2012;33:1437–44. doi: 10.1016/j.biomaterials.2011.10.056. [DOI] [PubMed] [Google Scholar]
- 95.Zhang W, Choi DS, Nguyen YH, et al. Studying cancer stem cell dynamics on PDMS surfaces for microfluidics device design. Scientific reports. 2013;3:2332. doi: 10.1038/srep02332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Feng S, Duan X, Lo PK, et al. Expansion of breast cancer stem cells with fibrous scaffolds. Integrative biology : quantitative biosciences from nano to macro. 2013;5:768–77. doi: 10.1039/c3ib20255k. [DOI] [PubMed] [Google Scholar]
- 97.Park HJ, Yang K, Kim MJ, et al. Bio-inspired oligovitronectin-grafted surface for enhanced self-renewal and long-term maintenance of human pluripotent stem cells under feeder-free conditions. Biomaterials. 2015;50:127–39. doi: 10.1016/j.biomaterials.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 98.Muth CA, Steinl C, Klein G, et al. Regulation of hematopoietic stem cell behavior by the nanostructured presentation of extracellular matrix components. PloS one. 2013;8:e54778. doi: 10.1371/journal.pone.0054778. [DOI] [PMC free article] [PubMed] [Google Scholar]